Abstract
As SARS-CoV-2 variants of concern (VOC) reduce the effectiveness of existing anti-COVID therapeutics, it is increasingly critical to identify highly potent neutralizing antibodies (nAbs) that bind to conserved regions across multiple variants, especially beta, delta, and omicron variants. Using single-cell sequencing with biochemical methods and pseudo-typed virus neutralization experiments, here we report the characterization of a potent nAb BD-218, identified from an early screen of patients recovering from the original virus. We have determined the cryo-EM structure of the BD-218/spike protein complex to define its epitope in detail, which revealed that BD-218 interacts with a novel epitope on the receptor-binding domain (RBD) of the spike protein. We concluded that BD-218 is a highly effective and broadly active nAb against SARS-CoV-2 variants with promising potential for therapeutic development.
Keywords: SARS-CoV-2, Neutralizing antibody
Abbreviations: VOC, Variants of Concern; RBD, Receptor Binding Domain; nAbs, Neutralizing Antibodies; SPR, Surface Plasmon Resonance
1. Introduction
The ongoing COVID-19 pandemic caused by SARS-CoV-2 has been spreading globally [1] for almost three years, leading to the emergence of different virus mutants over time. Emerging SARS-CoV-2 variants of concern (VOCs) have developed resistance to neutralizing antibodies, including some clinical antibodies that are used as therapeutics. Mutations on the receptor-binding domain (RBD) of the spike protein have likely led to increased transmissibility and a partial escape from humoral immunity induced by vaccines made from the original strain of SARS-CoV-2 [2], [3], [4]. The most widely circulating omicron variant has 15 mutations of the RBD [5], [6], in particular, L452 substitutions have led to omicron sublineages with higher transmission advantage over previously-emerged variants [7]. Although three to four doses of vaccines have been reported to be limited effective on delta and omicron variants [8], [9], [10], controlling this pandemic has been remaining a critical issue.
To restrict the further spread of variants and hospitalization rate, efforts to improve vaccine effectiveness—such as maximizing vaccine uptake with at least three doses [11] and improving vaccine design should continue. Alongside these efforts, effective therapeutics against severe disease namely, SARS-CoV-2 nAbs, which have shown promising therapeutic efficacy for COVID-19 patients should continue to be developed. With the emergence of increasingly transmissible variants, like delta and omicron variants [7], the need for continued screening and characterization of more nAbs has correspondingly increased. Research into nAbs will aid in the development of broad vaccine design against concerning variants; furthermore, the lessons learned from SARS-CoV-2 can also be applied to the fight against other emerging, infectious pathogens.
Here, we have characterized a series of potent nAbs in detail from previously recovered COVID-19 patients [12], [13], one of which is BD-218. Surface plasmon resonance (SPR) experiments showed strong affinities between BD-218 and the RBDs of several circulating variants. Moreover, we identified that BD-218 could efficiently neutralize pseudo-typed viruses with different circulated and circulating mutations. We further investigated the mechanism by which BD-218 targets the circulating variants' RBDs by solving their cryo-EM complex structure and comparing it with nine antibody-based drugs or reported potent antibodies. Together, our results demonstrated that BD-218 recognizes a novel and robust epitope within concerning variants and has strong potential as a broad-spectrum nAb drug to treat COVID-19.
2. Materials and methods
2.1. Protein expression and purification
The spike protein (S-6P: S-HexaPro) expression construct was obtained from Dr. Junyu Xiao's lab; it encodes the spike ectodomain (residues 1–1208) with six stabilizing Pro substitutions (F817P, A892P, A899P, A942P, K986P, and V987P) and a ‘GSAS’ substitution at the furin cleavage site (residues 682–685), as previously described [13]. The S-6P plasmid was transfected into HEK293F cells at a cell density of 106 cells/mL and expressed for four days. The S-6P protein was purified using the Ni-NTA resin followed by the Superose 6 Increase 10/300 gel filtration column (Cytiva, Marlborough, MA, USA), and eluted using the final buffer containing 25 mM Tris (pH 8.0) and 150 mM NaCl.
