Abstract
Objective
To propose standardized MRI-proton density fat fraction (PDFF) cutoff values for diagnosing hepatic steatosis, evaluated using contemporary PDFF measuring methods in a large population of healthy adults, using histologic fat fraction (HFF) as the reference standard.
Materials and Methods
A retrospective search of electronic medical records between 2015 and 2018 identified 1063 adult donor candidates for liver transplantation who had undergone liver MRI and liver biopsy within a 7-day interval. Patients with a history of liver disease or significant alcohol consumption were excluded. Chemical shift imaging-based MRI (CS-MRI) PDFF and high-speed T2-corrected multi-echo MR spectroscopy (HISTO-MRS) PDFF data were obtained. By temporal splitting, the total population was divided into development and validation sets. Receiver operating characteristic (ROC) analysis was performed to evaluate the diagnostic performance of the MRI-PDFF method. Two cutoff values with sensitivity > 90% and specificity > 90% were selected to rule-out and rule-in, respectively, hepatic steatosis with reference to HFF ≥ 5% in the development set. The diagnostic performance was assessed using the validation set.
Results
Of 921 final participants (624 male; mean age ± standard deviation, 31.5 ± 9.0 years), the development and validation sets comprised 497 and 424 patients, respectively. In the development set, the areas under the ROC curve for diagnosing hepatic steatosis were 0.920 for CS-MRI-PDFF and 0.915 for HISTO-MRS-PDFF. For ruling-out hepatic steatosis, the CS-MRI-PDFF cutoff was 2.3% (sensitivity, 92.4%; specificity, 63.0%) and the HISTO-MRI-PDFF cutoff was 2.6% (sensitivity, 88.8%; specificity, 70.1%). For ruling-in hepatic steatosis, the CS-MRI-PDFF cutoff was 3.5% (sensitivity, 73.5%; specificity, 88.6%) and the HISTO-MRI-PDFF cutoff was 4.0% (sensitivity, 74.7%; specificity, 90.6%).
Conclusion
In a large population of healthy adults, our study suggests diagnostic thresholds for ruling-out and ruling-in hepatic steatosis defined as HFF ≥ 5% by contemporary PDFF measurement methods.
Keywords: Proton density fat fraction, Chemical shift imaging-based magnetic resonance imaging, High-speed T2-corrected multi-echo magnetic resonance spectroscopy, Hepatic steatosis, Threshold value
INTRODUCTION
As nonalcoholic fatty liver disease (NAFLD) has gained clinical importance, quantification of liver fat to diagnose and monitor NAFLD is emerging as an active area of research. Proton density fat fraction (PDFF) measured via MRI is accepted as a noninvasive, reliable, accurate, and quantitative biomarker for hepatic fat with excellent inter- and intraobserver agreement [1,2,3,4]. PDFF can be estimated using either chemical shift imaging-based MRI (CS-MRI) or MR spectroscopy (MRS). As MRI sequences dedicated to PDFF measurements have recently improved, commercially available advanced MRI sequences have enabled the widespread use of PDFF.
PDFF from CS-MRI and MRS demonstrate a strong correlation with the histologic fat fraction (HFF), and both are expressed as percentages [5]. However, PDFF values are not directly interchangeable with HFF as they are fundamentally different measures, such that PDFF is calculated from the signal intensity ratio of triglycerides and water, whereas HFF is a visual and semiquantitative estimation of the area occupied by fat vacuoles in the cross-section of a liver biopsy [6,7]. The clinically recommended limits of hepatic steatosis based on HFF ≥ 5% of hepatocytes [8] cannot be directly used as a cutoff value for PDFF values.
Studies have suggested that, with HFF as reference standard, the threshold PDFF value to diagnose hepatic steatosis is 5.1%–6.4% [9,10]. However, these studies included a limited number of participants (fewer than 50 participants) with normal liver histology, and the suggested thresholds were not validated in a separate population. A population-based approach in a study of 345 participants who had no identifiable risk factors for hepatic steatosis suggested a PDFF cutoff value of 5.56% for hepatic steatosis [11]; this value has been applied in some clinical studies [12,13,14,15]. However, this previous study lacked pathological validation and was based on a traditional MRS method under free-breathing conditions, vulnerable to erroneous data acquisition, and line broadening. The traditional MRS method is now replaced with updated MRI techniques using CS-MRI and high-speed T2-corrected multi-echo (HISTO) MRS obtained within a single breath hold [16]. A recent phantom-based study revealed that PDFF measurements can be affected by sequences, imager vendors, and field strength [17]. Thus, PDFF values based on updated MRI methods may differ from the PDFF values obtained using a previous method.
Therefore, this study aimed to propose cutoff values for diagnosing hepatic steatosis using contemporary PDFF measurement methods in a large population of healthy adults using HFF as the reference standard.
MATERIALS AND METHODS
This retrospective study was approved by our Institutional Review Board, which waived the requirement for informed consent from patients (IRB No. 2018-0931).
