Abstract
Atmospheric ammonia (NH3) and ammonium (NH4+) can substantially influence air quality, ecosystems, and climate. NH3 volatilization from fertilizers and wastes (v-NH3) has long been assumed to be the primary NH3 source, but the contribution of combustion-related NH3 (c-NH3, mainly fossil fuels and biomass burning) remains unconstrained. Here, we collated nitrogen isotopes of atmospheric NH3 and NH4+ and established a robust method to differentiate v-NH3 and c-NH3. We found that the relative contribution of the c-NH3 in the total NH3 emissions reached up to 40 ± 21% (6.6 ± 3.4 Tg N yr−1), 49 ± 16% (2.8 ± 0.9 Tg N yr−1), and 44 ± 19% (2.8 ± 1.3 Tg N yr−1) in East Asia, North America, and Europe, respectively, though its fractions and amounts in these regions generally decreased over the past decades. Given its importance, c-NH3 emission should be considered in making emission inventories, dispersion modeling, mitigation strategies, budgeting deposition fluxes, and evaluating the ecological effects of atmospheric NH3 loading.
Subject terms: Element cycles, Atmospheric chemistry, Geochemistry
By integrating nitrogen isotope systematics of ammonia emissions and transformations in the atmosphere, this study quantified the combustion-related ammonia emission and uncovered its importance for mitigating strategies of ammonia pollution.
Introduction
Ammonia (NH3) is a highly water-soluble and reactive gas in the atmosphere1. The uptake of NH3 within clouds and rain and onto atmospheric aerosols causes the formation of ammonium (NH4+) within atmospheric particulates and precipitation (denoted as p-NH4+ and w-NH4+, respectively)1–3. Over the last century, urbanization and industrial and agricultural intensification have greatly increased NH3 production and have led to a continuous increase in the NH3 emission amounts and deposition fluxes4–8. Globally, the NH3 emission has increased from 20.6 Tg N yr−1 in 1860 to 58.2 Tg N yr−1 in 1993 and may double to 118.0 Tg N yr−1 by 20504. Since the 1950s, East Asia, North America, and Europe have been three regions of high NH3 emissions9,10, with total emission amounts of 10.7 ± 0.4, 3.7 ± 0.2, and 3.5 ± 0.3 Tg N yr−1 during 2000–2015, respectively, according to emission inventory data (Supplementary Table 1). As a result, atmospheric NH3 concentrations (averaging 2.9 ± 2.4, 1.4 ± 1.8, 1.2 ± 1.3 μg m−3, respectively11) and NHx deposition (the sum of NH3, p-NH4+, and w-NH4+) (averaging 12.0, 4.7, and 6.9 Tg N yr−1, respectively12,13) in the above three regions also remain high. In human-disturbed areas, excessive NH3 has promoted secondary aerosol production and air pollution2–4. Elevated NHx concentrations and deposition have caused negative impacts on ecosystem structure and function (e.g., biodiversity declines, soil acidification, water eutrophication2,3,14) and huge economic loss15–17.
There are two major groups of atmospheric NH3 emission sources. One is NH3 volatilization from NH4+-containing substrates (mainly fertilized and natural soils, animal wastes, and natural and N-polluted water) (denoted as v-NH3)2,9,18,19. The dissolved NH3 volatilizes from liquid-phase substrates containing NH4+ at favorable pH, temperature, and pressure conditions20. The other is NH3 emission from combustion-related sources (mainly coal combustion, vehicle exhausts, and biomass burning) (denoted as c-NH3)21–24. NH3 would be released from industrial coal combustion, heavy-duty and light-duty diesel vehicles equipped with selective catalytic or non-catalytic reduction systems because of excessive urea/NH3 used for the catalytic degradation of nitrogen oxides (NOx)25,26. It is also produced via steam reforming from hydrocarbons27 and or catalytic reaction of nitric oxide with molecular hydrogen28 in light and medium-dust gasoline vehicles equipped with three-way catalytic converters, relating to the catalyst temperatures and air-to-fuel ratios29. The biomass N, typically as amides (R-(C = O)-NH-R’) and amines (R-NH2), can produce NH3 under poor mixing conditions during biomass burning30. So far, there have been direct observations on emission factors of various v-NH3 sources to budget the v-NH3 emission amount (Supplementary Table 1; Supplementary Fig. 1a). According to statistical emission inventories, the v-NH3 is the dominant source of regional NH3 emissions, accounting for 94 ± 1%, 90 ± 1%, and 95 ± 1% of the total emission in East Asia during 2001–2015, North America during 1970–2019, and Europe during 1970–2018, respectively (Supplementary Fig. 1b). In contrast, it has long been difficult to estimate the c-NH3 emission because of limited data on emission factors of c-NH3 sources and uncertainties associated with the amount of combusted materials, especially biomasses22,23.
However, evidence from laboratory simulations, in-situ observations, satellite observations, and emission inventories points to an underestimation of c-NH3 emissions relative to previous assessments (summarized in Supplementary Table 2). First, laboratory simulations found that biomass burning and light-duty diesel vehicles equipped with selective catalytic reduction are important NH3 sources and have been overlooked (Supplementary Table 2). Second, ground observations found that ambient NH3 concentrations at sites impacted by biomass burning, traffic pollution, industrial pollution, or urban pollution were enhanced by a factor of 1.4–20 compared to unpolluted sites (Supplementary Table 2). Third, satellite observations revealed that the NH3 from biomass burning controlled seasonal variations of surface NH3 concentrations in the southern and high-emission regions of China, the USA, and Europe in the northern hemisphere (Supplementary Table 2). Spatially, satellite observations also identified 13 hotspots of urban NH3 pollution and 266 hotspots of industrial NH3 pollution from coal combustion and coal-related industries (Supplementary Table 2). Fourth, according to emission inventories, the total c-NH3 emissions from transportation (1.3 Tg N yr−1)23, biomass burning (8.2 Tg N yr−1)31, and other combustion sources (6.3 Tg N yr−1)10 has reached up to 15.9 Tg N yr−1, which accounts for 30% of the global NH3 emission (54.3 Tg N yr−1). Additionally, isotopic evidence demonstrated that c-NH3 had reached 29–62% in NH3 of the ambient atmosphere32–34 and 45–90% of the NH4+ deposition in cities of China and the USA35,36. All the above evidence suggests that the relative importance and amount of regional c-NH3 emissions are still open questions and should be re-evaluated.
Here we collated observation data of natural N isotopes (expressed as δ15N, δ15N = (15N/14N)sample/(15N/14N)standard −1, where atmospheric N2 is used as the standard) of primary v-NH3 and c-NH3 emission sources (Supplementary Fig. 2), NH3 gas in the ambient atmosphere (a-NH3), NH4+ in atmospheric particulates (p-NH4+) and precipitation (w-NH4+) in East Asia, North America, and Europe (Fig. 1–3; Supplementary Figs. 3 & 4). In combination with the collected data of a-NH3 and p-NH4+ concentrations observed in the above regions, we evaluated δ15N differences of the initial NH3 mixture of v-NH3 and c-NH3 (i-NH3) to a-NH3, p-NH4+, or w-NH4+, respectively (Supplementary Figs. 5–9; detailed in Methods). Then, based on the source δ15N, δ15N difference, δ15N observation of a-NH3, p-NH4+, and w-NH4+, and the Stable Isotope Analysis in R model (i.e., the SIAR model; detailed in Methods), we established a set of isotopic methods to calculate relative contributions between v-NH3 and c-NH3 in above study regions (Fig. 4 & 5; Supplementary Figs. 10–12). Finally, using regional mean fraction values and emission amounts of v-NH3, we recalculated the amounts of c-NH3 and total NH3 emissions in each region (Fig. 5; Supplementary Figs. 13 & 14).
Fig. 1. Conceptual framework of atmospheric NH3 and NH4+.
