Abstract
Background
The aim of this study is to investigate chronological changes of lower urinary tract symptoms (LUTS) in patients with prostate cancer who underwent low-dose-rate brachytherapy (LDR-BT) followed by the insertion of SpaceOAR® system (SpaceOAR).
Methods
In this retrospective study, 483 patients with localized prostate cancer underwent LDR-BT at the Gifu University Hospital between August 2004 and December 2020. SpaceOAR was inserted in 30 patients after LDR-BT (SpaceOAR group), and 453 patients received LDR-BT alone (non-SpaceOAR group). The International Prostate Symptom Score (IPSS), Overactive Bladder Symptom Score (OABSS), quality of life due to urinary symptoms (IPSS-QOL), and uroflowmetry (UFM), including maximum flow rate (Qmax), voided volume, and post-voided residual urine (PVR), were evaluated before LDR-BT, and at 1, 3, 6, 9, and 12 months after LDR-BT. The outcomes were chronological changes in IPSS, OABSS, and IPSS-QOL compared to pretreatment values and those of covariates in relation to UFM.
Results
The IPSS, OABSS, IPSS-QOL, Qmax, and voided volume were not significantly associated with either group. According to the PVR interaction effect, the insertion of SpaceOAR was significantly affected by chronological changes in PVR (P = 0.001). Three months after LDR-BT, PVR in the SpaceOAR group was significantly higher than that in the non-SpaceOAR group (49.8 mL vs. 30.5 mL; P = 0.002).
Conclusion
SpaceOAR use may temporally increase PVR; however, IPSS, OABSS, IPSS-QOL, Qmax, and voided volume were not significantly associated with LUTS before and after LDR-BT. The combination of LDR-BT and SpaceOAR may be acceptable for treating patients with prostate cancer regarding the chronological changes in LUTS after brachytherapy.
Keywords: Prostate cancer, Low-dose-rate brachytherapy, SpaceOAR® system, Lower urinary tract symptoms
1. Introduction
Prostate cancer (PCa) is the second most common cancer and sixth leading cause of cancer-associated mortality among men worldwide in 2020.1 In Japan, PCa has the highest incidence of male malignancy.2 Low-dose-rate brachytherapy with iodine-125 (LDR-BT) is a definitive therapeutic option for localized and/or advanced PCa with excellent oncological outcomes as well as radical prostatectomy (RP) or external beam radiation therapy (EBRT).3,4 Additionally, the combination of LDR-BT and EBRT allows dose escalation for the prostate. Therefore, LDR-BT with or without EBRT has potential advantages to avoid biochemical or clinical recurrence for all-risk patients with PCa.5, 6, 7 However, the escalation of radiation doses may increase acute and/or long-term treatment-related complications, such as gastrointestinal (GI) toxicities.8 Several studies reported that maximal reduction in the rectal dose is very important to prevent serious GI toxicities.7,9, 10, 11
The SpaceOAR® System (SpaceOAR) (Augmenix Inc., Waltham, MA, USA) is a synthetic polyethylene glycol hydrogel injected between the prostate and rectum, which moves the rectum away from the prostate to reduce irradiation of the anterior rectal wall.8,12 Furthermore, SpaceOAR led to a reduction in GI events by reducing rectal exposure.8,12 Therefore, SpaceOAR reduced GI toxicity and helped improve bowel symptoms.13 Additionally, several studies reported that SpaceOAR may decrease the rate of genitourinary (GU) toxicity, especially lower urinary tract symptoms (LUTS), because of the reduction of irradiation dose for the penile bulb and bladder in patients with PCa who received LDR-BT.13, 14, 15 For this reason, the insertion of SpaceOAR causes anatomical changes in the pelvic organs.16 Until today, the chronological changes of LUTS in patients with PCa who received the combination of LDR-BT and SpaceOAR therapy remain unclear.
We aimed to evaluate the chronological changes of LUTS in patients with PCa who were treated with LDR-BT followed by the insertion of SpaceOAR.
2. Materials and methods
2.1. Patients
In this study, we retrospectively analyzed 483 consecutive patients with PCa who were diagnosed with clinical T1c/T2/T3a PCa according to the 2010 American Joint Committee on Cancer Staging Manual17 and underwent LDR-BT at the Gifu University Hospital between August 2004 and February 2020. All patients were stratified according to the classification model proposed by the National Comprehensive Cancer Network guidelines (version 4, 2018) into very low-, low-, favorable intermediate-, poor intermediate-, high-, and very high-risk groups.18 Patients with lymph node involvement, distant metastasis, history of transurethral prostate resection, or uroflowmetry (UFM) assessment with a maximum flow rate (Qmax) of <10 mL/s were excluded from this study. SpaceOAR was inserted in 30 patients before LDR-BT (SpaceOAR group), and 453 patients received LDR-BT alone (non-SpaceOAR group).
