Highlights
-
•
This is the first study that examined the prediction of maximal oxygen uptake with an outdoor hiking test including a natural slope.
-
•
We transferred a maximal laboratory test to a submaximal field test.
-
•
The project aimed to appreciate natural and safe cardio-trekking trails for the sustainable promotion of cross-generational and health-oriented tourism.
-
•
The calculated predictive model achieved similar predictive power compared to previously published work.
Keywords: Borg scale, Cardiovascular health, Exercise testing, Field test, Hiking, Prediction model
Abstract
Maximum oxygen uptake (V̇O2max), the gold standard measure of cardiorespiratory fitness (CRF), supports cardiovascular risk assessment and is mainly assessed during maximal spiroergometry. However, for field use, submaximal exercise tests might be appropriate and feasible. There have been no studies attempting a submaximal test protocol involving uphill hiking. This study aimed to develop and validate a 1-km cardio-trekking test (CTT) controlled by heart rate monitoring and Borg’s 6–20 rating of perceived exertion (RPE) scale to predict V̇O2max outdoors. Healthy participants performed a maximal incremental treadmill walking laboratory test and a submaximal 1-km CTT on mountain trails in Austria and Germany, and V̇O2max was assessed with a portable spirometry device. Borg’s RPE scale was used to control the exercise intensity of the CTT. All subjects wore a chest strap to measure heart rate (HR). A total of 134 participants (median age: 56.0 years [IQR: 51.8–63.0], 43.3 % males) completed both testing protocols. The prediction model is based on age, gender, smoking status, weight, mean HR, altitude difference, duration, and the interaction between age and duration (R2 = 0.65, adj. R2 = 0.63). Leave-one-out cross-validation revealed small shrinkage in predictive accuracy (R2 = 0.59) compared to the original model. Submaximal exercise testing using uphill hiking allows for practical estimation of V̇O2max in healthy adults. This method may allow people to engage in physical activity while monitoring their CRF to avert unnecessary cardiovascular events.
1. Introduction
Cardiovascular diseases are the leading cause of mortality around the world (Roth et al., 2019, World Health Organization, 2022). Regular physical activity is beneficial in reducing the effect of risk factors and preventing cardiovascular diseases (Wen et al., 2011, Visseren et al., 2021). Moreover, physical inactivity and a sedentary lifestyle are leading causes of cardiovascular and all-cause morbidity and mortality (Blair, 2009); therefore, it is crucial to interrupt the vicious circle of a sedentary lifestyle and physical inactivity.
Cardiorespiratory fitness (CRF) is an essential component and a strong predictor of health-related physical fitness which measures performance-related abilities. The most important and widely used measure of CRF is maximum oxygen uptake (V̇O2max) (Ferguson, 2014). Higher physical activity level has positive effects on CRF, resulting in better health outcomes (Myers et al., 2015). It is known that aerobic exercise and resistance training particularly improve cardiovascular fitness and functional capacity (Seals et al., 2014) and reduce the risk of mortality (Blair et al., 1989). The most commonly used method to assess V̇O2max is the metabolic analysis done during maximal effort exercise testing. The measurement of V̇O2max is the best point of reference for CRF and demands a maximal effort while testing (Evans et al., 2015). Maximal exercise stress tests are highly accurate and well established to provide important preventive medical and clinical data (Ferguson, 2014, Niebauer, 2020); however, they are often limited to laboratory settings as they demand additional monitoring equipment (e.g. an electrocardiogram (ECG) device) (Noonan and Dean, 2000). Reaching maximal exertion is only advisable under supervision for cardiac patients and should be symptom limited. Nonetheless, it should be considered that maximal exercise testing may increase the risk of adverse cardiac events (Arena et al., 2007, Ross et al., 2016). Alternatively, submaximal testing protocols, although inferior to maximal exercise tests, may be used clinically depending on the objective of the intervention. The common submaximal test protocols, such as the one-mile track walk (Kline et al., 1987) or the single-stage submaximal treadmill walking test (Ebbeling et al., 1991), involve walking either on a flat track or on a treadmill. Hitherto, no studies have assessed submaximal testing while hiking on outdoor uphill terrain.
Natural environments are often places for recreational and physical activities, such as hiking in mountainous areas (Tyrväinen et al., 2005), which is the most widely-practiced leisure activity in mountain regions (Fredman and Tyrväinen, 2010). Overexertion in hiking can lead to cardiovascular events and death, especially in people with low fitness levels (Niebauer and Burtscher, 2021) who may overestimate their fitness. Such scenarios may be averted by assessing an individual’s CRF.
Therefore, this study aimed to develop and validate a standardized submaximal 1-km cardio-trekking test (CTT) controlled by Borg’s rate of perceived exertion (RPE) scale to predict V̇O2max in healthy adults in two alpine regions of Germany and Austria.
2. Methods
2.1. Study design
This observational study is a part of the “Connect2Move” study, which is a European project aimed at appreciating natural and evidence-based cardio-trekking trails for the sustainable promotion of cross-generational and health-oriented tourism. The study was funded by the European Regional Development Fund, INTERREG V-A Program Austria-Bavaria 2014–2020. The methods of this cross-sectional cross-border study have been published previously (Mayr et al., 2022).
The study protocol complied with the Declaration of Helsinki and its current amendments and was approved by the Ethical Committee of the State of Salzburg (EK-Nr.:1090/2020) and the Ethics Committee of the Medical Faculty of the Technical University of Munich (527/20S). Both committees also controlled the data curator's guidelines for the protection of human subjects concerning safety and privacy. The study was registered with the Clinical trials registry (ClinicalTrials.gov; Reg no: NCT05226806). All participants provided written informed consent for voluntary participation.
