Abstract
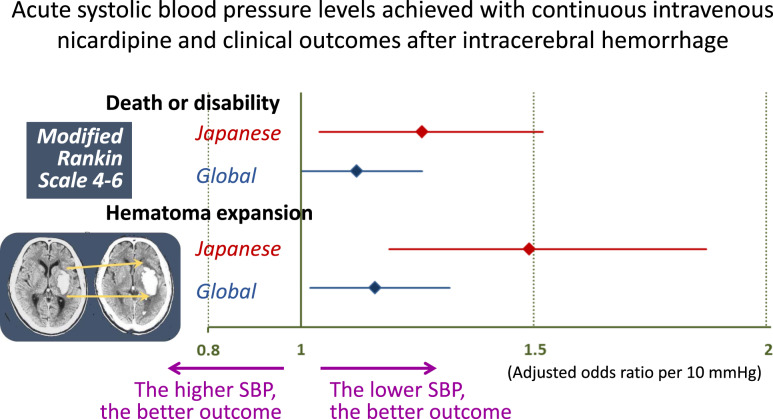
The effects of acute systolic blood pressure levels achieved with continuous intravenous administration of nicardipine for Japanese patients with acute intracerebral hemorrhage on clinical outcomes were determined. A systematic review and individual participant data analysis of articles were performed based on prospective studies involving adults developing hyperacute intracerebral hemorrhage who were treated with intravenous nicardipine. Outcomes included death or disability at 90 days, defined as the modified Rankin Scale score of 4–6, and hematoma expansion, defined as an increase 6 mL or more from baseline to 24 h computed tomography. Of the total 499 Japanese patients (age 64.9 ± 11.8 years, 183 women, initial BP 203.5 ± 18.3/109.1 ± 17.2 mmHg) studied, death or disability occurred in 35.6%, and hematoma expansion occurred in 15.6%. Mean hourly systolic blood pressure during the initial 24 h was positively associated with death or disability (adjusted odds ratio 1.25, 95% confidence interval 1.03–1.52 per 10 mmHg) and hematoma expansion (1.49, 1.18–1.87). These odds ratios were relatively high as compared to the reported ones for overall global patients of this individual participant data analysis [1.12 (95% confidence interval 1.00–1.26) and 1.16 (1.02–1.32), respectively]. In conclusion, lower levels of systolic blood pressure by continuous intravenous nicardipine were associated with lower risks of hematoma expansion and 90-day death or disability in Japanese patients with hyperacute intracerebral hemorrhage. The impact of systolic blood pressure lowering on better outcome seemed to be stronger in Japanese patients than the global ones.
Keywords: Acute stroke, Antihypertensive therapy, Blood pressure, Hypertension, Intracranial hemorrhage
Introduction
Acute lowering of elevated blood pressure (BP) is one of the few promising therapies for acute intracerebral hemorrhage (ICH) [1–3] that reportedly has relatively high incidence in Asia, including Japan [4]. Nicardipine, a dihydropyridine-derived calcium channel blocker is rapidly titratable, has a short half-life and few side effects, and produces peripheral arterial vasodilatation without affecting cardiac conduction pathways and, accordingly, has a low risk of bradydysrhythmias [5]. Thus, this agent is recommended as a first-line agent for acute cerebrovascular emergencies. We performed a meta-analysis using individual participant data (IPD) and showed that a lower level of mean hourly systolic BP (SBP) achieved during the initial 24 h by continuous intravenous administration of nicardipine was associated with lower risks of early hematoma expansion and 90-day death or disability in acute ICH [6]. In the study, SBP was especially strongly associated with the both risks in Asian participants, showing significant treatment-by-subgroup (Asians versus non-Asians) interactions.
Japanese participants accounted for 39% of overall participants in the meta-analysis. In the present study using the same IPD database, we determined the effects of acute SBP levels achieved with intravenous nicardipine for Japanese ICH patients on clinical outcomes.
Methods
This meta-analysis using IPD is registered with PROSPERO (CRD42020213857). Details of the study design have been reported previously [6]. Briefly, PubMed was searched for relevant articles published before October 1, 2020 with the search terms regarding ICH, BP, and nicardipine. Prospectively-registered clinical trials or observational studies involving adults who started to receive intravenous injection of nicardipine within 12 h after onset of ICH, whose hourly SBP levels were available during the first 24 h, and whose functional outcome at 90 days or later was assessed using the modified Rankin Scale (mRS, ranging from 0 to 6 with higher scores indicating worse functional disability) with >20 patients enrolled were eligible for inclusion. Two reviewers (SY and MF-D) independently reviewed articles for inclusion.
Ethical approval was obtained from all participating sites for all studies included in the IPD analysis, and patients or their legal representatives provided written, informed consent according to national and local regulations. Ethical approval to perform the present IPD analysis was obtained from the site of the corresponding author. All data in the dataset are de-identified.
