Abstract
Depression is gradually becoming a primary mental disease threatening human health. Therefore, there is an urgent need to clarify the pathogenesis of depression and identify new effective natural antidepressants. This study aimed to investigate the antidepressant effects of baicalin and explore its potential mechanism in a mouse model of depression induced by chronic unpredictable mild stress (CUMS). Following a 6-week exposure to CUMS, mice were treated with baicalin (10 mg/kg) or fluoxetine (10 mg/kg) for 4 weeks by oral gavage. A sucrose preference test and a forced swimming test were performed to evaluate depression-like behaviors, and the levels of adenosine triphosphate (ATP) in the prefrontal cortex were measured. Moreover, gene expression and enzyme activities related to ATP production, and mitochondrial function, were monitored. The results indicated that baicalin and fluoxetine could alleviate CUMS-induced depression-like behaviors of mice. In addition, baicalin significantly elevated the ATP content and the expression of genes hexokinase 1 (Hk1), pyruvate dehydrogenase E1 alpha 1 (Pdha-1), isocitrate dehydrogenase (Idh), peroxisome proliferator-activated receptor, gamma, coactivator 1 alpha (Pgc-1α), and sirtuin-1 (Sirt1) in the prefrontal cortex. Furthermore, baicalin increased the activity of the respiratory chain complexes I and V as well as the mitochondrial membrane potential. In conclusion, baicalin may exert its antidepressant effect partly by upregulating the expression of some genes coding for enzymes involved in the glycolysis and the tricarboxylic acid cycle, and improving the mitochondrial function to enhance the ATP level in the brain.
Keywords: Depression, Baicalin, ATP, Mitochondrion, Prefrontal cortex
Depression; Baicalin; ATP; Mitochondrion; Prefrontal cortex.
1. Introduction
Depression manifests mainly as persistent low mood, anhedonia, energy loss, and sleep disturbance [1, 2]. Chronic external stress has become a primary cause of depression in humans. Growing amounts of patients with depression will inevitably entail a great burden on family and society [3]. First-line clinical antidepressants, despite being widely used, present various disadvantages, such as slow onset, low cure rate, and serious adverse reactions [4]. Therefore, there is an urgent need to develop new, effective antidepressants.
Increasing evidence has shown that the pathogenesis of depression is exceptionally complicated [5]. Previous studies mainly focused on the hypothesis of “monoamines deficiency,” “hyperactivation of hypothalamic-pituitary-adrenal (HPA) axis,” “inflammatory response,” and “neurotrophic factor deficiency” [6, 7, 8, 9]. In recent years, the correlation between energy metabolism disorder and depression has been widely studied [10]. In addition, previous studies have demonstrated that depression is closely associated with mitochondrial dysfunction [11, 12]. In particular, recent studies have reported that the low levels of adenosine triphosphate (ATP) in an animal model of depression [13, 14]. And the lower energy levels in the brain of patients with depression and animal models could be significantly reversed by antidepressants [15, 16, 17, 18]. Moreover, direct intracerebroventricular injection of ATP into the brain could substantially improve depression-like behaviors in mice exposed to chronic unpredictable mild stress (CUMS) [10]. These findings suggest that improving the energy levels in the brain may represent a new strategy for treating depression.
Baicalin, a flavonoid that can cross the blood-brain barrier, possesses multiple biological functions, including neuroprotective, anti-apoptotic and anti-inflammatory functions [19, 20, 21, 22]. Our previous study has shown that baicalin can alleviate the CUMS-induced depression-like behaviors by inhibiting cyclooxygenase-2 (COX-2) and reducing prostaglandin E2 (PGE2) levels in hippocampus and prefrontal cortex in rat [23]. In addition, the inhibition of COX-2/PGE2 signaling will promote glucose metabolism and mitochondrial respiration to produce ATP [24], suggesting that the antidepressant effects of baicalin may relate to the increased energy production. However, whether baicalin can improve the brain energy level in the depressed animal is unclear.
The main purpose of the present study was to investigate the effects of baicalin on the levels of ATP, mitochondrial membrane potential (MMP), and the activity of respiratory chain complexes I–V in the prefrontal cortex, because mitochondrial oxidative phosphorylation is the main pathway to produce ATP. Moreover, the effects of baicalin on the mRNA levels of several key enzymes in glycolysis and TCA cycle were also investigated.
2. Materials and methods
2.1. Animals
Male C57BL/6N mice (6 weeks, 18–20 g) were purchased from Beijing Vital River Laboratory Animal Technology (Beijing, China). Mice were individually housed in cage (310 × 230 × 157 mm) under a standard 12 h light and 12 h dark cycle at 23–25 °C and 40–60 % humidity. Mice were acclimatized to the environment for 1 week and free access to water and food before use. All animal experiments were performed in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and were approved by the Committee of Animal Care of Henan University of Chinese Medicine (DWLL16020024).
2.2. Drug administration
Before the start of the experiment, we conducted the preliminary experiments to determine the required sample size according to statistical principles [25], and our result of preliminary experiments verified the validity of 6-week CUMS followed by 4-week treatment when the sample size was set to 9. The mice were randomly divided into two groups: a control group (n = 9) and a stress group (n = 27). The stress group received CUMS for 6 weeks and kept the control group in a separate room without any stimulation. After 6 weeks, the stress group was subdivided into three groups based on equal sucrose preference: a CUMS group, a baicalin group (10 mg/kg), and a fluoxetine group (10 mg/kg). The dose of baicalin was selected from our previous study, in which 10 mg/kg of baicalin was found to exert an antidepressant effect in mice [23, 26]. All drugs were suspended in saline and administered by oral gavage daily in a volume of 10 mL/kg for 4 weeks. The CUMS group and control group received an equal volume of saline. The CUMS procedure was continued during the drug administration period.
2.3. CUMS procedure
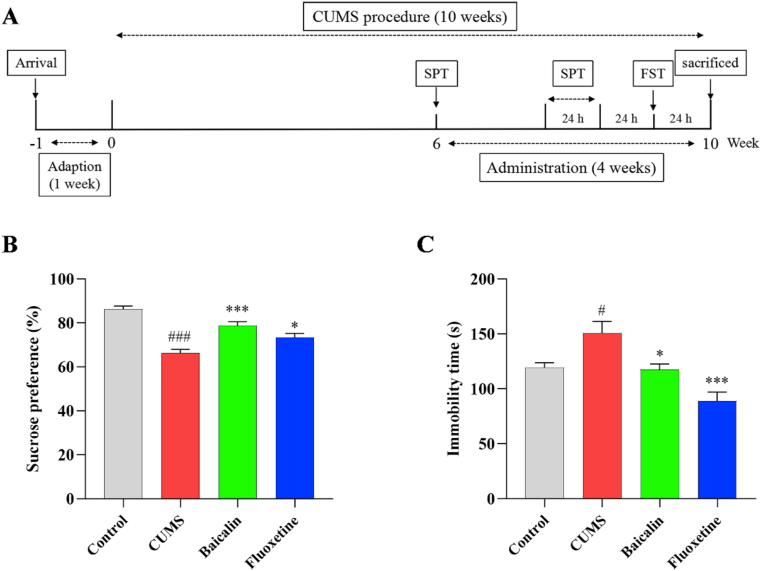
The CUMS procedure was slightly modified from that previously described by Willner et al. [27]. Briefly, the stress group mice were individually fed in a cage and received 1–2 different stimuli every day. Stressors including day and night reversal for 24 h, food deprivation for 12 h, tilting of the cage by 45° for 24 h, exposure to cold (4 °C) or hot (45 °C) water for 4 min, exposure to white noise (90 dB) for 12 h, housing in soiled cage for 24 h, restraining for 3 h, exposure to flashlight (150 flashes/min) for 6 h, and pair-housing for 2 h. The schedule of the experimental procedure was displayed in Figure 1A.
