Abstract
Objective
The purpose of this study was to compare the effects of oral hypoglycaemic drugs (HDs) on cognitive function and biomarkers of mild cognitive impairment (MCI) and Alzheimer's disease (AD) through a network meta-analysis of randomized controlled trials (RCTs).
Methods
We conducted systematic searches for English- and Chinese-language articles in the PubMed, Medline, Embase, Cochrane Library and Google Scholar databases, with no date restrictions. We performed a network meta-analysis, which we report here according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The 16 studies included a total of 3,081 patients. We selected the Mini-Mental State Examination (MMSE), the Alzheimer's Disease Assessment Scale-Cognitive section (ADAS-Cog), the Alzheimer's Disease Cooperative Study Activities of Daily Living section (ADCS-ADL) and amyloid beta (Aβ) 42 as the outcome measures for analysis and comparison.
Result
We selected seven treatments and assessed the clinical trials in which they were tested against a placebo control. Of these treatments, intranasal insulin 20 IU (ITSN20), glucagon-like peptide-1 (GLP-1), and dipeptidyl peptidase 4 inhibitor (DPP-4) were associated with significantly improved MMSE scores (7 RCTs, 333 patients, 30≥MMSE score≥20: mild) compared with placebo [standardized mean difference (SMD) 1.11, 95% confidence interval (CI) (0.87, 1.35); SMD 0.75, 95% CI (0.04, 1.41); and SMD 4.08, 95% CI (3.39, 4.77), respectively]. Rosiglitazone 4 mg (RLZ4), rosiglitazone 10 mg (RLZ10), intranasal insulin 40 IU (ITSN40), and ITSN20 significantly decreased ADAS-Cog scores (11 RCTs, 4044 patients, 10 ≤ ADAS-Cog scores ≤ 30: mild and moderate) compared with placebo [SMD −1.40, 95% CI (−2.57, −0.23), SMD −3.02, 95% CI (−4.17, −1.86), SMD −0.92, 95% CI (−1.77, −0.08), SMD −1.88, 95% CI (−3.09, −0.66)]. Additionally, ITSN20 and ITSN40 significantly improved ADCS-ADL scores (2 RCTs, 208 patients, ADCS-ADL scale score ≤ 10: mild) compared with placebo [SMD 0.02, 95% CI (0.01, 0.03), and SMD 0.04, 95% CI (0.03, 0.05), respectively]. In the 16 included studies, the degree of AD was classified as mild or moderate. For mild cognitive impairment, DPP-4 performed best, but for mild to moderate impairment, ITSN40 had excellent performance.
Conclusion
Various HDs can improve the cognitive function of MCI and AD patients. Different drug regimens brought different degrees of improvement, which may be related to their dosage, duration, and mechanism of action.
Systematic review registration
Keywords: hypoglycemic drugs, cognitive function, Alzheimer's disease, mild cognitive impairment, a systematic review, network meta-analysis
Introduction
Alzheimer's disease (AD) is a type of neurodegenerative dementia characterized by an impaired ability to encode and store new memories in the early stage, followed by the gradual degradation of cognition and behaviour to the point of clinical manifestations (1–3). As currently understood, the mechanism of AD dementia consists mainly of the cleavage of amyloid precursor protein (APP), the deposition of amyloid beta (Aβ), and the accumulation of hyperphosphorylated tau protein. The above factors lead to decreased synaptic strength and neurodegeneration (4, 5). Mild cognitive impairment (MCI) is an abnormal mental condition between normal cognitive status and dementia. The main clinical manifestations of MCI are a decline in cognitive function, a deficit in episodic memory, and a decline in the ability to carry out complex daily activities. Although they do not meet the diagnostic criteria for dementia, elderly patients with MCI are a high-risk group for AD (6, 7).
Diabetes is one of the most prevalent metabolic diseases in the world, according to statistics reported by the World Health Organization, the number of diabetes cases worldwide is expected to reach 366 million by 2030(8, 9). A large amount of epidemiological evidence supports an association between diabetes and MCI and AD. The cognitive decline rate of diabetes patients is twice the rate associated with normal aging, and the diabetes patients also have an elevated risk of MCI (10). A meta-analysis found that the relative risk of MCI in patients with diabetes compared to those without was 1.21 (11). Biessels et al. (12) showed that the risk of AD in diabetes patients was almost twice that of nondiabetic patients of the same age. Poor blood glucose control and a long duration of diabetes are risk factors for AD (13). Indeed, two large-scale national population studies with a follow-up time of approximately 10 years have confirmed that these features are risk factors (14).
Although numerous studies have been carried out to develop drugs for the treatment of MCI and AD, no effective cure has been found; current treatments can only reduce symptoms and delay disease progression. Therefore, it is necessary to research and develop drugs with stronger efficacy and novel mechanisms of action (15). The hypothesis that hypoglycaemic drugs (HDs) can improve MCI and AD has been widely considered. It has been found that intranasal insulin (ITSN), metformin (MTN), pioglitazone (PLZ), rosiglitazone (RLZ), glucagon-like peptide-1 (GLP-1) and dipeptidyl peptidase 4 inhibitor (DPP-4) agonists can improve the metabolism and nutrition of synapses, nerves and glia, alleviate neuroinflammatory reactions, and regulate memory and other cognitive and emotional functions, mainly due to insulin sensitization and other direct effects independent of the insulin signalling mechanism of the above drugs (16).
To study the potential use of HD in the treatment of MCI and AD, we selected ITSN, MTN, PLZ, RLZ, GLP-1 and DPP-4 as intervention measures, including ITSN 20 IU (ITSN20) and ITSN 40 IU (ITSN40), RLZ 2 mg (RLZ2), RLZ 4 mg (RLZ4), RLZ 8 mg (RLZ8), and RLZ 10 mg (RLZ10), and reviewed relevant randomized clinical trials to evaluate the efficacy of HDs in patients with AD and MCI by network meta-analysis (NMA).
Materials and methods
Registration
The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the following registration number: CRD42022355924.
Search strategy and data extraction
We strictly adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) reporting guidelines (17, 18). The databases Public Medicine (PubMed), Medline, Excerpta Medica Database (Embase), Cochrane Library and Google Scholar were searched as of June 2022. The following MESH terms were applied to search for relevant literature: “Hypoglycemic drugs” OR “Hypoglycemic Agent” OR “Hypoglycemic Agent” OR “Antihyperglycemic Agent” OR “Antidiabetic Drug” OR “Intranasal insulin” OR “Metformin” OR “Dimethylbiguanidine” OR “Glucophage” OR “Metformin” OR “Hydrochloride” OR “Metformin HCl” OR “Pioglitazone” OR “Pioglitazone Hydrochloride” OR “Rosiglitazone” OR “Rosiglitazone Maleate” OR “Glucagon like peptide GLP-1 receptor” OR “DPP-4” OR “Dipeptidyl-Peptidase IV Inhibitors” OR “DPP-4 Inhibitor”, and “Alzheimer's Disease”, “Mild Cognitive Impairment”.