The BD-218 Fab heavy chain and light chain sequences were cloned into pcDNA3.1 plasmids with a signal peptide and C-terminal His6-tag. Plasmids with the heavy chain and light chain were mixed at a 1:1 ratio and transfected into HEK293F cells using polythylenimine. After incubation for four days, the conditioned media were collected, concentrated, and exchanged into the binding buffer containing 25 mM Tris (pH 8.0) and 150 mM NaCl. The BD-218 Fab was then purified using the Ni-NTA resin and Superdex 200 Increase column (Cytiva).
2.2. Cryo-EM sample preparation and data collection
Holey‑carbon gold grids (Quantifoil, R1.2/1.3) were glow-discharged for 45 s using a Solarus 950 Plasma Cleaner (Gatan Inc., Berwyn, PA, USA) with a 4:1 O2/H2 ratio. We sufficiently mixed 4 μL S-6P (~0.2 mg/mL) and 0.5 μL BD-218 Fab (1.2 mg/mL) at room temperature, and then quickly applied the mixture onto the glow-discharged grids. The grids were then blotted with filter paper (Whatman No. 1) at 4 °C and 100 % humidity and injected onto liquid ethane using a Vitrobot Mark IV System (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The grids were first screened using a 200 kV Talos Arctica transmission electron microscope equipped with a Ceta camera (Thermo Fisher Scientific, Inc.). Data collection was carried out using a Titan Krios electron microscope (Thermo Fisher Scientific, Inc.) operated at 300 kV. Movies were recorded on a K2 Summit direct electron detector (Gatan Inc.) using SerialEM software [14], in the super-resolution mode at a nominal magnification of 130,000 and an exposure rate of 7.125 e−/Å2/s. The defocus range was set from −0.7 to −1.5 μm. The micrographs were dose-fractioned into 32 frames with a total exposure time of 8 s and a total electron exposure of 57 e−/Å2.
2.3. Cryo-EM data processing
The workflow of data processing is shown in Supplementary Fig. S1. Motion correction with 4633 stacks was carried out using MotionCor2 [15], and all movies were binned 2-fold, resulting in a pixel size of 1.055 Å/pixel. The defocus values were estimated with the Gctf program [16]. A total of 417,369 particles were auto-picked using the AutoPick node in Relion 3.1, with the EMD-30374 map as a reference [17], and then subjected to 2D classification without symmetry restriction, which yielded 297,084 good particles. The good particles were selected and subjected to heterogeneous refinement using cryoSPARC [18]. The good particles were further selected and subjected to non-uniform without symmetry, resulting in a three-dimensional reconstruction of the whole structure and a map with an average resolution of 3.74 Å. To improve the map quality for the interface between the S-6P protein of SARS-CoV-2 and BD-218, the dataset was subject to focused refinement with an adapted mask on the region of the RBD/BD-218 sub-complex. Finally, the dataset was re-centered on the interface between RBD and BD-218, resulting in a local refinement map with an average resolution of 4.01 Å. The resolution was estimated with the gold-standard Fourier shell correlation 0.143 criteria [19] with high resolution noise substitution.
2.4. Model building and structure refinement
For structure building of the S-6P protein with BD-218, an initial model was first obtained from the PDB as a template (PDB ID: 6xm4). The template was flexibly fitted into the whole cryo-EM map of the complex using UCSF Chimera [20]. Additionally, a Fab model and an RBD model, generated as templates from our previous work, were docked into the local refined map. Finally, all coordinates were merged with the S-6P protein and further manually adjusted using Coot [21]. Each residue of the complex was manually checked, and real space refinement was performed in Phenix [22]. Statistics associated with data collection, 3D reconstruction, and model building are summarized in Supplementary Table S1.
2.5. Surface plasmon resonance
A Biacore T200 (Cytiva) was used to measure and compare the dissociation coefficients between antibodies and the RBD of SARS-CoV-2 and its mutants. Fabs of BD-218 were captured to 200–300 RU on a Series S Sensor Chip CM5 (Cytiva). Next, serial 2-fold dilutions of SARS-CoV-2 RBD and mutants were injected, with concentrations from 20 to 0.63 nM. All proteins were exchanged into running buffer containing 10 mM HEPES (pH 7.4), 150 mM NaCl, 3 mM EDTA, and 0.005 % (v/v) P20. The final data were processed using the Biacore Evaluation Software and fitted to a 1:1 binding model.