Study Population
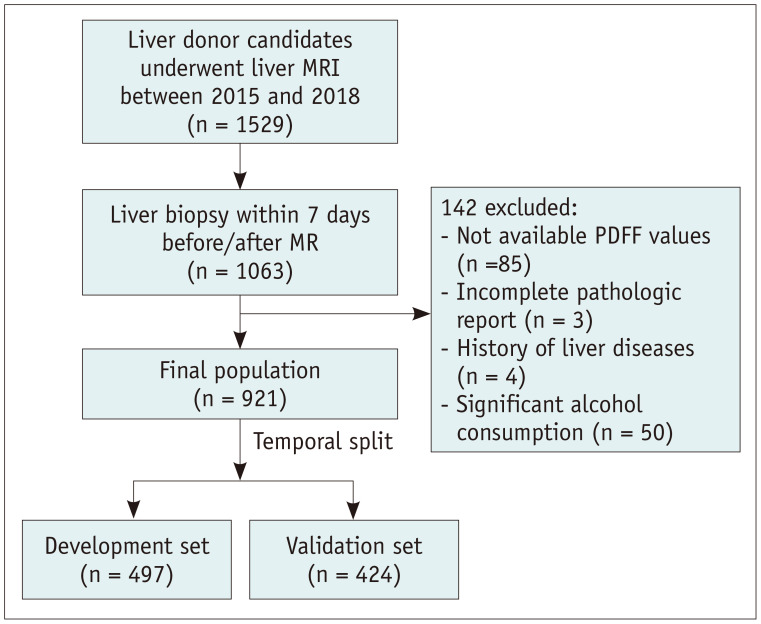
Among the donor candidates who had undergone liver MRI between 2015 and 2018 for living-donor liver transplantation at our institution, participants who underwent liver biopsy within a 7-day interval from MRI examinations were selected. Clinical data, including demographic information such as sex, age, alcohol history, and laboratory test results performed on the date closest to MRI, were obtained from the medical records. At our institution, screening serologic tests and liver MRI were performed on the same day as the routine donor work-up. The exclusion criteria were as follows: unavailable PDFF values, incomplete pathological report, histology of liver disease, and history of significant alcohol consumption (≥ 30 g/day in males and ≥ 20 g/day in females) (Fig. 1) [11].
Fig. 1. Flowchart of the patient inclusion process.
PDFF = proton density fat fraction
MRI Acquisition Techniques and PDFF Measurement
All patients were imaged using a 3T MRI system (Magnetom Skyra, Siemens) with a 32-channel body matrix coil. As a routine liver MRI pulse sequence for living liver donor work-up, MRI techniques for hepatic fat quantification were performed before intravenous administration of contrast media.
In CS-MRI, the whole-liver volume was acquired within a single breath-hold (scan time of 16–20 seconds) using a multi-echo 3D spoiled gradient-echo acquisition, which is an investigational variant of “hybrid multi-step adaptive fitting approach with multi-echo volume interpolated breath-hold examination acquisition,” which in consequence generates a hepatic PDFF map [18,19,20]. The parameters were as follows: repetition time (TR), 9.0 ms; echo time (TE), 1.09, 2.49, 3.69, 4.92, 6.15, and 7.38 ms; flip angle, 4; slice thickness, 3.5 mm; receiver bandwidth, 1080 Hz/pixel; field of view, 380 × 380 mm; parallel imaging factor, 2 × 2. The images were processed using commercially available software (Syngo MR D13, Siemens) to create water/fat images, water/fat R2* maps, effective R2* maps, and water/fat percentage maps [18,20]. One radiologist (with 5 years of clinical experience in MRI), blinded to the clinical profiles, values on HISTO-MRS-PDFF, and histologic results, interpreted the MRI images. PDFF values from CS-MRI (CS-MRI-PDFF) were measured at two different 30 × 30-mm regions of interest (ROIs), which were manually placed in a region similar to those indicated by the MRS measurements. The average of two measurements was used as the representative PDFF value for each participant.
HISTO-MRS was performed using a modified stimulated-echo acquisition sequence at TEs of 12, 24, 36, 48, and 72 ms. HISTO-MRS was performed twice in each patient. Each acquisition was completed during one breath-hold (approximately 15 seconds). The parameters were as follows: TR = 3000 ms; mixing time = 10 ms; flip angle = 90°; and receiver bandwidth = 1200 Hz/pixel. Experienced radiologic technicians manually placed ROIs (30 × 30 × 30 mm3) per participant in triplanar localizing images in the right hepatic lobe away from the large vessels, large bile ducts, and focal hepatic lesions. HISTO-MRS data were automatically post-processed using Syngo MR D13 software (Siemens). The T2 values of water and fat were calculated separately, and T2 correction was applied for both water and fat peaks to obtain an accurate hepatic fat fraction. Subsequently, T2-corrected fat fractions were computed using the equation (M0lipid/[M0lipid + M0water]) × 100%, where M0 is the equilibrium magnetization [16]. The measurements were presented as percentages. Two HISTO-MRS were obtained from each patient, and the average of the two measurements was used as the representative PDFF value for each patient.
