Abstract
Vaccines for P. falciparum will need to contain both T- and B-cell epitopes. Conserved epitopes are the most desirable, but they are often poorly immunogenic. The major merozoite surface protein 1 (MSP-1) is currently a leading vaccine candidate antigen. In this study, six peptides from conserved or partly conserved regions of MSP-1 were evaluated for immunogenicity in B10 congenic mice. Following immunization with the peptides, murine T cells were tested for the ability to proliferate in vitro and antibody responses to MSP-1 were evaluated in vivo. The results showed that one highly conserved sequence (MSP-1#1, VTHESYQELVKKLEALEDAV; located at amino acid positions 20 to 39) and one partly conserved sequence (MSP-1#23, GLFHKEKMILNEEEITTKGA; located at positions 44 to 63) contained both T- and B-cell epitopes. Immunization of mice with these peptides resulted in T-cell proliferation and enhanced production of antibody to MSP-1 upon exposure to merozoites. MSP-1#1 stimulated T-cell responses in three of the six strains of mice evaluated, whereas MSP-1#23 was immunogenic in only one strain. Immunization with the other four peptides resulted in T-cell responses to the peptides, but none of the resulting peptide-specific T cells recognized native MSP-1. These results demonstrate that two sequences located in the N terminus of MSP-1 can induce T- and B-cell responses following immunization in a murine model. Clearly, these sequences merit further consideration for inclusion in a vaccine for malaria.
A vaccine against the asexual stages of Plasmodium falciparum will need to contain both T- and B-cell epitopes, probably from several different antigens. One important antigen under consideration is the major merozoite surface protein (MSP-1), which is expressed on the surface of merozoites. Studies have demonstrated that immunization with either the intact molecule or various constructs containing segments of MSP-1 can induce partial or complete protection in mice, monkeys, and possibly humans (5, 7, 8, 15, 20). Antibodies are thought to be a major effector mechanism, since monoclonal antibodies against epitopes at both the 83-kDa amino end and the 19-kDa carboxyl terminus of MSP-1 inhibit P. falciparum growth in vitro (1, 11). In addition, passive transfer of a monoclonal antibody against the MSP-1 of P. yoelii protects mice against challenge with this rodent malaria parasite (12). Thus, identification of conserved B-cell and helper T-cell sites within MSP-1 is of importance for vaccine development.
To date, over 40 sequences (11 to 22 amino acids in length) of MSP-1 have been reported to contain CD4+-T-cell epitopes. Many of the epitopes that have been studied are located at the C-terminal end of MSP-1 (blocks 13 to 17). The majority of these epitopes are present in dimorphic regions and differ in their immunogenicity and level of major histocompatibility complex restriction (4, 6, 14, 17, 19, 25). It is hypothesized that additional T-cell epitopes that are important for the induction of growth-inhibitory antibodies are present in MSP-1 (25). Thus, a search for additional T-cell epitopes of MSP-1, preferably from conserved regions of the molecule, is warranted.
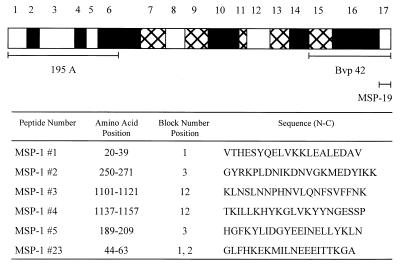
In 1994, Quakyi et al. (17) evaluated five conserved MSP-1 sequences and one partly conserved sequence (i.e., the 13 amino acids at the amino terminus are conserved). The six sequences are located in blocks 1 to 12 as follows: in block 1 (MSP-1#1), between blocks 1 and 2 (MSP-1#23), in block 3 (MSP-1#2 and MSP-1#5), and in block 12 (MSP-1#3 and MSP-1#4) (see Fig. 1). Based on T-cell proliferation, more exposed than nonexposed Caucasians responded to MSP-1#1 and MSP-1#2, whereas approximately equal numbers responded to MSP-1#3, MSP-1#4, MSP-1#5, and MSP-1#23. Thus, it is possible that T-cell responses observed to the latter peptides were initially induced by antigens or pathogens other than those associated with malaria. As a means of further evaluating these peptides, a total of 35 human T-cell clones were produced against MSP-1#1 (5 clones), MSP-1#2 (18 clones), and MSP-1#4 (12 clones). Surprisingly, only one of the peptide-specific clones was able to respond to P. falciparum merozoites. Thus, all six peptides are capable of inducing a positive proliferative response, but only one of them generated T-cell clones that also recognized native antigen. Since T-cell epitopes incorporated into a subunit vaccine must induce T cells that can be activated by natural infection with the parasite, the immunogenicity of these peptides was further evaluated.
FIG. 1.
Schematic representation of P. falciparum MSP-1. P. falciparum MSP-1 has been divided in 17 blocks based on amino acid sequence homology (22). Open boxes represent regions with high homology, hatched boxes represent regions with intermediate homology, and solid boxes represent regions with low homology. Peptides and fragments study are indicated. Amino acid positions are based on those in reference 13 adapted from reference 22. Block number positions are based on reference 22.
In the present study, six strains of B10 congenic mice were immunized with the MSP-1 peptides and the resulting primed lymphocytes were tested in vitro for the ability to proliferate when cultured with either the peptides or P. falciparum merozoites. In addition, the antibody response to recombinant and purified MSP-1 was evaluated in mice that were first primed with the MSP-1 peptides. The results showed that two of the sequences, namely, MSP-1#1 and MSP-1#23, which are located in block 1 and between blocks 1 and 2, respectively, stimulated T-cell responses and enhanced antibody production upon exposure to P. falciparum merozoites. Thus, these two sequences contain both T- and B-cell epitopes and are able to induce MSP-1-specific cellular and humoral immune responses when injected into mice. Therefore, these sequences should be seriously considered when designing a subunit vaccine for malaria.