2.6. Pseudo-typed virus neutralization assay
The pseudo-typed virus neutralization assays were performed using Huh-7 cell lines. Pseudo-typed viruses were prepared as previously described [12]. Various concentrations of antibodies (3-fold serial dilution using DMEM) were mixed with the same volume of SARS-CoV-2 pseudo-typed virus in a 96-well plate. The mixture was incubated for 1 h at 37 °C and supplied with 5 % CO2. Pre-mixed Huh-7 cells were added to all wells and incubated for 24 h at 37 °C and supplied with 5 % CO2. After incubation, the supernatants were removed, and D-Luciferin reagent (Invitrogen, Waltham, MA, USA) was added to each well; luciferase activity was measured using an EnSight microplate spectrophotometer (PerkinElmer, Waltham, MA, USA). The inhibition rate was calculated by comparing the OD value to the negative and positive control wells. The EC50 values were determined with a four-parameter logistic regression using Origin (OriginLab).
3. Results
3.1. BD-218 strongly binds to RBDs and potently neutralizes spike proteins of concerning variants
The third complementarity-determining region of the heavy chain (CDR-H3) of BD-218 is encoded by the VH4–34 lineage gene, which is an essential germline gene in humans [23]. Sequence alignment analysis reveals that BD-218 is not conserved with other previously reported high-efficiency nAbs or antibody-based drugs against SARS-CoV-2 (Fig. 1A,B). The non-conserved sequence of BD-218 may lead to a different epitope on the RBD. As we previously reported, BD-218 was a high-efficiency antibody against the wildtype of SARS-CoV-2 (Cao et al., 2020). To evaluate BD-218's activity against global variants of concern, such as the alpha, beta, delta, and other variants [24], SPR experiments were carried out to measure the binding affinity between BD-218 and these major variants. As expected, BD-218 binds to the RBD of variants with high affinity; we observed a very small KD value of 0.16 nM with the delta variant, which was similar to those observed with the wildtype, gamma, and kappa variants (Fig. 1C). Additionally, to evaluate the capacity of BD-218 to neutralize lentiviral particles pseudo-typed with several variants' spike proteins, we performed neutralization assays with pseudo-virus expressing wildtype, and several other variants of concern. Consistent with SPR results, the BD-218 displayed significant potency against the delta spike-based pseudo-virus (EC50 of 0.145 μg/mL), as well as the wildtype, gamma, and kappa variants (Fig. 1D). Furthermore, the latest omicron sublineages pseudo-typed viruses were also used to detect the neutralization activity of BD-218. neutralization capacity of BD-218 against omicron variants decreased marginally but remained strong compared to most other nAbs in the pseudo-typed viral neutralization assay investigations (Fig. 1D). For example, BD-218 could neutralize the BA.5, the most widely circulating omicron sublineage in the world. Moreover, BD-218 can also provide neutralizing activity to the BF.7 starin, which is widespreading in China. Although BD-218 cannot neutralize omicron sublineage XBB, it might be used with other highly active nAbs to offer higher and more general neutralizing activity.
Fig. 1.
BD-218 strongly binds to receptor binding domains (RBDs) and potently neutralizes spike proteins of major SARS-CoV-2 variants. A and B. Sequence alignment of heavy chain and light chain CDR regions within BD-218, DXP-604, LY-CoV555, LY-CoV016, REGN-10933, REGN-10987, AZD-8895, AZD-1061, VIR-7831, and BD-368-2. C. Surface plasmon resonance sensorgrams of neutralizing antibodies binding to the RBD of wildtype, gamma, delta, delta plus, and kappa variants. All analyses were performed using serial two-fold dilutions of purified RBDs as the analyte, starting from 20 nM to 0.625 nM. D. Neutralization potency of BD-218 on wildtype, gamma, delta, kappa and some circulated and circulating omicron sublineage of SARS-CoV-2 pseudo-typed virus. The yellow color indicates the neutralization activity fell slightly, and the red color indicates the neutralization activity decreased significantly. Data were obtained from a representative neutralization experiment, which contains three replicates. Data are represented as mean ± SD.