The study protocol was approved by the institutional review board of Gifu University (number: 2018-169).
2.2. Treatment
Patients were implanted with loose 125I radioactive seeds (Oncoseed, Nihon Mediphysics, Tokyo, Japan) using a Mick applicator (Mick Radio-Nuclear Instruments, Bronx, NY, USA) or with linked seeds using the ProLink® delivery system (C. R. Bard, Inc., Murray Hill, NJ, USA) and a real-time transrectal ultrasound-guided trans-perineal technique.19 The prescribed minimum peripheral doses were 145 Gy for patients who underwent LDR-BT alone and 104 Gy for those who underwent LDR-BT combined with EBRT. EBRT (40 Gy in 2 Gy fractions) was administered to the prostate and seminal vesicles within 1 month of LDR-BT. Patients with very low- and low-risk PCa with pretreatment prostate volume (PV) > 50 mL were administered neoadjuvant androgen deprivation therapy for at least 3 months for downsizing. Patients with favorable intermediate- and poor intermediate-risk PCa were treated with a combination of LDR-BT with 125I and/or EBRT and/or received androgen deprivation therapy (ADT) for 9 months. Patients with high- and very-high-risk PCa underwent combined LDR-BT with 125I and EBRT and ADT for 24 months. EBRT was performed 4 weeks after LDR-BT at a prescription dose of 40 Gy (2 Gy fractions limited to the prostate/seminal vesicle field). Patients were routinely administered α-1 blockers after LDR-BT to reduce the risk of urinary retention or LUTS.
In all cases, seed implantation was performed after preplanning using modified peripheral loading techniques with a Mick applicator (Mick Radio-Nuclear Instruments, Bronx, NY, USA) or the ProLink® delivery system (C. R. Bard, Inc., Murray Hill, NJ, USA).20
2.3. SpaceOAR placement
SpaceOAR was inserted into the space between the prostate and anterior rectal wall immediately after LDR-BT to minimize artifacts in ultrasound imaging and avoid potential pubic arch interference when placing the seeds.
2.4. Postdosimetric evaluation
Therapeutic planning and post-implant dosimetric evaluations were performed using the updated American Association of Physicists in Medicine Task Group 43 formalism and Variseed version 7.1 (Varian Medical Systems, Palo Alto, CA, USA).
A postimplant dosimetric study using computed tomography and magnetic resonance imaging was performed 1 month after LDR-BT. The dosimetric parameters analyzed in this study were the minimum dose received by 90% of the target volume (D90), percentage of target volume receiving a minimum of 100% of the prescribed dose (V100), minimum dose received 30% of the urethral volume (UD30), rectal volume receiving 100% of the prescribed dose (V100), and rectal volume receiving 150% of the prescribed dose (V150).
2.5. Follow-up schedule
The International Prostate Symptom Score (IPSS), Overactive Bladder Symptom Score (OABSS), quality of life due to urinary symptoms (IPSS-QOL), UFM, voided volume (VV), and postvoided residual urine (PVR) were measured before LDR-BT and at 1, 3, 6, 9, and 12 months after LDR-BT.
2.6. Statistical analysis
The endpoints of this study were chronological changes in IPSS, OABSS, and IPSS-QOL compared to pretreatment values and chronological changes of covariates in relation to UFM. Patient characteristics are described as the median and interquartile range (IQR) for continuous variables and frequency (percentage) for categorical variables. Fisher's exact test was used to compare categorical variables, and Mann–Whitney U test was used to compare continuous variables. Linear mixed-effect models were used to analyze the longitudinal data and to assess the least square mean differences between the two groups at the time of each measurement. The interaction term between the SpaceOAR group and the period was incorporated into the model to evaluate the effect modification on changes in outcomes over time. In addition, the age, body mass index (BMI), ADT, number of seeds, prostate volume, National Comprehensive Cancer Network risk classification, and baseline value of the outcome were treated as covariates in each model. All analyses used a 5% two-sided significant level and were performed using R software version 3.6.3 (www.r-project.org) with “lme4” package.
3. Results
3.1. Patient characteristics
The patient characteristics are listed in Table 1. The median age of the patients was 66 years (IQR: 62–71 years). The median initial PSA was 6.5 ng/mL (IQR: 5.1–9.1 ng/mL), and the Gleason score was 7 (IQR: 6–7). A total of 368 patients underwent neoadjuvant ADT prior to LDR-BT. EBRT combined with LDR-BT was performed in 209 patients.
Table 1.
