Abstract
Objective:
Assessment of the results of the ProtekDuo cannula applied for dedicated right ventricular support with oxygenator in ARDS secondary to COVID-19.
Methods:
Systematic literature search in NHS library, Medline (Pubmed) and EMBASE using appropriate keywords as well as PICOS and PRISMA approach.
Results:
Out of 285 publications found, 5 publications met the search criteria and were included in this review. A total of 194 patients with ARDS secondary to COVID-19 underwent ProtekDuo placement to establish a combination of respiratory [veno-venous extracorporeal membrane oxygenation (V-V ECMO)] and right ventricular support. Patients treated using the ProtekDuo cannula had survival rates between 59% and 89% throughout the five studies, and a significant survival benefit when compared to an invasive ventilation group or compared to dual site V-V ECMO or other double lumen ECMO cannulas. One study focused on extubation and discontinuation of ventilator support, which could be achieved in 100% of ProtekDuo patients. An association for reduced incidence of acute kidney injury (AKI) and use of continuous renal replacement therapy (CRRT) could be shown when the ProtekDuo was used.
Conclusion:
Only limited literature is available for the ProtekDuo in V-P ECMO configuration in the setting of COVID-19 ARDS and should be interpreted with caution. Data on the ProtekDuo is suggestive for lower rates of mortality, AKI and CRRT as compared to other respiratory support modalities.
Keywords: ECLS, extracorporeal life support, extracorporeal membrane oxygenation, percutaneous, RVAD, right ventricular assist device, ProtekDuo
Introduction
The ProtekDuo (LivaNova PLC, London, UK) is a single-site, double-lumen cannula that is inserted percutaneously into the right internal jugular vein (RIJV) and advanced through the right heart until the distal tip of the cannula rests in the main pulmonary artery (PA). When in proper position, the cannula’s proximal fenestrations in the right atrium (RA) drain venous blood toward the extracorporeal membrane oxygenation (ECMO) system which is reinfused, after proper blood-related gas exchange, through distal fenestrations in the main PA downstream the pulmonic valve. The site of inflow and outflow, therefore, allow bypassing of the right ventricle (RV), making the cannula an effective percutaneous right ventricular assist device (RVAD). The ProtekDuo comes in two sizes, 29 and 31 French (Fr) and approximates a blood flow of 4–5 liters per minute (LPM), utilizing a centrifugal pump.1
For cannula placement, an 8 French (Fr) introducer sheath is first inserted into the RIJV and then a pulmonary artery catheter (PAC) or pulmonary wedge pressure catheter is floated through the sheath into the right PA. Thereafter, a Lunderquist® Extra-Stiff (Cook, Bloomington, USA) or Amplatz Super Stiff™ (Boston Scientific, Malborough, MA, USA) exchange guidewire (both 0.035″ × 260 cm) is inserted through the PAC which is removed while keeping the wire in the PA position. Serial dilators may be used and the ProtekDuo cannula then inserted over the wire under fluoroscopy into its position in the main PA. The implanted double-lumen cannula can then be connected with an extracorporeal circuit which provides only blood drainage and reinfusion, therefore unloading the right atrium and RV, thereby supporting the right heart-related circulation (RVAD configuration) or combining RV and respiratory support by introducing an oxygenator in the same circuit (OxyRVAD or V-P ECMO configuration). Any commercially available and approved extracorporeal circuit, oxygenator, and pump may be sufficient for use. It should be secured like any other large bore cannula.1 If fluoroscopy is not available, a transesophageal echocardiogram may be a useful tool for an ECMO retrieval team that cannulates in remote small hospitals without catheter laboratory or other technical capacity.