In addition, we reviewed numerous references from the retrieved articles and sought out other literature materials, such as research reports and conference reports. The search scope was limited to randomized controlled trials (RCTs) in humans. The reference lists of included articles were reviewed, and relevant studies were sought as comprehensively as possible to avoid omissions. Working independently, the two reviewers (XC and CL) reviewed the titles and abstracts, summarized the search results, and applied the inclusion and exclusion criteria. XC and CL used the Cochrane Guidelines (19) to assess the risk of bias and the quality of the included trials. If there was a disagreement between the two reviewers, the third author (SJ) made the final decision.
Study selection
We followed the PICOS (population, interventions, comparisons outcomes, study designs) when defining the eligibility criteria. The studies included in the review met the following conditions: (1) the patients met their country's diagnostic standard for MCI or AD, (2) the intervention measure was an HD, and the control was a placebo, (3) the outcome measures included the Mini-Mental State Examination (MMSE), the Alzheimer's Disease Assessment Scale Cognitive subscale (ADAS-Cog), and/or the Alzheimer's Disease Cooperative Study Activities of Daily Living subscale (ADCS-ADL), and (4) the study was an RCT.
The exclusion criteria were as follows: (1) the subjects were not human (2) the article was a review or an in vitro cell experiment, (3) HDs were used in the control group, (4) cognitive function outcome indicators were not included, and (5) the data were incomplete.
Outcome measures
In contrast to a traditional meta-analysis, our NMA does not extract the relevant results of each study separately but rather extracts, combines, and analyses results from across RCTs. The outcome measures included the MMSE to measure intelligence and the degree of cognitive impairment, the ADAS-Cog score to detect the level of cognitive ability, and the ADCS-ADL scale score to detect the ability to perform activities of daily living.
Statistical analysis
We applied Stata 17.0 software to the extracted continuous variables for NMA and generated the standardized mean difference (SMD) with its 95% confidence interval (CI) or the odds ratio (OR) with its 95% CI. The statistical heterogeneity criteria for the application of the fixed-effects model were I2 < 50%, p > 0.01. If these criteria were not met, the random-effects model was used. Publication bias and small-sample effects were assessed by funnel plots. Each result was ranked using the surface under the cumulative ranking curve (SUCRA). The higher the SUCRA value, the better the curative effect that may be achieved. A matrix was developed to compare all interventions and detect whether the SUCRA difference between each pair of interventions reached a significant level. The consistency or inconsistency of these relationships was evaluated to enhance the stability of the results. The threshold for statistical significance was p < 0.05. Subgroup analysis by treatment duration was completed with Review Manager 5.3 software.
Results
Literature search and included studies
A total of 3,762 studies were selected from the five databases according to the search strategy. After the duplicate articles were removed and the titles and abstracts were screened, 55 studies remained; these studies were then evaluated in full-text form. Thirty-nine studies were excluded based on the full text. Ultimately, there were 16 eligible studies that included 3081 patients meeting the inclusion criteria for the NMA; 2870 patients were diagnosed with AD, and 211 patients were diagnosed with MCI (Figure 1). Information on the included studies is listed in Table 1.
Figure 1.
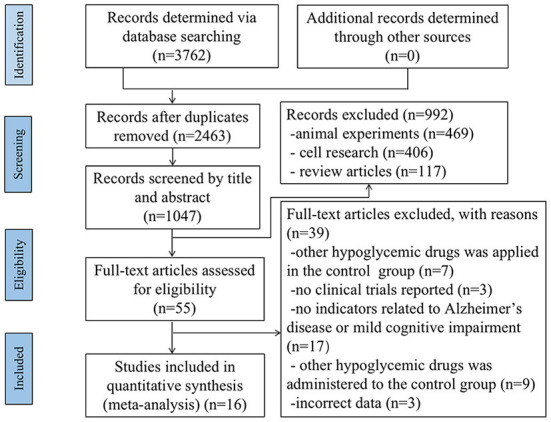
PRISMA flow diagram for search and selection of eligible studies included in the network meta-analysis.
Table 1.
The information about the included studies.
| References | Randomized sample size:I/C | Intervention (/once) | Control | Treatment duration (Month) | Cognitive assessment |
|---|---|---|---|---|---|
| Suzanne Craft (20) | 36, 38/30 | 20 IU of Insulin 40 IU of Insulin |
Placebo | 48 M | ADAS-Cog score ADCS-ADL scale score |
| Suzanne Craft (21) | 12/12 | 20 IU of Insulin | Placebo | 4 M | MMSE score |
| Maja Mustapica (22) | 33, 32/26 | 20 IU of Insulin 40 IU of Insulin |
Placebo | 4 M | ADAS-Cog score |
| Amy Claxtona (23) | 36, 38/30 | 20 IU of Insulin 40 IU of Insulin |
Placebo | 4 M | ADAS-Cog score ADCS-ADL scale score |
| José A. Luchsinger (24) | 40/40 | 500 mg Metformin | Placebo | 32 M | MMSE score ADAS-Cog score |
| Michael Rosenbloom (25) | 19/16 | 20 IU of Insulin | Placebo | 6 M | ADAS-Cog score |
| David S. Geldmacher (26) | 15/14 | 15 mg Pioglitazone | Placebo | 18 M | ADAS-Cog score |
| Haruo Hanyu (27) | 15/17 | 15 mg−30 mg Pioglitazone | Placebo | 6 M | MMSE score |
| TomohikoSato (28) | 21/21 | 15 mg−30 mg Pioglitazone | Placebo | 6 M | MMSE score ADAS-Cog score |
| Risner (29) | 127,130/122 | 2 mg Rosiglitazone 4 mg Rosiglitazone 8 mg Rosiglitazone |
Placebo | 6 M | ADAS-Cog score |
| C. Harrington (30) | 473, 459/461 | 2 mg Rosiglitazone 8 mg Rosiglitazone |
Placebo | 12 M | ADAS-Cog score |
| Michael Gold (31) | 162, 156/159 | 2 mg Rosiglitazone 8 mg Rosiglitazone |
Placebo | 6 M | ADAS-Cog score |
| Michael C. Irizarrya (32) | 733/856 | Rosiglitazone | Placebo | 6 M | ADAS-Cog score |
| Qiang Li (33) | 24/21 | 0.6 mg−1.8 mg GLP-1 | Placebo | 3 M | MMSE score |
| Roger J. Mullins (34) | 11/10 | GLP-1 | Placebo | 18 M | MMSE score ADAS-cog score |
| Jujun Xue (35) | 30/30 | 100 mg Sitagliptin | Placebo | 6 M | MMSE score |
I/C, Intervention/Control; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive section; ADCS-ADL, Alzheimer's Disease Cooperative Study-Activities of Daily Living Scale; MMSE, Mini-mental State Examination; GLP-1Glucagon-like Peptide-1.