Patient characteristics
| Non-SpaceOAR group (n = 453) | SpaceOAR group (n = 30) | P | |
|---|---|---|---|
| Age (year, median, interquartile range) | 66.0 (62.0-71.0) | 67.0 (61.0-71.3) | 0.56 |
| Initial prostate-specific antigen (ng/mL, median, interquartile range) | 6.4 (5.0-9.0) | 7.3 (5.6-11.2) | 0.28 |
| Clinical T stage (number, %) | |||
| T1c | 244 (53.9) | 6 (20.0) | 0.004 |
| T2a | 126 (27.8) | 13 (43.3) | |
| T2b | 26 (5.7) | 5 (16.7) | |
| T2c | 46 (10.2) | 4 (13.3) | |
| T3a | 11 (2.4) | 2 (6.7) | |
| Gleason score (median, interquartile range) | 7 (6–7) | 7 (7–7) | 0.004 |
| Risk classification (number, %) | |||
| Very low-risk | 21 (4.6) | 0 | <0.001 |
| Low-risk | 147 (32.5) | 0 | |
| Favorable intermediate-risk | 208 (45.9) | 9 (30.0) | |
| Poor intermediate-risk | 29 (6.4) | 6 (20.0) | |
| High-risk | 46 (10.2) | 14 (46.7) | |
| Very high-risk | 2 (0.4) | 1 (3.3) | |
| Body mass index (kg/m2, median, interquartile range) | 23.5 (21.9–25.3) | 24.1 (22.0–25.7) | 0.37 |
| Prostate volume at LDR-BT (mL, median, interquartile range) | 22.5 (17.7–29.1) | 26.1 (21.1–32.5) | 0.002 |
| Inserted seed (number, median, interquartile range) | 64 (51.0–78.0) | 65 (56.0–79.0) | 0.25 |
| Neoadjuvant androgen deprivation therapy (number, %) | 341 (75.3) | 27 (90.0) | 0.045 |
| External beam radiation therapy (number, %) | 192 (42.4) | 17 (56.7) | 0.13 |
| Preoperative International Prostate Symptom score (median, interquartile range) | 5 (3–9) | 7 (3–10) | 0.33 |
| Preoperative Overactive Bladder Symptom score (median, interquartile range) | 3 (2–4) | 3 (2–5) | 0.38 |
| Preoperative quality of life due to urinary (median, interquartile range) | 2 (1–3) | 3 (1–4) | 0.25 |
| Preoperative maximal urinary flow rate (mL/s, median, interquartile range) | 17.6 (14.5–21.8) | 15.1 (11.6–19.9) | 0.029 |
| Preoperative voiding volume (mL, median, interquartile range) | 250 (183.0–349.0) | 177.8 (125.8–292.5) | 0.018 |
| Preoperative postvoided residual urine (mL, median, interquartile range) | 15 (3.8–30.0) | 30 (10.0–56.3) | <0.001 |
Abbreviations: LDR-BT = low-dose-rate brachytherapy; n = number; SpaceOAR = SpaceOAR® System.
3.2. Patients’ dosimetric data
Table 2 shows the dosimetric data. There were no significant differences in D90, V100, or UD30 between the SpaceOAR and non-SpaceOAR groups. However, RV100 and RV150 were significantly lower in the SpaceOAR group than in the non-SpaceOAR group (P < 0.001 and P = 0.034, respectively).
Table 2.
Patient dosimetric data.
| Non-SpaceOAR group (n = 453) | SpaceOAR group (n = 30) | P | |
|---|---|---|---|
| The minimum dose received by 90% of the target volume (Gy, median, interquartile range) | |||
| LDR-BT alone | 173.4 (161.5–183.1) | 172.5 (166.0–181.0) | 0.35 |
| LDR-BT + EBRT | 125.2 (115.5–134.3) | 120.5 (118.3–120.5) | 0.98 |
| The percentage of target volume receiving minimum of 100% of prescribed dose (%, median, interquartile range) | 96.4 (94.4–97.9) | 97.0 (96.0–97.8) | 0.12 |
| The minimum dose received 30% of urethral volume (%, median, interquartile range) | |||
| LDR-BT alone | 212.2 (195.4–235.8) | 192.9 (181.9–203.5) | 0.34 |
| LDR-BT + EBRT | 157.5 (142.6–179.0) | 139.5 (130.4–139.5) | 0.27 |
| The rectal volume receiving 100% of the prescribed dose (mL, median, interquartile range) | 0.22 (0.08–0.77) | 0 (0–0.003) | <0.001 |
| The rectal volume receiving 150% of the prescribed dose (mL, median, interquartile range) | 0 (0–0.05) | 0 (0–0) | 0.034 |
Abbreviations: EBRT = external beam radiation therapy; LDR-BT = low-dose-rate brachytherapy; n = number; SpaceOAR = SpaceOAR® System.