MATERIALS AND METHODS
Synthetic peptides.
The sequences of the six peptides used in this study and their positions in MSP-1 are shown in Fig. 1. These peptides, which have been described previously (16, 17), were synthesized by R. Houghten, Torrey Pines Institute of Molecular Studies (San Diego, Calif.) and by AnaSpec (San Jose, Calif.). Purity was assessed by high-pressure liquid chromatography and determined to be >90%. Lyophilized peptides were dissolved in water (or, when needed, in 5 mM HCl or 5 mM NaOH) at 10 mg/ml, and molarities were calculated.
Preparation of P. falciparum merozoite extract.
P. falciparum parasites (3D7 clone of the NF54 strain) (26) were cultured using a modification of the method of Trager and Jensen (24). In brief, asynchronous P. falciparum parasites were cultured in human O+ erythrocytes (Interstate Blood Bank, Memphis, Tenn.) in RPMI 1640 medium supplemented with 10% human O+ serum (Interstate Blood Bank), 50 mg of hypoxanthine (Sigma, St. Louis, Mo.) per ml, and 2 μg of gentamicin (GIBCO, Grand Island, N.Y.) per ml. Cultures were maintained at a 5% packed-cell volume in the presence of 3% O2, 7% CO2, and 90% N2. Infected erythrocytes (RBC) were purified by Percoll density gradient centrifugation as previously described (1). Since MSP-1 is found only on schizonts and merozoites, fractions containing ≥90% schizonts and merozoites were pooled and stored at −80°C. These preparations are referred to as P. falciparum merozoite extract. Uninfected O+ human RBC from the same source were used as a negative control.
Preparation of purified MSP-1 protein and the 195A and C-terminal fragments.
MSP-1 was purified from P. falciparum cultures (FUP strain) using monoclonal antibody affinity chromatography as previously described (2). Purified recombinant fragments of MSP-1 were also used (Fig. 1), including the N-terminal 50-kDa fragment (195A) (10), the C-terminal 42-kDa fragment (Bvp42) (3), and the C-terminal 19-kDa fragment (YMSP-19, the E-KNG variant) (10).
Mouse strains.
In this study, 6- to 12-week-old female B10 congenic mice with the following H-2 haplotypes were used: C57BL/10 (H-2b), B10.D2 (H-2d), B10.A(4R) (H-2h4), B10.A(5R) (H-2i5), B10.S(9R) (I-As I-Eβsαk), and B10.BR (H-2k). BALB/cJ (H-2d) mice were also used. The mice were purchased from the Jackson Laboratories (Bar Harbor, Maine), except for the B10.S(9R) mice, which were kindly provided by J. Berzofsky (National Institutes of Health).
Immunization protocols for assessing T-cell proliferation.
Three different immunization protocols were used. First, three mice per group were immunized at the base of the tail with the P. falciparum merozoite extract, containing 107 merozoites per mouse emulsified in complete Freund's adjuvant (CFA). In the second set of immunizations, three mice per group received a mixture of the six peptides, with 83.5 μM each peptide (approximately 21 μg of peptide per mouse), emulsified in CFA. Control mice received phosphate-buffered saline emulsified in CFA. Previous titration studies had shown that immunization with 83.5 μM peptide resulted in optimal proliferative responses. In the third set of experiments, B10.D2 and BALB/c mice (three mice per group) were immunized with each peptide individually. Each mouse received 83.5 μM either peptide (MSP-1#1, MSP-1#3, MSP-1#4, MSP-1#5, or MSP-1#23) or diluent emulsified in CFA at the base of the tail. Ten days later, proliferation assays were performed.
Proliferation assays using murine lymph node cells.
Cells from draining inguinal and periaortic lymph nodes were removed and pooled from mice within each group, and single-cell suspensions were prepared. The cells were cultured in 96-well flat-bottom microtiter plates (Costar Corp., Cambridge, Mass.) at 4 × 105 cells per 200 μl of complete medium. The culture medium consisted of equal volumes of RPMI 1640 (GIBCO, Grand Island, N.Y.) and Eagle's Hanks amino acids (Biofluids, Rockville, Md.) supplemented with 10% fetal calf serum (JRH Biosciences, Lenexa, Kans.) and 10−5 M 2-mercaptoethanol (Bio-Rad, Inc., Richmond, Calif.). The cells were cultured in quadruplicate in the presence of either medium alone (unstimulated control), tuberculin purified protein derivative at 2 μg/well (Connaught Laboratories, Willowdale, Calif.), the peptides at 0.1, 1.0, or 10 μM per well (approximately 0.063, 0.63, and 6.3 μg/ml, respectively), an aliquot of the merozoite extract (104 frozen-thawed merozoites/well), or an extract of uninfected human RBC (103/well). After 96 h of incubation, the cells were pulsed with 0.5 μCi of [3H]thymidine (185 MBq) (ICN, Irvine, Calif.) for 14 to 18 h. The cells were harvested, and incorporation of [3H]thymidine was determined using a 1205 Betaplate liquid scintillation counter (Wallac Inc., Gaithersburg, Md.).
The geometric mean stimulation index (SI), i.e., the geometric mean counts per minute in stimulated wells/geometric mean counts per minute in unstimulated wells, was calculated. In calculating the SI, outlier analysis was employed to exclude nonrepresentative data from the calculations. An outlier value was defined as one that was beyond the mean ± 4 standard deviations. A response was considered to be positive when the SI was equal or greater than 2.7 and the P value between unstimulated and stimulated cells was <0.01. A computer program, BETA, was used for the data analysis with QuatroPro software (Corel Word Perfect, version 8.0).
Identification of B-cell epitopes.
