Abstract
The intracellular human pathogens Legionella pneumophila and Mycobacterium tuberculosis reside in altered phagosomes that do not fuse with lysosomes and are only mildly acidified. The L. pneumophila phagosome exists completely outside the endolysosomal pathway, and the M. tuberculosis phagosome displays a maturational arrest at an early endosomal stage along this pathway. Rab5 plays a critical role in regulating membrane trafficking involving endosomes and phagosomes. To determine whether an alteration in the function or delivery of Rab5 could play a role in the aberrant development of L. pneumophila and M. tuberculosis phagosomes, we have examined the distribution of the small GTPase, Rab5c, in infected HeLa cells overexpressing Rab5c. Both pathogens formed phagosomes in HeLa cells with molecular characteristics similar to their phagosomes in human macrophages and multiplied in these host cells. Phagosomes containing virulent wild-type L. pneumophila never acquired immunogold staining for Rab5c, whereas phagosomes containing an avirulent mutant L. pneumophila (which ultimately fused with lysosomes) transiently acquired staining for Rab5c after phagocytosis. In contrast, M. tuberculosis phagosomes exhibited abundant staining for Rab5c throughout its life cycle. To verify that the overexpressed, recombinant Rab5c observed on the bacterial phagosomes was biologically active, we examined the phagosomes in HeLa cells expressing Rab5c Q79L, a fusion-promoting mutant. Such HeLa cells formed giant vacuoles, and after incubation with various particles, the giant vacuoles acquired large numbers of latex beads, M. tuberculosis, and avirulent L. pneumophila but not wild-type L. pneumophila, which consistently remained in tight phagosomes that did not fuse with the giant vacuoles. These results indicate that whereas Rab5 is absent from wild-type L. pneumophila phagosomes, functional Rab5 persists on M. tuberculosis phagosomes. The absence of Rab5 on the L. pneumophila phagosome may underlie its lack of interaction with endocytic compartments. The persistence of functional Rab5 on the M. tuberculosis phagosomes may enable the phagosome to retard its own maturation at an early endosomal stage.
Following phagocytosis, phagosomes containing inert particles follow an intracellular pathway that mirrors the stages of the endosomal-lysosomal pathway (16, 17, 34, 35). At early time points after phagocytosis there is a rapid sorting of membrane proteins and recycling of many plasma membrane proteins to the plasma membrane (34, 35). The early phagosomes of inert particles rapidly acquire markers of early endosomes, including the mannose receptor and Rab5 (16, 17, 35). Subsequently, the phagosome loses Rab5 and the markers of early endosomes and acquires Rab7 and markers associated with late endosomes, such as lysosome-associated membrane glycoproteins (LAMPs), and cathepsin D (16, 17, 35). With still more time and maturation, the phagosome fuses with secondary lysosomes, acquires higher concentrations of acid hydrolases and LAMPs, and loses the Rab7-GTPase but acquires other, as-yet-unidentified, small GTPases (17, 27).
The pathways of the intracellular parasites Legionella pneumophila and Mycobacterium tuberculosis deviate from the above pathway of inert particles in that they reside and multiply in phagosomes that resist acidification and fusion with lysosomes (4, 12, 15, 25, 26, 44). The pathways of L. pneumophila and M. tuberculosis also differ from one another (12, 13). Whereas phagosomes containing wild-type L. pneumophila do not acquire the transferrin receptor or any other markers of the endosomal-lysosomal pathway studied to date (13), phagosomes containing virulent M. tuberculosis show a persistent interaction with early endosomes, as evidenced by the presence of transferrin receptor (13) and acquisition of exogenously added transferrin (14). The mechanisms underlying the altered maturation of L. pneumophila and M. tuberculosis phagosomes have not been determined.
Since Rab-GTPases play a pivotal role in the regulation of membrane trafficking within eukaryotic cells, we considered the possibility that a disruption in the function or delivery of Rab-GTPases to L. pneumophila or M. tuberculosis phagosomes could play a role in the altered development of these phagosomes. Rab-GTPases are low-molecular-weight members of the Ras superfamily that regulate docking and fusion between different subcellular organelles (21, 31, 33). Over 30 different Rab-GTPases have been identified, and it is likely that each compartment of the endocytic and secretory pathways in eukaryotic cells has a unique subset of Rab-GTPases. The Rab-GTPases cycle between GTP-bound and GDP-bound forms. In the GTP-bound form, the molecule is in the “on” configuration and permits fusion between vesicles bearing homologous Rab-GTPases. The GDP-bound form is the “off” configuration, which does not permit fusion. In addition to cycling between on and off configurations, the Rab-GTPases also cycle between membrane-bound and soluble forms. The GDP-bound, but not the GTP-bound, form of a Rab protein can be extracted in a reversible fashion from a membrane-bound form to a soluble cytosolic form by the Rab chaperon protein, Rab-GDP dissociation inhibitor (GDI). Acquisition of a particular Rab-GTPase by a membrane requires that the membrane have specific receptor mechanisms for the Rab-GTPase (5, 42). In addition, functional activity of a Rab-GTPase on the membrane in promoting membrane fusion events requires specific effector machinery (39). The specific receptor and effector mechanisms for Rab-GTPases have not been completely elucidated. Rab5 is present on early endosomes (11) and on phagosomes immediately after phagocytosis (17, 27) and regulates membrane-trafficking events involving these compartments (2, 6, 9, 40). There are three isoforms of Rab5 (A, B, and C), all of which are present on the early endosomal compartment and regulate membrane-trafficking events involving this compartment (6, 9, 11, 40). Each of these three isoforms is also transiently associated with maturing latex bead phagosomes in mouse macrophages (17).
The role of Rab-GTPases in human cells infected with either L. pneumophila or M. tuberculosis has not been studied. Nor has the role of Rab5 in cells of any type infected with these pathogens been studied. However, Via et al. (43) examined Rab-GTPases in mouse bone marrow macrophages infected with Mycobacterium bovis BCG, an avirulent form of a mycobacterial species related to M. tuberculosis. Using biochemical techniques to study a population of phagosomes isolated from infected cells, these investigators found that M. bovis BCG phagosomes acquire Rab5 but not Rab7 or LAMP-1. As M. bovis BCG is not pathogenic for mice or humans, the relevance of studies of this organism to the cell biology of the highly pathogenic species M. tuberculosis is unclear.
As a step towards determining whether M. tuberculosis or L. pneumophila disrupts the maturation of its phagosome by altering the function or distribution of Rab-GTPases on the phagosome, we have examined the distribution of Rab5 in human HeLa cells infected with these pathogens. We shall demonstrate that Rab5 expression on phagosomes containing L. pneumophila or M. tuberculosis deviates from that on phagosomes containing inert particles or avirulent bacteria. Whereas phagosomes containing inert particles or avirulent bacteria transiently display Rab5, phagosomes containing wild-type L. pneumophila do not display Rab5, and phagosomes containing virulent M. tuberculosis exhibit a persistent display of Rab5.
MATERIALS AND METHODS
Reagents and antibodies.
Glutaraldehyde was purchased from Polysciences (Warrington, Pa.); PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], methylcellulose, polyvinylpyrrolidone, and paraformaldehyde were purchased from Sigma Chemical Co. (St. Louis, Mo.); and Dulbecco's phosphate-buffered saline (PBS) was from GIBCO Laboratories (Santa Clara, Calif.). Dulbecco's modified Eagles medium (DMEM) was purchased from Irvine Scientific Co. (Santa Ana, Calif.).
Mouse monoclonal antibody to the human transferrin receptor (immunoglobulin G1 [IgG1]) was purchased from AMAC (Westbrook, Maine). Mouse monoclonal antibody to LAMP-1 (H4A3, IgG1) was obtained from the Hybridoma Bank of the University of Iowa, Iowa City. Isotypic mouse myeloma control proteins were obtained from Cappel Organon Teknica (West Chester, Pa.). Rabbit antibody to mycobacterial lipoarabinomannan (LAM) was prepared as described previously (13). Rabbit antibody to L. pneumophila lipopolysaccharide (LPS) was prepared by immunizing rabbits with LPS purified from L. pneumophila Ph1 in Freund's adjuvant (18). Purified rabbit anti-mouse IgG antibody was obtained from Sigma Chemical Company. Reactivity of this commercial antibody to mycobacterial antigens was eliminated by three consecutive overnight adsorptions to an excess of heat-killed M. tuberculosis. Protein A colloidal gold conjugates (5, 10, and 15 nm) were provided by G. Posthuma (Utrecht University, Utrecht, The Netherlands).
Bacteria.
