Abstract
Background and Aims
A third of patients with primary biliary cholangitis (PBC) experience poorly understood cognitive symptoms, with a significant impact on quality of life (QOL), and no effective medical treatment. Allopregnanolone, a neurosteroid, is a positive allosteric modulator of gamma-aminobutyricacid-A (GABA-A) receptors, associated with disordered mood, cognition, and memory. This study explored associations between allopregnanolone and a disease-specific QOL scoring system (PBC-40) in PBC patients.
Method
Serum allopregnanolone levels were measured in 120 phenotyped PBC patients and 40 age and gender-matched healthy controls. PBC subjects completed the PBC-40 at recruitment. Serum allopregnanolone levels were compared across PBC-40 domains for those with none/mild symptoms versus severe symptoms.
Results
There were no overall differences in allopregnanolone levels between healthy controls (median = 0.03 ng/ml (IQR = 0.025)) and PBC patients (0.031 (0.42), p = 0.42). Within the PBC cohort, higher allopregnanolone levels were observed in younger patients (r (120) = −0.53, p < 0.001) but not healthy controls (r (39) = −0.21, p = 0.21). Allopregnanolone levels were elevated in the PBC-40 domains, cognition (u = 1034, p = 0.02), emotional (u = 1374, p = 0.004), and itch (u = 795, p = 0.03). Severe cognitive symptoms associated with a younger age: severe (50 (12)) vs. none (60 (13); u = 423 p = 0.001).
Conclusion
Elevated serum allopregnanolone is associated with severe cognitive, emotional, and itch symptoms in PBC, in keeping with its known action on GABA-A receptors. Existing novel compounds targeting allopregnanolone could offer new therapies in severely symptomatic PBC, satisfying a significant unmet need.
1. Introduction
Primary biliary cholangitis (PBC) is a slowly progressive autoimmune liver disease resulting in obstructive cholangitis of the small intrahepatic ducts [1]; left untreated it leads to chronic cholestasis and progressive fibrosis. Fatigue and cognitive symptoms are frequently reported by patients with PBC [2, 3], and do not correlate with disease severity [2]. The PBC-40 [4], a fully validated disease-specific quality of life measure, suggests that clinically significant fatigue and/or cognitive symptoms affect over 50% and 30% of the PBC population, respectively [3, 5]. Patients presenting with symptoms are typically younger and may have more aggressive disease [6], whilst fatigue related to PBC is associated with increased all-cause mortality [7]. Recent data exploring health utility confirms that the greatest impact on functional status in PBC comes from fatigue and cognitive symptoms [8], with resultant social isolation giving rise to additional burdens and worsening quality of life [9].
Fatigue itself is complex and multi-faceted. Increasingly, it is recognised that fatigue associated with PBC has central and peripheral components, which are considered distinct pathological entities, but with subgroups of patients expressing both phenotypes [5]. Central fatigue results in “brain fog,” as a consequence of central nervous system (CNS) changes [2], whilst peripheral fatigue, “feeling like my batteries are running down,” may relate to a peripheral bioenergetic cause [10]. Cognitive symptoms in PBC, therefore, manifest in cognitive dysfunction, with difficulties relating to cognition and memory [2, 5], and are frequently associated with emotional symptoms [5]. Sleep disturbance is also common in PBC [11, 12], particularly in those with fatigue [3].
There are emerging data to suggest that even early disease-stage PBC patients with cognitive symptoms exhibit both anatomical and functional central nervous system (CNS) changes and autonomic dysfunction [13–18]. There are pronounced changes in PBC patients with cognitive symptoms [19], occurring as early as 6 months after disease diagnosis [20] with the lack of symptom improvement following liver transplantation suggesting that CNS damage may become irreversible at some point in the disease process [21–23].
Neurosteroids (synthesised in the brain from cholesterol independently of endocrine gland function) affect CNS function. Gamma-aminobutyricacid-A (GABA-A) receptors are the major biological target of the inhibitory neurotransmitter γ-aminobutyric acid and can be modulated by progesterone derivatives. Evidence shows them to be involved in altered sleep cycles [24], impaired cognition and memory [25, 26], fatigue in humans [27, 28], and mood disorders including premenstrual dysphoric disorder [24, 29, 30].
Allopregnanolone (3A-hydroxy-5A-pregnan-20-one), a neurosteroid and progesterone derivative, contributes to GABAergic tone [31], with elevated levels in the brain tissue of patients who died of hepatic encephalopathy (HE) [32, 33]. It has been hypothesised that fatigue related to PBC and hepatitis C (CHC) might be secondary to increased progesterone metabolites [28]. Serum allopregnanolone was found to be 80% higher in patients with PBC and CHC with significant fatigue when compared to age-matched controls, as measured using the fatigue impact scale (FIS) [34].
Cognitive symptoms remain a significant problem for many patients with PBC, with a detrimental impact on quality of life [8, 35, 36], no effective treatments [21, 37], and limited understanding of the underlying pathogenic mechanisms [38]. In this study, the relationship between PBC-associated cognitive symptoms and allopregnanolone was explored to examine whether this potentially modifiable pathway could be a target for novel therapies.
2. Methodology
2.1. Recruitment
A total of 160 serum samples were selected for allopregnanolone analysis. Of these, 120 samples were from patients with a confirmed clinical diagnosis of PBC (based on standard diagnostic criteria) [39]. This PBC cohort (comprised of 90 samples from the Birmingham and Newcastle Cohort (BANC) and 30 samples from the UK-PBC group) was phenotyped at the time of study recruitment. A further 40 healthy controls (age and gender-matched) were taken from the environmental risk factors for the aetiology of autoimmune liver disease (E-AILD). These cohorts were designed to support many diverse subprojects to explore the pathology of disease in PBC. For this pilot exploratory study, the sample size was defined by the availability of stored serum samples, with paired clinical data, in the described cohorts. All the samples used in this study had prior ethical approval for use, along with valid written informed consent obtained at the time of recruitment. This research was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice Guidelines and the Declaration of Helsinki.
The BANC cohort was established as a proof-of-concept resource recruited from Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK, and the Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK. It is comprised of a cross-sectional group of patients with an established diagnosis of PBC recruited between 2015 and 2017.
The UK-PBC Cohort (https://www.UK-PBC.com) is a large, prospective national cross-sectional cohort of PBC patients with detailed clinical data collection and was established to undertake studies of treatment efficacy in PBC. Within the cohort is a nested subcohort, termed the UK-PBC Nested Cohort, with additional bio-fluid sampling and banking to accompany the clinical data collection [40]. This nested cohort was designed to characterise the cellular and molecular response in PBC and facilitate the development of second-line therapies and biomarkers for more accurate stratification of treatment response and disease progression (REC ref 14/NW/1146). Recruitment was undertaken in 18 research centres, over 5 years, from 2014 to 2019.
The E-AILD cohort was established to identify disease clustering of patients within the northeast of England who had a confirmed diagnosis of autoimmune liver disease (PBC, autoimmune hepatitis, and primary sclerosing cholangitis). In conjunction, 105 healthy age and gender-matched volunteers were recruited, with optional serum samples taken. Recruitment occurred from 2015 to 2017.
All PBC patients at the point of recruitment to the relevant cohort had undertaken symptom stratification using the self-reporting PBC-40 questionnaire, which was contemporaneous with the time of serum sample collection. The PBC-40 categorises symptom severity as “none,” “mild,” “moderate” and “severe” in the following 6 domains: “cognition”; “emotional”; “itch”; “fatigue”; “social” and “general symptoms.” This score has been well described previously and the cut-offs for each domain and severity are validated [3, 4, 16] and defined in Table 1. Patients with “none” or “mild” symptoms are comparable to those found in the general population [3].
