Abstract
Objectives
A conflicting body of evidence suggests localized periodontal inflammation spreads systemically during pregnancy inducing adverse pregnancy outcomes. This systematic review and meta-analysis aim to specifically evaluate the relationship between periodontitis and preeclampsia.
Methods
Electronic searches were carried out in Medline, Pubmed, Embase, Lilacs, Cochrane Controlled Clinical Trial Register, CINAHL, ClinicalTrials.gov, and Google Scholar with no restrictions on the year of publication. We identified and selected observational case–control and cohort studies that analyzed the association between periodontal disease and preeclampsia. This meta-analysis was conducted following the PRISMA checklist and MOOSE checklist. Pooled odds ratios, mean difference, and 95% confidence intervals were calculated using the random effect model. Heterogeneity was tested with Cochran’s Q statistic.
Results
Thirty studies including six cohort- and twenty-four case–control studies were selected. Periodontitis was significantly associated with increased risk for preeclampsia (OR 3.18, 95% CI 2.26 – 4.48, p < 0.00001), especially in a subgroup analysis including cohort studies (OR 4.19, 95% CI 2.23 – 7.87, p < 0.00001). The association was even stronger in a subgroup analysis with lower-middle-income countries (OR 6.70, 95% CI 2.61 – 17.19, p < 0.0001).
Conclusions
Periodontitis appears as a significant risk factor for preeclampsia, which might be even more pronounced in lower-middle-income countries. Future studies to investigate if maternal amelioration of periodontitis prevents preeclampsia might be warranted.
Keywords: Periodontitis, Periodontal disease, Preeclampsia, Pre-eclampsia, Hypertension, Pregnancy outcome
Significance
The most recent systematic review and meta-analysis on the relationship between periodontal disease and preeclampsia were published in 2013 and could only detect the statistical differences in a subgroup of case-control studies. For that reason, we conducted this study to re-evaluate this association, especially with regard to cohort study design. Furthermore, we take socioeconomic factors into consideration. This systematic review and meta-analysis not only empower the positive association between periodontitis and preeclampsia during pregnancy by the larger sample size and the data synthesis of cohort studies but also point out the considerable difference in lower-middle-income countries.
Introduction
Preeclampsia is the onset of pregnancy-related hypertensive disorder and proteinuria arising most commonly after 20 weeks of gestation, which could lead to eclampsia and induce maternal and perinatal morbidity, and mortality. The prevalence of preeclampsia is between 2 to 8% of all pregnancies worldwide (Duley, 2009). Preeclampsia affected pregnancies had a higher risk of poor maternal outcomes including cerebrovascular bleeding, HELLP syndrome, eclampsia, poorer outcomes of their offspring including premature birth, intrauterine growth restriction, and the complications may manifest over years postpartum (Hung et al., 2018; Turbeville & Sasser, 2020).
Contributing to USD 6.4 billion short-term estimated costs for preeclamptic pregnancies in US healthcare system, USD 1.03 billion were spent on maternal healthcare and USD 1.15 billion were expended for infants born to these women while the remaining expenses were for peripartum and postpartum care (Stevens et al., 2017).
Herein, managing risk factors of preeclampsia is important to improve maternal and perinatal outcomes and lessen the burden on the health economic aspects.
Depending on geographical regions approximately 14.2 and 54.8% of pregnant women suffer from periodontal disease (Alchalabi et al., 2013; Gesase et al., 2018; Govindasamy et al., 2017). Especially, periodontitis, a more severe type of periodontal diseases affecting 11% of the pregnant women, can cause the destruction of periodontal tissue and cause systemic dissemination of bacteria and other inflammatory mediators (Bui et al., 2019; Piscoya et al., 2012). Systemic inflammatory processes triggered by focal periodontal infections have been attributed to cardiovascular, cerebrovascular diseases and respiratory diseases.(Winning & Linden, 2015) Periodontitis has independently been linked to several pregnancy complications such as preterm birth, low birth weight, and gestational diabetes (Abariga & Whitcomb, 2016; Corbella et al., 2012).
Socioeconomic status is a recognized factor associated with medical outcomes, including pregnancy outcomes (Kivimäki et al., 2020). Women with lower socioeconomic status are at a higher risk of pregnancy complications such as gestational diabetes, preterm delivery, and preeclampsia (Bo et al., 2002; Peacock et al., 1995; Silva et al., 2008). Women with high socioeconomic status have a statistically significant reduced risk of preeclampsia with an odds ratio of 0.899 (95% CI, 0.862–0.937, p < 0.001) compared to women with lower socioeconomic statuses (Ross et al., 2019). At the same time, the proportion of periodontitis in pregnancy is linked to low socioeconomic status with 42.6%, compared to high socioeconomic status with 15.0%.
Two previous meta-analyses both published in 2013 reported positive associations between preeclampsia and periodontitis with OR 2.17, 95% CI 1.38–3.41, p = 0.008 and OR of 2.79, 95% CI 2.01–3.01, p < 0.0001, but did not consider socioeconomic factors (Sgolastra et al., 2013; Wei et al., 2013). Since then further case–control and cohort studies have been published on this research topic (Lafaurie et al., 2018; Soucy-Giguère et al., 2016; Varshney & Gautam, 2014). Nonetheless, the causal relationship between periodontal disease and preeclampsia remains unclear (Kunnen et al., 2010; Lavigne & Forrest, 2020). In this review, we included all available new studies, to re-evaluate the potential association between periodontitis and preeclampsia and also to take socioeconomic factors into consideration (Australian Institute of Health and Welfare 2010. Socioeconomic variation in periodontitis among Australian adults 2004–06.)
Methods
Eligibility Criteria
The studies were screened according to the following inclusion criteria:
Study design was either case–control or prospective cohort study;
Studies analysing the association between periodontal disease and preeclampsia;
Study population was pregnant women without systemic diseases;
Preeclampsia was defined as the development of blood pressure of ≥ 140/90 mmHg after 20 weeks of gestation, combined with proteinuria of at least 1 + on midstream urine specimen or catheter specimen;
Periodontitis was either diagnosed ≥ 2 sites with PD ≥ 4 mm and CAL ≥ 3 mm, not on the same site or one site with PD ≥ 5 mm at the same site or evaluated the progression by clinical periodontal parameters including periodontal pocket depth, clinical attachment loss and bleeding on probing (Eke et al., 2012). The progression was measured by pocket depth (mm), clinical attachment level (mm) at baseline and delivery time;
Data was presented in such a way that Odds Ratio and 95% Confidence Interval could be calculated.
Studies were excluded if they did not report adequate data on periodontal or preeclamptic conditions or outcome of interest or did not meet the inclusion criteria.
Information Sources
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines with the checklist of 27 items to conduct our study (Moher et al., 2009). We also adopted the MOOSE checklist for Meta-analysis of Observational studies (Stroup et al., 2000). A systematic search of the electronic database including Medline (from 1950), Pubmed (from 1946), Embase (from 1949), Lilacs, Cochrane Controlled Clinical Trial Register, CINAHL, ClinicalTrials.gov and Google Scholar (from 1990) to identify relevant articles.
Search Strategy
We used the following search terms: periodontitis, periodontal disease, preeclampsia, pre-eclampsia, pregnancy outcomes, pregnancy complications, and hypertension. The combinations of search terms were used to explore above databases. The search strategy was peer reviewed by two independent reviewers (QA and LD). The reference lists of relevant articles were also scanned for appropriate studies. No language restrictions were adopted in either the search or study selection. No search for unpublished literature was carried out. Authors were contacted for translation and information.
Study Selection
Two independent reviewers (LD and HN) reviewed the titles, abstracts and methods of retrieved results to assess for the eligibility criteria. When there was a disagreement in a selection process between reviewers, consensus with the third reviewer (QA) was obtained.
Data Extraction
Data extraction was carried out using a standardized extraction form, collecting information on the first author’s name, publication year, study design, number of cases, number of controls, total sample size, country, national income group, mean age, the risk of estimates or data used to calculate the risk estimates, CIs or data used to generate CI. According to the World Bank classification of countries which is based on Gross National Income per capita, groups of national income per year are (according to World Bank classification (Country and Lending Groups & The World Bank Group, 2011)):
Low income: $995 or less;
Lower-middle-income: $996–$3945;
Upper-middle-income: $3946–$12,195;
High income: $12,196 or more.
The researchers cross-checked all extracted data and discussed if there were disagreements.
Assessment of Risk of Bias
Risk of bias was executed using the Newcastle Ottawa Scale by two reviewers (QA and LD) (Lo et al., 2014) with disagreements resolved by consensus attainment between reviewers. This scale has three components including Selection, Comparability and Outcome/Exposure assessment with maximum overall score of nine. Studies were rated as low risk of bias if they received nine score, moderate risk of bias if they received seven or eight score and high risk of bias if they received less than seven scores.
Data Synthesis
Data were imported in a statistical software (RevMan, Version 5, 2008, The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). Pooled Odds Ratios, mean difference, and 95% Confidence Intervals were calculated for the association between periodontitis and preeclampsia using a random effects model. The pooled effect was considered significant if p-value was less than 0.05. Forest plots for primary analysis and subgroup analysis show the raw data, Odds Ratio and CIs, Means and SDs for the chosen effect, heterogeneity statistic (I2), total number of participants per group, overall Odds Ratio and Mean difference.