M. tuberculosis Erdman (ATCC 35801), a highly virulent strain, was obtained from the American Type Culture Collection (Manassas, Va.). The organism was passaged through guinea pig lung to maintain virulence as described previously (13). Before an experiment, a vial of guinea pig lung homogenate was rapidly thawed at 37°C, and the bacteria were cultured on 7H11 agar at 37°C with 5% CO2 and 100% humidity. Seven to 8 days later, bacteria were scraped from 20 to 40 plates into 10 to 20 ml of DMEM containing 10% fetal bovine serum (FBS) and 5% human serum from type AB blood. A suspension containing predominantly single bacilli was prepared by sonicating the bacteria in a water bath (model 9; Astrason, Plainview, N.Y.) for 60 s, sedimenting any remaining clumps of organisms by centrifugation at 20 × g for 10 min and removing an aliquot of the predominantly single-bacillus suspension from the top of the tube. The concentration of organisms was determined by the measurement of optical density at 540 nm and by counting in a Petroff-Hausser chamber. Viability of the organisms was determined by plating serial dilutions of the infecting inoculum on 7H11 agar. Viability ranged from 67 to 86% in these experiments.
L. pneumophila Philadelphia 1 was grown in embryonated hens' eggs, harvested, tested for viability and contaminants, and stored at −70°C, as previously described (26). The egg yolk-grown L. pneumophila was cultured one time only on charcoal-yeast extract (CYE) agar, harvested after 4 days of growth, and used immediately. The avirulent mutant L. pneumophila 25D was prepared and maintained as described previously (24). This strain has been shown to bear a mutation in the dot-icm virulence locus (30, 37).
Cloning, expression, and purification of recombinant Rab5 and preparation of antisera.
To clone the human rab5 genes, we screened a human fetal lung cDNA library (Invitrogen) by colony hybridization with a cDNA probe (ATCC 84765) encoding the 3′ third of a rab5-like gene. The probe was labeled with [α-32P]dCTP (Amersham) by the random-priming method. Prehybridization and hybridization were carried out at 42°C in a solution containing 2× PIPES, 50% deionized formamide, 0.5% (wt/vol) sodium dodecyl sulfate, and 100 μg of denatured salmon sperm DNA per ml. Positive clones were selected after three rounds of colony hybridization and analyzed by restriction enzyme digestions. The identities of the positive clones were confirmed by sequencing both strands of DNA in opposite directions. The rab5 sequence obtained (GenBank accession no. AF141304) was identical to the previously published sequences for human rab5c (22) except for two nucleotide changes in the eighth codon, resulting in an alanine rather than an arginine. This single amino acid change was confirmed by nucleotide-sequencing analyses of four independently derived rab5c clones. The human rab5c gene is highly homologous to the canine rab5c sequence as well as to rab5a and rab5b sequences (8, 10). The cDNA for the complete rab5c gene was amplified by PCR and cloned into expression vectors for Escherichia coli. The rab5c gene was cloned into pET15 between NdeI and XhoI cutting sites. The construct was under the control of the T7lac promoter with an amino-terminal sequence coding for a thrombin-cleavable His6 tag. High-level expression of Rab5c in E. coli BL21(DE3)plysS was induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and the recombinant proteins were purified to homogeneity from sonicated cell pellet extracts by a combination of nickel affinity and gel filtration chromatography. The His6 tag was removed by thrombin cleavage (Thrombin Cleavage kit; Novagen), and residual Rab5c still bearing the His6 tag was removed by a second round of nickel affinity chromatography. The resulting material was found by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to exhibit a single 25-kDa band by Coomassie blue staining. Rabbit polyclonal antibodies to human Rab5c were raised by immunizing New Zealand White rabbits three times, 3 weeks apart, with 1 mg of recombinant protein (purified from E. coli) in Syntex adjuvant (1). This adjuvant was used to avoid the production of antibodies to mycobacterial antigens present in Freund's adjuvant. The first immunization was supplemented with 100 μg of N-acetylmuramyl-l-alanyl-d-isoglutamine (Sigma Chemical Co.). Rabbits immunized with the recombinant proteins yielded high-titer-specific polyclonal antisera to human Rab5c. The resulting polyclonal antibodies were affinity purified by binding to recombinant E. coli Rab5c, eluted with glycine-HCl (pH 2.5) containing 0.1% bovine serum albumin (BSA) carrier protein, and immediately neutralized with Tris-HCl (pH 8.0). The purified antibodies reacted equally well with geranylated and nongeranylated Rab5c and did not cross-react with L. pneumophila or M. tuberculosis antigens. Antisera to Rab5c did not cross-react with Rab7.
Stable transfection of human cell lines with Rab5c and a GTPase-deficient, constitutively active Rab5c mutant.
To facilitate the immunolocalization of Rab5 in infected cells, we developed a stably transfected human HeLa cell line that overexpresses the Rab5c isoform of Rab5 (HeLa-Rab5c). We cloned the human rab5c gene into pTRE, transfected the recombinant plasmids into HeLa Tet-off cells (Clontech) by calcium phosphate precipitation, and selected stably transfected clones with hygromycin in the presence of tetracycline.
GTPase-deficient, fusion-promoting mutant forms of Rab5 and other Rab-GTPases have been previously described (9, 31). We prepared the corresponding rab5c Q79L mutant by PCR-based mutagenesis by published methods (28, 40) using the mutant primer 5′-GGGACACAGCTGGACTGGAGCGGTATCACAGCC-3′ (the mutated nucleotide is underlined) and pTRE forward and reverse sequences (5′-TCCAGCCTCCGCGGCCCC-3′ and 5′-TCATCAATGTATCTTATCATGTCT-3′, respectively) as outer primers in the amplifications. The mutant construct was confirmed by DNA sequencing. HeLa cells stably transfected with the constitutively active rab5c were selected as described above for the wild-type rab5c.
Preparation of a HeLa cell line stably transfected with transferrin receptor.
A 2.4-kb EcoRI fragment containing the human transferrin receptor gene was released from pGEM1-TR (provided by Marino Zerial, Heidelberg, Germany) and subcloned into pcDNA3.1/Zeo(+) (Invitrogen). HeLa-Rab5c cells were transfected with pcDNA3.1/Zeo(+)-TR by calcium phosphate precipitation. Transfected cells were maintained in complete DMEM containing hygromycin (100 μg/ml) and tetracycline (5 μg/ml) and were selected with zeomycin (200 μg/ml) for 3 to 4 weeks. Zeomycin-resistant clones were isolated and screened for coexpression of Rab5c and the transferrin receptor. Immunofluorescence microscopy demonstrated colocalization of Rab5c and the transferrin receptor, consistent with published observations of other investigators (11).
Assessment of intracellular growth of M. tuberculosis and L. pneumophila in monolayers of THP-1 or HeLa cells.
Monolayers of HeLa cells (105 cells/well) or THP-1 cells (4 × 105 cells/well) were cultured to confluency in 2-cm2 tissue culture wells for 2 days in RPMI 1640 (THP-1) or DMEM (HeLa) with 10% FBS without tetracycline. The THP-1 cells were differentiated with phorbol myristic acid (0.16 nM). Monolayers were coincubated with either M. tuberculosis (106/ml) or L. pneumophila (2 × 107 or 2 × 108/ml). Monolayers were washed with culture medium and incubated in fresh medium at 37°C. The number of CFU of M. tuberculosis and L. pneumophila was determined at sequential time points using the methods described by Hirsch et al. (23) and Horwitz and Silverstein (26), respectively. The culture supernatant and the cell lysates from each time point were combined, serially diluted, and plated on CYE agar plates.
Infection of monolayers of HeLa cells with M. tuberculosis and L. pneumophila.
Stably transfected HeLa cells were plated at a density of 2.5 × 106 per 75-cm2 culture flask or at a density of 106 per 10-cm-diameter tissue culture plate. Optimal Rab5c expression was obtained by omitting tetracycline from the culture medium 1 to 3 days before the cells were to be fixed. Monolayers were cultured without antibiotics in DMEM (low glucose) with 10% fetal bovine serum (certified tetracycline negative; Clontech). In experiments designed to examine early time points after infection, stably transfected HeLa cells were plated in 10-cm-diameter petri plates in DMEM containing 10% heat-inactivated FBS without tetracycline. Two days later, the plates were chilled on ice, and L. pneumophila (2 × 109/ml) or M. tuberculosis (4 × 108/ml) was added to the plates at 0°C. The plates were centrifuged for 20 min at 1,160 × g in a biohazard-safe rotor, incubated at 37°C for 15 or 30 min (L. pneumophila) or 2 h (M. tuberculosis), and either fixed immediately or washed extensively and incubated for an additional 15 min to 4 h prior to fixation. In experiments designed to examine later time points after infection, the HeLa cells were coincubated with L. pneumophila (2 × 109/ml) or M. tuberculosis (4 × 108/ml) together with 1-μm-diameter latex beads (1:500 dilution of a 2.5% solid suspension) at 37°C. After coincubation at 37°C for 1 to 2 h, the monolayers were washed extensively with culture medium to remove noningested bacteria and beads, the medium was replaced with fresh DMEM with 10% fetal calf serum, and the monolayers were incubated for 6 h to 2 days prior to fixation.
Immunoelectron microscopy.
