Abstract
The Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network originated over 20 years ago to foster research to optimize the care of critically ill infants and children. Over this period, PALISI has seen two major evolutions: formalization of our network infrastructure and a broadening of our clinical research focus. First, the network is unique in that its activities and meetings are funded by subscriptions from members who now comprise a multidisciplinary group of investigators from over 90 PICUs all over the United States (US) and Canada, with collaborations across the globe. In 2020, the network converted into a standalone, nonprofit organizational structure (501c3), making the PALISI Network formally independent of academic and clinical institutions or professional societies. Such an approach allows us to invest in infrastructure and future initiatives with broader opportunities for fund raising. Second, our research investigations have expanded beyond the original focus on sepsis and acute lung injury, to incorporate the whole field of pediatric critical care, for example, efficient liberation from mechanical ventilator support, prudent use of blood products, improved safety of intubation practices, optimal sedation practices and glucose control, and pandemic research on influenza and COVID-19. Our network approach in each field follows, where necessary, the full spectrum of clinical and translational research, including: immunobiology studies for understanding basic pathologic mechanisms; surveys to explore contemporary clinical practice; consensus conferences to establish agreement about literature evidence; observational prevalence and incidence studies to measure scale of a clinical issue or question; case control studies as preliminary best evidence for design of definitive prospective studies; and, randomized controlled trials for informing clinical care. As a research network, PALISI and its related subgroups have published over 350 peer-reviewed publications from 2002 through September 2022.
THE PALISI NETWORK
The term “Evidence-Based Medicine” was first coined in 1991 (1) and opened up a discipline that was immediately relevant to rigorous scientific evidence for ICU practice. As such, ICU-focused research networks in the United States, Canada, Europe, Australia, and other countries published results of pivotal randomized controlled trials (RCTs) in critically ill adults. However, at that time, pediatric critical care was a young field (2), and RCTs were rarely conducted in the PICU; even when they were, the validity of their findings were often questioned because of small sample size and insufficient power (3). By 1998, there was a Boston Children’s Hospital-led RCT evaluating three methods of weaning children from mechanical ventilator support across 10 centers (4) called the Pediatric Acute Lung Injury and Sepsis Investigator’s (PALISI) Network. This collaborative expanded, holding its first meeting in 2002 in Stowe, Vermont. The meeting brought together the leadership and participants of three additional ongoing RCTs about use of Calfactant for acute hypoxemic respiratory failure (5), prone positioning in acute respiratory distress syndrome (ARDS) (6), and liberal versus restrictive red blood cell transfusion thresholds in the critically ill (7). The PALISI Network had achieved its goal of increasing the highest quality evidence supporting pediatric critical care medicine (PCCM); as shown in Table 1, by 2021, the network had published 12 RCTs (4–15).
TABLE 1.
Randomized Controlled Trials in Critically Ill Children From the Pediatric Acute Lung Injury and Sepsis Investigator’s Network
| Year (Reference no.) | Countries | Study Interventions (Study Name) | Patients (Sites) | Funding |
|---|---|---|---|---|
| 2002 (4) | United States | Comparison of three mechanical ventilation weaning methods: pressure support, volume support, and usual care | 182 (10) | Foundation and Industrya |
| 2005 (5) | United States | Endotracheal calfactant versus placebo for acute lung injury | 153 (21) | Industry |
| 2005 (6) | United States | Prone positioning for pediatric acute lung injury | 102 (7) | NIH |
| 2007 (7) | United States, Canada, and UK Belgium | Noninferiority trial of transfusion hemoglobin threshold of 7 versus 9.5 mg/dL (Transfusion Requirements in the Pediatric Intensive Care Unit Study) | 637 (19) | CIHR |
| 2012 (11) | United States, Chile | Lucinactant for acute hypoxemic respiratory failure in children < 2 years of age | 165 (36) | Industry |
| 2013 (13) | United States, Canada, Israel Australia, New Zealand, South Korea | Endotracheal calfactant versus placebo for acute lung injury | 110 (24) | Industry |
| 2015 (8) | United States | Cluster randomized trial of sedation protocols in children with acute respiratory failure | 2449 (31) | NIH |
| 2017 (12) | United States | Targets for managing critical illness-related hyperglycemia: glucose 80–110 vs 140–180 mg/dL (Heart and Lung Failure-Pediatric INsulin Titration Trial) | 713 (35) | NIH and Industrya |
| 2018 (10) | United States | Calfactant for acute lung injury in pediatric stem cell and oncology patients | 43 (17) | U. S. Food and Drug Administration and Industrya |
| 2019 (9) | United States, Canada, France | Comparison of fresh versus standard issue packed red blood cells on multiple organ dysfunction syndrome (Age of Blood in Children in Pediatric Intensive Care Units study) | 1538 (50) | CIHR, NIH, and Programme Hospitalier Recherche Clinique (France) |
| 2019 (14) | United States | Continuous vs bolus gastric feeds in mechanically ventilated children in the PICU | 158 (7) | Foundation |
| 2021 (15) | United States | Central venous catheter-related early thrombosis with enoxaparin in critically ill children | 51 (7) | NIH |
CIHR = Canadian Institutes of Health Research, NIH = National Institutes of Health.