3.3. Chronological changes in IPSS, OABSS, IPSS-QOL, UFM, and PVR
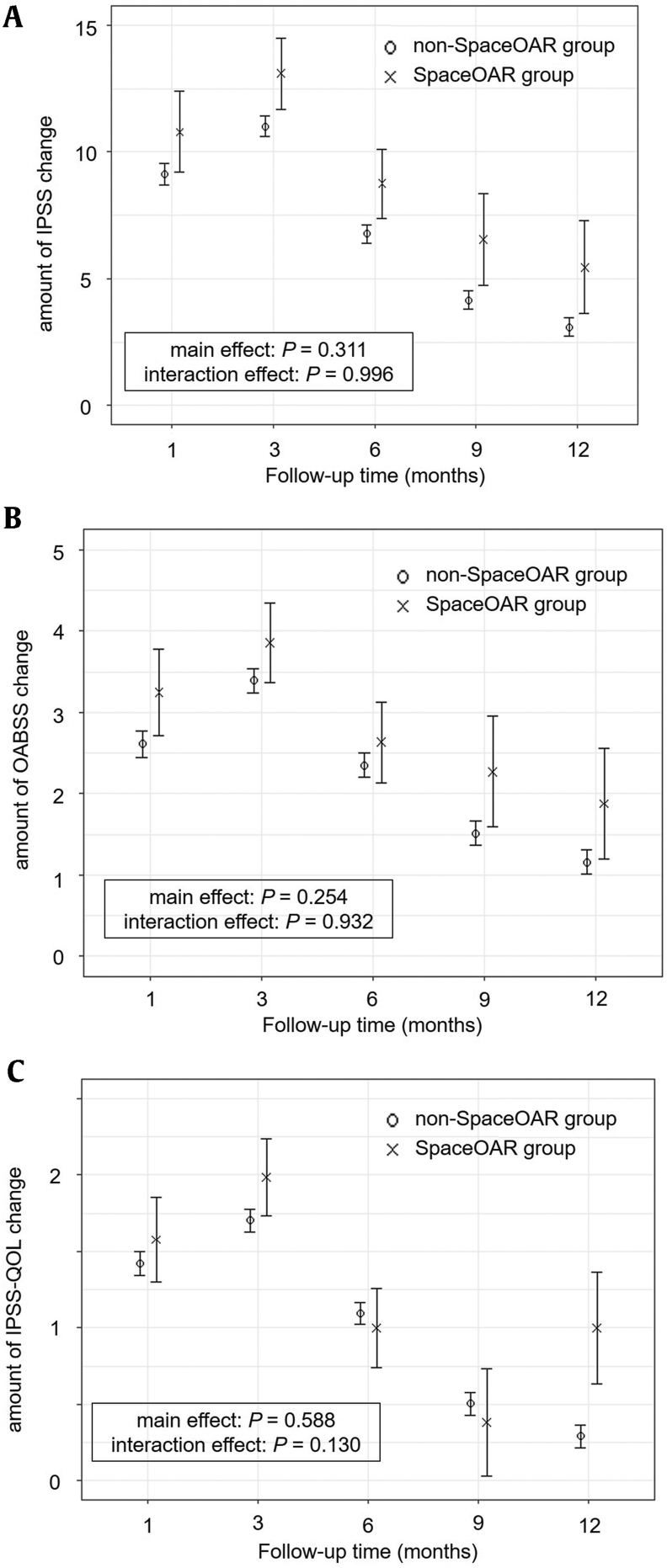
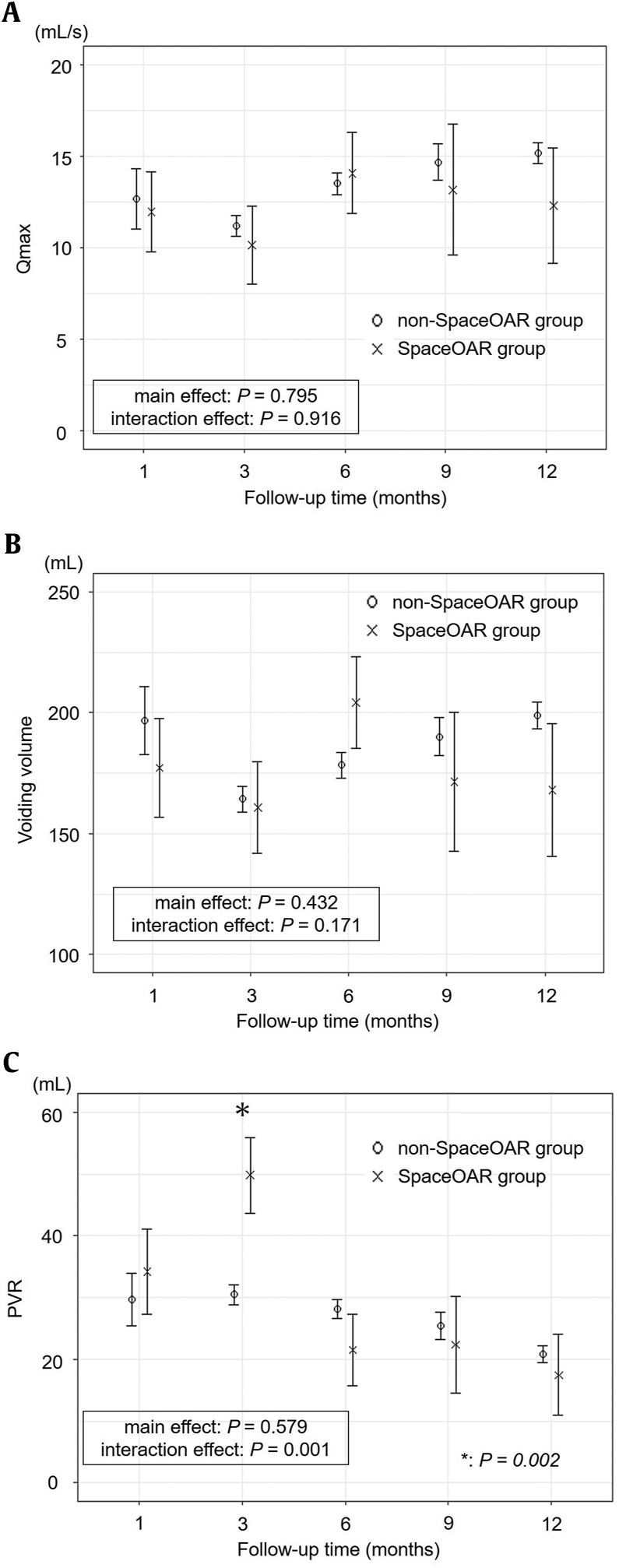
Linear mixed-effects models of chronological changes in IPSS, OABSS, and IPSS-QOL are shown in Fig. 1. The IPSS, OABSS, and IPSS-QOL increased at 3 months after LDR-BT, and the scores decreased at 12 months in both groups. The follow-up time was significantly associated with changes in IPSS, OABSS, and IPSS-QOL (P < 0.001, P < 0.001, and P < 0.001, respectively). During the follow-up period, the IPSS, OABSS, and IPSS-QOL scores were not significantly associated with either group (P = 0.311, P = 0.254, and P = 0.588, respectively). The interaction between the IPSS, OABSS, and IPSS-QOL scores and group was not significant (P = 0.996, P = 0.932, and P = 0.130, respectively). Fig. 2 shows chronological changes in Qmax, VV, and PVR. Although transient deterioration of Qmax and VV was observed, all factors recovered 6–9 months after LDR-BT in both groups. The follow-up time was significantly associated with changes in Qmax, VV, and PVR (P < 0.001, P < 0.001, and P < 0.001, respectively). Qmax and VV were not significantly associated in either group (P = 0.795 and P = 0.432, respectively). The interaction between the Qmax and VV and group was not significant (P = 0.916 and P = 0.171, respectively).
Figure 1.