Subgroup analysis was carried out according to the study design (case–control or cohort), definition of periodontitis (defined by pocket depth (PD) and/or clinical attachment loss (Taghzouti et al.)), mean CAL, mean PD, national income (high-income or middle-income or low-income countries).
Heterogeneity was tested with Cochran’s Q statistic, with p < 0.10 indicating heterogeneity, and quantified the degree of heterogeneity using the I2 statistic, which represents the percentage of the total variability across studies which is due to heterogeneity. I2 values of 25, 50 and 75% corresponded to low, moderate and high degrees of heterogeneity respectively (Higgins & Thompson, 2002). We quantified publication bias using the Egger’s regression model with the effect of bias assessed using the fail-safe number method (Egger et al., 1997) The fail-safe number was the number of studies that we would need to have missed for our observed result to be nullified to statistical non-significance at the p < 0.05 level. Publication bias is generally regarded as a concern if the fail-safe number is less than 5n + 10, with n being the number of studies included in the meta-analysis (Orwin, 1983) Publication bias was assessed using Stata (16.1, StataCorp LLC, College Station, TX).
Results
Study Selection
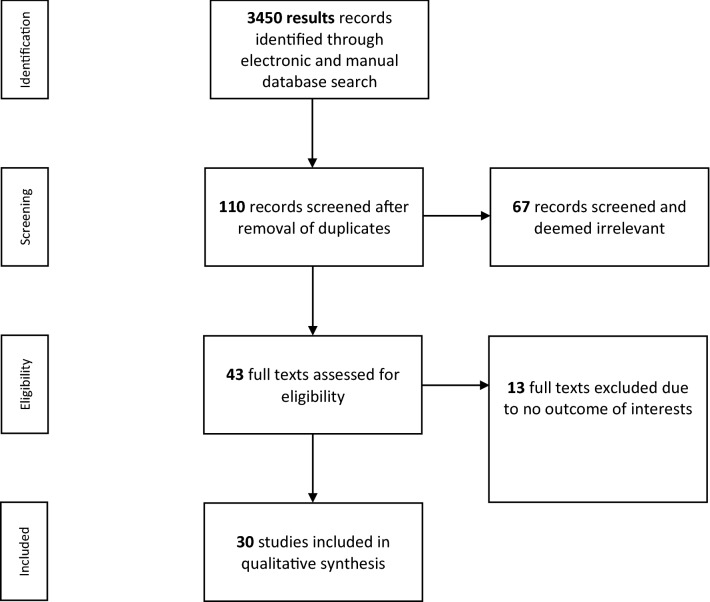
A total of 3450 articles were found through the manual and electronic searches. We searched clinicaltrials.gov however, we could not find eligible articles from this source to include in our study. After duplicates’ removal, we screened 110 records for relevance. Sixty-seven papers were excluded on a basis of evaluation of the title and abstract, leaving 43 articles to be assessed for eligibility. Of these, thirty articles were included in the quantitative analysis. A PRISMA flow diagram is provided in Fig. 1. Eventually, the selection process led to the inclusion of 9650 participants in this systematic review and meta-analysis.
Fig. 1.
Flow diagram of study selection
Study Characteristics
Table 1 depicts the characteristics of the included studies. Six cohort studies were included (Boggess et al., 2003; Ha et al., 2014; Horton et al., 2010; Kumar et al., 2013; Lee et al., 2016; Soucy-Giguère et al., 2016), whilst the remaining studies were case – control (Canakci et al., 2004, 2007; Chaparro et al., 2013; Contreras et al., 2006; Cota et al., 2006; Desai et al., 2015; Ha et al., 2011; Hirano et al., 2012; Jaiman et al., 2018; Khader et al., 2006; Khalighinejad et al., 2017; Kunnen et al., 2007; Lafaurie et al., 2018; Lohsoonthorn et al., 2009; Moura da Silva et al., 2012; Pattanashetti et al., 2013; Politano et al., 2011; Pralhad et al., 2013; Sayar et al., 2011; Shetty et al., 2009; Siqueira et al., 2008; Taghzouti et al., 2012; Varshney & Gautam, 2014; Yaghini et al., 2012). The definitions and evaluation of periodontitis varied slightly among these studies with the assessment of bleeding on probing, while the definition of preeclampsia was presented consistent as blood pressure ≥ 140/90 mmHg and proteinuria during second trimester of gestation. The oral examination was conducted at different timepoints among studies, within two days of childbirth(Boggess et al., 2003; Canakci et al., 2004, 2007; Cota et al., 2006; Desai et al., 2015; Ha et al., 2014; Horton et al., 2010; Jaiman et al., 2018; Khader et al., 2006; Khalighinejad et al., 2017; Kumar et al., 2013; Lohsoonthorn et al., 2009; Moura da Silva et al., 2012; Pattanashetti et al., 2013; Politano et al., 2011; Pralhad et al., 2013; Sayar et al., 2011; Shetty et al., 2009; Siqueira et al., 2008; Taghzouti et al., 2012; Varshney & Gautam, 2014; Yaghini et al., 2012), within 7 days after the delivery(Ha et al., 2011; Hirano et al., 2012; Lafaurie et al., 2018), 3 months postpartum(Kunnen et al., 2007), during the second trimester of pregnancy(Chaparro et al., 2013; Contreras et al., 2006; Lee et al., 2016; Soucy-Giguère et al., 2016). Nineteen out of thirty studies reported the implementation of calibration with the intra- and inter-examiner variability which showed the agreement of 85% and above(Boggess et al., 2003; Canakci et al., 2004, 2007; Cota et al., 2006; Ha et al., 2011, 2014; Horton et al., 2010; Jaiman et al., 2018; Khalighinejad et al., 2017; Kunnen et al., 2010; Lafaurie et al., 2018; Lee et al., 2016; Lohsoonthorn et al., 2009; Moura da Silva et al., 2012; Politano et al., 2011; Sayar et al., 2011; Siqueira et al., 2008; Taghzouti et al., 2012).
Table 1.
Descriptions of included studies
| No | References | Country | Design | Participants and age | Definition of PE | Definition of PD | Examination Time | Finding (conclusion) | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | (Boggess et al., 2003) | US | Prospective cohort |
39 exposed 763 unexposed |
BP ≥ 140/90 on 2 separate occasions, and ≥ 1 + proteinuria on catheterized urine specimen |
PD ≥ 4 and CAL ≥ 3 mm without BOP Mild: PD ≥ 4 mm or BOP on 1–15 teeth Severe: PD ≥ 4 mm on > 15 teeth Disease progression: ≥ 4 sites that increased ≥ 2 mm in PD, resulting in ≥ 4 mm in PD |
At the first or second prenatal visit and then repeated within 48 h antepartum Enrolled at < 26 weeks’gestation and followed until delivery |
Women were at higher risk for preeclampsia if they had severe periodontal disease at delivery or if they had periodontal disease progression during pregnancy |
Severe periodontal disease: 2.4 (1.1- 5.3) Periodontal disease progression: 2.1 (1.0 – 4.4) |
| 2 | (Canakci et al., 2004) | Turkey | Case–control | 41 cases,41 controls | BP ≥ 140/90 mmHg and proteinuria ≥ 300 mg/24 h or 2 + proteinuria on dip sticks, on 2 occasions ≥ 6 h apart if 24 h urine specimen is unavailable | ≥ 4 teeth with ≥ 1 sites with PD ≥ 4 mm and BOP + and CAL ≥ 3 mm at the same site | within 48 h prior to delivery | multiple logistic regression results showed that pre-eclamptic patients were 3.47 times more likely to have periodontal disease than normotensive patients | 3.47 (1.07–11.95) |
| 3 | (Contreras et al., 2006) | Colombia | Case–control | 130 case, 243 controls | 2 + proteinuria, confirmed by ≥ 0.3 g proteinuria/24 h and hypertension (≥ 140/ 90 mmHg) |
≥ 4 sites showed PD ≥ 4 mm), CAL ≥ 4 mm, and bleeding on probing Incipient: CAL from 4 to 5 Moderate/severe: CAL ≥ 6 mm |
between 26 to 36 weeks of pregnancy | Chronic periodontal disease was significantly associated with preeclampsia in pregnant women | 3.0 (1.91—4.87) |
| 4 | (Cota et al., 2006) | Brazil | Case–control | 109 cases, 479 controls | BP > 140/90 mm Hg and ≥ 1 + proteinuria after 20 weeks of gestation | ≥ 4 teeth with ≥ 1 sites with a PD ≥ 4 mm and CAL ≥ 3 mm at the same site | Within 48 h of delivery | Maternal periodontitis was determined to be associated with an increased risk of preeclampsia | 1.88 (1.1—3.0) |
| 5 | (Khader et al., 2006) | Jordan | Case–control |
115 cases 230 controls |
Preeclampsia was defined as the development of blood pressure of ‡140/90 mmHg after 20 weeks of gestation, combined with proteinurea of at least 1 + on a midstream urine specimen or on a catheter specimen, provided urinary tract infection was not the contributing factor to the proteinuria in women who were known to be normotensive and nonproteinuric before pregnancy or in early pregnancy |
Not mentioned | Within 24 h after delivery | This study did not support the association between periodontal parameters and preeclampsia | |
| 6 | (Kunnen et al., 2007) | Netherlands | Case–control | 17 cases, 35 controls | DBP ≥ 90 mmHg on 2 occasions and proteinuria ≥ 30 mg/dl (or 1 + on a urine dip stick) on ≥ 2 random specimens collected ≥ 4 h apart |
PD ≥ 4 mm Mild PD: BOP and PD ≥ 4 mm on 1–15 sites Severe: BOP and PD ≥ 4 mm on > 15 sites |
