Highlights
-
•
Real-world adherence to cancer screening remained suboptimal and low over time.
-
•
Notable screening disparities were observed among certain subpopulations.
-
•
Efforts to increase screening and reduce cancer health disparities remain critical.
Keywords: Cancer, Screening, Adherence, Cancer prevention, Early detection
Abstract
This study aimed to comprehensively assess breast, colorectal, cervical, lung, and prostate cancer screening rates and trends in the United States over time among individuals for whom screening is recommended by the United States Preventive Services Task Force (USPSTF). This retrospective study was conducted in two-year intervals from January 1, 2008 to February 29, 2020, using Optum’s de-identified Clinformatics® Data Mart Database, which includes Medicare Advantage and commercially insured members. Screening-eligible individuals, who had not previously had the cancer being screened and met USPSTF criteria for screening, were identified at various time points within the study timeframe for relevant screening tests within five cancer types: breast, colorectal, cervical, lung, and prostate. In the 2020 analysis period, patients who were eligible for cancer screening included: breast: 1,620,588; colorectal: 2,763,736; cervical: 1,371,506; lung: 1,491,594; prostate: 1,126,249. Breast and cervical cancer screening prevalence rates were highest (64.4% and 63.8%, respectively), followed by colorectal (29.5%), prostate (11.7%), and lung (3.8%). Black/African American individuals and Hispanics had moderately low screening rates for cervical (58.6%) and breast (61.8%) cancer, respectively; Hispanics had the lowest screening rates for prostate cancer (6.1%). Those residing in the West had lower screening rates for breast (58.9%), cervical (62.1%), and prostate (5.6%) cancer. Screening rates remained stable over time for breast, colorectal, and lung cancer, and changed significantly for cervical (-9.5%, 2012–2020) and prostate (+7.3%, 2008–2020) cancer. Real-world cancer screening rates remain suboptimal and low, and efforts to increase screening uptake and reduce cancer health disparities remain critical.
1. Introduction
Cancer remains one of the leading causes of death in the United States (US), despite recent improvements in mortality due to increased detection and treatment options (Center for Disease Control and Prevention, 2021). Early detection and diagnosis of cancer are vital in reducing the number of cancer-related deaths, as the 5-year survival rates for many common cancers are significantly lower when patients are diagnosed in later stages. For instance, the 5-year survival rates for localized versus metastatic breast cancer are 99% vs 27%, 92% vs 17% for cervical cancer, 90% vs 14% for colorectal cancer (CRC), 57% vs 5% for lung cancer, and >99% vs 31% for prostate cancer (Brill, 2020).
Regular cancer screenings in the general asymptomatic population can lead to timelier detection of unrecognized (pre-clinical) cancer or pre-cancerous lesions, thereby helping to avoid later-stage diagnoses (World Health Organization, 2017), which are often costlier and more invasive, and can lead to poor clinical outcomes (eg, increased mortality and morbidity) and higher healthcare resource utilization (World Health Organization, 2020, National Cancer Institute, 2021, Clarke et al., 2020, Gildea et al., 2017, Kakushadze et al., 2017, Siegel et al., 2021). The United States Preventive Services Task Force (USPSTF) strongly recommends preventive screenings for asymptomatic individuals who may be at a higher risk of developing cancer based on individual risk factors—such as age, gender, and smoking history—across individual cancer types such as breast (Siu, 2016), cervical (Curry et al., 2018), colorectal (Bibbins-Domingo et al., 2016), and lung (Moyer, 2014), and promotes informed, individual decision making for prostate cancer screening (Grossman et al., 2018).
Recent data on the cancer screening prevalence in the US has largely been sourced from self-reported surveys, including the Behavioral Risk Factor Surveillance System (BRFSS) survey and the National Health Interview Survey (NHIS). In the 2018 BRFSS survey, approximately 78% of eligible women reported having a mammogram within the past two years, 80% of eligible women reported receiving a Pap test within the past three years, 70% of eligible respondents reported fully meeting the USPSTF recommendations for CRC screening, 14%–19% of eligible respondents with a history of smoking reported undergoing a low-dose computed tomography (LDCT) scan within the past year, and 33% of eligible men reported having a prostate-specific antigen (PSA) test within the past two years (Narayan et al., 2021, Zahnd and Eberth, 2019, Centers for Disease Control and Prevention, 2018). Similarly, among a comparable population and timeframe, the 2019 NHIS data demonstrated that approximately 75% of eligible women reported being up to date with their breast and cervical cancer screening, and 67% of eligible adults reported having received either a home fecal occult blood test (FOBT) within the past year, a sigmoidoscopy within the past five years, or a colonoscopy within the past 10 years (Clarke et al., 2020).
Cancer screening prevalence estimates from self-reported survey data may not, however, reflect real-world adherence to guideline-based screening recommendations (Anderson et al., 2019, Bonafede et al., 2019, Cronin et al., 2009, Ferrante et al., 2008). In one large population-based claims analysis using data from 2010 to 2015 (Bonafede et al., 2019), screening prevalence rates from real-world practice were lower than self-reported data (Cronin et al., 2009, Ferrante et al., 2008, National Center for Health Statistics, 2017). Breast and cervical cancer screenings remained underutilized among the commercially and Medicaid-insured populations. Screening rates were substantially lower than in the 2013 NHIS and did not meet public health screening goals outlined in Healthy People 2020 (Office of Disease Prevention and Health Promotion, 2020), which is a 10-year target guide for national health promotion and disease prevention to “achieve health equity, eliminate disparities, and improve the health of all groups” by increasing target screening rates for cervical, colorectal, and breast cancer to 93.0%, 70.5%, and 81.1%, respectively. Claims-based estimates for mammography, which required at least three mammography claims within six years to qualify as screened in eligible women aged 40 to 59 years, were approximately 19 to 40 percentage points lower than in the NHIS data; similarly, estimates for cervical cancer screening, which required at least two cervical cancer screening claims within six years to qualify as screened in eligible women aged 30 to 59 years, were approximately 22 to 44 percentage points lower (Bonafede et al., 2019).