Chronological changes in the lower urinary tract symptom scores using linear mixed-effect model; (a) IPSS, (b) OABSS, (c) IPSS-QOL. Both in the non-SpaceOAR and SpaceOAR groups, IPSS, OABSS, and IPSS-QOL increased at 3 months after LDR-BT, and the scores decreased at 12 months. There was no significant difference in IPSS, OABSS, and IPSS-QOL over time with and without SpaceOAR insertion (P = 0.996, P = 0.932, and P = 0.130, respectively).
Figure 2.
Chronological changes in the UFM and PVR using linear mixed-effect model; (a) Qmax, (b) VV, (c) PVR. Although transient deterioration of Qmax and VV were observed, all factors recovered at 6 to 9 months after LDR-BT in the non-SpaceOAR and SpaceOAR groups. There was no significant difference in Qmax and VV over time with and without SpaceOAR insertion (P = 0.916, and P = 0.171, respectively). The use of SpaceOAR significantly affected chronological change in PVR (P = 0.001). The least square mean of PVR in the SpaceOAR group was significantly increased compared with the non-SpaceOAR group at 3 months after LDR-BT (49.8 mL vs. 30.5 mL, P = 0.002).
The change in PVR over time differed between the insertion and noninsertion of SpaceOAR (P = 0.001). Interestingly, the chronological changes in PVR were significantly different between the insertion and noninsertion of the SpaceOAR according to the interaction for group and PVR (P = 0.001). The least square mean of PVR in the SpaceOAR group was significantly higher than that in the non-SpaceOAR group at 3 months after LDR-BT (49.8 mL vs. 30.5 mL, P = 0.002).