Table 1.
Defined scores ranges and cut-offs for PBC-40 domains.
| PBC -40 domain (number of items) | None | Mild | Moderate | Severe |
|---|---|---|---|---|
| Cognitive impairment (6) | <6 | 7–15 | 16–21 | >22 |
| Emotional (3) | <3 | 4–7 | 8–11 | >12 |
| Itch (3) | <3 | 4–8 | 9–11 | >11 |
| Fatigue (11) | <11 | 12–28 | 29–39 | >40 |
| Social (10) | <10 | 11–28 | 29–40 | >41 |
| General symptoms (7) | <7 | 8–18 | 19–25 | >26 |
2.2. Allopregnanolone Assay
A liquid chromatography–tandem mass spectrometry method was developed for the analysis of allopregnanolone in human plasma or serum (Admescope, Oulu, Finland). The samples were prepared for analysis by supported liquid extraction with ethyl acetate, followed by derivatization with hydroxylamine, as previously described [41].
Standard sample spiking solutions were prepared by diluting 1 mg/ml stock solution of allopregnanolone in dimethyl sulfoxide into acetonitrile at concentrations of 2 pg/ml to 100 ng/ml. 20 μl of spiking solutions were added into 180 μl of active charcoal purified human serum for standard and quality control samples in a 2 ml 96-well plate. After spiking, 10 μl of internal standard solution (100 ng/ml allopregnanolone-D5 in 50% methanol) was added to each sample and mixed for 5 minutes at 1000 rpm. Finally, the plate was centrifuged at 2200 g for 1 minute and 200 μl of the sample was transferred onto Novum simplified liquid extraction max (Phenomenex) plate and the samples were absorbed into the plate material using a vacuum at <5 in Hg for 5–10 seconds. The plate was allowed to stand for 5 minutes (without vacuum), after which two times 500 μl of ethyl acetate was allowed to flow through each well. Finally, a vacuum was applied for 20–30 seconds to allow the remaining ethyl acetate to flow through the plate. The collection plate was dried under nitrogen flow (20 L/h N2, 50°C) for 20 minutes, until dry. Finally, 150 μl of 100 mM hydroxylamine in 50% methanol was added into the wells and the plate was incubated at 65°C for 60 minutes with shaking (600 rpm) to derivatize allopregnanolone into oxime-derivative for improved analytical sensitivity [41]. Finally, the plate was centrifuged for 5 minutes at 2200 g and the supernatants were transferred into a clean 1 ml 96-well plate for analysis.
Increased sensitivity was reached by the use of UniSpray ion source in a XEVO-TQ-S triple quadrupole mass spectrometer. The obtained detection limit was 0.002 ng/mL and the quantitation limit was 0.005 ng/mL, i.e., 0.006 and 0.015 nmol/L, respectively. The method accuracies in the range of 0.005 to 10 ng/mL were 91.3–107.5% (n = 4). Similarly, the precision (n = 4) was determined to be 10.5–2.5% in the concentration range of 0.005 to 10 ng/mL.
2.3. Statistical Analyses
Baseline characteristics of the PBC cohort, healthy controls, and serum allopregnanolone levels were analysed. This included serum allopregnanolone levels in healthy controls versus the PBC cohort, and according to age and gender within each group.
Biochemical parameters (bilirubin, alanine aminotransferase (ALT), alkaline phosphatase (ALP), albumin, platelets, and prothrombin time) were recorded for all PBC patients at the time of recruitment. PBC patients were classified as a “responder” or “nonresponder” according to the POISE [42] criteria (responder defined as ALP ≤1.67 × upper limit normal (ULN) and/or bilirubin ≤1 × ULN) and were also classified as “normal” or “abnormal” liver function tests (normally defined as bilirubin, ALT and ALP all ≤1 × ULN) for additional analysis. Treatment with first-line therapy, Ursodeoxycholic acid (UDCA), was recorded at the time of recruitment, with a therapeutic dose considered to be 13–15 mg/kg daily, as per national and international guidelines [39]. The duration of treatment with UDCA was also available. Staging of the disease, in terms of cirrhosis and its potential complications, was recorded, with evidence from biochemistry, transient elastography, imaging, or histopathology.
All analyses were undertaken using SPSS (version 26) using the nonparametric tests, Mann–Whitney U, Spearman Rho, and Chi-square. Data are presented as the median value and interquartile range (IQR) unless otherwise specified, with the significance level set at p < 0.05.
3. Results
160 samples were successfully extracted, with one healthy control sample being excluded from further analysis due to significant variance. All other samples were included.
The basic demographics of the two groups (PBC patients and healthy controls) are summarised in Table 2. There was no significant difference in age between healthy controls and PBC patients (median 60 years IQR (15) versus 59 (13), p=0.33) or gender (U = 2236.5, p=0.35). Serum allopregnanolone levels did not statistically differ between healthy controls and PBC patients (0.03 ng/ml (0.025) versus 0.031 (0.042), U = 2541.5, p=0.42) and there was no significant difference between serum allopregnanolone levels according to gender (males (0.025 (0.033) versus females 0.032 (0.037), U = 898, p=0.57).
Table 2.
Allopregnanolone levels (ng/ml) by, group, gender, and age.
| Group | Gender | Number | Age∗ | Allopregnanolone (ng/ml)∗ | Significance+ |
|---|---|---|---|---|---|
| Healthy | Male | 4 (10%) | 60 (20) | 0.042 (0.088) | |
| Female | 35 (90%) | 60 (15) | 0.030 (0.023) | ||
| Both | 39 | 60 (15) | 0.030 (0.025) | r (39) = −0.21 p=0.21 | |
|
| |||||
| PBC | Male | 7 (5.8%) | 59 (18) | 0.025 (0.045) | |
| Female | 113 (94.2%) | 59 (13) | 0.032 (0.042) | ||
| Both | 120 | 59 (13) | 0.031 (0.042) | r (120) = −0.53 p < 0.001 | |
∗ median [IQR]; +analysis using Spearman's rho; bold denotes statistical significance.
Serum allopregnanolone levels did not vary with age in the healthy control group (p=0.21); however, within the PBC cohort, younger age was predictive of significantly higher serum allopregnanolone levels (p < 0.001) (Table 2 and Figure 1).
Figure 1.
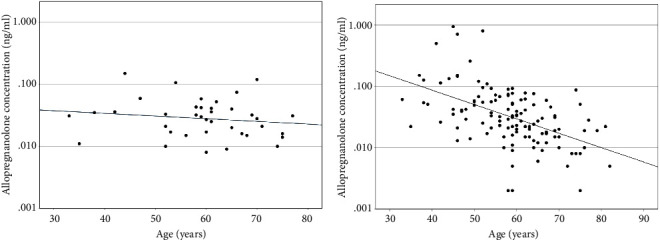
Serum allopregnanolone levels (ng/ml) according to age (years) (a) healthy cohort and (b) PBC cohort.