before 34 weeks of pregnancy and 3 months postpartum | These results indicate that Caucasian women with a recent history of early-onset pre-eclampsia have a worse periodontal condition, as compared with women with uncomplicated deliveries | 7.9 (1.9–32.8) |
| 7 | (Canakci et al., 2007) | Turkey | Case–control | 20 Mild PE, 18 Severe PE, 21 Controls |
DBP ≥ 90 mmHg and proteinuria(300 mg/24 h urine sample) and the presence of edema Mild: BP ≥ 140/90 mmHg on ≥ 2 occasions 6 h apart, with or without proteinuria Severe: SBP ≥ 160 or DBP ≥ 110 mmHg on 2 occasions ≥ 6 h apart and proteinuria ≥ 5 g/24 h urine sample or ≥ 3 l on dip stick in ≥ 2 random clean-catch samples ≥ 4 h apart |
Mild: BOP and ≥ 4 mm PD on 1–15 sites Severe:: BOP and ≥ 4 mm PD on ≥ 15sites |
within 48 h preceding delivery | The results of multivariate logistic regression showed a highly significant association between mild to severe pre-eclampsia and severe periodontal disease | Severe PD: 3.78 (1.77–12.74) |
| 8 | (Siqueira et al., 2008) | Brazil | Case–control | 164 cases, 1042 controls | BP > 90 mmHg on 2 occasions after 20 GW and ≥ 1 + proteinuria | ≥ 4 mm and CAL ≥ 3 mm at the same site in ≥ 4 teeth | within 48 h of delivery | Maternal periodontitis is a risk factor associated with preeclampsia | 1.52 ( 1.01—2.29) |
| 9 | (Lohsoonthorn et al., 2009) | Thailand | Case–control | 150 cases, 150 controls | BP ≥ 140/90 mmHg and proteinuria ≥ 30 mg/dl (or 1 + on a urine dip stick) on ≥ 2 random specimens collected ≥ 4 h apart |
Mild: ≥ 1 teeth with interproximal sites showing ≥ 4 mm CAL and ≥ 4 mm PD Moderate: ≥ 2 nonadjacent teeth with interproximal sites showing ≥ 5 mm CAL and ≥ 4 mm PD Severe: ≥ 2 nonadjacent teeth with interproximal sites showing ≥ 6 mm CAL and ≥ 4 mm PD |
within 48 h after delivery | This study provides no convincing evidence that periodontal disease is associated with preeclampsia risk among Thai women | Severe PD: 0.92 ( 0.26–3.28) |
| 10 | (Horton et al., 2010) | US | Prospective cohort |
34 exposed(pree-clampsia) 757 unexposed (non pree-clampsia) |
BP > 140/90 mmHg and ≥ 1 + proteinuria on a catheterized urine specimen |
Mild: < 15 sites with the presence of one or more pockets ≥ 4 mm or one or more pockets with bleeding Moderate/severe: ≥ 15 sites demonstrated a probing depth ≥ 4 mm |
< 26 weeks of gestation | Among women with periodontal disease, the presence of 8-isoprostane ≥ 75th percentile did not significantly increase the odds for the development of preeclampsia |
2.08 (0.65–6.60) |
| 11 | (Shetty et al., 2009) | India | Case–control |
30 cases 100 controls |
BP > 140/90 mmHg on more than 2 occasions 4 h apart and 1 + or more proteinuria by Dipstick on a random urine sample |
CAL of ≥ 3 mm and a PD of ≥ 4 mm (The teeth examined were 16, 22, 24, 36, 42, and 44) |
within 48 h of delivery | periodontitis both at enrolment and within 48 h of delivery may be associated with an increased risk of preeclampsia |
Enrolment:5.78 (2.41– 13.89) Delivery: 20.15 (4.55–89.29) |
| 12 | (Politano et al., 2011) | Brazil | Case–control |
58 cases 58 controls |
increase in systolic arterial pressure (≥ 140 mmHg) and/or diastolic pressure (≥ 90 mmHg) and proteinuria (≥ 300 mg/24 h), after 20 wk of gestation |
two or more sites showed pocket formation (≥ 4 mm), clinical attachment level (≥ 4 mm) and bleeding on probing |
after 20 wk of gestation | periodontal disease may increase the risk of pre-eclampsia |
3.73 (1.32–10.58) |
| 13 | (Ha et al., 2011) | Korea | Case–control |
16 cases 48 controls |
BP > 140/90 mm Hg on two separate occasions and ≥ 1 + proteinuria on a random sample of urine |
Localized periodontitis: periodontal clinical attachment loss ≥ 3.5 mm on two or three sites not on the same tooth Generalized periodontitis: CAL ≥ 3.5 mm on ≥ 4 sites not on the same tooth |
5 days after delivery | preeclampsia could be associated with the maternal periodontal condition and interdental cleaning |
Localized periodontitis: 4.79 (1.02 – 29.72) Generalized periodontitis: 6.60 (1.25 –41.61) |
| 14 | (Sayar et al., 2011) | Iran | Case–control |
105 cases 105 controls |
blood pressure ≥ 140/90 mmHg and proteinuria + 1 |
No definition specified (results based on periodontal parameters) Mild: CAL ≤ 2 mm Moderate to Severe: CAL ≥ 3 mm |
48 h after child delivery | Preeclamptic cases significantly had higher attachment loss and gingival recession than the control group |
4.1 (1.5–11.5) |
| 15 | (Taghzouti et al., 2012) | Canada | Case–control |
92 cases 245 controls |
BP ≥ 140/90 mm Hg on two occasions ≥ 4 h apart after 20 weeks of gestation, and 0.3 g proteinuria on a 24-h urine collection, or ≥ 1 on a dipstick | periodontitis is defined as ≥ 4 sites exhibiting PD ≥ 5 mm and CAL ≥ 3 mm at the same sites | within 48 h after delivery | This study does not support the hypothesis of an association between periodontal disease and preeclampsia | 1.13 (0.59—2.17) |
| 16 | (Chaparro et al., 2013) | Chile | Case–control |
11 cases 43 controls |
During the second and third trimester of pregnancy, BP > 140/90 and proteinuria, which was considered to be present when one 24-h urine collection showed a total protein excretion ≥ 300 mg |
PD ≥ 4 mm and CAL ≥ 3 mm at the same site of ≥ 4 teeth, inflammation and bleeding on probing (BOP) |
Blood samples were collected at enrolment Gingival crevicular fluid samples were collected between 11–14 wk |
Preeclamptic women shows increased levels of IL-6 in GCF and CRP in plasma during early pregnancy. Periodontal disease could contribute to systemic inflammation in early pregnancy via a local increase of IL-6 and the systemic elevation of CRP. Therefore, both inflammatory markers could be involved in the relationship between periodontal disease and pre-eclampsia | 1.36 (0.252–7.372) |
| 17 | (Hirano et al., 2012) | Japan | Case–control |
18 cases 109 controls |
hypertension (systolic blood pressure > 140 mmHg and/or diastolic blood pressure > 90 mmHg) with proteinuria ( 300 mg/day) occurring after the 20th week of gestation, but being resolved by the 12th postpartum week | having over 60% of sites with CAL ≥ 3 mm | 5 days after labor | No statistically significant association between any of the periodontal clinical parameters or the presence of periodontitis and preeclampsia |
1.7 (1.1–2.7) |
| 18 | (Kumar et al., 2013) | India | Prospective cohort |
35 exposed 305 unexposed |
systolic blood pressure 140 mm of mercury and diastolic blood pressure 90 mm of mercury at two occasions at least 4 h apart after 20 weeks of gestation in a woman with previously normal blood pressure along with development of proteinuria | clinical attachment loss and probing depth 4 ≥ mm in one or more sites were diagnosed as those with periodontitis | 14–20 weeks period of gestation and the time of delivery | Maternal periodontitis is associated with an increased risk of pre-eclampsia |
7.48 (2.72–22.42) |
| 19 | Da (Silva et al., 2008) | Brazil | Case–control |
284 cases 290 controls |
a systolic blood pressure ≥ 140 mmHg or a diastolic pressure ≥ 90 mmHg and proteinuria ≥ 300 mg/24 h or 2 + on dipsticks, developed after week 20 of gestation in previously normotensive females | ≥ 4 teeth with ≥ 1 sites with a PD ≥ 4 mm and AL ≥ 3 mm in the same site | within 48 h of childbirth | periodontitis was a risk factor for preeclampsia |
8.60 ( 3.92–18.88) |
| 20 | (Pralhad et al., 2013) | India | Case–control |
100 cases 100 controls |
The resting blood pressure was ≥ 140/90 mmHg after 20 weeks of gestation with or without associated proteinuria |
Any of the following is present: 1) OHI > 3 (Hosseinpoor et al.); 2) GI > 1 (moderate-to-severe gingival inflammation); 3) mean PD > 4 mm; and 4) CAL > 3 mm |