The primary efficacy outcome was moderately severe or severe disability or death at 90 days as defined by an mRS score of 4–6, hereafter referred to as “death or disability”. Only patients with the prestroke mRS score <4 were included for this analysis. The secondary efficacy outcome was hematoma expansion, defined as an increase in volume >6 cm3 from baseline to the 24 h CT scan [7]. Safety outcomes were any serious adverse events (SAEs) within 90 days and SAEs related to cardiac and renal dysfunction within 72 h.
Statistical analysis
The mean level of hourly SBP between 1 h and 24 h after entry/randomization was determined in each patient. When SBP was measured more than once in an hour, the average value was taken as the hourly level. Patients with SBP data available for <4 timepoints were excluded from the analysis.
As an IPD meta-analysis to determine any significant independent associations of mean hourly SBP levels or their quartile levels with outcomes, the logistic regression model was adjusted for the variables used in our previous studies; [6] for sex, age, and study groups (model 1) or further for baseline SBP, baseline National Institutes of Health Stroke Scale (NIHSS) score (ranging from 0 to 42 with higher scores indicating worse neurological deficits), baseline hematoma volume, hematoma site (lobar or not), and onset-to-randomization time (<180 min or ≥180 min, model 2). Patients with unavailable data for the 90-day mRS score and those undergoing surgical procedures before the 24 h CT were excluded from the initial analysis of the primary and secondary efficacy outcomes, respectively. As the additional analyses, the former patients were regarded as having mRS scores of 4–6 and the latter were regarded as having hematoma expansion and included in the analyses. Effect modification by predefined subgroups on the association between mean hourly SBP and outcomes was identified by logistic regression using the multiplicative interaction terms (SBP × subgroups).
A value of p < 0.15 was considered significant for assessment of the treatment-by-subgroup interaction. In the other comparisons, a value of p < 0.05 was considered significant. Statistical analysis was performed using JMP version 16 software (SAS Institute).
Results
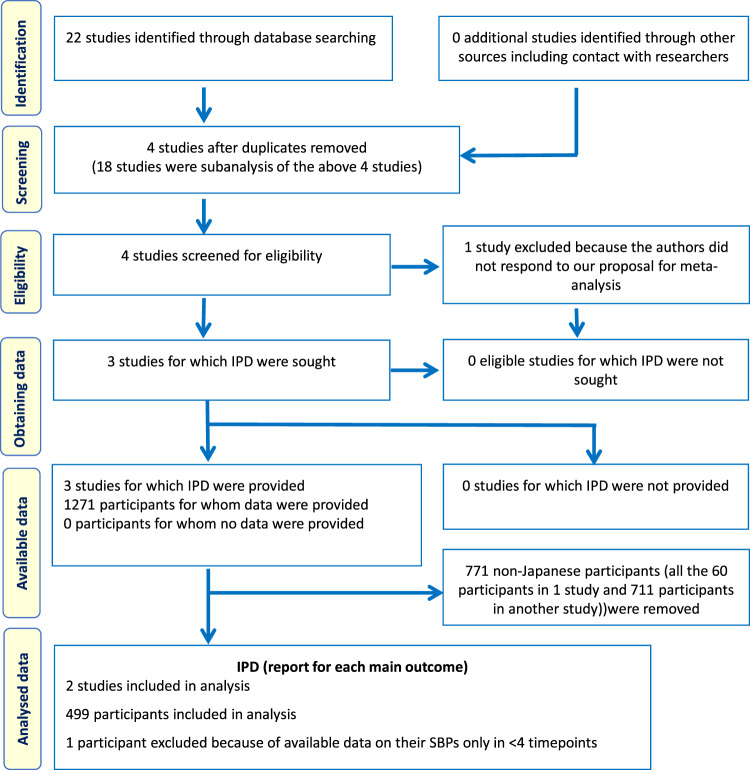
Three studies met the eligibility criteria: Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH)-1 [8, 9], ATACH-2 [2, 10], and Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-IntraCerebral Hemorrhage (SAMURAI-ICH, Fig. 1) [11]. Since ATACH-1 did not include Japanese patients, the present analysis was performed using the other two. Both studies enrolled adult patients with hyperacute spontaneous supratentorial ICH whose initial parenchymal hematoma volume was <60 mL, initial SBP was >180 mmHg, and initial Glasgow Coma Scale score was ≥5 and treated them with intravenous nicardipine during the initial 24 h using the titration method common to both studies (Supplementary Table 1). SBP was measured at least every 15 min during the first hour and every 15–30 min in ATACH-2 and at least every 60 min in SAMURAI-ICH during the remainder of the first 24 h. Major differences in methods were maximal allowed time from symptom onset to initiation of nicardipine treatment (4.5 h in ATACH-2 and 3 h in SAMURAI-ICH) and target SBP range during the first 24 h (two arms of 140–179 and 110–139 mmHg in ATACH-2 and a single arm of 120–160 mmHg in SAMURAI-ICH).