Monolayers were fixed with 2% paraformaldehyde in 0.1 M PIPES (pH 7.3) containing 6% sucrose for 2 h at 4°C. Aldehydes were quenched with 10 mM glycine in PBS, and the cells were scraped into PBS with 0.1% BSA, pelleted by centrifugation, embedded in 10% gelatin at 37°C, cryoprotected with 20% polyvinylpyrrolidone in 2.3 M sucrose, and frozen in liquid nitrogen. Cryosections were collected on drops of 2.3 M sucrose and 2% methylcellulose (1:1) (W. Liou and J. Slot, 13th International Congress on Electron Microscopy, p. 253–254, 1994), transferred to Formvar-coated nickel grids, and blocked with 1% BSA and 0.1% fish skin gelatin in 0.05 M HEPES (pH 7.5) containing 0.3 M NaCl for 1 h at 4°C. Immunogold double and triple labelling were performed as described by Slot et al. (38). Sections were embedded in 1.8% methylcellulose–0.4% uranyl acetate (20). Consecutive phagosomes were photomicrographed at a magnification of ×14,000 using a JEOL 100 CX II electron microscope. Measurements of the number of gold particles per micron of membrane and per square micron of cytoplasm were determined from the negatives with a Numonics 2220 digitizer tablet and Sigma Scan software (Jandel Scientific).
RESULTS
Establishment of a model human cell system suitable for evaluating Rab5 expression on phagosomes.
We have found that the endogenous levels of Rab5 in normal human monocytes, monocyte-derived macrophages, and cell lines are too low to be detected reliably by immunofluorescence or immunoelectron microscopy. Therefore, to undertake studies of the distribution and function of Rab5 on phagosomes containing intracellular pathogens, we cloned the rab5 gene from a human fetal lung library. Although we found four independent clones with identical Rab5c sequences, we found no clones corresponding to either Rab5a or Rab5b in the human fetal lung cDNA library, despite our use of probes that would have detected such clones. We first sought to overexpress the gene in macrophage-like cell lines (U937, THP-1, and HL60), since macrophages are the natural host cells of L. pneumophila and M. tuberculosis. However, we were unable to achieve stable high-level expression compatible with immunofluorescence or immunoelectron microscopy studies in these cell lines. We therefore prepared a HeLa cell line capable of inducible expression of the human rab5c gene. Long-term overexpression of Rab-GTPases is often associated with toxicity and loss of expression by the cell line (7, 31). To circumvent this problem, we used a tetracycline-regulated expression system (7, 19). Expression of Rab5c by the isolated clones was found to be tightly regulated by tetracycline, with strong expression evident from 24 to 72 h after removal of tetracycline from the culture medium (data not shown).
To confirm that the study of L. pneumophila and M. tuberculosis phagosomes in infected HeLa-Rab5c cells is relevant to understanding the pathogenesis of L. pneumophila and M. tuberculosis infection of macrophages, the natural host cells of these pathogens in humans, we investigated the extent to which the interaction of the pathogens with HeLa-Rab5c cells resembles their interaction with human macrophages. We specifically examined (i) the capacity of the two pathogens to multiply within HeLa-Rab5c cells and (ii) the pattern of expression of endolysosomal markers on the phagosomes of the two pathogens in HeLa-Rab5c cells.
(i) Uptake and growth of L. pneumophila and M. tuberculosis in parental HeLa Tet-off cells and HeLa cells overexpressing Rab5c.
Although HeLa cells are poorly phagocytic, adequate uptake of L. pneumophila and M. tuberculosis can be obtained by increasing the multiplicity of infection (MOI) relative to that used when infecting more phagocytic cells. By immunofluorescence microscopy, 12% of HeLa Tet-off cells had associated bacteria after incubation with L. pneumophila for 2 h at an MOI of 2,000:1. By electron microscopy, approximately 1 in 15 HeLa Tet-off cells contained an L. pneumophila phagosome in the plane of the section. By immunofluorescence microscopy, 7% of HeLa Tet-off cells had associated bacteria after incubation for 2 h at an MOI of 400:1, and by electron microscopy, approximately 1 in 20 HeLa cells contained an M. tuberculosis phagosome in the plane of the section.
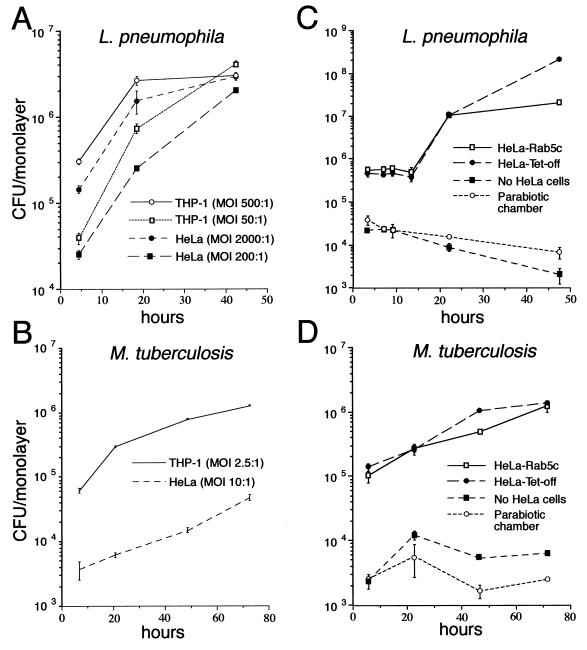
L. pneumophila adheres five times more avidly to THP-1 cells than to HeLa Tet-off cells (Fig. 1A). Similarly, M. tuberculosis adheres 15 times more avidly to monolayers of THP-1 cells than to comparable monolayers of HeLa Tet-off cells (Fig. 1B). However, once taken up by the HeLa Tet-off cells, L. pneumophila and M. tuberculosis grow at rates comparable to that in THP-1 cells (Fig. 1C and D). M. tuberculosis grows with a doubling time of approximately 19 h in HeLa Tet-off cells and 15 h in THP-1 cells (i.e., 1 log in 3 days, matching the growth rate that we have previously observed for M. tuberculosis in THP-1 cells and human monocyte-derived macrophages) (13, 29). L. pneumophila grows in HeLa Tet-off cells and THP-1 cells with an initial doubling time of approximately 3 h, which is also very similar to its previously published doubling time of 2 to 3 h in human monocyte-derived macrophages (26).
FIG. 1.
Growth of L. pneumophila and M. tuberculosis in THP-1, HeLa Tet-off, and HeLa-Rab5c cells. Monolayers of THP-1 macrophage-like cells, HeLa Tet-off cells, and HeLa-Rab5c cells expressing Rab5c were coincubated with L. pneumophila at a high MOI (2 × 108/ml) or a low MOI (2 × 107/ml) for 1 h (A), with L. pneumophila (2 × 108/ml) for 2 h (C), with M. tuberculosis (106/ml) for 2 h (B), or with M. tuberculosis (107/ml) for 2 h (D) at 37°C, washed, and incubated in fresh medium at 37°C. At sequential times thereafter, the monolayers were lysed and combined with the culture supernatant, and the number of CFU was determined by plating serial dilutions on CYE (A and C) or 7H11 (B and D) agar plates. The capacity of the bacteria to grow extracellularly in the culture medium was assessed by inoculating L. pneumophila (C) or M. tuberculosis (D) into wells containing only the culture medium or into parabiotic chambers in which the bacteria were separated from the HeLa cells by a 0.2-μm-pore-size filter. Although the bacteria are taken up much less efficiently by HeLa cells, they multiply, once inside, with a similar doubling time in HeLa cells and THP-1 cells (A and B). Overexpression of Rab5c does not alter the intracellular growth rate of L. pneumophila or M. tuberculosis in HeLa cells (C and D). The bacteria do not grow in the absence of cell monolayers or when separated from the monolayer in a parabiotic chamber (C and D). Data shown are the means ± the standard deviations of triplicate determinations.
To determine whether overexpression of Rab5c alters the rate of intracellular growth of M. tuberculosis or L. pneumophila in HeLa Tet-off cells, we compared the intracellular growth of M. tuberculosis and L. pneumophila in HeLa Tet-off and HeLa-Rab5c 1 day after withdrawal of tetracycline (Fig. 1C and D). The growth rate of L. pneumophila and M. tuberculosis in cells overexpressing Rab5c was equal to the growth rate in parental nontransfected HeLa Tet-off cells. When separated from HeLa Tet-off cells by a 0.2-μm-pore-size filter in a parabiotic chamber (Transwells; Costar), neither L. pneumophila nor M. tuberculosis grew in the same culture medium. Thus, once inside parental HeLa Tet-off cells or HeLa-Rab5c cells overexpressing Rab5c, L. pneumophila and M. tuberculosis multiply at a rate comparable to that in human macrophages.
(ii) Distribution of transferrin receptor and LAMP-1 on L. pneumophila or M. tuberculosis phagosomes in HeLa-Rab5c cells.