4. Discussion
LDR-BT is a standard treatment modality for T1c-T3a PCa with excellent long-term biochemical recurrence-free survival.21 However, approximately 90% of patients who undergo LDR-BT experience GU toxicities.22 Previous studies reported the incidence of acute and late GU toxicities to be 35–67% and 22–55%, respectively.23 GU toxicities affect the patient's QOL after RT.24 Several studies revealed the chronological changes of LUTS after LDR-BT.25,26 Iinuma et al. reported long-term changes of LUTS in PCa patients who underwent LDR-BT.25 The IPSS, OABSS, and IPSS-QOL were worsened immediately after LDR-BT compared to preoperative scores, and symptoms improved with time and returned to baseline after 18–36 months.25 Onishi et al. investigated chronological changes of LUTS in patients who received LDR-BT for PCa treatment using the IPSS, OABSS, IPSS-QOL, UFM, and PVR.26 The IPSS, OABSS, and IPSS-QOL increased at 3 months following LDR-BT compared with baseline and returned to baseline after 12–48 months.26 The Qmax and VV of UFM and PVR were worst at 3 months after LDR-BT and gradually improved.26 There are limited data on the LUTS after RT with SpaceOAR. Alshak et al. evaluated LUTS according to patient-reported symptoms and AUA-SS after stereotactic body radiation therapy with SpaceOAR in patients with PCa.27 Self-reported LUTS showed no statistical significant difference between the non-SpaceOAR and SpaceOAR groups.27 Patient-reported urinary frequency (38% vs. 68%) and nocturia (8% vs. 35%) were both less common in the SpaceOAR group compared to the non-SpaceOAR group.27 In our study, both in the non-SpaceOAR and SpaceOAR groups, the IPSS, OABSS, and IPSS-QOL increased at 3 months after LDR-BT, and the scores decreased at 12 months. Although transient deterioration of Qmax and VV was observed, all factors recovered 6–9 months after LDR-BT in both groups. SpaceOAR did not show significant differences in the relationship between follow-up time and the IPSS, OABSS, IPSS-QOL, Qmax, and VV (P = 0.311, P = 0.254, P = 0.588, P = 0.795, P = 0.432, respectively). However, the use of SpaceOAR significantly affected chronological changes in PVR (P = 0.001). The least square mean of PVR in the SpaceOAR group was significantly higher than that in the non-SpaceOAR group at 3 months after LDR-BT (49.8 mL vs. 30.5 mL, P = 0.002). PVR is a controversial part of routine clinical assessment in males with LUTS.28,29 The use of a PVR threshold of 50 mL has a positive predictive value of 63% and a negative predictive value of 52% as a predictor of bladder outlet obstruction.30 In our study, the PVR at 3 months after LDR-BT was significantly increased in the SpaceOAR group; however, the PVR was less than 50 mL in both groups. It is possible that the IPSS, OABSS, and IPSS-QOL were not affected because the PVR was less than 50 mL. Based on our results, the use of SpaceOAR may increase PVR temporally, with no significant change in LUTS after treatment with LDR-BT.
SpaceOAR was originally created to reduce irradiation of the anterior rectal wall therefore reducing GI toxicities.12 Several studies have reported that SpaceOAR significantly decreased the irradiation dose of rectum in patients with PCa who were treated with LDR-BT.16,31 Morita et al. reported that RV100 and RV150 were significantly lower in the SpaceOAR group compared to the non-SpaceOAR group (RV100: 0.001 cc vs. 0.025 cc, RV150: 0.026 cc vs. 0.318 cc, P < 0.001).16 Zhang et al. evaluated the rectal dose reduction in patients with PCa who underwent a combination of volumetric modulated arc therapy and LDR-BT with the insertion of SpaceOAR.31 Significant decreases of the doses were observed in patients with SpaceOAR, which were on average 34.5, 28.4, 20.6 (P < 0.01), and 8.5 Gy (P < 0.05) to rectal wall volume of 0.5, 1, 2, and 5 cm3, respectively.31 In this study, RV100 and RV150 were significantly lower in the SpaceOAR group than in the non-SpaceOAR group (P < 0.001, P = 0.034, respectively).
This study has some limitations. First, it was a retrospective study, and as such, has an inherent potential for bias. Second, this was a single-institution, non-randomized study. Third, a relatively small number of patients were enrolled, and the follow-up period was relatively short. Fourth, the impact of EBRT on LUTS was not evaluated in this study.
To the best of our knowledge, this is the first study to evaluate chronological changes in the IPSS, OABSS, IPSS-QOL, UFM, and PVR as assessment tools for LUTS after LDR-BT with SpaceOAR. The use of SpaceOAR may temporally increase PVR; however, IPSS, OABSS, IPSS-QOL, Qmax, and VV were not significantly associated with LUTS before and after LDR-BT. The combination of LDR-BT and SpaceOAR may be an acceptable treatment option in patients with PCa regarding the chronological change in LUTS after brachytherapy. Future prospective multicenter clinical trials with longer follow-up periods are needed.
Financial support
None.