All participants completed a maximal incremental walking test on a treadmill (h/p/cosmos Sports & Medical GmbH, Nussdorf-Traunstein, Germany) and a submaximal 1-km CTT in the field. Two independent working groups in Austria and Germany conducted both laboratory and field testing. For the Austrian participants, the Ludwig Boltzmann Institute for Digital Health and Prevention, Salzburg carried out the laboratory investigations at the University Institute of Sports Medicine Salzburg (424 m) in Austria and the field tests were implemented in Werfenweng (902 m), Austria. For the German participants, the Technical University of Munich conducted their research in the St. Irmingard Klinik, Clinic for Cardiology, Prien am Chiemsee (533 m) in Germany and the field tests were done in Aschau im Chiemgau (615 m), Germany.
2.2. Laboratory testing
The investigators obtained the participants’ medical history and collected fasting venous blood samples. After completing the cardiac examinations (Mayr et al., 2022), the participants underwent a maximal exercise test on the treadmill to evaluate their aerobic capacity. Each participant was fitted with the portable spirometry device K5 (COSMED Deutschland GmbH, Fridolfing, Germany) to measure the respiratory gas exchange throughout the exercise testing; the K5 dynamic mixing chamber mode was used for the gas analysis (Winkert et al., 2021). The K5 was calibrated before use each time. The participant’s heart rate (HR) was measured using a Garmin chest strap (Garmin, Olathe, Kansas, United States of America). In addition, the participants wore a 12-lead electrocardiogram device (Amedtec Medizintechnik Aue GmbH, Aue-Bad Schlema, Germany) to measure exercise-related ECG changes. All methods of HR measurement were started synchronically. The modified Bruce protocol (Bruce et al., 1973) was used as the treadmill test protocol. The participants were instructed to walk for as long as possible without holding on to the rail and not running. Participants were asked to score their RPE on a 6–20 point Borg scale (Borg, 1982) at the end of every stage of the Bruce protocol and after test termination. The test was stopped immediately if the participant reached maximal exhaustion or started running on the treadmill.
2.3. Cardiovascular risk score
Three cardiovascular risk scores were calculated for each participant: the Framingham Risk Score (Wilson et al., 1998), the PROCAM (Prospective Cardiovascular Münster Study) Score (Assmann et al., Dec 2007), and the HeartScore of the European Society of Cardiology (Conroy et al., 2003).
2.4. Submaximal 1-km CTT
At least 24 h after the laboratory testing, the participants performed an outdoor submaximal 1-km CTT controlled by Borg’s 6–20 RPE scale in the field testing areas. Each participant was fitted with the portable spirometry device K5 to measure the respiratory gas exchange throughout the exercise testing. The trekking paths were at medium altitudes (Austria: highest altitude 1040 m, Germany: highest altitude 730 m) and were chosen from easily accessible and passable hiking trails in the respective region. The trails were both forest roads without any difficulties like roots and rocks. In Austria, the trail covered a length of 1090 m and an altitude difference of 130 m, while in Germany, the trail covered a length of 1030 m and an altitude difference of 90 m. The slope reached a maximum of 26 % for both trails. The height profiles for both regions are presented in Fig. 1. The intensity of the 1-km CTT was controlled subjectively by using the Borg RPE scale. The participants were instructed to reach a submaximal effort with a maximum value of 15 (hard) perceived on the scale throughout the whole test. A more detailed description of the 1-km CTT is provided in the previously published study (Mayr et al., 2022).
Fig. 1.
A: Height profile of the 1-km cardio-trekking test trail in Aschau im Chiemgau, Germany. B: Height profile of the 1-km cardio-trekking test trail in Werfenweng, Austria.
2.5. Statistical analysis
2.5.1. Descriptive and inferential statistics
All data were corrected by identifying errors or outliers. Subjects with implausible results, a K5 crash or HR failures were excluded. Data from participants who performed both laboratory and field testing were used for the statistical analyses. To ensure maximum exertion during the treadmill test, participants who achieved at least 2 of the following 4 criteria were included in the analysis: 1) maximal Borg value (Borgmax) ≥ 18; 2) respiratory exchange ratio (RER) ≥ 1.1; 3) maximal HR (HRmax) ≥ 85 % of the age-predicted HRmax (using the equation: 220–age); 4) levelling-off oxygen consumption despite an increasing workload, increase in O2 ≤ 150 mL·min−1 (Kline et al., 1987, Hi et al., 2021). V̇O2peak, the highest value of V̇O2 attained upon the maximal incremental walking test, was classified as V̇O2max. Due to the dynamic mixing chamber mode of the K5, which uses a rolling average of over 30 s, the V̇O2 values the highest 10 s of the rolling average.
All variables were tested for normal distribution using the Kolmogorov-Smirnov test. For the descriptive analyses, means and standard deviations (SD) for normally distributed and median and interquartile range (IQR, 25th–75th percentile) for non-normal distribution were reported. Both study groups were tested on mean differences using Student’s t-tests and Mann-Whitney U tests if the distribution was non-normal. Within-group differences between laboratory and field tests were assessed using paired samples t-tests. All statistical analyses were performed using IBM SPSS Statistics version 28 (IBM Inc., Chicago, Illinois), and p values < 0.05 (two-sided) were considered statistically significant.
2.5.2. Predictor model for CRF (V̇O2max)
An interpretable and explainable regression approach was chosen for the estimation of the CRF (V̇O2max). Multiple linear regressions were used to generate multivariate V̇O2max regression equations for the participants who completed both laboratory and field testing. A field estimation model, based on the submaximal 1-km CTT measurements, was created to check if the V̇O2max from the laboratory could be estimated with the data collected from the field measurements.