Pairwise meta-analysis
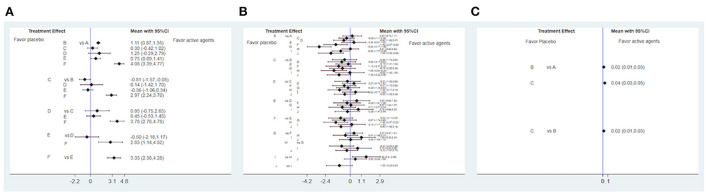
Several HDs affect cognitive function, as determined through a comparative analysis of MMSE scores (Figure 2A); the higher the MMSE score, the better the patient's cognitive function. DPP-4 represented a significant improvement over ITSN20, MTN, PLZ and GLP-1 (SMD 2.97, 95% CI (2.24, 3.70); SMD 3.78, 95% CI (2.78, 4.78); SMD 2.83, 95% CI (1.14, 4.52); and SMD 3.33, 95% CI (2.38, 4.28), respectively); a network map is shown in Figure 3A. Compared with ITSN20, MTN had a significant disadvantage [SMD −0.81, 95% CI (−1.57, −0.05)].
Figure 2.
Forest plot efficacy of hypoglycemic drugs (HD) with placebo (A) Forest plot efficacy of HD with placebo in improving MMSE score. A: placebo, B: intranasal insulin, C: metformin, D: pioglitazone, E: glucagon-like peptide-1 (GLP-1), F: dipeptidyl peptidase 4 inhibitor (DPP-4). (B) Forest plot efficacy of HD with placebo in improving ADAS-Cog score. A: placebo, B: metformin, C: pioglitazone, D: glucagon-like peptide-1, E: rosiglitazone 2 mg, F: rosiglitazone 4 mg, G: rosiglitazone 10 mg, H: rosiglitazone 8 mg, I: intranasal insulin 20 IU, J: intranasal insulin 40 IU. (C) Forest plot efficacy of HD with placebo in improving ADCS-ADL scale score. A: placebo, B: intranasal insulin 20 IU, C: intranasal insulin 40 IU.
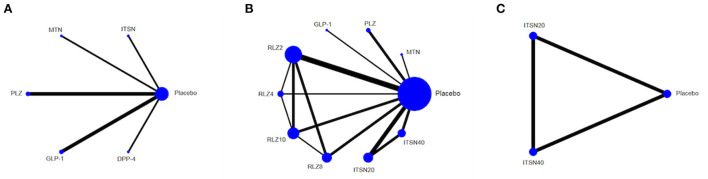
Figure 3.
Network map for hypoglycemic drugs (HD) with placebo in improving MMSE score. (A) ADAS-Cog score, (B) ADCS-ADL scale score, (C) Lines connect the interventions that have been studied in head-to-head comparisons in eligible RCTs. The width of the lines represents the total number of RCTs for each pairwise comparison. The size of each node is proportional to the number of randomized participants. Metformin (MTN), Pioglitazone (PLZ), Intranasal insulin 20 IU (ITSN20), Intranasal insulin 40 IU (ITSN40), Rosiglitazone 2 mg (RLZ2), Rosiglitazone 4 mg (RLZ4), Rosiglitazone 8 mg (RLZ8), Rosiglitazone 10 mg (RLZ10), Glucagon-like peptide-1 (GLP-1), Dipeptidyl peptidase 4 inhibitor (DPP-4).
The ADAS-Cog score was used to assess memory, language, operating ability and attention; the smaller the difference was, the better the effect of the intervention in improving cognitive function. Analysis and comparison of ADAS-Cog scores (Figure 2B) showed that RLZ8 was significantly lower than MTN and RLZ2 (SMD −1.59, 95% CI (−2.84, −0.34), and SMD −1.40, 95% CI (−2.57, −0.23), respectively]; a network map is shown in Figure 3B.
The ADCS-ADL score is mainly used to assess the ability to carry out common tasks in daily life. The higher the score, the greater the ability to complete these tasks. Analysis of the effect of HDs on the ADCS-ADL scale score showed that ITSN20 and ITSN40 significantly outperformed placebo [SMD 0.02, 95% CI (0.01, 0.03), and SMD 0.04, 95% CI (0.03, 0.05), respectively], and ITSN40 significantly outperformed ITSN20 [SMD 0.02, 95% CI (0.01, 0.03)] (Figure 2C). A network map is shown in Figure 3C.
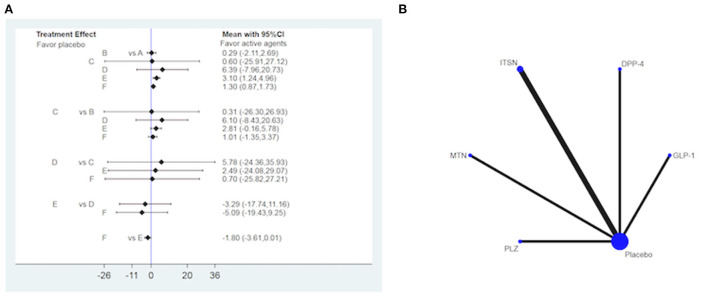
We selected Aβ42 as the blood biochemical index to test AD and MCI. GLP-1 and DPP-4 performed better than placebo [SMD 3.10, 95% CI (1.24, 4.96), and SMD 1.3, 95% CI (0.87, 1.73), respectively] (Figure 4).
Figure 4.
Forest plot (A) and network map (B) for hypoglycemic drugs (HD) with placebo in improving Aβ42. (A) A: placebo, B: intranasal insulin, C: metformin, D: pioglitazone, E: glucagon-like peptide-1, F: dipeptidyl peptidase 4 inhibitor. (B) Lines connect the interventions that have been studied in head-to-head comparisons in eligible RCTs. The width of the lines represents the total number of RCTs for each pairwise comparison. The size of each node is proportional to the number of randomized participants. Metformin (MTN), Pioglitazone (PLZ), Intranasal insulin (ITSN), Glucagon-like peptide-1 (GLP-1), Dipeptidyl peptidase 4 inhibitor (DPP-4).
Network meta-analysis of treatment groups
Mini-mental state examination
The indirect comparison of the effects of various intervention measures on MCI and AD cognitive function by MMSE, ADAS-Cog and ADCS-ADL shows that DPP-4 and MTN have higher significant effects.