Fig. 1.
PRISMA individual participant data flow diagram
All 211 patients enrolled in SAMURAI-ICH and 289 of 1000 patients enrolled in ATACH-2 were Japanese. Of these, one patient with available data on their hourly SBPs only for <4 timepoints from ATACH-2 was excluded. Thus, 499 patients (183 women, 64.9 ± 11.8 years of age) were finally analyzed. The baseline characteristics of the patients are shown in Table 1. The trend of hourly SBP is shown in Supplementary Fig. 1. Mean baseline systolic/diastolic BP was 203.5 ± 18.3/109.1 ± 17.2 mmHg. Median hourly SBP between 1 h and 24 h was 134.3 mmHg (interquartile range 127.5–145.1 mmHg); these three values were the cutoffs of the SBP quartiles.
Table 1.
Patients’ baseline characteristics
| N = 499 | |
|---|---|
| Women | 183 (36.7%) |
| Age, y | 64.9 ± 11.8 |
| Asian race | 499 (100%) |
| History of stroke/transient ischemic attack | 66 (13.2%) |
| Hypertension | 403 (80.9%) |
| Dyslipidaemia | 125 (25.2%) |
| Diabetes mellitus | 70 (14.1%) |
| Current smoking | 153 (30.7%) |
| Glasgow Coma Scale score | 15 [13–15] |
| National Institutes of Health Stroke Scale score | 11 [7–16] |
| Hematoma volume, mL | 9.4 [5.0–18.1] |
| Hematoma location | |
| Lobar | 32 (6.4%) |
| Basal gangliaa | 271 (54.3%) |
| Thalamus | 197 (39.4%) |
| Intraventricular hemorrhage | 104 (20.8%) |
| Onset-to-randomization, min | 120 [90–165] |
n (%), mean ± standard deviation or median [interquartile range]
aMainly putaminal hematoma. Patients with mix hematoma in both putamen and thalamus is counted here. A patient with multiple hematomas in putamen, thalamus, and lobe is counted twice in “Lobar” and “Basal ganglia”
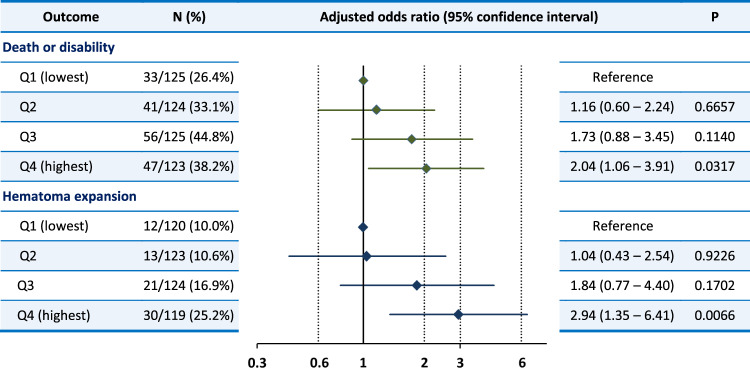
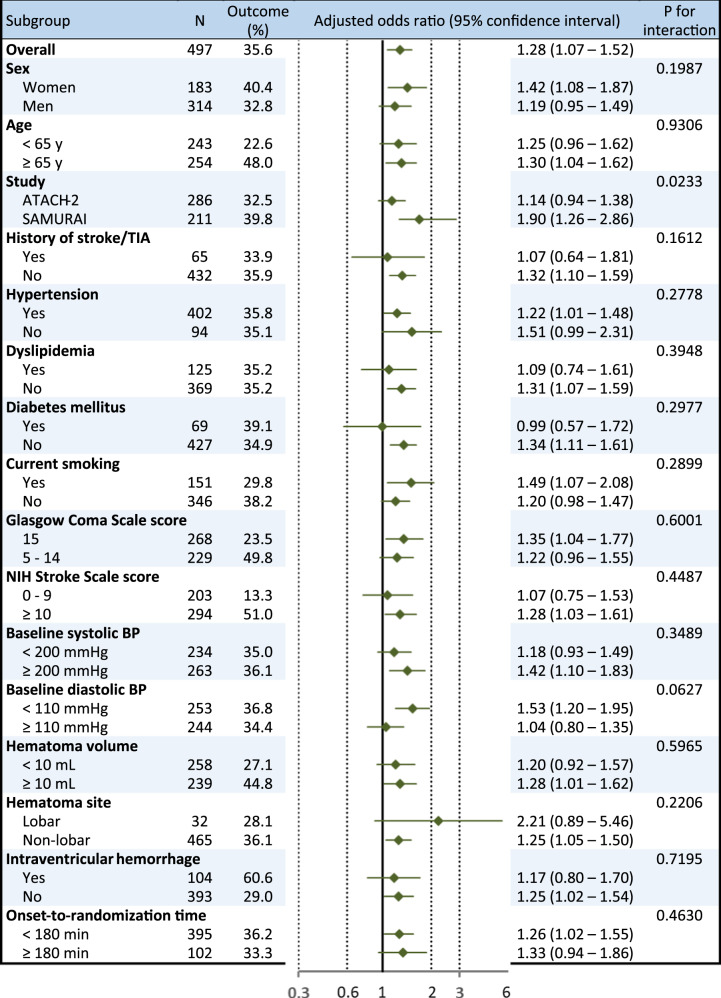
At 90 days, the mRS score was 0 in 30 patients, 1 in 93, 2 in 98, 3 in 99, 4 in 142, 5 in 29, 6 (death) in 7, and unavailable in 2. The proportion for the primary efficacy outcome of death or disability was 35.6% after exclusion of 2 patients with prestroke mRS of 4 (Table 2). After multivariable adjustment, a higher mean hourly SBP significantly increased the risk of death or disability [adjusted odds ratio (aOR) 1.25, 95% confidence interval (CI) 1.03–1.52 per 10 mmHg in model 2, Table 2]. The result was almost identical when two patients with unavailable data for the mRS score were regarded as having mRS scores of 4–6 and included. In the analysis using the quartiles of mean hourly SBP, the risk of