Histopathologic Analysis
Ultrasound-guided percutaneous liver biopsy was performed for preoperative donor work-up. Biopsy samples of approximately 1.5-cm length were obtained from two different sites in the right hepatic lobe using an 18-gauge needle (Stericut 18G coaxial; TSK Laboratory) under local anesthesia. All specimens were reviewed by pathologists with more than five years of experience. HFF was determined as the fraction of hepatocytes that contained fat droplets on hematoxylin-eosin-stained specimens collected from pathologic reports. Hepatic steatosis was categorized based on the percentage of fat within the hepatocytes: grade 0 (healthy, < 5%), grade 1 (mild, 5%–33%), grade 2 (moderate, 34%–66%), and grade 3 (severe, > 66%) [21].
Statistical Analysis
To determine and validate the optimal threshold of PDFF values for diagnosing hepatic steatosis, the final study population was temporally divided into two groups based on the date of the MRI (before or after January 1, 2017); these two groups were used as the development and validation sets, respectively. Differences in baseline characteristics between the development and validation sets were assessed using the independent t test or Mann–Whitney U test for continuous variables and the chi-square test for categorical variables. The correlation between CS-MRI-PDFF and HFF and that between HISTO-MRS-PDFF and HFF were evaluated using Pearson’s correlation.
In the development set, receiver operating characteristic (ROC) analysis was performed, and dual cutoff values were selected for the diagnosis of hepatic steatosis (HFF ≥ 5%) with 90% sensitivity to rule-out and 90% specificity to rule-in hepatic steatosis. The corresponding sensitivity, specificity, positive predictive value, and negative predictive value of the cutoffs were calculated for the development and validation sets. Additionally, previously reported thresholds of 5.1%, 5.6%, and 6.4% were applied to our validation set to test the diagnostic performance of these threshold values appearing in published studies [9,10,11].
The repeatability of the PDFF value on MRI was calculated using the intraclass correlation coefficients (ICCs) for CS-MRI-PDFF and HISTO-MRS-PDFF. ICC values less than 0.5, between 0.5 and 0.75, between 0.75 and 0.9, and greater than 0.90 were considered poor, moderate, good, and excellent correlation, respectively [22]. The correlation of the PDFF values measured on CS-MRI and HISTO-MRS was evaluated using ICC and the 95% Bland-Altman limit of agreement (LOA) of their mean difference. All statistical analyses were performed using the SPSS version 21 (IBM Corp.) and MedCalc statistical software (MedCalc software, version 18.2.1).
RESULTS
Study Participants
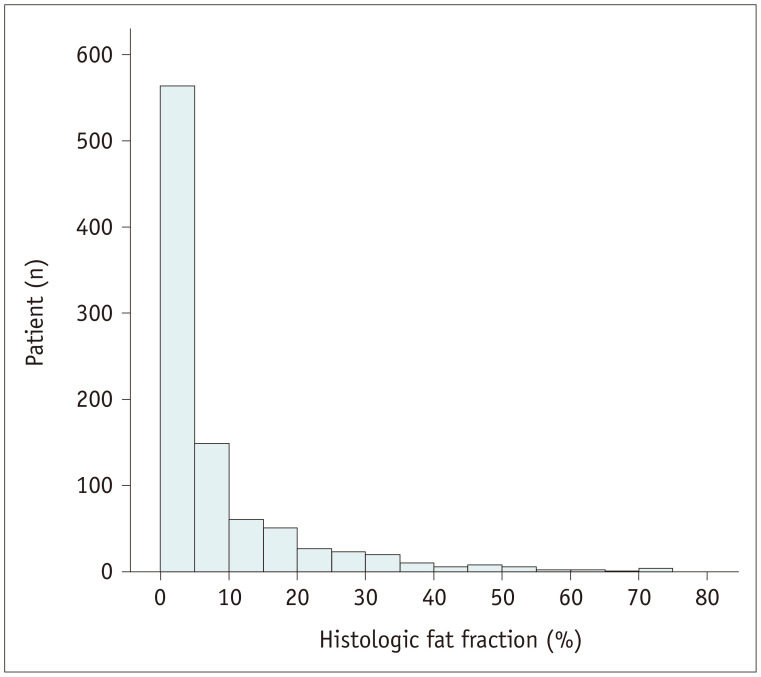
Among 1529 participants who had undergone liver MRI in the stated time period, 1063 underwent liver biopsy within 7 days before or after the MRI; 142 were excluded for the following reasons: unavailable PDFF values (n = 85), incomplete pathologic reports (n = 3), history of liver diseases (n = 4), and significant alcohol consumption (n = 50). Finally, 921 participants (mean age, 31.5 ± 9.0 years; range, 18–67 years) comprising 624 men (mean age, 30.0 ± 8.2 years) and 297 female (mean age, 34.9 ± 9.6 years) were included in the analyses. Their mean body mass index was 24.3 ± 3.1 kg/m2. Overall, 61.1% (563/921) of the participants had grade 0 hepatic steatosis, followed by grade 1 (35.3% [325/921]), grade 2 (3.3% [30/921]), and grade 3 (0.3% [3/921]) hepatic steatosis. The mean HFF was 6.5% ± 10.5% (median 2.0%). The distribution of HFF is presented in Figure 2. The majority of clinical and laboratory characteristics, except for sex, did not significantly differ between the development and validation sets (Table 1). Although a significant difference was observed in CS-MRI-PDFF, it could be considered clinically negligible, as the difference was only 0.2% and attributable to an exaggeration of the statistical significance causes by the large number of cases. CS-MRI-PDFF and HISTO-MRS-PDFF strongly correlated with the histologic fraction (r = 0.866 and 0.872, respectively; p < 0.001 for both; Supplementary Fig. 1).