Figure 1.
Effects of baicalin on the SPT and FST in CUMS mice. All values are presented as means ± SEM (n = 9). (A) schematic timeline of experimental procedures. (B) The sucrose preference in the SPT. (C) The immobility time in the FST. ###p < 0.001 and #p < 0.05 as compared with the control group; ∗∗∗p < 0.001 and ∗p < 0.05 as compared with the CUMS group.
2.4. Behavioral tests
The sucrose preference test (SPT) and forced swimming test (FST) evaluated depression-like behaviors. Xiaohui Jin performed these behavioral tests, and the results were analyzed by Ming Bai, both of whom were blinded to the experimental conditions.
SPT was performed according to the protocol described in Liu et al. [28]. Pre-test, all mice were trained to consume two bottles of 1% sucrose solution for 24 h. Then, all the sucrose solution bottles were replaced with fresh sucrose solution and water for another 24 h. After the training, the mice received 12 h of water and food deprivation, and then the SPT was performed. Briefly, weigh the bottles containing sucrose solution or fresh water and given them to the mice for 24 h. To avoid any position preference, the two bottles were replaced every 12 h. At the end of the test, all bottles were weighed again. Sucrose preference was calculated as follows: sucrose preference (%) = sucrose intake/(sucrose intake + water intake) ×100%.
FST was carried out according to the protocol described in Porsolt et al. [29]. The mice were placed in a 13 cm × 25 cm (diameter × height) glass cylinder filled with 10 cm of water (23–25 °C). The mice were forced to swim in the water for 6 min, and a camera was used to record the immobility time of the mice in the last 4 min.
2.5. Collection and distribution of brain tissues
All mice were recovered for 24 h at the end of the behavioral test. Thirty minutes after the last administration, all mice were sacrificed by decapitation after deep anesthesia with isoflurane. The prefrontal cortex was collected immediately, frozen by liquid nitrogen, and stored at −80 °C until analysis. The prefrontal cortices were collected from each group (two tissues in each mouse), and the tissues were distributed as follows for subsequent analyses: ATP content, five tissues; gene expression, five tissues; MMP and activity of the respiratory chain complexes I–V, five tissues. Moreover, two tissues were used to determine the efficiency of mitochondrial extraction and the detection limit of the kit. The tissues were randomly selected from each group, and we ensured that the tissues used for each analysis came from different mice.
2.6. Extraction of mitochondrion
The prefrontal cortices were homogenized in a 10-fold volume lysis buffer (SM0020, Solarbio, Beijing, China). The homogenate was then centrifuged at 1000× g for 5 min, and the supernatants were centrifuged at 1000× g for another 5 min. Next, the supernatants were centrifuged at 12,000× g for 10 min, and then collected and used as cytoplasmic extracts. The precipitate was then resuspended in wash buffer and centrifuged at 1000× g for 5 min. The supernatant was centrifuged again at 12,000× g for 10 min. Finally, the precipitate was resuspended in store buffer and considered a highly pure mitochondrial extract, which was used to detect the level of MMP and the activity of mitochondrial respiratory chain complexes.
2.7. Biochemical analyses
The measurement of ATP content. An ATP kit (S0027; Beyotime Biotechnology, Shanghai, China) was used for ATP detection. The prefrontal cortex was weighed and homogenized in pre-cooled lysate (10 μL/mg). The lysate was centrifuged at 12,000× g and 4 °C for 5 min. After the supernatant was fully mixed with the detection reagent, luminescence signal was detected by Cytation 5 (Biotek, Winooski, VT, USA), and ATP content was calculated using standard curve.
The measurement of MMP. A JC-10 kit (CA1310, Solarbio) was used for MMP measurement. The prepared JC-10 solution was mixed with purified mitochondria at a 9:1 ratio. Fluorescence signals were detected using Cytation 5 at excitation/emission wavelengths of 485/590 nm.
The measurement of mitochondrial complexes activity. To measure the activity of the mitochondrial complexes I and II, appropriate kits (ab109721, ab109908, Abcam, Cambridge, UK) were used. The purified mitochondria were reacted with the reagent, and then the absorbance was detected at 450 nm or 600 nm wavelength using Cytation 5. For complexes III–V, the BC3245, BC0945, and BC1445 kits (Solarbio) were used. Then, an appropriate volume of extraction buffer was added to the purified mitochondria, and ultrasonic crushing (power 20%, 5 s on/10 s off for 15 times) was performed. Finally, the absorbance was detected at 550 nm or 660 nm wavelength using Cytation 5.
Protein concentration was determined using a BCA kit (CW0014S, CWBIO, Beijing, China). Absorbance was measured using Cytation 5. Concentrations were calculated using standard curve.
2.8. Quantitative real-time polymerase chain reaction
Total RNA was extracted from the prefrontal cortex, and cDNA was synthesized using a commercial kit following the manufacturer's instructions (DP451, Tiangen, Beijing, China). Real-time PCR reactions were performed in the ABI-Q6 system (Applied Biosystems, Waltham, MA, USA) using the SYBR Green kit (208054, Qiagen, Hilden, Germany). The primers were synthesized by Invitrogen (Carlsbad, CA, USA). The primer sequences are listed in Table 1 β-actin was used to normalize the expression levels.
Table 1.