Industry provided devices and/or therapeutics but did not fund patient enrollment.
In this Special Article for PCCM, we describe the PALISI Network perspective and approach to developing a multicenter clinical research collaboration. In particular, our two main evolutions are as follows: development of our infrastructure, and an expanded scope of our research work.
PALISI ORGANIZATION AND STRUCTURE
PALISI was largely modeled on the Canadian Critical Care Trials Group, which is an investigator-led clinical trials group with multiple programs of research (https://www.ccctg.ca). Membership of the PALISI Network is voluntary and, since its inception, nonrestrictive with the goal of being interdisciplinary, and inclusive of physicians, nurse scientists, respiratory therapists, pharmacists, and research coordinators. Our pediatric subspecialties include pediatric critical care, infectious disease, hematology, oncology, immunology, and pharmacology, among others.
The PALISI Network is maintained by volunteers. Our leaders are elected by members to serve in roles such as Chair, Vice-Chair, Treasurer, Executive Committee member, or Scientific-Steering Committee member. From its inception, we have also required that entrance to PALISI semiannual meetings and general membership would need a subscription—either from a site or by an individual. These funds are used to pay for meeting expenses and a part-time administrative director. Regarding our semiannual meetings (which included virtual meetings during the 2020 pandemic and now hybrid meetings), they are designed to provide opportunities for investigators to present research ideas, discuss more developed research protocols, and obtain supportive feedback from colleagues. To further foster collaboration between investigators and centers, our meetings are also structured around social activities (e.g., skiing, sightseeing, and dining) that promote informal dialogue, comradery, and networking. Overall, our aim is to engender collaboration between experts in multiple clinical research domains, sharing of preliminary data to optimize funding success, creation of common data repositories, and develop standard protocols for patient care and outcome assessment.
In early 2020, the PALISI Network elected to convert from an ad hoc, academic-institution-related group, to become a standalone US Internal Revenue Code 501(c) (3), nonprofit organization that is formally separated from institutional links. Such an approach—deemed “charitable status” in other countries—now allows the PALISI Network organization the ability to invest in its infrastructure and future initiatives in the manner that best suits the network. Furthermore, philanthropy has now become an option to further expand funding opportunities.
PALISI RESEARCH APPROACH
Initially, the focus of PALISI Network studies was on children suffering from life-threatening inflammatory disorders such as acute lung injury or ARDS, sepsis, bronchiolitis, and multiple organ failure. From its inception, PALISI had multiple investigative goals including promoting collaborative clinical research, defining optimal supportive care, preventing disease-associated complications, identifying therapies to shorten the course and severity of illness, and optimizing patient comfort.
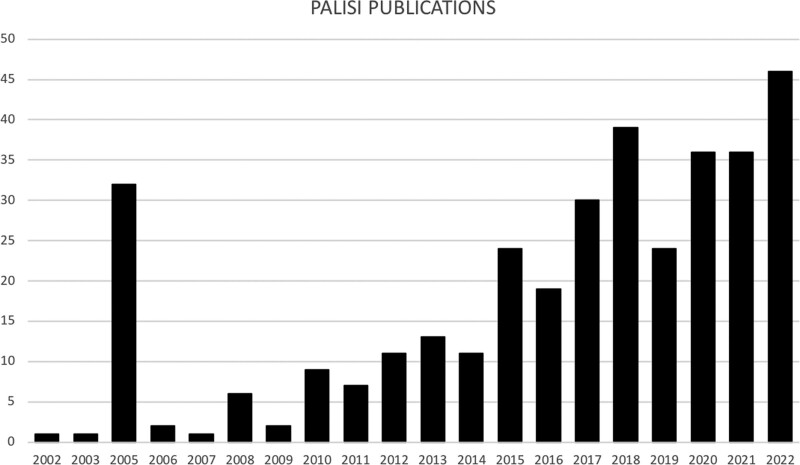
Regarding subject matter or focus, the 47 attendees at the 2002 inaugural meeting were formed into eight different “working groups.” Over subsequent meetings, more special interests were proposed and adopted by PALISI, and working groups became formal “subgroups.” Now, we have expanded our interests into all areas of pediatric critical care. In our research, we now have a tried and repeatedly tested network approach, in which the full spectrum in clinical investigation can be examined, including: 1) immunobiology studies for understanding basic pathologic mechanisms, 2) surveys of contemporary clinical practice to identify what clinicians are doing, and 3) consensus conferences to establish what our thought leaders agree about literature evidence, for example, observational and retrospective studies, systematic reviews, and guideline statements and recommendations; observational prevalence and incidence studies to measure scale of a clinical issue or question; case control studies as preliminary best evidence for design of definitive prospective studies; and, ultimately, RCTs for informing clinical care. Of course, wider communication and publication is an important and necessary output of all this work. In addition, from 2002 to 2022, articles published on behalf of PALISI as a “Corporate-Author” include 140 publications with 65 (46%) in PCCM, and when expanded to include formally recognized PALISI subgroups, this number respectively rises to 352 and 144 (41%). Publications have steadily risen over time (Fig. 1). In the subsections below, we provide examples of the methodologies used in the PALISI Network approach, in which comprehensive literature reviews, data synthesis, and preliminary research studies lead on to further definitive work, providing evidence to support clinical practice.