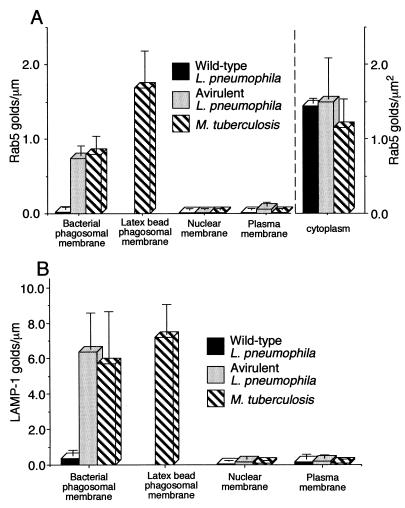
To determine if phagosomes in infected HeLa-Rab5c cells have molecular characteristics similar to phagosomes in infected human macrophages, we studied transferrin receptor expression on M. tuberculosis phagosomes and LAMP-1 expression on both L. pneumophila and M. tuberculosis phagosomes. Consistent with our published observations with human monocyte-derived macrophages (13), we found that in HeLa-Rab5c cells the majority of M. tuberculosis phagosomes stably transfected with the transferrin receptor gene stain positively for the transferrin receptor (Fig. 2). Also consistent with our previous observations of human macrophages (13), we found little or no LAMP-1 on phagosomes containing wild-type L. pneumophila (Fig. 3A) or live M. tuberculosis (Fig. 3B) in HeLa-Rab5c cells but intense staining on phagosomes containing either the avirulent mutant L. pneumophila (Fig. 3A) or heat-killed M. tuberculosis or latex beads (Fig. 3B) in these cells. These results confirmed that L. pneumophila and M. tuberculosis phagosomes in HeLa-Rab5c cells do not fuse with lysosomes and that overexpression of Rab5c in HeLa cells does not fundamentally alter the membrane-trafficking properties of L. pneumophila or M. tuberculosis phagosomes. We concluded from these sets of studies that, while uptake of L. pneumophila and M. tuberculosis into HeLa Tet-off cells is much less efficient than that into macrophages, both the intracellular rates of bacterial growth and the interaction of the phagosomes with the endolysosomal pathway in these host cells are very similar. This implied that lessons learned from studying L. pneumophila and M. tuberculosis phagosomes in HeLa-Rab5c cells were likely to apply to phagosomes of these pathogens in macrophages.
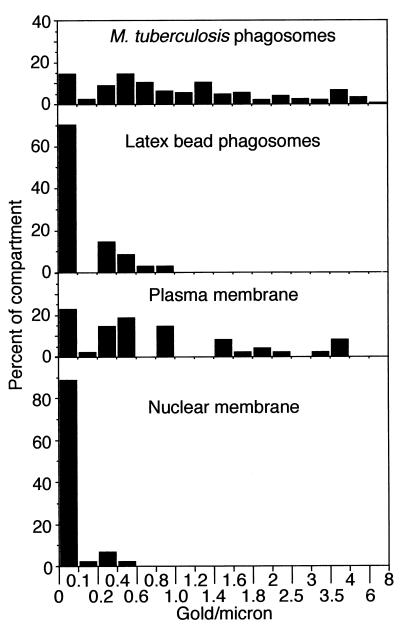
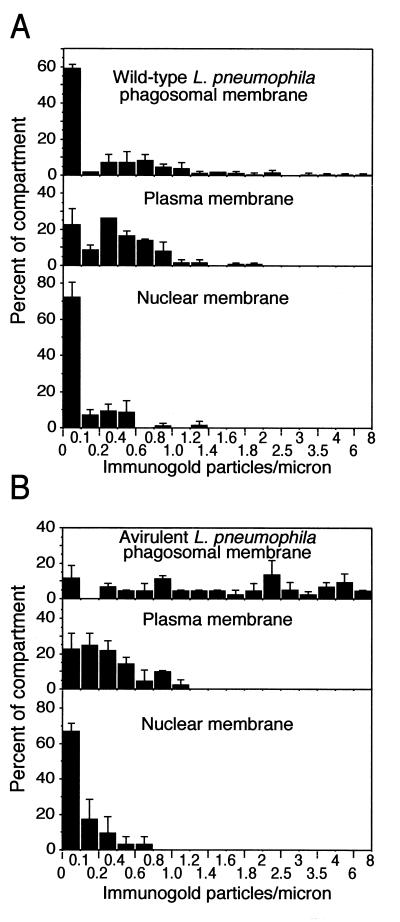
FIG. 2.
Distribution of staining for the human transferrin receptor in HeLa-Rab5c cells 2 days after infection with M. tuberculosis. HeLa-Rab5c cells stably transfected with the transferrin receptor gene were coincubated with M. tuberculosis and latex beads for 2 h, washed extensively, incubated at 37°C for 2 days, fixed, processed for cryoimmunoelectron microscopy, and stained by immunogold for the transferrin receptor. The number of transferrin immunogold particles per micrometer of phagosomal membrane, nuclear membrane, and plasma membrane was enumerated. Data are the percentages of the specified compartment whose membranes contain the indicated number of gold particles per micrometer. A total of 59 M. tuberculosis phagosomes and 48 latex bead phagosomes were evaluated.
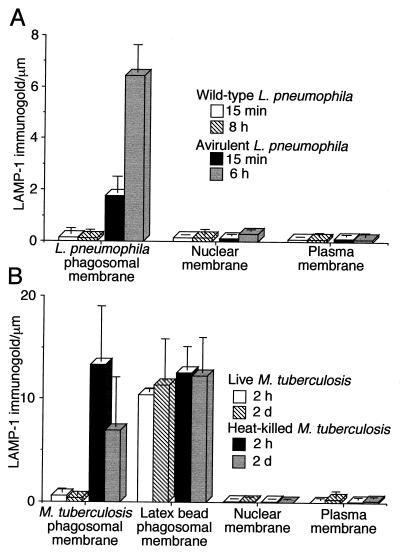
FIG. 3.
Quantitation of LAMP-1 immunogold staining in HeLa-Rab5c cells infected with L. pneumophila or M. tuberculosis. (A) HeLa-Rab5c cells were coincubated with wild-type or avirulent L. pneumophila for 15 min at 37°C and fixed immediately or coincubated at 37°C for 30 min, washed, and incubated for 6 or 8 h and then fixed. (B) HeLa-Rab5c cells expressing Rab5c were coincubated with latex beads and either live or heat-killed M. tuberculosis for 2 h and either fixed immediately or washed, incubated for 2 days at 37°C, and then fixed. After fixation, all cells were processed for cryoimmunoelectron microscopy and stained for LAMP-1. LAMP-1-bound immunogold particles were enumerated on phagosomal, nuclear, and plasma membranes. Data shown represent the mean and standard deviation of gold particle counts on at least 20 cells (each with at least one phagosome) on each of at least three electron microscopy grids. Wild-type L. pneumophila lacks LAMP-1 at both 15 min and 8 h (A). In contrast, avirulent L. pneumophila phagosomes have a modest level of LAMP-1 at 15 min and stain intensely for LAMP-1 at 6 h. Phagosomes containing live M. tuberculosis have very little LAMP-1, whereas phagosomes containing heat-killed M. tuberculosis and latex beads stain intensely for LAMP-1 at both 2 h and 2 days (B). The nuclear membrane and plasma membrane have negligible staining for LAMP-1 and serve as internal negative controls (A and B).
Distribution of Rab5c on phagosomes containing wild-type and avirulent L. pneumophila in HeLa-Rab5c cells.
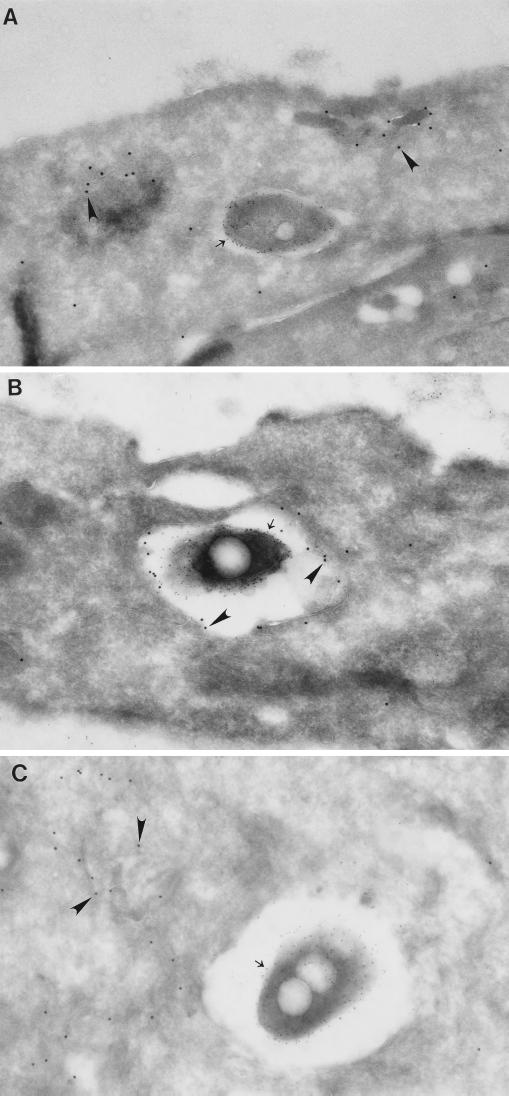
Two days after removal of tetracycline from the culture medium, 90% of HeLa-Rab5c cells had abundant immunogold staining for Rab5c on cytoplasmic vesicles (>1 gold particle/μm2), with an average level of 4 gold particles/μm2. As expected, we found that the Rab5c immunogold particles colocalized extensively with early endosomes labeled kinetically with 5-nm BSA-bound gold particles in the Rab5c-overexpressing cells (data not shown). Parental HeLa Tet-off cells, on the other hand, lacked significant staining for Rab5c (of 20 consecutive cells, none had more than 0.5 gold particle/μm2; mean level of immunogold staining, 0.14 gold particle/μm2). In HeLa-Rab5c cells infected with wild-type L. pneumophila, the majority of phagosomes had little or no detectable Rab5c at all time points examined, from 15 min to 18 h after phagocytosis. At 15 min, the earliest time point at which examination was feasible, 60% of the phagosomes had no detectable staining for Rab5c (Fig. 4 to 6A). In marked contrast, 90% of phagosomes containing the avirulent mutant L. pneumophila 25D did stain positive for Rab5c at 15 min (Fig. 4, 7B, and 8B). As typically occurs with phagosomes that mature to phagolysosomes, expression of Rab5c on mutant L. pneumophila phagosomes was transient, as staining was absent by 6 h of infection (Fig. 4 and 6C).