This study aims to assess breast, colorectal, cervical, lung, and prostate cancer screening rates and trends in the US over time among individuals for whom screening is recommended by the USPSTF. This study is the first to comprehensively assess the real-world prevalence of different guideline-recommended screening modalities across multiple cancer types and in different subpopulations, using the most recent large scale claims data in an effort to understand whether current screening rates are sufficiently meeting expert recommendations and national public health goals.
2. Methods
2.1. Data source
Data were obtained from Optum’s de-identified Clinformatics® Data Mart Database, which captures fully adjudicated pharmacy and medical claims data for more than 100 million unique enrollees across the US with commercial insurance and Medicare Advantage. De-identified patient-level data was provided on patient demographics and comorbidities in compliance with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act of 1996. This study was granted a review exemption from the WIRB-Copernicus Group Institutional Review Board.
2.2. Study design
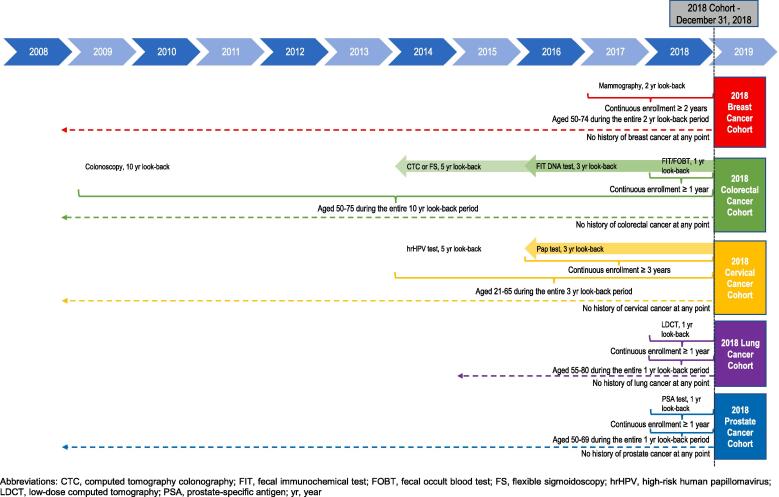
This observational, retrospective study consisted of an analysis time frame that ranged from January 1, 2008 to February 29, 2020. Cancer screening prevalence was assessed cross-sectionally every other year, as applicable, for breast, cervical, colorectal, lung, and prostate cancer. Following the USPSTF recommendations for cancer screening (Supplementary Table S1), individuals who were eligible to receive screening were identified and assessed whether they had received the recommended screening modality within a specific time interval (ie, look-back period). For instance, the 2018 breast cancer screening cohort consisted of eligible women aged 50 to 74 years who received a mammography during the two-year lookback period from January 1, 2017 to December 31, 2018 (Fig. 1). For cancer types with multiple screening modalities (eg, CRC) with different screening intervals, individuals were considered screened if they received at least one of the modalities (eg, colonoscopy) within its recommended time interval.
Fig. 1.
Study Design: 2018 Cancer Screening Cohorts Example. Abbreviations: CTC, computed tomography colonography; FIT, fecal immunochemical test; FOBT, fecal occult blood test; FS, flexible sigmoidoscopy; hrHPV, high-risk human papillomavirus; LDCT, low-dose computed tomography; PSA, prostate-specific antigen; yr, year.
2.3. Study population
At each given time point from January 1, 2008 to February 29, 2020, individual cohorts were comprised of eligible individuals who met the USPSTF screening criteria (Supplementary Tables S1-2), had no previous history of cancer, and had continuous insurance eligibility for at least the entire lookback period (Fig. 1, Supplementary Table S2). When there were multiple recommended screening modalities for a given cancer type, the minimum continuous enrollment was required based on the shortest screening interval (eg, one year for CRC screening based on the fecal immunochemical test [FIT] or FOBT). Cohorts were comprised of patients within the age ranges eligible for screening, as outlined in Supplementary Table S1. Given the recommended starting age for CRC screening is age 50 years, the screening-eligible population consisted of individuals between 60 and 75 years in 2020, in order to accommodate a 10-year lookback period for a colonoscopy.
2.4. Statistical analysis
Demographic and clinical characteristics were analyzed descriptively for each cancer screening-eligible cohort at each applicable time point. Individuals’ age, sex, primary insurance type, geographic region, and race/ethnicity, were assessed. Charlson Comorbidity Index (CCI) scores, which predict mortality risk and are defined by 17 medical conditions (using enhanced International Classification of Diseases, Ninth Revision [ICD-9] diagnosis codes), were also calculated for each patient, with a higher CCI score indicating greater comorbidity burden (Concept: Charlson Comorbidity Index., 2021, Quan et al., 2005). CCI scores were calculated using data from a 1-year period between January 1 and December 31 of the year in which the cohort was being assessed. History of diabetes, obesity status, and smoking was also collected.
ICD-9, International Classification of Diseased, Tenth Revision (ICD-10), and Current Procedural Terminology (CPT) codes indicative of cancer screening-specific procedure codes were used to identify those who received cancer screening (Supplementary Table S3). Codes indicating cancer diagnostic procedures (eg, diagnostic mammography) were not included. Of note, the lung cancer screening-eligible population consisted of smokers using codes indicative of tobacco dependence or cessation from published literature (Huo et al., 2018) (Supplementary Table S4).