3.2. The structural binding mechanism of BD-218 neutralizes concerning variants efficiently
To investigate the molecular mechanism by which BD-218 neutralizes SARS-CoV-2, we determined the complex structure of the nAb BD-218 Fab form with the S-HexaPro (S-6P) protein of SARS-CoV-2 using the single particle method, at an overall resolution of 3.74 Å (Fig. 2A, Supplementary Fig. S1, and Supplementary Table S1). The spike protein exhibited an asymmetric conformation, consistent with previous observations [13], [25], with one RBD in an ‘up’ conformation bound to BD-218, and two RBDs in a ‘down’ conformation in the complex structure (Fig. 2A). This complex structure suggests that the binding of a single BD-218 to the RBD in the ‘up’ state prevents this ‘up’ RBD from binding to ACE2. Together, our data have shown that BD-218 retains broad neutralization activity against concerning variants.
Fig. 2.
Structural basis of BD-218 bound to spike protein. A. Cryo-EM structure of the S-6P trimer in complex with BD-218 Fab reconstructed at 3.74 Å. The three RBDs are highlighted as follows: ‘up’ in orange and ‘down’ in green and purple-blue. The fragment variable (Fv) region of BD-218 is shown in grey (light chain) and salmon pink (heavy chain). B. Schematic of BD-218 bound to SARS-CoV-2 spike protein., including the interaction between BD-218 Fab and the RBD is shown. BD-218 is shown in the cartoon, whereas RBD is shown in the spheres view. Main regions in BD-218 that interact with RBD are highlighted using thicker ribbons. C. Interactions between CDRH1, CDRH2, and RBD. A dashed line indicates major interactions. D. Interactions between CDRL3 and RBD. E. Schematic shows the two hydrophobic cores on RBD that are recognized by BD-218. The red color indicates hydrophobic surface, and the white color indicates hydrophilic surface. F. Superimposition of the structure of RBD in the S-6P/BD-218 complex and the RBD/ACE2 complex (PDB: 6m0j). BD-218 would inhibit ACE2 binding to the RBD via VH.
BD-218's broad neutralization activity indicates that the antibody recognizes a conserved region in the RBD of the different variants. To find out the structural basis of the BD-218/RBD binding interface in detail, we used local refinement to account for the conformational dynamics of BD-218 relative to the RBD and obtained a cryo-EM reconstruction of this binding interface with 4.01 Å resolution (Fig. 2B-D). BD-218 recognizes an epitope towards the center of the spike protein trimer, which sterically hinders BD-218 from binding to the ‘down’ RBDs by the adjacent protomer (Fig. 2B).
We have adopted the RBD nomenclature defined by Dejnirattsai et al. [26], in which the antibody-binding sites of the RBD were divided into six groups: left shoulder, neck, right shoulder, left flank, chest, and right flank. BD-218 binds to the neck of the RBD. The epitope of BD-218 overlaps with the ACE2 binding sites on the RBD to a large extent (Figs. 2C,D and 3A). Consistent with our neutralization assay analyses, BD-218 can competitively inhibit the ‘up’ RBD conformation from binding to ACE2. Three regions in BD-218 are primarily involved in interacting with the RBD: heavy chains CDRH1 and CDRH2, and light chain CDRL3 (Fig. 2B). Among the prominent interactions, D31 on CDRH1 forms hydrogen bonds with Y453 of RBD, D50 of CDRH2 contacts Y489 on RBD, and S92 of CDRL3 interacts with S477 of RBD via its hydroxy group (Fig. 2C and D). The aromatic residues of the RBD are particularly crucial for the recognition between BD-218 and RBD. The aromatic groups of Y505 and Y453 are recognized by CDRH1, and the aromatic group of Y489 is involved in the interaction with CDRH2 (Fig. 2C). BD-218 recognizes two hydrophobic cores on the RBD, which are formed by the hydrophobic residues mentioned above via hydrophobic interactions (Fig. 2E). Furthermore, a structural superimposition of the S-6P/BD-218 and RBD/ACE2 complexes reveals a notable clash between the variable domain on the heavy chain (VH) of the BD-218 Fab and ACE2 (Fig. 2F). Additionally, we noted substitutions on the RBD that are likely to lead to viral immune system evasion, such as L452R and E484K. The L452R substitution leads to increased transmissibility in the majority of omicron lineages; it breaks down the hydrophobic core of the RBD, which disrupts the hydrophobic interaction between antibodies and the RBD. The E484K substitution breaks the salt bridge interaction between antibodies and the RBD. However, BD-218 recognizes the RBD independently of L452 and E484; therefore, the neutralization capacity of BD-218 is not affected by the concerning variants with these substitutions. The complex structure reveals the epitope of BD-218 and provides further atomic insights into the critical interactions between BD-218 and the RBD.