Fig. 2. Histogram showing the histologic fat fraction distribution.
Table 1. Baseline Characteristics of the Final Study Population.
| Characteristic | Total (n = 921) | Development Set (n = 497) | Validation Set (n = 424) | P (Development vs. Validation Sets) | |
|---|---|---|---|---|---|
| Age, years | 31.5 ± 9.0 | 31.2 ± 8.7 | 31.9 ± 9.3 0.256 | ||
| Sex | < 0.001 | ||||
| Male | 624 (67.8) | 361 (72.6) | 263 (62.0) | ||
| Female | 297 (32.2) | 136 (27.4) | 161 (38.0) | ||
| BMI, kg/m2 | 24.3 ± 3.1 | 24.3 ± 3.2 | 24.3 ± 3.2 | 0.969 | |
| AST, IU/mL, median (IQR) | 19.0 (16.0, 22.0) | 19.0 (16.0, 22.0) | 18.0 (16.0, 22.0) | 0.741 | |
| ALT, IU/mL | 20.2 ± 12.3 | 20.3 ± 12.5 | 20.1 ± 12.2 | 0.744 | |
| FPG, mg/dL, median (IQR) | 92.0 (87.0, 98.0) | 91.0 (86.0, 97.0) | 65.0 (87.0, 99.0) | 0.013 | |
| γ-GT, IU/mL, median (IQR) | 17.0 (12.0, 26.0) | 17.0 (12.0, 26.0) | 17.0 (12.0, 26.0) | 0.515 | |
| TG, mg/dL, median (IQR) | 89.0 (62.0, 133.0) | 87.0 (61.0, 130.0) | 95.0 (64.5, 136.0) | 0.096 | |
| HDL-C, mg/dL | 54.4 ± 13.7 | 53.8 ± 13.7 | 55.2 ± 13.7 | 0.123 | |
| CS-MRI-PDFF, %, median (IQR) | 2.6 (1.8, 4.7) | 2.5 (1.7, 4.5) | 2.7 (1.9, 5.0) | 0.039 | |
| HISTO-MRS-PDFF, %, median (IQR) | 2.9 (1.6, 5.3) | 2.9 (1.6, 5.1) | 2.9 (1.6, 5.4) | 0.980 | |
| Histologic fat fraction, % | 6.5 ± 10.5 | 6.6 ± 10.9 | 6.3 ± 10.0 | 0.590 | |
| Hepatic steatosis, % | 0.806 | ||||
| Grade 0 (< 5) | 563 (61.1) | 309 (62.2) | 254 (59.9) | ||
| Grade 1 (5–33) | 325 (35.3) | 169 (34.0) | 156 (36.8) | ||
| Grade 2 (34–66) | 30 (3.3) | 17 (3.4) | 13 (3.1) | ||
| Grade 3 (> 66) | 3 (0.3) | 2 (0.4) | 1 (0.2) | ||
Data are presented as mean ± standard deviation or n (%), unless indicated otherwise. ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, CS-MRI-PDFF = chemical-shift-imaging-based MRI-proton density fat fraction, FPG = fasting plasma glucose, γ-GT = gamma-glutamyltransferase, HDL-C = high-density lipoprotein cholesterol, HISTO-MRS-PDFF = high-speed T2-corrected multi-echo MR spectroscopy proton density fat fraction, IQR = interquartile range, TG = triglyceride
Diagnostic Performance and Dual Thresholds of PDFF for Ruling-Out and Ruling-In Hepatic Steatosis
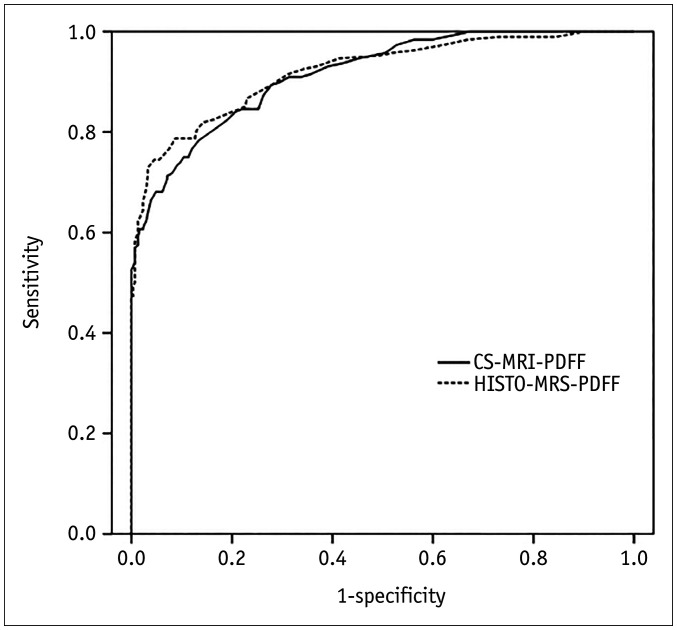
In the development set, the area under ROC (AUC) for diagnosing hepatic steatosis was 0.920 (95% confidence interval [CI], 0.892–0.942) for CS-MRI-PDFF and 0.915 (95% CI, 0.887–0.938) for HISTO-MRS-PDFF (Fig. 3). The diagnostic performance of the MRI-PDFF method is summarized in Table 2. From the development set, the threshold of CS-MRI-PDFF to rule-out hepatic steatosis was determined as 2.3% and that to rule-in was 3.5%. For the ruling-out threshold of CS-MRI-PDFF, the corresponding sensitivity and specificity were 92.3% and 63.0%, respectively, in the validation set. For the ruling-in threshold, sensitivity and specificity were 73.5% and 88.6%, respectively, in the case of HISTO-MRS-PDFF, the selected thresholds were 2.6% to rule-out and 4.0% to rule-in hepatic steatosis in the development set. When these cutoffs were applied to the validation set, the corresponding sensitivity and specificity for the ruling-out criteria of HISTO-MRS-PDFF were 88.8% and 70.1%, respectively, whereas the sensitivity and specificity for the ruling-in criteria were 74.7% and 90.6%, respectively.