Cancer screening prevalence, or the proportion of eligible individuals who received at least one screening modality, was assessed cross‑sectionally at multiple time points between January 2008 and June 2020 for relevant screening tests across the five cancer screening cohorts. Subgroup analyses of the most recent 2020 screening-eligible cohorts to determine cancer screening rates by race, primary insurance type, and geographic region were also conducted to better understand current populations with disparities in cancer screening. Linear regression models were used to estimate time trends in cancer screening rates and the proportions of individuals with comorbidities.
Standard descriptive summary statistics were run as relevant and statistically appropriate for categorical and continuous variables. All data management and analyses were conducted using SAS Studio, version 3.8 (SAS Institute Inc).
2.5. Sensitivity analyses
Additional analyses were conducted to assess screening rates among individuals with varying continuous enrollment eligibility, given certain cancer types have multiple screening modalities and intervals. Cervical cancer and CRC screening-eligible individuals were required to be continuously enrolled for at least 5 and 10 years, respectively, to account for the selection of fewer individuals with the same exposure period and amount of time to be screened for co-testing and colonoscopy, respectively.
In addition, CRC screening rates were assessed using an inclusive code list of both screening and diagnostic procedures.
3. Results
In the 2020 analysis period, patients who met the study criteria and were included in the analysis as eligible for cancer screening were as follows: breast, 1,620,588; colorectal, 2,763,736; cervical, 1,371,506; lung, 1,491,594; prostate, 1,126,249). Among the screening-eligible population, the majority (52.9%) of individuals were white and approximately 40.8% were from the Southern region of the US in each of the cohorts (Table 1). A greater proportion of individuals eligible for breast (58.2%), colorectal (73.5%), and lung (78.3%) cancer screening had Medicare Advantage, while a greater proportion of those eligible for cervical (91.6%) and prostate (58.1%) cancer screening had commercial insurance. Nearly 48.2% of individuals eligible for cancer screening for all 2020 cohorts had a CCI score of at least 1, and at least 35.0%, 27.2%, and 39.3% had a history of obesity, diabetes, or smoking, respectively.
Table 1.
Characteristics of Cancer Screening-Eligible Patients, 2020.
| Breast | Colorectal | Cervical | Lung | Prostate | |
|---|---|---|---|---|---|
| Eligible, n | 1,620,588 | 2,763,736 | 1,371,506 | 1,491,594 | 1,126,249 |
| Gender, n (%)a | |||||
| Female | 1,620,588 (100.0%) | 1,529,524 (55.3%) | 1,371,506 (100.0%) | 712,246 (47.8%) | 0 (0.0%) |
| Male | 0 (0.0%) | 1,234,090 (44.7%) | 0 (0.0%) | 779,292 (52.2%) | 1,126,249 (100.0%) |
| Unknown | 0 (0.0%) | 122 (0.0%) | 0 (0.0%) | 56 (0.0%) | 0 (0.0%) |
| Race, n (%)a | |||||
| Asian | 57,260 (3.5%) | 84,433 (3.0%) | 80,757 (5.9%) | 25,463 (1.7%) | 32,890 (2.9%) |
| Black/African American | 168,543 (10.4%) | 228,489 (8.3%) | 142,101 (10.4%) | 149,626 (10.0%) | 82,283 (7.3%) |
| Hispanic | 166,645 (10.3%) | 255,527 (9.3%) | 184,121 (13.4%) | 113,314 (7.6%) | 105,532 (9.4%) |
| White | 964,312 (59.5%) | 1,463,235 (52.9%) | 885,910 (64.6%) | 908,033 (60.9%) | 637,885 (56.6%) |
| Unknown | 263,828 (16.3%) | 732,052 (26.5%) | 78,617 (5.7%) | 295,158 (19.8%) | 267,659 (23.8%) |
| Primary Insurance, n (%)a | |||||
| Commercial | 673,398 (41.6%) | 723,863 (26.2%) | 1,256,498 (91.6%) | 319,468 (21.4%) | 654,661 (58.1%) |
| Medicare Advantage | 943,008 (58.2%) | 2,031,882 (73.5%) | 114,422 (8.3%) | 1,168,526 (78.3%) | 467,836 (41.5%) |
| Multiple | 4,182 (0.3%) | 7,991 (0.3%) | 586 (0.0%) | 3,600 (0.2%) | 3,752 (0.3%) |
| Geographic Region, n (%)a | |||||
| Northeast | 177,560 (11.0%) | 329,529 (11.9%) | 125,752 (9.2%) | 174,963 (11.7%) | 120,644 (10.7%) |
| South | 692,908 (42.8%) | 1,144,081 (41.4%) | 559,184 (40.8%) | 662,240 (44.4%) | 478,665 (42.5%) |
| West | 372,333 (23.0%) | 680,915 (24.6%) | 280,984 (20.5%) | 306,870 (20.6%) | 236,837 (21.0%) |
| Midwest | 353,615 (21.8%) | 565,396 (20.5%) | 350,349 (25.5%) | 337,886 (22.7%) | 264,813 (23.5%) |
| Multiple | 7,677 (0.5%) | 13,393 (0.5%) | 8,213 (0.6%) | 8,215 (0.6%) | 5,239 (0.5%) |
| Unknown | 16,495 (1.0%) | 30,422 (1.1%) | 47,024 (3.4%) | 1,420 (0.1%) | 20,051 (1.8%) |
| CCI, n (%)a | |||||
| 0 | 884,072 (54.6%) | 1,337,613 (48.4%) | 1,047,807 (76.4%) | 443,553 (29.7%) | 623,129 (55.3%) |
| 1 | 363,445 (22.4%) | 645,319 (23.4%) | 213,981 (15.6%) | 362,651 (24.3%) | 244,618 (21.7%) |
| 2 | 178,024 (11.0%) | 354,543 (12.8%) | 63,597 (4.6%) | 257,881 (17.3%) | 121,674 (10.8%) |
| ≥3 | 195,047 (12.0%) | 426,261 (15.4%) | 46,121 (3.4%) | 427,509 (28.7%) | 136,828 (12.2%) |
| Ever Obese, n (%)a, b | |||||
| Yes | 612,022 (37.8%) | 919,920 (33.3%) | 414,527 (30.2%) | 621,062 (41.6%) | 361,507 (32.1%) |
| No | 1,008,566 (62.3%) | 1,843,816 (66.7%) | 956,979 (69.8%) | 870,532 (58.4%) | 764,742 (67.9%) |
| Ever Diabetes, n (%)a | |||||
| Yes | 427,234 (26.4%) | 826,528 (29.9%) | 156,856 (11.4%) | 558,331 (37.4%) | 307,516 (27.3%) |
| No | 1,193,354 (73.6%) | 1,937,208 (70.1%) | 1,214,650 (88.6%) | 933,263 (62.6%) | 818,733 (72.7%) |
| Ever Smoker, n (%)a | |||||
| Yes | 427,354 (26.4%) | 783,879 (28.4%) | 249,980 (18.2%) | 1,491,594 (100.0%) | 338,611 (30.1%) |
| No | 1,193,234 (73.6%) | 1,979,857 (71.6%) | 1,121,526 (81.8%) | 0 (0.0%) | 787,638 (69.9%) |
aPercentages may not add to 100% due to rounding.