The sequence of the primers for qRT-PCR.
| Gene name | Primer sequence | Accession number |
|---|---|---|
| Hk | Forward: 5′- CTACCCGGAGTTGTTCTGCT-3′ | NM_013820.3 |
| Reverse: 5′-CCCTAAGTCTCACTCCTGCC-3′ | ||
| Pfk | Forward: 5′- TGAGGATGGCTGGGAGAACT-3′ | NM_008826.5 |
| Reverse: 5′-TGAACCACCAGATCCTTCACG-3′ | ||
| Pk | Forward: 5′- CTAGCGGTCCTTGGACTTCAGG-3′ | NM_001378869.1 |
| Reverse: 5′-ACAAATGATGCCAGTGTTGCG-3′ | ||
| Pdha-1 | Forward: 5′- TCTGTCGGTTCCCAGTCCA-3′ | NM_008810.3 |
| Reverse: 5′-CGTTTCCTTTTCACAGCACAT-3′ | ||
| Idh | Forward: 5′- ATTAGACGCCAGCCAGTCG-3′ | NM_001111320.1 |
| Reverse: 5′-AGAAGTCGGTCCAAGAGAGC-3′ | ||
| Ogdh | Forward: 5′- CCGTGCCCGCTGACATTATCT-3′ | NM_001252282.1 |
| Reverse: 5′-AGGCCATAGAACCCTCCTACTG-3′ | ||
| Cs | Forward: 5′- TTTGTCTACCCTTCCCCTCA-3′ | NM_026444.4 |
| Reverse: 5′-CAGGATGAGTTCTTGGCTCC-3′ | ||
| Pgc-1α | Forward: 5′- TGAAAAAGCTTGACTGGCGTC-3′ | NM_008904.2 |
| Reverse: 5′-ACCAGAGCAGCACACTCTATG-3′ | ||
| Sirt1 | Forward: 5′- CGATGACAGAACGTCACACG-3′ | NM_019812.3 |
| Reverse: 5′-ATTGTTCGAGGATCGGTGCC-3′ | ||
| β-Actin | Forward: 5′- ACTGAGCTGCGTTTTACACC-3′ | NM_007393.5 |
| Reverse: 5′-GCCTTCACCGTTCCAGTTTTT-3′ |
2.9. Western blot
The mitochondrial and cytoplasmic proteins of the prefrontal cortex were extracted using an appropriate kit (SM0020, Solarbio) and quantified using a BCA kit (CW0014S, CWBIO, Beijing, China). Protein samples were mixed with loading buffer and boiled at 99 °C for 5 min. Protein samples (10 μg) were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto polyvinylidene difluoride (PVDF) membranes (IPVH00010, Millipore, Billerica, MA, USA) for blocking in 5% skim milk (BD Biosciences, Franklin Lakes, NJ, USA). The membranes were incubated with polyclonal rabbit primary antibodies in blocking solution at 4 °C overnight, including anti-GAPDH (1:60.000, KC-5G5, Kangchen, Shanghai, China) and anti-COXIV (1:1000, 4844, Cell Signaling Technology, Danvers, MA, USA) antibodies. The membranes were then washed three times with PBST and incubated with goat anti-rabbit secondary antibody (1:120,000, KC-RB-025, Kangchen) for 30 min at room temperature. Immunoblotting bands were visualized by incubation with ECL reagent (WBLUF0100, Merck Millipore, Darmstadt, Germany) and exposed to an X-ray film (6535876, Kodak, Rochester, NY, USA).
2.10. Statistical analysis
Statistical analysis was performed using SPSS version 24.0. Data are expressed as the mean ± SEM. Differences between the control and CUMS groups were analyzed by Student's t-test. Data relative to the CUMS, baicalin, and fluoxetine groups were analyzed by one-way ANOVA followed by Tukey's post hoc test. Statistical significance was set at p < 0.05.
3. Results
3.1. Effects of baicalin on the SPT and FST in CUMS mice
To investigate the effects of baicalin on CUMS-induced depression-like behaviors in mice. In the SPT (Figure 1B), sucrose preference was significantly reduced in the CUMS group compared with that in the control group [t (16) = 9.025, p < 0.001]; this effect was partially reversed by treatment [F (2, 24) = 12.818, p < 0.001; baicalin, p < 0.001 and fluoxetine, p < 0.05]. As shown in Figure 1C, the immobility time in the FST was longer in the CUMS group [t (16) = −2.723, p < 0.05]. However, both baicalin and fluoxetine reduced immobility time [F (2, 24) = 13.892, p < 0.001; baicalin, p < 0.05 and fluoxetine, p < 0.001]. In addition, baicalin had no effect on normal mice (Figure S1). These results indicated that a mouse model of depression was successfully established and confirmed that baicalin can exert antidepressant effects in a CUMS mouse model.
3.2. Effects of baicalin on the ATP levels in the prefrontal cortex in CUMS mice
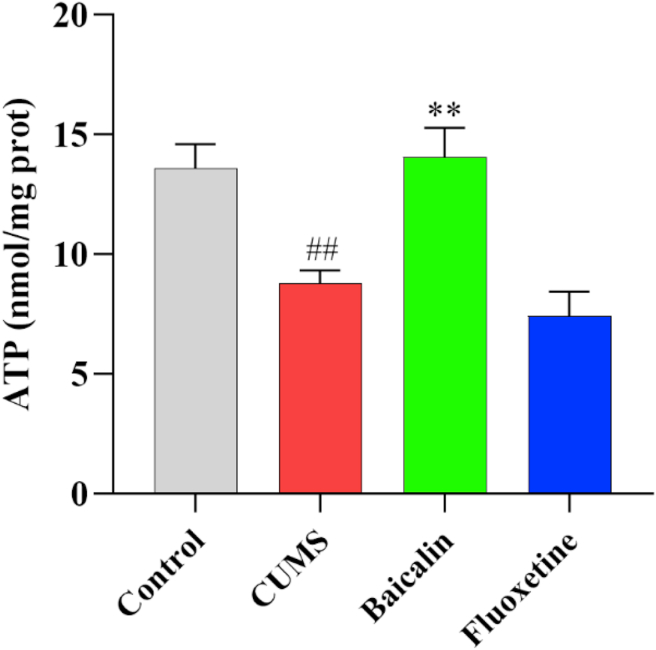
To investigate whether the antidepressant effects of baicalin were related to the regulation of the energy level in the brain, ATP levels in the prefrontal cortex were detected. Figure 2 shows that the ATP levels of the prefrontal cortex were significantly reduced in the CUMS group [t (8) = 4.148, p < 0.01]. Such reduction was significantly reversed by baicalin treatment [F (2, 12) = 12.705, p < 0.01; baicalin, p < 0.01]. Interestingly, the ATP level was also increased in normal mice [t (10) = −3.475, p < 0.01] (Figure S2). These results indicate that baicalin can increase ATP levels, and thus energy level, in the prefrontal cortex of CUMS mice.
Figure 2.
Effects of baicalin on the ATP levels in the prefrontal cortex in CUMS mice. All values are presented as means ± SEM (n = 5). ##p < 0.01 as compared with the control group; ∗∗p < 0.01 as compared with the CUMS group.