Younger PBC subjects were significantly more likely to suffer from “severe” symptoms as compared to those classified as “none” or “mild” when stratified by age in 5 of the PBC-40 domains: “cognition” (none/mild = 60 years (13 years) and severe = 50.5 (12); p = 0.001), “emotional” (none/mild = 64 (11) and severe = 57 (12); p < 0.001), “itch” (none/mild = 59 (13) and severe = 50.5 (13); p = 0.011), “fatigue” (none/mild = 60 (10), severe = 57.5 (13); p = 0.045), and “social” (none/mild = 64 (19) and severe = 57 (10); p = 0.040) (Table 3 and Figure 2). There was no statistically significant difference according to age for the “general symptoms” domain.
Table 3.
Age (years) and allopregnanolone (ng/ml) by PBC-40 domain.
| PBC-40 domain | Severity | Age (years) | Significance+ | Allopregnanolone level (ng/ml) | Significance+ |
|---|---|---|---|---|---|
| Cognition | None/mild | 60 (13) | p < 0.001 u = 423 | 0.024 (0.036) | p=0.019 u = 1034 |
| Severe | 50.5 (12) | 0.042 (0.044) | |||
|
| |||||
| Emotional | None/mild | 64 (11) | p < 0.001 u = 536 | 0.024 (0.038) | p=0.004 u = 1374 |
| Severe | 57 (12) | 0.039 (0.056) | |||
|
| |||||
| Itch | None/mild | 59 (13) | p=0.011 u = 315 | 0.030 (0.039) | p=0.03 u = 795 |
| Severe | 50.5 (13) | 0.050 (0.101) | |||
|
| |||||
| Fatigue | None/mild | 60 (10) | p=0.045 u = 602 | 0.027 (0.035) | p=0.50 u = 885 |
| Severe | 57.5 (13) | 0.035 (0.047) | |||
|
| |||||
| Social | None/mild | 64 (19) | p=0.04 u = 184 | 0.025 (0.036) | p=0.64 u = 277 |
| Severe | 57 (10) | 0.022 (0.031) | |||
|
| |||||
| General symptoms | None/mild | 59 (15) | p=0.6 u = 205 | 0.029 (0.040) | p=0.67 u = 214 |
| Severe | 57.5 (7) | 0.025 (0.084) | |||
Median (IQR); +analysis using Mann–Whitney U; bold denotes statistical significance.
Figure 2.
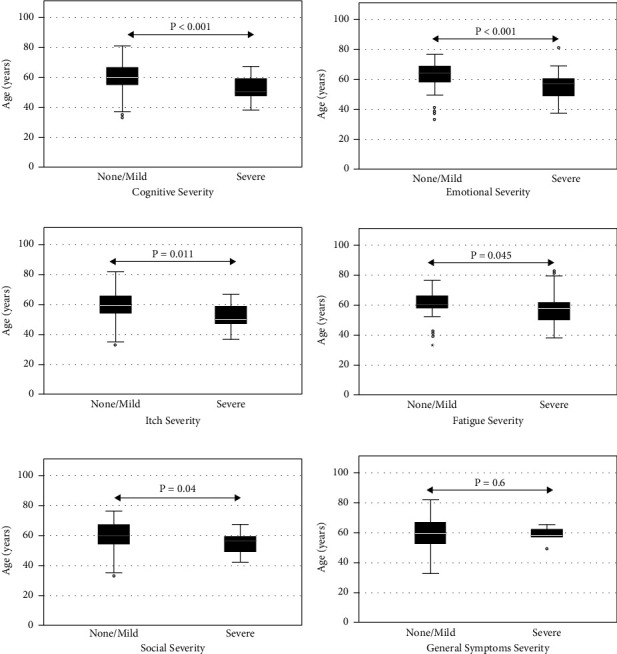
Symptom severity (none/mild vs. severe) as defined by the PBC-40 questionnaire domains, according to age (years). (a) Cognitive; (b) emotional; (c) itch; (d) fatigue; (e) social; (f) general symptoms.
Serum allopregnanolone levels in the PBC group were significantly higher for those with “severe” symptoms when compared to those who had “none” or “mild” symptoms in the 3 domains, “cognition” (none/mild = 0.024 ng/ml (0.036) and severe = 0.042 (0.044); p = 0.019), “emotional” (none/mild = 0.024 (0.038) and severe = 0.039 (0.056); p = 0.004), and “itch” (none/mild = 0.03 (0.039) and severe = 0.050 (0.101); p = 0.03) (Table 3 and Figure 3).
Figure 3.
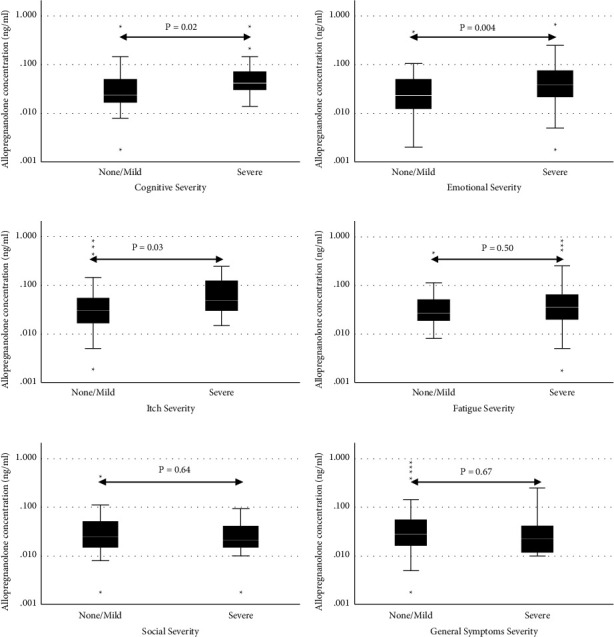
Symptom severity (none/mild vs. severe) as defined by the PBC-40 questionnaire domains, according to allopregnanolone levels (ng/ml). (a) Cognitive; (b) emotional; (c) itch; (d) fatigue; (e) social; (f) general symptoms.
Full details on UDCA treatment were available for 93/120 (78%) of PBC patients. Of these, 37/93 (40%) patients were on a therapeutic dose (13–15 mg/kg/day) of UDCA, whilst 56/93 (60%) of patients were considered subtherapeutic. There was no statistically significant difference in allopregnanolone levels according to total daily UDCA dose (r(102) = 0.162, p=0.10) or in those classified as therapeutic or subtherapeutic (Table 4).
Table 4.
Allopregnanolone (ng/ml) levels, by UDCA therapy, POISE criteria, liver blood tests, and cirrhosis.
| Status | Number of participants | Allopregnanolone (ng/ml)∗ | Significance+ |
|---|---|---|---|
| UDCA therapy | 93 | ||
| Therapeutic dose | 37 (40%) | 0.033 (0.044) | p=0.58 u = 965 |
| Subtherapeutic dose | 56 (60%) | 0.031 (0.045) | |
|
| |||
| POISE criteria | |||
| Responder | 79 (72%) | 0.025 (0.043) | p=0.141 u = 1446 |
| Nonresponder | 31 (28%) | 0.037 (0.49) | |
|
| |||
| Abnormal LFTs | |||
| Normal | 42 (38%) | 0.022 (0.032) | p=0.075 u = 1717 |
| Abnormal | 68 (62%) | 0.036 (0.048) | |
|
| |||
| Cirrhosis | |||
| Noncirrhotic | 99 (90%) | 0.031 (0.041) | p=0.098 u = 379 |
| Cirrhotic | 11 (10%) | 0.012 (0.049) | |
∗ median (IQR); POISE criteria: ALP ≤ 1.67 × upper limit normal (ULN) and/or bilirubin ≤ 1 × ULN; LFT: liver function tests; +analysis using Mann–Whitney U.