within 72 h of their hospital admission for delivery | Periodontal disease is more prevalent in females with pregnancy hypertension |
5.5 (2.7–11.4) |
| 21 | (Yaghini et al., 2012) | Iran | Case–control |
26 cases 25 controls |
blood pressure > 140/90 mmHg and > or = 1 + proteinuria on a catheterized urine specimen |
Periodontal assessment was carried out using these indices: Clinical attachment loss, Gingival bleeding index, plaque index | 48 h after delivery | Maternal periodontal disease during pregnancy is not associated with preeclampsia | |
| 22 | (Pattanashetti et al., 2013) | India | Case–control |
100 cases 100 controls |
Pregnancy induced hypertension occurs after the 20th week of gestation and is characterized by: Hypertension – High blood pressure, usually higher than 140/90 mm Hg. The rise of blood pressure should be evident at least on two occasions, four or more hours apart Edema – Demonstration of pitting oedema over the ankles after 12 h of bed rest or rapid gain in weight of more than 1 lb per week or more than 5 lb a month in the later month of pregnancy may be the easiest evidence of preeclampsia Proteinuria – Presence of protein in 24 h urine with more than 1gm per litre in 2 or more midstream specimens obtained 6 h apart in the absence of urinary tract infection is considered significant |
-Mild periodontal disease: One or more sites with probing depth ≥ 3 mm that bleed upon probing but less than 25 sites with probing depth ≥ 4 mm -Moderate/Severe: 15 or more sites with periodontal probing ≥ 4 mm -Worsening periodontal status was defined as four or more sites had increased by at least 2 mm in pocket depth between the two oral health examinations |
Sixth month of pregnancy and within 48 h post-partum | Pregnant women with preeclampsia are at greater risk for preterm delivery if periodontal disease is present during pregnancy or progress during pregnancy and also the rate of preterm delivery is more in preeclamptic women having moderate to severe periodontal disease | Periodontitis in the case group was 72%, in control group was 62%, p < 0.001 |
| 23 | (Ha et al., 2014) | Korea | Prospective cohort |
13 exposed 270 unexposed |
BP > 140/90 mmHg on two separate occasions, and at least 1 + proteinuria on a random urine screen after the 20th week of pregnancy |
CAL ≥ 4.0 mm on two or more sites on different teeth |
21–24 weeks of gestation and 5 days after delivery |
Periodontitis increased the risk of preeclampsia among never-smokers |
4.51 (1.13–17.96) |
| 24 | (Varshney & Gautam, 2014) | India | Case–control |
20 cases 20 controls |
BP ≥ 140/90 mm of Hg on two separate occasions after 20 week of gestation and ≥ 1 + proteinuria | PD ≥ 4 mm and CAL ≥ 3 mm at the same site on at least 4 different non-neighboring teeth | within the 48 h after delivery | Maternal clinical periodontal disease at delivery is associated with an increased risk for the development of pre-eclampsia, independent of the effects of maternal age, race, smoking, gestational age at delivery |
4.33 (1.15- 16.32) |
| 25 | (Desai et al., 2015) | India | Case–control |
120 cases 1120 controls |
Blood pressure ≥ 140/90 mm Hg on two separate occasions after week 20 of gestation | PD ≥ 4 mm and CAL ≥ 3 mm at the same site in at least four teeth | 48 h after delivery | Maternal clinical periodontal disease at delivery is associated with an increased risk for the development of pre-eclampsia, independent of the effects of maternal age, race, smoking, gestational age at delivery | 19.898 (7.80–48.94) |
| 26 | (Soucy-Giguère et al., 2016) | Canada | Prospective cohort |
11 exposed 237 unexposed |
Not mentioned |
the presence of at least one site with probing depths ≥ 4 mm and ≥ 10% bleeding on probing |
in the seven days following amniocentesis | Pregnant women with periodontal disease were more likely to develop preeclampsia | RR 5.89 (1.24–28.05) |
| 27 | (Lee et al., 2016) | Korea | Prospective cohort |
15 exposed 313 unexposed |
BP > 140/90 mmHg on two separate occasions with at least 1 + proteinuria on a random urine screen after the 20th week of pregnancy | two or more inter-proximal sites with CAL ≥ 4 mm that were not on the same tooth | at 21–24 weeks of gestation | The association was much stronger in women with both obesity and periodontitis | 15.94 (3.31–76.71) |
| 28 | (Khalighinejad et al., 2017) | USA | Case–control |
50 cases 50 controls |
A systolic blood pressure ≥ 140 mm HG or a diastolic pressure ≥ 90 mm HG and proteinuria > 300 mg/24 h developed after the 20th week of gestation |
The presence of 4 or more teeth with 1 or more sites with PD ≥ 4 mm and with clinical attachment loss ≥ 3 mm at the same site |
Before delivery | Apical periodontitis was significantly more prevalent in the experimental group | 2.23 (1.92–6.88) |
| 29 | (Lafaurie et al., 2018) | Colombia | Case–control |
76 cases 304 controls |
not mentioned | the patients were classified according to the presence of periodontal pockets (code 3: periodontal pockets of 4-5 mm or code 4: periodontal pockets > 5 mm) | during the first week after birth | Periodontal pockets presence was not associated with preeclampsia | 5.46 (1.84- 16.1) |
| 30 | (Jaiman et al., 2018) | India | Case–control |
15 cases 15 controls |
preeclampsia as the appearance of a diastolic blood pressure ≥ 90 mmHg mercury measured at two different occasions at least 4 h apart in combination with proteinuria (≥ 300 mg/24 h or + 1 dipstick) developing after a gestational age of 20 weeks in a previously normotensive woman | According to the criteria of Löe and Silness | 24 h before delivery | The preeclamptic women were associated with significantly higher periodontitis and lower fetal birth weight than normotensive women | Periodontitis in case group was 93.3% and in control group was 33.3% (p < 0.05) |
BP blood pressure, DBP diastolic blood pressure, SBP systolic blood pressure, GW gestational week, PD pocket depth, CAL clinical attachment loss, OHI oral hygiene index, GI gingival index, BOP bleeding on probing, GCF gingival crevicular fluid
The sample size ranged from 40 participants (Varshney & Gautam, 2014) to 1240 subjects (Desai et al., 2015). Seven studies reported no evidence of an association between periodontitis and preeclampsia (Chaparro et al., 2013; Hirano et al., 2012; Horton et al., 2010; Khalighinejad et al., 2017; Lafaurie et al., 2018; Lohsoonthorn et al., 2009; Pattanashetti et al., 2013; Shetty et al., 2009; Taghzouti et al., 2012), while the remaining studies reported a positive association. Studies which had controlled for factors such as age, weight, smoking or occupation were reported(Boggess et al., 2003; Canakci et al., 2004, 2007; Cota et al., 2006; Desai et al., 2015; Ha et al., 2011, 2014; Hirano et al., 2012; Horton et al., 2010; Khader et al., 2006; Kumar et al., 2013; Kunnen et al., 2007; Lafaurie et al., 2018; Lee et al., 2016; Lohsoonthorn et al., 2009; Moura da Silva et al., 2012; Politano et al., 2011; Pralhad et al., 2013; Sayar et al., 2011; Shetty et al., 2009; Siqueira et al., 2008; Soucy-Giguère et al., 2016; Taghzouti et al., 2012).
Risk of Bias of Included Studies
Newcastle Ottawa Scale was used to evaluate the quality of evidence of these reports. Two reviewers marked the scores for each paper based on the tool provided by the Scale. Nine studies (Ha et al., 2011, 2014; Jaiman et al., 2018; Khader et al., 2006; Khalighinejad et al., 2017; Lee et al., 2016; Moura da Silva et al., 2012; Pattanashetti et al., 2013; Soucy-Giguère et al., 2016) obtained the maximum score in Selection outcome while fourteen studies were marked with maximum score in the Comparability outcome and none of the studies could achieve ultimately 3 marks in the Exposure outcome. Table 2 describes the evaluation of risk of bias for this review.
Table 2.