3.3. Effects of baicalin on the mRNA levels of several key glycolysis-related enzymes in the prefrontal cortex in CUMS mice
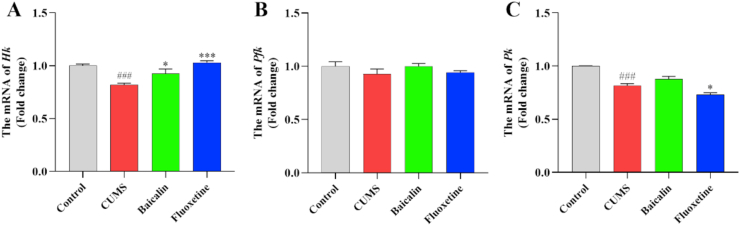
To investigate the efficacy of baicalin on glycolysis in the prefrontal cortex, we detected the expression of three genes encoding key enzymes of glycolysis. As shown in Figure 3, The expression of Hk and Pk was significantly downregulated in the CUMS group [t (8) = 7.744, p < 0.001; t (8) = 9.478, p < 0.001] (Figure 3A, C). However, the expression of Hk was significantly increased in the treatment groups [F (2, 12) = 13.961, p < 0.001; baicalin, p < 0.05 and fluoxetine, p < 0.001], and baicalin had no effects in the Hk in normal mice (Figure S3), whereas that of Pk was significantly decreased in the treatment group [F (2, 12) = 11.157, p < 0.01; fluoxetine, p < 0.05]. Both baicalin and fluoxetine had no effect on the mRNA levels of Pfk (Figure 3B). There results suggest that baicalin might upregulate the expression of Hk to produce ATP.
Figure 3.
Effects of baicalin on the mRNA levels of several key glycolysis-related enzymes in the prefrontal cortex in CUMS mice. The mRNA levels were normalized to β-actin, and subsequently represents as fold change relative to control group. All values are presented as means ± SEM (n = 5). ###p < 0.001 as compared with the control group; ∗∗∗p < 0.001 and ∗p < 0.05 as compared with the CUMS group. Hk: Hexokinase; Pfk: Phosphofructokinase; Pk: Pyruvate kinase.
3.4. Effects of baicalin on the mRNA levels of several key TCA cycle-related enzymes in the prefrontal cortex in CUMS mice
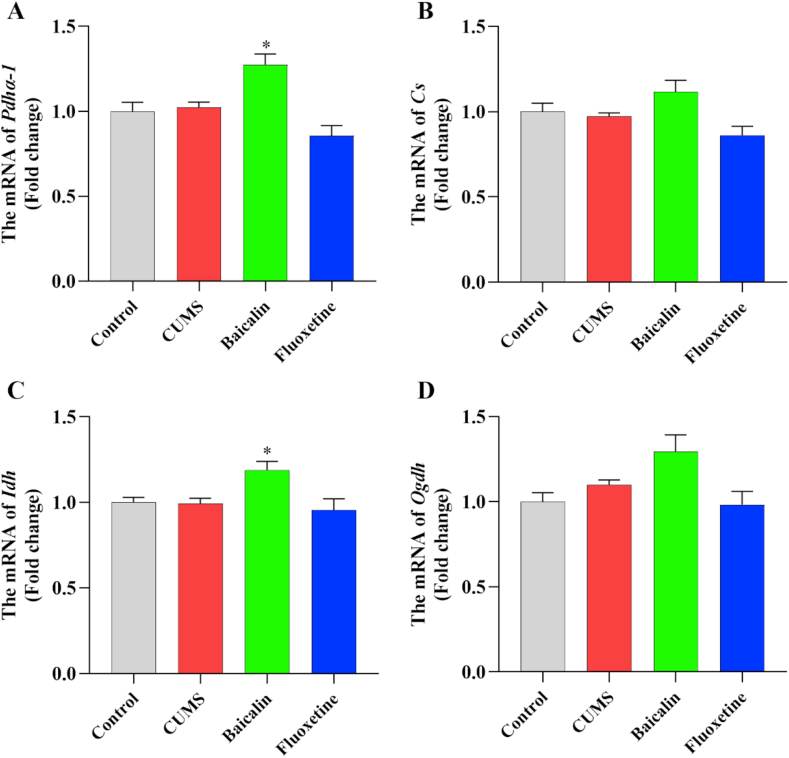
To investigate whether baicalin affects the activity of the TCA cycle in the prefrontal cortex, the expression of four genes encoding key enzymes in the TCA cycle was detected. The results are presented in Figure 4. There were no significant differences in the expression of these genes in the CUMS and the control groups (Figure 4A–D). However, the expression of Pdha-1 and Idh was significantly upregulated in the treatment groups compared to the CUMS group [F (2, 12) = 15.815, p < 0.001; baicalin, p < 0.05. F (2, 12) = 6.083, p < 0.05; baicalin, p < 0.05] (Figure 4A, C), and baicalin had no effects in the Pdha-1 and Idh in normal mice (Figure S3). Moreover, the expression of Cs and Ogdh was slightly upregulated in the baicalin group, although not significantly [F (2, 12) = 6.315, p < 0.05; baicalin, p = 0.152 and fluoxetine, p = 0.311. F (2, 12) = 4.498, p < 0.05; baicalin, p = 0.196 and fluoxetine, p = 0.520] (Figure 4B, D). These results indicated that baicalin may upregulate the expression of Pdha-1 and Idh, thereby promoting the production of ATP.
Figure 4.
Effects of baicalin on the mRNA levels of several key TCA cycle-related enzymes in the prefrontal cortex in CUMS mice. The mRNA levels were normalized to β-actin, and subsequently represents as fold change relative to control group. All values are presented as means ± SEM (n = 5). ∗p < 0.05 as compared with the CUMS group. Pdha-1: pyruvate dehydrogenase E1 alpha 1; Cs: Citrate synthase; Idh: Isocitrate dehydrogenase; Ogdh: Oxoglutarate dehydrogenase.
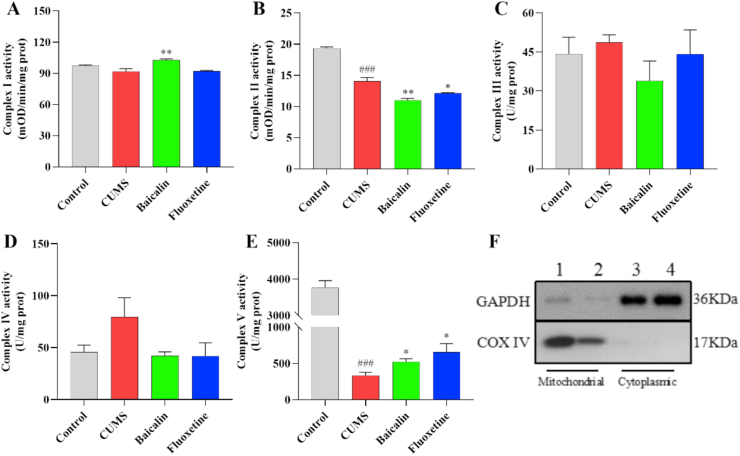
3.5. Effects of baicalin on the activity of complex I–V in the prefrontal cortex in CUMS mice
To investigate whether reduced energy levels in depressed mice resulted from the impairment of the mitochondrial ETC, the activity of the five mitochondrial respiratory chain complexes was assessed. The results are shown in Figure 5A–E. The activity of complexes II and V were significantly reduced in the CUMS group [t (8) = 8.454, p < 0.001; t (8) = 17.686, p < 0.001]. Interestingly, the activity of complex I was significantly increased by baicalin treatment [F (2, 12) = 12.992, p < 0.001; baicalin, p < 0.01], but that of complex II was further reduced in the treatment group [F (2, 12) = 16.153, p < 0.001; baicalin, p < 0.01 and fluoxetine, p < 0.05]. In addition, the activity of complex V was significantly increased in the treatment groups [F (2, 12) = 4.636, p < 0.05; baicalin, p < 0.05 and fluoxetine, p < 0.05]. In normal mice, baicalin reduced the activity of complex II [t (8) = 4.957, p < 0.01], but did not affect complex I and V (Figure S4). These results suggest that the effect of baicalin on complex I may be responsible for contributing to the observed elevated ATP levels. Determination of the purity of extracted mitochondria by western blotting (Figure 5F).