Fig. 3. ROC curves for diagnosing hepatic steatosis based on CS-MRI-PDFF and HISTO-MRS-PDFF.
The AUC values of CS-MRI-PDFF and HISTO-MRS-PDFF were 0.920 and 0.915, respectively. AUC = area under the curve, CS-MRI-PDFF = chemical-shift-imaging-based MRI-proton density fat fraction, HISTO-MRS-PDFF = high-speed T2-corrected multi-echo MR spectroscopy proton density fat fraction, ROC = receiver operating characteristic
Table 2. Cutoff Values of PDFF for Hepatic Steatosis (Histologic Fat Fraction ≥ 5%) and Their Corresponding Diagnostic Performance in the Development Set and Validation Set.
| Indices | Cutoffs (%) | Development Set | Sensitivity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Sensitivity | Specificity | PPV | NPV | ||
| CS-MRI-PDFF | 2.3* | 91.5 (172/188) | 68.9 (213/309) | 64.2 (172/268) | 93.0 (213/229) | 92.4 (157/170) | 63.0 (160/254) | 62.6 (157/251) | 92.5 (160/173) |
| 3.5† | 78.7 (148/188) | 91.3 (282/309) | 84.6 (148/175) | 87.6 (282/322) | 73.5 (125/170) | 88.6 (225/254) | 81.2 (125/154) | 83.3 (225/270) | |
| HISTO-MRS-PDFF | 2.6* | 91.0 (171/188) | 68.6 (212/309) | 63.8 (171/268) | 92.6 (212/229) | 88.8 (151/170) | 70.1 (178/254) | 66.5 (151/227) | 90.4 (178/197) |
| 4.0† | 73.9 (139/188) | 90.3 (279/309) | 82.25 (139/169) | 85.1 (279/328) | 74.7 (127/170) | 90.6 (230/254) | 84.1 (127/151) | 84.3 (230/273) | |
Results are presented as percentages (number of patients/total number of patients assessed). *Cutoff for 90% sensitivity, †Cutoff for 90% specificity. CS-MRI-PDFF = chemical-shift-imaging-based MRI-proton density fat fraction, HISTO-MRS-PDFF = high-speed T2-corrected multi-echo MR spectroscopy proton density fat fraction, NPV = negative predictive value, PPV = positive predictive value
On applying previously suggested CS-MRI-PDFF cutoff values of 5.1% [10] and 6.4% [9] to our validation set, the sensitivities were 54.1% (92/170) and 38.2% (65/170), respectively, and the specificities were 100% for both cutoff values. On applying a previously reported MRS-PDFF threshold value (5.6%) [11] to the current validation set, the sensitivity was 54.7% (93/170) and the specificity was 96.9% (246/254).
Repeatability and Correlation of CS-MRI-PDFF and HISTO-MRS-PDFF
The repeatability between the first and second measurements of PDFF values were both excellent in CS-MRI (ICC, 0.999; 95% CI, 0.998–0.999) and HISTO-MRS (ICC, 0.995; 95% CI, 0.994–0.995). Correlation between CS-MRI-PDFF and HISTO-MRS-PDFF in the same participants were excellent as well (ICC, 0.973; 95% CI, 0.969–0.976). The Bland-Altman 95% LOA revealed significant biases (mean bias ± 1.96 × standard deviation [SD], -0.4 ± 2.4; p < 0.001) between CS-MRI-PDFF and HISTO-MRS-PDFF (Fig. 4). The biases increased when the PDFF value was high.
Fig. 4. Bland-Altman plot showing the relationships between CS-MRI-PDFF and HISTO-MRS-PDFF.