The SUCRA was used to rank the interventions in terms of their effects on MMSE scores. DPP-4 was the best treatment (100%), followed by ITSN (65%), PLZ (62%), GLP-1 (44.8%), MTN (22.6%), and placebo (5.5%). ITSN20 was superior to MTN and placebo [SMD 0.81, 95% CI (0.05, 1.57), (SMD 1.11, 95% CI (0.87, 1.35)], and GLP-1 was superior to placebo [SMD 0.75, 95% CI (0.09, 1.41)] (Table 2).
Table 2.
Matrix of pairwise comparison among hypoglycemic drugs on MMSE score (shown as mean difference and 95% confidence intervals).
| DPP-4 | ITSN20 | PLZ | GLP-1 | MTN | Placebo | |
|---|---|---|---|---|---|---|
| SUCRA (%) | 100 | 65.1 | 62 | 44.8 | 22.6 | 5.5 |
| DPP-4 | 0 | −2.97 (−3.70, −2.24) | −2.83(−4.52, −1.14) | −3.33 (−4.28, −2.38) | −3.78 (−4.78, −2.78) | −4.08 (−4.77, −3.39) |
| ITSN20 | 2.97 (2.24, 3.70) | 0 | 0.14 (−1.42, 1.70) | −0.36 (−1.06, 0.34) | −0.81 (−1.57, −0.05) | −1.11 (−1.35, −0.87) |
| PLZ | 2.83 (1.14, 4.52) | −0.14 (−1.70, 1.42) | 0 | −0.50 (−2.18, 1.17) | −0.95 (−2.65, 0.75) | −1.25 (−2.79, 0.29) |
| GLP-1 | 3.33 (2.38, 4.28) | 0.36(−0.34, 1.06) | 0.50 (−1.17, 2.18) | 0 | −0.45 (−1.43, 0.53) | −0.75 (−1.41, −0.09) |
| MTN | 3.78 (2.78, 4.78) | 0.81 (0.05, 1.57) | 0.95 (−0.75, 2.65) | 0.45 (−0.53, 1.43) | 0 | −0.30 (−1.02, 0.42) |
| Placebo | 4.08 (3.39, 4.77) | 1.11 (0.87, 1.35) | 1.25 (−0.29, 2.79) | 0.75 (0.09, 1.41) | 0.30 (−0.42, 1.02) | 0 |
SUCRA, Surface under the cumulative ranking curve; ITSN20, Intranasal insulin 20IU; MTN, Metformin; PLZ, Pioglitazone;, RLZ2, Rosiglitazone 2 mg; RLZ4, Rosiglitazone 4 mg; RLZ8, Rosiglitazone 8mg; RLZ10, Rosiglitazone 10 mg; GLP-1, Glucagon-like Peptide-1; DPP-4, Dipeptidyl Peptidase 4 Inhibitor; MMSE, Mini-mental State Examination.
ADAS-Cog score
As determined by the SUCRA, MTN was the best treatment to improve ADAS-Cog scores. The treatments, from most to least effective, were as follows: MTN (86.6%), placebo (82.4%), RLZ2 (79.6%), PLZ (61.6%), RLZ10 (52.2%), GLP-1 (47.6%), ITSN40 (34.8%), RLZ4 (31.8%), RLZ8 (12.9%), ITSN20 (10.5%). MTN significantly outperformed RLZ8 [SMD 1.59, 95% CI (0.34, 2.84)]. RLZ10, ITSN40, RLZ4, and ITSN20 were significantly worse than placebo [SMD 3.02, 95% CI (1.86, 4.17); SMD 1.88, 95% CI (1.66, 3.09); SMD 1.40, 95% CI (0.23, 2.57); and SMD 0.92, 95% CI (0.08, 1.77), respectively]. RLZ2 was significantly better than RLZ8 [SMD 1.40, 95% CI (0.23, 2.57)]. ITSN20 was better than RLZ8 [SMD 1.55, 95% CI (0.21, 2.90)] (Table 3).
Table 3.
Matrix of pairwise comparison among hypoglycemic drugs on ADAS-Cog score (shown as mean difference and 95% confidence intervals).
| MTN | Placebo | RLZ2 | PLZ | RLZ10 | GLP-1 | ITSN40 | RLZ4 | RLZ8 | ITSN20 | |
|---|---|---|---|---|---|---|---|---|---|---|
| SUCRA (%) | 86.6 | 82.4 | 79.6 | 61.6 | 52.2 | 47.6 | 34.8 | 31.8 | 12.9 | 10.5 |
| MTN | 0 | −0.20 (−1.11, 0.72) | −0.19 (−1.41, 1.04) | −0.55 (−1.74, 0.64) | −0.75 (−2.00, 0.49) | −0.82 (−2.17, 0.53) | −1.08 (−2.22, 0.06) | −1.12 (−2.37, 0.12) | −1.59 (−2.84, −0.34) | −0.03 (-1.42,1.35) |
| Placebo | 0.20 (−0.72, 1.11) | 0 | 0.36 (−0.81, 1.53) | −0.35 (−1.11, 0.40) | −3.02 (−4.17, −1.86) | −0.62 (−1.62, 0.37) | −1.88 (−3.09, −0.66) | −1.40 (−2.57, −0.23) | 0.01 (−0.80, 0.82) | −0.92 (-1.77,-0.08) |
| RLZ2 | 0.19 (−1.04, 1.41) | −0.36 (−1.53, 0.81) | 0 | −0.37 (−1.47, 0.74) | −0.57 (−1.74, 0.60) | −0.63 (−1.92, 0.65) | −0.90 (−1.95, 0.16) | −0.94 (−2.11, 0.23) | −1.40 (−2.57, −0.23) | 0.15 (-1.17,1.47) |
| PLZ | 0.55 (−0.64, 1.74) | 0.35 (−0.40, 1.11) | 0.37 (−0.74, 1.47) | 0 | −0.20 (−1.33, 0.93) | −0.27 (−1.52, 0.98) | −0.53 (−1.55, 0.48) | −0.57 (−1.70, 0.56) | −1.04 (−2.17, 0.10) | 0.52 (-0.77,1.80) |
| RLZ10 | 0.75 (−0.49, 2.00) | 3.02 (1.86, 4.17) | 0.57 (−0.60, 1.74) | 0.20 (−0.93, 1.33) | 0 | −0.07 (−1.37, 1.24) | −0.33 (−1.41, 0.75) | −0.37 (−1.21, 0.47) | −0.83 (−2.03, 0.36) | 0.72 (-0.62,2.06) |
| GLP-1 | 0.82 (−0.53, 2.17) | 0.62 (−0.37, 1.62) | 0.63 (−0.65, 1.92) | 0.27 (−0.98, 1.52) | 0.07 (−1.24, 1.37) | 0 | −0.26 (−1.47, 0.94) | −0.30 (−1.61, 1.00) | −0.77 (−2.07, 0.54) | 0.78 (-0.66,2.23) |
| ITSN40 | 1.08 (−0.06, 2.22) | 1.88 (0.66, 3.09) | 0.90 (−0.16, 1.95) | 0.53 (−0.48, 1.55) | 0.33 (−0.75, 1.41) | 0.26 (−0.94, 1.47) | 0 | −0.04 (−1.12, 1.04) | −0.50 (−1.59, 0.58) | 1.05 (-0.20,2.29) |