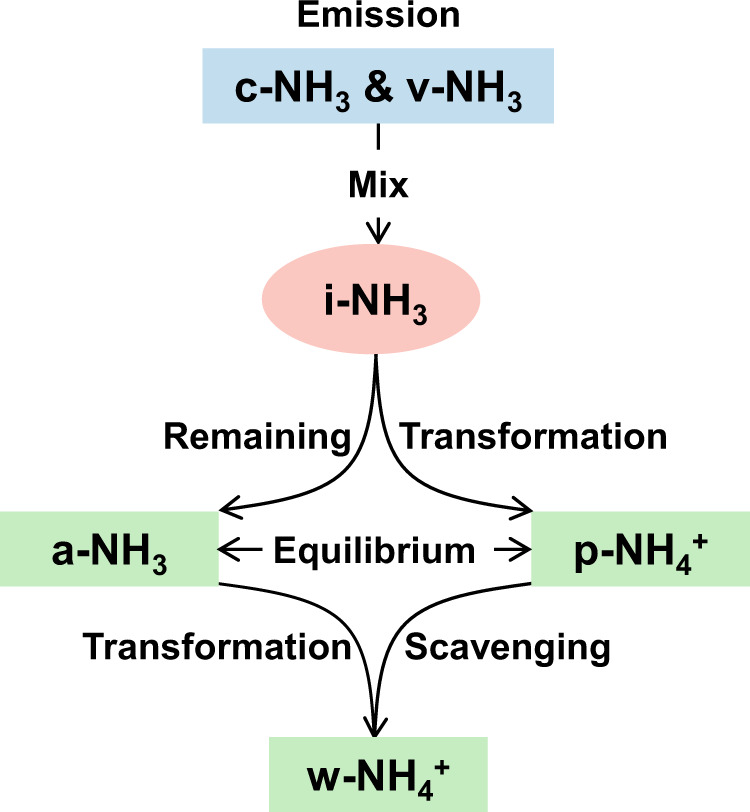
It shows the relationships among NH3 emissions from combustion-related sources (c-NH3) and volatilization sources (v-NH3), the initial mixture of c-NH3 and v-NH3 (i-NH3), ambient NH3 (a-NH3), particulate NH4+ (p-NH4+), and precipitation NH4+ (w-NH4+). The a-NH3 potentially includes the NH3 that has not been converted to p-NH4+ in earlier stages and the fresh NH3 emissions in relatively later periods.
Fig. 3. δ15N of ambient NH3 (a-NH3), particulate NH4+ (p-NH4+), and precipitation NH4+ (w-NH4+) (a) and the initial NH3 mixture from different sources (δ15Ni-NH3) (b) in East Asia, North America, and Europe.
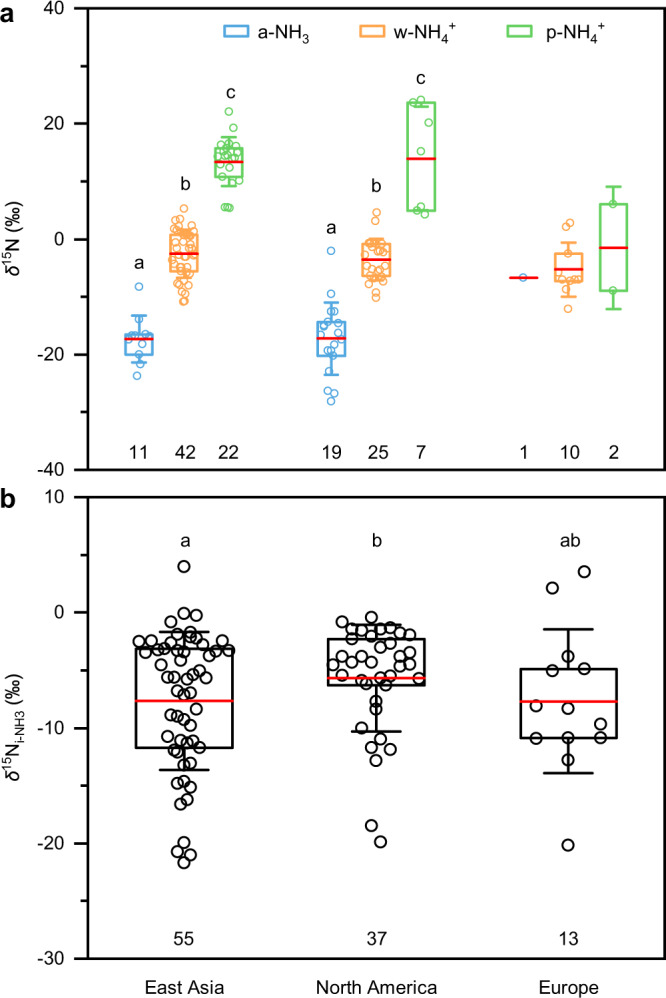
Each data point represents the site-based mean δ15N. Each box encompasses the 25th−75th percentiles, whiskers and red lines in boxes are the SD and mean values, respectively. The number below each box is that of observation sites. Note that the site with simultaneous a-NH3, w-NH4+, or p-NH4+ observation is counted as one site in sub-figure b. In sub-figure a, different letters (a, b, and c) above the boxes indicate the significant differences (p < 0.05) among species in the same region; in sub-figure b, different letters (a and b) above the boxes indicate the significant differences among the three regions. The δ15Na-NH3 based on the passive samplers have been calibrated by adding 15‰41. The δ15Ni-NH3 was calculated according to Eqs. (11–13) (detailed in Methods).
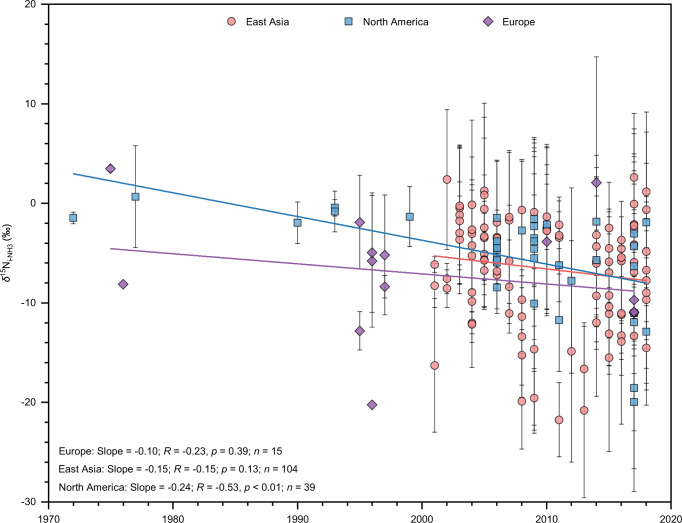
Fig. 4. Temporal variations of δ15N of the initial NH3 mixture from different sources (δ15Ni-NH3) in East Asia, North America, and Europe.
The mean ± SD of replicate measurements at each site in each year is shown. We counted the same site with different years as different observations, given that δ15N observations at a few sites have been conducted in different sampling years.
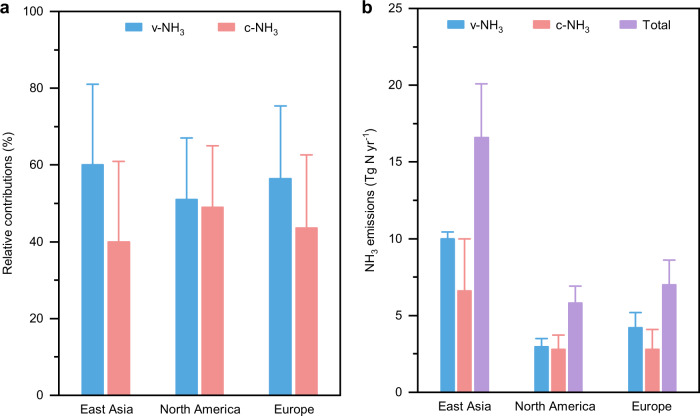
Fig. 5. Relative contributions of volatilization NH3 (v-NH3) and combustion-related NH3 (c-NH3) sources (a) and their emission amounts (b).
The ‘Total’ is the sum of v-NH3 and c-NH3. Mean±SD is shown.