Figure 1.
Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) network publications published by year from November 27, 2002, to September 20, 2022.
Scoping Reviews and Consensus Meetings
When a clinical problem is insufficiently defined or the research priorities have not been fully considered and debated, scoping reviews and consensus conferences can aid in lending clarity to what is known and what future research priorities should be. In the last 3 years, PALISI has focused on aspects of hemopoietic stem-cell transplantation (HCT) (16–18), transfusion and bleeding (19, 20), measuring outcomes (21–23), and clinical decision support (24). Table 2 shows other examples of consensus conferences and their links to prior or future PALISI Network studies.
TABLE 2.
Pediatric Acute Lung Injury and Sepsis Investigators Consensus Conference Topics, Publications and Potential Impact
| Years | Consensus Conference | Topics Covered | Journal |
|---|---|---|---|
| 2005 | International Sepsis Forum on Sepsis in Infants and Children | Definitions of sepsis, organ dysfunction, and specific infections in critically ill children focusing on specific populations and implications for future studies | Pediatr Crit Care Med May 2005; Supplement 3 |
| 2012–2014 (updating 2020–2022) | Pediatric Acute Lung Injury Consensus Conference | Definition of pediatric acute respiratory distress syndrome and Grading of Recommendations, Assessment, Development, and Evaluation method recommendations for its management | Pediatr Crit Care Med June 2015; 16:428–439 and Supplement |
| 2014–2017 | Transfusion and Anemia Expertise Initiative | Decision-making for RBC transfusion management and research priorities for transfusion in critically ill children including in nine specific populations | Pediatr Crit Care Med Sept 2018; 19:884–898 and Supplement |
| 2016–2017, and 2019 | Hepatic veno-occlusive disease after haemopoietic stem-cell transplantation | Diagnosis, grading, and treatment recommendations for children, adolescents, and young adults with sinusoidal obstructive syndrome | Biol Blood Marrow Transplant Nov/Dec 2017 and Feb 2018 |
| Lancet Haematol 2020; 7:e61–e72 | |||
| 2018 | CAR-T-cell therapy in pediatric oncology patients | Management guidelines for pediatric patients receiving CAR-T therapy | Nat Rev Clin Oncol 2019; 16:45–63 |
| 2017–2021 | Pediatric Organ Dysfunction Information Update Mandate | Single-organ dysfunction definitions for neurologic, cardiovascular, respiratory, gastrointestinal, acute liver, renal, hematologic, coagulation, endocrine, endothelial, and immune system dysfunction | Pediatrics 2022 January Supplement |
| 2018–2020 | PICU Research COS | Development of a core outcomes set for research and clinical programs for children in the PICU | Crit Care Med 2020; 48:1819–1828 |
| 2019–2022 | Pediatric ECMO Anticoagulation Collaborative | Develop consensus statements for management of pediatric patients receiving ECMO support | Ongoing, funded by National Institutes of Health R13HD104432 |
| 2019–2022 | Ventilator Liberation | Practice guidelines for liberating children from mechanical ventilator support | Am J Respir Crit Care Med 2022; Aug |
| 2020–2022 | Core Outcomes Measurement Set (PICU Core Outcomes Measurement Set | The core outcomes measurement set is to measure specific outcomes in the PICU COS | Pediatr Crit Care Med Aug 2022; 19:884–898 and Supplement |
| 2020–2022 | Transfusion and Anemia EXpertise Initiative-Control/Avoidance of Bleeding | Plasma and platelet transfusion strategies in critically Ill children following severe trauma, traumatic brain injury, and/or intracranial hemorrhage | Pediatr Crit Care Med Sept 2018; 19:884–898, Jan 2022; 23:34–51, and Supplement |
CAR-T = chimeric antigen receptor T cell, COS = core outcome set, ECMO = extracorporeal membrane oxygenation, TAXI = Transfusion Anemia Expertise Initiative.