The following variables were included in the analysis to generate the final model: gender (male or female), age (years), smoking status (current smoker, former smoker, never smoked), weight (kg), maximum Borg score of the 1-km CTT, mean HR during the 1-km CTT in beats per minute (bpm), altitude difference (meters), and duration for completing the 1-km CTT (minutes). To account for possible relationships between the variables, three interaction terms were also added–age*duration, weight*duration, and mean HR*age. The final model was selected based on a backward and forward stepwise approach using the R package MASS (Venables and Ripley, 2002). The linear model assumptions were checked with the global validation test (Peña and Global, 2006) and the R package gvlma (Peña et al., 2022). To analyze the goodness of fit and the precision of the regression model, the R-squared (R2) and the adjusted R-squared (adj. R2) values were computed. Furthermore, to assess predictive ability, we also report R2 of a leave-one-out cross-validation (Cross-Validation et al., 2010) performed with the caret package (Kuhn, 2022) to analyze how the model performed on independent data. We used the same variables and estimated 134 models, one person was omitted from each model. The model was then tested 134 times with one left-out person. We compared R2 of the cross-validated model to the original regression equation results to see how R2 shrinks with cross-validation.
These statistical analyses were performed using R software version 4.1.0 (R: A Language and Environment for Statistical Computing, Vienna, Austria); p values < 0.05 (two-sided) were considered statistically significant.
3. Results
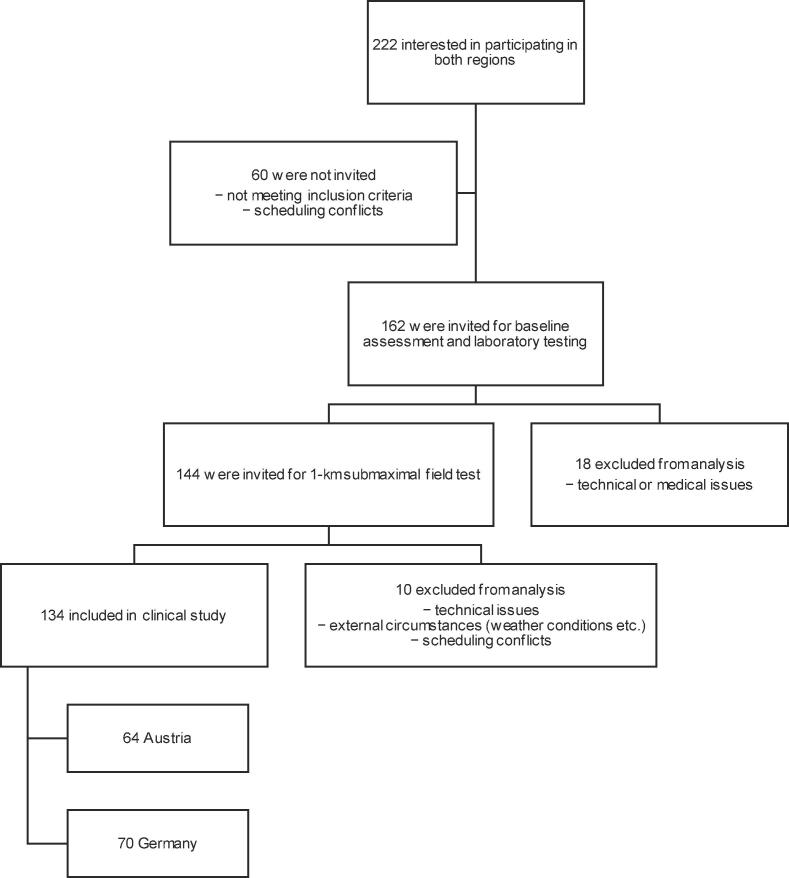
Overall, 222 people were interested in participating in both regions, of which 162 were invited for laboratory testing. In the next step, 144 participants took part in the 1-km CTT. In the end, data from 134 participants, including 64 participants from Austria and 70 from Germany, (median age: 56.0 years [IQR: 51.8–63.0], 43.3 % males, 5.2 % smokers) were used for the final analyses. A flowchart of the study is shown in Fig. 2.
Fig. 2.
Flowchart of the study.
The participants from both regions were comparable in terms of anthropometric data and blood pressure measurements. German participants had a significantly higher total cholesterol (median: 225 mg/dL [IQR: 200–253], p < 0.05) as compared to the Austrian participants (median: 209 mg/dL [IQR: 187–237]). Low-density lipoprotein (LDL) cholesterol was also significantly higher in the German sample (median LDL: 140 mg/dL [IQR: 120–170], p < 0.001) than in the Austrian one (median LDL: 112 mg/dL [IQR: 90–139]). Furthermore, significant differences were observed in both PROCAM Score and HeartScore between samples from the two regions (p < 0.05). Detailed baseline characteristics of all study participants are presented in Table 1.
Table 1.
Baseline characteristics of all participants from Germany and Austria.