Fig. 3.
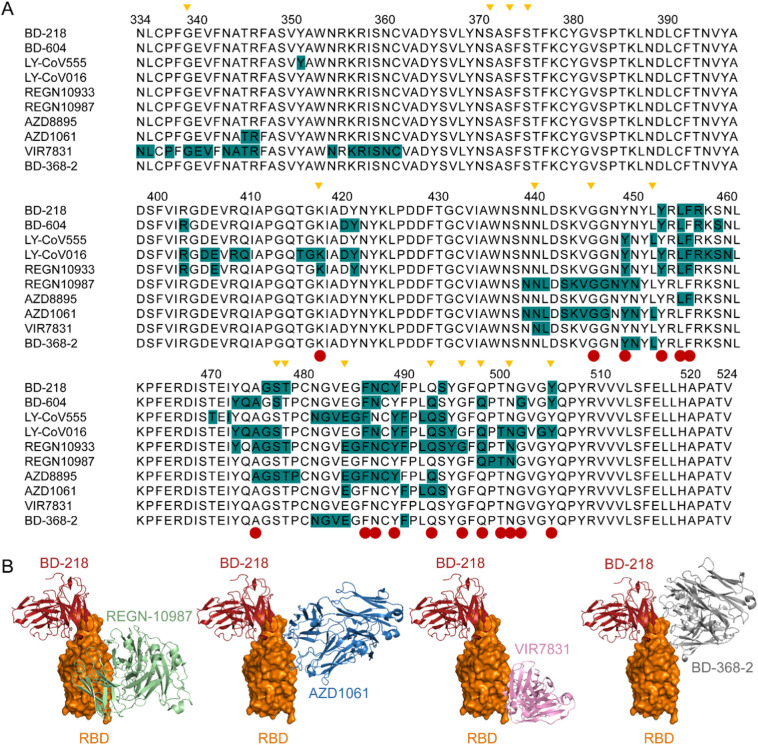
The conserved epitope of BD-218 compared to other potent neutralizing antibodies. A. Multiple sequence alignment showing the epitope footprints of BD-218 and other antibody drugs on the SARS-CoV-2 RBD highlighted in dark cyan. Red circle below the alignment indicates hACE2 contact residues on the SARS-CoV-2 RBD. Yellow inverted triangles indicate mutated sites on the RBD that have been reported in concerning variants. B. Combinations of BD-218 with REGN-10987, AZD1061, VIR7831, and BD-368-2, respectively, without spatial overlaps. BD-218 is shown in red cartoon and RBD is shown in orange surface form.
3.3. BD-218 has potent neutralization capability due to its recognition of a novel epitope compared with other high-efficiency nAbs
The cryo-EM structure of BD-218 with the spike protein reveals several conserved residues among the concerning variants as compared with previously reported high-efficiency nAbs and commercial antibodies. The main binding site of BD-218 consists of Y453, L455, F456, F486, N487, and Y489 (Fig. 3A); these residues are also recognized by many other potent nAbs, as well as ACE2 (Fig. 3A). These residues constitute a conserved recognition site and play a major role in the recognition of the RBD by BD-218 through hydrogen bonding and hydrophobic interactions. It is worth noting that, unlike other potent nAbs or commercial antibodies, BD-218 did not recognize the reported substituted residues on the RBD in different variants, including the omicron variant. Consequently, different reported mutations of concerning variants have minor impacts on the binding activity of BD-218 to the RBD, and the conserved amino acids contribute to a robust neutralization capability of BD-218.
In addition to the conserved recognition site, a novel amino acid combination contributes to the BD-218's potent binding activity. Unlike many other reported nAbs, BD-218 relies not only on the heavy chain to recognize the RBD but also on the light chain to provide a larger junction area. Y453 and C488 were recognized by the heavy chain of BD-218, while T478 was bound by the light chain of BD-218. The combination of these three amino acids has rarely been reported (Fig. 3A), and provides multiple hydrogen bond interactions with the antibody, causing BD-218 to bind a different location relative to reported nAbs. Furthermore, the conserved F486 and Y489 residues offer strong hydrophobic interactions with BD-218 (Figs. 2C,E and 3A). Combined, this novel combination and conserved recognition site demonstrate a potent neutralization activity for BD-218.