To determine if the peptides contained B-cell as well as T-cell epitopes, BALB/c mice were immunized with each peptide (83.5 μM) in CFA and then boosted twice with the same peptide (83.5 μM) in incomplete Freund's adjuvant (IFA). Approximately 14 days later, plasma was collected and tested by enzyme-linked immunosorbent assay (ELISA) as described below. Results are expressed as the mean of the optical density (OD) of the tested sample minus the mean OD of the background. ODs of >0.100 were considered positive. This value represents the mean OD of preimmune plasma plus 4 standard deviations.
Antibody production.
To determine if immunization with the peptides enhanced antibody production upon subsequent exposure to malarial parasites, BALB/c mice (six per group) were immunized with one of the peptides (83.5 μM) in CFA. Three weeks later, three of the six mice were injected intraperitoneally with 5 μg of MSP-1 per mouse emulsified in IFA and the other three mice were injected with an extract of 107 P. falciparum merozoites per mouse emulsified in IFA. As a control group, nine BALB/c mice received a primary immunization with PBS emulsified in CFA. Then, approximately 3 weeks later, three of the mice were injected with purified MSP-1 in IFA (5 μg/mouse), three mice received an extract of 107 P. falciparum merozoites per mouse in IFA, and three received PBS in IFA. Fourteen days later, plasma samples from mice within a group were pooled and stored at −80°C until tested.
ELISA.
Plasma samples from the above mice were assayed for binding to purified and recombinant fragments of MSP-1 (9). Plates were coated with the following antigen preparations: purified MSP-1 (0.38 μg/ml), 195A (0.2 μg/ml), Bvp42 (0.38 μg/ml)1, and YMSP-1 19 (0.38 μg/ml). Wells were blocked with 1% bovine serum albumin in borate buffer saline (BBS) (pH 8.0). Pooled plasma samples were diluted 1:50 and 1:100 with 1% bovine serum albumin in BBS and incubated in duplicate for 1 h at room temperature (RT). Wells were then washed with BBS supplemented with 0.5 M NaCl and incubated for 1 h with a 1:2,000 dilution of goat anti-mouse immunoglobulin G conjugated to peroxidase (Zymed Laboratories, Inc., San Francisco, Calif.). The wells were washed, 2,2′-azinobis(3-ethylbenzthiazolino sulfonic acid) (ABTS)-H2O2 (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) was added, and the OD was measured at 405 nm using a Dynatec ELISA reader.
Western blot analysis.
The plasma samples were also tested for binding to intact and processed MSP-1 by Western blotting. An extract of merozoites (1.5 μg/lane) was electrophoresed under nonreducing conditions in a 10% acrylamide–sodium dodecyl sulfate Bio-Rad minigel and transferred to a nitrocellulose membrane as previously described (23). The membranes were blocked overnight at 4°C with 10% dry milk (Carnation Co., Los Angeles, Calif.) in 0.1 M PBS (pH 7.2) containing 0.05% Tween 20 (PBS-Tween). They were then incubated for 2 h at RT with mouse plasma at a 1:40 dilution. After incubation, the membranes were washed three times with PBS-Tween, incubated for 1 h at RT with a 1:1,000 dilution of polyvalent anti-mouse Ig labeled with alkaline phosphatase (Sigma, St. Louis, Mo.), and washed three times. Then 5-chloro-4-bromo-3-indolylphosphate–nitroblue tetrazolium (BCIP/NBT) substrate (Kirkegaard and Perry Laboratories) was added for color development.
RESULTS
T-cell response to MSP-1 peptides in mice immunized with the extract of P. falciparum.
Three strains of mice were immunized with the extract of merozoites emulsified in CFA, and draining lymph node cells were subsequently incubated in vitro with different concentrations of the test peptides (Table 1). The experiment was repeated two to four times for each mouse strain, and similar results were obtained in each experiment. Positive T-cell proliferative responses were observed in vitro with purified protein derivative, an antigen present in CFA (SI, 4.2 to 9.4), and in response to the extract of merozoites (SI, 37.9 to 43.0), but responses to the control extract of human RBCs were negative (SI, 2.2 to 2.7). No response was obtained upon stimulation in vitro with any of the six peptides (SI, 0.6 to 2.1). Thus, immunization of mice once with malarial merozoite extract primed T cells for a recall response to the parasites but did not stimulate a response that was specific for any of the six MSP-1 peptides. These data are consistent with our hypothesis that T-cell epitopes from conserved regions of parasite antigens are weak immunogens.
TABLE 1.
SI of lymph node cells from mice immunized with an extract of P. falciparum and then incubated in vitro with peptides and controls
| Peptide | SI for mouse straina:
|
|||||
|---|---|---|---|---|---|---|
| C57BL/10
|
B10.BR
|
BALB/c
|
||||
| 1 μMb | 5 μMb | 1 μM | 5 μM | 1 μM | 5 μM | |
| MSP-1#1 | 1.8 ± 0.4 | 1.5 ± 0.2 | 1.0 ± 0.2 | 0.6 ± 0.3 | 1.0 ± 0.1 | 1.6 ± 0.4 |
| MSP-1#2 | 1.9 ± 0.2 | 1.6 ± 0.2 | 1.2 ± 0.3 | 1.1 ± 0.3 | 0.9 ± 0.1 | 1.8 ± 0.3 |
| MSP-1#3 | 1.4 ± 0.4 | 1.4 ± 0.1 | 1.1 ± 0.4 | 0.8 ± 0.4 | 1.1 ± 0.2 | 1.1 ± 0.2 |
| MSP-1#4 | 2.1 ± 0.4 | 1.4 ± 0.1 | 1.8 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.1 | 2.1 ± 0.3 |
| MSP-1#5 | 1.5 ± 0.4 | 1.2 ± 0.4 | 0.9 ± 0.2 | 0.7 ± 0.4 | 0.6 ± 0.1 | 1.3 ± 0.1 |
| MSP-1#23 | 1.6 ± 0.3 | 1.6 ± 0.1 | 1.4 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.2 | 1.5 ± 0.2 |
| PPD | 4.4 ± 1.0 | 4.2 ± 1.2 | 9.4 ± 3.2 | |||
| RBC | 2.7 ± 0.6 | 2.2 ± 0.3 | 2.4 ± 0.8 | |||
| Extractc | 43 ± 1.9 | 42.8 ± 4.1 | 37.9 ± 7.4 | |||
Results are expressed as mean SI ± standard error of the mean from two to four experiments. Positive responses are shown in bold type.