Results and Discussion
δ15N signatures of v-NH3 and c-NH3
δ15N for v-NH3 (δ15Nv-NH3) average −18.9 ± 4.2‰, significantly lower than c-NH3 (δ15Nc-NH3, averaging 8.0 ± 4.4‰) (Supplementary Fig. 2b, detailed in Methods). The volatilization of NH3 includes three main steps, i.e., the equilibrium of NH4+ ↔ NH3, the diffusion of NH3 to and away from volatilization sites37. The overall isotope effects range from −60‰ to −30‰ (depending on temperature, pH, and cation-exchange capacities of substrates20,38–40, causing the low δ15Nv-NH3. Differently, δ15Nc-NH3 would assemble or be slightly higher than δ15N of burning materials that are relatively 15N-enriched41. Distinct δ15Nv-NH3 and δ15Nc-NH3 provide a unique tool to differentiate the relative contributions between v-NH3 and c-NH3 in the SIAR isotope mass-balance model.
δ15N differences between a-NH3, p-NH4+, or w-NH4+ and i-NH3
The δ15N of the initial NH3 mixture of v-NH3 and c-NH3 emissions (δ15Ni-NH3) integrate their δ15N signatures and fractional contributions (Fv-NH3 and Fc-NH3, respectively) (Fig. 1, Eq. (1)).
| 1 |
where Fv-NH3 + Fc-NH3 = 1. However, because NH3 is very reactive and readily transformed into NH4+, it is difficult in reality, if not impossible, to directly measureδ15Ni-NH342,43.
Practically, site-based δ15N values of a-NH3, p-NH4+, and w-NH4+ have been widely measured (Fig. 2). However, their δ15N, namely δ15Na-NH3, δ15Np-NH4+, and δ15Nw-NH4+, cannot be directly used as δ15Ni-NH3 to calculate Fv-NH3 and Fc-NH3 (Eq. (1)). First, the i-NH3 mixture of v-NH3 and c-NH3 emissions will be partially converted to p-NH4+ and w-NH4+ (Fig. 1). The conversion of NH3 to p-NH4+ has significant isotope effects as a result of either kinetic isotope fractionations (εk) during the unidirectional reaction of NH3 → p-NH4+ or equilibrium isotope fractionations (εeq) during reversible reactions of NH3 ↔ p-NH4+ (Supplementary Table 3). Accordingly, δ15Na-NH3 and δ15Np-NH4+ differ from δ15Ni-NH3 and thus cannot be directly used in Eq. (1)34,35. Second, precipitation scavenges both a-NH3 and p-NH4+ via the rainout and washout processes (Fig. 1), but the preferential wet scavenge between a-NH3 and p-NH4+ can potentially cause differences between δ15Nw-NH4+ and δ15Ni-NH344. Consequently, δ15Nw-NH4+ cannot be directly used to calculate Fv-NH3 and Fc-NH3 in Eq. (1) either. According to both simultaneous observations at the same sites (Supplementary Table 4) and non-synchronous observations in the same regions (Fig. 3a), δ15N in NH4+ (particularly p-NH4+) are generally higher than δ15Na-NH3, which is generally negative (Fig. 3a). The main reason is that large equilibrium isotope fractionations occur during the transformation of NH3 to NH4+ (Supplementary Table 3), leading to substantial differences (denoted as 15∆) of δ15Na-NH3, δ15Np-NH4+, or δ15Nw-NH4+ to the corresponding δ15Ni-NH3 (15∆a-NH3, 15∆p-NH4+, and 15∆w-NH4+, respectively). In this work, we developed a new set of methods to constrain 15∆a-NH3, 15∆p-NH4+, and 15∆w-NH4+, and then reconstruct δ15Ni-NH3 (detailed in Methods).
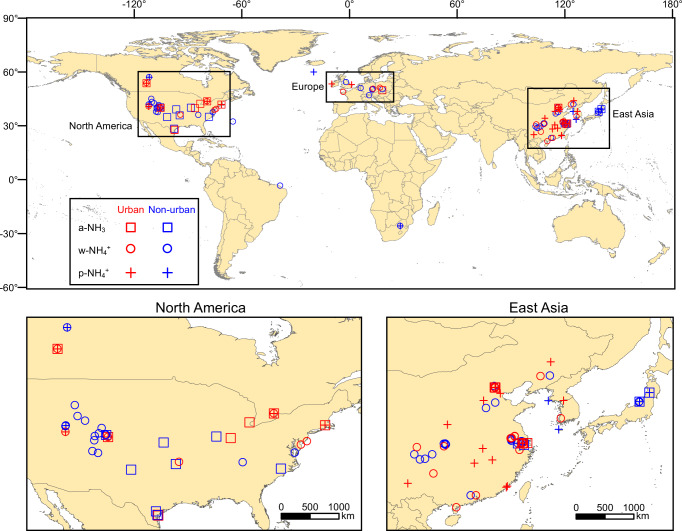
Fig. 2. Global and regional maps with the observation sites for δ15N of ambient NH3 (a-NH3), particulate NH4+ (p-NH4+), and precipitation NH4+ (w-NH4+) in East Asia, North America, and Europe.
Maps were created by using ArcGIS version 10.5 (Esri Inc., USA). The base map was download from https://hub.arcgis.com/datasets/esri::world-countries-generalized.
Generally, based on simultaneous observation data of seasonal mean Ca-NH3, Cp-NH4+, δ15Na-NH3, δ15Np-NH4+, and δ15Nw-NH4+ at six sites (Supplementary Table 5), we estimated corresponding δ15Ni-NH3, 15∆a-NH3, 15∆p-NH4+, and 15∆w-NH4+ (Eqs. (7–10); detailed in Methods). Then, we established the relationships between 15∆a-NH3, 15∆p-NH4+, or 15∆w-NH4+ and atmospheric NH3 conversion ratios (expressed as fp-NH4+, i.e., Cp-NH4+/(Ca-NH3 + Cp-NH4+)) (Supplementary Fig. 5). These relationships show that 15∆a-NH3, 15∆p-NH4+, and 15∆w-NH4+ decrease with an increase of fp-NH4+ (Supplementary Fig. 5). 15∆a-NH3 and 15∆p-NH4+ vary linearly with the reaction degree of an open system (i.e., fp-NH4+) (Supplementary Fig. 5a) consistent with the prediction of isotopic theory45. The 15∆w-NH4+ includes 15∆a-NH3 and 15∆p-NH4+ because precipitation scavenges a-NH3 and p-NH4+ via the rainout and washout processes (Supplementary Fig. 5b).
Meanwhile, we examined the impact of historical NOx and sulfur dioxide (SO2) reductions on mean annual fp-NH4+ (Supplementary Fig. 6a–f). The fp-NH4+ generally decreased from 1990 to 2017 in Europe and 2004 to 2018 in North America, but did not vary clearly from 2000 to 2018 in East Asia (Supplementary Fig. 7). Then, based on the relationships between 15∆a-NH3, 15∆p-NH4+, or 15∆w-NH4+ and fp-NH4+ (Supplementary Fig. 5) and mean annual fp-NH4+ values (Supplementary Fig. 7), we calculated mean annual 15∆a-NH3, 15∆p-NH4+, and 15∆w-NH4+ in each region (Supplementary Fig. 8), which were further used to calculate the corresponding δ15Ni-NH3 (Fig. 3b) of site-based δ15Na-NH3, δ15Np-NH4+, or δ15Nw-NH4+ (Fig. 3a) (detailed in Methods).
Spatial and temporal patterns of δ15Ni-NH3 variations
Spatially, δ15Ni-NH3 is higher in North America (−5.7 ± 4.6‰) than in Europe (−7.7 ± 6.3‰) and East Asia (−8.0 ± 6.0‰) (Fig. 3b). Because the δ15Nc-NH3 is distinctly higher than the δ15Nv-NH3 (Supplementary Fig. 2), the emission strength of c-NH3 relative to v-NH3 in North America is higher than that in East Asia and Europe. On the one hand, the emission inventories also show that the proportion of v-NH3 in North America is lower than that in East Asia and Europe (Supplementary Fig. 1b). On the other hand, because energy consumption is the primary source of c-NH3 and fertilizer consumption and animal manure are the source of v-NH3 (Supplementary Fig. 15a, b), the energy consumption ratio to fertilizer consumption and animal manure is higher in North America than in East Asia and Europe (Supplementary Fig. 15c).