Figure 5.
Effects of baicalin on the activity of complex I–V in the prefrontal cortex in CUMS mice. All values are presented as means ± EMD (n = 5). (A-E) Mitochondria electron transport chain complex I–V. (F) Purity of mitochondria. 1, 2 Mitochondrial. 3, 4 Cytoplasmic. ##p < 0.001 as compared with the control group; ∗∗p < 0.01 and ∗p < 0.05 as compared with the CUMS group.
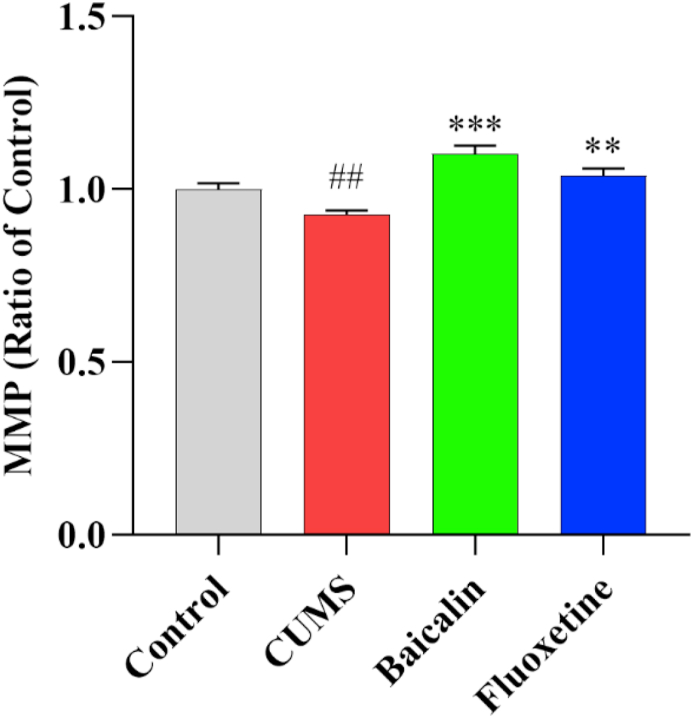
3.6. Effects of baicalin on the MMP level in the prefrontal cortex in CUMS mice
To further investigate the effects of baicalin on mitochondrial function, the mitochondrial membrane potential (MMP) was measured. The results are presented in Figure 6. The MMP was reduced in CUMS-exposed mice [t (8) = 3.409, p < 0.01]. As expected, both baicalin and fluoxetine increased MMP levels [F (2, 12) = 19.807, p < 0.001; baicalin, p < 0.001 and fluoxetine, p < 0.01]. These results suggest that baicalin may elevate energy levels by increasing the MMP in the prefrontal cortex of CUMS mice.
Figure 6.
Effects of baicalin on the MMP level in the prefrontal cortex in CUMS mice. All values are presented as means ± SEM (n = 5). ##p < 0.01 as compared with the control group; ∗∗∗p < 0.001 and ∗∗p < 0.01 as compared with the CUMS group.
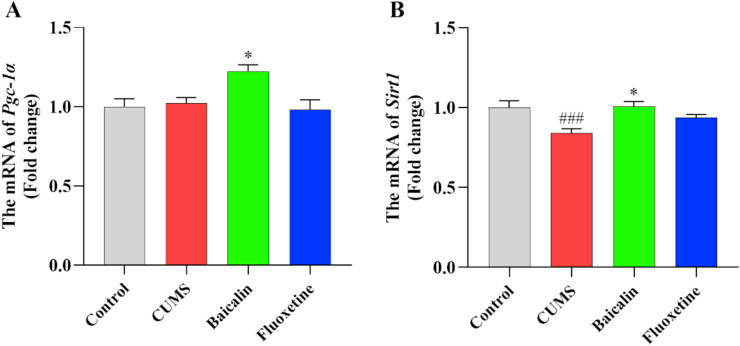
3.7. Effects of baicalin on the mRNA levels of Pgc-1α and Sirt1 in the prefrontal cortex in CUMS mice
To further clarify the dysfunctional energy metabolism in the prefrontal cortex of CUMS-induced depression mice at the transcription level, the expression of two key genes related to the regulation of mitochondrion activity was assessed by qPCR. Compared with the control group, the expression of Sirt1 was significantly downregulated in the CUMS group [t (8) = 5.183, p < 0.001] (Figure 7B). Contrarily, the expression of both Pgc-1α and Sirt1 was significantly upregulated in the baicalin treatment group [F (2, 12) = 7.840, p < 0.01, baicalin, p < 0.05. F (2, 12) = 7.111, p < 0.01; baicalin, p < 0.05] (Figure 7A, B). However, fluoxetine did not affect the expressions of these genes (Figure 7). In addition, baicalin had no effects in these genes in normal mice (Figure S3). These results suggest that baicalin might promote the Pgc-1α and Sirt1 expression to improve mitochondrial function in the prefrontal cortex of mice.
Figure 7.
Effects of baicalin on the mRNA levels of Pgc-1α and Sirt1 in the prefrontal cortex in CUMS mice. The mRNA levels were normalized to β-actin, and subsequently represents as fold change relative to control group. All values are presented as means ± SEM (n = 5). ###p < 0.001 as compared with the control group; ∗p < 0.05 as compared with the CUMS group. Pgc-1α: peroxisome proliferator-activated receptor, gamma, coactivator 1 alpha; Sirt1: Sirtuin-1.
4. Discussion
The antidepressant effect of baicalin has been widely demonstrated in our and other previous studies, suggesting that its mechanism of action may be related to brain-derived neurotrophic factor (BDNF), inflammation, and the HPA axis [22, 23, 24]. In the present study, the antidepressant effect of baicalin was confirmed in CUMS-exposed mice, as revealed by an increased sucrose preference and a reduced immobility time in the FST. Moreover, we found that baicalin significantly reversed CUMS-induced reduction of ATP levels in the prefrontal cortex, which may be related to its antidepressant effect.
In recent years, the relationship between abnormal energy metabolism and depression has received increasing attention. Imaging studies have revealed obvious dysregulation of energy metabolism in the brains of patients with depression [30, 31], these findings were also confirmed in the brain of chronic mild stress (CMS)-exposed rats [32]. In addition, baicalin has the effect of reducing oxidative stress and improving mitochondrial dysfunction [33, 34]. This may be closely related to the production of ATP and the treatment of depression.