Concentration of peptide used in vitro.
P. falciparum merozoite extract.
T-cell proliferative responses in congenic mice immunized with a pool of the six MSP-1 peptides.
Six different strains of B10 congenic mice were immunized with a pool of the six peptides, and draining lymph nodes cells were subsequently incubated in vitro with different concentrations of each of the peptides (Table 2). Cells from adjuvant control mice (diluent and CFA) proliferated in response to PPD but not in response to any of the peptides or the extract of merozoites (Table 2), thereby demonstrating that the peptides were not mitogenic and that the control mice did not possess memory cells due to previous exposure to cross-reactive antigens. All six of the peptides were able to stimulate a recall response in vitro in one or more strains of mice. As shown in Table 2, the extent of H-2 restriction, the concentration of peptide required to induce a recall proliferative response, and the SI differed among the strains of mice tested.
TABLE 2.
T-cell proliferative response of B10 congenic mice immunized with MSP-1 peptides
| Peptide | Mean SI fora:
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control B10 mice (I-Ab I-E−) (CFA + diluent)
|
Congenic mice immunized with the six MSP-1 peptides
|
||||||||||||||||||||
| C57BL/10 (I-Ab I-E−)
|
B10A.(5R) (I-Ab I-Eβbαk)
|
B10.BR (I-Ak I-Ek)
|
B10.A(4R) (I-Ak I-E−)
|
B10.D2 (I-Ad I-Ed)
|
B10.S(9R) (I-As I-Eβsαk)
|
||||||||||||||||
| 0.1 μMb | 1.0 μMb | 10 μMb | 0.1 μM | 1.0 μM | 10 μM | 0.1 μM | 1.0 μM | 10 μM | 0.1 μM | 1.0 μM | 10 μM | 0.1 μM | 1.0 μM | 10 μM | 0.1 μM | 1.0 μM | 10 μM | 0.1 μM | 1.0 μM | 10 μM | |
| MSP-1#1 | 1.2 | 1.0 | 1.3 | 1.1 | 1.1 | 1.3 | 0.8 | 0.8 | 1.9 | 2.0 | 1.1 | 5.0 | 4.3 | 2.9 | 3.1 | 1.0 | 1.4 | 2.7 | 1.7 | 1.7 | 2.0 |
| MSP-1#2 | 1.3 | 0.9 | 1.2 | 2.2 | 1.5 | 1.3 | 0.9 | 1.7 | 2.0 | 1.1 | 2.1 | 2.9 | 1.8 | 2.5 | 1.7 | 1.0 | 1.9 | 1.8 | 1.2 | 1.8 | 2.2 |
| MSP-1#3 | 1.2 | 0.8 | 1.0 | 14.6 | 18.3 | 5.6 | 4.6 | 9.1 | 16.2 | 12.2 | 27.7 | 24.8 | 9.8 | 9.8 | 9.8 | 1.7 | 2.9 | 10.9 | 3.2 | 8.9 | 8.2 |
| MSP-1#4 | 1.3 | 1.0 | 1.4 | 2.3 | 1.1 | 1.0 | 4.3 | 6.3 | 11.3 | 4.4 | 9.8 | 16.0 | 1.4 | 2.0 | 2.6 | 1.5 | 4.0 | 7.8 | 10.5 | 15.0 | 14.5 |
| MSP-1#5 | 0.9 | 0.9 | 0.9 | 1.2 | 1.1 | 1.0 | 1.1 | 0.8 | 1.2 | 2.6 | 2.2 | 2.7 | 2.2 | 3.3 | 3.6 | 1.2 | 4.9 | 10.8 | 2.9 | 2.8 | 3.3 |
| MSP-1#23 | 0.8 | 0.6 | 0.7 | 1.3 | 0.9 | 0.8 | 1.0 | 0.8 | 1.2 | 1.4 | 1.1 | 1.7 | 1.1 | 2.1 | 1.9 | 2.9 | 6.8 | 12.4 | 1.8 | 1.2 | 2.2 |
| PPD | 9.6 | 5.2 | 18.0 | 24.3 | 26.7 | 4.2 | 18.8 | ||||||||||||||
| Extractc | 2.3 | 4.2 | 2.1 | 4.1 | 4.1 | 1.5 | 4.8 | ||||||||||||||
Positive responses are indicated in bold type.
Concentration of peptide used in vitro.
P. falciparum merozoite extract.
Peptide MSP-1#3 was the least restricted and most immunogenic peptide. It primed for a recall T-cell proliferative response in all six strains of mice tested (Table 2), with SI ranging from 2.9 to 27.7 (mean, 11.0). In the majority of cases, cells from all strains proliferated in response to the smallest amount of peptide used (0.1 μM) and increased when larger amounts of peptide were added to the cultures. Two of the strains of mice studied, B10 and B10.A(4R), lack functional I-E class II molecules. Thus, positive recall responses in these strains indicated that MSP-1#3 can be presented by I-Ab and I-Ak class II molecules.
Peptide MSP-1#4 primed for a recall response in four of the six congenic strains, with SI ranging from 4.3 to 16.0 (mean, 8.8). Since B10 and B10.A(4R) mice lack I-E and since both strains were nonresponsive, the data suggest that MSP-1#4 was not presented by I-Ab and I-Ak. Therefore, the positive recall response in B10A(5R) (I-Ab I-Eb/k) and B10.BR (I-Ak I-Ek) was most probably due to presentation by I-Eb/k and I-Ek.