Complete biochemistry, disease response, and disease staging were available for 110/120 (92%) of PBC patients. Using the POISE criteria, 31/110 (28%) patients were classified as “nonresponders,” whilst 79/110 (72%) were classified as “responders.” Overall, “nonresponders” were significantly younger than “responders” (55 (11) versus 60 (13) years; u = 765, p=0.002), but levels of serum allopregnanolone were not significantly related to biochemical response status (Table 4). There was no statistically significant difference between response status and symptom severity in any of the PBC-40 domains, apart from itch (p=0.004) (Table 5). There were 68/110 (62%) PBC patients with at least one abnormality of their liver biochemistry (ALP, ALT, or bilirubin) but there was no statistically significant difference in serum allopregnanolone when grouped by “abnormal” or “normal” liver function tests (Table 4). There was no statistically significant difference between normal and abnormal liver blood tests and symptom severity in any of the PBC-40 domains (Table 5).
Table 5.
POISE response, liver blood tests, and cirrhosis by PBC-40 domain severity.
| PBC-40 domain | Severity | POISE response (n = 110) | Liver blood tests (n = 110) | Cirrhosis (n = 110) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Responders (n) | Nonresponders (n) | Significance+ | Normal (n) | Abnormal (n) | Significance+ | Noncirrhotic (n) | Cirrhotic (n) | Significance+ | ||
| Cognition | None/mild | 43 | 15 | p=0.30 X(1) = 1.11 | 20 | 38 | p=0.40 x(1) = 0.705 | 52 | 6 | p=0.43 X(1) = 0.63 |
| Severe | 15 | 9 | 6 | 18 | 20 | 4 | ||||
|
| ||||||||||
| Emotional | None/mild | 35 | 11 | p=0.35 X(1) = 0.89 | 17 | 29 | p=0.54 x(1) = 0.37 | 42 | 4 | p=0.46 X(1) = 0.55 |
| Severe | 24 | 12 | 11 | 25 | 31 | 5 | ||||
|
| ||||||||||
| Itch | None/mild | 71 | 18 | p=0.004 X(1) = 8.25 | 39 | 50 | p=0.07 x(1) = 3.23 | 83 | 6 | p=0.23 X(1) = 1.43 |
| Severe | 5 | 7 | 2 | 10 | 10 | 2 | ||||
|
| ||||||||||
| Fatigue | None/mild | 25 | 9 | p=0.72 X(1) = 0.13 | 13 | 21 | p=0.91 x(1) = 0.013 | 33 | 1 | p=0.43 X(1) = 0.628 |
| Severe | 30 | 13 | 17 | 26 | 40 | 3 | ||||
|
| ||||||||||
| Social | None/mild | 41 | 13 | p=0.70 X(1) = 0.15 | 19 | 54 | p=0.75 x(1) = 0.10 | 51 | 3 | p=0.015 X(1) = 5.93 |
| Severe | 7 | 3 | 3 | 7 | 7 | 3 | ||||
|
| ||||||||||
| General symptoms | None/mild | 54 | 19 | p=0.21 X(1) = 1.59 | 30 | 43 | p=0.24 x(1) = 1.39 | 67 | 6 | p=0.05 X(1) = 3.84 |
| Severe | 3 | 3 | 1 | 5 | 4 | 2 | ||||
POISE criteria: ALP ≤ 1.67 × upper limit normal (ULN) and/or bilirubin ≤ 1 × ULN; +analysis using Chi-square; bold denotes statistical significance.
A total of 11/110 (9%) of PBC patients had an established diagnosis of cirrhosis, but none of these had a diagnosis of hepatic encephalopathy. There was no significant difference in serum allopregnanolone levels between cirrhotic and noncirrhotic PBC patients (Table 4) but patients with cirrhosis were statistically more likely to score severely on the PBC-40 social domain (none/mild = 6/73 cirrhosis and severe 2/6 cirrhosis; p=0.015) (Table 5).
Logistic regression was performed to test the association between allopregnanolone and symptom severity in the 6 PBC-40 domains when corrected for age. Those with higher serum allopregnanolone levels were 5 (95% CI: 0.03–852; p=0.53) times more likely to score severely in the cognitive domain and 5.3 (95% CI: 0.02–1285; p=0.55) times more likely to score severely on the emotional domain, however, this was not statistically significant.
Differences in serum allopregnanolone between the PBC-40 domains stratified by “none/mild,” “moderate” and “severe” severity of symptoms were explored. Serum allopregnanolone levels were statistically significantly higher with increasing severity of symptoms in the emotional domain (none/mild = 0.024 ng/ml (0.038), moderate = 0.031 (0.046), and severe = 0.039 (0.056); p=0.015). Those with moderate symptoms in the social domain were associated with statistically significantly higher serum allopregnanolone levels, but not in the severe domain (none/mild = 0.025 ng/ml (0.036), moderate = 0.039 (0.063), and severe = 0.031 (0.031); p=0.02) (see Supplementary Figure 1 and Supplementary Table 1).
4. Discussion
Despite the improvements in disease-modifying therapies for PBC in recent years, there has been no therapeutic advance for the fatigue or cognitive symptoms that affect many patients [38]. It is well described that these symptoms have a marked impact on patients' quality of life and ability to undertake activities of daily living. Prognostically, those presenting with symptoms of fatigue (central and peripheral) and/or pruritus are typically younger, with evidence of more active disease and an increased risk of complications [6], whilst fatigue (central and peripheral) related to PBC is independently associated with an increased all-cause mortality [7]. Objective neuropsychiatric tests demonstrate impairment in memory and orientation in PBC patients with cognitive fatigue [43]. Whilst perceived cognitive symptoms, as quantified by self-reporting questionnaires such as the PBC-40 (used here), correlate to objective impairments in attention, concentration, visuomotor co-ordination, perception, and motor and mental speed [2]. This study has demonstrated an association between elevated serum allopregnanolone levels and increased symptom severity in PBC, suggesting that novel therapies that antagonise allopregnanolone, resulting in the modification of GABA-A receptor activity, may provide a potential therapeutic target in this area of significant unmet need.
Key findings from the study were that a younger age in the PBC group, along with severe cognitive, emotional, and itch symptoms were significantly associated with higher allopregnanolone levels. As seen in the PBC population as a whole, younger age was associated with worse biochemistry in PBC [44] (denoting higher risk disease [39]), but despite this, there was no correlation between allopregnanolone levels and the degree of biochemical abnormality (according to POISE criteria or normal versus abnormal liver blood tests), treatment with UDCA, or cirrhosis. This may suggest that it is a symptom, but not disease severity, that is linked to allopregnanolone levels.
In keeping with previous work, there were no significant differences in allopregnanolone levels in the healthy controls according to age or gender [45].
Previous studies have demonstrated elevated levels of serum allopregnanolone and pregnanolone in patients with PBC, with allopregnanolone most strongly associated with fatigue severity in PBC as assessed by the fatigue impact scale (FIS). The FIS is not disease-specific and includes assessments relating to both peripheral and central fatigue, and this may account for the correlation between fatigue scores and serum allopregnanolone. In this study, fatigue severity was not associated with elevated serum allopregnanolone levels. However, the PBC-40 is disease-specific (unlike the FIS) with specific domains relating to fatigue and cognitive symptoms, which may explain these differences. The association with serum allopregnanolone and emotional symptoms may be a direct consequence of elevated serum allopregnanolone on the CNS, but in PBC there is a complex interplay between symptom domains, with previous studies demonstrating a significant emotional burden associated with fatigue and cognitive symptoms [5, 9].