δ15Ni-NH3 decreased significantly between 1972 and 2018 in North America (p < 0.01) and decreased slightly from 2001 to 2018 in East Asia (p = 0.13) and 1974–2017 in Europe (p = 0.39) (Fig. 4). These results suggest that the emission strength of v-NH3 relative to c-NH3 generally increased during the past decades in our three study regions, especially in North America. This finding coincided with increasing fertilizer consumption and animal manure in East Asia and North America over the past decades (Supplementary Fig. 16).
Relative contributions and amounts of v-NH3 and c-NH3 emissions
We considered 15∆a-NH3, 15∆p-NH4+, and 15∆w-NH4+ (detailed in Methods) in Eq. (1) to establish a set of new isotope mass-balance equations to calculate Fv-NH3 and Fc-NH3 by using δ15Na-NH3, δ15Np-NH4+, or δ15Nw-NH4+ in each region (Eqs. (2–4), respectively).
| 2 |
| 3 |
| 4 |
Fv-NH3 and Fc-NH3 values were calculated by the SIAR model (detailed in Methods).
Fc-NH3 averages 40 ± 21% in East Asia, 49 ± 16% in North America, and 44 ± 19% in Europe (Fig. 5a), which confirms higher emission strength of c-NH3 relative to v-NH3 in North America than in East Asia and Europe. These estimations based on isotope methods are generally higher than the fractions of c-NH3 emissions in corresponding regions (5–10%; Supplementary Fig. 1b) or the globe based on emission inventories (30%10,23,31). One possible explanation is that the study regions are hotspots of globally high c-NH3 emissions24. Supportively, the total consumption of fossil fuels in these regions accounts for more than 60% of the global energy consumption (Supplementary Fig. 15a), though their areas account for only 24% of the worldwide land area46. Further, the spatial pattern of Fc-NH3 (North America > Europe > East Asia; Fig. 5a) assembles that of the energy consumption ratio to fertilizer consumption and animal manure (Supplementary Fig. 15c). Based on Fv-NH3 in our study and explicit amounts of v-NH3 (Av-NH3) in emission inventories (Fig. 5), we further estimated the amounts of c-NH3 (Ac-NH3) and total NH3 emissions (detailed in Methods), which average 6.6 ± 3.4 Tg N yr−1 and 16.6 ± 3.5 Tg N yr−1 in East Asia, 2.8 ± 0.9 Tg N yr−1 and 5.8 ± 1.1 Tg N yr−1 in North America, and 2.8 ± 1.3 Tg N yr−1 and 7.0 ± 1.6 Tg N yr−1 in Europe, respectively (Fig. 5b). The highest energy consumption in East Asia supports its highest Ac-NH3 among the three study regions (Fig. 5b & Supplementary Fig. 15a).
Our new estimates of total NH3 emission in Europe are very close to its total NHx deposition (Supplementary Fig. 13). In China and the United States, the NHx deposition fluxes could be explained by our updated total NH3 emissions (Supplementary Fig. 13). Lower deposition than the emission was observed in China and the United States because part of the NH3 emissions was diffused or deposited out of these polluted areas24,47. Before this work, total NH3 emissions based on statistical inventories in China, the United States, and Europe were all distinctly lower than the corresponding NHx deposition (Supplementary Fig. 13). Accordingly, our results provided new estimates on c-NH3 and total NH3 emissions. However, because the v-NH3 underestimation may still exist48, the contribution of the c-NH3 underestimation to the underestimation of total NH3 emissions and the mismatches between regional NH3 emissions and NHx deposition (Supplementary Fig. 13) remains uncertain.
Temporally, Fc-NH3 has decreased significantly (p < 0.01) over the past decades in North America (Supplementary Fig. 12a), leading to increasing ratios of Fv-NH3 to Fc-NH3 (p < 0.01) and also Av-NH3 to Ac-NH3 (p < 0.05) generally from lower than 1.0 to higher than 1.0 (Supplementary Figs. 12b & 14b). Fc-NH3 in East Asia and Europe has decreased slightly (p = 0.12 and p = 0.26, respectively) (Supplementary Fig. 12a), leading to slightly increasing ratios of Fv-NH3 to Fc-NH3 (p = 0.12 and p = 0.51, respectively) and also Av-NH3 to Ac-NH3 over the past decades (p = 0.25 and p = 0.61, respectively) (Supplementary Figs. 12b & 14b). In East Asia and North America, the temporally increasing v-NH3 emissions relative to c-NH3 are supported by the increasing fertilizer consumption and animal manure production (Supplementary Fig. 16). Emission inventories also showed the rapid increase of the relative contribution of v-NH3 in North America (Supplementary Fig. 1b). These temporal variations in North America revealed that the more dominant NH3 emission has shifted from c-NH3 to v-NH3 sources over the past decades. Rapidly increasing v-NH3 emissions may be one of the reasons for the shift from nitrate-dominated to NH4+-dominated N deposition in the United States7.
In this study, the non-urban Fc-NH3 does not change with the corresponding distance between the sampling site and the nearest urban area (Supplementary Fig. 11). Meanwhile, there are no significant differences in Fc-NH3 between urban (73 sites) and non-urban (65 sites) or between agricultural (32 sites) and non-agricultural sites (33 sites) (Supplementary Fig. 11). Similar numbers of replicate sites between the above surface environments reduce the risks of over- or under-estimating c-NH3 or v-NH3 contributions. Accordingly, the Fc-NH3 at urban or non-urban sites is not substantially influenced by local NH3 emissions but reflects the source diversity and transporting/mixing complexity of regional NH3 emissions. There has been much evidence from ground monitoring, satellite observations, and emission inventories to show the co-occurrence of c-NH3 and v-NH3 emissions and extensive NH3 or NH4+ transportation and mixing among landscapes. Firstly, v-NH3 sources (mainly from solid wastes and sewages) have comparable emission strengths with c-NH3 sources (mainly from fossil fuel combustion) in urban areas. Human excreta contributed 11.4% to the total NH3 emissions in Shanghai urban of eastern China49. The v-NH3 from urban green space contributed up to 60% to ambient NH3 in Qingdao in northern China50. The urban NH3 concentrations influenced by the v-NH3 from urban waste containers, sewage systems, humans, and open markets were 2.5 times higher than that of traffic-influenced urban areas in Spain51. Ambient NH3 concentrations in the Beijing urban peaked when fertilizer was intensively applied on the North China Plain52. Secondly, the c-NH3 emission (mainly wildfire, fossil fuel and crop residue combustion) is undoubtedly as significant as the v-NH3 (mainly fertilizer application and live stocks) in non-urban areas. For example, the c-NH3 from biomass burning control seasonal variations of surface NH3 concentrations in major disturbed regions of the Northern Hemisphere, which is even stronger in the Southern Hemisphere with frequent wildfires (Supplementary Table 2). Based on a data synthesis (Supplementary Table 2), ambient NH3 concentrations during wildfire smoke-impacted periods could be enhanced by a factor of 2–20 compared to periods with no wildfires. Besides, our results, as well as previous studies, show that v-NH3 contributed 58% (31–87%) for sites in Beijing urban34,35,53, while the c-NH3 accounted for 33% (25–69%) in the total NH4+ deposition of the cropland 370 km far from the Beijing urban11. Collectively, site-based δ15Na-NH3 and δ15NNH4+ represent mixing signatures and can be feasibly used to evaluate regional c-NH3 and v-NH3 emissions.