Observational Prevalence and Incidence Studies
When there are no preliminary or pilot data, generating contemporary observational data is an important next step in developing information for a proposal for an RCT. The network has carried out a number of population-based prevalence and incidence studies across a range of topics (Table 3). Among others, these have included sepsis, pediatric ARDS, hemoglobin and transfusion practices, and rehabilitation of children after critical illness. The early studies followed the true point-prevalence methodology, but expansion of these to include collection of longer-term outcome data and the study of incidence in place of prevalence had expanded this low-cost methodology of the collection of data from a large number of children and sites. The network has published a large number of these types of studies (Table 3), and the generation of these larger data sets have also led to multiple secondary publications, many led by junior investigators first learning the field of multicenter clinical research. PALISI has also described the successes and pitfalls of the use of point-prevalence methodology (25).
TABLE 3.
Pediatric Acute Lung Injury and Sepsis Investigators Observational Point Prevalence and Incidence Studies by Publication Year
| Year | Title | Subject | Acronym | Countries | Sites | Subjects | Journal |
|---|---|---|---|---|---|---|---|
| 2010 | Acute lung injury in children: Therapeutic practice and feasibility of international clinical trials | Acute lung injury | PALIVE | 12 | 59 | 165 | Pediatr Crit Care Med, Nov, 2010 |
| 2103 | A National Emergency Airway Registry for children | Tracheal intubation | NEAR4KIDS | 1 | 15 | 1,715 | Crit Care Med, Mar, 2013 |
| 2014 | A multinational study of thromboprophylaxis practice in critically ill children | Thromboprophylaxis | PROTRACT | 7 | 59 | 2,159 | Crit Care Med, May, 2014 |
| 2015 | Global epidemiology of pediatric severe sepsis: The Sepsis PRevalence, Outcomes and Therapies study | Sepsis | SPROUT | 26 | 128 | 569 | Am J Respir Crit Care Med, May, 2015 |
| 2015 | Indications and Effects of Plasma Transfusions in Critically Ill Children | Plasma transfusions | PlasmaTV | 21 | 101 | 443 | Am J Respir Crit Care Med, Jun, 2015 |
| 2017 | Pediatric Ventilator-Associated Infections: The Ventilator-Associated INfection Study | Ventilator-associated infections | VAIN | 3 | 47 | 229 | Pediatr Crit Care Med, Jan, 2017 |
| 2017 | The Prevalence of Acute critical Neurological Disease in Children: A Global Epidemiological Assessment Study | Neurologic insults | PANGEA | 23 | 107 | 924 | Pediatr Crit Care Med, Jan, 2017 |
| 2018 | Platelet Transfusion Practices in Critically Ill Children | Platelet transfusion | P3T | 16 | 82 | 559 | Crit Care Med, Aug, 2018 |
| 2019 | Paediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology | Pediatric acute respiratory distress syndrome | PARDIE | 27 | 145 | 744 | Lancet Resp Med, Feb, 2019 |
| 2020 | Physical Rehabilitation in Critically ill Children: A Multicenter Point Prevalence Study in the United States | Rehabilitation Acceleration | PARK-PICU | 1 | 82 | 1,769 | Crit Care Med, May, 2020 |
PALISI SUBGROUPS AND WORKING GROUPS
PALISI currently has 16 active official subgroups, and seven special interest groups (SIGs) centered around a specific research focus. Subgroups and SIGs are semiautonomous, but meet formally at PALISI to review ongoing research studies and to design future research, combined with additional virtual meetings. These groups are designed to be responsive to contemporary clinical questions that emerge out of a need to know what to do, including addressing emerging pandemic pathogens. Many of the groups have developed their own websites (Fig. 2), which are used to facilitate group communication, to educate the public about the group’s work, and to disseminate evidence. These subgroups have, in part, facilitated expansion of the network focus to other areas of clinical research. In the following five subsections, we provide some of the areas of this development, and lessons learned by the network.
Figure 2.
Examples of Pediatric Acute Lung Injury and Sepsis Investigators (PALISI)-related websites used to facilitate communication and disseminate evidence, including those of selected PALISI subgroups, special interest groups, and clinical studies. PROSpect = pediatric acute respiratory distress syndrome.
Patient Safety and Quality of Care—Using Epidemiology
At present, PALISI does not have a formal “Quality and Safety subgroup.” However, that has not stopped the development of a working group, which recently became an officially endorsed subgroup focused on the safety and quality of endotracheal intubations (the National Emergency Airway Registry for kids [NEAR4KIDS]) in children. The group has been publishing since 2012, predominantly using an epidemiologic approach to data gathering with the purpose of identifying gaps in practice, service delivery, and training. For example, the NEAR4KIDS program of research has identified high variability across PALISI centers in tracheal intubation practice and adverse events (26). A key learning point here is that epidemiology has led to the identification of a problem and the need to develop a systems-approach to solving that problem. Most recently, the program developed a tracheal intubation intervention bundle with the purpose of improving care in PICUs and emergency departments initially across the United States and Canada (https://www.research.chop.edu/near4kids). Examples of other PALISI topics focused on improving the quality of care of children in the PICU including ventilator-associated infections (27), nutrition delivery methods (14), and antibiotic stewardship (28).