death or disability gradually increased as SBP levels increased with the significant difference between the highest and lowest quartiles (aOR 2.04, 95% CI 1.06–3.91, Fig. 2). Study groups and diastolic BP modified the association between mean hourly SBP and the outcome (Fig. 3). Higher mean hourly SBP significantly increased the risk of death or disability in SAMURAI-ICH participants (aOR 1.90, 95% CI 1.26–2.86) and patients with diastolic BP < 110 mmHg (1.53, 1.20–1.95).
Table 2.
The association of mean hourly systolic blood pressure between 1 and 24 h with outcomes
| Outcome | N (%) | Crude | Adjusted. Model 1 | Adjusted. Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Odds ratioa | 95% CIa | P | Odds ratioa | 95% CIa | P | Odds ratioa | 95% CIa | P | ||
| Death or disability | 176/495 (35.6%) | 1.17 | 1.00–1.37 | 0.0507 | 1.28 | 1.07–1.52 | 0.0051 | 1.25 | 1.03–1.52 | 0.0224 |
| Adding 2 patients without available datab | 178/497 (35.8%) | 1.17 | 1.00–1.36 | 0.0538 | 1.28 | 1.08–1.52 | 0.0050 | 1.25 | 1.03–1.52 | 0.0221 |
| Hematoma expansion | 76/486 (15.6%) | 1.51 | 1.22–1.86 | 0.0001 | 1.45 | 1.16–1.81 | 0.0011 | 1.49 | 1.18–1.87 | 0.0005 |
| Adding 13 patients with emergent surgeryc | 89/499 (17.8%) | 1.44 | 1.18–1.75 | 0.0003 | 1.38 | 1.13–1.68 | 0.0015 | 1.40 | 1.14–1.73 | 0.0014 |
| Serious adverse events | 88/499 (17.6%) | 1.07 | 0.88–1.30 | 0.5114 | 1.11 | 0.90–1.36 | 0.3418 | 1.08 | 0.87–1.34 | 0.4892 |
| Cardio-renal serious adverse events | 7/499 (1.4%) | 1.02 | 0.54–1.92 | 0.9478 | — | — | ||||
Multivariable analysis is not done for cardio-renal serious adverse events because of a small event number
Model 1: adjusted for sex, age, and study group
Model 2: adjusted for sex, age, study group, baseline systolic blood pressure, baseline National Institutes of Health Stroke Scale score, baseline hematoma volume, lobar hematoma, and onset-to-randomization time
aper 10 mmHg
b2 patients added are regarded as having death or disability
c13 patients added are regarded as having hematoma expansion
Fig. 2.
The association of mean hourly systolic blood pressure quartiles with outcomes Systolic blood pressure range: Q1, <127.5 mmHg; Q2, 127.5–134.3 mmHg; Q3, 134.3–145.1 mmHg; Q4, ≥145.1 mmHg. Adjusted for sex, age, study group, baseline systolic blood pressure, baseline National Institutes of Health Stroke Scale score, baseline hematoma volume, lobar hematoma, and onset-to-randomization time (model 2)
Fig. 3.