bObesity is defined as subjects with a claim code indicating “obesity” or a BMI of ≥ 30 in the description.
Abbreviations: CCI, Charlson Comorbidity Index.
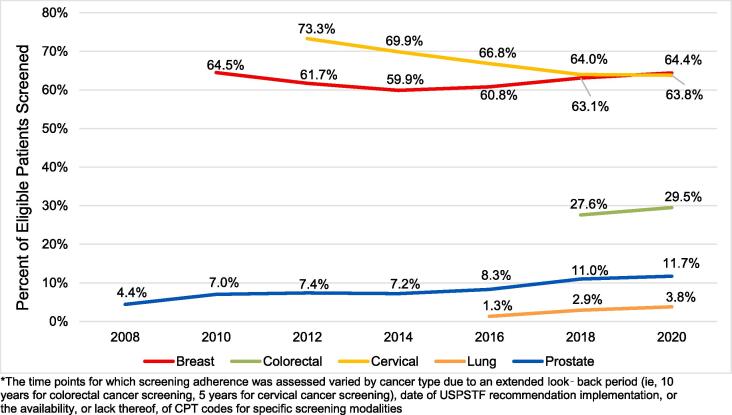
Cancer screening rates among eligible patients varied widely by cancer type. For the 2020 analysis period, the prevalence of breast and cervical cancer screening was the highest (64.4% and 63.8%, respectively), followed by colorectal (29.5%), prostate (11.7%), and lung (3.8%) cancer screening (Fig. 2).
Fig. 2.
Trends in Percent of Eligible Patients Screened for Cancer, 2008 to 2020.* *The time points for which screening adherence was assessed varied by cancer type due to an extended look‑back period (ie, 10 years for colorectal cancer screening, 5 years for cervical cancer screening), date of USPSTF recommendation implementation, or the availability, or lack thereof, of CPT codes for specific screening modalities.
Among the screening-eligible population in the 2020 cohorts, subgroup analyses of screening prevalence were conducted by race, primary insurance type, and geographic region (Table 2). In terms of race/ethnicity, Black/African American individuals had moderately low screening rates for cervical cancer (58.6%), particularly compared to White individuals (66.3%), while Hispanic individuals had moderately low screening rates for breast cancer (61.8%), compared to White and Black/African American individuals (>65%). Hispanic individuals also had the lowest prostate cancer screening rates (6.1%), compared to White and Black/African American individuals (>11%). With regard to primary insurance type, screening prevalence was lower among those with commercial insurance for prostate cancer (5.1%) and lower among those with Medicare Advantage for cervical cancer (37.5%). Additionally, those residing in the West had consistently lower cancer screening rates for breast (58.9%), cervical (62.1%), and prostate (5.6%) cancer, compared to those in other regions.
Table 2.
Percent of Patients Screened for Cancer by Race, Primary Insurance Type, and Geographical Regions, 2020.
| Cancer Screening Type | Breast | Colorectal | Cervical | Lung | Prostate |
|---|---|---|---|---|---|
| Screened, n | 1,043,099 | 813,890 | 874,697 | 56,963 | 131,787 |
| Race, n (%)a | |||||
| Asian | 35,864 (62.6%) | 27,718 (32.8%) | 50,876 (63.0%) | 829 (3.3%) | 2,213 (6.7%) |
| Black/African American | 110,103 (65.3%) | 69,788 (30.5%) | 83,284 (58.6%) | 4,894 (3.3%) | 9,853 (12.0%) |
| Hispanic | 103,054 (61.8%) | 79,046 (30.9%) | 109,355 (59.4%) | 2,098 (1.9%) | 6,422 (6.1%) |
| White | 631,336 (65.5%) | 467,918 (32.0%) | 587,327 (66.3%) | 35,147 (3.9%) | 72,934 (11.4%) |
| Unknown | 162,742 (61.7%) | 169,420 (23.1%) | 43,855 (55.8%) | 13,995(4.7%) | 40,365 (15.1%) |
| Primary Insurance Type, n (%)a | |||||
| Commercial | 419,706 (62.3%) | 200,424 (27.7%) | 831,432 (66.2%) | 10,166 (3.2%) | 33,161 (5.1%) |
| Medicare Advantage | 620,437 (65.8%) | 610,561 (30.0%) | 42,958 (37.5%) | 46,675 (4.0%) | 98,051 (21.0%) |
| Multiple | 2,956 (70.7%) | 2,905 (36.4%) | 307 (52.4%) | 122 (3.4%) | 575 (15.3%) |
| Geographic Region, n (%)a | |||||
| Northeast | 118,487 (66.7%) | 91,310 (27.7%) | 90,958 (72.3%) | 9,663 (5.5%) | 16,257 (13.5%) |
| South | 457,957 (66.1%) | 355,437 (31.1%) | 370,818 (66.3%) | 23,220 (3.5%) | 59,156 (12.4%) |
| West | 219,288 (58.9%) | 194,746 (28.6%) | 174,485 (62.1%) | 8,648 (2.8%) | 13,284 (5.6%) |
| Midwest | 241,412 (68.3%) | 167,779 (29.7%) | 230,498 (65.8%) | 15,085 (4.5%) | 42,318 (16.0%) |
| Multiple | 5,125 (66.8%) | 3,994 (29.8%) | 5,591 (68.1%) | 326 (4.0%) | 716 (13.7%) |
| Unknown | 830 (5.0%) | 624 (2.1%) | 2,347 (5.0%) | 21 (1.5%) | 56 (0.3%) |
aThe denominator consists of screen-eligible individuals with the corresponding patient characteristics in Table 1.