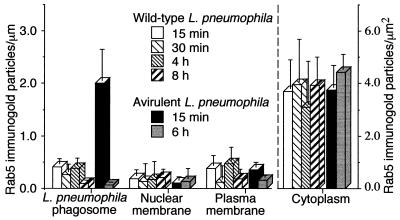
FIG. 4.
Quantitation of Rab5c immunogold staining in HeLa-Rab5c cells infected with wild-type or avirulent L. pneumophila. HeLa-Rab5c cells were coincubated with wild-type or avirulent L. pneumophila for 15 or 30 min and either fixed immediately or washed extensively, incubated for an additional 30 min to 8 h, and then fixed. After fixation, all cells were processed for cryoimmunoelectron microscopy, and Rab5c immunogold particles were enumerated on phagosomal, nuclear, and plasma membranes. Data shown are the means and standard deviations of gold counts on at least 20 cells (each with at least one phagosome) on each of at least three electron microscopy grids. (Left) At 15 min, Rab5c is scarce on wild-type L. pneumophila phagosomes but present on phagosomes containing avirulent L. pneumophila. Subsequently, Rab5c is absent or scarce on wild-type and avirulent L. pneumophila phagosomes. Rab5c is scarce on nuclear membranes and plasma membranes at all time points examined. (Right) As a control, Rab5c staining in the cytoplasm of the HeLa cells was quantitated and found to be comparable in the cells containing wild-type or avirulent L. pneumophila at all time points.
FIG. 6.
Phagosomes containing wild-type L. pneumophila but not avirulent L. pneumophila exclude Rab5c. Suspensions of wild-type (A) or avirulent (B and C) L. pneumophila were spun down onto monolayers of pTRE/rab5c-HeLa Tet-off cells at 4°C, incubated at 37°C for 15 min, and either fixed immediately (A and B) or washed, incubated in fresh culture medium for 6 h, and then fixed (C). Cells were processed for cryoimmunoelectron microscopy. Rab5c has been stained using 15-nm gold particles (arrowheads), and L. pneumophila LPS has been stained using 5-nm gold particles (arrows). Rab5c is absent from the wild-type L. pneumophila phagosome (A), despite the presence of Rab5c immunogold staining on vesicles adjacent to the phagosome. Rab5c is present on the avirulent L. pneumophila phagosome at 15 min (B) but is absent by 6 h (C). Magnifications, ×72,827 (A), ×38,235 (B), and ×50,664 (C).
FIG. 7.
Quantitation of Rab5c immunogold staining in HeLa-Rab5c cells infected with live or heat-killed M. tuberculosis. (A) Suspension of live or heat-killed M. tuberculosis together with 1-μm-diameter latex beads were centrifuged at 4°C onto monolayers of HeLa-Rab5c cells expressing Rab5c, incubated for 2 h at 37°C, and either fixed immediately (2-h time point) or washed extensively and incubated in fresh culture medium for 1 to 2 days and then fixed (1- or 2-day time point). The cells were processed for cryoimmunoelectron microscopy and stained for Rab5c. Rab5c-bound immunogold particles were enumerated on phagosomal, nuclear, and plasma membranes. Data shown are the means and standard deviations of gold counts on at least 20 cells (each with at least one phagosome) on each of at least three electron microscopy grids. (Left) Rab5c is present on phagosomes containing live M. tuberculosis at all time points but is scarce on phagosomes containing either heat-killed M. tuberculosis or latex beads, nuclear membranes, and plasma membranes. (Right) As a control, Rab5c staining in the cytoplasm of the HeLa cells was quantitated and found to be comparable in cells containing live or heat-killed M. tuberculosis. The level of staining in the cytoplasm at 2 h is somewhat less than that at 1 to 2 days, due to a shorter tetracycline-free induction period. (B) The distribution of staining for Rab5c in HeLa-Rab5c cells at the 2-day time point after coincubation with live M. tuberculosis and latex beads is shown (a total of 88 live M. tuberculosis phagosomes and 76 latex bead phagosomes were examined).
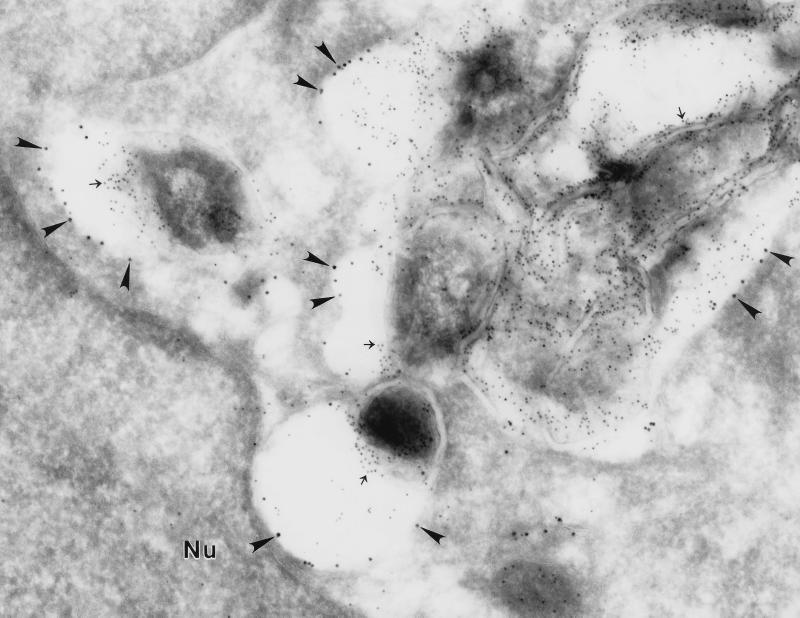
FIG. 8.
M. tuberculosis phagosomes in HeLa-Rab5c cells stain positively for Rab5c. HeLa-Rab5c cells were maintained and expanded in the presence of tetracycline (5 μg/ml). One day prior to infection with M. tuberculosis, tetracycline was removed from the culture medium to induce Rab5c expression. The HeLa cells were coincubated for 2 h with M. tuberculosis using an MOI of 400:1. Nonadherent bacteria and beads were washed away, and the monolayers were incubated for 2 additional days. Monolayers were fixed and processed for cryoimmunoelectron microscopy. Rab5c was stained with 15-nm immunogold particles (arrowheads) and is abundant on the M. tuberculosis phagosomal membrane. Mycobacterial LAM was stained with 5-nm gold particles and is present on the mycobacterial cell wall (arrows). Nu, nucleus. Magnification, ×55,700.
Distribution of Rab5c on phagosomes containing live and heat-killed M. tuberculosis in HeLa-Rab5c cells.
In HeLa-Rab5c cells infected with M. tuberculosis, the majority of M. tuberculosis phagosomes stained positively for Rab5c at all time points examined—from 2 h to 3 d after phagocytosis (Fig. 7 and 8). In contrast, phagosomes containing heat-killed M. tuberculosis in HeLa-Rab5c cells lacked significant staining for Rab5 at any of the time points examined, ranging from 2 h to 2 days (Fig. 7). At the MOIs used, heat-killed M. tuberculosis and latex beads were taken up inefficiently by HeLa cells. Because of this, we were unable to examine time points earlier than 2 h, times at which the phagosomes of inert particles or dead M. tuberculosis would be expected to have Rab5.
Also in contrast to phagosomes containing live M. tuberculosis, latex bead phagosomes in HeLa-Rab5c cells within the same monolayers usually lacked staining for Rab5c, even within cells that also contained Rab5c-immunopositive M. tuberculosis phagosomes (Fig. 7). Interestingly, however, latex bead phagosomes in HeLa-Rab5c cells that were very heavily infected with live M. tuberculosis did stain positively for Rab5c.