Associations of mean hourly systolic blood pressure with death or disability by subgroups. Adjusted for sex, age, and study groups
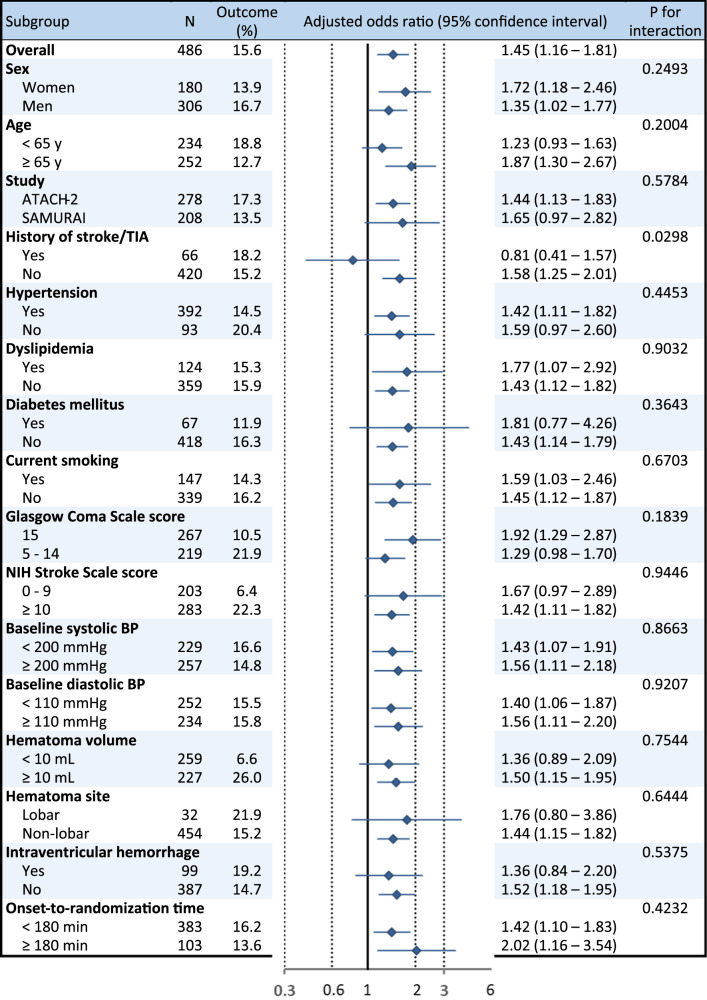
The secondary efficacy outcome of hematoma expansion was not assessed in 13 patients due to emergent surgery within 24 h that might affect hematoma size. The proportion of hematoma expansion in the other 486 patients was 15.6% (Table 2). Mean hourly SBP was positively and significantly associated with the risk of hematoma expansion (aOR 1.49, 95% CI 1.18–1.87 per 10 mmHg in model 2). The result was similar when all 13 patients with emergent surgery were regarded as having hematoma expansion (1.40, 1.14–1.73). In the analysis using the quartiles of mean hourly SBP, the risk of hematoma expansion gradually increased as SBP levels increased with the significant difference between the highest and lowest quartiles (aOR 2.94, 95% CI 1.35–6.41, Fig. 2). History of stroke/transient ischemic attack showed significant treatment-by-subgroup interactions in relation to hematoma expansion (Fig. 4).
Fig. 4.
Associations of mean hourly systolic blood pressure with hematoma expansion by subgroups. Adjusted for sex, age, and study groups
SAEs occurred in 17.6% within 90 days and cardio-renal SAEs in 1.4% within 72 h among all 499 patients (Table 2). Mean hourly SBP was not significantly associated with either any SAEs or cardio-renal SAEs.
Discussion
In the present IPD meta-analysis, the association of acute SBP levels achieved with continuous intravenous administration of nicardipine for Japanese ICH patients on clinical outcomes were explored. The major finding was that a lower level of mean hourly SBP achieved during the initial 24 h by nicardipine was associated with lower risks of hematoma expansion defined as an increase in volume >6 cm3 on the 24 h CT scan and moderately severe or severe disability or death at 90 days as defined by an mRS score of 4–6. The similar findings were obtained for both of these two outcomes in the analysis for the categorized mean hourly SBP level. The risk of SAEs did not change according to achieved SBP. The finding had been also proven in overall global ICH patients registered in the same IPD meta-analysis [6]. Interestingly, the impact of SBP lowering on better outcome seemed to be stronger in Japanese patients than the global ones.
Two large, global, randomized, controlled trials, the Second Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT2) and the ATACH-2 trial, were published in succession in the mid-2010’s [1, 2]. INTERACT2 demonstrated possibly better functional outcomes for acute ICH patients with early intensive lowering to a target SBP of <140 mmHg than the standard SBP target of <180 mmHg (odds ratio 0.87, 95% CI 0.75–1.01) [1]. In contrast, ATACH-2 did not show benefit in reducing the rate of death or disability between the two treatment groups with the same target SBP level as INTERACT2 (relative risk with intensive treatment 1.04, 95% CI 0.85–1.27) [2]. In a pooled analysis of these two trials, the level of SBP in the initial 24 h was continuously associated with the distribution of the mRS scores at 90 days [12]. SAMURAI-ICH was a kind of pilot study of ATACH-2 that aimed to prove the safety of nicardipine use in Japanese ICH patients in the period when intravenous nicardipine for hyperacute ICH patients was limited on the official label in Japan without scientific evidence [3, 11]. Study designs of SAMURAI-ICH were accordingly similar with that of ATACH-2.