|
All participants (N = 134) |
AUT (n = 64) |
GER (n = 70) |
p | |
|---|---|---|---|---|
| Age (years) | 56.0 [51.8–63.0] | 55.0 [51.0–59.8] | 57.5 [52.0–65.0] | 0.088‡ |
| Men (%) | 43.3 | 42.2 | 44.3 | |
| Smokers (%) | 5.2 | 6.3 | 4.3 | 0.337‡ |
| Anthropometrics | ||||
| Height (cm) | 171.6 [8.4] | 171.7 [8.4] | 171.6 [8.5] | 0.955† |
| Weight (kg) | 71.1 [13.3] | 70.5 [13.3] | 71.7 [13.4] | 0.609† |
| BMI (kg/m2) | 24.0 [3.4] | 23.8 [3.3] | 24.2 [3.5] | 0.467† |
| Blood pressure | ||||
| RRsys (mm Hg) | 120 [115–135] | 120 [110–130] | 125 [115–135] | 0.052‡ |
| RRdia (mm Hg) | 80 [70–85] | 80 [71–85] | 75 [70–80] | 0.054‡ |
| Lipid and glucose metabolism | ||||
| CHOL (mg/dL) | 216 [193–243] | 209 [187–237] | 225 [200–253] | 0.030*‡ |
| HDL (mg/dL) | 75 [63–92] | 78 [67–92] | 74 [60–92] | 0.383‡ |
| LDL (mg/dL) | 127 [102–150] | 112 [90–139] | 140 [120–170] | 0.000***‡ |
| TRI (mg/dL) | 77 [60–113] | 77 [61–116] | 78 [59–105] | 0.779‡ |
| GLU (mg/dL) | 91 [85–97] | 90 [85–98] | 91 [85–94] | 0.623‡ |
| Cardiovascular risk | ||||
| FRS score | 2.2 [0.8–5.8] | 1.6 [0.6–5.6] | 2.6 [1.0–6.7] | 0.066‡ |
| PR score | 1.1 [0.6–3.4] | 0.9 [0.4–2.3] | 1.6 [0.7–4.6] | 0.012*‡ |
| HS score | 1.0 [1.0–2.0] | 1.0 [0.0-–0.0] | 1.0 [1.0–3.0] | 0.038*‡ |
| FEV1 (L) | 3.0 [0.7] | 3.0 [0.7] | 3.1 [0.7] | 0.307† |
aData are shown as mean [SD] for normal distribution or median [IQR 25th-75th percentile] for non-normal distribution. *p < 0.05; **p < 0.01; ***p < 0.001; † Student’s t-test; ‡ Mann-Whitney U test. Abbreviations AUT = Austria, BMI = body mass index, CHOL = total cholesterol, FEV1 = forced expiratory volume in the 1st second, FRS score = Framingham risk score, GER = Germany, GLU = fasting blood glucose, HDL = high-density lipoprotein cholesterol, HS score = HeartScore of the European Society of Cardiology (ESC, LDL = low-density lipoprotein cholesterol, N = number of participants, PR score = PROCAM score, RRdia = diastolic blood pressure, RRsys = systolic blood pressure, TRI = triglycerides.
3.1. Exercise capacity
Exercise capacity of all participants who performed the treadmill test in the laboratory and the 1-km CTT in the field are presented in Table 2. Study participants achieved a mean relative V̇O2max of 38.3 [IQR: 34.2–43.1] mL·min−1·kg−1 during exercise testing in the laboratory, which, according to the American College of Sports Medicine’s guidelines for exercise testing and prescription, corresponds with a good (for 50–59-year-old men) and superior (for 50–59-year-old women) fitness level (Riebe et al., 2018). They showed a mean relative V̇O2peak of 37.3 ± 6.3 mL·min−1·kg−1 during the submaximal 1-km CTT outside. The V̇O2peak of all participants of the 1-km CTT was significantly lower than the V̇O2max of all participants during the maximal treadmill test in the laboratory (p < 0.001). Also, there was a statistically significant difference between the Austrian and German samples in the laboratory and field tests (p < 0.05). The Austrian participants had significantly better CRF in both tests (laboratory: median V̇O2max: 39.9 mL·min−1·kg−1 [IQR: 35.4–43.8], field: mean V̇O2peak: 38.5 ± 5.5 mL·min−1·kg−1) compared to the German participants (laboratory: median V̇O2max: 37.7 mL·min−1·kg−1 [IQR: 33.5–41.7], field: mean V̇O2peak: 36.2 ± 6.8 mL·min−1·kg−1). The median Borgmax during laboratory testing was 18 [IQR: 17–19] while the median peak Borg value (Borgpeak) in the field was 15 [IQR: 15–16]. Austrian participants had a significantly higher Borgpeak (median Borgpeak: 18 [IQR: 17–19], p < 0.001) in the laboratory than German participants (median Borgpeak: 17 [IQR: 16–18]). No significant differences were observed in the Borgpeak values for the field tests.
Table 2.
Performance parameters of maximal laboratory testing and submaximal field test.
|
All LAB (N = 134) |
LAB AUT (n = 64) |
LAB GER (n = 70) |
p (GER–AUT) |
All 1-km (N = 134) |
1-km AUT (n = 64) |
1-km GER (n = 70) |
p (GER–AUT) |
p (LAB–1-km) |
|
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 56.0 [51.8–63.0] | 55.0 [51.0–59.8] | 57.5 [52.0–65.0] | 0.088 | 56.0 [51.8–63.0] | 55.0 [51.0–59.8] | 57.5 [52.0–65.0] | 0.088 | |
| Men (%) | 43.3 | 42.2 | 44.3 | 43.3 | 42.2 | 44.3 | |||
| Exercise capacity | |||||||||
| VO2max/peak (mL·min−1·kg−1) | 38.3 [34.2–43.1] | 39.9 [35.4–43.8] | 37.7 [33.5–41.7] | 0.026*‡ | 37.3 [6.3] | 38.5 [5.5] | 36.2 [6.8] | 0.032*† | < 0.001***§ |
| HRmax/peak (bpm) | 165 [14] | 169 [12] | 161 [13] | < 0.001***† | 157 [15] | 162 [11] | 154 [16] | < 0.001***† | < 0.001***§ |
| HRavg (bmp) | 111 [13] | 112 [12] | 111 [13] | 0.706† | 135 [17] | 145 [12] | 126 [16] | < 0.001***† | < 0.001***§ |
| RERmax/peak | 1.1 [1.1–1.2] | 1.1 [1.0–1.2] | 1.1 [1.1–1.2] | 0.371‡ | 1.1 [1.0–1.2] | 1.1 [1.1–1.2] | 1.0 [1.0–1.1] | < 0.001***‡ | < 0.01**§ |
| Borgmax/peak | 18 [17–19] | 18 [17–19] | 17 [16–18] | < 0.001***‡ | 15 [15–16] | 16 [15–17] | 15 [15–16] | 0.227‡ | < 0.001***§ |
| Speedmax/peak (km/h) | 5.6 [5.0–5.9] | 5.8 [5.5–6.0] | 5.4 [4.9–5.8] | < 0.001***‡ | 6.4 [5.7–7.2] | 7.1 [6.7–7.4] | 5.7 [5.3–6.2] | < 0.001***‡ | < 0.001***§ |
| Time (sec) | 922.0 [93.9] | 948.0 [88.7] | 898.3 [92.2] | < 0.01**‡ | 778.7 [102.8] | 791.4 [105.1] | 767.0 [100.1] | 0.371‡ | < 0.001***§ |
| Stagemax | 6 [5–7] | 7 [6–7] | 5 [5–6] | < 0.001***‡ |
b Data are shown as mean [SD] for normal distribution or median [IQR 25th-75th percentile] for non-normal distribution.