Trends in screening-eligible patient characteristics were generally consistent over time (Supplementary Tables S5-9). However, the age distribution skewed slightly older over time for breast (2010 to 2020), lung (2016 to 2020), and prostate cancer (2008 to 2020). Among patients eligible for breast cancer screening, there was also a shift from having predominantly commercial insurance in 2010 to Medicare Advantage in 2020. While some cancer screening-eligible populations seemed to demonstrate fluctuations in race distribution, they stayed relatively constant when the “unknown” race category was not considered. Patients’ comorbidity status and history of obesity, diabetes, or smoking were also comparable. The rates of obesity, diabetes, and smoking increased significantly over time among individuals who were eligible for breast, cervical, and prostate cancer screening, suggesting a greater number of screening-eligible individuals with more comorbidities. Finally, cancer screening prevalence remained fairly stable over time for breast, colorectal, and lung cancer, with a statistically significant decrease in cervical cancer screening (-9.5%, over 9 years, 2012 to 2020, p<0.01) and a statistically significant increase in prostate cancer screening (+7.3%, over 13 years, 2008 to 2020, p<0.01) (Fig. 2).
3.1. Sensitivity analyses
Among cervical cancer and CRC screening-eligible individuals in 2020 who were continuously enrolled for at least 5 and 10 years, respectively (758,847, cervical; 151,493, colorectal), approximately 67.0% and 42.1% received cervical and CRC screening, respectively.
When using an inclusive code list of CRC screening and diagnostic procedures, the overall screening rate in 2018 was 34.2%.
4. Discussion
The results from this real-world study, the first to comprehensively assess cancer screening rates per USPSTF recommendations across multiple cancer types and over time, indicate that adherence to guideline-recommended cancer screening is generally lower than what is self-reported and below the new Healthy People 2030 targets (Office of Disease Prevention and Health Promotion, 2030). Overall, patient adherence to cancer screening is suboptimal, given relatively constant or low rates over time.
Breast cancer screening rates stayed constant over time from 2008 to 2020, which is consistent with their unchanging USPSTF guidelines over time. Despite previous updates to CRC screening guidelines, however, rates remained unchanged from 2018 to 2020 given they reflect adherence to any screening modality over a longer period.
Cervical cancer screening rates decreased considerably over time from 2008 to 2020, and while the exact reasons are unclear, this trend has been seen in other studies (Watson et al., 2018, MacLaughlin et al., 2019), and may represent a lack of knowledge regarding screening as well as a gap in healthcare provider recommendations (Suk et al., 2022). This decline may also be associated with changes in screening behaviors with the relatively recent availability of the HPV vaccine. Lung cancer screening rates remained mostly low and constant over time despite having been assessed in the general population, and adoption may have been slow since the USPSTF recommendation in 2013. These findings are consistent with other published literature (Clarke et al., 2020). Prostate cancer screening rates stayed relatively consistent over time until 2016 and increased slowly until 2020. This may result partly from changes in USPSTF recommendations (ie, from a grade D to C in 2018) and patient cohorts over time (eg, growing elderly population).
In comparison to recent national survey data (Narayan et al., 2021, Centers for Disease Control and Prevention, 2018, Fisher et al., 2022; National Center for Health Statistics, 2021), screening rates from the current analysis were approximately 11.5 to 13.5 percentage points lower for mammography, 12.2 to 40.0 percentage points lower for CRC screening, 10.2 to 16.2 percentage points lower for cervical cancer screening, 17.8 percentage points lower for LDCT scans, and 21.3 percentage points lower for PSA tests. This study’s findings are consistent with those of other studies in that self-reported cancer screening rates may generally be overestimating the true rates to varying degrees (Anderson et al., 2019, Bonafede et al., 2019, Cronin et al., 2009, Ferrante et al., 2008).
Screening rates observed in the current study were comparable to those from other claims-based studies. While breast and cervical cancer screening rates were comparable when accounting for the different time periods assessed and insurance-coverage mix of the population (Bonafede et al., 2019), CRC screening rates were lower by approximately 16.5 to 33.3 percentage points compared to existing studies in the literature (Bonafede et al., 2019, Cyhaniuk and Coombes, 2016). This difference in rates is likely due to differences in study methodology and screening code selection. When we conducted a sensitivity analysis reflecting individuals with at least 10 years of continuous enrollment, the CRC screening rate increased to 42.1% in 2020. With regards to the selection of cancer screening-specific codes, our sensitivity analyses conducted in 2018 CRC screening cohorts replicated as closely as possible the methodology described in another claims-based study that used a broader code list (Cyhaniuk and Coombes, 2016), and found that including codes that were not specific to screening resulted in a notable increase in the CRC screening rates which were more aligned with—although approximately-six percentage points higher than—previously published results (Cyhaniuk and Coombes, 2016). Thus, while this study may underestimate the number of screening procedures that are coded as diagnostic procedures, a more inclusive screening code list may contribute to an overestimation of screening prevalence and suggest more progress toward reaching national public health goals than warranted.