Peptide MSP-1#5 primed for a recall T-cell response in four strains of mice, with SI ranging from 2.7 to 10.8 (mean, 3.5). Recall responses by B10.A(4R) (I-Ak I-E−) and B10.BR (I-Ak I-Ek) showed that peptide MSP-1#4 can be presented by I-Ak.
The remaining three peptides (MSP-1#1, MSP-1#2, and MSP-1#23) primed for weaker recall responses than did the peptides described above. MSP-1#1 was weakly immunogenic in both I-Ak-positive strains and primed for a marginal recall response in B10.D2 mice (Table 2). In contrast, MSP-1#23 was able to prime for a recall T-cell response in B10.D2 mice at all peptide concentrations tested but not in the other five strains. Finally, peptide MSP-1#2 appeared to be the least immunogenic of the six peptides since it could prime for a recall response in only one strain (B10.BR) and only at the highest concentration of peptide used. Thus, immunization with the peptides could prime for a recall T-cell response to each of the peptides in one or more strains of mice.
A key question is, however, whether immunization with peptides induces a recall response to the parasite itself. As can be seen, cells from four of the six strains of peptide-primed mice proliferated when cultured with the P. falciparum merozoite extract (Table 2). Thus, in these four strains, one or more of the peptides activated T cells that recognized both the peptide and the native antigen. The two nonresponding strains, B10A.(5R) and B10.D2, developed peptide-specific responses to two and five peptides, respectively, but the activated T cells did not respond to the extract of merozoites. Therefore, the peptides differed not only in their level of H-2 restriction among the strains of mice but also in their ability to stimulate anti-merozoite MSP-1 responses.
Evaluating the influence of background genes on H-2d responses.
Since B10.D2 mice responded to five of the six peptides in vitro but not to the malaria extract, we sought to further evaluate the immunogenicity of these peptides in another H-2d strain. Accordingly, BALB/c (H-2d) mice were immunized with a mixture of the six MSP-1 peptides and the resulting T-cell proliferative response was evaluated. BALB/c mice responded to the same five peptides as did B10.D2 mice, namely, MSP-1#1, MSP-1#3, MSP-1#4, MSP-1#5, and MSP-1#23 (Table 3).
TABLE 3.
Comparison of the response in two H-2d strains of mice
| Peptide | Immunizations with the six MSP-1 peptidesa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| B10.D2
|
BALB/c
|
Immunizations with single peptides, BALB/c
|
|||||||
| 0.1 μMb | 1.0 μMb | 10 μMb | 0.1 μM | 1.0 μM | 10 μM | 0.1 μM | 10 μM | Extractb | |
| MSP-1 #1 | 1.0 | 1.4 | 2.7 | 2.6 | 8.8 | 14.9 | 3.3 | 3.0 | 2.1 |
| MSP-1 #2 | 1.0 | 1.9 | 1.8 | 1.1 | 1.2 | 2.4 | NDd | ND | ND |
| MSP-1 #3 | 1.7 | 2.9 | 10.9 | 1.2 | 3.6 | 11.4 | 2.0 | 8.1 | 1.9 |
| MSP-1 #4 | 1.5 | 4.0 | 7.8 | 1.3 | 3.2 | 3.9 | 2.8 | 2.5 | 1.5 |
| MSP-1 #5 | 1.2 | 4.9 | 10.8 | 1.6 | 5.1 | 6.4 | 2.1 | 4.0 | 1.8 |
| MSP-1 #23 | 2.9 | 6.8 | 12.4 | 1.3 | 4.7 | 3.6 | 9.9 | 5.2 | 3.0 |
Positive responses are shown in bold type.
Concentration of peptide used in vitro.
Cells cultured with the extract of P. falciparum merozoites.
ND, not done.
T-cell proliferative responses in BALB/c mice immunized with individual MSP-1 peptides.
BALB/c mice were immunized once with each peptide individually, and the draining lymph node cells were tested in vitro for proliferation in response to the homologous peptide and the extract of merozoites. The mice responded to the same peptides when used individually or pooled as an immunogen (Table 3), indicating that there was no competition among peptides when pooled as an immunogen. Mice immunized with peptides MSP-1#1, MSP-1#3, MSP-1#4, and MSP-1#5 failed to respond to the antigen extract, but a positive response to extract was induced by MSP-1#23 (Table 3). The merozoite response in mice immunized with MSP-1#1 did not meet the criteria for positivity but was significantly higher than that of the nonresponder strains (SI = 2.1; P = 0.03). Thus, immunization of BALB/c mice with individual peptides resulted in peptide-specific responses but only MSP-1#23 and possibly MSP-1#1 induced a merozoite-specific T-cell response.
Identification of B-cell epitopes within the peptides.
Since B-cell epitopes are often located adjacent to T-cell sites, we sought to determine if linear B-cell epitopes were present within the peptides. BALB/c mice were immunized three times with the individual peptides, and the resulting plasma samples were screened by ELISA for antibodies which reacted with affinity-purified P. falciparum MSP-1, recombinant 195A, and two C-terminal fragments. Peptides MSP-1#1 and MSP-1#23, both located at the amino terminus of the MSP-1 molecule, induced antibodies that reacted with both affinity-purified MSP-1 and the N-terminal 195A construct (Table 4). As expected these antibodies did not react with the MSP-1 C terminus (MSP-19 and BVp42). Peptides MSP-1#2, MSP-1#3, MSP-1#4, and MSP-1#5 did not induce antibodies to MSP-1 (data not shown). Thus, immunization of mice with MSP-1#1 and MSP-1#23 resulted in the production of antibodies that bound to recombinant and purified MSP-1.