During the COVID-19 pandemic, use of the ProtekDuo as RVAD was broadened by adding an oxygenator to the circuit to provide venovenous extracorporeal membrane oxygenation (OxyRVAD) for patients suffering from acute respiratory distress syndrome (ARDS). When considering the position paper of the Extracorporeal Life Support Organization (ELSO), the so called “ELSO Maastricht Treaty for ECLS Nomenclature: Abbreviations for cannulation configuration in extracorporeal life support,” this ECMO configuration is named venopulmonary (V-P) ECMO.2
Based on our own previous experience with the ProtekDuo as RVAD and V-P ECMO/OxyRVAD we were interested in further investigating the actual ProtekDuo-related results in COVID-19 patients.
Presently, the number of patients who require ECMO for COVID-19 infection went down to zero at our institution. Widely organized vaccination programs may be responsible for this development, and even though not yet conclusive, we do not anticipate many patients or studies to come up in the near future. Therefore, we aimed to conduct a systematic review of the available literature to determine the present level of evidence for its function as V-P ECMO/OxyRVAD in patients with ARDS secondary to COVID-19 infection.
Materials and methods
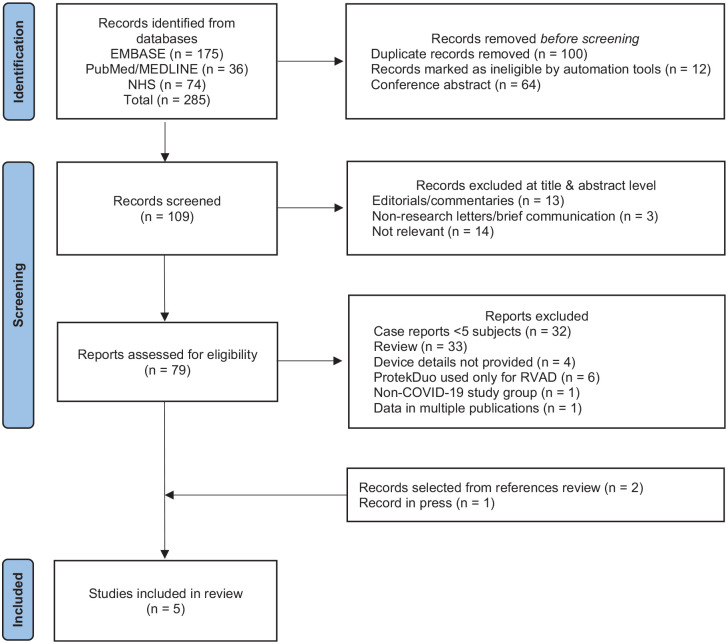
The PICOS approach (Participants, Intervention, Comparison, Outcome and Study Design) for selection of clinical studies has been used for our systematic literature search as recently described (Table 1).3 The PRISMA system (Preferred Reporting Items for Systematic Review and Meta-Analyses) has been used throughout the screening process to ensure clarity and transparency (Figure 1).4 The systematic literature search was performed in Medline (PubMed), EMBASE, and through the NHS (National Health Service) Library in the United Kingdom. The search strategy was developed and carried out supported by the NHS Library. The search included controlled vocabulary and free text terms such as: ProtekDuo or Protek Duo or percutaneous right ventricular assist device and oxygenator or ECMO or Extracorporeal Membrane Oxygenation or ECLS or Extra Corporeal Life Support and RVAD or RVAD/OXY or OxyRVAD. The literature was screened for any publication on the ProtekDuo, and a redundancy check was performed. The search strategy included all clinical studies. All authors participated in the study selection and determination of eligibility for inclusion in this systematic review. Discordances were addressed by consensus. Clinical guidelines, reviews, book chapters, editorials and letters to the editor were excluded as displayed in Figure 1. All publications relevant to the subject, however, were reviewed and contextually integrated in the discussion of this systematic review.
Table 1.
PICOS approach for the selection of studies in the systematic search process.
| Participants | Patients with ARDS secondary to COVID-19 |
| Intervention | ECMO with Protek Duo cannula |
| Comparison | With control group if available |
| Outcomes | Effectiveness of treatment in terms of survival rate and complications |
| Study design | Prospective and retrospective clinical studies, case series |
Figure 1.