Severe itch was significantly associated with nonresponder status in the PBC patients, as seen previously [46, 47], and this may be related to worse cholestasis, reflected in the corresponding biochemical parameters. Cirrhosis was associated with increased severity in the PBC-40 social domain, mirroring previous work showing cirrhosis to be associated with increased social isolation [48].
Reduced central nervous system (CNS) activation is associated with cognitive dysfunction in many conditions [49]. Different forms of transcranial magnetic stimulation (TMS) can be used to modulate cortical pathways and affect neuronal plasticity [50, 51]. In particular, paired pulse protocols with TMS result in the modulation of GABA-A receptors [50]. Reduced central activation and abnormal intracortical inhibition have been demonstrated with TMS in both transplanted and nontransplanted PBC patients with cognitive dysfunction, fatigue, and sleep disturbance, suggesting impaired intracortical inhibition as a potential cause [22]. This could be a consequence of elevated allopregnanolone in a subset of PBC patients, as demonstrated here, contributing to the inhibition of GABA-A receptors and thus cortical pathways due to its positive allosteric action. This is further supported by emerging evidence that changes in cortical networks occur within PBC patients with cognitive dysfunction and fatigue, with increases in resting-state functional MRI (rsfMRI) and resting-state functional connectivity (rsFC) demonstrated in areas of the brain involved in memory, learning, and emotional processing [52].
There is already data suggesting that modulation of GABA-A receptors and allopregnanolone may have therapeutic effects on cognition. In animal studies, reversal of induced dysfunction in spatial learning, memory, and motor co-ordination was achieved with GR3027 (a selective antagonist postulated to reverse the GABA-A receptor modification occurring secondary to elevated allopregnanolone) [53]. Human studies have demonstrated that injection of allopregnanolone has an adverse effect on memory [26] and decreases saccadic eye velocity and induces somnolence that was largely reversed using GR3027 [27]. Furthermore, a recent pharmacokinetics pilot study for the use of GR3027 (subsequently branded as golexanolone), in patients with covert hepatic encephalopathy secondary to cirrhosis demonstrated that golexanolone was safe and well tolerated and led to statistically significant improvements in daytime somnolence and electroencephalogram readings (delta and theta waves) when compared to placebo [54]. Given the emerging data that golexanolone improves cognitive symptoms, through the antagonistic effects of allopregnanolone on GABA-A receptors, a further study to confirm the findings presented here should be undertaken and, if validated, then a proof-of-concept trial to evaluate the effect of golexanolone on symptomatic PBC should be explored.
The correlation between severe itch and elevated serum allopregnanolone levels is an interesting finding. There is limited research into the effect of elevated serum allopregnanolone and itch of any aetiology. Early animal studies have demonstrated that injections of allopregnanolone result in dose-dependent marked scratching behaviour in atopic dermatitis mice, whilst administration of 2-methyl-5-HT (a tryptamine derivative closely related to serotonin that acts as a moderate 5HT3 agonist) almost completely suppressed the allopregnanolone-induced scratching [55]. Allopregnanolone had no effect on histamine-induced scratching. Selective serotonin reuptake inhibitors (SSRIs) form part of the treatment ladder for PBC-associated pruritus [39] and this study may provide insight into their mechanism of action. Associations with itch severity and the functional connections of the sensory and premotor cortices have previously been demonstrated in studies and suggest a potential central mechanism for itch in PBC [52]. Our understanding of itch in PBC remains incomplete and this discovery points to a potential mechanism with cholestasis that warrants further investigation as a potential treatment pathway.
The mechanism for elevated allopregnanolone levels in PBC remains unclear. Neurosteroids are synthesised in the nervous system de novo from cholesterol (the biosynthesis of which mostly occurs within the endoplasmic reticulum of hepatic cells and the adrenal glands) or from the accumulation of steroid precursors such as progesterone in the CNS [56]. Progesterone and oestradiol are both metabolised in the liver and have been demonstrated to be increased in end-stage liver disease, with levels of progesterone improving following transplantation [57]. However, a corresponding improvement in cognitive symptoms does not occur, suggesting that by this point CNS damage is irreversible. This phenomenon of irreversible cognitive impairment has been replicated in wild-type mice receiving continuous allopregnanolone treatment [58]. Translocator protein (TSPO), a nucleoside transporter located on the outer mitochondrial membrane, is upregulated in the CNS in response to neuro-inflammation, especially in microglial cells [59, 60]. TSPO regulates the transport of cholesterol into mitochondria, a rate-limiting step in the synthesis of neurosteroids, including allopregnanolone [59] (See Figure 4), whilst elevated serum cholesterol is commonly seen in PBC patients [61]. A potential mechanism resulting in neuro-inflammation in liver disease patients with central fatigue has previously been proposed [62]. Recent data using bile duct ligation (BDL) in rodents (an established model of cholestasis) supports these postulated mechanisms, with BDL rodents developing cognitive symptoms early in the disease process, which was associated with disruption of the blood-brain-barrier and hippocampal dysfunction [63]. This might suggest a mechanistic explanation for the increased allopregnanolone levels seen in this study and is an area that warrants further investigation.
Figure 4.
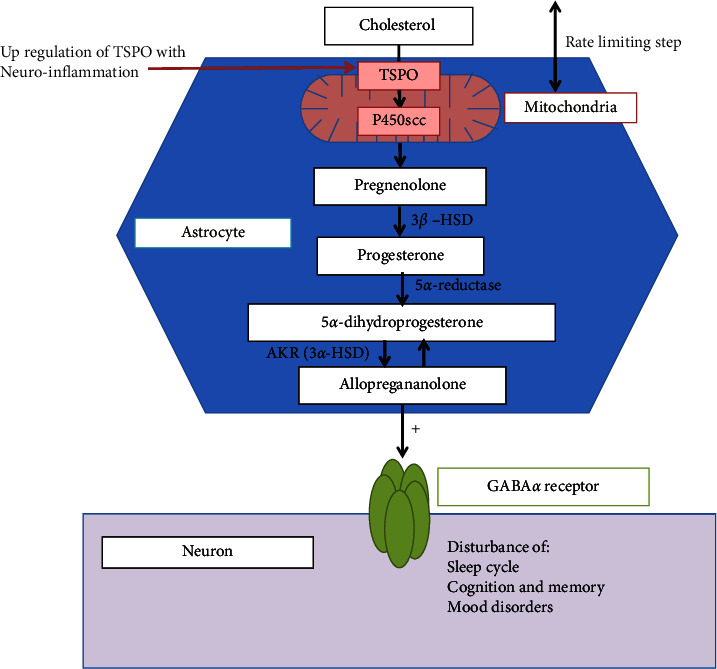
De novo synthesis of allopregnanolone in the CNS. Cholesterol is transported across the mitochondrial membrane by the nucleoside transporter, translocator protein (TSPO) [60], and converted to pregnenolone within the mitochondria of glial cells, such as astrocytes, by side chain cleavage of cytochrome P450 (P450scc). Pregnenolone is subsequently converted to progesterone by 3β-hydroxysteroid dehydrogenases (3β-HSD). 5α-reductase type 1, converts Progesterone to 5α-dihydroprogesterone (5α-DHP), 5α-DHP is then converted to Allopregnanolone by the aldo-keto reductases enzyme, 3α-hydroxysteroid dehydrogenase (3α-HSD). Allopregnanolone activates GABA-A receptor intracellular sites by lateral diffusion in the neuronal membrane, where it acts as a potent allosteric modulator. Allopregnanolone can eventually be reconverted back to 5α-DHP by 3α-HSD [56].