*p < 0.05; **p < 0.01; ***p < 0.001; † Student’s t-test; ‡ Mann-Whitney U test; § paired samples t-test.
Abbreviations: 1-km = submaximal 1-km CTT, AUT = Austria, Borgmax/peak = maximal/peak rating of perceived exertion, GER = Germany, HRavg = average heart rate, HRmax/peak = maximal/peak heart rate, LAB = laboratory, RERmax/peak = maximal/peak respiratory exchange ratio, Stagemax = maximal stage of the modified Bruce protocol, VO2max/peak = maximal/peak oxygen uptake.
Moreover, the Borgpeak of the submaximal 1-km CTT was significantly lower (median Borgpeak: 15 [IQR: 15–16], p < 0.001) than Borgmax of the maximal treadmill test in the laboratory (median Borgmax: 18 [IQR: 17–19]). The mean maximal HR (HRmax) in the laboratory was 165 ± 14 bpm and the mean peak HR (HRpeak) in the field was 157 ± 15 bpm. Austrian participants had a significantly higher HRmax in the laboratory testing (mean HRmax: 169 ± 12 bpm, p < 0.001) compared to German participants (mean HRmax: 161 ± 13 bpm). There was also a statistically significant difference in the HRpeak between both groups during the submaximal 1-km CTT (p < 0.01). The HRpeak value during submaximal 1-km CTT was significantly lower than HRmax during maximal treadmill tests (p < 0.001). An outlier value of V̇O2max = 79.8 mL·min−1·kg−1 was removed for the development of the predictor model for CRF.
3.2. Predicted CRF (V̇O2max)
Table 3 shows the results of the multiple linear regression model for the V̇O2max estimation, where V̇O2max was log-transformed to meet all assumptions of the global validation test.
Table 3.
Multiple linear regression model to predict log-transformed V̇O2max.
|
Log(V̇O2max) |
||||
|---|---|---|---|---|
| Predictor | Estimate | Std. error | t-value | Pr(>|t|) |
| (intercept) | 6.443 | 0.510 | 12.633 | < 0.001 |
| Gender (female) | −0.136 | 0.030 | −4.483 | < 0.001 |
| Smoker (formerly) | 0.019 | 0.023 | 0.854 | 0.395 |
| Smoker (yes) | −0.085 | 0.041 | −2.078 | 0.040 |
| Mean heart rate | −0.002 | 0.001 | −2.844 | < 0.001 |
| Altitude difference | 0.002 | 0.001 | 4.714 | 0.005 |
| Age | −0.029 | 0.008 | −3.4538 | 0.001 |
| Duration | −0.154 | 0.034 | −4.459 | < 0.001 |
| Weight | −0.005 | 0.001 | −4.657 | < 0.001 |
| Age*duration | 0.002 | 0.001 | 2.989 | 0.003 |
| Multiple R-squared: 0.6548, Adjusted R-squared: 0.630 F-statistic: 26.13 on 9 and 124 DF, p-value: < 2.2e-16 | ||||
For the final prediction of V̇O2max a bias correction was applied (Wooldridge and Publishing, 2013). We applied a forward and backward feature selection process by using the stepAIC function (Venables and Ripley, 2002). A stepwise algorithm approach leaves 8 of the 11 initial variables in the model: age, gender, smoking status, weight, mean HR, altitude difference, duration, and age*duration. The following resultant model equation was used for the log-transformed V̇O2max:
log(V̇O2max) = 6.442519 – 0.135635*female – 0.004856*weight + 0.019315*former smoker – 0.085353*current smoker – 0.002273*mean HR + 0.002818*altitude difference – 0.029075*age – 0.153760*duration + 0.001722*age*duration.
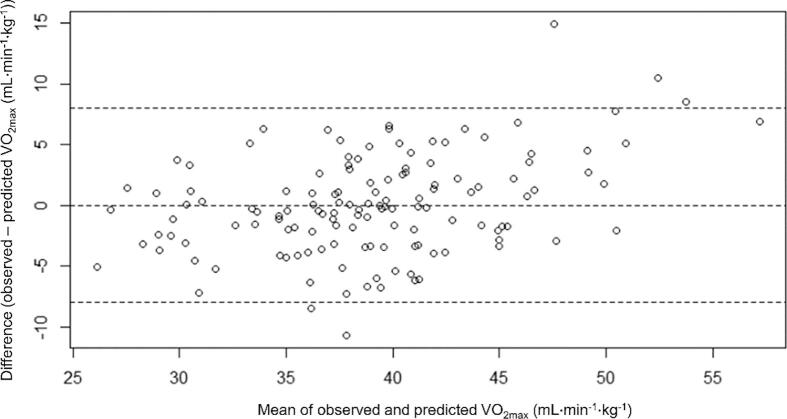
“Female” was replaced by 0 for men and 1 for women. “Former smoker” was replaced by 0 for non-smokers or current smokers and 1 for former smokers. “Current smoker” was replaced by 0 for non-smokers or former smokers and 1 for current smokers. The model showed an R2 of 0.65 and an adjusted R2 of 0.63. Fig. 3 presents a scatterplot of the predicted versus the observed V̇O2max values and Fig. 4 a Bland-Altman analysis. A normal probability plot is shown in Fig. 5.
Fig. 3.