Lung cancer screening rates may also have been underestimated in this study, given limitations with identifying the higher risk screening-eligible cohort though they were still considerably lower than self-reported numbers (Kee et al., 2021).
Understanding screening disparities is essential for planning public health interventions to promote screening and reduce cancer morbidity and mortality. Our study findings suggest greater potential disparities in cancer screening within the Black/African American and Hispanic subpopulations, and those residing in the West. Previous studies have observed both lower (Sengupta and Honey, 2020, DeSantis et al., 2019) and higher (American Cancer Society, 2019, Benavidez et al., 2021, Hall et al., 2018) cancer screening rates among Black/African American and Hispanic individuals compared to White individuals, which makes identifying vulnerable populations by race less clear. Of note, this literature is based on self-reported data, and studies have shown Black/African American and Hispanic individuals may overestimate some screenings, such as mammograms (Allgood et al., 2014, Rauscher et al., 2008).
Most of the literature on geographic disparities in cancer screening focuses on rural versus urban, which was not assessed in the present study. However, previous studies have noted lower screening rates in the West (Li et al., 2020, Okereke et al., 2019), potentially due to lower density of healthcare resources and population densities in the West compared with the Northeast (Li et al., 2020, Onega et al., 2014, Peipins et al., 2012).
The effects of primary insurance type on cancer screening rates were limited. Our study findings are not surprising—screening is lower in the commercially insured population for all cancer types with USPSTF recommendations in older age groups.
Several limitations exist within this study, including using claims data, which may have the potential for selection bias, as the study population consisted entirely of an insured population, which may not be completely generalizable to the US population. This analysis, however, is focused on, and limited to, real-world screening prevalence among an insured population, which is lower than previously self-reported survey data which can be overestimated. Prior research reported that lack of insurance is strongly associated with a lack of cancer screening and lower adherence to cancer screening (Fiscella et al., 2011, Freund et al., 2019). This is indicative of a clear unmet need in overall cancer screening adherence in both the insured and uninsured populations, and an area of focus for reducing disparities. Another limitation concerns the inability to identify the eligible lung cancer screening population. Detailed information on smoking status was not available in the database, other than individuals with a claim for tobacco dependence or cessation, of whom we assumed as smokers, or the higher risk lung cancer screening-eligible population. Finally, measurement and evaluation of screening disparities was limited.
Given this analysis was mostly descriptive, future studies should be conducted to identify which characteristics or variables are predictive of individuals receiving screening, and further evaluate screening disparities. While the actual rates of cancer screening may vary across our study, self-reported survey data, and other claims data, cancer screening prevalence remains below Healthy People 2020 targets. As data demonstrate that regular cancer screening can help avoid later-stage diagnoses (World Health Organization, 2017) and their potential increased cost, utilization, and poor clinical outcomes (World Health Organization, 2020, National Cancer Institute, 2021, Clarke et al., 2020, Gildea et al., 2017, Kakushadze et al., 2017, Siegel et al., 2021), it remains critical to improve cancer screening rates to meet the outlined goals and recommendations through better education, outreach, and access to resources, especially among populations with identified disparities. Advances in screening may also enable more people to access cancer screening, enable earlier cancer detection and treatment, and improve patient outcomes.
5. Conclusions
Real‑world cancer screening rates per USPSTF recommendations are lower than self‑reported adherence from survey data that is frequently cited to assess progress made toward Healthy People targets to improve national health and well-being. Additionally, despite long-established recommendations for cancer screening, screening rates have remained relatively unchanged over time for breast and CRC, very low for lung cancer, and variable for cervical (ie, decreasing) and prostate (ie, increasing) cancer. Though current cancer screening disparities among certain segments of the population are demonstrated, additional research may be needed to identify those that may be vulnerable. Hispanic and Black/African American populations, as well as those residing in the West may require more efforts to raise screening prevalence among those recommended for screening. Efforts to increase screening uptake and reduce cancer health disparities remain critical to align with expert recommendations, achieve national public health goals, and avoid related downstream suboptimal clinical outcomes and high healthcare costs.
6. Disclosure of competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. AK is an employee of GRAIL, LLC, who supported this study. AK reported stock or stock options in Illumina (parent company of GRAIL). ZC is an employee of GRAIL, LLC, who supported this study. KC is an employee of GRAIL, LLC, who supported this study. NM is an employee of BluePath Solutions, who received financial support from GRAIL, LLC for study-related research activities. MG is an employee of BluePath Solutions, who received financial support from GRAIL, LLC for study-related research activities. EF was an employee of BluePath Solutions at the time of the study conduction, who received financial support from GRAIL, LLC for study-related research activities. No other disclosures were reported.
Funding
This study was sponsored by GRAIL, LLC, a subsidiary of Illumina Inc., is currently held separate from Illumina, Inc. under the terms of the Interim Measures Order of the European Commission dated 29 October 2021. The sponsor had no role in the collection, management, and analysis of the data. The sponsor contributed to study design and data interpretation.