We defined the novel epitope of BD-218 through further structural analysis. The BD-218 recognition site is very similar to REGN-10933, which is an antibody-drug that has received emergency use authorization (EUA) from the FDA. Both antibodies primarily recognize Y453, L455, F456, F486, N487, and Y489. Like REGN-10933, this conserved epitope allows BD-218 to strengthen its neutralizing activity by pairing with other potent neutralizing antibodies. Therefore, we carried out structure superimpositions, which indicated that BD-218 could pair with REGN-10987, AZD1061, VIR7831, and BD-368-2, without steric hindrance (Fig. 3B).
4. Conclusion and discussion
We have characterized BD-218, obtained from screenings of recovered COVID-19 patients, as a potent nAb with broad activity against concerning variants of SARS-CoV-2. Structural analysis and biochemistry experiments demonstrated that BD-218 could block the ‘up’ RBD conformation, thereby exerting a potent neutralization effect, thus offering its potential use as an antibody-drug against currently circulating and other emerging SARS-CoV-2 variants.
An ideal anti-SARS-CoV-2 antibody would be resistant to viral escape, and highly protective through viral neutralization and antibody effector functions. Potent nAbs against SARS-CoV-2 were identified from recovered patients [12], [13], [27], [28], [29]. Among these previously screened antibodies, BD-218 performs effectively to neutralize the RBD of wildtype and major variants, including the widespread omicron variants [5], [6], [12], [30]. Most highly potent neutralizing antibodies recognize and bind to the left shoulder and neck of the RBD, which is also the ACE2 binding site [31], [32]; however, the high-frequency substitutions L452R and E484K that occur in many variants are also located on the neck and left shoulder. Therefore, concerning variants are likely to reduce the neutralizing activity of nAbs that recognize the neck and left shoulder of the RBD. Alternatively, BD-218 recognizes a different part of the neck and shoulder on the back of the RBD (Fig. 2A); since this recognition site is dominated by Y453, T478, and C488, the epitope of BD-218 involves neither L452 nor E484. Consequently, antibodies that bind to the neck position on the back of the RBD will not be affected by variants, in contrast to some VH3-53/66 germline-encoded antibodies. Although BD-218 is unable to neutralize a few omicron sublineages such as XBB, it might be paired with other highly active nAbs to offer higher and more general neutralizing efficacy.
BD-218 and some potent nAbs against SARS-CoV-2 recognize a conserved site of the RBD. There was no residue substitution and deletion reported on the conserved site, even in the omicron variant (Fig. 3A). Subsequently, antibodies like BD-218 that recognize these residues could provide potent neutralizing activity against concerning variants of SARS-CoV-2. Furthermore, this conserved site could also guide vaccine design and optimization. Robust vaccines could be developed based on the sequence and structure of the conserved epitope, which could result in increased circulation of nAbs like BD-218 that recognize this conserved site.
CRediT authorship contribution statement
Bo Wang: Data curation, Methodology, Formal analysis, Writing – original draft. Hua Xu: Data curation, Methodology, Formal analysis. Zi-teng Liang: Validation, Investigation. Tian-ning Zhao: Validation, Investigation. Xin Zhang: Data curation. Tian-bo Peng: Data curation. You-chun Wang: Conceptualization. Xiao-dong Su: Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank the National Center for Protein Sciences at Peking University in Beijing, China, for assistance with SPR assays. We thank the cryo-EM platform of Peking University for help with data collection. This project was supported by the National Key Research and Development Program of China (2021YFC2301301 to X.D.S, and 2021YFC2301402 to X.D.S), the Ministry of Science and Technology of China (2020YFC0848700), and the Qidong-SLS Innovation Fund (Both to X.D.S). Special thanks to Drs. Xiaoliang Xie, Junyu Xiao, and Yunlong Cao, for providing plasmid and IgG samples of the antibodies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijbiomac.2022.12.120.
Appendix A. Supplementary data
Supplementary material for Human Antibody BD-218 with Broad-Activities against Concerning Variants of SARS-CoV-2.
Data availability
Data will be made available on request.