Predicted versus observed V̇O2max values.
Fig. 4.
Bland-Altman plot of V̇O2max differences between observed and predicted values. The outer dashed lines are at ± 95 % limits of agreement.
Fig. 5.
Normal probability plot of the residuals.
The leave-one-out cross-validation showed an R-squared of 0.59 demonstrating a small shrinkage in predictive accuracy compared to the original model and therefore supporting the validity of the prediction model developed in the current study.
4. Discussion
The present study was the first to investigate a prediction of V̇O2max with an outdoor hiking test for healthy adults. Oja et al. (Oja et al., 1991) showed, that walking is an appropriate exercise method for cardiorespiratory fitness estimation. The population of the present study was older than in comparable studies (Kline et al., 1987, Oja et al., 1991, Cao et al., Feb 2013). With a correlation coefficient of r = 0.80 (standard error of estimate (SEE) = 4.2 mL·min−1·kg−1) between the predicted and measured V̇O2max, the present project is in line with previous studies (Evans et al., 2015, Kline et al., 1987, Oja et al., 1991). The calculated predictive model achieved similar or even better predictive power (Oja et al., 1991, Cao et al., Feb 2013, Peterson et al., 2003). The result of the cross-validation was comparable to previous studies (Jalili et al., 2018, Webb et al., 2014).
We conducted this study to help promote physical activity adapted to personal fitness levels, especially for hiking. In this context, the study is intended to raise awareness of possible cardiovascular risk factors. Cardiological and sports medicine examinations should not be replaced here but supplemented with the 1-km CTT as an additional preventive measure.
4.1. On-field reproduction of the treadmill test
Although the objective results of the performance parameters showed significant differences between the maximal incremental walking test in the laboratory and the submaximal 1-km CTT in the field, the differences between the V̇O2, HR, and RER data were not physiologically relevant. Even though the field results were expected to be lower due to the target submaximal intensity (Borg 15), there was a good reproduction of the treadmill test conditions in the 1-km CTT. Grazzi et al. (Grazzi et al., 2017) had successfully reproduced a 1-km laboratory treadmill walking test in an outdoor test, but they had used a flat track with cardiac outpatients. Like our study in a healthy population, the authors also reported similar V̇O2peak results for both test designs. In the patient population, Chiaranda et al. (Chiaranda et al., 2012) developed a valid V̇O2max estimation method for cardiac patients with a 1-km treadmill walking test. They achieved nearly the same correlation coefficients between the predicted and measured V̇O2max as we did. Moreover, their prediction has proven to be a strong predictor of survival in patients with cardiovascular disease (Grazzi et al., 2014).
Although the field test was subjectively described as less intense than the laboratory test, the objective measurement of the RER showed similar intensity for both tests. This suggests that the participants rated their effort lower in the outdoor test than on the treadmill. Exercise performed in a natural environment may feel easier, and it has been established that participants tend to walk faster outdoors when they chose their walking speed and describe a lower RPE (Focht, 2009). Such informal feedback from the participants in the present study indicates that people feel more comfortable in nature than on the treadmill, which could have been a reason for their lower RPE in the field test despite the objective exercise intensity being the same as during the treadmill test. Dasilva et al. (Dasilva et al., 2011) have also confirmed the positive influence of environmental settings on the self-perception of physical performance.
The self-paced fitness test developed outdoors had to be performed at a submaximal intensity level to avoid overexertion and reduce the risk of cardiac events. Self-paced protocols have been reported as a reliable way to measure CRF (Beltz et al., 2016). The use of submaximal hiking as a test method in this study with healthy participants controlled by Borg’s RPE scale proved to be technically feasible, even with older participants. The instruction of “start to walk with a normal, self-selected walking pace and increase your walking speed after 200 m up to a maximum value of 15 (‘hard’) on the Borg scale” was sufficient for acceptable performance in most cases. Occasionally, in very steep sections of the trail, participants’ pace had to be down-regulated to avoid exceeding exhaustion above Borg 15. The 1-km CTT was accompanied by a team member of the study group. Future studies may try administering the test independently without any coaching from experts. Consistent verbal instructions or signs to control the submaximal intensity while testing may help standardize the test procedure.
4.2. Characteristics of the trail
The following features characterized both trails–a mean length of 1000 m ± 100 m, an altitude difference of 100 ± 30 m, a maximum slope of 26 %, safe trail conditions in a natural environment, and no highly frequented paths with sufficient security to perform the 1-km CTT. The incline in the trail helped to achieve a score of 15 (‘hard’) on the Borg scale while walking since healthy and active people might experience difficulties reaching this score in flat areas without running. Therefore, the test should ideally be performed uphill. Additionally, the administration of the 1-km CTT is possible during most of the year, excluding the peak winter months, when performed on a traversable and safe hiking trail. This project aims to expand the scope of this 1-km CTT to other alpine regions by respecting the characteristics of the trails in the 2 pilot regions; further investigations in this area are warranted.
In summary, this project provides a new valid and standardized test method to predict CRF in healthy adults ≥45 years of age with an outdoor hiking test. The 1-km CTT represents a simple and feasible way to promote cardiovascular health which can be performed without any laboratory equipment, other than an HR monitor. Future studies are recommended to assess the potential of the 1-km CTT in different settings.
4.3. Conclusion
V̇O2max is an important indicator of CRF and the strongest predictor for cardiovascular as well as all-cause morbidity and mortality. Therefore, it is of utmost interest to develop valid and feasible clinical tests. The gold standard is a maximal test using a spiroergometry device. The current study describes a new submaximal uphill walking test method for healthy adults in the field to predict V̇O2max while hiking. The exercise intensity is patient-controlled using Borg’s RPE scale. The present project shows that the 1-km CTT is a valid tool for healthy adults above 45 years to predict outdoor CRF. Furthermore, the test is simple, low-risk, does not require special laboratory equipment and enables healthy subjects to estimate V̇O2max with wearable consumer-grade sensors only.