| RLZ4 | 1.12 (−0.12, 2.37) | 1.40 (0.23, 2.57) | 0.94 (−0.23, 2.11) | 0.57 (−0.56, 1.70) | 0.37 (−0.47, 1.21) | 0.30 (−1.00, 1.61) | 0.04 (−1.04, 1.12) | 0 | −0.47 (−1.66, 0.73) | 1.09 (-0.25,2.43) |
| RLZ8 | 1.59 (0.34, 2.84) | −0.01 (−0.82, 0.80) | 1.40 (0.23, 2.57) | 1.04 (−0.10, 2.17) | 0.83 (−0.36, 2.03) | 0.77 (−0.54, 2.07) | 0.50 (−0.58, 1.59) | 0.47 (−0.73, 1.66) | 0 | 1.55 (0.21,2.90) |
| ITSN20 | 0.03 (−1.35, 1.42) | 0.92 (0.08, 1.77) | −0.15 (−1.47, 1.17) | −0.52 (−1.80, 0.77) | −0.72 (−2.06, 0.62) | −0.78 (−2.23, 0.66) | −1.05 (−2.29, 0.20) | −1.09 (−2.43, 0.25) | −1.55 (−2.90, −0.21) | 0 |
SUCRA, Surface under the cumulative ranking curve; ITSN20, Intranasal insulin 20IU; ITSN40, Intranasal insulin 40IU; MTN, Metformin; PLZ, Pioglitazone; RLZ2, Rosiglitazone 2 mg; RLZ4, Rosiglitazone 4 mg; RLZ8, Rosiglitazone 8 mg; RLZ10, Rosiglitazone 10 mg; GLP-1, Glucagon-like Peptide-1; ADAS-Cog score, Alzheimer's Disease Assessment Scale-Cognitive section.
ADCS-ADL scale score
By SUCRA ranking analysis, placebo (100%) was superior to ITSN20 (50.1%) and ITSN40 (0.1%) [SMD −0.02, 95% CI (−0.03, −0.01), and SMD −0.04, 95% CI (−0.05, −0.03), respectively)](Table 4).
Table 4.
Matrix of pairwise comparison among hypoglycemic drugs on ADCS-ADL scale score (shown as mean difference and 95% confidence intervals).
| Placebo | ITSN20 | ITSN40 | |
|---|---|---|---|
| SUCRA (%) | 99.8 | 50.1 | 0.1 |
| Placebo | 0 | 0.02 (0.01,0.03) | 0.04 (0.03,0.05) |
| ITSN20 | −0.02 (−0.03, −0.01) | 0 | 0.02 (0.01,0.03) |
| ITSN40 | −0.04 (−0.05, −0.03) | −0.02 (−0.03, −0.01) | 0 |
SUCRA, Surface under the cumulative ranking curve; ITSN20, Intranasal insulin 20IU; ITSN40, Intranasal insulin 40IU; ADCS-ADL, Alzheimer's Disease Cooperative Study-Activities of Daily Living Subscal.
Adverse events
Of the 16 studies included in the network meta-analysis, nine mentioned the occurrence of adverse events. Among them, oedema, rhinitis, dizziness, and diarrhoea were the most common (Table 5).
Table 5.
Adverse events included in the literature.
| Adverse events | Control(n) | Intervention(n) |
|---|---|---|
| Rhinitis | Placebo 3 | ITSN 17 |
| Headache/Dizziness | Placebo 22 | ITSN 19 |
| RLZ 35 | ||
| Musculoskeletal Injury/Pain | Placebo 10 | ITSN 12 |
| RLZ 25 | ||
| Nose bleed/ Nasal irritation | Placebo 26 | ITSN 68 |
| Gastointestinal Symptoms/Diarrhea | Placebo 32 | ITSN 62 |
| Fall | Placebo 31 | ITSN 5 |
| RLZ 31 | ||
| Rash | Placebo 2 | ITSN 3 |
| Upper respiratory tract infection | Placebo 2 | ITSN 3 |
| Respiratory/sinus symptoms | Placebo 3 | ITSN 7 |
| Dental | Placebo 1 | ITSN 3 |
| Infection | Placebo 0 | ITSN 2 |
| Cardiovascular | Placebo 0 | ITSN 2 |
| Nausea | Placebo 4 | ITSN 5 |
| Cold symptoms | Placebo 14 | PLZ 14 |
| Glucose level low/asymptomatic | Placebo 4 | PLZ 4 |
| Swelling/edema of leg/ Peripheral edema | Placebo 11 | PLZ 7 |
| RLZ 106 | ||
| Anemia | Placebo 4 | PLZ 3 |
| RLZ 26 | ||
| Fatigue | Placebo 5 | PLZ 3 |
| Increased confusion | Placebo 5 | PLZ 3 |
| Insomnia | Placebo 2 | RLZ 5 |
| Other (stroke, breast cancer, weakness, ophthalmic, hypoglycemia, hematological, hearing loss, endocrine) | Placebo 18 | ITSN 70 |
MTN, Metformin; ITSN, Intranasal insulin; PLZ, Pioglitazone; RLZ, Rosiglitazone.
Sensitivity and publication bias
Sensitivity analysis showed that any single study or cluster study with certain characteristics had little effect on the change in SMD and its corresponding 95% CI. Significant publication bias was not reported by Egger's regression test or Begg's adjusted rank correlation test.
Discussion
According to current studies, most HDs have a considerable positive effect on the cognitive function of patients with AD and MCI. Only GLP-1 and DPP-4 have an inhibitory effect on the accumulation of Aβ42, and the impact on other biomarker indicators still needs more research. For the three different scoring standards of cognitive function, the efficacy of various HDs was different. In our study, for MMSE, DPP-4 was better than other HDs. For ADAS-Cog scores, 8 mg RLZ was better than MTN and 2 mg RLZ. For the ADCS-ADL scale, the curative effect of ITSN 40 IU was better than that of ITSN 20 IU.