ATP is the most important energy source of the cell and its levels directly reflect the cellular energy status. Previous studies have shown lower ATP levels in the brains of patients with depression and in animal models [35, 36]. In particular, Jun et al. reported that calcium homeostasis modulator family protein 2 (Calhm2) knockout mice displayed a low ATP content in the brain and exhibited depression-like behavior [37]. Moreover, Cao et al. demonstrated that lateral intracerebroventricular and intraperitoneal injection of ATP, or an increase in endogenous ATP released by astrocytes, could significantly improve depression-like behaviors in mice induced by chronic social defeat stress (CSDS) [10]. In addition, increasing ATP levels could alleviate synaptic damage, increase spine density and neural activity in the hippocampus, and improve depression-like behavior in CUMS-exposed mice [10, 37].
Altogether, these studies have shown that increasing ATP levels might represent a new strategy for treating depression. In the present study, we found that the ATP content of the prefrontal cortex was significantly reduced in the CUMS group, and that this effect was reversed by baicalin. However, baicalin increased the ATP level but did not affect the behaviors in normal mice. This may be due to the difference in the age of the mice used in the two experiments. In CUMS mice, both production and consumption of ATP were impaired [38]. The results indicated that baicalin improved energy metabolism. Our results suggested that baicalin-mediated increase in ATP content is beneficial for maintaining the function of neurons, this phenomenon may partly explain the antidepressant effect of baicalin.
Glycolysis is essential for ATP production in most organisms [39]. Reduced glycolysis has been observed in various neurodegenerative diseases, such as depression, Alzheimer's disease, and amyotrophic lateral sclerosis [40, 41]. HK, PFK, and PK are key enzymes in the glycolytic pathway [42], and the expression of the genes in the prefrontal cortex was measured to examine the effect of baicalin on glycolytic activity. We found that baicalin did not affect the mRNA levels of Pk and Pfk, but significantly upregulated the mRNA levels of Hk. The upregulation of mRNA levels may increase protein levels and further increase enzymatic activity, accelerating glycolysis. Therefore, we speculated that baicalin may promote glycolysis by upregulating the mRNA level of Hk. However, the changes in mRNA levels are not always consistent with changes in protein levels and enzymatic activity, which cannot fully reflect the level of glycolysis. Therefore, more studies focusing on the protein levels and enzyme activity to HK are needed in the future.
Under aerobic conditions, the TCA cycle and coupled oxidative phosphorylation are the main pathways for ATP production in mitochondria. Previous studies reported that mitochondrial function is strongly connected to depression [43]. Other researchers have reported that chronic stress can inhibit oxidative phosphorylation in mitochondria, disrupt the MMP, and damage the mitochondrial structures in various regions of mouse brains, such as the hippocampus and the prefrontal cortex [44, 45]. In addition, disruption of the TCA cycle has been shown to reduce ATP production in a CMS-induced rat model of depression [46]. We found that baicalin upregulated the mRNA levels of Pdha-1 and Idh, but not Cs and Ogdh. Similar to glycolysis, we speculated that baicalin may promote the TCA cycle by upregulating the mRNA levels of Pdha-1 and Idh. However, it is necessary to detect the changes in protein expression and enzyme activity level to clarify the effect of baicalin on the TCA cycle.
The electrons generated during the TCA cycle, carried by nicotinamide adenine dinucleotide (NADH) and nicotinamide adenine dinucleotide phosphate (NAPDH), are delivered to the mitochondrial intermembrane space by the proton pump of the ETC; the resulting proton gradient is used by ATP synthase to generate ATP [47]. Mitochondrial ETC dysfunction is closely related to depression [48, 49]. In the present study, baicalin was found to upregulate the activity of complexes I and V, but to repress that of complex II. Together, the mitochondrial respiratory chain complexes I and II provide the electrons needed for the ETC-mediated reactions, which represent the main mechanism of energy production in mitochondria. Nevertheless, complex I is often the starting point of such process [50], although both complexes I and II represent entry points for electrons in the respiratory chain. These two complexes recognize different substrates, so that they do not interfere with each other during electron transfer along the respiratory chain. However, most of the electrons in the respiratory chain are provided by complex I, not complex II [47]. In our study, baicalin was found to promote the activity of complex I, while inhibiting that of complex II. In addition, baicalin reduced the activity of complex II, increased ATP level and did not affect the complex I and V in normal mice suggested the baicalin inhibited the activity of complex II to increase the role of complex I in the TCA cycle, thereby increased the ATP level. Therefore, we speculate that the effect of baicalin on improving depression-like behaviors by increasing ATP levels may be achieved by increasing the flux of complexes I to V and inhibiting the flux of complexes II to V. Unfortunately, this study failed to further explore its plausibility. Such phenomenon may represent one of the mechanisms by which baicalin increases ATP levels and exerts an antidepressant effect.
Altogether, these results suggest that baicalin promoted the activity of TCA cycle and mitochondrial complex I, thereby increasing the ATP content. It is well known that the MMP depends on electron transfer by the respiratory chain. And complex V will use the potential to produce ATP. A previous study reported a significantly reduced MMP in the prefrontal cortex of CUMS-induced mice [44]. In the present study, baicalin and fluoxetine increased the MMP in the CUMS group. Previous studies have reported that fluoxetine can alter mitochondrial function and ATP levels [49]. These results suggest that baicalin may exert its antidepressant effect by activating complex I and V to enhance MMP and then increase ATP content.
Sirt1 and Pgc-1α are two key genes closely related to mitochondrial function, biogenesis, and oxidative stress damage [51]. Previous studies have shown an association between depression and decreased the mRNA level of Sirt1 [52]. Pgc-1α, a downstream target gene of Sirt1, is closely linked to the mitochondrial biogenesis [53]. Notably, increased expression of Pgc-1α can significantly alleviate mitochondrial dysfunction and reduce neuronal damage [54]. In addition, previous studies have reported that the activation of the SITR1/PGC-1α pathway plays a major role in mitochondrial function and neuroprotection [55]. In our study, baicalin was found to significantly increase the mRNA level of Sirt1 and Pgc-1α. This result suggested that baicalin might activate the SIRT1/PGC-1α pathway to promote energy metabolism.
5. Conclusions
In summary, the present study demonstrated that baicalin could improve CUMS-induced depression-like behaviors of mice and increase ATP levels. Additionally, baicalin upregulated the mRNA levels of Hk, Pdha-1, Idh, Pgc-1α, and Sirt1. Baicalin also improved mitochondrial function, promoted the activity of complexes I and V, and increased the MMP. These findings unclosed the possible antidepressant mechanism of baicalin and provided a new strategy for developing novel antidepressants. In order to promote the clinical use of baicalin, more studies need to be performed in the future.
Declarations
Author contribution statement
Shuai-Fei Lu: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Cai-Yin Li; Xiao-Hui Jin, Lei-Lei Zhu: Performed the experiments; Analyzed and interpreted the data.