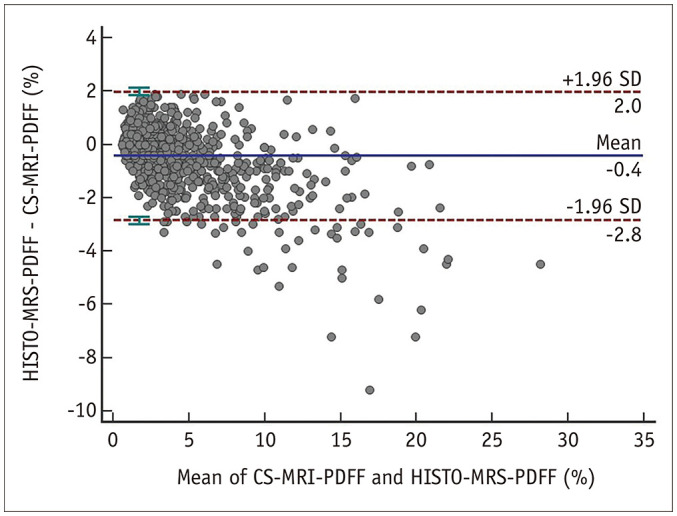
The X-axis represents the mean of CS-MRI-PDFF and HISTO-MRS-PDFF, and the Y-axis represents the differences between them, calculated as HISTO-MRS-PDFF minus CS-MRI-PDFF. The Bland-Altman plot reveals a bias of -0.4, with a 95% limit of agreement, ranging from -2.8 to 2.0. CS-MRI-PDFF = chemicalshift-imaging-based MRI-proton density fat fraction, HISTO-MRS-PDFF = high-speed T2-corrected multi-echo MR spectroscopy proton density fat fraction, SD = standard deviation
DISCUSSION
In this study, we suggested cutoff values for diagnosing hepatic steatosis measured by contemporary PDFF measuring methods in a large population of 921 healthy adults, using HFF as a reference standard. To rule-out hepatic steatosis, the CS-MRI-PDFF cutoff was 2.3% (sensitivity, 92.4%; specificity, 63.0%) and the HISTO-MRI-PDFF cutoff was 2.6% (sensitivity, 88.8%; specificity, 70.1%). To rule-in hepatic steatosis, the CS-MRI-PDFF cutoff was 3.5% (sensitivity, 73.5%; specificity, 88.6%) and the HISTO-MRI-PDFF cutoff was 4.0% (sensitivity, 74.7%; specificity, 90.6%). The cutoff values proposed by previous studies (i.e., 5.1%, 5.6%, and 6.4% of MRI-PDFF) [9,10,11] revealed 38.2%–54.7% of sensitivity with almost 100% specificity when applied to our validation set.
In our study, dual MRI-PDFF cutoff values were used to diagnose hepatic steatosis, instead of a single cutoff approach with the highest Youden’s index. One of these cutoffs was based on 90% sensitivity, which defined a normal control group by excluding most participants with hepatic steatosis (ruling-out cutoff). In our study, the ruling-in cutoff of CS-MRI-PDFF was 2.3% and that of HISTO-MRI-PDFF was 2.6%, showing approximately 90% sensitivity and 60%–70% specificity. Based on 90% specificity, the other cutoff was intended for identifying participants with hepatic steatosis with high confidence (ruling-in cutoff). We determined 3.5% as the ruling-in cutoff of CS-MRI-PDFF and 4.0% as that of HISTO-MRS-PDFF, showing approximately 90% specificity and 70% sensitivity. This dual cutoff value approach is gaining increasing usage for staging hepatic fibrosis or steatosis [23,24], as it is versatile and can be used according to clinical needs. For example, if a study intends to build a cohort of participants with hepatic steatosis, the ruling-in cutoff is better. Conversely, when a normal control group is needed for a trial, the ruling-out cutoff is more suitable. However, the cost of MRI is higher, and its availability is more limited than that of other imaging modalities such as ultrasound or transient elastography [25,26]. Thus, MRI-PDFF as a first-line examination method for the screening of hepatic steatosis in daily practice is limited. However, in the research setting, MRI-PDFF plays an important role owing to its high diagnostic performance. Therefore, our cutoff values may help define an appropriate population according to the study purpose.
Regardless of ruling-in or ruling-out cutoffs for hepatic steatosis, our analysis-estimated threshold values were lower than previously reported values (5.1%–6.4%) [9,10,11]. This inconsistency may be partially attributable to the implementation of different sequences, imager vendors, and field strengths, which would have affected the PDFF measurements [17]. Therefore, these factors must be considered when applying the threshold PDFF value. The MRI techniques used in our study were based on recently updated techniques, with a shorter acquisition time and were less prone to technical errors. Thus, we surmise that the threshold values from our study are time relevant and reliable. Moreover, two studies suggesting MRI-PDFF thresholds based on histological reference standards included few healthy adult participants. Since they initially recruited patients with elevated liver function test results [10] or NAFLD [9], the inclusion of normal HFF might have resulted from sampling bias, which could have increased the PDFF values and thresholds in these studies. Considering the significant decrease in their sensitivities (38.2%–54.7%) when applied to our validation set containing a large population of healthy adult participants, using a lower threshold may help diagnose hepatic steatosis more sensitively in clinical practice. Although MRI-PDFF strongly correlated with HFF, they are not directly interchangeable, as they are fundamentally different measures. The overall MRI-PDFF values were lower than those of HFF in our study; previous studies have also reported similar results [5].