Risk of bias in included studies based on Newcastle–Ottawa scale
| Study, year | Selection (Max 4*) | Comparability (Max 2*) | Exposure (Max 3*) | Risk of bias |
|---|---|---|---|---|
| Boggess 2003 | *** | * | ** | High |
| Canakci 2004 | *** | ** | ** | Moderate |
| Contreras 2006 | *** | * | ** | High |
| Cota 2006 | *** | * | ** | High |
| Khader 2006 | **** | ** | ** | Moderate |
| Kunnen 2007 | *** | ** | ** | Moderate |
| Canakci 2007 | *** | ** | ** | Moderate |
| Siqueira 2008 | *** | * | ** | High |
| Lohsoonthorn 2009 | *** | ** | ** | Moderate |
| Horton 2010 | *** | * | ** | High |
| Shetty 2010 | *** | ** | ** | Moderate |
| Politano 2011 | *** | ** | ** | Moderate |
| Ha 2011 | **** | ** | ** | Moderate |
| Sayar 2011 | *** | ** | ** | Moderate |
| Taghzouti 2012 | *** | ** | ** | Moderate |
| Chaparro 2012 | *** | * | ** | High |
| Hirano 2012 | *** | * | ** | High |
| Kumar 2012 | *** | * | ** | High |
| da Silva 2012 | **** | ** | ** | Moderate |
| Pralhad 2012 | *** | ** | ** | Moderate |
| Yaghini 2012 | *** | ** | ** | Moderate |
| Pattanashetti 2013 | **** | ** | ** | Moderate |
| Ha 2014 | **** | ** | ** | Moderate |
| Varshney 2014 | *** | ** | ** | Moderate |
| Desai 2015 | *** | ** | ** | Moderate |
| Soucy-Giguere 2015 | **** | ** | *** | Low |
| Lee 2016 | **** | * | ** | Moderate |
| Khalighinejad 2017 | **** | ** | ** | Moderate |
| Lafaurie 2018 | *** | * | ** | High |
| Jaiman 2018 | **** | ** | ** | Moderate |
Synthesis of Results
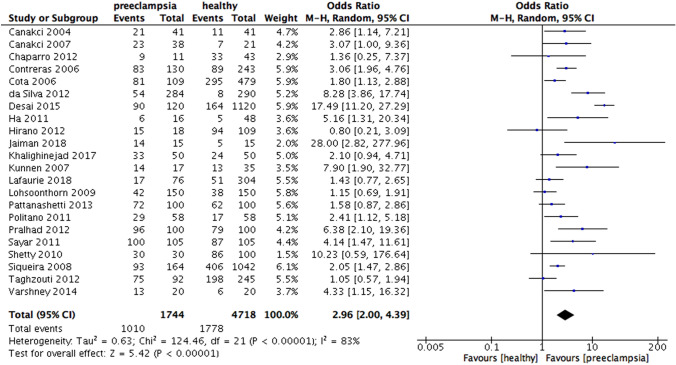
The results of the meta-analysis showed that periodontitis was associated with increased risk for preeclampsia (OR 3.18, 95% CI 2.26 – 4.48, p < 0·00,001; Fig. 2). The heterogeneity was high (I2 = 81%, p < 0.00001) revealing a significant variation among studies.
Fig. 2.
Forest plot for the association between periodontitis and preeclampsia
Subgroup Analysis
According to the study type, the results revealed the increased risk of preeclampsia in periodontitis patients in the cohort studies (OR 4.19, 95% CI 2.23 – 7.87, p < 0.00001; Fig. 3) and in case–control studies (OR 2.96, 95% CI 2.00 – 4.39, p < 0.00001; Fig. 4). Heterogeneity was moderate for cohort (I2 = 55%, p = 0.05) but high for case–control (I2 = 83%, p < 0.00001).
Fig. 3.
Forest plot for the subgroup analysis according to the type of study design (cohort study)
Fig. 4.
Forest plot for the subgroup analysis according to the type of study design (case–control study)
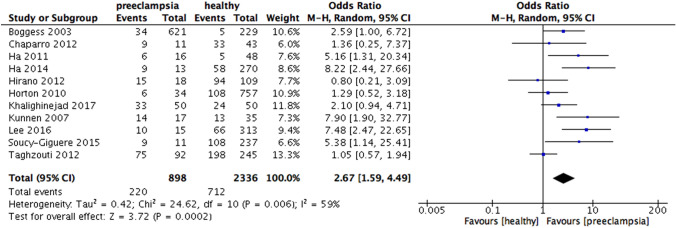
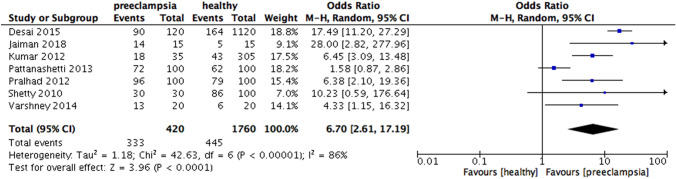
When analyzing according to the national income, increased risk of preeclampsia were found in periodontitis group in high-income countries (OR 2.67, 95% CI 1.59 – 4.49, p = 0.0002; Fig. 5), upper middle-income countries (OR 2.40, 95% CI 1.73 – 3.31, p < 0.00001; Fig. 6) and especially lower-middle income countries (OR 6.7, 95% CI 2.61 – 17.19, p < 0.0001; Fig. 7). Heterogeneity in the group of high-income, upper middle-income and lower middle-income countries were moderate (I2 = 59%, p = 0.006; I2 = 64%, p = 0.003; I2 = 86%, p < 0.00001, respectively) (Fig. 8).
Fig. 5.
Forest plot for the subgroup analysis according to the national income (high income countries)
Fig. 6.
Forest plot for the subgroup analysis according to the national income (upper middle-income countries)
Fig. 7.
Forest plot for the subgroup analysis according to the national income (lower middle- income countries)
Fig. 8.
Forest plot for the subgroup analysis according to the definition of periodontitis (PD alone)
When the results were analyzed according to the definition of periodontitis, an increased risk of preeclampsia was observed in all subgroups, including PD only (OR 3.13, 95% CI 1.51 – 6.50, p = 0.002), CAL and PD (OR 3.30, 95% CI 2.02 – 5.41, p < 0.00001), CAL alone (OR 2.74, 95% CI 1.50 – 5.01, p = 0.001). Heterogeneity was moderate for the subgroups in which periodontitis was defined by PD alone (I2 = 59%, p = 0.03) and CAL alone (I2 = 66%, p = 0.01), while significantly high heterogeneity was found in the subgroup which periodontitis was defined by CAL and PD ( I2 = 86%, p < 0.00001) (Fig. 9).
Fig. 9.
Forest plot for the subgroup analysis according to the definition of periodontitis (PD and CAL)
When analyzing the periodontal condition between both groups, mean CAL was statistically higher in the preeclamptic patients than in the healthy group (MD = 0.62, 95% CI 0.27 – 0.98, p = 0.0006). Likewise, the preeclamptic group had a statistically higher mean PD compared to healthy group (MD = 0.79, 95% CI -0.47 – 1.11, p < 0.00001). The heterogeneity was 98% in both subgroup analysis, (I2 = 98%, p < 0.00001) (Figs. 10, 11 and 12).
Fig. 10.
Forest plot for the subgroup analysis according to the definition of periodontitis (CAL alone)
Fig. 11.
Forest plot for the subgroup analysis of mean CAL between preeclamptic and healthy groups
Fig. 12.
Forest plot for the subgroup analysis of mean PD between preeclamptic and healthy groups
Publication Bias
The funnel plot for the association between periodontitis and preeclampsia revealed the symmetry (Fig. 13). No publication bias was found.
Fig. 13.

Funnel plot for the association between periodontitis and preeclampsia
Discussion
The aim of this meta-analysis was to re-evaluate the potential association between preeclampsia and periodontitis. The results confirm that periodontitis is a risk factor for preeclampsia, which was similar to the finding of a meta-analysis in 2013 by Sgolastra et al. (Sgolastra et al., 2013). Our review has fifteen additional studies with three more cohort studies considerably increasing the sample size and hence generating more robust effect sizes and significance levels.
By stratifying according to study designs, periodontitis and preeclampsia showed significant associations in both case–control and cohort studies, whereas Sgolastra et al. (Sgolastra et al., 2013) could not report the statistical significance in the subgroup analysis of cohort studies (OR 2.2, 95% CI 0.66 – 7.36, p = 0.2). As a review of cohort studies provides higher level of evidence compared to case–control studies (Guyatt et al., 2000) our data considerably strengthens the evidence of a positive association between preeclampsia and periodontitis. Moreover, the heterogeneity in an analysis of cohort studies in our study (I2 = 55%, p = 0.05) was substantially lower than in a study by Sgolastra et al. (I2 = 89%, p = 0.0001), again strengthening the reliability of our results (Sgolastra et al., 2013).
When analyzed according to the definition of periodontitis, three subgroup analysis with studies defining periodontitis by PD alone, CAL and PD and CAL alone showed statistically significant differences, whereas the previous meta-analysis showed only significance with a subgroup analyzing periodontitis by CAL and PD. This could be explained by the number of studies included in our review was more than in the previous analysis, thus, providing a more comprehensive finding. However, according to the most recent case definition developed by the Centre for Disease Control and Prevention in partnership with the American Academy of Periodontology, the diagnostic criteria of periodontitis is at least 2 interproximal sites with the minimum of attachment loss of 3 mm and at least 2 interproximal sites with the minimum pocket depth of 4 mm (not on the same tooth) or one site with pocket depth ≥ 5 mm (Eke et al., 2012). Moreover, pregnant women who were preeclamptic had higher mean CAL and PD, however, the heterogeneity in both subgroup analysis was high, indicating significant variations among these studies in each subgroup. The high heterogeneity reported could result from the difference in the periodontal probes used in the dental examination in each study.