TABLE 4.
Immunization with peptide results in the production of antibodies that bind to purified (native) and recombinant MSP-1
| Mouse antiserum raised against: | Antigen used in ELISA | ELISA OD valuea at dilution of:
|
|
|---|---|---|---|
| 1:50 | 1:250 | ||
| MSP-1 #1 | Purified MSP-1 | 0.55 | 0.191 |
| Recombinant 195A | 1.01 | 0.656 | |
| YMSP1-19 | 0.009 | 0.001 | |
| Bvp42 | 0.009 | 0.002 | |
| MSP-1 #23 | Purified MSP-1 | 0.141 | 0.052 |
| Recombinant 195A | 0.144 | 0.063 | |
| YMSP1-19 | 0 | 0 | |
| Bvp42 | 0.003 | 0 | |
Positive results are shown in bold type.
Determining if the peptides prime for enhanced antibody production.
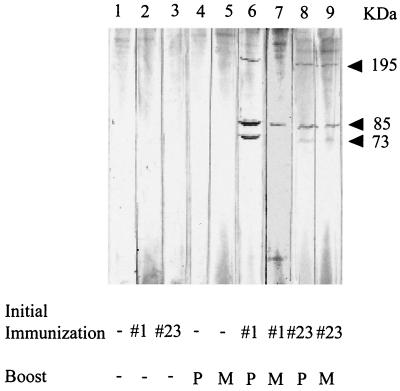
To determine if immunizations with MSP-1#1 and MSP-1#23 would enhance antibody production upon exposure to P. falciparum merozoites, groups of BALB/c mice were immunized with each peptide and then boosted with either affinity-purified MSP-1 or freeze-thawed merozoites which express native MSP-1. Plasma samples from these mice were screened by Western blotting using an extract of merozoites (Fig. 2). MSP-1-specific antibodies were not detected in mice immunized only once with either peptide MSP-1#1 or MSP-1#23 (Fig. 2, lanes 2 and 3) or in mice receiving a single injection of purified MSP-1 or merozoites (lanes 4 and 5). However, mice that were initially injected with peptide MSP-1#1 or MSP-1#23 and then with affinity-purified MSP-1 or merozoites (lanes 6 to 9) produced antibodies that reacted with the 195-kDa protein and/or two smaller processed fragments of MSP-1 of ∼85 and 73 kDa (Fig. 2). Thus, mice immunized only once with either MSP-1#1 or MSP-1#23 were able to produce an enhanced MSP-1-specific antibody response following exposure to native intact MSP-1, whereas a single injection of peptide or of native MSP-1 resulted in no antibody production.
FIG. 2.
Western blots of antibody produced in BALB/c mice. Mice were first immunized with either the diluent (−), MSP-1#1, or MSP-1#23. Approximately 3 weeks later, the mice were boosted with either diluent (−), 5 μg of purified MSP-1 (P), or 107 merozoites (M). Plasma samples were collected 14 days later. An extract of merozoites was electrophoresed and transferred to nitrocellulose. Strips were incubated with a 1:40 dilution of the plasma. Arrows show bands corresponding to the molecular masses of MSP-1 and its processed products.
DISCUSSION
Antigenic variation is thought to be one of the major mechanisms by which plasmodial parasites escape the immune response of the host. Thus, the coevolution of malarial parasites and the human population has most probably led to the selection of immunodominant variable epitopes and subdominant conserved epitopes, since immunodominance of conserved epitopes would prevent parasite survival. Recent data suggest that immunity to P. falciparum in humans is largely the result of immune responses that develop after years of continuous exposure to weak, conserved immunogens (21). If, however, relevant conserved epitopes which induce protective responses can be identified, these epitopes may be included in a vaccine designed to accelerate the acquisition of immunity.
Previous studies have identified five conserved and one partly conserved sequences in MSP-1 that are capable of inducing proliferative T-cell responses in humans who have been exposed to P. falciparum malaria (17). The present study sought to further evaluate these peptides and determine if they could induce T- and B-cell responses when used as a “peptide-based vaccine” in mice. Six strains of B10 congenic mice were immunized with the peptides, and primed T cells were tested in vitro for proliferation in response to the peptides and an extract of merozoites. All six peptides were able to induce strong recall T-cell proliferation in response to the peptide itself in one or more strains of mice (Table 2). Thus, each peptide contains one or more T-cell epitopes. The peptides, however, differed in their immunogenicity and level of major histocompatibility complex restriction (Tables 2 and 3).
The peptides also differed in their ability to induce T cells that recognize intact MSP-1. As demonstrated in Table 2, four of the strains of mice immunized with the peptides had T cells that recognized native MSP-1 whereas the other two did not. Therefore, in some mouse strains the peptides induced T cells that recognized both the peptide and native MSP-1 whereas in other strains the peptides were immunogenic but did not induce merozoite-specific responses. For example, B10 mice responded in vitro to peptide MSP-1#3 and the merozoite extract but not to the other five peptides (Table 2). Thus, peptide MSP-1#3-primed T cells in B10 mice recognized both the peptide and the corresponding sequence in native MSP-1. On the other hand, B10.A(5R) primed with the mixture of six peptides responded to MSP-1#3 and MSP-1#4 in vitro yet did not respond to the merozoite extract containing MSP-1. Therefore, MSP-1#3 would be useful in a vaccine for B10 but not B10.A(5R) mice, since peptides included in a malarial vaccine must induce T cells that recognize the native protein. Thus, identification of immunologically relevant T-cell epitopes must take into consideration both parasite and host factors.