8. Authors’ contributions
EF, MG, and NM had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: AK, MG, EF, NM, ZC, and KC; Acquisition, analysis, or interpretation of data: AK, MG, EF, NM, ZC, and KC; Drafting of the manuscript: AK, MG, EF, NM, ZC, and KC; Critical revisions of the manuscript for important intellectual content: AK, MG, EF, NM, ZC, and KC; Statistical analysis: EF.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2022.102046.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Allgood K.L., Rauscher G.H., Whitman S., Vasquez-Jones G., Shah A.M. Validating self-reported mammography use in vulnerable communities: findings and recommendations. Cancer Epidemiol. Biomarkers Prev. 2014;23(8):1649–1658. doi: 10.1158/1055-9965.EPI-13-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society. (2019). Cancer facts & figures for African Americans 2019-2021. Atlanta, GA.
- Anderson J., et al. Department of Veterans Affairs (US); Washington (DC): 2019. Evidence Brief: Accuracy of Self-report for Cervical and Breast Cancer Screening. Accessed September 28, 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539386/ [PubMed] [Google Scholar]
- Benavidez G.A., Zgodic A., Zahnd W.E., Eberth J.M. Disparities in meeting USPSTF breast, cervical, and colorectal cancer screening guidelines among women in the United States. Prev. Chronic Dis. 2021;18 doi: 10.5888/pcd18.200315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbins-Domingo K., Grossman D.C., Curry S.J., Davidson K.W., Epling J.W., García F.A.R., Gillman M.W., Harper D.M., Kemper A.R., Krist A.H., Kurth A.E., Landefeld C.S., Mangione C.M., Owens D.K., Phillips W.R., Phipps M.G., Pignone M.P., Siu A.L. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2016;315(23):2564. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- Bonafede M.M., Miller J.D., Pohlman S.K., Troeger K.A., Sprague B.L., Herschorn S.D., Winer I.H. Breast, cervical, and colorectal cancer screening: patterns among women with medicaid and commercial insurance. Am J Prev Med. 2019;57(3):394–402. doi: 10.1016/j.amepre.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill J.V. Screening for cancer: the economic, medical, and psychosocial issues. Am. J. Manag. Care. 2020;26(14 Suppl):S300–S306. doi: 10.37765/ajmc.2020.88534. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. (2021). An Update on Cancer Deaths in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Division of Cancer Prevention and Control.
- Centers for Disease Control and Prevention National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health: BRFSS Prevalence & Trends Data. 2018. https://www.cdc.gov/brfss/brfssprevalence/ Accessed September 28, 2021.
- Clarke C.A., Hubbell E., Kurian A.W., Colditz G.A., Hartman A.-R., Gomez S.L. Projected reductions in absolute cancer-related deaths from diagnosing cancers before metastasis, 2006–2015. Cancer Epidemiol. Biomark. Prev. 2020;29(5):895–902. doi: 10.1158/1055-9965.EPI-19-1366. [DOI] [PubMed] [Google Scholar]
- Concept: Charlson Comorbidity Index. (2021). Quan's - ICD-9-CM and ICD-10 Charlson SAS Code. Last updated 2021. Accessed September 28, 2021. Available from: http://mchp-appserv.cpe.umanitoba.ca/viewConcept.php?conceptID=1098.
- Cronin K.A., Miglioretti D.L., Krapcho M., Yu B., Geller B.M., Carney P.A., Onega T., Feuer E.J., Breen N., Ballard-Barbash R. Bias associated with self-report of prior screening mammography. Cancer Epidemiol. Biomarkers Prev. 2009;18(6):1699–1705. doi: 10.1158/1055-9965.EPI-09-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry S.J., Krist A.H., Owens D.K., Barry M.J., Caughey A.B., Davidson K.W., Doubeni C.A., Epling J.W., Kemper A.R., Kubik M., Landefeld C.S., Mangione C.M., Phipps M.G., Silverstein M., Simon M.A., Tseng C.-W., Wong J.B. Screening for cervical cancer: US preventive services task force recommendation statement. JAMA. 2018;320(7):674. doi: 10.1001/jama.2018.10897. [DOI] [PubMed] [Google Scholar]
- Cyhaniuk A., Coombes M.E. Longitudinal adherence to colorectal cancer screening guidelines. Am J Manag Care. 2016;22(2):105–111. [PubMed] [Google Scholar]
- DeSantis C.E., Miller K.D., Goding Sauer A., Jemal A., Siegel R.L. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211–233. doi: 10.3322/caac.21555. [DOI] [PubMed] [Google Scholar]
- Ferrante J.M., Ohman-Strickland P., Hahn K.A., Hudson S.V., Shaw E.K., Crosson J.C., Crabtree B.F. Self-report versus medical records for assessing cancer-preventive services delivery. Cancer Epidemiol. Biomarkers Prev. 2008;17(11):2987–2994. doi: 10.1158/1055-9965.EPI-08-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiscella K., et al. Eliminating disparities in cancer screening and follow-up of abnormal results: what will it take? J. Health Care Poor Underserved. 2011;22(1):83–100. doi: 10.1353/hpu.2011.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D.A., Princic N., Miller-Wilson L.-A., Wilson K., DeYoung K., Ozbay A.B., Limburg P. Adherence to fecal immunochemical test screening among adults at average risk for colorectal cancer. Int. J. Colorectal Dis. 2022;37(3):719–721. doi: 10.1007/s00384-021-04055-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund K.M., Reisinger S.A., LeClair A.M., Yoon G.H., Al-Najar S.M., Young G.S., González E.T., Oliveri J.M., Paskett E.D. Insurance stability and cancer screening behaviors. Health Equity. 2019;3(1):177–182. doi: 10.1089/heq.2018.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildea T.R., et al. A retrospective analysis of delays in the diagnosis of lung cancer and associated costs. Clinicoecon Outcomes Res. 2017;9:261–269. doi: 10.2147/ceor.S132259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman D.C., Curry S.J., Owens D.K., Bibbins-Domingo K., Caughey A.B., Davidson K.W., Doubeni C.A., Ebell M., Epling J.W., Kemper A.R., Krist A.H., Kubik M., Landefeld C.S., Mangione C.M., Silverstein M., Simon M.A., Siu A.L., Tseng C.-W. Screening for prostate cancer: US preventive services task force recommendation statement. JAMA. 2018;319(18):1901. doi: 10.1001/jama.2018.3710. [DOI] [PubMed] [Google Scholar]