Ji-Duo Shen: Contributed reagents, materials, analysis tools.
Ming Bai: Analyzed and interpreted the data.
Yu-Cheng Li: Conceived and designed the experiments; Wrote the paper.
Er-Ping Xu: Conceived and designed the experiments; Contributed reagents, materials, analysis tools.
Funding statement
Shuai-fei Lu was supported by National Natural Science Foundation of China [81673629 & 81973739], Natural Science Excellent Youth Fund of Henan Province [202300410249], Scientific and Technological Innovation Talents in Universities of Henan Province [18HASTIT050], Traditional Chinese Medicine Science Special Project of Henan Province [2018ZYZD09 & 2021ZY2149].
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Yucheng Li, Email: Liyucheng@hactcm.edu.cn.
Erping Xu, Email: Xuerping0371@163.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Belmaker R.H., Agam G. Major depressive disorder. N. Engl. J. Med. 2008;358(1):55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 2.Willner P., Scheel-Kruger J., Belzung C. The neurobiology of depression and antidepressant action. Neurosci. Biobehav. Rev. 2013;37(10):2331–2371. doi: 10.1016/j.neubiorev.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Secretariat T. World Health Organization; 2012. Global burden of mental Disorders and the Need for a Comprehensive, Coordinated Response from Health and Social Sectors at the Country Level; p. 6. EB130. [Google Scholar]
- 4.Westergren T., Narum S., Klemp M. Critical appraisal of adverse effects reporting in the 'treatment for adolescents with depression study (TADS) BMJ Open. 2019;9(3) doi: 10.1136/bmjopen-2018-026089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malhi Gin S., John Mann J. Depression. Lancet. 2018;392(10161):2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 6.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J. Psychiatry Neurosci. 2004;29(3):185–193. [PMC free article] [PubMed] [Google Scholar]
- 7.Koo J.W., Russo S.J., Ferguson D., et al. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl. Acad. Sci. U. S. A. 2010;107(6):2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duman R.S., Monteggia L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatr. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Schildkraut J.J., Kety S.S. Biogenic amines and emotion. Science. 1967;156(3771):21–37. doi: 10.1126/science.156.3771.21. [DOI] [PubMed] [Google Scholar]
- 10.Cao X., Li L.P., Wang Q., et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med. 2013;19(6):773–777. doi: 10.1038/nm.3162. [DOI] [PubMed] [Google Scholar]
- 11.Bansal Y., Kuhad A. Mitochondrial dysfunction in depression. Curr. Neuropharmacol. 2016;14(6):610–618. doi: 10.2174/1570159X14666160229114755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma S., Akundi R.S. Mitochondria: a connecting link in the major depressive disorder Jigsaw. Curr. Neuropharmacol. 2019;17(6):550–562. doi: 10.2174/1570159X16666180302120322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Detka J., Kurek A., Kucharczyk M., et al. Brain glucose metabolism in an animal model of depression. Neuroscience. 2015;295:198–208. doi: 10.1016/j.neuroscience.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 14.Guo W., Tang Z.Y., Cai Z.Y., et al. Iptakalim alleviates synaptic damages via targeting mitochondrial ATP-sensitive potassium channel in depression. FASEB J. 2021;35(5) doi: 10.1096/fj.202100124RR. [DOI] [PubMed] [Google Scholar]
- 15.Glombik K., Detka J., Kurek A., et al. Impaired brain energy metabolism: involvement in depression and hypothyroidism. Front. Neurosci. 2020;14 doi: 10.3389/fnins.2020.586939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park D.I., Novak B., Yan Y., et al. Paroxetine binding and activation of phosphofructokinase implicates energy metabolism in antidepressant mode of action. J. Psychiatr. Res. 2020;129:8–14. doi: 10.1016/j.jpsychires.2020.05.033. [DOI] [PubMed] [Google Scholar]
- 17.Weckmann K., Deery M.J., Howard J.A., et al. Ketamine's antidepressant effect is mediated by energy metabolism and antioxidant defense system. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-16183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam R.W., Wajsbrot D.B., Meier E., et al. Effect of desvenlafaxine 50 mg and 100 mg on energy and lassitude in patients with major depressive disorder: a pooled analysis. J. Psychopharmacol. 2017;31(9):1204–1214. doi: 10.1177/0269881117719261. [DOI] [PubMed] [Google Scholar]
- 19.Tarragó T., Kichik N., Claasen B., et al. Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor. Bioorg. Med. Chem. 2008;16(15):7516–7524. doi: 10.1016/j.bmc.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 20.Shen J.D., Ma L.G., Hu C.Y., et al. Berberine up-regulates the BDNF expression in hippocampus and attenuates corticosterone-induced depressive-like behavior in mice. Neurosci. Lett. 2016;614:77–82. doi: 10.1016/j.neulet.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Sowndhararajan K., Deepa P., Kim M., et al. Neuroprotective and cognitive enhancement potentials of baicalin: a review. Brain Sci. 2018;8 doi: 10.3390/brainsci8060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z., Cheng J., Liu J. Baicalin protects human OA chondrocytes against IL-1beta-induced apoptosis and ECM degradation by activating autophagy via MiR-766-3p/AIFM1 Axis. Drug Des. Dev. Ther. 2020;14:2645–2655. doi: 10.2147/DDDT.S255823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y.C., Shen J.D., Li J., Wang R., et al. Chronic treatment with baicalin prevents the chronic mild stress-induced depressive-like behavior: involving the inhibition of cyclooxygenase-2 in rat brain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;40:138–143. doi: 10.1016/j.pnpbp.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Minhas P.S., Latif-Hernandez A., McReynolds M.R., et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature. 2021;590(7844):122–128. doi: 10.1038/s41586-020-03160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sneddon L.U., Halsey L.G., Bury N.R. Considering aspects of the 3Rs principles within experimental animal biology. J. Exp. Biol. 2017;220(Pt 17):3007–3016. doi: 10.1242/jeb.147058. [DOI] [PubMed] [Google Scholar]
- 26.Li Y.C., Wang L.L., Pei Y.Y., et al. Baicalin decreases SGK1 expression in the hippocampus and reverses depressive-like behaviors induced by corticosterone. Neuroscience. 2015;311:130–137. doi: 10.1016/j.neuroscience.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Willner P., Towell A., Sampson D., et al. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93(3):358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 28.Liu M.Y., Yin C.Y., Zhu L.J., et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018;13(7):1686–1698. doi: 10.1038/s41596-018-0011-z. [DOI] [PubMed] [Google Scholar]