In previous PBC studies, pregnanolone metabolites (isopregnanolone, epipregnanolone, and tetrahydrodoxycorticosterone) were undetectable [34, 64]. Analysis of other steroidal molecules, such as isopregnanolone, the precursor steroids in the synthesis of allopregnanolone described in Figure 4, and the associated degradation molecules, were beyond the scope of this pilot study, where the primary outcome was to confirm the previously found association of elevated allopregnanolone and symptoms in PBC but would be an interesting area for future research.
This study utilised a representative cohort of PBC patients with features in keeping with previous studies such as younger age associated with worse biochemistry [23], higher rate of UDCA nonresponse in younger patients [3, 6, 23], and more severe symptoms in younger PBC patients [3, 6]. Whilst there was an increased odds ratio for higher serum allopregnanolone in those with severe cognitive and emotional symptoms when corrected for age, these did not reach statistical significance, however, this may be affected by the relatively small sample size in each group.
There are limitations to this study that must be acknowledged. Allopregnanolone levels may be affected by normal physiological conditions such as the menstrual cycle, pregnancy (including the postpartum period), and menopause along with conditions such as social isolation, acute stress, and depression [56, 65]. This study did not take into account the effects of these potential influences or the impact of concomitant medications that patients may have been taking. These potential confounding factors for increased allopregnanolone levels were outside the scope of this pilot study. Whilst there were no significant differences in allopregnanolone levels according to gender in either group, the relatively small number of men in this study (in keeping with the epidemiology of PBC) makes it difficult to draw conclusions. Likewise, it is possible that the lack of significant difference in allopregnanolone levels according to age in the healthy controls could be biased due to the relatively small cohort size.
5. Conclusion
It remains unclear why some patients with PBC suffer from cognitive symptoms, whilst others do not. Disease severity bares no relationship to cognitive symptom burden, yet cognition and fatigue have a significant impact on QOL, are associated with increased all-cause mortality, and are an important unmet need for patients. Younger age is strongly associated with more severe cognitive symptoms in PBC, but age is not a modifiable risk factor. It has previously been postulated that abnormal peripheral signalling pathways exist between the diseased liver and brain resulting in changes in neurotransmission within the brain. We have demonstrated elevated allopregnanolone levels in a cohort of PBC patients with severe symptoms, as defined by the fully validated PBC-40 symptoms questionnaire, in relation to cognition, emotion, and itch. The activation of GABA-A receptors by neurosteroids may represent a potentially modifiable factor in cognitive symptoms in PBC and, given the advent of treatments capable of modulating neurosteroids, may offer a potential future therapy to address a significant unmet need.
Acknowledgments
This work was supported by Umecrine Cognition AB, Solna, Sweden.
Data Availability
All presented data are available upon request, pending approval of proposed use and signed data access agreement. Application for access to data to be made via the corresponding author.
Conflicts of Interest
Torbjörn Bäckström is a board member and shareholder of Umecrine Cognition AB. Magnus Doverskog and Maja Johansson are current or recent employees of Umecrine Cognition AB. Professor David Jones has consulted for Intercept, Abbot, Calliditas, and GSK and received lecture fees from Intercept, Falk, Abbot, and Calliditas and received grant funding from Intercept. Dr. Jessica Dyson has received speaker fees from Dr. Falk Pharma and Intercept.
Supplementary Materials
Differences in serum allopregnanolone and the PBC-40 domains stratified by “none/mild,” “moderate” and “severe” severity of symptoms are outlined in supplementary figure 1 and supplementary Table 1. Supplementary Figure 1: symptom severity (None/Mild vs. Moderate vs. Severe) as defined by the PBC-40 questionnaire domains, according to allopregnanolone levels (ng/ml). (A) Cognitive; (B) emotional; (C) itch; (D) fatigue; (E) social; (F) general symptoms. Supplementary Table 1: serum allopregnanolone (ng/ml) by PBC-40 domain severity.
References
- 1.Carey E. J., Ali A. H., Lindor K. D. Primary biliary cirrhosis. The Lancet . 2015;386(10003):1565–1575. doi: 10.1016/s0140-6736(15)00154-3. [DOI] [PubMed] [Google Scholar]
- 2.Newton J. L., Hollingsworth K. G., Taylor R., et al. Cognitive impairment in primary biliary cirrhosis: symptom impact and potential etiology. Hepatology . 2008;48(2):541–549. doi: 10.1002/hep.22371. [DOI] [PubMed] [Google Scholar]
- 3.Mells G. F., Pells G., Newton J. L., et al. Impact of primary biliary cirrhosis on perceived quality of life: the UK-PBC national study. Hepatology . 2013;58(1):273–283. doi: 10.1002/hep.26365. [DOI] [PubMed] [Google Scholar]
- 4.Jacoby A., Rannard A., Buck D., et al. Development, validation, and evaluation of the PBC-40, a disease specific health related quality of life measure for primary biliary cirrhosis. Gut . 2005;54(11):1622–1629. doi: 10.1136/gut.2005.065862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phaw N. A., Dyson J. K., Mells G., Jones D. Understanding fatigue in primary biliary cholangitis. Digestive Diseases and Sciences . 2020;66 doi: 10.1007/s10620-020-06502-0. [DOI] [PubMed] [Google Scholar]
- 6.Quarneti C., Muratori P., Lalanne C., et al. Fatigue and pruritus at onset identify a more aggressive subset of primary biliary cirrhosis. Liver International . 2015;35(2):636–641. doi: 10.1111/liv.12560. [DOI] [PubMed] [Google Scholar]
- 7.Jones D. E., Al-Rifai A., Frith J., Patanwala I., Newton J. L. The independent effects of fatigue and UDCA therapy on mortality in primary biliary cirrhosis: results of a 9 year follow-up. Journal of Hepatology . 2010;53(5):911–917. doi: 10.1016/j.jhep.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Rice S., Albani V., Minos D., et al. Effects of primary biliary cholangitis on quality of life and health care costs in the United Kingdom. Clinical Gastroenterology and Hepatology . 2021;19(4):768–776. doi: 10.1016/j.cgh.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Dyson J. K., Wilkinson N., Jopson L., et al. The inter-relationship of symptom severity and quality of life in 2055 patients with primary biliary cholangitis. Alimentary Pharmacology & Therapeutics . 2016;44(10):1039–1050. doi: 10.1111/apt.13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollingsworth K. G., Newton J. L., Robinson L., Taylor R., Blamire A. M., Jones D. E. Loss of capacity to recover from acidosis in repeat exercise is strongly associated with fatigue in primary biliary cirrhosis. Journal of Hepatology . 2010;53(1):155–161. doi: 10.1016/j.jhep.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Newton J. L., Gibson G. J., Tomlinson M., Wilton K., Jones D. Fatigue in primary biliary cirrhosis is associated with excessive daytime somnolence. Hepatology . 2006;44(1):91–98. doi: 10.1002/hep.21230. [DOI] [PubMed] [Google Scholar]