Implications and uncertainties
This study demonstrates that c-NH3 emissions have been considerably underestimated in regions with significant anthropogenic sources. In the process, the new estimates of c-NH3 emissions have brought measured NHx deposition data into closer agreement with emissions. Our conclusion about the underestimation of c-NH3 emission has important implications for control measures since the current efforts have been focused almost exclusively on agricultural sources. Although the marginal abatement cost of NH3 emissions is only 10% of the global NOx emission, with 162 billion US dollars net benefit, the reduction can effectively improve air pollution and its negative impact54,55. For example, measures to reduce c-NH3 emissions should be considered in the methods for mitigating v-NH3 emissions by improved farm management practices with N use reductions, deep machine placement of fertilizer, enhanced-efficiency fertilizer use, and enhanced manure management in the agriculture sector56,57. The revelation of high c-NH3 emissions also implies that the potential, costs, and impacts of NH3 emissions reduction need to be re-assessed. Steps to reduce c-NH3 emissions may alleviate the pressure of reducing agricultural NH3 emissions and achieve ‘win-win’ outcomes for agricultural production and food supply, human and environmental health2,56.
The uncertainty of this study lies in the technical difficulty in measuring δ15N for all NH3 emission sources in each region. It is even more challenging to conduct simultaneous observations on Ca-NH3, Cp-NH4+, δ15Na-NH3, δ15Np-NH4+, and δ15Nw-NH4+ among landscapes. As a result, a limited number of data is available in terms of observation sites and their spatial distribution in the three regions, and the corresponding data comparison among the three regions is preliminary in the current stage. Moreover, δ15N observations focus largely on the middle- and low-latitudes and low altitudes of the Northern Hemisphere but rare on the high mountains and high latitudes of the Northern Hemisphere and the Southern Hemisphere. More observations on the concentration and δ15N parameters of NH3 and NH4+ in both source emissions and deposition are necessary to improve the isotope source apportionment, especially in remote areas. Finally, despite the updated regional c-NH3 and total NH3 emissions, future in-depth cooperation among different observational and modeling methods should be encouraged.
Methods
δ15N of major NH3 emission sources
We collected the δ15N data of major NH3 emission sources (Supplementary Fig. 2a) from 17 relevant publications (by December 2020). For c-NH3, the proportional contributions of global NH3 emissions from vehicle exhausts (ve-NH3), coal combustion (cc-NH3), and biomass burning (bb-NH3) (1.3, 6.3, and 8.2 Tg N yr−1, respectively10,23,31) in total c-NH3 emissions (15.9 Tg N yr−1) are 8%, 40%, and 52%, respectively (Eq. (5)). Accordingly, we calculated the δ15Nc-NH3 by a mass-balance method (Eq. (5)).
| 5 |
For v-NH3, global NH3 emissions from fertilizer application (fa-NH3) and waste materials (wm-NH3) account for 56% and 44% of the total v-NH3 emission, respectively10. Similarly, we calculated the δ15Nv-NH3 by Eq. (6).
| 6 |
The standard deviation (SD) of δ15Nc-NH3 and δ15Nv-NH3 is propagated errors estimated using the Monte Carlo method (MCM). Briefly, we ran 10000 trials for the MCM in the software of Microsoft Excel-Add-In and calibrated the SD to match the corresponding true values.
Atmospheric δ15Na-NH3, δ15Np-NH4+, and δ15Nw-NH4+ observations
Keywords used for the search are ‘nitrogen isotope’, ‘ammonia/NH3’, ‘ammonium/NH4+’, ‘rainfall’, ‘rain’, ‘rain water’, ‘precipitation’, ‘aerosol’, and ‘particulate’. The databases include the Web of Science (http://isiknowledge.com), Google Scholar (http://scholar.google.com.hk), and Baidu Scholar (http://xueshu.baidu.com). By December 2020, there are 18 publications on δ15Na-NH3 (listed in Supplementary Text 1), 43 publications on δ15Nw-NH4+ (listed in Supplementary Text 2), and 28 publications on δ15Np-NH4+ (listed in Supplementary Text 3). Data in the figures of these publications were extracted using the software of Web Plot Digitizer (Version 4.2, San Francisco, California, USA).
Spatial distributions of the sites with δ15Na-NH3, δ15Np-NH4+, and δ15Nw-NH4+ observations are shown in Fig. 2. When counting the same site with observations in different years as one site only, there are 387 measurements of δ15Na-NH3 at 32 sites (including 12 sites in East Asia, 19 sites in North America, and one site in Europe), 857 measurements of δ15Np-NH4+ at 33 sites (including 22 sites in East Asia, seven sites in North America, two sites in Europe, one site in Africa, and one site in Atlantic), and 1540 measurements of δ15Nw-NH4+ at 80 sites (including 42 sites in East Asia, 25 sites in North America, ten sites in Europe, one site in South America, one site in Africa, and one site in Atlantic) (Fig. 2 & Supplementary Fig. 3). The surface land types of observation sites were identified according to descriptions in original publications. There are 73 urban sites (mainly constructed lands) and 65 non-urban sites (mainly including 32 agricultural sites and 33 non-agricultural sites) with δ15Na-NH3, δ15Np-NH4+, or δ15Nw-NH4+ observations in the study areas of East Asia, North America, and Europe (Supplementary Fig. 4a, b).
We only analyzed the data in the major areas of East Asia during 2001–2018, North America during 1972–2018, and Europe during 1974–2017 due to the sparsity of available data out of these areas (Fig. 2 & 4). It should be noted that analytical methods of 15Na-NH3, δ15Np-NH4+, and δ15Nw-NH4+ differ among studies, including converting to 1) N2 as an end product using the Elemental Analyzer combustion method or 2) N2O as an end product using the bromate oxidation and azide or hydroxylamine reduction, or using the persulfate oxidation and denitrifier method58, but such difference would not change the spatiotemporal patterns of δ15Ni-NH3 (Fig. 3 & 4) because the analytical accuracy is generally better than ±0.7‰. In addition, 9%, 16%, and 75% of the δ15N observations were conducted across cooler seasons, warmer seasons, and the whole year, respectively. The seasonal differences in NH3 emissions would not substantially influence the spatiotemporal patterns of δ15Ni-NH3 (Fig. 3 & 4). Moreover, all observation sites in this study are more than 1 km away from obvious local emission sources, excluding the influence of a single source.
Atmospheric Ca-NH3 and Cp-NH4+ observations
‘Atmospheric ammonia/NH3’, ‘particulate ammonium/NH4+’, and ‘aerosol ammonium/NH4+’ were used as keywords to search publications for the concentration of a-NH3 and p-NH4+ (Ca-NH3 and Cp-NH4+, respectively) in the same databases as described above. There are 107 publications published by July 2021 (listed in Supplementary Text 4) with simultaneous observations on Ca-NH3 and Cp-NH4+. To describe temporal variations of Cp-NH4+/(Ca-NH3 + Cp-NH4+) values (i.e., fp-NH4+ values) in each region (Supplementary Fig. 7), we counted the same site with different years as different observations because few sites observed Ca-NH3 and Cp-NH4+ for many years. We only used data with an observation period exceeding six months to improve the estimation of annual fp-NH4+ values. According to this criterion, there are 262 sites in East Asia during 1993–2018, 459 sites in North America during 1986–2018, and 1018 sites in Europe during 1981–2017 (Supplementary Fig. 7).
Differences of δ15Na-NH3, δ15Np-NH4+, or δ15Nw-NH4+ from i-NH3
Based on simultaneous observations of seasonal Ca-NH3, Cp-NH4+, δ15Na-NH3, δ15Np-NH4+, and δ15Nw-NH4+ values at the same sites (Supplementary Table 5), we calculated the δ15Ni-NH3 by the following isotope mass-balance equation (Eq. (7)).
| 7 |
where fa-NH3 = Ca-NH3 / (Ca-NH3 + Cp-NH4+) and fp-NH4+ = Cp-NH4+ / (Ca-NH3 + Cp-NH4+).