4.4. Limitations of the study
The maximal exertion in the laboratory could be limited by the modified Bruce protocol which requires only walking and does not allow running. The last stages of the protocol consist of speed and incline combinations which may be uncomfortable as they might become too fast to walk. As a result, some participants were biomechanically limited, but not physiologically, and therefore could not reach their V̇O2max. Consequently, more harmonious stage changes with lower speed, which are adapted to walking, could be advantageous. Since the natural environment affects the RPE, it could be important for CVD prevention to set the RPE target of the 1-km CTT lower than 15 to avoid maximal exertion in the field test. The present study examined a fit study group that hiked regularly, which may lead to the conclusion that the formula cannot be used for inexperienced hikers. Further investigations are therefore required. Additionally, our prediction model of CRF is limited to trekking paths at medium altitudes.
CRediT authorship contribution statement
Laura Eisenberger: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization. Barbara Mayr: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – review & editing. Maximilian Beck: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – review & editing. Verena Venek: Methodology, Software, Data curation, Writing – review & editing. Christina Kranzinger: Methodology, Software, Validation, Formal analysis, Data curation, Writing – review & editing. Andrea Menzl: Conceptualization, Investigation, Writing – review & editing. Inga Jahn: Investigation, Writing – review & editing. Mahdi Sareban: Conceptualization, Investigation, Writing – review & editing, Supervision. Renate Oberhoffer-Fritz: Conceptualization, Resources, Writing – review & editing, Supervision. Josef Niebauer: Conceptualization, Resources, Writing – review & editing, Supervision. Birgit Böhm: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – review & editing, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The study was funded by the European Regional Development Fund, INTERREG V-A Program Austria-Bavaria 2014-2020 (Project AB296).
Data availability
Data will be made available on request.
References
- Arena R., Myers J., Williams M.A., et al. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation. 2007;116(3):329–343. doi: 10.1161/circulationaha.106.184461. [DOI] [PubMed] [Google Scholar]
- Assmann G., Schulte H., Cullen P., Seedorf U. Assessing risk of myocardial infarction and stroke: new data from the Prospective Cardiovascular Münster (PROCAM) study. Eur. J. Clin. Invest. 2007;37(12):925–932. doi: 10.1111/j.1365-2362.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- Beltz N.M., Gibson A.L., Janot J.M., Kravitz L., Mermier C.M., Dalleck L.C. Graded exercise testing protocols for the determination of VO(2)max: historical perspectives, progress, and future considerations. J. Sports Med. (Hindawi Publ. Corp.) 2016;2016 doi: 10.1155/2016/3968393. 3968393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair S.N. Physical inactivity: the biggest public health problem of the 21st century. Br. J. Sports Med. 2009;43(1):1–2. [PubMed] [Google Scholar]
- Blair S.N., Kohl H.W., 3rd, Paffenbarger R.S., Jr., Clark D.G., Cooper K.H., Gibbons L.W. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- Borg G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- Bruce R.A., Kusumi F., Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am. Heart J. 1973;85(4):546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- Cao Z.B., Miyatake N., Aoyama T., Higuchi M., Tabata I. Prediction of maximal oxygen uptake from a 3-minute walk based on gender, age, and body composition. J. Phys. Activity Health. 2013;10(2):280–287. doi: 10.1123/jpah.10.2.280. [DOI] [PubMed] [Google Scholar]
- Chiaranda G., Myers J., Mazzoni G., et al. Peak oxygen uptake prediction from a moderate, perceptually regulated, 1-km treadmill walk in male cardiac patients. J. Cardiopulm. Rehabilit. Prevent. 2012;32(5):262–269. doi: 10.1097/HCR.0b013e3182663507. [DOI] [PubMed] [Google Scholar]
- Conroy R.M., Pyörälä K., Fitzgerald A.P., et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur. Heart J. 2003;24(11):987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- Leave-One-Out Cross-Validation. In: Sammut C, Webb GI, eds. Encyclopedia of Machine Learning. Springer US; 2010:600-601.
- Dasilva S.G., Guidetti L., Buzzachera C.F., et al. Psychophysiological responses to self-paced treadmill and overground exercise. Med. Sci. Sports Exerc. 2011;43(6):1114–1124. doi: 10.1249/MSS.0b013e318205874c. [DOI] [PubMed] [Google Scholar]
- Ebbeling C.B., Ward A., Puleo E.M., Widrick J., Rippe J.M. Development of a single-stage submaximal treadmill walking test. Med. Sci. Sports Exerc. 1991;23(8):966–973. [PubMed] [Google Scholar]
- Evans H.J.L., Ferrar K.E., Smith A.E., Parfitt G., Eston R.G. A systematic review of methods to predict maximal oxygen uptake from submaximal, open circuit spirometry in healthy adults. J. Sci. Med. Sport. 2015;18(2):183–188. doi: 10.1016/j.jsams.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Ferguson, B., 2014. ACSM’s Guidelines for Exercise Testing and Prescription 9th Ed. 2014. J Can Chiropr Assoc. 58(3):328–328.