Conflicts of interest
The authors have no conflicts of interest to declare and received no financial support for this study.
References
- 1.Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Pineros M., et al. International Agency for Research on Cancer; 2020. Global Cancer Observatory: Cancer Today. [Google Scholar]
- 2.National Cancer Registry (Ministry of Health, Labour and Welfare), tabulated by Cancer Information Service. National Cancer Center; Japan: 2021. [Google Scholar]
- 3.Tanaka N., Asakawa I., Anai S., Hirayama A., Hasegawa M., Konishi N., et al. Periodical assessment of genitourinary and gastrointestinal toxicity in patients who underwent prostate low-dose-rate brachytherapy. Radiat Oncol. 2013;8:25. doi: 10.1186/1748-717X-8-25. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallis C.J.D., Saskin R., Choo R., Herschorn S., Kodama R.T., Satkunasivam R., et al. Surgery Versus Radiotherapy for Clinically-localized Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2016;70(1):21–30. doi: 10.1016/j.eururo.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 5.D'Amico A.V., Whittington R., Malkowicz S.B., Schultz D., Blank K., Broderick G.A., et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 6.Shiraishi Y., Yorozu A., Ohashi T., Toya K., Seki S., Yoshida K., et al. Dose constraint for minimizing grade 2 rectal bleeding following brachytherapy combined with external beam radiotherapy for localized prostate cancer: rectal dose-volume histogram analysis of 457 patients. Int J Radiat Oncol Biol Phys. 2011;81(3):e127–e133. doi: 10.1016/j.ijrobp.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka N., Asakawa I., Hasegawa M., Fujimoto K. Low-dose-rate brachytherapy for prostate cancer: A 15-year experience in Japan. Int J Urol. 2020;27(1):17–23. doi: 10.1111/iju.14098. [DOI] [PubMed] [Google Scholar]
- 8.Price J.G., Stone N.N., Stock R.G. Predictive factors and management of rectal bleeding side effects following prostate cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2013;86(5):842–847. doi: 10.1016/j.ijrobp.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 9.Katayama N., Yorozu A., Maruo S., Kojima S., Ohashi T., Tanaka N., et al. Predictive factors of rectal toxicity after permanent iodine-125 seed implantation: Prospective cohort study in 2339 patients. Brachytherapy. 2016;15(6):736–745. doi: 10.1016/j.brachy.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Wallner K., Roy J., Harrison L. Dosimetry guidelines to minimize urethral and rectal morbidity following transperineal I-125 prostate brachytherapy. Int J Radiat Oncol Biol Phys. 1995;32(2):465–471. doi: 10.1016/0360-3016(94)00599-G. [DOI] [PubMed] [Google Scholar]
- 11.Taniguchi T., Iinuma K., Kato D., Takai M., Maekawa Y.M., Nakane K., et al. Predictive factors of rectal hemorrhage in patients with localized prostate cancer who underwent low-dose-rate brachytherapy. Int J Clin Oncol. 2020;25(9):1711–1717. doi: 10.1007/s10147-020-01713-x. [DOI] [PubMed] [Google Scholar]
- 12.Mariados N., Sylvester J., Shah D., Karsh L., Hudes R., Beyer D., et al. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2015;92(5):971–977. doi: 10.1016/j.ijrobp.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 13.Hwang M.E., Black P.J., Elliston C.D., Wolthuis B.A., Smith D.R., Wu C.C., et al. A novel model to correlate hydrogel spacer placement, perirectal space creation, and rectum dosimetry in prostate stereotactic body radiotherapy. Radiat Oncol. 2018;13(1):192. doi: 10.1186/s13014-018-1135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelefsky M.J., Pinitpatcharalert A., Kollmeier M., Goldman D.A., McBride S., Gorovets D. Early Tolerance and Tumor Control Outcomes with High-dose Ultrahypofractionated Radiation Therapy for Prostate Cancer. Eur Urol Oncol. 2020;3(6):748–755. doi: 10.1016/j.euo.2019.09.006. at al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuccia F., Mazzola R., Nicosia L., Figlia V., Giaj-Levra N., Ricchetti F., et al. Impact of hydrogel peri-rectal spacer insertion on prostate gland intra-fraction motion during 1.5 T MR-guided stereotactic body radiotherapy. Radiat Oncol. 2020;15(1):178. doi: 10.1186/s13014-020-01622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morita M., Fukagai T., Hirayama K., Yamatoya J., Noguchi T., Ogawa Y., et al. Placement of SpaceOAR hydrogel spacer for prostate cancer patients treated with iodine-125 low-dose-rate brachytherapy. Int Urol. 2020;27(1):60–66. doi: 10.1111/iju.14123. [DOI] [PubMed] [Google Scholar]