PRISMA flow diagram of the systematic search. Adjusted from Page et al. BMJ 2021.15
Results
We identified a total of 285 publications, of which 175 were found in EMBASE, 36 in Medline (PubMed) and 74 through the NHS library. A total of 100 duplicates and 64 conference abstracts were discarded, while 12 records were marked as ineligible by automation tools. A total of 176 papers were eliminated and 109 remaining records were screened.
In the screening phase, 16 publications were excluded for being editorials or letters to the editor and 14 publications were deemed irrelevant to the subject, leaving 79 articles to be assessed for eligibility. Further exclusion criteria were applied for case reports with less than 5 subjects, review articles, articles that did not provide sufficient details about the devices used, articles in which the ProtekDuo was used for RVAD only or for non-COVID-19 related respiratory support, and articles with data spread over multiple publications. In total, an additional 77 articles were excluded. Lastly, reviews of reference lists and availability of articles in press resulted in the addition of two articles. The final review included five articles, one was a large case series of 40 patients and the others were retrospective studies.
In the five selected studies, a total of 194 patients underwent ProtekDuo placement in combination with an oxygenator (V-P ECMO/OxyRVAD configuration) for the treatment of ARDS due to COVID-19. The ProtekDuo showed survival rates between 59 and 89% throughout the five studies and was suggestive for a survival benefit when compared to an invasive ventilation group9 or compared to dual site V-V ECMO or other double-lumen ECMO cannulas.10 One study focused on extubation and discontinuation of ventilator support, which could be achieved in 100% of ProtekDuo patients.8 In addition, an association of reduced incidence of acute kidney injury (AKI) and consecutive use of continuous renal replacement therapy (CRRT) could be demonstrated when the ProtekDuo was used (Table 2).
Table 2.
Protek Duo for RVAD+ECMO.
| First author, study design | Use of Protek Duo | Comparison | Patients included | Important outcomes |
|---|---|---|---|---|
| Cain et al.,9 Retrospective cohort | RVAD+ECMO for COVID-19 ARDS | Protek Duo for RVAD+ECMO compared to invasive mechanical ventilation alone | 39 adult patients 18 with Protek Duo for RVAD+ECMO 21 with IMV alone |
In-hospital mortality: Total 13(33%), IMV = 11 (52.4%),
RVAD+ECMO = 2 (11.1%), p = 0.008 30-day mortality: Total 10 (25.6%), IMV = 9(42.9%%), RVAD+ECMO = 1 (5.6%), p = 0.011 ICU LOS: 13 (6–27), IMV = 11.5(6–22.5), RVAD+ECMO = 21 (9–36), p = 0.067 AKI:15 (38.5%), IMV = 15 (71.4%), RVAD+ECMO = 0 (0%), p ⩽ 0.001 Duration of IMV:7.5 (1–22), IMV = 10 (5–20), RVAD+ECMO = 5(1–34), p = 0.44 |
| Mustafa et al.,8 Case series | RVAD+ECMO for COVID-19 ARDS | None | 40 adult patients | Ventilator discontinuation: 40 (100%) ECMO initiation to extubation: 13 ± 2.6 days Weaned from ECMO: 32 (80%) Hospital discharge: 29 (73%) Mortality: 6 (15%) |
| Saeed et al.,10 Multicenter retrospective study | RVAD+ECMO for COVID-19 ARDS | Compared dual-site (femoral vein-femoral vein or femoral vein-internal jugular vein) to single, dual-lumen cannula in internal jugular vein with tip positioned in the pulmonary artery (Protek Duo), and (3) single, dual-lumen cannula in internal jugular vein advanced through the SVC into the right atrium with tip positioned in the IVC (Crescent or Avalon cannulas) | 435 adult patients 99 (23%) had Protek Duo cannulation, 247 (57%) had dual site cannulation, 89 (20%) had single site IVC cannulation |