Previous studies have shown that PLZ 15–30 mg, an HD, has an extraordinary promoting cognitive effect for MCI/AD (36). However, many measurement standards are not insufficient to evaluate how HD improves cognitive function. To the best of our knowledge, our network meta-analysis was the first to compare six types of HD-ameliorated MCI/AD and analysed the different criteria for evaluating cognitive function by pairwise comparison.
Both hippocampus and connected limbic brain structures in the brain are memory-forming regions with a high density of insulin receptors. Insulin signals contribute to neuronal plasticity and regulate the cognitive function and memory function of the brain (37). The intranasal pathway was capable of delivering insulin to the central nervous system with relatively no systemic absorption and associated peripheral side effects. Intranasal insulin (ITSN) rapidly accumulates in the cerebrospinal fluid and is efficiently transported to the brain (38). Studies have shown (39, 40) that insulin is involved in synaptic plasticity in the brain, i.e., long-term potentiation (LTP) and long-term depression (LTD), and the establishment of the hippocampal memory locus depends on LTP and LTD. Insulin regulates LTD by inducing internalization of the glutamate AMPA receptor. ITSN enhances long-term declarative memory without hypoglycaemic side effects and enhances functional connectivity between the prefrontal cortex and the hippocampus in diabetes mellitus type 2 (37, 41). Previous studies (42, 43) have shown that ITSN40 can improve memory and cognitive function, with few negative effects, which was consistent with our analysis that ITSN40 was more effective and safer than ITSN 20.
Metformin (MTN), a HD commonly used in most health guidelines, can cross the blood–brain barrier (BBB) and is involved in cognitive improvement (44). Previous studies (45, 46) showed that MTN not only significantly reduced the hyperphosphorylation of tau and APPc99 but also improved the learning and memory performance of SAMP8 and APP/PS1 transgenic mice based on Morris water maze and Y-maze results. MTN can significantly reduce β-secretase1 (BACE1) protein expression and activity, reducing BACE1 cleavage products and Aβ (47). The AMPK pathway in human neural stem cells (NCS) can be activated by MTN, which is considered a potential therapeutic target of AD (48). Saliu et al. (49) confirmed that MTN at a dose of 500 mg/kg can significantly reduce AChE activity in the brains of streptozotocin-induced diabetic rats. MTN has many ways to improve neural cells and can effectively improve the cognitive impairment of patients with type 2 diabetes clinically. In our study, the metformin group had lower (better) ADAS-Cog scores (p = 0.02), but other biological indicators were not mentioned.
Pioglitazone (PLZ) and rosiglitazone (RLZ) are insulin sensitizers of thiazolidinedione nuclear peroxisome proliferator activated receptor γ (PPARγ) agonists. PLZ was found to reduce glial inflammation and Aβ levels in the brains of transgenic mice, enhancing microglial uptake of Aβ in a PPARγ-dependent manner (50, 51). RLZ can reduce the expression of inflammatory cytokines and improve cognitive function by inhibiting the activation of NF-κB signalling in the hippocampus (52). RLZ can regulate several processes related to AD, such as reducing tau and amyloid pathology and inhibiting inflammation (53). For the treatment of MCI/AD with RLZ, the selection of dose was particularly important. In our analysis, RLZ was divided into 2, 4, 8, and 10 mg, and the dose was not directly proportional to the effect. In terms of the ADAS-Cog score, 2 mg had a better efficacy than 10 mg, but 10 mg was better than 4 and 8 mg.
Glucagon-like peptide-1 (GLP-1) is derived from the glucagon gene. It is produced in the central nervous system (CNS), mainly in the brain stem, and then transported to a large number of areas of the central nervous system (54). GLP-1 enhances central insulin resistance, promotes the growth of synapses and neurons, and prevents oxidative damage (55). In an AD mouse model, GLP-1 agonists reduce the level of AD pathological markers, including oligomer Aβ and Aβ plaque load, reduce the activation of microglia, and improve memory behaviour (56). In our study, the GLP group had better performance on all cognitive tests, which included the total learning (p = 0.039), animal naming test (p = 0.025), and MMSE (p = 0.001). For blood glucose indicators, the concentrations of FBG and HbA1c in the GLP group and the control group decreased significantly, but there was no significant difference between the two groups.
Dipeptidyl peptidase-4 (DPP-4) inhibitors exist in blood plasma and cerebrospinal fluid and exert their multifunctional functions through the influence of various signalling pathways, inducing and regulating inflammatory and immune processes (57). DPP-4 inhibitors have recently been shown to have important neuroprotective effects, reversing the pathophysiological processes of AD and improving the cognitive abilities of AD animal models and patients (58). It has also been suggested (59) that DPP-4 inhibitors tend to improve MCI. The number of related studies involving DPP-4 inhibitors in our included literature was small, so further studies are needed. Antonio et al. (60) used DPP-4+metformin as an intervention measure in their study, and the results showed that the combination of two HDs had a better effect in improving the MMSE score than metformin alone (p < 0.001). Through a further search of the database, we found that there were few clinical studies of the combined application of two HDs in the treatment of MCI/AD. Therefore, large sample and multi-centre RCTs are needed. Clinically, the combination of HD can be used to improve the symptoms of MCI/AD patients, especially those with type 2 diabetes.
Limitations
First, the sample size included in the network meta-analysis was relatively small, and there were few studies with comparable original outcome indicators. Second, there were few biological indicators related to MCI/AD, and the analysis was not thorough enough. Finally, the observation time, drug dose and duration of medication of the RCTs included in our study were different. It is not ruled out that some unused databases have literature matching the inclusion criteria, which may affect the results.