Our study demonstrated a strong correlation between CS-MRI-PDFF and HISTO-MRS-PDFF (ICC = 0.973), consistent with the results of a recent meta-analysis [4]. Concurrently, a small but significant bias was observed between CS-MRI-PDFF and HISTO-MRS-PDFF (mean bias ± 1.96 × SD, -0.4 ± 2.4; p < 0.001), as revealed on the Bland-Altman 95% LOA. In Jang et al. [17] study, when comparing the PDFFs acquired from the same vendor and field strength, a significant bias was identified between CS-MRI-PDFF and HISTO-MRS-PDFF (3.69%). The Bland-Altman plots in the present study revealed that larger PDFF values indicated greater differences between the two values, which is consistent Jang et al. [17] study as well. Thus, although the two values are well-correlated, they are not completely interchangeable, especially when analyzing and interpreting high PDFF values. However, as noted in our study, the cutoff values of PDFF were as low as approximately 2%–4%, and these did not significantly differ between the two MRI-derived PDFF values.
Our study had several limitations. First, it was a retrospective study conducted at a single institution. Additionally, the study population was somewhat limited in number and ethnic diversity was not considered. Although this was the largest study to estimate the PDFF range in a healthy adult population, further studies with more ethnically diverse populations are warranted. Second, we only obtained MRIs from one vendor with one field strength and tested only in the internal validation set, which may undermine the generalizability of our cutoff values. Detailed and precise threshold values for each image vendor and field strength should be addressed in subsequent studies.
In conclusion, we investigated the cutoff values of CS-MRI-PDFF and HISTO-MRS-PDFF with currently used MRI protocols in a large population of healthy adults, using HFF as the reference standard. We have proposed dual diagnostic thresholds of CS-MRI-PDFF and HISTO-MRS-PDFF for ruling-out and ruling-in hepatic steatosis defined as HFF ≥ 5%. For ruling-out hepatic steatosis, the CS-MRI-PDFF and the HISTO-MRI-PDFF cutoffs were 2.3% and 2.6%, respectively. For ruling-in hepatic steatosis, the cutoffs of CS-MRI-PDFF HISTO-MRI-PDF were 3.5% and 4.0%, respectively.
Footnotes
Conflicts of Interest: So Yeon Kim and Seung Soo Lee who is on the editorial board of the Korean Journal of Radiology was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
- Conceptualization: So Yeon Kim, Ji Hun Kang, Seung Soo Lee.
- Data curation: Sohee Park, Jae Hyun Kwon, So Yeon Kim, Ji Hun Kang, Jung Il Chung, Hye Young Jang.
- Formal analysis: Sohee Park, Jae Hyun Kwon, So Yeon Kim, Ji Hun Kang.
- Investigation: Sohee Park, Jae Hyun Kwon, So Yeon Kim, Ji Hun Kang, Jung Il Chung, Hye Young Jang.
- Methodology: Sohee Park, Jae Hyun Kwon, So Yeon Kim, Ji Hun Kang, Jong Keon Jang.
- Project administration: So Yeon Kim.
- Resources: So Yeon Kim, Jong Keon Jang, Ju Hyun Shim.
- Software: Sohee Park, Jae Hyun Kwon, So Yeon Kim.
- Supervision: So Yeon Kim, Jong Keon Jang, Ju Hyun Shim, Seung Soo Lee, Kyoung Won Kim, Gi-Won Song.
- Validation: Sohee Park, Jae Hyun Kwon, So Yeon Kim, Jung Il Chung, Jong Keon Jang, Hye Young Jang.
- Visualization: Sohee Park, Jae Hyun Kwon.
- Writing—original draft: Sohee Park, Jae Hyun Kwon, So Yeon Kim.
- Writing—review & editing: Sohee Park, Jae Hyun Kwon, So Yeon Kim, Jung Il Chung, Jong Keon Jang, Hye Young Jang, Ju Hyun Shim, Seung Soo Lee, Kyoung Won Kim, Gi-Won Song.
Funding Statement: None
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2022.0334.