- 12.Montagnese S., Nsemi L. M., Cazzagon N., et al. Sleep-Wake profiles in patients with primary biliary cirrhosis. Liver International . 2013;33(2):203–209. doi: 10.1111/liv.12026. [DOI] [PubMed] [Google Scholar]
- 13.Keresztes K. Autonomic and sensory nerve dysfunction in primary biliary cirrhosis. World Journal of Gastroenterology . 2004;10(20):p. 3039. doi: 10.3748/wjg.v10.i20.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kempler P., Varadi A., Kadar E., Szalay F. Autonomic and peripheral neuropathy in primary biliary cirrhosis: evidence of small sensory fibre damage and prolongation of the QT interval. Journal of Hepatology . 1994;21(6):1150–1151. doi: 10.1016/s0168-8278(05)80640-3. [DOI] [PubMed] [Google Scholar]
- 15.Newton J. L., Allen J., Kerr S., Jones D. E. J. Reduced heart rate variability and baroreflex sensitivity in primary biliary cirrhosis. Liver International . 2006;26(2):197–202. doi: 10.1111/j.1478-3231.2005.01214.x. [DOI] [PubMed] [Google Scholar]
- 16.Newton J. L., Hudson M., Tachtatzis P., et al. Population prevalence and symptom associations of autonomic dysfunction in primary biliary cirrhosis. Hepatology . 2007;45(6):1496–1505. doi: 10.1002/hep.21609. [DOI] [PubMed] [Google Scholar]
- 17.Newton J. L., Davidson A., Kerr S., et al. Autonomic dysfunction in primary biliary cirrhosis correlates with fatigue severity. European Journal of Gastroenterology and Hepatology . 2007;19(2):125–132. doi: 10.1097/01.meg.0000252629.96043.67. [DOI] [PubMed] [Google Scholar]
- 18.Horsfield M. A. Magnetization transfer imaging in multiple sclerosis. Journal of Neuroimaging . 2005;15:58S–67S. doi: 10.1177/1051228405282242. [DOI] [PubMed] [Google Scholar]
- 19.Forton D. M. Fatigue and primary biliary cirrhosis: association of globus pallidus magnetisation transfer ratio measurements with fatigue severity and blood manganese levels. Gut . 2004;53(4):587–592. doi: 10.1136/gut.2003.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grover V. P. B., Southern L., Dyson J. K., et al. Early primary biliary cholangitis is characterised by brain abnormalities on cerebral magnetic resonance imaging. Alimentary Pharmacology & Therapeutics . 2016;44(9):936–945. doi: 10.1111/apt.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carbone M., Bufton S., Monaco A., Griffiths L., Jones D. E., Neuberger J. M. The effect of liver transplantation on fatigue in patients with primary biliary cirrhosis: a prospective study. Journal of Hepatology . 2013;59(3):490–494. doi: 10.1016/j.jhep.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Mcdonald C., Newton J., Lai H. M., Baker S. N., Jones D. E. Central nervous system dysfunction in primary biliary cirrhosis and its relationship to symptoms. Journal of Hepatology . 2010;53(6):1095–1100. doi: 10.1016/j.jhep.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 23.Pells G., Mells G. F., Carbone M., et al. The impact of liver transplantation on the phenotype of primary biliary cirrhosis patients in the UK-PBC cohort. Journal of Hepatology . 2013;59(1):67–73. doi: 10.1016/j.jhep.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology . 2003;28(2):139–168. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- 25.Johansson I.-M., Birzniece V., Lindblad C., Olsson T., Backstrom T. Allopregnanolone inhibits learning in the Morris water maze. Brain Research . 2002;934(2):125–131. doi: 10.1016/s0006-8993(02)02414-9. [DOI] [PubMed] [Google Scholar]
- 26.Kask K., Backstrom T., Nilsson L. G., Sundstrom-Poromaa I. Allopregnanolone impairs episodic memory in healthy women. Psychopharmacology . 2008;199(2):161–168. doi: 10.1007/s00213-008-1150-7. [DOI] [PubMed] [Google Scholar]
- 27.Johansson M., Mansson M., Lins L. E., Scharschmidt B., Doverskog M., Backstrom T. GR3027 reversal of neurosteroid-induced, GABA-Areceptor-mediated inhibition of human brain function: an allopregnanolone challenge study. Psychopharmacology (Berl) . 2018;235(5):1533–1543. doi: 10.1007/s00213-018-4864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy B. Elevated levels of some neuroactive progesterone metabolites, particularly isopregnanolone, in women with chronic fatigue syndrome. Psychoneuroendocrinology . 2004;29(2):245–268. doi: 10.1016/s0306-4530(03)00026-x. [DOI] [PubMed] [Google Scholar]
- 29.Walton N., Maguire J. Allopregnanolone-based treatments for postpartum depression: why/how do they work? Neurobiology of Stress . 2019;11 doi: 10.1016/j.ynstr.2019.100198.100198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bäckström T., Bixo M., Strömberg J. GABAA receptor-modulating steroids in relation to women’s behavioral health. Current Psychiatry Reports . 2015;17(11):p. 92. doi: 10.1007/s11920-015-0627-4. [DOI] [PubMed] [Google Scholar]
- 31.Jones E. A. Ammonia, the GABA neurotransmitter system, and hepatic encephalopathy. Metabolic Brain Disease . 2002;17(4):275–281. doi: 10.1023/a:1021949616422. [DOI] [PubMed] [Google Scholar]
- 32.Ahboucha S., Pomier-Layrargues G., Mamer O., Butterworth R. F. Increased levels of pregnenolone and its neuroactive metabolite allopregnanolone in autopsied brain tissue from cirrhotic patients who died in hepatic coma. Neurochemistry International . 2006;49(4):372–378. doi: 10.1016/j.neuint.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Ahboucha S., Layrargues G. P., Mamer O., Butterworth R. F. Increased brain concentrations of a neuroinhibitory steroid in human hepatic encephalopathy. Annals of Neurology . 2005;58(1):169–170. doi: 10.1002/ana.20534. [DOI] [PubMed] [Google Scholar]
- 34.Ahboucha S., Butterworth R. F., Pomier-Layrargues G., Vincent C., Hassoun Z., Baker G. B. Neuroactive steroids and fatigue severity in patients with primary biliary cirrhosis and hepatitis C. Neuro-Gastroenterology and Motility . 2008;20(6):671–679. doi: 10.1111/j.1365-2982.2007.01080.x. [DOI] [PubMed] [Google Scholar]
- 35.Jopson L., Jones D. E. Fatigue in primary biliary cirrhosis: prevalence, pathogenesis and management. Digestive Diseases . 2015;33(2):109–114. doi: 10.1159/000440757. [DOI] [PubMed] [Google Scholar]
- 36.Goldblatt J., Taylor P. J., Lipman T., et al. The true impact of fatigue in primary biliary cirrhosis: a population study. Gastroenterology . 2002;122(5):1235–1241. doi: 10.1053/gast.2002.32993. [DOI] [PubMed] [Google Scholar]
- 37.Khanna A., Jopson L., Howel D., et al. Rituximab is ineffective for treatment of fatigue in primary biliary cholangitis: a phase 2 randomized controlled trial. Hepatology . 2019;70(5):1646–1657. doi: 10.1002/hep.30099. [DOI] [PubMed] [Google Scholar]
- 38.Wetten A., Jones D. E. J., Dyson J. K. Specific considerations for the management of primary biliary cholangitis: are the drug treatment options good enough? Expert Opinion on Pharmacotherapy . 2021;22(15):1949–1953. doi: 10.1080/14656566.2021.1940135. [DOI] [PubMed] [Google Scholar]