90-day in hospital mortality for entire cohort:
55% Unadjusted 90-day in hospital mortality: dual site = 60%, single site to PA = 41%, single site IVC = 61%, p = 0.06 Adjusted (clinical and center factors) 90-day in hospital mortality was lower in single site PA (HR 0.52, p = 0.029) and similar in single site IVC (HR 0.98, p = 0.86) compared to dual site Single site PA cannulation had longer duration of ECMO compared to other modes Single site PA had shorter mechanical ventilation and more commonly discharged home |
| Smith et al.,11 Retrospective cohort | RVAD+ECMO for COVID-19 ARDS | Protek Duo for RVAD+ECMO compared to other V-V ECMO configuration and different eras of Protek use |
N = 54 38 (70.4%) had Protek Duo and 16 (29.6%) had VV ECMO Compared 2 eras of Protek pts: ERA 1 = Mar 1–Jul 6, 2020 ERA 2 = Jul 7, 2020–Mar 1, 2021 Pts treated with Protek: ERA 1 = 18 ERA 2 = 20 |
The total in-hospital mortality was 42.6% (39.5% V-P ECMO, 50.0%
V-V ECMO). Cumulative mortality 120-days post-cannulation was
45.7% (V-V ECMO 60.8%, V-P ECMO 40.0%) Era 2 patients experienced a longer intubation duration (3.0 vs 24.0 days, p = 0.026), higher incidences of reintubation (27.8 vs 60.0%, p = 0.046), in-hospital mortality (16.7% vs 60.0%, p = 0.006), RRT (0.0% vs 50.0%, p < 0.001), and infection (61.1% vs 95.0%, p = 0.016) due to increased rate of secondary bacterial pneumonia (22.2% vs 85.0%, p < 0.001). Era 2 patients were significantly less likely to be discharged home (72.2% vs 5.6%, p < 0.001). Era 2 patients suffered significantly more cannula-associated complications (25.0% vs 0.0%, p = 0.048) Era 2 patients experienced significantly more “major” bleeding events (22.2% vs 60.0%, p = 0.025). Era 2 patients had a much higher cumulative incidence of mortality (60.4%) compared to Era 1 patients (16.2%) |
| El Banayosy et al.,12 Retrospective cohort | RVAD+ECMO for COVID-19 ARDS | None | 9 adult patients (initial configuration: 2 V-P, 6 V-V, 1 V-A; mode for Protek: 4 V-P, 1 V-VP, 4 both | Survival: 67% ECMO duration: 55 ± 29 days The ProtekDuo was placed in a late stage after V-V ECMO when either right heart failure or oxygenation problems occurred. |
Discussion
The group of Zwischenberger first described the placement of a single-site, percutaneous, double-lumen cannula for RVAD in an ovine model in 2015.5 Over the following years, numerous authors have reported cases in which the ProtekDuo has been utilized in multiple configurations including its original configuration as RVAD, RVAD with oxygenator for ECMO (V-P ECMO/OxyRVAD), left ventricular assist device (LVAD), biventricular assist device (BiVAD) or ECPELLA 2.0 when either combined with a durable LVAD or any of the multiple available Impella® devices. It had also been used as double-lumen drainage cannula for cardiopulmonary bypass (CPB), and in other ECMO configurations, such as veno-pulmonary (V-P), venovenous-pulmonary (VV-P), and in veno-venopulmonary (V-VP) ECMO. These configurations and technical aspects have been described by our group elsewhere in detail.6,7 The focus of this systematic review is to explore the results related to the use of the ProtekDuo, as part of V-P ECMO configuration circuit, since several publications have recently been published supporting this extracorporeal cardio-respiratory support modality during the COVID-19 pandemic for ARDS.