Then, we calculated the differences between δ15Ni-NH3 and δ15Na-NH3 (15∆a-NH3, Eq. (8)), between δ15Ni-NH3 and δ15Np-NH4+ (15∆p-NH4+, Eq. (9)), between δ15Ni-NH3 and δ15Nw-NH4+ (15∆w-NH4+, Eq. (10)) for the same sites with simultaneous observations of seasonal Ca-NH3, Cp-NH4+, δ15Na-NH3, δ15Np-NH4+, and δ15Nw-NH4+ values (Supplementary Table 5).
| 8 |
| 9 |
| 10 |
Based on the relationships in Supplementary Fig. 5 and mean annual fp-NH4+ values in Supplementary Fig. 7, we calculated the mean annual 15∆a-NH3, 15∆p-NH4+, and 15∆w-NH4+ in each region (Supplementary Fig. 8). Because no clear trends were observed in fp-NH4+ between 2001–2018 in East Asia and before implementing emission reduction measures in Europe (i.e., 1971–1989 in this study) and in North America (i.e., 1971–2004 in this study)59–61 (Supplementary Fig. 7), we calculated isotope effect values by using the mean fp-NH4+ during above-mentioned years in the corresponding region (Supplementary Fig. 8). In other words, the same values are assumed for 15∆a-NH3, 15∆p-NH4+, or 15∆w-NH4+ during these years in the region (Supplementary Fig. 8).
To examine the applicability of our method for estimating and calibrating 15∆ values, we used the mean annual 15∆ values and simultaneous δ15Na-NH3 and δ15Np-NH4+ observations, δ15Na-NH3 and δ15Nw-NH4+ observations, or δ15Np-NH4+ and δ15Nw-NH4+ observations at the same sites (Supplementary Table 4) to calculate their corresponding δ15Ni-NH3 (denoted as δ15Ni-NH3(a-NH3), δ15Ni-NH3(p-NH4+), or δ15Ni-NH3(w-NH4+); Eq. (11 − 13), respectively).
| 11 |
| 12 |
| 13 |
We found that differences between δ15Ni-NH3(a-NH3) and δ15Ni-NH3(p-NH4+), δ15Ni-NH3(a-NH3) and δ15Ni-NH3(w-NH4+), δ15Ni-NH3(p-NH4+) and δ15Ni-NH3(w-NH4+) are negligible, averaging 0.4 ± 2.3‰, −0.2 ± 1.3‰, and −0.4 ± 2.5‰, respectively (Supplementary Fig. 9). This demonstrates that the mean annual 15∆a-NH3, 15∆p-NH4+, and 15∆w-NH4+ values (Supplementary Fig. 8) estimated by the mean annual fp-NH4+ values (Supplementary Fig. 7) can be used to calculate corresponding δ15Ni-NH3 (Fig. 3b) of site-based δ15Na-NH3, δ15Np-NH4+, or δ15Nw-NH4+ (Fig. 3a) and to estimate the source contributions using the SIAR model.
Relative contributions and amounts of v-NH3 and c-NH3 emissions
Site-based Fv-NH3 and Fc-NH3 values of each region (Eqs. (2−4)) were calculated using the SIAR model62. Because each of our calculations has only two end members, the SIAR model can determine the Fv-NH3 and Fc-NH3 values. Moreover, this model allows us to incorporate isotope effects (by inputting mean annual 15∆a-NH3, 15∆p-NH4+, and 15∆w-NH4+ in our calculations; Supplementary Fig. 8), the variabilities in δ15N of both sources (by inputting mean ± SD of δ15Nv-NH3 and δ15Nc-NH3; Supplementary Fig. 2b) and the mixture (by inputting all replicate measurements of δ15Na-NH3, δ15Np-NH4+, or δ15Nw-NH4+ at each site) into the source contributions. In each run, the percentage data (n = 10000) output from the SIAR model was used to calculate the mean ± SD values of corresponding Fv-NH3 and Fc-NH3 at each site, then site-based mean ± SD values of Fv-NH3 and Fc-NH3 (Supplementary Figs. 10 & 11) were used to calculate the mean ± SD values of each region (Fig. 5a). The mean annual Fv-NH3 and Fc-NH3 were calculated by inputting all replicate measurements of δ15Na-NH3, δ15Np-NH4+, or δ15Nw-NH4+ in each year at each site (Fig. 4; Supplementary Fig. 12).
Based on the amount of the v-NH3 emission (Av-NH3), we calculated corresponding amounts of total NH3 emissions (Atotal, Eq. (14)) and the c-NH3 emission (Ac-NH3, Eq. (15)).
| 14 |
| 15 |
Regional mean ± SD of Av-NH3 and Fv-NH3 were used to calculate regional mean ± SD of Atotal and Ac-NH3 (Fig. 5). The annual Ac-NH3 (Supplementary Fig. 14a) was calculated using the mean Av-NH3 (Supplementary Fig. 1a) and mean ± SD of site-based Fv-NH3 values in each year (Supplementary Fig. 12). The SDs of Atotal and Ac-NH3 values were propagated errors estimated by using the same MCM described above.
Statistical analyses
The SPSS 18.0 software package (SPSS Science, Chicago, USA) and Origin 2016 statistical package (OriginLab Corporation, USA) for Windows were used for data analyses in this study. The Tukey honest significant difference (Tukey HSD) and the least significant difference (LSD) tests of the one-way analysis of variance (ANOVA) were used to identify significant differences in δ15N among a-NH3, w-NH4+, and p-NH4+ (Fig. 3a), East Asia, North America, and Europe (Fig. 3b), major sources (Supplementary Fig. 2), and urban and non-urban sites or agricultural and non-agricultural sites (Supplementary Fig. 4), in Fc-NH3 values among East Asia, North America, and Europe (Supplementary Fig. 10), and urban and non-urban sites or agricultural and non-agricultural sites (Supplementary Fig. 11a). Linear regressions were used to examine correlations between sampling years and δ15Ni-NH3 (Fig. 4), 15∆a-NH3, 15∆p-NH4+, or 15∆w-NH4+ and fp-NH4+ values (Supplementary Fig. 5), fp-NH4+ values and NOx and SO2 emissions (Supplementary Fig. 6), Fc-NH3 values at non-urban sites with the corresponding distances from the edge of the nearest urban area (Supplementary Fig. 11b), sampling years and Fc-NH3 or Fv-NH3/Fc-NH3 ratio (Supplementary Fig. 12), and sampling years and Ac-NH3 or Av-NH3/Ac-NH3 ratio (Supplementary Fig. 14). Statistically significant differences were set at p < 0.05 or as otherwise stated. The maps with the observation sites for δ15N (Fig. 2) were plotted with ArcGIS 10.5 software (Esri Inc., USA).
Supplementary information
Acknowledgements
This study was supported by the National Natural Science Foundation of China (42125301 (X.Y.L.), 41730855 (X.Y.L.), and 42073005 (W.S.)), and the Coordinated Research Project of IAEA (F32008 (X.Y.L.)). We want to thank all researchers who reported and kindly provided us with precious data on concentrations and isotopes of atmospheric NH3 and NH4+. We sincerely thank Prof. Keisuke Koba of Kyoto University for the initial discussion in Tianjin University.
Source data
Author contributions
X.Y.L. designed the research. Z.L.C., W.S. and X.Y.L. conducted the research (data collections and analyses) and co-wrote the manuscript. C.C.H., X.J.L., G.Y.C., W.W.W., G.M., C.Q.L. and D.F. commented on the manuscript.
Peer review
Peer review information
Nature Communications thanks Shaoneng He, Jun Li and Yanan Shen for their contribution to the peer review of this work.
Data availability
The data underlying the findings of this study are provided in the Source Data file. Source data are provided with this paper.
Code availability
The SPSS package can be downloaded from https://www.ibm.com/products/spss-statistics. The Origin 2016 statistical package can be downloaded from https://www.originlab.com. The ArcGIS package can be downloaded from https://www.esri.com/en-us/arcgis/about-arcgis/overview. The source code for SIAR used in this paper is openly available from https://rdrr.io/cran/siar.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zhi-Li Chen, Wei Song.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-35381-4.