Effect of overexpression of the Rab5c GTPase-deficient, constitutively active mutant on L. pneumophila and M. tuberculosis phagosomes.
Whereas the majority of L. pneumophila phagosomes do not acquire detectable Rab5c, a minority do acquire some of the overexpressed Rab5c. It is possible that this low level of association is an artifact of overexpression and that the Rab5c on the phagosomes is either not truly intimately associated with the phagosome or not functional, due, for example, to an absence of downstream effectors. Likewise, although the M. tuberculosis phagosomes appeared to recruit Rab5c very avidly, it is possible that the recombinant Rab5c recruited to the M. tuberculosis phagosome is either not truly incorporated into the membrane or not biologically functional. It has been demonstrated that overexpression of constitutively active mutant forms of Rab5 leads to dramatic enlargement of early endosomes (9, 39, 40). To determine whether the overexpressed Rab5c is specifically associated with the bacterial phagosomes and whether it is biologically functional in these sites, we examined the effect of overexpression of the GTPase-deficient, constitutively active Rab5c Q79L mutant on M. tuberculosis and L. pneumophila phagosomes, reasoning that if the Rab5c is functional on the bacterial phagosomes, then there would be enlargement of the bacterial phagosomes. On the other hand, if Rab5c was not truly incorporated into the phagosomal membrane or was not biologically functional, then there would be no alteration in the phagosomal membrane.
Two days after withdrawal of tetracycline to induce expression of the mutant Rab5c, 77.5% of the HeLa-Rab5c Q79L cells had developed large vacuoles measuring over 2 μm in diameter. Immunogold staining revealed that these large vacuoles stained positively for Rab5c. To our surprise, many of these vacuoles also stained moderately to intensely for LAMP-1; in contrast, little or no colocalization of Rab5c with LAMP-1 was observed in cells overexpressing wild-type Rab5c. The LAMP-1 in the large vacuoles was present both on the walls of the vacuoles and also on membranes within the vacuoles. When wild-type or avirulent L. pneumophila was spun down onto monolayers of cells overexpressing the mutant Rab5c and fixed after a 30-min incubation, wild-type L. pneumophila was only rarely found within the large vacuoles (only 1 of 60 consecutive L. pneumophila phagosomes). Instead, the tight morphology of the L. pneumophila phagosome appeared unchanged, and the L. pneumophila phagosome continued to exclude both Rab5c and LAMP-1 (Fig. 9 and 10A). In contrast, when avirulent L. pneumophila was found inside HeLa cells that contained a large vacuole, without exception (40 of 40 consecutive avirulent L. pneumophila phagosomes) the avirulent L. pneumophila was found within the large vacuole. Typically, even at this early 30-min time point, dozens of avirulent L. pneumophila cells were found together within a phagosome (Fig. 10B). Vacuoles containing the avirulent L. pneumophila stained positively for both Rab5c and LAMP-1 (Fig. 9 and 10B).
FIG. 9.
Quantitation of Rab5c and LAMP-1 immunogold staining in HeLa-Rab5c Q79L cells infected with L. pneumophila or M. tuberculosis. Monolayers of HeLa cells expressing Rab5c Q79L were coincubated with L. pneumophila or with M. tuberculosis and latex beads, fixed, and processed for immunoelectron microscopy after 30-min or 2-h incubations (respectively). The number of Rab5c-bound (A) and LAMP-1-bound (B) immunogold particles was enumerated on phagosomes, plasma membranes, and nuclear membranes. In the case of the M. tuberculosis-infected cells, vacuoles that contained only latex beads were scored as latex bead phagosomes. Vacuoles that contained both latex beads and M. tuberculosis were scored as M. tuberculosis phagosomes. Latex bead phagosomes were not scored for the L. pneumophila infected cells, due to inadequate uptake in the 30 min of coincubation. Data shown are the means and standard deviations of gold counts on at least 20 cells (each with at least one phagosome) on each of at least two electron microscopy grids.
FIG. 10.
The phenotype of M. tuberculosis phagosomes and avirulent L. pneumophila phagosomes but not wild-type L. pneumophila phagosomes is altered by expression of the constitutively active Rab5c Q79L mutant. Suspensions of wild-type L. pneumophila (A), avirulent L. pneumophila (B), or M. tuberculosis and latex beads (D) were added to monolayers of HeLa cells expressing Rab5c Q79L and centrifuged at 1,160 × g for 20 min at 4°C, incubated at 37°C for either 30 min (L. pneumophila [A and B]) or 2 h (M. tuberculosis [D]), fixed, and processed for cryoimmunoelectron microscopy. Rab5c was stained with 15-nm immunogold particles (large arrowheads), LAMP-1 was stained with 10-nm immunogold particles (small arrowheads), and L. pneumophila LPS (A and B) or mycobacterial LAM (C and D) was stained with 5-nm immunogold particles (arrows). (A) Wild-type L. pneumophila resides in a morphologically tight phagosome that lacks immunogold staining for Rab5c and LAMP-1, which are present on an adjacent large vacuole (∗). (B) A large vacuole contains numerous avirulent L. pneumophila and stains positive for both Rab5c and LAMP-1. (C and D) M. tuberculosis resides in large vacuoles that stain positively for Rab5c and for LAMP-1. M. tuberculosis often shares the large vacuole with latex beads (D). Nu, nucleus. Magnifications, ×37,310 (A), ×37,310 (B), ×37,310 (C), and ×42,770 (D).
When live M. tuberculosis and latex beads were coincubated with the HeLa-Rab5c Q79L cells for 2 h, M. tuberculosis was found to enter the large vacuoles (Fig. 10C) that stained positively for Rab5c and that were also frequently positive for LAMP-1 (Fig. 9). Within HeLa-Rab5c Q79L cells that contained large vacuoles, 21% (8 of 46) of the M. tuberculosis phagosomes were of a normal, tight morphology and 79% (38 of 46) were unusually large and spacious. Within these cells, a similar percentage of latex bead phagosomes were within tight phagosomes (23%; 7 of 30) versus large spacious vacuoles (77%; 23 of 30). Interestingly, M. tuberculosis and latex beads were often found together in the large spacious vacuoles within HeLa-Rab5c Q79L cells (Fig. 10D). In striking contrast, we have only rarely observed M. tuberculosis and latex beads coresiding within a single phagosome in human monocyte-derived macrophages, and we have not observed coresidence of M. tuberculosis and latex beads in any of hundreds of M. tuberculosis phagosomes examined in HeLa cells overexpressing wild-type Rab5c.
Both L. pneumophila and M. tuberculosis multiplied in the HeLa-Rab5c Q79L cells. However, we were precluded from obtaining accurate measurements of their growth rates in these cells because during the long culture period required for this assessment there was significant loss of the HeLa cells from the monolayer.
DISCUSSION
Our initial studies established the relevance of the HeLa-Rab5c model to understanding phagosome trafficking of the intracellular pathogens L. pneumophila and M. tuberculosis. First, once inside HeLa-Rab5c cells, these bacteria multiplied at rates equivalent to that in human macrophages. Second, just as in human macrophages, (i) the wild-type L. pneumophila phagosome in HeLa-Rab5c cells excluded markers of the endolysosomal pathway, (ii) the M. tuberculosis phagosome in HeLa-Rab5c cells readily incorporated transferrin receptors but generally excluded LAMP-1, and (iii) phagosomes in HeLa-Rab5c cells containing avirulent L. pneumophila, heat-killed M. tuberculosis, and latex beads stained intensely for LAMP-1. Additional studies of Rab5c distribution in HeLa-Rab5c cells lent further support to the use of this model for studies of phagosome-endosome interaction. Our finding that Rab5c was transiently displayed on phagosomes in HeLa-Rab5c cells containing avirulent L. pneumophila, which mature to phagolysosomes, lent further support to the relevance of this model, since other studies have similarly reported transient expression of Rab5 immediately after phagocytosis on phagosomes that mature to phagolysosomes (17, 27). Our subsequent studies showed that Rab5c is absent on L. pneumophila phagosomes but persistently expressed on M. tuberculosis phagosomes. The latter finding with M. tuberculosis parallels that of Alvarez-Dominguez et al. (2) with a hemolysin-deficient strain of Listeria monocytogenes. In contrast to wild-type L. monocytogenes, the mutant strain fails to lyse the phagosomal membrane and enter the cytoplasm. Instead, it remains intraphagosomal in mouse J774 cells. Like virulent M. tuberculosis in our study, the mutant L. monocytogenes phagosome in the study by Alvarez-Dominguez et al. (2) exhibits a maturational arrest manifest by persistent expression of Rab5 on the phagosome and persistent interaction with early endosomes in an in vitro assay. Antibodies to Rab5 blocked the capacity of these L. monocytogenes phagosomes to interact with early endosomes, underscoring the importance of Rab5 in mediating phagosome-endosome interaction.