The ProtekDuo with oxygenator may be beneficial in ARDS due to its default V-P ECMO position with drainage of venous blood from the RA and return of arterialized blood into the PA. Considering its average blood flow of 4.5 LPM, it mostly achieves sufficient flow and oxygenation, especially since two cardiac valves are in between both cannula openings and prevent recirculation. For the rare cases of high body mass index with increased need for blood flow and oxygenation, reconfiguration to V-VP ECMO as developed and described by Maybauer et al. has been shown to be effective in providing up to 7 LPM of oxygenated blood flow, with approximately 40% of the blood flow bypassing the RV.6
In 2020, Mustafa et al. presented the first experience with V-P ECMO for patients with ARDS secondary to COVID-19. The group presented a case series of 40 patients in which they reported an average duration of mechanical ventilation of 13 days, 80% (32 patients) rate of ECMO weaning, and 73% (29 patients) survival rate.8 Similarly, good results were reported by Cain et al.9 who compared 39 patients in two groups: V-P ECMO (18 patients) and invasive mechanical ventilation (IMV, 21 patients). Their group reported a significant reduction of in-hospital (52.4% vs 11.1%, p = 0.0008) and 30-day mortality rates (42.9% vs 5.6%, p = 0.011) in favor of the V-P ECMO group without any device related complications. In addition, while the occurrence of acute kidney injury (AKI) was not evident in the V-P ECMO group at all, the IMV group had 15 cases of AKI (71.4%, p < 0.001).
In 2022, a large multicenter retrospective study including 435 adult patients was published by Saeed et al.10 This group compared the dual-site versus single-site cannulation approach. For dual site they used femoral vein to femoral vein or femoral vein to internal jugular vein access. For single site they used the ProtekDuo with its tip in the pulmonary artery, or Crescent/Avalon cannulas with their tip positioned in the inferior vena cava (IVC). Of 435 adult patients, 99 (23%) had Protek Duo cannulation, 247 (57%) had dual site cannulation, and 89 (20%) had single site IVC cannulation. The 90-day in hospital mortality for the entire cohort was 55% with an unadjusted 90-day in hospital mortality of 60% for dual site, 41% for ProtekDuo, and 61% IVC. After adjusting for clinical and center factors, the 90-day in-hospital mortality was significantly lower for ProtekDuo (HR 0.52, p = 0.029) and similar in single site IVC (HR 0.98, p = 0.86) compared to dual site. However, the ProtekDuo cannulation had longer duration of ECMO compared to other modes, but had shorter mechanical ventilation and patients were more commonly discharged home.
Smith et al.11 investigated a cohort of 54 patients, comparing the ProtekDuo with V-V ECMO through 1 year of the pandemic. Sixteen (29.6%) of their patients received V-V ECMO and 38 (70.4%) V-P ECMO after a median time of 7 days from admission to cannulation. Their median ECMO support time was 30.5 days (V-V ECMO 35.0 days vs V-P ECMO 26.0 days). In this study, the total in-hospital mortality was 42.6% with 39.5% for V-P ECMO and 50.0% for V-V ECMO. The total cumulative mortality after 120-days post-cannulation was 45.7%, with 60.8% for V-V ECMO and 40.0% for V-P ECMO. The authors concluded ECMO support for COVID-19 was beneficial and that V-P ECMO support demonstrated consistent advantages in survival compared to V-V ECMO.