Hematoma expansion was reported to greatly attenuate by deep and rapid SBP lowering [12–15]. The positive and significant association between mean hourly SBP and the risk of hematoma expansion was also evident in the overall participants of the present IPD meta-analysis mainly from the United States and East Asia [6]. The impact of the association was somewhat different; the aOR was 1.16 (95% CI 1.02–1.32 per 10 mmHg) in the overall result and 1.49 (1.18–1.87) in the present one limited to Japanese patients. As compared to the overall participants, Japanese had relatively short time delay from onset to randomization (median 120 min versus 168 min), suggesting the higher rate of active continuous bleeding at randomization in the Japanese cohort [16–18]. For such patients with short time delay, intensive SBP lowering may be much protective against hematoma expansion. In the analysis of SAMURAI-ICH alone, the lowest quartile of time from imaging to target SBP < 160 mmHg (<38 min) was negatively associated with hematoma expansion [19]. When the present patients <120 min time delay were separately analyzed, the aOR for the association of SBP with hematoma expansion was 1.60 (95% CI 1.09–2.36). In addition, etiological mechanism of spontaneous ICH is considered to be more hypertensive and less amyloid-angiopathic in Japanese than western population [20]. This may be another reason for strong association of SBP level and hematoma expansion.
The impact of the association between mean hourly SBP and the risk of death or disability at 90 days was also somewhat stronger in the present Japanese patients (aOR 1.25, 95% CI 1.03–1.52) relative to the overall participants of the IPD meta-analysis (1.12, 1.00–1.26) [6]. It seems to reflect the known finding that hematoma expansion is strongly related to poor functional outcomes [21]. When the present patients with <120 min time delay were separately analyzed, the aOR for the association of SBP with death or disability was 1.63 (95% CI 1.19–2.21) and showed a significant treatment-by-subgroup interaction as compared to those ≥120 min delay (aOR 1.14, 95% CI 0.93–1.41, P for interaction = 0.1211).
Subgroup analyses shown in Figs. 3 and 4 did not show the similar tendency. Although history of stroke/transient ischemic attack modified the association of mean hourly SBP with hematoma expansion, it did not modify those with death or disability. A small number of patients with the history might cause the statistical bias. Subgroups divided by baseline diastolic BP level (<110 mmHg versus ≥110 mmHg) had the similar association of SBP with hematoma expansion, but showed a significant treatment-by-subgroup interaction in relation to death or disability. The similar finding was shown in the global participants of the IPD meta-analysis [6].
The limitations of the IPD meta-analysis used here were described elsewhere, including the difficulty in generalization of results to very severe ICH patients because of exclusion of patients with supratentorial hemorrhage ≥60 mL in volume and those with infratentorial hemorrhage from the study [6]. In addition, merging the datasets of the randomized ATACH-2 and non-randomized SAMURAI-ICH together might cause potential bias. In the present subgroup analysis, the association of SBP with death or disability differed between ATACH-2 and SAMURAI-ICH (P for interaction = 0.0233), although the association of SBP with hematoma expansion was not so different between the two studies.
In conclusion, the present Japanese patients with acute ICH extracted from the database of our IPD meta-analysis showed somewhat stronger association of the lower mean SBP level within the initial 24 h with better imaging and functional outcomes as compared to the global cohort from the same meta-analysis. Acute SBP lowering was not associated with the increase in SAEs. However, we should still take care of the previous lesson from ATACH-2 that intensive SBP lowering is potentially harmful among patients with a decreased estimated glomerular filtration rate, that are relatively common in Japanese [22].
Supplementary information
Funding
Partly funded by Japan Agency for Medical Research and Development (AMED: JP22lk0201094 and JP22lk0201109).