- 39.Hirschfield G. M., Dyson J. K., Alexander G. J. M., et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut . 2018;67(9):1568–1594. doi: 10.1136/gutjnl-2017-315259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liaskou E., Patel S. R., Webb G., et al. Increased sensitivity of Treg cells from patients with PBC to low dose IL-12 drives their differentiation into IFN-γ secreting cells. Journal of Autoimmunity . 2018;94:143–155. doi: 10.1016/j.jaut.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 41.Keski-Rahkonen P., Huhtinen K., Poutanen M., Auriola S. Fast and sensitive liquid chromatography–mass spectrometry assay for seven androgenic and progestagenic steroids in human serum. The Journal of Steroid Biochemistry and Molecular Biology . 2011;127(3-5):396–404. doi: 10.1016/j.jsbmb.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Bowlus C. L., Pockros P. J., Kremer A. E., et al. Long-Term obeticholic acid therapy improves histological endpoints in patients with primary biliary cholangitis. Clinical Gastroenterology and Hepatology . 2020;18(5):1170–1178. doi: 10.1016/j.cgh.2019.09.050. [DOI] [PubMed] [Google Scholar]
- 43.Floreani A., Marchiori M., Bonato S., Zucchetto M., Naccarato R., Chiaramonte M. Cognitive assessment in primary biliary cirrhosis: a case-control study. American Journal of Gastroenterology . 1995;90(2):250–253. [PubMed] [Google Scholar]
- 44.Carbone M., Mells G. F., Pells G., et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology . 2013;144(3):560–569. doi: 10.1053/j.gastro.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Genazzani A. R., Petraglia F., Bernardi F., et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. Journal of Clinical Endocrinology and Metabolism . 1998;83(6):2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- 46.Leuschner M. Characterisation of patients with primary biliary cirrhosis responding to long term ursodeoxycholic acid treatment. Gut . 2000;46(1):121–126. doi: 10.1136/gut.46.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vleggaar F. P., van Buuren H. R., Zondervan P. E., ten Kate F. J. W., Hop W. C. J. Jaundice in non-cirrhotic primary biliary cirrhosis: the premature ductopenic variant. Gut . 2001;49(2):276–281. doi: 10.1136/gut.49.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaughn-Sandler V., Sherman C., Aronsohn A., Volk M. L. Consequences of perceived stigma among patients with cirrhosis. Digestive Diseases and Sciences . 2014;59(3):681–686. doi: 10.1007/s10620-013-2942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zwarts M. J., Bleijenberg G., Van Engelen B. G. M. Clinical neurophysiology of fatigue. Clinical Neurophysiology . 2008;119(1):2–10. doi: 10.1016/j.clinph.2007.09.126. [DOI] [PubMed] [Google Scholar]
- 50.Di Lazzaro V., Rothwell J., Capogna M. Noninvasive stimulation of the human brain: activation of multiple cortical circuits. The Neuroscientist . 2018;24(3):246–260. doi: 10.1177/1073858417717660. [DOI] [PubMed] [Google Scholar]
- 51.Rossi S., Hallett M., Rossini P. M., Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology . 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mosher V. A. L., Swain M. G., Pang J. X. Q., et al. Primary biliary cholangitis alters functional connections of the brain’s deep gray matter. Clinical and Translational Gastroenterology . 2017;8(7):p. e107. doi: 10.1038/ctg.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johansson M., Agusti A., Llansola M., et al. GR3027 antagonizes GABAA receptor-potentiating neurosteroids and restores spatial learning and motor coordination in rats with chronic hyperammonemia and hepatic encephalopathy. American Journal of Physiology - Gastrointestinal and Liver Physiology . 2015;309(5):G400–G409. doi: 10.1152/ajpgi.00073.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montagnese S., Lauridsen M., Vilstrup H., et al. A pilot study of golexanolone, a new GABA-Areceptor-modulating steroid antagonist, in patients with covert hepatic encephalopathy. Journal of Hepatology . 2021;75(1):98–107. doi: 10.1016/j.jhep.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Fujii M., Ohgami S., Asano E., et al. Brain allopregnanolone induces marked scratching behaviour in diet-induced atopic dermatitis mouse model. Scientific Reports . 2019;9(1):p. 2364. doi: 10.1038/s41598-019-38858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paul S. M., Pinna G., Guidotti A. Allopregnanolone: from molecular pathophysiology to therapeutics. A historical perspective. Neurobiology of Stress . 2020;12 doi: 10.1016/j.ynstr.2020.100215.100215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aller R., de Luis D. A., Moreira V., et al. The effect of liver transplantation on circulating levels of estradiol and progesterone in male patients: parallelism with hepatopulmonary syndrome and systemic hyperdynamic circulation improvement. Journal of Endocrinological Investigation . 2001;24(7):503–509. doi: 10.1007/bf03343883. [DOI] [PubMed] [Google Scholar]
- 58.Bengtsson S. K., Johansson M., Backstrom T. Long-term continuous allopregnanolone elevation causes memory decline and hippocampus shrinkage, in female wild-type B6 mice. Hormones and Behavior . 2016;78:160–167. doi: 10.1016/j.yhbeh.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 59.Papadopoulos V., Baraldi M., Guilarte T. R., et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends in Pharmacological Sciences . 2006;27(8):402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Jacobs A. H., Tavitian B., the InmiND consortium Noninvasive molecular imaging of neuroinflammation. Journal of Cerebral Blood Flow and Metabolism . 2012;32(7):1393–1415. doi: 10.1038/jcbfm.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jahn C. E., Schaefer E. J., Taam L. A., et al. Lipoprotein abnormalities in primary biliary cirrhosis. Gastroenterology . 1985;89(6):1266–1278. doi: 10.1016/0016-5085(85)90642-0. [DOI] [PubMed] [Google Scholar]
- 62.Swain M. G., Jones D. E. J. Fatigue in chronic liver disease: new insights and therapeutic approaches. Liver International . 2019;39(1):6–19. doi: 10.1111/liv.13919. [DOI] [PubMed] [Google Scholar]
- 63.Gee L. M. V., Barron-Millar B., Leslie J., et al. Anti–cholestatic therapy with obeticholic acid improves short-term memory in bile duct ligated mice. American Journal Of Pathology . 2022;S0002-9440 doi: 10.1016/j.ajpath.2022.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Ahboucha S., Pomier-Layrargues G., Vincent C., et al. Reduced plasma dehydroepiandrosterone sulfate levels are significantly correlated with fatigue severity in patients with primary biliary cirrhosis. Neurochemistry International . 2008;52(4-5):569–574. doi: 10.1016/j.neuint.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 65.Turkmen S., Backstrom T., Wahlstrom G., Andreen L., Johansson I. M. Tolerance to allopregnanolone with focus on the GABA-A receptor. British Journal of Pharmacology . 2011;162(2):311–327. doi: 10.1111/j.1476-5381.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differences in serum allopregnanolone and the PBC-40 domains stratified by “none/mild,” “moderate” and “severe” severity of symptoms are outlined in supplementary figure 1 and supplementary Table 1. Supplementary Figure 1: symptom severity (None/Mild vs. Moderate vs. Severe) as defined by the PBC-40 questionnaire domains, according to allopregnanolone levels (ng/ml). (A) Cognitive; (B) emotional; (C) itch; (D) fatigue; (E) social; (F) general symptoms. Supplementary Table 1: serum allopregnanolone (ng/ml) by PBC-40 domain severity.
Data Availability Statement
All presented data are available upon request, pending approval of proposed use and signed data access agreement. Application for access to data to be made via the corresponding author.