Measuring Longer Term Clinical Outcomes in Patients Enrolled in Trials
Optimizing supportive care in critically ill children is a major goal of PALISI members. The randomized evaluation of sedation titration for respiratory failure (RESTORE) study evaluated the safety and effectiveness of a nurse-led sedation protocol on duration of mechanical ventilation, sedative and opioid use, and patient comfort (8). This cluster RCT carried out in 2009–2013, across 31 PICUs, with 2,449 patients, failed to show a difference between groups in its primary end point of ventilator-free days. However, the intervention was associated with a different sedation experience; specifically, patients were safely managed in a more awake and calm state while intubated receiving fewer days of opioid exposure and sedative classes. In addition, the dataset (available at https://biolincc.nhlbi.nih.gov/studies/restore/) and children enrolled in the RESTORE trial have proved to be an invaluable resource for understanding the late cognitive, functional, and health-related quality-of-life outcomes after critical illness in childhood (29). This framework included a website (http://afterpicu.com) for patients and families. One of the many learning points from RESTORE is that evaluation of longer term clinical outcomes can be built into the design of an RCT.
Transfusion and Use of Blood Products—Organizational Cross-Discipline Interaction
The pediatric critical care blood research network (BloodNet; http://bloodnetresearch.org) has become an active and productive subgroup of PALISI. This group is mainly comprised of hematologists, transfusion medicine, and pediatric critical care specialists. To date, the BloodNet investigators have conducted a range of studies and outputs for the field, including surveys (30, 31), point-prevalence studies (32), prospective observational studies (33, 34), RCTs (15), development of assessment scales for bleeding (19), and consensus conferences in the areas of transfusion medicine (20, 35). The key lesson learned here is that there are some areas in pediatric critical care in which research development requires cross-discipline interactions and working together, and the transfusion and blood product subgroup is an exemplar of this approach.
Critical Illness After Hematopoietic Stem Cell Transplant—Institution Cross-Discipline Membership
Children who become critically ill after receiving hematopoietic stem cell transplant or other cell-based therapies have very high mortality in the PICU. The PALISI HCT-Cancer Immunotherapy Subgroup focuses on improving outcomes in this vulnerable population. This subgroup has conducted surveys (36), observational studies about management (37), and recovery (38), and produced a consensus-based approach to use of extracorporeal membrane oxygenation (ECMO) (18). A key lesson learned here is that each center involved in this subgroup provides both a stem cell transplant expert and a pediatric intensivist who contribute to research questions and study development.
Pandemic Preparedness—A Public Health Responsive Framework and Infrastructure
Acute lung injury from influenza virus infection has been a focus of PALISI since 2008. The Pediatric Intensive Care Influenza (PICFLU) subnetwork of over 35 sites (https://picflu.org) enrolled children during the 2009 influenza pandemic (39). PICFLU has now been funded by the U.S. Centers for Disease Control and Prevention (CDC), the National Institutes of Health (NIH), and the National Institute of Allergy and Infectious Diseases, with a focus on host genetics and disease severity, influenza vaccine effectiveness in preventing severe illness, the role of bacterial coinfection, and the identification of biomarkers associated with ARDS and septic shock. A key lesson that we have learnt is that on creating an infrastructure such as the PALISI PICFLU Emerging Pathogens (PICFLU-EP) group, with the ability to perform real-time surveillance, we are able to be responsive to emergent national, public health need if a novel influenza virus or other severe respiratory pathogen emerges. For example, the PICFLU-EP was recently triggered by the CDC to conduct multiple public health investigations on the effect of the severe acute respiratory syndrome coronavirus 2 pandemic causing COVID-19. Rebranded as Overcoming COVID-19 (https://overcomecovid.org) and expanding to 65 pediatric sites across the United States, long-term preparedness allowed rapid study of a newly emerged life-threatening postinfectious complication called Multisystem Inflammatory Syndrome in Children (40).
Other PALISI Subgroups and Special Interest Groups (https://www.palisi.org/subgroups)
Expanding the focus of PALISI across the span of critical care medicine, additional PALISI subgroups and interest groups not shown in Figure 1 include Pediatric ECMO, Social Determinants of Health, Chronic Critical Illness and Long-Term Ventilation, Pediatric Respiratory and Mechanical Ventilation, ExCelLInce in Pediatric Implementation SciencE (ECLIPSE), Bronchiolitis and Codetection, pediatric nutrition, and PEdiatric Research Collaborative on Critical Ultrasound. Examples of additional interest groups focused on specific studies that meet separately during the PALISI meeting and report interim progress to the overall group including the Stress Hydrocortisone in Septic Shock (RCT NCT03401398) and PediAtric ReseArch of Drugs, Immunoparalysis and Genetics during Multiple organ dysfunction syndrome (PARADIGM, NIH R01HD095976).