References
- 1.Sutton, M. A., Reis, S. & Baker, S. M. H. Atmospheric Ammonia: Detecting Emission Changes and Environmental Impacts. (Springer, Netherlands, 2009).
- 2.Sutton MA, Oenema O, Erisman JW, Leip A, Winiwarter W. Too much of a good thing. Nature. 2011;472:159–161. doi: 10.1038/472159a. [DOI] [PubMed] [Google Scholar]
- 3.Zhang RY, et al. Formation of urban fine particulate matter. Chem. Rev. 2015;115:3803–3855. doi: 10.1021/acs.chemrev.5b00067. [DOI] [PubMed] [Google Scholar]
- 4.Galloway JN, et al. Nitrogen cycles: past, present, and future. Biogeochemistry. 2004;70:153–226. doi: 10.1007/s10533-004-0370-0. [DOI] [Google Scholar]
- 5.Fowler D, et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B. 2013;368:20130164. doi: 10.1098/rstb.2013.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu XJ, et al. Enhanced nitrogen deposition over China. Nature. 2013;494:459–462. doi: 10.1038/nature11917. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, et al. Increasing importance of deposition of reduced nitrogen in the United States. Proc. Natl Acad. Sci. USA. 2016;113:5874–5879. doi: 10.1073/pnas.1525736113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu GR, et al. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019;12:424–429. doi: 10.1038/s41561-019-0352-4. [DOI] [Google Scholar]
- 9.Clarisse L, Clerbaux C, Dentener F, Hurtmans D, Coheur PF. Global ammonia distribution derived from infrared satellite observations. Nat. Geosci. 2009;2:479–483. doi: 10.1038/ngeo551. [DOI] [Google Scholar]
- 10.Emissions Database for Global Atmospheric Research (EDGAR). Data were downloaded from https://edgar.jrc.ec.europa.eu/overview.php?v=431 (accessed on 16 October 2020) (2016).
- 11.Feng SJ, et al. Overlooked nonagricultural and wintertime agricultural NH3 emissions in Quzhou County, North China Plain: Evidence from 15N-stable isotopes. Environ. Sci. Technol. Lett. 2022;9:127–133. doi: 10.1021/acs.estlett.1c00935. [DOI] [Google Scholar]
- 12.Ge Y, Vieno M, Stevenson DS, Wind P, Heal MR. A new assessment of global and regional budgets, fluxes, and lifetimes of atmospheric reactive N and S gases and aerosols. Atmos. Chem. Phys. 2022;22:8343–8368. doi: 10.5194/acp-22-8343-2022. [DOI] [Google Scholar]
- 13.Holland EA, Braswell BH, Sulzman J, Lamarque JF. Nitrogen deposition onto the United States and western Europe: Synthesis of observations and models. Ecol. Appl. 2005;15:38–57. doi: 10.1890/03-5162. [DOI] [Google Scholar]
- 14.Joyce EE, et al. Highly concentrated atmospheric inorganic nitrogen deposition in an urban, coastal region in the US. Environ. Res. Commun. 2020;2:081001. doi: 10.1088/2515-7620/aba637. [DOI] [Google Scholar]
- 15.Zhang X, et al. Societal benefits of halving agricultural ammonia emissions in China far exceed the abatement costs. Nat. Commun. 2020;11:4357. doi: 10.1038/s41467-020-18196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodkind AL, Tessum CW, Coggins JS, Hill JD, Marshall JD. Fine-scale damage estimates of particulate matter air pollution reveal opportunities for location-specific mitigation of emissions. Proc. Natl Acad. Sci. USA. 2019;116:8775–8780. doi: 10.1073/pnas.1816102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Grinsven HJM, et al. Costs and benefits of nitrogen for Europe and implications for mitigation. Environ. Sci. Technol. 2013;47:3571–3579. doi: 10.1021/es303804g. [DOI] [PubMed] [Google Scholar]
- 18.Erisman JW, Sutton MA, Galloway J, Klimont Z, Winiwarter W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008;1:636–639. doi: 10.1038/ngeo325. [DOI] [Google Scholar]
- 19.Stokstad E. Ammonia pollution from farming may exact hefty health costs. Science. 2014;343:238. doi: 10.1126/science.343.6168.238. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Lollar BS, Li H, Wortmann UG, Lacrampe-Couloume G. Ammonium stability and nitrogen isotope fractionations for NH4+–NH3(aq)–NH3(gas) systems at 20–70 °C and pH of 2–13: Applications to habitability and nitrogen cycling in low-temperature hydrothermal systems. Geochim. Cosmochim. Acta. 2012;84:280–296. doi: 10.1016/j.gca.2012.01.040. [DOI] [Google Scholar]
- 21.Myles LT. Underestimating ammonia. Nat. Geosci. 2009;2:461–462. doi: 10.1038/ngeo565. [DOI] [Google Scholar]
- 22.Li Q, et al. Gaseous ammonia emissions from coal and biomass combustion in household stoves with different combustion efficiencies. Environ. Sci. Technol. Lett. 2016;3:98–103. doi: 10.1021/acs.estlett.6b00013. [DOI] [Google Scholar]
- 23.Meng WJ, et al. Improvement of a global high-resolution ammonia emission inventory for combustion and industrial sources with new data from the residential and transportation sectors. Environ. Sci. Technol. 2017;51:2821–2829. doi: 10.1021/acs.est.6b03694. [DOI] [PubMed] [Google Scholar]
- 24.Van Damme M, et al. Industrial and agricultural ammonia point sources exposed. Nature. 2018;564:99–103. doi: 10.1038/s41586-018-0747-1. [DOI] [PubMed] [Google Scholar]
- 25.Felix JD, Elliott EM, Gish TJ, McConnell LL, Shaw SL. Characterizing the isotopic composition of atmospheric ammonia emission sources using passive samplers and a combined oxidation-bacterial denitrifier approach. Rapid Commun. Mass Sp. 2013;27:2239–2246. doi: 10.1002/rcm.6679. [DOI] [PubMed] [Google Scholar]
- 26.Bishop GA, Stedman DH. Reactive nitrogen species emission trends in three light-/medium-duty United States fleets. Environ. Sci. Technol. 2015;49:11234–11240. doi: 10.1021/acs.est.5b02392. [DOI] [PubMed] [Google Scholar]
- 27.Whittington BI, Jiang CJ, Trimm DL. Vehicle exhaust catalysis: I. The relative importance of catalytic oxidation, steam reforming and water-gas shift reactions. Catal. Today. 1995;26:41–45. doi: 10.1016/0920-5861(95)00093-U. [DOI] [Google Scholar]
- 28.Barbier Jr J, Duprez D. Steam effects in three-way catalysis. Appl. Catal. B Environ. 1994;4:105–140. doi: 10.1016/0926-3373(94)80046-4. [DOI] [Google Scholar]
- 29.Heeb NV, Saxer CJ, Forss AM, Brühlmann S. Trends of NO-, NO2-, and NH3-emissions from gasoline-fueled Euro-3- to Euro-4-passenger cars. Atmos. Environ. 2008;42:2543–2554. doi: 10.1016/j.atmosenv.2007.12.008. [DOI] [Google Scholar]
- 30.Behera SN, Sharma M, Aneja VP, Balasubramanian R. Ammonia in the atmosphere: a review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ. Sci. Pollut. Res. 2013;20:8092–8131. doi: 10.1007/s11356-013-2051-9. [DOI] [PubMed] [Google Scholar]
- 31.Andreae MO. Emission of trace gases and aerosols from biomass burning—an updated assessment. Atmos. Chem. Phys. 2019;19:8523–8546. doi: 10.5194/acp-19-8523-2019. [DOI] [Google Scholar]
- 32.Chang YH, et al. Assessing contributions of agricultural and nonagricultural emissions to atmospheric ammonia in a Chinese megacity. Environ. Sci. Technol. 2019;53:1822–1833. doi: 10.1021/acs.est.8b05984. [DOI] [PubMed] [Google Scholar]
- 33.Berner AH, David Felix J. Investigating ammonia emissions in a coastal urban airshed using stable isotope techniques. Sci. Total Environ. 2020;707:134952. doi: 10.1016/j.scitotenv.2019.134952. [DOI] [PubMed] [Google Scholar]