- Focht B.C. Brief walks in outdoor and laboratory environments: effects on affective responses, enjoyment, and intentions to walk for exercise. Res. Q. Exerc. Sport. 2009;80(3):611–620. doi: 10.1080/02701367.2009.10599600. [DOI] [PubMed] [Google Scholar]
- Fredman P., Tyrväinen L. Frontiers in nature-based tourism. Scandin. J. Hospit. Tourism. 2010;10(3):177–189. doi: 10.1080/15022250.2010.502365. [DOI] [Google Scholar]
- Grazzi G., Myers J., Bernardi E., et al. Association between VO₂ peak estimated by a 1-km treadmill walk and mortality. A 10-year follow-up study in patients with cardiovascular disease. Int. J. Cardiol. 2014;173(2):248–252. doi: 10.1016/j.ijcard.2014.02.039. [DOI] [PubMed] [Google Scholar]
- Grazzi G., Chiaranda G., Myers J., et al. Outdoor reproducibility of a 1-km treadmill walking test to predict peak oxygen uptake in cardiac patients. J. Cardiopulm. Rehabilit. Prevent. 2017;37(5):347–349. doi: 10.1097/hcr.0000000000000266. [DOI] [PubMed] [Google Scholar]
- Hi Y., Cho W., Lee D.H., Suh S.-H., Jeon J.Y. Development of a new submaximal walk test to predict maximal oxygen consumption in healthy adults. Sensors (Basel, Switzerland) 2021;21 doi: 10.3390/s21175726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili M., Nazem F., Sazvar A., Ranjbar K. Prediction of maximal oxygen uptake by six-minute walk test and body mass index in healthy boys. J. Pediatrics. 2018;200:155–159. doi: 10.1016/j.jpeds.2018.04.026. [DOI] [PubMed] [Google Scholar]
- Kline G.M., Porcari J.P., Hintermeister R., et al. Estimation of VO2max from a one-mile track walk, gender, age, and body weight. Med. Sci. Sports Exerc. 1987;19(3):253–259. [PubMed] [Google Scholar]
- Kuhn, M., Caret: Classification and Regression Training. R package version 6.0-88. Accessed 07.03.2022, https://CRAN.R-project.org/package=caret.
- Mayr B., Beck M., Eisenberger L., et al. Valorization of natural cardio trekking trails through open innovation for the promotion of sustainable cross-generational health-oriented tourism in the connect2move project: protocol for a cross-sectional study. JMIR Res. Protocols. 2022;11(7):e39038. doi: 10.2196/39038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., McAuley P., Lavie C.J., Despres J.-P., Arena R., Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog. Cardiovasc. Dis. 2015;57(4):306–314. doi: 10.1016/j.pcad.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Niebauer J. Call for truly maximal ergometries during clinical routine. Eur. J. Preventive Cardiol. 2020;26(7):728–730. doi: 10.1177/2047487319831875. [DOI] [PubMed] [Google Scholar]
- Niebauer J., Burtscher M. Sudden cardiac death risk in downhill skiers and mountain hikers and specific prevention strategies. Int. J. Environ. Res. Public Health. 2021;18(4) doi: 10.3390/ijerph18041621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan V., Dean E. Submaximal exercise testing: clinical application and interpretation. Phys. Ther. 2000;80(8):782–807. doi: 10.1093/ptj/80.8.782. [DOI] [PubMed] [Google Scholar]
- Oja P., Laukkanen R., Pasanen M., Tyry T., Vuori I. A 2-km walking test for assessing the cardiorespiratory fitness of healthy adults. Int. J. Sports Med. 1991;12(4):356–362. doi: 10.1055/s-2007-1024694. [DOI] [PubMed] [Google Scholar]
- Peña E.A., Slate E.H. Global validation of linear model assumptions. J. Am. Stat. Assoc. 2006;101(473):341. doi: 10.1198/016214505000000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña, E.A., Slate, E.H., gvlma: Global Validation of Linear Models Assumptions. R package version 1.0.0.3. Accessed 22.02.2022, https://CRAN.R-project.org/package=gvlma.
- Peterson M.J., Pieper C.F., Morey M.C. Accuracy of VO2 max prediction equations in older adults. Med. Sci. Sports Exerc. 2003;35(1):145–149. doi: 10.1097/00005768-200301000-00022. [DOI] [PubMed] [Google Scholar]
- Riebe, D., Ehrman, J.K., Liguori, G., Magal, M., eds. ACSM's guidelines for exercise testing and prescription. 10th ed. Wolters Kluwer; 2018.
- Ross R., Blair S.N., Arena R., et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653–e699. doi: 10.1161/cir.0000000000000461. [DOI] [PubMed] [Google Scholar]
- Roth, G.A., Mensah, G.A., Johnson, C.O., et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 Study. J. Am. College Cardiol. 2020;76(25):2982-3021. doi:10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed]
- Seals D.R., Edward F., Lecture A.D. The remarkable anti-aging effects of aerobic exercise on systemic arteries. J. Appl. Physiol. (Bethesda, Md: 1985) 2014;117(5):425–439. doi: 10.1152/japplphysiol.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrväinen L., Pauleit S., Seeland K., Vries S. Urban Forests and Trees. Springer; 2005. Benefits and uses of urban forests and trees; pp. 81–114. [Google Scholar]
- Venables W.N., Ripley B.D. fourth ed. Springer; 2002. Modern Applied Statistics with S. [Google Scholar]
- Visseren F.L.J., Mach F., Smulders Y.M., et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021;42(34):3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- Webb, C., Vehrs, P.R., George, J.D., Hager, R., 2014. Estimating VO2max Using a Personalized Step Test. Measurement in Physical Education and Exercise Science. 2014;18(3):184-197. doi:10.1080/1091367X.2014.912985.
- Wen C.P., Wai J.P.M., Tsai M.K., et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–1253. doi: 10.1016/s0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- Wilson P.W., D'Agostino R.B., Levy D., Belanger A.M., Silbershatz H., Kannel W.B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Winkert K, Kirsten J, Kamnig R, Steinacker JM, Treff G. Differences in V̇O2max measurements between breath-by-breath and mixing-chamber mode in the COSMED K5. Int. J. Sports Physiol. Performance. 2021;16(9):1335–1340. doi:10.1123/ijspp.2020-0634. [DOI] [PubMed]
- Wooldridge, J.M., Publishing, S.-W.E., 2013. Introductory Econometrics. 5th ed. ed. Upper Level Economics Titles. Cengage Learning US; 912.
- World Health Organization, 2022. Cardiovascular diseases. World Health Organization. Accessed 25th of April, 2022 https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.