TRAINING THE NEXT GENERATION OF INVESTIGATORS
One of the primary aims of the PALISI Network is to mentor the next generation of clinical investigators in pediatric critical care. We support this aim by encouraging PALISI members at our sites to bring PICU fellows or other trainees to our meetings, and learn about the wealth of collaborators, experts, and comradery in our field. The network also has a Clinical Research Course focused on the key research skills that each investigator-in-training should learn. Of note, the HCT-subgroup also runs a training symposium for fellows, nurses, and advanced practice providers in the field of HCT and cancer immunology. Now, the PALISI course also includes topics relevant to new investigators, for example, building a research program, how to get started in a career of clinical research, approaches to funding and grant writing, and the importance of mentorship. The course has additional important opportunities for attendees: to observe subgroups and working groups in action; to practice writing skills in producing research specific aims, with individualized feedback from senior PALISI investigators; and to learn about leadership. Finally, the course has a rotation of mid-level investigators as course leaders, thus providing national leadership experience to this group of individuals.
Finally, PALISI has started something new for junior investigators. We provide an “Early (self-defined) Investigator Showcase,” in which attendees have the option to submit a proposal for consideration at the meeting. A selection committee chooses the proposals to be presented: first to the course faculty and attendees, where they receive feedback and suggestions on improvement, and then, the refined versions of these proposals are presented at the main meeting the following day for further feedback.
PALISI IN THE FUTURE
In the more than 20 years since PALISI was formed, its members have produced a significant domain of research work, and an updated list of publications can be accessed on its website (https://www.palisi.org/our-work). The name PALISI connects us with our historic origins, but our research network has expanded to include all things related to pediatric critical care. One measure of success of the network was recently highlighted by the high-profile representation of the PALISI Network ranking in the top five on four measures of research influence based on RCTs related to the field of pediatric critical care examining 415 RCTs from 43 countries (41).
As PALISI moves forward, continued expansion of individual and institutional members is a goal. Regarding our program, we envisage that new investigator-led subgroups will develop in methodologies such as proteomic and genomics. The newly formed implementation science subgroup (ECLIPSE) will have a major role in developing ways to move the network discoveries into sustainable practice. The PALISI research coordinator subgroup is working to standardize our research practices. We also anticipate expansion of new topic areas for subgroups such as complex critical illness, and late morbidities like altered quality of life after the PICU and postintensive care syndrome.
Finally, like our colleagues in adult critical care, we see that the scale of the sample size needed for RCTs in critical care is, in general, beyond the reach of a single research network. To this end, it is important that our community develops a track record and ways to collaborate across international borders. PALISI is currently enrolling patients into the PRone and OScillation Pediatric Clinical Trial for pediatric ARDS (PROSpect, NCT03896763) study, which is a collaboration between PICUs in North America, Australia and New Zealand, and Europe and Asia (i.e., PALISI, the Australia and New Zealand intensive care society, the European Society of Pediatric and Neonatal Intensive Care society, and the Pediatric Acute and Critical Care Medicine Asian Network).
ACKNOWLEDGMENTS
We thank the contribution of the following additional individuals who held major leadership roles in the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Douglas Willson, MD, Daniel Levin, MD, Jerry Zimmerman, MD, Barry Markovitz, MD. We thank the numerous individuals have contributed time on the PALISI Scientific Steering Committee and in developing and leading the PALISI Network groups. We also thank Ms. Anyssa Queen for her services coordinating the PALISI Network and its meetings.
Footnotes
Drs. Randolph, Cheifetz, and Thompson are prior Chairs of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network (Randolph 2002–2010, Cheifetz 2016–2019, and Thompson 2011–2016). Dr. Thomas is the current Chair of the PALISI Network.