References
- 1.Kang GH, Cruite I, Shiehmorteza M, Wolfson T, Gamst AC, Hamilton G, et al. Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging. 2011;34:928–934. doi: 10.1002/jmri.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–1940. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol. 2016;65:1006–1016. doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokoo T, Serai SD, Pirasteh A, Bashir MR, Hamilton G, Hernando D, et al. Linearity, bias, and precision of hepatic proton density fat fraction measurements by using MR imaging: a meta-analysis. Radiology. 2018;286:486–498. doi: 10.1148/radiol.2017170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannas P, Kramer H, Hernando D, Agni R, Cunningham AM, Mandal R, et al. Quantitative magnetic resonance imaging of hepatic steatosis: validation in ex vivo human livers. Hepatology. 2015;62:1444–1455. doi: 10.1002/hep.28012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim B, Kim SY, Kim KW, Jang HY, Jang JK, Song GW, et al. MRI in donor candidates for living donor liver transplant: technical and practical considerations. J Magn Reson Imaging. 2018;48:1453–1467. doi: 10.1002/jmri.26257. [DOI] [PubMed] [Google Scholar]
- 7.Roldan-Valadez E, Favila R, Martínez-López M, Uribe M, Ríos C, Méndez-Sánchez N. In vivo 3T spectroscopic quantification of liver fat content in nonalcoholic fatty liver disease: correlation with biochemical method and morphometry. J Hepatol. 2010;53:732–737. doi: 10.1016/j.jhep.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 8.European Association for the Study of Obesity (EASO); European Association for the Study of Diabetes (EASD); European Association for the Study of the Liver (EASL) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121–1140. doi: 10.1007/s00125-016-3902-y. [DOI] [PubMed] [Google Scholar]
- 9.Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422–431. doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kühn JP, Hernando D, Muñoz del Rio A, Evert M, Kannengiesser S, Völzke H, et al. Effect of multipeak spectral modeling of fat for liver iron and fat quantification: correlation of biopsy with MR imaging results. Radiology. 2012;265:133–142. doi: 10.1148/radiol.12112520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 12.Bril F, Barb D, Portillo-Sanchez P, Biernacki D, Lomonaco R, Suman A, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65:1132–1144. doi: 10.1002/hep.28985. [DOI] [PubMed] [Google Scholar]
- 13.Rehm JL, Wolfgram PM, Hernando D, Eickhoff JC, Allen DB, Reeder SB. Proton density fat-fraction is an accurate biomarker of hepatic steatosis in adolescent girls and young women. Eur Radiol. 2015;25:2921–2930. doi: 10.1007/s00330-015-3724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767–775. doi: 10.1148/radiol.10100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokoo T, Bydder M, Hamilton G, Middleton MS, Gamst AC, Wolfson T, et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology. 2009;251:67–76. doi: 10.1148/radiol.2511080666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pineda N, Sharma P, Xu Q, Hu X, Vos M, Martin DR. Measurement of hepatic lipid: high-speed T2-corrected multiecho acquisition at 1H MR spectroscopy--a rapid and accurate technique. Radiology. 2009;252:568–576. doi: 10.1148/radiol.2523082084. [DOI] [PubMed] [Google Scholar]
- 17.Jang JK, Lee SS, Kim B, Cho ES, Kim YJ, Byun JH, et al. Agreement and reproducibility of proton density fat fraction measurements using commercial MR sequences across different platforms: a multivendor, multi-institutional phantom experiment. Invest Radiol. 2019;54:517–523. doi: 10.1097/RLI.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 18.Zhong X, Nickel MD, Kannengiesser SA, Dale BM, Kiefer B, Bashir MR. Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn Reson Med. 2014;72:1353–1365. doi: 10.1002/mrm.25054. [DOI] [PubMed] [Google Scholar]
- 19.Kim KY, Song JS, Kannengiesser S, Han YM. Hepatic fat quantification using the proton density fat fraction (PDFF): utility of free-drawn-PDFF with a large coverage area. Radiol Med. 2015;120:1083–1093. doi: 10.1007/s11547-015-0545-x. [DOI] [PubMed] [Google Scholar]
- 20.Bashir MR, Zhong X, Nickel MD, Fananapazir G, Kannengiesser SA, Kiefer B, et al. Quantification of hepatic steatosis with a multistep adaptive fitting MRI approach: prospective validation against MR spectroscopy. AJR Am J Roentgenol. 2015;204:297–306. doi: 10.2214/AJR.14.12457. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 22.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferraioli G, Maiocchi L, Lissandrin R, Tinelli C, De Silvestri A, Filice C. Ruling-in and ruling-out significant fibrosis and cirrhosis in patients with chronic hepatitis C using a shear wave measurement method. J Gastrointestin Liver Dis. 2017;26:139–143. doi: 10.15403/jgld.2014.1121.262.fer. [DOI] [PubMed] [Google Scholar]
- 24.Ahn Y, Yun SC, Lee SS, Son JH, Jo S, Byun J, et al. Development and validation of a simple index based on non-enhanced CT and clinical factors for prediction of non-alcoholic fatty liver disease. Korean J Radiol. 2020;21:413–421. doi: 10.3348/kjr.2019.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J, Lee JM, Lee G, Jeon SK, Joo I. Quantitative evaluation of hepatic steatosis using advanced imaging techniques: focusing on new quantitative ultrasound techniques. Korean J Radiol. 2022;23:13–29. doi: 10.3348/kjr.2021.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon SK, Lee JM, Joo I, Park SJ. Quantitative ultrasound radiofrequency data analysis for the assessment of hepatic steatosis in nonalcoholic fatty liver disease using magnetic resonance imaging proton density fat fraction as the reference standard. Korean J Radiol. 2021;22:1077–1086. doi: 10.3348/kjr.2020.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.