Conclusion
The study provides a theoretical basis for the effect of different doses of HDs on MCI/AD cognitive function. Such large-scale, multi-centre and repetitive studies are necessary, and the specific mechanisms of different HDs to improve MCI/AD of different degrees need to be further studied.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
X-CW and C-LC: completed the data analysis. X-CW, C-LC, and H-CL: wrote and revised the manuscript. S-JZ and C-LC: conceived and designed the study. X-CW: received financial support. Y-FC and S-JQ: reviewed the full text and approved the final manuscript submitted. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Zhaoqing University Excellent Young Teachers' scientific research ability improvement project (No. YQ202102) and Science and Technology Innovation Guidance Project of Zhaoqing Science and Technology Bureau (No. 2022040311001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1018027/full#supplementary-material
References
- 1.Li F, Huang W, Zhang X. Efficacy and safety of different regimens for primary open-angle glaucoma or ocular hypertension: a systematic review and network meta-analysis. Acta Ophthalmol. (2018) 96:e277–84. 10.1111/aos.13568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soria Lopez JA, González HM, Léger GC. Alzheimer's disease. Handb Clin Neurol. (2019) 167:231–55. 10.1016/B978-0-12-804766-8.00013-3 [DOI] [PubMed] [Google Scholar]
- 3.Brayne C, Davis D. Making Alzheimer's and dementia research fit for populations. Lancet. (2012) 380:1441–3. 10.1016/S0140-6736(12)61803-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. (2015) 385:2255–63. 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 5.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. (2002) 297:353–6. 10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- 6.Vega JN, Newhouse PA. Mild cognitive impairment: diagnosis, longitudinal course, and emerging treatments. Curr Psychiatry Rep. (2014) 16:490. 10.1007/s11920-014-0490-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jongsiriyanyong S, Limpawattana P. Mild cognitive impairment in clinical practice: a review article. Am J Alzheimers Dis Other Demen. (2018) 33:500–7. 10.1177/1533317518791401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. (2004) 27:1047–53. 10.2337/diacare.27.5.1047 [DOI] [PubMed] [Google Scholar]
- 9.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. (2018) 14:591–604. 10.1038/s41574-018-0048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simó R, Ciudin A, Simó-Servat O, Hernández C. Cognitive impairment and dementia: a new emerging complication of type 2 diabetes-The diabetologist's perspective. Acta Diabetol. (2017) 54:417–24. 10.1007/s00592-017-0970-5 [DOI] [PubMed] [Google Scholar]
- 11.Yu ZW Li X, Wang Y, Fu YH, Gao XY. Association between lipid accumulation product and mild cognitive impairment in patients with type 2 diabetes. J Alzheimers Dis. (2020) 77:367–74. 10.3233/JAD-200332 [DOI] [PubMed] [Google Scholar]
- 12.Biessels GJ, Staekenborg S, Brunner E, Brayne C. Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. (2006) 5:64–74. 10.1016/S1474-4422(05)70284-2 [DOI] [PubMed] [Google Scholar]
- 13.Wong RH, Scholey A, Howe PR. Assessing premorbid cognitive ability in adults with type 2 diabetes mellitus–a review with implications for future intervention studies. Curr Diab Rep. (2014) 14:547. 10.1007/s11892-014-0547-4 [DOI] [PubMed] [Google Scholar]
- 14.Wang KC, Woung LC, Tsai MT, Liu CC, Su YH, Li CY. Risk of Alzheimer's disease in relation to diabetes: a population-based cohort study. Neuroepidemiology. (2012) 38:237–44. 10.1159/000337428 [DOI] [PubMed] [Google Scholar]
- 15.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. (2011) 7:263–9. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muñoz-Jiménez M, Zaarkti A, García-Arnés JA, García-Casares N. Antidiabetic drugs in alzheimer's disease and mild cognitive impairment: a systematic review. Dement Geriatr Cogn Disord. (2020) 49:423–34. 10.1159/000510677 [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. (2012) 69:29–38. 10.1001/archneurol.2011.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craft S, Claxton A, Baker LD, Hanson AJ, Cholerton B, Trittschuh EH, et al. Effects of regular and long-acting insulin on cognition and Alzheimer's disease biomarkers: A pilot clinical trial. J Alzheimers Dis. (2017) 57:1325–34. 10.3233/JAD-161256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mustapic M, Tran J, Craft S, Kapogiannis D. Extracellular vesicle biomarkers track cognitive changes following intranasal insulin in Alzheimer's Disease. J Alzheimers Dis. (2019) 69:489–98. 10.3233/JAD-180578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claxton A, Baker LD, Wilkinson CW, Trittschuh EH, Chapman D, Watson GS, et al. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer's disease. J Alzheimers Dis. (2013) 35:789–97. 10.3233/JAD-122308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luchsinger JA, Perez T, Chang H, Mehta P, Steffener J, Pradabhan G, et al. Metformin in amnestic mild cognitive impairment: Results of a pilot randomized placebo controlled clinical trial. J Alzheimers Dis. (2016) 51:501–14. 10.3233/JAD-150493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbloom M, Barclay TR, Kashyap B, Hage L, O'Keefe LR, Svitak A, et al. A phase ii, single-center, randomized, double-blind, placebo-controlled study of the safety and therapeutic efficacy of intranasal glulisine in amnestic mild cognitive impairment and probable mild Alzheimer's Disease. Drugs Aging. (2021) 38:407–15. 10.1007/s40266-021-00845-7 [DOI] [PubMed] [Google Scholar]
- 26.Geldmacher DS, Fritsch T, McClendon MJ, Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch Neurol. (2011) 68:45–50. 10.1001/archneurol.2010.229 [DOI] [PubMed] [Google Scholar]
- 27.Hanyu H, Sato T, Kiuchi A, Sakurai H, Iwamoto T. Pioglitazone improved cognition in a pilot study on patients with Alzheimer's disease and mild cognitive impairment with diabetes mellitus. J Am Geriatr Soc. (2009) 57:177–9. 10.1111/j.1532-5415.2009.02067.x [DOI] [PubMed] [Google Scholar]
- 28.Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-? agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. (2011) 32:1626–33. 10.1016/j.neurobiolaging.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 29.Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, et al. Rosiglitazone in Alzheimer's Disease Study Group. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J. (2006) 6:246–54. 10.1038/sj.tpj.6500369 [DOI] [PubMed] [Google Scholar]
- 30.Harrington C, Sawchak S, Chiang C, Davies J, Donovan C, Saunders AM, et al. Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer's disease: two phase 3 studies. Curr Alzheimer Res. (2011) 8:592–606. 10.2174/156720511796391935 [DOI] [PubMed] [Google Scholar]
- 31.Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer's disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. (2010) 30:131–46. 10.1159/000318845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irizarry MC, Webb DJ, Bains C, Barrett SJ, Lai RY, Laroche JP, et al. Predictors of placebo group decline in the Alzheimer's disease Assessment Scale-cognitive subscale (ADAS-Cog) in 24 week clinical trials of Alzheimer's disease. J Alzheimers Dis. (2008) 14:301–11. 10.3233/jad-2008-14304 [DOI] [PubMed] [Google Scholar]