- 29.Porsolt R.D., Brossard G., Hautbois C., et al. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2001 doi: 10.1002/0471142301.ns0810as14. Chapter 8, Unit 8 10A. [DOI] [PubMed] [Google Scholar]
- 30.Kahl K.G., Atalay S., Maudsley A.A., et al. Altered neurometabolism in major depressive disorder: a whole brain (1)H-magnetic resonance spectroscopic imaging study at 3T. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2020;101 doi: 10.1016/j.pnpbp.2020.109916. [DOI] [PubMed] [Google Scholar]
- 31.Ernst J., Hock A., Henning A., et al. Increased pregenual anterior cingulate glucose and lactate concentrations in major depressive disorder. Mol. Psychiatr. 2017;22(1):113–119. doi: 10.1038/mp.2016.73. [DOI] [PubMed] [Google Scholar]
- 32.Khan A.R., Hansen B., Wiborg O., et al. Diffusion MRI and MR spectroscopy reveal microstructural and metabolic brain alterations in chronic mild stress exposed rats: a CMS recovery study. Neuroimage. 2018;167:342–353. doi: 10.1016/j.neuroimage.2017.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong J., Li G., Xu H., et al. Baicalin ameliorates chronic mild stress-induced depression-like behaviors in mice and attenuates inflammatory cytokines and oxidative stress. Br. J. Med. Biol. Res. = Revista brasileira de pesquisas medicas e biologicas. 2019;52(7) doi: 10.1590/1414-431X20198434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H., Cong X., Sui J., et al. Baicalin enhances the thermotolerance of mouse blastocysts by activating the ERK1/2 signaling pathway and preventing mitochondrial dysfunction. Theriogenology. 2022;178:85–94. doi: 10.1016/j.theriogenology.2021.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Karabatsiakis A., Schonfeldt-Lecuona C. Depression, mitochondrial bioenergetics, and electroconvulsive therapy: a new approach towards personalized medicine in psychiatric treatment - a short review and current perspective. Transl. Psychiatry. 2020;10(1):226. doi: 10.1038/s41398-020-00901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolar D., Kleteckova L., Brozka H., et al. Mini-review: brain energy metabolism and its role in animal models of depression, bipolar disorder, schizophrenia and autism. Neurosci. Lett. 2021:136003. doi: 10.1016/j.neulet.2021.136003. [DOI] [PubMed] [Google Scholar]
- 37.Jun M., Xiaolong Q., Chaojuan Y., et al. Calhm2 governs astrocytic ATP releasing in the development of depression-like behaviors. Mol. Psychiatr. 2018;23(4):1091. doi: 10.1038/mp.2017.254. [DOI] [PubMed] [Google Scholar]
- 38.Luo J., Tang C., Chen X., et al. Impacts of aerobic exercise on depression-like behaviors in chronic unpredictable mild stress mice and related factors in the AMPK/PGC-1 alpha pathway. Int. J. Environ. Res. Publ. Health. 2020;17(6):2042. doi: 10.3390/ijerph17062042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu X., Ke S., Wang Q., et al. Energy metabolism in major depressive disorder: recent advances from omics technologies and imaging. Biomed. Pharmacother. 2021;141 doi: 10.1016/j.biopha.2021.111869. [DOI] [PubMed] [Google Scholar]
- 40.Xie X., Shen Q., Yu C., et al. Depression-like behaviors are accompanied by disrupted mitochondrial energy metabolism in chronic corticosterone-induced mice. J. Steroid Biochem. Mol. Biol. 2020;200 doi: 10.1016/j.jsbmb.2020.105607. [DOI] [PubMed] [Google Scholar]
- 41.Bell S.M., Burgess T., Lee J., et al. Peripheral glycolysis in neurodegenerative diseases. Int. J. Mol. Sci. 2020;21(23) doi: 10.3390/ijms21238924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hipkiss A.R. Aging, alzheimer's disease and dysfunctional glycolysis; similar effects of too much and too little. Aging Dis. 2019;10(6):1328–1331. doi: 10.14336/AD.2019.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Streck E.L., Goncalves C.L., Furlanetto C.B., et al. Mitochondria and the central nervous system: searching for a pathophysiological basis of psychiatric disorders. Br. J. Psychiatry. 2014;36(2):156–167. doi: 10.1590/1516-4446-2013-1224. [DOI] [PubMed] [Google Scholar]
- 44.Gong Y., Chai Y., Ding J.H., et al. Chronic mild stress damages mitochondrial ultrastructure and function in mouse brain. Neurosci. Lett. 2011;488(1):76–80. doi: 10.1016/j.neulet.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Ling-Hu T., Liu S.B., Gao Y., et al. Stable isotope-resolved metabolomics reveals the abnormal brain glucose catabolism in depression based on chronic unpredictable mild stress rats. J. Proteome Res. 2021;20(7):3549–3558. doi: 10.1021/acs.jproteome.1c00155. [DOI] [PubMed] [Google Scholar]
- 46.Shao W.H., Chen J.J., Fan S.H., et al. Combined metabolomics and proteomics analysis of major depression in an animal model: perturbed energy metabolism in the chronic mild stressed rat cerebellum. OMICS. 2015;19(7):383–392. doi: 10.1089/omi.2014.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sousa J.S., D'Imprima E., Vonck J. Mitochondrial respiratory chain complexes. Subcell. Biochem. 2018;87:167–227. doi: 10.1007/978-981-10-7757-9_7. [DOI] [PubMed] [Google Scholar]
- 48.Rezin G.T., Goncalves C.L., Daufenbach J.F., et al. Acute administration of ketamine reverses the inhibition of mitochondrial respiratory chain induced by chronic mild stress. Brain Res. Bull. 2009;79(6):418–421. doi: 10.1016/j.brainresbull.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Villa R.F., Ferrari F., Bagini L., et al. Mitochondrial energy metabolism of rat hippocampus after treatment with the antidepressants desipramine and fluoxetine. Neuropharmacology. 2017;121:30–38. doi: 10.1016/j.neuropharm.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 50.Willems P.H., Rossignol R., Dieteren C.E., et al. Redox homeostasis and mitochondrial dynamics. Cell Metabol. 2015;22(2):207–218. doi: 10.1016/j.cmet.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Tang B.L. Sirt1 and the mitochondria. Mol. Cell. 2016;39(2):87–95. doi: 10.14348/molcells.2016.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lo Iacono L., Visco-Comandini F., Valzania A., et al. Adversity in childhood and depression: linked through SIRT1. Transl. Psychiatry. 2015;5:e629. doi: 10.1038/tp.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li P.A., Hou X., Hao S. Mitochondrial biogenesis in neurodegeneration. J. Neurosci. Res. 2017;95(10):2025–2029. doi: 10.1002/jnr.24042. [DOI] [PubMed] [Google Scholar]
- 54.Jia L., Wang J., Cao H., et al. Activation of PGC-1alpha and mitochondrial biogenesis protects against prenatal hypoxic-ischemic brain injury. Neuroscience. 2020;432:63–72. doi: 10.1016/j.neuroscience.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 55.Fanibunda S.E., Deb S., Maniyadath B., et al. Serotonin regulates mitochondrial biogenesis and function in rodent cortical neurons via the 5-HT2A receptor and SIRT1-PGC-1alpha axis. Proc. Natl. Acad. Sci. U. S. A. 2019;116(22):11028–11037. doi: 10.1073/pnas.1821332116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.