Several mechanisms have been proposed for the link between periodontitis and preeclampsia. Higher levels of some periodontal pathogens such as P.gingivalis and F. nucleatum were found in placenta of patients with preeclampsia (Barak et al., 2007). Moreover, inflammatory responses including the shifting of Th2 toward Th1, increasing oxidative stress, anti-angiogenic proteins, vascular endothelial growth factor receptor 1 and complement C5a could potentially enhance the development of preeclampsia (Nourollahpour Shiadeh et al., 2017). Ananth et al. has reported the association between intrauterine growth restriction and maternal periodontitis (Ananth et al., 2018). Since severe and early onset preeclampsia were associated significantly with fetal growth restriction, this could contribute to the mechanism underlying the association between preeclampsia and periodontitis (Odegård et al., 2000). Furthermore, the mechanisms might be a reflection of dietary patterns. Recently, some evidence has indicated that pathogenesis of preeclampsia involves maternal gut microbiota, specifically, high-fiber diet which promote short chain fatty acid production and are associated with reduced risk of preeclampsia (Hu et al., 2019). Similarly, high-fiber foods such as fruit and grains have been linked to the reduction of the progression of periodontal disease, suggesting the role of dietary intake in the potential relationship between preeclampsia and periodontal disease (Hu et al., 2019; Schwartz et al., 2012). However, future studies are required to elucidate these hypotheses.
When analyzing the association between periodontitis and preeclampsia according to national income, this review revealed the significant difference in the subgroup analysis of high-income and upper-middle-income countries (OR = 2.67 and OR = 2.40, respectively). Moreover, the subgroup analysis with lower-middle-income countries, which generated an Odds ratio of 6.70, indicated the considerable significance in the relationship between periodontitis and preeclampsia in this specific country group. The high heterogeneity was observed in the subgroup of lower-middle-income countries which implies the variation between included countries. Countries were categorized as lower-middle-income using national gross income per capita, thereby being subject to variation even within each individual country. In other words, using national income as a proxy may result in this variation and therefore, suggested the individual level approach for future studies to tackle this issue. Moreover, lower middle-income countries have poorer oral health condition compared to upper-middle and high-income country groups, which may indicate the inequalities in oral health care (Bastani et al., 2021; Watt & Sheiham, 1999). Inequalities can stem from unjust provision of services or inappropriate access and become more pronounced by the fact that most dental treatment is funded by out-of-pocket payments (Listl et al., 2015).
Socioeconomic inequalities in access to oral health care accounted for 60% in lower-middle-income countries (Hosseinpoor et al., 2012). Therefore, improving access to oral health services for pregnant women in lower-middle-income countries is notably important to lessen the risk of having preeclampsia. Additionally, the allocation of resources for oral care might need more investigations and strategic management to eliminate the disparities in oral health care (Arevalo & Tomar 2019). Social and cultural determinants include biological, behavioral, cultural, social and political aspects should also be focused to thoroughly eradicate the inequalities(Patrick et al., 2006).
We used Newcastle Ottawa Scale to evaluate the risk of bias and found twenty studies with moderate risk of bias and ten remaining studies with a high risk of bias. Exposure bias in this study was due to the non-response rate which was not described clearly in these studies. No publication bias was detected. Sensitivity analysis resulted in no change to the finding of the study.
The strength of this systematic review and meta-analysis includes the large sample size of 9650 subjects. Six cohort studies comprising 2840 subjects were analyzed and revealed the statistically significant difference. Because systematic reviews of prospective cohort studies generate more reliable evidence, our study provided an updated systematic review and meta-analysis and confirmed the association between periodontitis and preeclampsia (Hillier et al., 2011). Furthermore, by stratifying into subgroup analysis of national income, our review has pointed out the association between these two diseases differed according to economical inequalities, thus, providing recommendation for health policy improvement. Pregnant women in low socioeconomical areas should be given access to oral healthcare services and encouraged to have their periodontal health checked and treated during pregnancy to potentially lower the risk of preeclampsia and other pregnancy complications. Jeffcoat et al. reported non-surgical periodontal therapy could significantly reduce the medical costs for pregnant women by 73.7% (Jeffcoat et al., 2014). We acknowledged few limitations in our study. Firstly, the heterogeneity of the overall analysis for the association between periodontitis and preeclampsia was high, pointing out the variations among studies included. The synthesis of cohort and case–control studies in our review may explain for this high heterogeneity. Secondly, the general consensus in the definition and diagnosis of periodontitis was not clear enough which could influence the results of our meta-analysis. Additionally, because the intraoral examinations were conducted at different time points, the diagnosis of periodontitis may be impacted. Deteriorated periodontium was observed more in the third trimester compared to the second trimester and overall periodontal health was improved postpartum (González-Jaranay et al., 2017; Kashetty et al., 2018). Thereby, future clinical studies should consider the time point of seven days of the elivery when conducting periodontal examinationsand confirm our results. Moreover, because our analysis was based on national income, new research with individual-level data of socioeconomic factors is recommended for a more informative conclusion.
Conclusions and Implications
This meta-analysis confirms previous findings of an association between periodontitis and preeclampsia. However, this study includes fifteen more recent publications, which resulted in a larger effect size of the association, specifically, for lower-middle-income countries in comparison to high and upper-middle-income countries. Our results warrant future studies to investigate the mechanisms of this association and whether targeted interventions to prevent or treat periodontitis preconception or during pregnancy can lead to better pregnancy outcomes.
Abbreviations
- PD
Pocket depth
- CAL
Clinical attachment loss
Author Contributions
QAL Data curation, data analysis, quality assessment of articles, writing-original, and draft preparation. RA methodology, writing-editing, and supervision. KMC methodology, writing-editing, and supervision. NTN data curation, methodology, and writing-review. LDTY data curation, and quality assessment of articles. HNV data curation, and quality assessment of articles. AY writing-review and editing. GC writing-review and editing. JE methodology, writing-review and editing, and supervision. RN conceptualization and project administration.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research was funded by the Scholarship provided by the Chair of Lifespan Oral Health to Quynh Anh Le.
Data Availability
Data will be available upon request.
Code Availability
Not applicable.
Declarations
Conflict of interest
The authors report no conflict of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abariga SA, Whitcomb BW. Periodontitis and gestational diabetes mellitus: a systematic review and meta-analysis of observational studies. BMC Pregnancy and Childbirth. 2016 doi: 10.1186/s12884-016-1145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alchalabi HA, Al Habashneh R, Jabali OA, Khader YS. Association between periodontal disease and adverse pregnancy outcomes in a cohort of pregnant women in Jordan. Clinical and Experimental Obstetrics and Gynecology. 2013;40(3):399–402. [PubMed] [Google Scholar]
- Ananth CV, Andrews HF, Papapanou PN, Ward AM, Bruzelius E, Conicella ML, Albert DA. History of periodontal treatment and risk for intrauterine growth restriction (IUGR) BMC Oral Health. 2018;18(1):161. doi: 10.1186/s12903-018-0623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo O, Tomar SL. Perpetual inequities in access to dental care: Government or professional responsibility? Oral Health in America Removing the Stain of Disparity. 2019 doi: 10.2105/9780875533063ch04. [DOI] [Google Scholar]
- Australian Institute of Health and Welfare 2010. Socioeconomic variation in periodontitis among Australian adults 2004–06.