the ATACH Trial Investigators
Adnan I. Qureshi4, Yuko Y. Palesch5, Kazunori Toyoda6, Kazuyuki Nagatsuka6, Masatoshi Koga6, Masafumi Ihara6, Yongjun Wang7, Nobuyuki Sakai8, Takayuki Hara9, Zhimin Wang10, Jiann-Shing Jeng11, Sachin Agarwal12, Kiwon Lee12, Stephan A. Mayer12, M. Fareed K. Suri13, Qaisar A. Shah14, Jawad F. Kirmani15, Adnan I. Qureshi16, Haitham Hussein16, Jill M. Novitzke16, Cathie Witzel16, Bo Connelly16, Saqib A. Chaudhry16, Emily I. Abbott16, Erik T. Maland16, Kathryn A. France16, Basit Rahim16, Zachariah Miller16, Alfredo J. Caceres16, Logan J. Brau16, Mushtaq H. Qureshi16, Jessy K. Thomas16, Mohammad R. Afzal16, Norrita Rech16, Yuko Y. Palesch17, Renee Martin17, Wenle Zhao17, Lydia Foster17, Jaime Speiser17, Catherine Dillon17, Jaemyung Kim17, Cassidy Conner17, Adam Henry17, Kristina Hill17, Kristen Clasen17, Christy Cassarly17, Daniel F. Hanley18, Carlos S. Kase18, J. Ricardo Carhuapoma18, Nichol McBee18, Claudia Moy19, Scott Janis19, J. Claude HemphillIII19, Brian L. Hoh19, Mario Zucharello19, Michael K. Parides19
the SAMURAI Investigators
Kazunori Toyoda6, Kazuomi Kario20, Michito Namekawa20, Jyoji Nakagawara21, Kenji Kamiyama21, Eisuke Furui22, Ryo Itabshi22, Yukako Yazawa22, Yoshiaki Shiokawa23, Kazutoshi Nishiyama23, Yasuhiro Hasegawa24, Hisanao Akiyama24, Satoshi Okuda25, Tomoko Noda25, Hioshi Yamagami26, Kenichi Todo26, Kazumi Kimura27, Kensaku Shibazaki27, Yoshiki Yagita27, Yasushi Okada28, Tomonaga Matsushita28, Kazuyuki Nagatsuka6, Takanari Kitazono29, Teruyuki Hirano30, Masatoshi Koga6, Shoji Arihiro6, Shoichiro Sato6, Masaki Naganuma6, Koichiro Maeda6, Mayumi Mori6, Tomohisa Nezu6, Tetsuya Miyagi6, Kaoru Endo6, Masato Osaki6, Junpei Kobayashi6, Takuya Okata6, Yuki Sakamoto6, Eijirou Tanaka6, Haruka Kanai6, Azusa Tokunaga6, Kazuo Minematsu6
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Members of the ATACH Trial Investigators and the SAMURAI Investigators are listed below Funding.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kazunori Toyoda, Email: toyoda@ncvc.go.jp.
the ATACH Trial Investigators:
Adnan I. Qureshi, Yuko Y. Palesch, Kazunori Toyoda, Kazuyuki Nagatsuka, Masatoshi Koga, Masafumi Ihara, Yongjun Wang, Nobuyuki Sakai, Takayuki Hara, Zhimin Wang, Jiann-Shing Jeng, Sachin Agarwal, Kiwon Lee, Stephan A. Mayer, M Fareed K. Suri, Qaisar A. Shah, Jawad F. Kirmani, Adnan I. Qureshi, Haitham Hussein, Jill M. Novitzke, Cathie Witzel, Bo Connelly, Saqib A. Chaudhry, Emily I. Abbott, Erik T. Maland, Kathryn A. France, Basit Rahim, Zachariah Miller, Alfredo J. Caceres, Logan J. Brau, Mushtaq H. Qureshi, Jessy K. Thomas, Mohammad R. Afzal, Norrita Rech, Yuko Y. Palesch, Renee Martin, Wenle Zhao, Lydia Foster, Jaime Speiser, Catherine Dillon, Jaemyung Kim, Cassidy Conner, Adam Henry, Kristina Hill, Kristen Clasen, Christy Cassarly, Daniel F. Hanley, Carlos S. Kase, J. Ricardo Carhuapoma, Nichol McBee, Claudia Moy, Scott Janis, J. Claude Hemphill, III, Brian L. Hoh, Mario Zucharello, and Michael K. Parides
the SAMURAI Investigators:
Kazuomi Kario, Michito Namekawa, Jyoji Nakagawara, Kenji Kamiyama, Eisuke Furui, Ryo Itabshi, Yukako Yazawa, Yoshiaki Shiokawa, Kazutoshi Nishiyama, Yasuhiro Hasegawa, Hisanao Akiyama, Satoshi Okuda, Tomoko Noda, Hioshi Yamagami, Kenichi Todo, Kazumi Kimura, Kensaku Shibazaki, Yoshiki Yagita, Yasushi Okada, Tomonaga Matsushita, Takanari Kitazono, Teruyuki Hirano, Shoji Arihiro, Shoichiro Sato, Masaki Naganuma, Koichiro Maeda, Mayumi Mori, Tomohisa Nezu, Tetsuya Miyagi, Kaoru Endo, Masato Osaki, Junpei Kobayashi, Takuya Okata, Yuki Sakamoto, Eijirou Tanaka, Haruka Kanai, Azusa Tokunaga, and Kazuo Minematsu
Supplementary information
The online version contains supplementary material available at 10.1038/s41440-022-01046-4.