References
- 1.E. Callaway D. Cyranoski S. Mallapaty E. Stoye J. Tollefson (Nature Publishing Group, 2020). [DOI] [PubMed]
- 2.Lupala C.S., Ye Y., Chen H., Su X.-D., Liu H. Mutations on RBD of SARS-CoV-2 omicron variant result in stronger binding to human ACE2 receptor. Biochem. Biophys. Res. Commun. 2022;590:34–41. doi: 10.1016/j.bbrc.2021.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M., et al. Reduced sensitivity of the SARS-CoV-2 lambda variant to monoclonal antibodies and neutralizing antibodies induced by infection and vaccination. Emerg.MicrobesInfect. 2022;11:18–29. doi: 10.1080/22221751.2021.2008775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L., et al. Ten emerging SARS-CoV-2 spike variants exhibit variable infectivity, animal tropism, and antibody neutralization. Commun.Biol. 2021;4:1196. doi: 10.1038/s42003-021-02728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y., et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by omicron infection. Nature. 2022;608:593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y., et al. 2022. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. bioRxiv, 2022.2004.2030.489997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemet I., et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection. N. Engl. J. Med. 2021;386:492–494. doi: 10.1056/NEJMc2119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez Bernal J., et al. <sb:contribution><sb:title>Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta)</sb:title></sb:contribution> <sb:host><sb:issue><sb:series><sb:title>variant</sb:title></sb:series></sb:issue></sb:host>. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2108891. [DOI] [Google Scholar]
- 10.Garcia-Beltran W.F., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 omicron variant. Cell. 2022;185:457–466.e454. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muecksch F., et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost. Nature. 2022 doi: 10.1038/s41586-022-04778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y., et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients' B cells. Cell. 2020;182:73–84 e16. doi: 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du S., et al. Structurally resolved SARS-CoV-2 antibody shows high efficacy in severely infected hamsters and provides a potent cocktail pairing strategy. Cell. 2020;183:1013–1023.e1013. doi: 10.1016/j.cell.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mastronarde D.N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Zheng S.Q., et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 2016;193:1–12. doi: 10.1016/j.jsb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zivanov J., et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife. 2018;7 doi: 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Punjani A., Rubinstein J.L., Fleet D.J., Brubaker M.A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods. 2017;14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal P.B., Henderson R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 2003;333:721–745. doi: 10.1016/j.jmb.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Pettersen E.F., et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 21.Emsley P., Lohkamp B., Scott W.G., Cowtan K. Features and development of coot. Acta Crystallogr. Sect. D. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liebschner D., et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in phenix. Acta Crystallogr. Sect. D. 2019;75:861–877. doi: 10.1107/S2059798319011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schickel J.-N., et al. Self-reactive VH4-34-expressing IgG B cells recognize commensal bacteria. J. Exp. Med. 2017;214:1991–2003. doi: 10.1084/jem.20160201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L., et al. Ultrapotent antibodies against diverse and highly transmissible SARS-CoV-2 variants. Science. 2021 doi: 10.1126/science.abh1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan R., et al. Structural basis for bivalent binding and inhibition of SARS-CoV-2 infection by human potent neutralizing antibodies. Cell Res. 2021;31:517–525. doi: 10.1038/s41422-021-00487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dejnirattisai W., et al. The antigenic anatomy of SARS-CoV-2 receptor binding domain. Cell. 2021;184:2183–2200.e2122. doi: 10.1016/j.cell.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asarnow D., et al. Structural insight into SARS-CoV-2 neutralizing antibodies and modulation of syncytia. Cell. 2021;184:3192–3204.e3116. doi: 10.1016/j.cell.2021.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes C.O., et al. Structures of human antibodies bound to SARS-CoV-2 spike reveal common epitopes and recurrent features of antibodies. Cell. 2020;182:828–842. e816. doi: 10.1016/j.cell.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ju B., et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 30.Xu H., et al. Structure-based analyses of neutralization antibodies interacting with naturally occurring SARS-CoV-2 RBD variants. Cell Res. 2021 doi: 10.1038/s41422-021-00554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lan J., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 32.Wrapp D., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for Human Antibody BD-218 with Broad-Activities against Concerning Variants of SARS-CoV-2.
Data Availability Statement
Data will be made available on request.