- 34.Zhang YY, et al. Persistent nonagricultural and periodic agricultural emissions dominate sources of ammonia in urban Beijing: Evidence from 15N stable isotope in vertical profiles. Environ. Sci. Technol. 2020;54:102–109. doi: 10.1021/acs.est.9b05741. [DOI] [PubMed] [Google Scholar]
- 35.Pan YP, et al. Fossil fuel combustion-related emissions dominate atmospheric ammonia sources during severe haze episodes: Evidence from 15N-stable isotope in size-resolved aerosol ammonium. Environ. Sci. Technol. 2016;50:8049–8056. doi: 10.1021/acs.est.6b00634. [DOI] [PubMed] [Google Scholar]
- 36.Liu XY, et al. Stable isotope analyses of precipitation nitrogen sources in Guiyang, southwestern China. Environ. Pollut. 2017;230:486–494. doi: 10.1016/j.envpol.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Högberg P. 15N natural abundance in soil-plant systems. Tansley Review No 95. N. Phytol. 1997;137:179–203. doi: 10.1046/j.1469-8137.1997.00808.x. [DOI] [PubMed] [Google Scholar]
- 38.Heaton THE. Isotopic studies of nitrogen pollution in the hydrosphere and atmosphere: A review. Chem. Geol. 1986;59:87–102. doi: 10.1016/0168-9622(86)90059-X. [DOI] [Google Scholar]
- 39.Frank DA, Evans RD, Tracey BF. The role of ammonia volatilization in controlling the natural 15N abundance of a grazed grassland. Biogeochemistry. 2004;68:169–178. doi: 10.1023/B:BIOG.0000025736.19381.91. [DOI] [Google Scholar]
- 40.Walters WW, Chai J, Hastings MG. Theoretical phase resolved ammonia–ammonium nitrogen equilibrium isotope exchange fractionations: Applications for tracking atmospheric ammonia gas-to-particle conversion. ACS Earth Space Chem. 2019;3:79–89. doi: 10.1021/acsearthspacechem.8b00140. [DOI] [Google Scholar]
- 41.Walters WW, et al. Characterizing the spatiotemporal nitrogen stable isotopic composition of ammonia in vehicle plumes. Atmos. Chem. Phys. 2020;20:11551–11567. doi: 10.5194/acp-20-11551-2020. [DOI] [Google Scholar]
- 42.Heaton THE, Spiro B, Robertson SMC. Potential canopy influences on the isotopic composition of nitrogen and sulphur in atmospheric deposition. Oecologia. 1997;109:600–607. doi: 10.1007/s004420050122. [DOI] [PubMed] [Google Scholar]
- 43.Kawashima H, Ono S. Nitrogen isotope fractionation from ammonia gas to ammonium in particulate ammonium chloride. Environ. Sci. Technol. 2019;53:10629–10635. doi: 10.1021/acs.est.9b01569. [DOI] [PubMed] [Google Scholar]
- 44.Zheng XD, Liu XY, Song W, Sun XC, Liu CQ. Nitrogen isotope variations of ammonium across rain events: Implications for different scavenging between ammonia and particulate ammonium. Environ. Pollut. 2018;239:392–398. doi: 10.1016/j.envpol.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 45.Fry B. Stable Isotope Ecology. Ch. 7 (Springer, New York, 2006)
- 46.World Bank Open Data. Data were downloaded from https://data.worldbank.org.cn (accessed on 28 June 2021) (2021)
- 47.Zhao YH, et al. Atmospheric nitrogen deposition to China: A model analysis on nitrogen budget and critical load exceedance. Atmos. Environ. 2017;153:32–40. doi: 10.1016/j.atmosenv.2017.01.018. [DOI] [Google Scholar]
- 48.Zhang XM, et al. Ammonia emissions may be substantially underestimated in China. Environ. Sci. Technol. 2017;51:12089–12096. doi: 10.1021/acs.est.7b02171. [DOI] [PubMed] [Google Scholar]
- 49.Chang YH, Deng CR, Dore AJ, Zhuang GS. Human excreta as a stable and important source of atmospheric ammonia in the megacity of Shanghai. PLoS One. 2015;10:e0144661. doi: 10.1371/journal.pone.0144661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teng XL, et al. Identification of major sources of atmospheric NH3 in an urban environment in Northern China during wintertime. Environ. Sci. Technol. 2017;51:6839–6848. doi: 10.1021/acs.est.7b00328. [DOI] [PubMed] [Google Scholar]
- 51.Pandolfi M, et al. Summer ammonia measurements in a densely populated Mediterranean city. Atmos. Chem. Phys. 2012;12:7557–7575. doi: 10.5194/acp-12-7557-2012. [DOI] [Google Scholar]
- 52.Gu MN, et al. Is fertilization the dominant source of ammonia in the urban atmosphere? Sci. Total. Environ. 2022;838:155890. doi: 10.1016/j.scitotenv.2022.155890. [DOI] [PubMed] [Google Scholar]
- 53.Chang YH, Liu XJ, Deng CR, Dore AJ, Zhuang GS. Source apportionment of atmospheric ammonia before, during, and after the 2014 APEC summit in Beijing using stable nitrogen isotope signatures. Atmos. Chem. Phys. 2016;16:11635–11647. doi: 10.5194/acp-16-11635-2016. [DOI] [Google Scholar]
- 54.Gu BJ, et al. Abating ammonia is more cost-effective than nitrogen oxides for mitigating PM2.5 air pollution. Science. 2021;374:758–762. doi: 10.1126/science.abf8623. [DOI] [PubMed] [Google Scholar]
- 55.Liu M, et al. Ammonia emission control in China would mitigate haze pollution and nitrogen deposition, but worsen acid rain. Proc. Natl Acad. Sci. USA. 2019;116:7760–7765. doi: 10.1073/pnas.1814880116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu XJ, et al. Environmental impacts of nitrogen emissions in China and the role of policies in emission reduction. Philos. Trans. R. Soc. A. 2020;378:20190324. doi: 10.1098/rsta.2019.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo Y, et al. Air quality, nitrogen use efficiency and food security in China are improved by cost-effective agricultural nitrogen management. Nat. Food. 2020;1:648–658. doi: 10.1038/s43016-020-00162-z. [DOI] [PubMed] [Google Scholar]
- 58.Bhattarai N, et al. δ15N-stable isotope analysis of NHx: An overview on analytical measurements, source sampling and its source apportionment. Front. Env. Sci. Eng. 2021;15:1–11. doi: 10.1007/s11783-021-1414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber RJ, Guo H, Russell AG, Nenes A. High aerosol acidity despite declining atmospheric sulfate concentrations over the past 15 years. Nat. Geosci. 2016;9:282–285. doi: 10.1038/ngeo2665. [DOI] [Google Scholar]
- 60.Tan J, Fu JS, Seinfeld JH. Ammonia emission abatement does not fully control reduced forms of nitrogen deposition. Proc. Natl Acad. Sci. USA. 2020;117:9771–9775. doi: 10.1073/pnas.1920068117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen Z, et al. Changes of nitrogen deposition in China from 1980 to 2018. Environ. Int. 2020;144:106022. doi: 10.1016/j.envint.2020.106022. [DOI] [PubMed] [Google Scholar]
- 62.Parnell AC, Inger R, Bearhop S, Jackson AL. Source partitioning using stable isotopes: Coping with too much variation. PLoS One. 2010;5:e9672. doi: 10.1371/journal.pone.0009672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying the findings of this study are provided in the Source Data file. Source data are provided with this paper.
The SPSS package can be downloaded from https://www.ibm.com/products/spss-statistics. The Origin 2016 statistical package can be downloaded from https://www.originlab.com. The ArcGIS package can be downloaded from https://www.esri.com/en-us/arcgis/about-arcgis/overview. The source code for SIAR used in this paper is openly available from https://rdrr.io/cran/siar.