In contrast to the above-mentioned studies where V-P ECMO was the initial configuration, the most recent study by the group of Maybauer showed that V-P or V-VP ECMO configuration was established weeks after the onset of ARDS and ECMO initiation. This selected group of patients still displayed good outcomes with a survival rate of 67%12 and the ProtekDuo has been shown to be a game changer when used in patients with ARDS secondary to COVID-19.13 However, the available data is scarce and may have institutional bias. It should therefore be considered with caution. The use of this cannula is also not without risk. The bend in the cannula could potentially lead to cannula fracture and even though extremely rare, it could lead to right coronary artery obstruction depending on the position in the RV, as described by Unger et al.14
Conclusion
It should be borne in mind that the amount of published literature and evidence for use of the ProtekDuo cannula in patients with ARDS secondary to COVID-19 is limited. However, the number of patients requiring ECMO support for COVID-19 ARDS has now decreased to zero. This may be due to herd immunity through infection or widespread vaccination programs and/or decrease in virulence. Future large cohort studies on COVID-19 and ECMO cannot be predicted at this time. Therefore, we aimed to summarize and present the available data. The ProtekDuo contributed to reduced mortality, reduced acute kidney injury, and consecutively reduced need for continuous renal replacement therapy. Therefore, many authors of the above-mentioned papers suggest using the ProtekDuo as first line cannula in the setting of COVID-19 ARDS. Investigations of the ProtekDuo in other causes of ARDS are warranted to compare its use in different etiologies of ARDS.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Marc O Maybauer  https://orcid.org/0000-0003-2406-655X
https://orcid.org/0000-0003-2406-655X
References
- 1.Maybauer MO, Koerner MM, Swol J, et al. The novel ProtekDuo ventricular assist device: configurations, technical aspects, and present evidence. Perfusion. Epub ahead of print 26 May 2022. DOI: 10.1177/02676591221090607 [DOI] [PubMed] [Google Scholar]
- 2.Broman LM, Taccone FS, Lorusso R, et al. The ELSO Maastricht Treaty for ECLS nomenclature: abbreviations for cannulation configuration in extracorporeal life support - a position paper of the Extracorporeal Life Support Organization. Crit Care 2019; 23(1): 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geli J, Capoccia M, Maybauer DM, et al. Argatroban anticoagulation for adult Extracorporeal Membrane Oxygenation: A systematic review. J Intensive Care Med 2022; 37(4): 459–471. [DOI] [PubMed] [Google Scholar]
- 4.Moher D, Liberati A, Tetzlaff J, et al.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Jones C, Ballard-Croft C, et al. Development of a double-lumen cannula for a percutaneous RVAD. ASAIO J 2015; 61(4): 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maybauer MO, Koerner MM, Mihu MR, et al. The ProtekDuo as double lumen return cannula in V-VP ECMO configuration: A first-in-man method description. Ann Card Anaesth 2022; 25(2): 217–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maybauer MO, Koerner MM, Harper MD, et al. The ProtekDuo as double lumen arterial return cannula in extracorporeal membrane oxygenation. Int J Artif Organs 2021; 44(9): 623. [Google Scholar]
- 8.Mustafa AK, Alexander PJ, Joshi DJ, et al. Extracorporeal membrane oxygenation for patients with COVID-19 in severe respiratory failure. JAMA Surg 2020; 155(10): 990–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cain MT, Smith NJ, Barash M, et al. Extracorporeal membrane oxygenation with right ventricular assist device for COVID-19 ARDS. J Surg Res 2021; 264: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saeed O, Stein LH, Cavarocchi N, et al. Outcomes by cannulation methods for venovenous extracorporeal membrane oxygenation during COVID-19: a multicenter retrospective study. Artif Organs 2022; 46(8): 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith NJ, Park S, Zundel MT, et al. Extracorporeal membrane oxygenation for COVID-19: an evolving experience through multiple waves. Artif Organs 2022; 46: 2257–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Banayosy AM, El Banayosy A, Brewer JM, et al. The ProtekDuo for percutaneous V-P and V-VP ECMO in patients with COVID-19 ARDS. Int J Artif Organs 2022; 45: 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maybauer MO, Lorusso R, Swol J.The ProtekDuo cannula for extracorporeal membrane oxygenation: A game changer in COVID-19! Artif Organs 2022; 46: 2107–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unger ED, Sweis RN, Bharat A.Unusual complication of a right ventricular support-extracorporeal membrane oxygenation Cannula. JAMA Cardiol 2021; 6(6): 723–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 74: 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]