The present study in mice revealed that peptides MSP-1#1 and MSP-1#23 contain both B-cell and T-helper-cell epitopes that collaborate in the production of antibodies to MSP-1 (Table 4; Fig. 2). MSP-1#1 is a particularly relevant peptide because it is completely conserved among all P. falciparum strains that have been sequenced and is located immediately after the signal peptidase site at amino acid 19 of block 1 (13). Previous studies have determined that activation of a human T-cell clone produced against this peptide resulted in gamma interferon production and inhibition of parasite growth in vitro (17). In the present study, we found that following a single immunization of mice with the MSP-1#1 peptide, antibodies to MSP-1 were induced upon exposure to P. falciparum merozoites. We also found that mice immunized three times with this peptide produced antibodies that reacted with both recombinant and purified MSP-1. The induction of IgG MSP-1-specific antibodies supports the conclusion that this conserved sequence contains both helper T- and B-cell epitopes. Studies by Ramasamy et al. (18) showed that 67% of the Weheragala population, who live in a malaria-endemic area of Sri Lanka, had antibodies to peptide P109, which is identical to MSP-1#1 except that it has an additional cysteine at the carboxyl terminus. Recent studies also found that more than 80% of adults living in Adropodume, a village in the Ivory Coast, have antibodies to peptide MSP-1#1 (I. A. Quakyi, unpublished data). Thus, mouse and human data demonstrate that peptide MSP-1#1 is an important and immunologically relevant sequence that should be seriously considered for inclusion in a subunit vaccine for malaria.
MSP-1#23 is located between blocks 1 and 2 of the MSP-1 molecule (Fig. 1). Analyses of sequences from different P. falciparum isolates show that the first 13 amino acids in the sequence are conserved whereas the remaining 7 are variant. In the present study, peptide MSP-1#23 induced peptide-specific and parasite-specific T-cell responses in only H-2d mice (Tables 2 and 3) and thus appears to be quite H-2 restricted (Table 2). It also contains a B-cell epitope, since mice immunized once with this peptide and then subsequently injected with merozoites produced antibodies that reacted with intact MSP-1 as well as processed fragments of 85 and 73 kDa (Fig. 2). Further studies are needed to determine if the T- and B-cell epitopes in MSP-1#23 are located in the conserved or variant regions. Peptide MSP-1#23 is located immediately adjacent to the SAQ(SGT)5 tripeptide repeat sequence (22), a region considered to be the target of mABCE2, which inhibits P. falciparum growth in vitro (11). It remains to be determined if antibodies to the B-cell epitope(s) in MSP-1#23 can also block parasite growth or if perhaps they might compete with potentially protective antibodies. Results from this study show that further investigation of the conserved region of MSP-1#23 is warranted.
In the present study, there was little evidence that peptides MSP-1#3, MSP-1#4, and MSP-1#5 contain epitopes that induce T-cell responses which can be boosted by intact MSP-1 in immunized BALB/c mice. There is also little evidence that they contain helper T- or B-cell epitopes for antibody production. These three sequences, however contain T-cell sites recognized by mice and induce positive T-cell responses in a population of malaria-exposed individuals (17). Thus, the importance of these peptides remain to be established.
In conclusion, one conserved region (MSP-1#1) and one partly conserved region (MSP-1#23) of MSP-1 have been identified that may be appropriate for inclusion in a trial vaccine for malaria. Both regions contain helper T-cell and B-cell epitopes that support the production of antibody to MSP-1. In this study, peptides MSP-1#1 and MSP-1#23 were identified primarily by their ability to provide help for antibody production to MSP-1 and not by T-cell proliferation per se. The other four peptides examined appear to be of less value as potential vaccine candidate peptides. The study also begins to reveal the complexity of identifying important T-cell epitopes for inclusion in a vaccine for malaria. The results of this study support the conclusions that conserved peptides may be immunogenic but that only a few are able to stimulate the production of memory T cells that are activated upon exposure to P. falciparum merozoites. The results further suggest that the conserved epitopes examined in this study should be classified as subdominant or weakly immunogenic. When mice were immunized once with merozoites, none of the peptides induced recall responses (Table 1). However, following one immunization with the peptide, MSP-1-specific T-cell responses were detected. Subdominancy of these peptides implies that immunization with them could potentially induce protective immune responses not elicited readily by exposure to whole parasites during a natural infection. Therefore, the MSP-1#1 and MSP-1#23 peptides merit further consideration for inclusion in a subunit vaccine for malaria that is designed to accelerate the rate of acquisition of immunity.
ACKNOWLEDGMENTS
This work was supported primarily by grant DPE-5979-A-00-0034AD from USAID with supplemental support from NIH grants R21-AI37943 and N01-AI45242.
We thank C. Hurley for her helpful comments, A. Zhou for statistical analysis, and M. Hickey for help with preparation of the manuscript.