- Barak S, Oettinger-Barak O, Machtei EE, Sprecher H, Ohel G. Evidence of periopathogenic microorganisms in placentas of women with preeclampsia. Journal of Periodontology. 2007;78(4):670–676. doi: 10.1902/jop.2007.060362. [DOI] [PubMed] [Google Scholar]
- Bastani P, Mohammadpour M, Mehraliain G, Delavari S, Edirippulige S. What makes inequality in the area of dental and oral health in developing countries? A scoping review. Cost Effectiveness and Resource Allocation. 2021;19(1):54. doi: 10.1186/s12962-021-00309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo S, Menato G, Bardelli C, Lezo A, Signorile A, Repetti E, Massobrio M, Pagano G. Low socioeconomic status as a risk factor for gestational diabetes. Diabetes & Metabolism. 2002;28(2):139–140. [PubMed] [Google Scholar]
- Boggess KA, Lieff S, Murtha AP, Moss K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstetrics and Gynecology. 2003;101(2):227–231. doi: 10.1016/s0029-7844(02)02314-1. [DOI] [PubMed] [Google Scholar]
- Bui FQ, Almeida-da-Silva CLC, Huynh B, Trinh A, Liu J, Woodward J, Asadi H, Ojcius DM. Association between periodontal pathogens and systemic disease. Biomedical Journal. 2019;42(1):27–35. doi: 10.1016/j.bj.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canakci V, Canakci CF, Canakci H, Canakci E, Cicek Y, Ingec M, Ozgoz M, Demir T, Dilsiz A, Yagiz H. Periodontal disease as a risk factor for pre-eclampsia: A case control study. The Australian & New Zealand Journal of Obstetrics & Gynaecology. 2004;44(6):568–573. doi: 10.1111/j.1479-828X.2004.00323.x. [DOI] [PubMed] [Google Scholar]
- Canakci V, Canakci CF, Yildirim A, Ingec M, Eltas A, Erturk A. Periodontal disease increases the risk of severe pre-eclampsia among pregnant women. Journal of Clinical Periodontology. 2007;34:639–645. doi: 10.1111/j.1600-051X.2007.01105.x. [DOI] [PubMed] [Google Scholar]
- Chaparro A, Sanz A, Quintero A, Inostroza C, Ramirez V, Carrion F, Figueroa F, Serra R, Illanes SE. Increased inflammatory biomarkers in early pregnancy is associated with the development of pre-eclampsia in patients with periodontitis: A case control study. Journal of Periodontal Research. 2013;48(3):302–307. doi: 10.1111/jre.12008. [DOI] [PubMed] [Google Scholar]
- Contreras A, Herrera JA, Soto JE, Arce RM, Jaramillo A, Botero JE. Periodontitis is associated with preeclampsia in pregnant women. Journal of Periodontology. 2006;77(2):182–188. doi: 10.1902/jop.2006.050020. [DOI] [PubMed] [Google Scholar]
- Corbella S, Taschieri S, Francetti L, De Siena F, Del Fabbro M. Periodontal disease as a risk factor for adverse pregnancy outcomes: A systematic review and meta-analysis of case-control studies. Odontology. 2012;100(2):232–240. doi: 10.1007/s10266-011-0036-z. [DOI] [PubMed] [Google Scholar]
- Cota LO, Guimarães AN, Costa JE, Lorentz TC, Costa FO. Association between maternal periodontitis and an increased risk of preeclampsia. Journal of Periodontology. 2006;77(12):2063–2069. doi: 10.1902/jop.2006.060061. [DOI] [PubMed] [Google Scholar]
- Country and Lending Groups, & The World Bank Group. (2011). Country Income Groups (World Bank Classification)http://data.worldbank.org/about/country-classifications/country-and-lending-groups
- Desai K, Desai P, Duseja S, Kumar S, Mahendra J, Duseja S. Significance of maternal periodontal health in preeclampsia. Journal of International Society of Preventive & Community Dentistry. 2015;5(2):103–107. doi: 10.4103/2231-0762.155734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duley L. The global impact of pre-eclampsia and eclampsia. Seminars in Perinatology. 2009;33(3):130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey SG, Schneider M, Minder C. Bias in metaanalysis detected by a simple, graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. Journal of Periodontology. 2012;83(12):1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesase N, Miranda-Rius J, Brunet-Llobet L, Lahor-Soler E, Mahande MJ, Masenga G. The association between periodontal disease and adverse pregnancy outcomes in Northern Tanzania: A cross-sectional study. African Health Sciences. 2018;18(3):601–611. doi: 10.4314/ahs.v18i3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Jaranay M, Téllez L, Roa-López A, Gómez-Moreno G, Moreu G. Periodontal status during pregnancy and postpartum. PLoS ONE. 2017;12(5):e0178234. doi: 10.1371/journal.pone.0178234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindasamy R, Dhanasekaran M, Varghese S, Balaji V, Karthikeyan B, Christopher A. Maternal risk factors and periodontal disease: A cross-sectional study among postpartum mothers in Tamil Nadu [Original Article] Journal of Pharmacy and Bioallied Sciences. 2017;9(5):50–54. doi: 10.4103/jpbs.JPBS_88_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Haynes RB, Jaeschke RZ, Cook DJ. Users' guides to the medical literature: XXV. evidence-based medicine: Principles for applying the users' guides to patient care. JAMA. 2000;284:1290–1296. doi: 10.1001/jama.284.10.1290. [DOI] [PubMed] [Google Scholar]
- Ha JE, Jun JK, Ko HJ, Paik DI, Bae KH. Association between periodontitis and preeclampsia in never-smokers: A prospective study. Journal of Clinical Periodontology. 2014;41(9):869–874. doi: 10.1111/jcpe.12281. [DOI] [PubMed] [Google Scholar]
- Ha JE, Oh KJ, Yang HJ, Jun JK, Jin BH, Paik DI, Bae KH. Oral health behaviors, periodontal disease, and pathogens in preeclampsia: A case-control study in Korea. Journal of Periodontology. 2011;82(12):1685–1692. doi: 10.1902/jop.2011.110035. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a metaanalysis. Statistics in Medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hillier S, Grimmer-Somers K, Merlin T, Middleton P, Salisbury J, Tooher R, Weston A. FORM: An Australian method for formulating and grading recommendations in evidence-based clinical guidelines. BMC Medical Research Methodology. 2011;11:23. doi: 10.1186/1471-2288-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano E, Sugita N, Kikuchi A, Shimada Y, Sasahara J, Iwanaga R, Tanaka K, Yoshie H. The association of Aggregatibacter actinomycetemcomitans with preeclampsia in a subset of Japanese pregnant women. Journal of Clinical Periodontology. 2012;39:229–238. doi: 10.1111/j.1600-051X.2011.01845.x. [DOI] [PubMed] [Google Scholar]
- Horton AL, Boggess KA, Moss KL, Beck J, Offenbacher S. Periodontal disease, oxidative stress, and risk for preeclampsia. Journal of Periodontology. 2010;81(2):199–204. doi: 10.1902/jop.2009.090437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinpoor AR, Itani L, Petersen PE. Socio-economic inequality in oral healthcare coverage: Results from the World Health Survey. Journal of Dental Research. 2012;91(3):275–281. doi: 10.1177/0022034511432341. [DOI] [PubMed] [Google Scholar]
- Hu M, Eviston D, Hsu P, Mariño E, Chidgey A, Santner-Nanan B, Wong K, Richards JL, Yap YA, Collier F, Quinton A, Joung S, Peek M, Benzie R, Macia L, Wilson D, Ponsonby A-L, Tang MLK, O’Hely M, Daly NL, Mackay CR, Dahlstrom JE, Saffery R, Allen KJ, Ranganathan S, Burgner D, Harrison LC, Sly P, Dwyer T, Vuillermin P, Nanan R, The BISIG. Decreased maternal serum acetate and impaired fetal thymic and regulatory T cell development in preeclampsia. Nature Communications. 2019;10(1):3031. doi: 10.1038/s41467-019-10703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T-H, Hsieh T. Risk of abnormal fetal growth in women with early- and late-onset preeclampsia. Pregnancy Hypertension. 2018;12:201–206. doi: 10.1016/j.preghy.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Jaiman G, Nayak PA, Sharma S, Nagpal K. Maternal periodontal disease and preeclampsia in Jaipur population. Journal of Indian Society of Periodontology. 2018;22(1):50–54. doi: 10.4103/jisp.jisp_363_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffcoat MK, Jeffcoat RL, Gladowski PA, Bramson JB, Blum JJ. Impact of periodontal therapy on general health: Evidence from insurance data for five systemic conditions. American Journal of Preventive Medicine. 2014;47(2):166–174. doi: 10.1016/j.amepre.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Kashetty M, Kumbhar S, Patil S, Patil P. Oral hygiene status, gingival status, periodontal status, and treatment needs among pregnant and nonpregnant women: A comparative study. J Indian Soc Periodontol. 2018;22(2):164–170. doi: 10.4103/jisp.jisp_319_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader YS, Jibreal M, Al-Omiri M, Amarin Z. Lack of association between periodontal parameters and preeclampsia. Journal of Periodontology. 2006;77(10):1681–1687. doi: 10.1902/jop.2006.050463. [DOI] [PubMed] [Google Scholar]
- Khalighinejad N, Aminoshariae A, Kulild JC, Mickel A. Apical periodontitis, a predictor variable for preeclampsia: A case-control study. Journal of Endodontics. 2017;43(10):1611–1614. doi: 10.1016/j.joen.2017.05.021. [DOI] [PubMed] [Google Scholar]
- Kivimäki M, Batty GD, Pentti J, Shipley MJ, Sipilä PN, Nyberg ST, Suominen SB, Oksanen T, Stenholm S, Virtanen M, Marmot MG, Singh-Manoux A, Brunner EJ, Lindbohm JV, Ferrie JE, Vahtera J. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: A multi-cohort study. The Lancet Public Health. 2020;5(3):e140–e149. doi: 10.1016/S2468-2667(19)30248-8. [DOI] [PubMed] [Google Scholar]