Dr. Randolph’s institution received funding from Centers for Disease Control and Prevention (CDC) and National Institute of Allergy and Infectious Diseases, and she received funding from UptoDate. Drs. Randolph and Flori received support for article research from National Institutes of Health (NIH). Dr. Bembea’s institution received funding from the NIH/National Institute of Neurological Disorders and Stroke and Grifols Investigator Sponsored Research Grant. Dr. Cheifetz received funding from Philips, Medtronic, UptoDate, and Tim Peters and Co. Dr. Curley’s institution received funding from National Institute of Child Health and Human Development and National Heart, Lung, and Blood Institute. Dr. Flori’s institution received funding from CDC, Society of Critical Care Medicine, and NIH; she received funding from Aerogen Pharma and Lucira Health; and she disclosed she is an Executive Committee Member for Pediatric Acute Lung Injury and Sepsis Investigators Network. Dr. Khemani received funding from Orange Med and Nihon Kohden. Dr. Nishisaki’s institution received funding from Agency for Healthcare Research and Quality (AHRQ) R03HS026939, AHRQ R18HS024511, and AHRQ R18HS022464, and he received unrestricted grant support by Chiesi, Inc to describe neonatal surfactant administration. Dr. Lacroix’s institution received funding from Canadian Institutes of Health Research. Dr. Thomas received funding from Bayer AG. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Guyatt G: Evidence-based medicine. ACP J Club 1991; 114:A-16 [Google Scholar]
- 2.Randolph AG, Gonzales CA, Cortellini L, et al. : Growth of pediatric intensive care units in the United States from 1995 to 2001. J Pediatr 2004; 144:792–798 [DOI] [PubMed] [Google Scholar]
- 3.Randolph AG, Lacroix J: Randomized clinical trials in pediatric critical care: Rarely done but desperately needed. Pediatr Crit Care Med 2002; 3:102–106 [DOI] [PubMed] [Google Scholar]
- 4.Randolph AG, Wypij D, Venkataraman ST, et al. : Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: A randomized controlled trial. JAMA 2002; 288:2561–2568 [DOI] [PubMed] [Google Scholar]
- 5.Willson DF, Thomas NJ, Markovitz BP, et al. : Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: A randomized controlled trial. JAMA 2005; 293:470–476 [DOI] [PubMed] [Google Scholar]
- 6.Curley MA, Hibberd PL, Fineman LD, et al. : Effect of prone positioning on clinical outcomes in children with acute lung injury: A randomized controlled trial. JAMA 2005; 294:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacroix J, Hebert PC, Hutchison JS, et al. : Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 2007; 356:1609–1619 [DOI] [PubMed] [Google Scholar]
- 8.Curley MA, Wypij D, Watson RS, et al. : Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: A randomized clinical trial. JAMA 2015; 313:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spinella PC, Tucci M, Fergusson DA, et al. : Effect of fresh vs standard-issue red blood cell transfusions on multiple organ dysfunction syndrome in critically Ill pediatric patients: A randomized clinical trial. JAMA 2019; 322:2179–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas NJ, Spear D, Wasserman E, et al. : CALIPSO: A randomized controlled trial of calfactant for acute lung injury in pediatric stem cell and oncology patients. Biol Blood Marrow Transplant 2018; 24:2479–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas NJ, Guardia CG, Moya FR, et al. : A pilot, randomized, controlled clinical trial of lucinactant, a peptide-containing synthetic surfactant, in infants with acute hypoxemic respiratory failure. Pediatr Crit Care Med 2012; 13:646–653 [DOI] [PubMed] [Google Scholar]
- 12.Agus MS, Wypij D, Hirshberg EL, et al. : Tight glycemic control in critically Ill children. N Engl J Med 2017; 376:729–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willson DF, Thomas NJ, Tamburro R, et al. : Pediatric calfactant in acute respiratory distress syndrome trial. Pediatr Crit Care Med 2013; 14:657–665 [DOI] [PubMed] [Google Scholar]
- 14.Brown AM, Fisher E, Forbes ML: Bolus vs continuous nasogastric feeds in mechanically ventilated pediatric patients: A pilot study. JPEN J Parenter Enteral Nutr 2019; 43:750–758 [DOI] [PubMed] [Google Scholar]
- 15.Faustino EVS, Shabanova V, Raffini LJ, et al. : Efficacy of early prophylaxis against catheter-associated thrombosis in critically ill children: A Bayesian phase 2b randomized clinical trial. Crit Care Med 2021; 49:e235–e246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahadeo KM, Bajwa R, Abdel-Azim H, et al. : Diagnosis, grading, and treatment recommendations for children, adolescents, and young adults with sinusoidal obstructive syndrome: An international expert position statement. Lancet Haematol 2020; 7:e61–e72 [DOI] [PubMed] [Google Scholar]