Our study is the first to examine the role of Rab-GTPases in human cells infected with L. pneumophila and the first to examine Rab5 in L. pneumophila-host cell interaction. Roy et al. (36) recently reported persistence of low levels of Rab7 on a minority of phagosomes containing wild-type L. pneumophila and transient acquisition of Rab7 by phagosomes containing avirulent dotA L. pneumophila mutants in mouse bone marrow macrophages. However, Rab5 was not examined in this study.
Our study is also the first to examine the role of Rab-GTPases in human cells infected with virulent M. tuberculosis. Via et al. (43) reported that isolated M. bovis BCG phagosomes from mouse J774 macrophages have persistent staining for Rab5 and do not acquire Rab4 or Rab7. These findings with M. bovis BCG in mouse macrophages are in agreement with our findings with the virulent Erdman strain of M. tuberculosis in the human HeLa cell line. Our two studies used very different but complementary methodologies. Via et al. used biochemical techniques to study a population of phagosomes isolated from infected cells. An advantage of this approach is that it allows the pooling of information from a very large number of phagosomes. However, the results can be distorted by contamination from other organelles, loss or gain of markers during the lengthy isolation procedure, and sample heterogeneity, in which case, high levels of staining on some phagosomes could skew the average level of staining. We used the cryosection immunogold technique to study the distribution of Rab-GTPases on individual phagosomes in fixed cells. This approach has the advantage of allowing the direct visualization of Rab5 on individual phagosomes and an assessment of the degree of heterogeneity of the phagosomes. This approach allowed us to conclude that the majority of M. tuberculosis phagosomes stain positive for Rab5, whereas the majority of L. pneumophila phagosomes do not.
Our study is the first to report on the subcellular distribution of human Rab5c. Previously, canine Rab5c overexpressed in BHK cells, as well as canine Rab5a and Rab5b, has been shown to localize to early endosomes (8, 11). We show here that human Rab5c localizes to early endosomes, early avirulent L. pneumophila phagosomes, and the M. tuberculosis phagosome, which has early endosomal properties (13, 14). We observe relatively little Rab5c on the plasma membrane. Therefore, it is unlikely that the Rab5c that we observe on the M. tuberculosis phagosome is derived from the plasma membrane. The Rab-GTPases cycle extensively between cytoplasm and membrane-bound forms, and it is likely that in these cells that overexpress Rab5c, the majority of the Rab5c observed on the M. tuberculosis phagosomes is recruited from the cytoplasmic pool, although some of it may also be derived from interaction with early endosomes.
We considered the possibility that the persistent recruitment of Rab5c to the M. tuberculosis phagosome might somehow be an artifact of the overexpression of Rab5c in the HeLa cells and that the Rab5c was not functionally integrated into the phagosomal membrane. Several findings argue against this possibility. First, the scarcity of Rab5c on latex bead phagosomes, wild-type L. pneumophila phagosomes, and late avirulent L. pneumophila phagosomes is consistent with a specific recruitment of recombinant Rab5c to the M. tuberculosis phagosome. Second, the presence of transferrin receptor (an early endosomal marker) on the M. tuberculosis phagosome 2 days after infection is consistent with persistence of functional Rab5c on the M. tuberculosis phagosome. Third, the dramatic changes in the M. tuberculosis phagosome resulting from overexpression of the fusion-promoting Rab5c Q79L mutant indicate that the Rab5c on the M. tuberculosis phagosome is functional. The M. tuberculosis phagosome in the Rab5c Q79L mutant is markedly different from that of a normal M. tuberculosis phagosome. Whereas a normal M. tuberculosis phagosome is tight, lacks LAMP-1, and does not fuse with latex bead compartments, the M. tuberculosis phagosome in the HeLa-Rab5c Q79L cell is spacious and LAMP-1 positive and often contains latex beads. In view of these phenotypic differences, it is very unlikely that M. tuberculosis disrupts the maturation of its phagosome by inhibiting GTP hydrolysis by the Rab5 on its phagosome. Although our studies using the GTPase-deficient, constitutively active Rab5 exclude inhibition of GTP hydrolysis as the mechanism by which M. tuberculosis disrupts the maturation of its phagosome, the persistence of wild-type Rab5 on the phagosome is likely to be an important mechanism in maintaining the capacity of the phagosome to interact with early endosomes and in maintaining early endosomal properties. Hence, these studies imply that M. tuberculosis blocks the maturation of its phagosome by disrupting events downstream of Rab5 acquisition and effector action, for example, by disrupting the acquisition or function of Rab7 effectors.
Whereas the M. tuberculosis phagosome undergoes dramatic phenotypic changes in HeLa cells expressing the GTPase-deficient, constitutively active Rab5c mutant, the wild-type L. pneumophila phagosome remains morphologically tight and continues to exclude Rab5 and LAMP-1. With regard to the mechanisms underlying the altered maturation of the L. pneumophila phagosome, our observations that the L. pneumophila phagosome (i) does not acquire wild-type Rab5c, (ii) does not acquire the constitutively active Rab5c Q79L mutant, and (iii) does not enter the large Rab5c Q79L-positive vacuoles strongly suggest that the L. pneumophila phagosome never acquires functional receptors for Rab5. The consequent exclusion of Rab5 from the L. pneumophila phagosome is likely to be an important aspect underlying the failure of the L. pneumophila phagosome to mature along the endocytic pathway.
The presence of LAMP-1 on the giant vacuoles in HeLa-Rab5c Q79L cells was unexpected, as the presence of LAMP-1 on the swollen endosomes of Rab5c Q79L mutant-expressing cells has not previously been reported. Possible explanations for this phenomenon are that (i) the high fusiogenicity of the Rab5c Q79L endosomes promotes their fusion with late endosomal and lysosomal compartments or (ii) late endosomal-lysosomal proteins may normally traffic transiently through the early endosome and be present at very low levels in early endosomal compartments, but overexpression of the Rab5c Q79L mutant retards their trafficking and causes them to accumulate in these compartments.
Prior studies have found no difference in the subcellular distribution or functional role of Rab5a, Rab5b, or Rab5c in endocytosis (8, 11). Similarly, all three isoforms of Rab5 have been found on early latex bead phagosomes in mouse macrophages (17). Nevertheless, Alvarez-Dominguez and Stahl (3) recently examined the effect of antisense oligonucleotides to Rab5a and Rab5c on the maturation of phagosomes containing hemolysin-deficient L. monocytogenes in human monocyte-derived macrophages and found that antisense oligonucleotides to Rab5a, but not Rab5c, disrupted maturation of the bacterial phagosome. These data suggest that Rab5a plays a more important role than Rab5c in human macrophages under the conditions studied. However, it is possible that the greater role of Rab5a over Rab5c in the studies by Alvarez-Dominguez and Stahl reflect higher levels of endogenous expression in Rab5a than in Rab5c, rather than a fundamental biological difference in their functions. It is likely that various Rab5 isoforms are expressed at different levels in different cell types under various conditions, and the type of Rab5 isoform that is dominant in mediating a biological function may vary accordingly. We found no cDNA clone corresponding to Rab5a in our probe of a human fetal lung library but found four clones corresponding to Rab5c, suggesting that in human fetal lung, Rab5c may be expressed at higher levels than Rab5a.
When permeabilized cells are coincubated with a particular Rab-GTPase, the Rab-GTPase inserts into those membranes that have receptors for the particular Rab-GTPase (41). Likewise, when a particular Rab-GTPase is overexpressed in a cell, the Rab-GTPase will be delivered to the membranes that have receptors for that Rab-GTPase. When a constitutively active Rab-GTPase is overexpressed, it will lead to phenotypic changes in any membrane-bound compartments that have both the receptors for the Rab-GTPase and the appropriate effector machinery to allow downstream functions of the Rab-GTPase to proceed. Therefore, the fact that we observe recruitment of Rab5c to the phagosomes containing latex beads, M. tuberculosis, and avirulent L. pneumophila indicates that these phagosomes do have receptors that allow the recruitment of Rab5c. That we observe dramatic phenotypic changes in the morphology of these phagosomes (but not the wild-type L. pneumophila phagosome, which does not recruit Rab5c) in cells expressing the constitutively active Rab5c Q79L indicates that Rab5c can also have a major role in phagosomal development when it is overexpressed.
In conclusion, our study shows that the expression of Rab5 on L. pneumophila and M. tuberculosis phagosomes deviates from the typical pattern of expression on phagosomes containing inert particles. The latter phagosomes display transient expression of Rab5 as the phagosomes mature along the endolysosomal pathway, culminating in the formation of a phagolysosome. In contrast, the L. pneumophila phagosome does not display Rab5, and the M. tuberculosis phagosome displays Rab5 persistently. The absence of Rab5 on the L. pneumophila phagosome may underlie its lack of interaction with the endocytic pathway. The persistence of functional Rab5 on the M. tuberculosis phagosome is undoubtedly important in allowing the phagosome to maintain interaction with early endosomes and preserve early endosomal properties; it may be an important factor underlying the arrested maturation of the phagosome.
FIG. 5.