- Hall I.J., Tangka F.K.L., Sabatino S.A., Thompson T.D., Graubard B.I., Breen N. Patterns and trends in cancer screening in the United States. Prev Chronic Dis. 2018;15 doi: 10.5888/pcd15.170465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo J., Yang M., Tina Shih Y.-C. Sensitivity of claims-based algorithms to ascertain smoking status more than doubled with meaningful use. Value Health. 2018;21(3):334–340. doi: 10.1016/j.jval.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Kakushadze Z., et al. Estimating cost savings from early cancer diagnosis. Data. 2017;2(3):30. https://www.mdpi.com/2306-5729/2/3/30 Retrieved from. [Google Scholar]
- Kee D., Wisnivesky J., Kale M.S. Lung cancer screening uptake: analysis of BRFSS 2018. J Gen Intern Med. 2021;36(9):2897–2899. doi: 10.1007/s11606-020-06236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Ji J., Besculides M., Bickell N., Margolies L.R., Jandorf L., Taioli E., Mazumdar M., Liu B. Factors associated with mammography use: A side-by-side comparison of results from two national surveys. Cancer Med. 2020;9(17):6430–6451. doi: 10.1002/cam4.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaughlin K.L., Jacobson R.M., Radecki Breitkopf C., Wilson P.M., Jacobson D.J., Fan C., St. Sauver J.L., Rutten L.J.F. Trends over time in Pap and Pap-HPV cotesting for cervical cancer screening. J. Womens Health (Larchmt) 2019;28(2):244–249. doi: 10.1089/jwh.2018.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer V.A. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014;160(5):330–338. doi: 10.7326/m13-2771. [DOI] [PubMed] [Google Scholar]
- Narayan A.K., Gupta Y., Little B.P., Shepard J.O., Flores E.J. Lung cancer screening eligibility and use with low-dose computed tomography: Results from the 2018 Behavioral Risk Factor Surveillance System cross-sectional survey. Cancer. 2021;127(5):748–756. doi: 10.1002/cncr.33322. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. (2021). Cancer Trends Progress Report. Accessed September 28, 2021. Available from: https://progressreport.cancer.gov.
- National Center for Health Statistics. (2017). Health, United States, 2016. Hyattsville, MD. [PubMed]
- National Center for Health Statistics. (2021). Health, United States, 2019. Hyattsville, MD. [PubMed]
- Office of Disease Prevention and Health Promotion. (2020). Healthy People. Accessed September 28, 2021. Available from: https://www.healthypeople.gov/2020.
- Office of Disease Prevention and Health Promotion. (2030). Healthy People. Accessed August 10, 2022. Available from: https:/health.gov/healthypeople.
- Okereke I.C., Nishi S., Zhou J., Goodwin J.S. Trends in lung cancer screening in the United States, 2016–2017. J. Thorac. Dis. 2019;11(3):873–881. doi: 10.21037/jtd.2019.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onega T., Hubbard R., Hill D., Lee C.I., Haas J.S., Carlos H.A., Alford-Teaster J., Bogart A., DeMartini W.B., Kerlikowske K., Virnig B.A., Buist D.S.M., Henderson L., Tosteson A.N.A. Geographic access to breast imaging for US women. J Am Coll Radiol. 2014;11(9):874–882. doi: 10.1016/j.jacr.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peipins L.A., Miller J., Richards T.B., Bobo J.K., Liu T.a., White M.C., Joseph D., Tangka F., Ekwueme D.U. Characteristics of US counties with no mammography capacity. J. Commun. Health. 2012;37(6):1239–1248. doi: 10.1007/s10900-012-9562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.-C., Saunders L.D., Beck C.A., Feasby T.E., Ghali W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- Rauscher G.H., et al. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2008;17(4):748–757. doi: 10.1158/1055-9965.Epi-07-2629. [DOI] [PubMed] [Google Scholar]
- Sengupta R., Honey K. AACR cancer disparities progress report 2020: Achieving the bold vision of health equity for racial and ethnic minorities and other underserved populations. Cancer Epidemiol. Biomarkers Prev. 2020;29(10) doi: 10.1158/1055-9965.EPI-20-0269. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- Siu A.L. Screening for breast cancer: U.S. preventive services task force recommendation statement. Ann. Intern. Med. 2016;164(4):279–296. doi: 10.7326/m15-2886. [DOI] [PubMed] [Google Scholar]
- Suk R., Hong Y.-R., Rajan S.S., Xie Z., Zhu Y., Spencer J.C. Assessment of US preventive services task force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw. Open. 2022;5(1) doi: 10.1001/jamanetworkopen.2021.43582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M., Benard V., Flagg E.W. Assessment of trends in cervical cancer screening rates using healthcare claims data: United States, 2003–2014. Prev. Med. Rep. 2018;9:124–130. doi: 10.1016/j.pmedr.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2017. Guide to Cancer Early Diagnosis. [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2020. WHO Report On Cancer: Setting Priorities, Investing Wisely And Providing Care For All. [Google Scholar]
- Zahnd W.E., Eberth J.M. Lung cancer screening utilization: A behavioral risk factor surveillance system analysis. Am. J. Prev. Med. 2019;57(2):250–255. doi: 10.1016/j.amepre.2019.03.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.