- 33.Li Q, Jia M, Yan Z, Li Q, Sun F, He C, et al. Activation of glucagon-like peptide-1 receptor ameliorates cognitive decline in type 2 diabetes mellitus through a metabolism-independent pathway. J Am Heart Assoc. (2021) 20:e020734. 10.1161/JAHA.120.020734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullins RJ, Mustapic M, Chia CW, Carlson O, Gulyani S, Tran J, et al. A pilot study of exenatide actions in alzheimer's disease. Curr Alzheimer Res. (2019) 16:741–52. 10.2174/1567205016666190913155950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue J, Wang C, Pan C, Xing H, Xu L, Chen X, et al. Effectof DPP-4 inhibitor on elderly patients with T2DM combined with MCI. Exp Ther Med. (2020) 19:1356–62. 10.3892/etm.2019.8339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao B, Rosenblat JD, Brietzke E, Park C, Lee Y, Musial N, et al. Comparative efficacy and acceptability of antidiabetic agents for Alzheimer's disease and mild cognitive impairment: a systematic review and network meta-analysis. Diabetes Obes Metab. (2018) 20:2467–71. 10.1111/dom.13373 [DOI] [PubMed] [Google Scholar]
- 37.Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, et al. Intranasal insulin improves memory in humans. Psycho-neuroendocrinology. (2004) 29:1326–34. 10.1016/j.psyneuen.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 38.Hallschmid M. Intranasal insulin. J Neuroendocrinol. (2021) 33:e12934. 10.1111/jne.12934 [DOI] [PubMed] [Google Scholar]
- 39.Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. (2018) 14:168–81. 10.1038/nrneurol.2017.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goh JJ, Manahan-Vaughan D. Role of inhibitory autophosphorylation of calcium/calmodulin-dependent kinase II (αCAMKII) in persistent (>24 h) hippocampal LTP and in LTD facilitated by novel object-place learning and recognition in mice. Behav Brain Res. (2015) 285:79–88. 10.1016/j.bbr.2014.01.022 [DOI] [PubMed] [Google Scholar]
- 41.Jauch-Chara K, Friedrich A, Rezmer M, Melchert UH, Scholand-Engler H, Hallschmid M, et al. Intranasal insulin suppresses food intake via enhancement of brain energy levels in humans. Diabetes. (2012) 61:2261–8. 10.2337/db12-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Craft S, Raman R, Chow TW, Rafii MS, Sun CK, Rissman RA, et al. Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and alzheimer disease dementia: a randomized clinical trial. JAMA Neurol. (2020) 77:1099–09. 10.1001/jamaneurol.2020.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, et al. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer's disease dementia. J Alzheimers Dis. (2015) 44:897–906. 10.3233/JAD-141791 [DOI] [PubMed] [Google Scholar]
- 44.Lv WS, Wen JP, Li L, Sun RX, Wang J, Xian YX, et al. The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain Res. (2012) 1444:11–9. 10.1016/j.brainres.2012.01.028 [DOI] [PubMed] [Google Scholar]
- 45.Niehoff ML, Roby DA, McKee A, Morley JE. Metformin improves learning and memory in the samp8 mouse model of Alzheimer's disease. J Alzheimers Dis. (2019) 68:1699–710. 10.3233/JAD-181240 [DOI] [PubMed] [Google Scholar]
- 46.Lu XY, Huang S, Chen QB, Zhang D, Li W, Ao R, et al. Metformin ameliorates Aβ pathology by insulin-degrading enzyme in a transgenic mouse model of Alzheimer's disease. Oxid Med Cell Longev. (2020) 2020:2315106. 10.1155/2020/2315106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markowicz-Piasecka M, Sikora J, Szydłowska A, Skupień A, Mikiciuk-Olasik E, Huttunen KM. Metformin-a future therapy for neurodegenerative diseases : theme: drug discovery, development and delivery in alzheimer's disease guest. Pharm Res. (2017) 34:2614–27. 10.1007/s11095-017-2199-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mima Y, Kuwashiro T, Yasaka M, Tsurusaki Y, Nakamura A, Wakugawa Y, et al. Impact of metformin on the severity and outcomes of acute ischemic stroke in patients with type 2 diabetes melli-tus. J Stroke Cerebrovasc dis. (2016) 25:436–46. 10.1016/j.jstrokecerebrovasdis.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 49.Saliua JA, Oboh G, Omojokun OS, Rochad J, Schetinger MR, Guterries J, et al. Effect of dietary supplementation of Padauk (Pterocarpus soyauxii) leaf on high fat diet/streptozotocin induced diabetes in rats' brain and platelets. Biomed Pharmacother. (2016) 84:1194–201. 10.1016/j.biopha.2016.10.043 [DOI] [PubMed] [Google Scholar]
- 50.Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, et al. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain. (2005) 128:1442–53. 10.1093/brain/awh452 [DOI] [PubMed] [Google Scholar]
- 51.Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, et al. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer's disease. J Neurosci. (2003) 23:7504–9. 10.1523/JNEUROSCI.23-20-07504.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fei L, Yong-Jun H, Zhang-Min M, Bing X, Shuang W, Qian-Qian S, et al. Rosiglitazone attenuates memory impairment in aged rat with diabetes by inhibiting NF-kappa B signal pathway activation. Exp Clin Endocrinol Diabetes. (2015) 123:536–42. 10.1055/s-0035-1559607 [DOI] [PubMed] [Google Scholar]
- 53.O'Bryant SE, Zhang F, Petersen M, Johnson L, Hall J, Rissman RA, et al. Precision medicine approach to treating Alzheimer's disease using rosiglitazone therapy: a biomarker analysis of the REFLECT trials. J Alzheimers Dis. (2021) 81:557–68. 10.3233/JAD-201610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yildirim Simsir I, Soyaltin UE, Cetinkalp S. Glucagon like peptide-1 (GLP-1) likes Alzheimer's disease. Diabetes Metab Syndr. (2018) 12:469–75. 10.1016/j.dsx.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 55.Zuzga DS, Francis JS, Fitzsimons HL, Jiao X. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. (2003) 9:1173–9. 10.1038/nm919 [DOI] [PubMed] [Google Scholar]
- 56.Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease- associated Aβ oligomers. J Clin Invest. (2012) 122:1339–53. 10.1172/JCI57256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gali CC, Khatwal RB. Dubala A, Chinni S, Holsinger RM. Saxagliptin: a dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer's disease. Neuropharmacology. (2013) 72:291–300. 10.1016/j.neuropharm.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 58.Angelopoulou E, Piperi C. DPP-4 inhibitors: a promising therapeutic approach against Alzheimer's disease. Ann Transl Med. (2018) 6:255. 10.21037/atm.2018.04.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rizzo MR, Barbieri M, Boccardi V, Angellotti E, Marfella R, Paolisso G. Dipeptidyl peptidase-4 inhibitors have protective effect on cognitive impairment in aged diabetic patients with mild cognitive impairment. J Gerontol A Biol Sci Med Sci. (2014) 69:1122–31. 10.1093/gerona/glu032 [DOI] [PubMed] [Google Scholar]
- 60.Borzì AM, Condorelli G, Biondi A, Basile F, Vicari ESD, Buscemi C, et al. Effects of vildagliptin, a DPP-4 inhibitor, in elderly diabetic patients with mild cognitive impairment. Arch Gerontol Geriatr. (2019) 84:103896. 10.1016/j.archger.2019.06.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.