Distribution of staining for Rab5c in HeLa-Rab5c cells fixed immediately after a 15-min coincubation with either wild-type or avirulent L. pneumophila. HeLa-Rab5c cells were coincubated with wild-type (A) or avirulent (B) L. pneumophila for 15 min, fixed, and processed for cryoimmunoelectron microscopy. The number of gold particles per micrometer of membrane on phagosomal, plasma, and nuclear membranes was enumerated. Data shown are the means ± standard deviations of the distributions from two separate experiments.
ACKNOWLEDGMENTS
We are grateful to Birgitta Sjostrand and to Chalermchai Chaloyphian for expert technical assistance.
This work was supported by a Research Grant from the American Lung Association and by grants AI-31338 and AI-35275 from the National Institutes of Health. M.A.H. is the Gordon MacDonald Scholar at the University of California, Los Angeles. During the time that this work was performed, D.L.C. was supported by a Young Investigator Award from the Infectious Diseases Society of America.
REFERENCES
- 1.Allison A C, Byars N E. Adjuvant formulation for use in vaccines to elicit both cell-mediated and humoral immunity. J Immunol Methods. 1986;95:157–168. doi: 10.1016/0022-1759(86)90402-3. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Dominguez C, Barbieri A, Beron W, Wandinger-Ness A, Stahl P D. Phagocytosed live Listeria monocytogenes influences Rab5-regulated in vitro phagosome-endosome fusion. J Biol Chem. 1996;271:13834–13843. doi: 10.1074/jbc.271.23.13834. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Dominguez C, Stahl P D. Increased expression of Rab5a correlates directly with accelerated maturation of Listeria monocytogenes phagosomes. J Biol Chem. 1999;274:11459–11462. doi: 10.1074/jbc.274.17.11459. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong J A, Hart P D. Response of cultured macrophages to M. tuberculosis with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayad N, Hull M, Mellman I. Mitotic phosphorylation of rab4 prevents binding to a specific receptor or endosome membranes. EMBO J. 1997;16:4497–4507. doi: 10.1093/emboj/16.15.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbieri M, Li G, Colobo M, Stahl P. Rab5, an early acting endosomal GTPase, supports in vitro endosome fusion without GTP hydrolysis. J Biol Chem. 1994;269:18720–18722. [PubMed] [Google Scholar]
- 7.Bottger G, Nagelkerken B, van der Sluijs P. Rab4 and Rab7 define distinct nonoverlapping endosomal compartments. J Biol Chem. 1996;271:29191–29197. doi: 10.1074/jbc.271.46.29191. [DOI] [PubMed] [Google Scholar]
- 8.Bucci C, Lutcke A, Steele-Mortimer O, Olkkonen V, Dupree P, Chiariello M, Bruni C, Simons K, Zerial M. Cooperative regulation of endocytosis by three Rab5 isoforms. FEBS Lett. 1995;366:65–71. doi: 10.1016/0014-5793(95)00477-q. [DOI] [PubMed] [Google Scholar]
- 9.Bucci C, Parton R, Mather I H, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 10.Chavrier P, Simons K, Zerial M. The complexity of the Rab and Rho GTP-binding subfamilies revealed by a PCR cloning approach. Gene. 1992;112:261–264. doi: 10.1016/0378-1119(92)90387-5. [DOI] [PubMed] [Google Scholar]
- 11.Chavrier P, Parton R G, Hauri H P, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 12.Clemens D L. Characterization of the Mycobacterium tuberculosis phagosome. Trends Microbiol. 1996;4:113–118. doi: 10.1016/0966-842X(96)81528-9. [DOI] [PubMed] [Google Scholar]
- 13.Clemens D L, Horwitz M A. Characterization of the M. tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemens D L, Horwitz M A. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J Exp Med. 1996;184:1–7. doi: 10.1084/jem.184.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowle A, Dahl R, Ross E, May M. Evidence that vesicles containing living virulent M. tuberculosis or M. avium in cultured human macrophages are not acidic. Infect Immun. 1991;59:1823–1831. doi: 10.1128/iai.59.5.1823-1831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desjardins M. Biogenesis of phagolysosomes: the “kiss and run” hypothesis. Trends Cell Biol. 1995;5:183–186. doi: 10.1016/s0962-8924(00)88989-8. [DOI] [PubMed] [Google Scholar]
- 17.Desjardins M, Huber L A, Parton R G, Griffiths G. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J Cell Biol. 1994;124:677–688. doi: 10.1083/jcb.124.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabay J, Horwitz M A. Isolation and characterization of the cytoplasmic and outer membranes of the Legionnaires' disease bacterium (Legionella pneumophila) J Exp Med. 1985;161:409–422. doi: 10.1084/jem.161.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths G, McDowall A, Back R, Dubochet J. On the preparation of cryosections for immunocytochemistry. J Ultrastruct Res. 1984;89:65–78. doi: 10.1016/s0022-5320(84)80024-6. [DOI] [PubMed] [Google Scholar]
- 21.Hall A. The cellular functions of small GTP-binding proteins. Science. 1990;249:635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- 22.Han H, Sudo K, Inazawa I, Nakamura Y. Isolation and mapping of a human gene (rabL) encoding a small GTP-binding protein homologous to the Ras-related RAB gene. Cytogenet Cell Genet. 1996;73:137–139. doi: 10.1159/000134325. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch C S, Ellner J J, Russell D G, Rich E A. Complement receptor mediated uptake and TNF-alpha-mediated growth inhibition of M. tuberculosis by human alveolar macrophages. J Immunol. 1993;152:743–753. [PubMed] [Google Scholar]
- 24.Horwitz M A. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J Exp Med. 1987;166:1310–1328. doi: 10.1084/jem.166.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwitz M A, Maxfield F R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984;99:1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz M A, Silverstein S. The Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Investig. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jahraus A, Storrie B, Griffiths G, Desjardins M. Molecular characterization of phagosomes. J Cell Sci. 1994;107:145–157. doi: 10.1242/jcs.107.1.145. [DOI] [PubMed] [Google Scholar]
- 28.Landt O, Grunert H, Hahn U. A general method for rapid site-directed mutagenesis using the polymerase chain reaction. Gene. 1990;96:125–128. doi: 10.1016/0378-1119(90)90351-q. [DOI] [PubMed] [Google Scholar]
- 29.Lee B-Y, Horwitz M A. Identification of macrophage and stress-induced proteins of Mycobacterium tuberculosis. J Clin Investig. 1995;96:245–249. doi: 10.1172/JCI118028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marra A, Blander S, Horwitz M, Shuman H. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc Natl Acad Sci USA. 1992;89:9607–9611. doi: 10.1073/pnas.89.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meresse S, Gorvel J, Chavrier P. The Rab7 GTPase resides on a vesicular compartment connected to lysosomes. J Cell Sci. 1995;108:3349–3358. doi: 10.1242/jcs.108.11.3349. [DOI] [PubMed] [Google Scholar]
- 32.Nuoffer C, Balch W. GTPases: multifunctional molecular switches regulating vesicular traffic. Annu Rev Biochem. 1994;63:949–990. doi: 10.1146/annurev.bi.63.070194.004505. [DOI] [PubMed] [Google Scholar]
- 33.Pfeffer S. Rab-GTPases: master regulators of membrane trafficking. Curr Biol. 1994;6:522–526. doi: 10.1016/0955-0674(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 34.Pitt A, Mayorga L S, Stahl P D, Schwartz A L. Alterations in the protein composition of maturing phagosomes. J Clin Investig. 1992;90:1978–1983. doi: 10.1172/JCI116077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitt A, Mayorga L S, Schwartz A L, Stahl P D. Transport of phagosomal components to an endosomal compartment. J Biol Chem. 1992;267:126–132. [PubMed] [Google Scholar]
- 36.Roy C, Berger K, Isberg R. L. pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 37.Sadosky A, Wiater L, Shuman H. Identification of L. pneumophila genes required for growth within and killing of human macrophages. Infect Immun. 1993;61:5361–5373. doi: 10.1128/iai.61.12.5361-5373.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slot J, Geuze H, Gigengack S, Lienhard G, James D E. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 40.Stenmark H, Parton R, Steele-Mortimer O, Lucte A, Gruenberg J, Zerial M. Inhibition of Rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1995;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ullrich O, Horuchi H, Alexandrov K, Zerial M. Use of Rab-GDP dissociation inhibitor for solubilization and delivery of Rab proteins to biological membranes in permeabilized cells. Methods Enzymol. 1995;257:243–252. doi: 10.1016/s0076-6879(95)57029-2. [DOI] [PubMed] [Google Scholar]
- 42.Ullrich O, Horiuchi H, Bucci C, Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994;368:157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- 43.Via L E, Deretic D, Ulmer R, Hibler N, Huber L, Deretic V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by Rab5 and Rab7. J Biol Chem. 1997;272:13326–13331. doi: 10.1074/jbc.272.20.13326. [DOI] [PubMed] [Google Scholar]
- 44.Xu S, Cooper A, Sturgill-Koszycki S, van Heyningen T, Chatterjee D, Orme I, Allen P, Russell D. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153:2568–2578. [PubMed] [Google Scholar]