REFERENCES
- 1.Blackman M J, Heidrich H G, Donachie S, McBride J S, Holder A. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang S P, Hui G S N, Siddiiqui W A. Generalized immunological recognition of the major surface antigen (gp 195) of Plasmodium falciparum. Proc Natl Acad Sci USA. 1989;86:6343–6347. doi: 10.1073/pnas.86.16.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang S P, Gibson H L, Lee-Ng C T, Barr P J, Hui G S N. An authentic carboxyl terminal fragment of Plasmodium falciparum gp 195 expressed by recombinant baculovirus induces antibodies that completely inhibit parasite growth. J Immunol. 1992;149:548–555. [PubMed] [Google Scholar]
- 4.Crisanti A, Muller H-M, Hilbich C, Sinigaglia F, Matilde H, McKay M, Scaife J, Beyreuther K, Bujard H. Epitopes recognized by human T cells map within the conserved part of GP 190 of P. falciparum. Science. 1988;240:324–1326. doi: 10.1126/science.2453924. [DOI] [PubMed] [Google Scholar]
- 5.Daly T, Long C A. A recombinant 15-kilodalton carboxy-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect Immun. 1993;61:2462–2467. doi: 10.1128/iai.61.6.2462-2467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egan A, Waterfall M, Pinder M, Holder A, Riley E. Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1. Evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect Immun. 1997;65:3024–3031. doi: 10.1128/iai.65.8.3024-3031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etlinger H M, Caspers P, Matile H, Schoenfeld H-J, Stuber D, Takas B. Ability of recombinant or native proteins to protect monkeys against heterologous challenge with Plasmodium falciparum. Infect Immun. 1991;59:3498–3503. doi: 10.1128/iai.59.10.3498-3503.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrera A, Rosero F, Herrera S, Caspers P, Rotman D, Sinigaglia F, Creta U. Protection against malaria in Aotus monkeys immunized with a recombinant blood-stage antigen fused to a universal T-cell epitope: correlation of serum gamma interferon levels and protection. Infect Immun. 1992;60:154–158. doi: 10.1128/iai.60.1.154-158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui G S N, Chang S P, Gibson H, Hashimoto A, Hashiro C, Barr P J, Kotani S. Influence of adjuvant on the antibody specificity to Plasmodium falciparum major merozoite surface protein, gp195. J Immunol. 1991;147:3935–3941. [PubMed] [Google Scholar]
- 10.Kaslow D C, Hui G S N, Kumar S. Expression and antigenicity of Plasmodium falciparum major merozoite protein (MSP 1-19) variants secreted from Saccharomyces cerevisiase. Mol Biochem Parasitol. 1994;63:283. doi: 10.1016/0166-6851(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 11.Locher C P, Tam L Q, Chang S P, McBride J S, Siddiqui W A. Plasmodium falciparum: gp195 tripeptide repeat-specific monoclonal antibody inhibits parasite growth in vitro. Exp Parasitol. 1996;84:74–83. doi: 10.1006/expr.1996.0091. [DOI] [PubMed] [Google Scholar]
- 12.Majarian W R, Daly T M, Weidanz W P, Long C A. Passive protection against murine malaria with IgG3 monoclonal antibody. J Immunol. 1984;132:3131–3137. [PubMed] [Google Scholar]
- 13.Miller L H, Roberts T, Shahabuddin M, McCutchan T F. Analysis of sequence diversity in Plasmodium falciparum merozoite surface protein-1 (MSP-1) Mol Biochem Parasitol. 1993;59:1–14. doi: 10.1016/0166-6851(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 14.Ohta N, Iwaki K, Itoh M, Fu J, Nakashima S, Hato M, Tolle R, Bujard H, Saitoh A, Tanable K. Epitope analysis of human T-cell response to MSP-1 of Plasmodium falciparum in malaria-nonexposed individuals. Int Arch Allergy Immunol. 1997;114:15–22. doi: 10.1159/000237637. [DOI] [PubMed] [Google Scholar]
- 15.Patarroyo M E, Romero P, Torrez M L, Clavijo P, Moreno A, Martinez A, Rodriquez R, Guzman F, Cabezas E. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987;328:629–632. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- 16.Quakyi I A, Taylor D W, Johnson A H, Allotey J B, Berzoksky J A, Miller L H, Good M F. Development of a malaria T cell vaccine for blood stage immunity. Scand J Immunol. 1992;36(Suppl. 11):9–16. doi: 10.1111/j.1365-3083.1992.tb01611.x. [DOI] [PubMed] [Google Scholar]
- 17.Quakyi I A, Currier J, Fell A, Taylor D W, Roberts T, Houghten R A, England R D, Berzofsky J A, Miller L H, Good M F. Analysis of human T cell clones specific for conserved peptide sequences within malaria proteins: paucity of clones responsive to intact parasites. J Immunol. 1994;153:2082–2092. [PubMed] [Google Scholar]
- 18.Ramasamy R, Nagendran K, Mamasamay M S. Antibodies to epitopes on merozoite and sporozoite surface antigens as serologic markers of malaria transmission: studies at a site in the dry zone of Sri Lanka. Am J Trop Med Hyg. 1994;50:537–547. doi: 10.4269/ajtmh.1994.50.537. [DOI] [PubMed] [Google Scholar]
- 19.Rzepczyk M C, Ramasamy R, Mutch D A, Ho P C-L, Battistutta D, Anderson K L, Parkinson D, Doran T J, Honeyman M. Analysis of human T cell response to two Plasmodium falciparum merozoite surface antigens. Eur J Immunol. 1989;19:1797–1802. doi: 10.1002/eji.1830191006. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui W A, Tam L Q, Kramer K H, Hui G S N, Case S E, Yamaga K M, Chang S P, Chan E B T, Kan S C. Merozoite coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1987;84:3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith T, Charlwood J D, Kitua A Y, Masanja H, Mwankusye S, Alonso P L, Tanner M. Relationships of malaria morbidity with exposure to Plasmodium falciparum in young children in a highly endemic area. Am J Trop Med Hyg. 1998;59:252–257. doi: 10.4269/ajtmh.1998.59.252. [DOI] [PubMed] [Google Scholar]
- 22.Tanabe K, Mackay M, Goman M, Scaife J G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biochem. 1987;195:273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- 23.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trager W, Jensen B J. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 25.Udhayakumar V, Anyona D, Kariuki S, Shi Y P, Bloland P B, Branch O H, Weiss W, Nahlen B L, Kaslow D C, Lal A A. Identification of T and B cell epitopes recognized by humans in the C-terminal 42 kDa domain of the Plasmodium falciparum merozoite surface protein (MSP-1) J Immunol. 1994;154:6022–6030. [PubMed] [Google Scholar]
- 26.Walliker D, Quakyi I A, Wellems T E, McCutchan T F, Szarfman A, London W T, Corcoran L M, Burkot T, Caruter R. Genetic analysis of the human parasite Plasmodium falciparum. Science. 1987;236:1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]