- Kumar A, Basra M, Begum N, Rani V, Prasad S, Lamba AK, Verma M, Agarwal S, Sharma S. Association of maternal periodontal health with adverse pregnancy outcome. The Journal of Obstetrics and Gynaecology Research. 2013;39(1):40–45. doi: 10.1111/j.1447-0756.2012.01957.x. [DOI] [PubMed] [Google Scholar]
- Kunnen A, Blaauw J, van Doormaal JJ, van Pampus MG, van der Schans CP, Aarnoudse JG, van Winkelhoff AJ, Abbas F. Women with a recent history of early-onset pre-eclampsia have a worse periodontal condition. Journal of Clinical Periodontology. 2007;34(3):202–207. doi: 10.1111/j.1600-051X.2006.01036.x. [DOI] [PubMed] [Google Scholar]
- Kunnen A, van Doormaal JJ, Abbas F, Aarnoudse JG, van Pampus MG, Faas MM. Periodontal disease and pre-eclampsia: A systematic review. Journal of Clinical Periodontology. 2010;37(12):1075–1087. doi: 10.1111/j.1600-051X.2010.01636.x. [DOI] [PubMed] [Google Scholar]
- Lafaurie GI, Gómez LA, Montenegro DA, De Avila J, Tamayo MC, Lancheros MC, QuicenoNoriega TTG, Grueso LA, Cepeda ML. Periodontal condition is associated with adverse perinatal outcomes and premature rupture membranes in low income pregnant women in Bogota, Colombia: A case control study. The Journal of Maternal-Fetal & Neonatal Medicine : THe Official Journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2018;33(1):16–23. doi: 10.1080/14767058.2018.1484092. [DOI] [PubMed] [Google Scholar]
- Lavigne SE, Forrest JL. An umbrella review of systematic reviews of the evidence of a causal relationship between periodontal disease and adverse pregnancy outcomes: A position paper from the Canadian Dental Hygienists Association. Canadian Journal of Dental Hygienists. 2020;54(2):92–100. [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Ha JE, Bae KH. Synergistic effect of maternal obesity and periodontitis on preterm birth in women with pre-eclampsia: A prospective study. Journal of Clinical Periodontology. 2016;43(8):646–651. doi: 10.1111/jcpe.12574. [DOI] [PubMed] [Google Scholar]
- Listl S, Galloway J, Mossey PA, Marcenes W. Global Economic Impact of Dental Diseases. Journal of Dental Research. 2015;94(10):1355–1361. doi: 10.1177/0022034515602879. [DOI] [PubMed] [Google Scholar]
- Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Medical Research Methodology. 2014 doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohsoonthorn V, Kungsadalpipob K, Chanchareonsook P, Limpongsanurak S, Vanichjakvong O, Sutdhibhisal S, Sookprome C, Wongkittikraiwan N, Kamolpornwijit W, Jantarasaengaram S, Manotaya S, Siwawej V, Barlow WE, Fitzpatrick AL, Williams MA. Maternal periodontal disease and risk of preeclampsia: A case-control study. American Journal of Hypertension. 2009;22(4):457–463. doi: 10.1038/ajh.2008.365. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Journal of Clinical Epidemiology. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Moura da Silva G, Coutinho SB, Piscoya MD, Ximenes RA, Jamelli SR. Periodontitis as a risk factor for preeclampsia. Journal of Periodontology. 2012;83(11):1388–1396. doi: 10.1902/jop.2012.110256. [DOI] [PubMed] [Google Scholar]
- Nourollahpour Shiadeh M, Behboodi Moghadam Z, Adam I, Saber V, Bagheri M, Rostami A. Human infectious diseases and risk of preeclampsia: An updated review of the literature. Infection. 2017;45(5):589–600. doi: 10.1007/s15010-017-1031-2. [DOI] [PubMed] [Google Scholar]
- Odegård RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Preeclampsia and fetal growth. Obstetrics and Gynecology. 2000;96(6):950–955. [PubMed] [Google Scholar]
- Orwin R. A fail-safe N for effect size in meta-analysis. Journal of Educational Statistics. 1983;8:157–159. [Google Scholar]
- Patrick DL, Lee RSY, Nucci M, Grembowski D, Jolles CZ, Milgrom P. Reducing oral health disparities: A focus on social and cultural determinants. BMC Oral Health. 2006;6(1):S4. doi: 10.1186/1472-6831-6-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanashetti JI, Nagathan VM, Rao SM. Evaluation of periodontitis as a risk for preterm birth among preeclamptic and non-preeclamptic pregnant women - a case control study. Journal of Clinical and Diagnostic Research : JCDR. 2013;7(8):1776–1778. doi: 10.7860/JCDR/2013/6497.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock JL, Bland JM, Anderson HR. Preterm delivery: Effects of socioeconomic factors, psychological stress, smoking, alcohol, and caffeine. BMJ. 1995;311(7004):531–535. doi: 10.1136/bmj.311.7004.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscoya MD, Ximenes RA, Silva GM, Jamelli SR, Coutinho SB. Periodontitis-associated risk factors in pregnant women. Clinics (são Paulo, Brazil) 2012;67(1):27–33. doi: 10.6061/clinics/2012(01)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politano GT, Passini R, Nomura ML, Velloso L, Morari J, Couto E. Correlation between periodontal disease, inflammatory alterations and pre-eclampsia. Journal of Dental Research. 2011;46:505–511. doi: 10.1111/j.1600-0765.2011.01368.x. [DOI] [PubMed] [Google Scholar]
- Pralhad S, Thomas B, Kushtagi P. Periodontal disease and pregnancy hypertension: A clinical correlation. Journal of Periodontology. 2013;84(8):1118–1125. doi: 10.1902/jop.2012.120264. [DOI] [PubMed] [Google Scholar]
- Ross KM, Dunkel Schetter C, McLemore MR, Chambers BD, Paynter RA, Baer R, Feuer SK, Flowers E, Karasek D, Pantell M, Prather AA, Ryckman K, Jelliffe-Pawlowski L. Socioeconomic status, preeclampsia risk and gestational length in black and white women. Journal of Racial and Ethnic Health Disparities. 2019;6(6):1182–1191. doi: 10.1007/s40615-019-00619-3. [DOI] [PubMed] [Google Scholar]
- Sayar F, Sadat Hoseini M, Abbaspour S. Effect of periodontal disease on preeclampsia. Iranian Journal of Public Health. 2011;40(3):122–127. [PMC free article] [PubMed] [Google Scholar]
- Schwartz N, Kaye EK, Nunn ME, Spiro A, 3rd, Garcia RI. High-fiber foods reduce periodontal disease progression in men aged 65 and older: The Veterans Affairs normative aging study/Dental Longitudinal Study. Journal of the American Geriatrics Society. 2012;60(4):676–683. doi: 10.1111/j.1532-5415.2011.03866.x. [DOI] [PubMed] [Google Scholar]
- Sgolastra F, Petrucci A, Severino M, Gatto R, Monaco A. Relationship between periodontitis and pre-eclampsia: A meta-analysis. PLoS ONE. 2013;8(8):e71387. doi: 10.1371/journal.pone.0071387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty M, Shetty PK, Ramesh A, Thomas B, Prabhu S, Rao A. Periodontal disease in pregnancy is a risk factor for preeclampsia. Acta Obstetricia Et Gynecologica Scandinavica. 2009;89(5):718–721. doi: 10.3109/00016341003623738. [DOI] [PubMed] [Google Scholar]
- Silva, L. M., Coolman, M., Steegers, E. A. P., Jaddoe, V. W. V., Moll, H. A., Hofman, A., Mackenbach, J. P., & Raat, H. (2008). Low socioeconomic status is a risk factor for preeclampsia: the Generation R Study. Journal of Hypertension, 26(6). https://journals.lww.com/jhypertension/Fulltext/2008/06000/Low_socioeconomic_status_is_a_risk_factor_for.20.aspx [DOI] [PubMed]
- Siqueira FM, Cota LO, Costa JE, Haddad JP, Lana AM, Costa FO. Maternal periodontitis as a potential risk variable for preeclampsia: A case-control study. Journal of Periodontology. 2008;79(2):207–215. doi: 10.1902/jop.2008.070174. [DOI] [PubMed] [Google Scholar]
- Soucy-Giguère L, Tétu A, Gauthier S, Morand M, Chandad F, Giguère Y, Bujold E. Periodontal disease and adverse pregnancy outcomes: a prospective study in a low-risk population. Journal of Obstetrics and Gynaecology Canada. 2016;38(4):346–350. doi: 10.1016/j.jogc.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Stevens W, Shih T, Incerti D, Ton TGN, Lee HC, Peneva D, Macones GA, Sibai BM, Jena AB. Short-term costs of preeclampsia to the United States health care system. American Journal of Obstetrics & Gynecology. 2017;217(3):237–248.e216. doi: 10.1016/j.ajog.2017.04.032. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Taghzouti N, Xiong X, Gornitsky M, Chandad F, Voyer R, Gagnon G, Leduc L, Xu H, Tulandi T, Wei B, Sénécal J, Velly AM, Salah MH, Fraser WD. Periodontal disease is not associated with preeclampsia in Canadian pregnant women. Journal of Periodontology. 2012;83(7):871–877. doi: 10.1902/jop.2011.110342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbeville HR, Sasser JM. Preeclampsia beyond pregnancy: Long-term consequences for mother and child American Journal of Physiology. Renal Physiology. 2020;318(6):F1315–F1326. doi: 10.1152/ajprenal.00071.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney S, Gautam A. Poor periodontal health as a risk factor for development of pre-eclampsia in pregnant women. Journal of Indian Society of Periodontology. 2014;18(3):321–325. doi: 10.4103/0972-124X.134569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt R, Sheiham A. Inequalities in oral health: A review of the evidence and recommendations for action. British Dental Journal. 1999;187:6–12. doi: 10.1038/sj.bdj.4800191. [DOI] [PubMed] [Google Scholar]
- Wei BJ, Chen YJ, Yu L, Wu B. Periodontal disease and risk of preeclampsia: A meta-analysis of observational studies. PLoS ONE. 2013;8(8):e70901. doi: 10.1371/journal.pone.0070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winning L, Linden GJ. Periodontitis and Systemic Disease. BDJ Team. 2015;2(10):15163. doi: 10.1038/bdjteam.2015.163. [DOI] [Google Scholar]
- Yaghini J, Mostajeran F, Afshari E, Naghsh N. Is periodontal disease related to preeclampsia? Dental Research Journal. 2012;9(6):770–773. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available upon request.
Not applicable.