- 17.Mahadeo KM, Khazal SJ, Abdel-Azim H, et al. : Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol 2019; 16:45–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zinter MS, McArthur J, Duncan C, et al. : Candidacy for extracorporeal life support in children after hematopoietic cell transplantation: A position paper from the pediatric acute lung injury and sepsis investigators network’s hematopoietic cell transplant and cancer immunotherapy subgroup. Pediatr Crit Care Med 2022; 23:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nellis ME, Tucci M, Lacroix J, et al. : Bleeding assessment scale in critically ill children (BASIC): Physician-driven diagnostic criteria for bleeding severity. Crit Care Med 2019; 47:1766–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nellis ME, Karam O, Valentine SL, et al. : Executive summary of recommendations and expert consensus for plasma and platelet transfusion practice in critically ill children: From the Transfusion and Anemia EXpertise Initiative-Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med 2022; 23:34–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlton EF, Pinto N, Smith M, et al. : Overall health following pediatric critical illness: A scoping review of instruments and methodology. Pediatr Crit Care Med 2021; 22:1061–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fink EL, Maddux AB, Pinto N, et al. : A core outcome set for pediatric critical care. Crit Care Med 2020; 48:1819–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinto NP, Maddux AB, Dervan LA, et al. : A core outcome measurement set for pediatric critical care. Pediatr Crit Care Med 2022 Aug 29. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dziorny AC, Heneghan JA, Bhat MA, et al. : Clinical decision support in the PICU: Implications for design and evaluation. Pediatr Crit Care Med 2022 [DOI] [PubMed] [Google Scholar]
- 25.Weiss SL, Fitzgerald JC, Faustino EV, et al. : Understanding the global epidemiology of pediatric critical illness: The power, pitfalls, and practicalities of point prevalence studies. Pediatr Crit Care Med 2014; 15:660–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nett S, Emeriaud G, Jarvis JD, et al. : Site-level variance for adverse tracheal intubation-associated events across 15 North American PICUs: A report from the national emergency airway registry for children*. Pediatr Crit Care Med 2014; 15:306–313 [DOI] [PubMed] [Google Scholar]
- 27.Shein SL, Karam O, Beardsley A, et al. : Development of an antibiotic guideline for children with suspected ventilator-associated infections. Pediatr Crit Care Med 2019; 20:697–706 [DOI] [PubMed] [Google Scholar]
- 28.Fontela PS, Quach C, Karim ME, et al. : Determinants of antibiotic tailoring in pediatric intensive care: A national survey. Pediatr Crit Care Med 2017; 18:e395–e405 [DOI] [PubMed] [Google Scholar]
- 29.Watson RS, Beers SR, Asaro LA, et al. : Association of acute respiratory failure in early childhood with long-term neurocognitive outcomes. JAMA 2022; 327:836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faustino EV, Patel S, Thiagarajan RR, et al. : Survey of pharmacologic thromboprophylaxis in critically ill children. Crit Care Med 2011; 39:1773–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demaret P, Karam O, Labreuche Bst J, et al. : How 217 pediatric intensivists manage anemia at PICU discharge: Online responses to an international survey. Pediatr Crit Care Med 2020; 21:e342–e353 [DOI] [PubMed] [Google Scholar]
- 32.Nellis ME, Goel R, Karam O, et al. : International study of the epidemiology of platelet transfusions in critically ill children with an underlying oncologic diagnosis. Pediatr Crit Care Med 2019; 20:e342–e351 [DOI] [PubMed] [Google Scholar]
- 33.Bateman ST, Lacroix J, Boven K, et al. : Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med 2008; 178:26–33 [DOI] [PubMed] [Google Scholar]
- 34.Leonard JC, Josephson CD, Luther JF, et al. : Life-threatening bleeding in children: A prospective observational study. Crit Care Med 2021; 49:1943–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valentine SL, Bembea MM, Muszynski JA, et al. : Consensus recommendations for RBC transfusion practice in critically ill children from the pediatric critical care transfusion and anemia expertise initiative. Pediatr Crit Care Med 2018; 19:884–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghafoor S, Fan K, Di Nardo M, et al. : Extracorporeal membrane oxygenation candidacy in pediatric patients treated with hematopoietic stem cell transplant and chimeric antigen receptor T-cell therapy: An international survey. Front Oncol 2021; 11:798236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowan CM, McArthur J, Hsing DD, et al. : Acute respiratory failure in pediatric hematopoietic cell transplantation: A multicenter study. Crit Care Med 2018; 46:e967–e974 [DOI] [PubMed] [Google Scholar]
- 38.Moffet JR, Mahadeo KM, McArthur J, et al. : Acute respiratory failure and the kinetics of neutrophil recovery in pediatric hematopoietic cell transplantation: A multicenter study. Bone Marrow Transplant 2020; 55:341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Randolph AG, Vaughn F, Sullivan R, et al. : Critically ill children during the 2009-2010 influenza pandemic in the United States. Pediatrics 2011; 128:e1450–e1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldstein LRR, Rose EB, Horwitz SM, et al. : Multisystem inflammatory syndrome in U.S. children and adolescents. New Engl J Med 2020; 383:334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duffett M, Brouwers M, Meade MO, et al. : Research collaboration in pediatric critical care randomized controlled trials: A social network analysis of coauthorship. Pediatr Crit Care Med 2020; 21:12–20 [DOI] [PubMed] [Google Scholar]