References
- 1.Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–65. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Palesch YY, Barsan WG, Hanley DF, Hsu CY, Martin RL, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375:1033–43. doi: 10.1056/NEJMoa1603460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toyoda K, Koga M. as the SAMURAI Investigators. Controlling blood pressure soon after intracerebral hemorrhage: The SAMURAI-ICH Study and its successors. Hypertens Res. 2022;45:583–90. doi: 10.1038/s41440-022-00866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1:e259–81. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens WB. Blood pressure control in acute cerebrovascular disease. J Clin Hypertens (Greenwich) 2011;13:205–11. doi: 10.1111/j.1751-7176.2010.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toyoda K, Yoshimura S, Fukuda-Doi M, Qureshi AI, Martin RH, Palesch YY, et al. Intensive blood pressure lowering with nicardipine and outcomes after intracerebral hemorrhage: An individual participant data systematic review. Int J Stroke. 2022;17:494–505. doi: 10.1177/17474930211044635. [DOI] [PubMed] [Google Scholar]
- 7.Al-Shahi Salman R, Frantzias J, Lee RJ, Lyden PD, Battey TWK, Ayres AM, et al. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol. 2018;17:885–94. doi: 10.1016/S1474-4422(18)30253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qureshi AI. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH): rationale and design. Neurocrit Care. 2007;6:56–66. doi: 10.1385/NCC:6:1:56. [DOI] [PubMed] [Google Scholar]
- 9.Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) investigators. Antihypertensive treatment of acute cerebral hemorrhage. Crit Care Med. 2010;38:637–48. doi: 10.1097/CCM.0b013e3181b9e1a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care. 2011;15:559–76. doi: 10.1007/s12028-011-9538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koga M, Toyoda K, Yamagami H, Okuda S, Okada Y, Kimura K, et al. Systolic blood pressure lowering to 160 mmHg or less using nicardipine in acute intracerebral hemorrhage: a prospective, multicenter, observational study (the Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-Intracerebral Hemorrhage study) J Hypertens. 2012;30:2357–64. doi: 10.1097/HJH.0b013e328359311b. [DOI] [PubMed] [Google Scholar]
- 12.Moullaali TJ, Wang X, Martin RH, Shipes VB, Robinson TG, Chalmers J, et al. Blood pressure control and clinical outcomes in acute intracerebral haemorrhage: a preplanned pooled analysis of individual participant data. Lancet Neurol. 2019;18:857–64. doi: 10.1016/S1474-4422(19)30196-6. [DOI] [PubMed] [Google Scholar]
- 13.Arima H, Huang Y, Wang JG, Heeley E, Delcourt C, Parsons M, et al. Earlier blood pressure-lowering and greater attenuation of hematoma growth in acute intracerebral hemorrhage: INTERACT pilot phase. Stroke. 2012;43:2236–8. doi: 10.1161/STROKEAHA.112.651422. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto Y, Koga M, Yamagami H, Okuda S, Okada Y, Kimura K, et al. Systolic blood pressure after intravenous antihypertensive treatment and clinical outcomes in hyperacute intracerebral hemorrhage: the Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-IntraCerebral Hemorrhage study. Stroke. 2013;44:1846–51. doi: 10.1161/STROKEAHA.113.001212. [DOI] [PubMed] [Google Scholar]
- 15.Carcel C, Wang X, Sato S, Stapf C, Sandset EC, Delcourt C, et al. Degree and timing of intensive blood pressure lowering on hematoma growth in intracerebral hemorrhage: Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial-2 results. Stroke. 2016;47:1651–3. doi: 10.1161/STROKEAHA.116.013326. [DOI] [PubMed] [Google Scholar]
- 16.Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.STR.28.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Broderick JP, Brott TG, Tomsick T, Barsan W, Spilker J. Ultra-early evaluation of intracerebral hemorrhage. J Neurosurg. 1990;72:195–9. doi: 10.3171/jns.1990.72.2.0195. [DOI] [PubMed] [Google Scholar]
- 18.Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27:1783–7. doi: 10.1161/01.STR.27.10.1783. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi Y, Koga M, Sato S, Yamagami H, Todo K, Okuda S, et al. Early achievement of blood pressure lowering and hematoma growth in acute intracerebral hemorrhage: Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-Intracerebral Hemorrhage Study. Cerebrovasc Dis. 2018;46:118–24. doi: 10.1159/000492728. [DOI] [PubMed] [Google Scholar]
- 20.Yakushiji Y, Tanaka J, Wilson D, Charidimou A, Noguchi T, Kawashima M, et al. Proportion of intracerebral haemorrhage due to cerebral amyloid angiopathy in the East and West: Comparison between single hospital centres in Japan and the United Kingdom. J Neurol Sci. 2020;416:117037. doi: 10.1016/j.jns.2020.117037. [DOI] [PubMed] [Google Scholar]
- 21.Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–81. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda-Doi M, Yamamoto H, Koga M, Doi Y, Qureshi AI, Yoshimura S, et al. Impact of renal impairment on intensive blood-pressure-lowering therapy and outcomes in intracerebral hemorrhage: results from ATACH-2. Neurology. 2021;97:e913–e921. doi: 10.1212/WNL.0000000000012442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.