- 17.Edge S.B., Byrd D.R., Compton C.C., Fritz A.G., Greene F.L., Trotti A. III, editors. AJCC Cancer Staging Manual. 7th ed. Springer; DelongchampsNew York: 2010. American Joint Committee on Cancer (AJC) Prostate; pp. 457–468. [Google Scholar]
- 18.Carroll P.H., Mohler J.L. NCCN Guidelines Updates: Prostate Cancer and Prostate Cancer Early Detection. J Natl Compr Cancer Netw. 2018;16(55):620–623. doi: 10.6004/jnccn.2018.0036. [DOI] [PubMed] [Google Scholar]
- 19.Stone N.N., Gerber N.K., Blacksburg S., Stone J., Stock R.G. Factors influencing urinary symptoms 10 years after permanent prostate seed implantation. J Urol. 2012;187(1):117–123. doi: 10.1016/j.juro.2011.09.045. [DOI] [PubMed] [Google Scholar]
- 20.Ohashi T., Yorozu A., Toya K., Saito S., Momma T., Nagata H., et al. Comparison of intraoperative ultrasound with postimplant computed tomography--dosimetric values at Day 1 and Day 30 after prostate brachytherapy. Brachytherapy. 2007;6(4):246–253. doi: 10.1016/j.brachy.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Mohler J.L., Armstrong A.J., Bahnson R.R., D'Amico A.V., Davis B.J., Eastham J.A., et al. Prostate Cancer, Version 1.2016. J Natl Compr Cancer Netw. 2016;14(1):19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 22.Vuolukka K., Auvinen P., Palmgren J.E., Voutilainen T., Aaltomaa S., Kataja V. Long-term efficacy and urological toxicity of low-dose-rate brachytherapy (LDR-BT) as monotherapy in localized prostate cancer. Brachytherapy. 2019;18(5):583–588. doi: 10.1016/j.brachy.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka N., Yorozu A., Kikuchi T., Higashide S., Kojima S., Ohashi T., et al. Genitourinary toxicity after permanent iodine-125 seed implantation: The nationwide Japanese prostate cancer outcome study of permanent iodine-125 seed implantation (J-POPS) Brachytherapy. 2019;18(4):484–492. doi: 10.1016/j.brachy.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Sanguedolce F., Sancho Pardo G., Mercadé Sanchez A., Balaña Lucena J., Pisano F., Cortez J.C., et al. Radiation-induced haemorrhagic cystitis after prostate cancer radiotherapy: factors associated to hospitalization and treatment strategies. Prostate Int. 2021;9(1):48–53. doi: 10.1016/j.prnil.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iinuma K., Nakano M., Kato T., Kato D., Takai M., Maekawa Y.M., et al. Assessment of Long-term Changes in Lower Urinary Tract Symptoms in Patients With Prostate Cancer Who Underwent Low-dose-rate Prostate Brachytherapy. Urology. 2020;142:213–220. doi: 10.1016/j.urology.2020.04.106. [DOI] [PubMed] [Google Scholar]
- 26.Onishi K., Tanaka N., Miyake M., Nakai Y., Anai S., Torimoto K., et al. Changes in lower urinary tract symptoms after iodine-125 brachytherapy for prostate cancer. Clin Transl Radiat Oncol. 2018;14:51–58. doi: 10.1016/j.ctro.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alshak M.N., Eidelberg A., Diaz S.M., Stoddard M.D., Formenti S., Nagar H., et al. Natural history of lower urinary tract symptoms among men undergoing stereotactic body radiation therapy for prostate cancer with and without a Rectal Hydrogel Spacer. World J Urol. 2022;40(5):1143–1150. doi: 10.1007/s00345-022-03953-0. [DOI] [PubMed] [Google Scholar]
- 28.Abrams P. New words for old: lower urinary tract symptoms for "prostatism". BMJ. 1994;308(6934):929–930. doi: 10.1136/bmj.308.6934.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gravas S., Cornu J.N., Gacci M., Gratzke C., Herrmann T.R.W., Mamoulakis C., et al. European Association of Urology.; 2018. EAU guidelines on management of non-neurogenic male lower urinary tract symptoms (LUTS), incl. benign prostatic obstruction (BPO) [Google Scholar]
- 30.Oelke M., Höfner K., Jonas U., de la Rosette J.J., Ubbink D.T., Wijkstra H. Diagnostic accuracy of noninvasive tests to evaluate bladder outlet obstruction in men: detrusor wall thickness, uroflowmetry, postvoid residual urine, and prostate volume. Eur Urol. 2007;52(3):827–834. doi: 10.1016/j.eururo.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H., Wang L., Riegel A.C., Antone J., Potters L., Lee L., et al. Biological effective dose in analysis of rectal dose in prostate cancer patients who underwent a combination therapy of VMAT and LDR with hydrogel spacer insertion. J Appl Clin Med Phys. 2022 doi: 10.1002/acm2.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]