Abstract
An essential step in bacterial transformation is the uptake of DNA into the periplasm, across the thick peptidoglycan cell wall of Gram-positive bacteria, or the outer membrane and thin peptidoglycan layer of Gram-negative bacteria. ComEA, a DNA-binding protein widely conserved in transformable bacteria, is required for this uptake step. Here we determine X-ray crystal structures of ComEA from two Gram-positive species, Bacillus subtilis and Geobacillus stearothermophilus, identifying a domain that is absent in Gram-negative bacteria. X-ray crystallographic, genetic, and analytical ultracentrifugation (AUC) analyses reveal that this domain drives ComEA oligomerization, which we show is required for transformation. We use multi-wavelength AUC (MW-AUC) to characterize the interaction between DNA and the ComEA DNA-binding domain. Finally, we present a model for the interaction of the ComEA DNA-binding domain with DNA, suggesting that ComEA oligomerization may provide a pulling force that drives DNA uptake across the thick cell walls of Gram-positive bacteria.
Subject terms: Bacterial structural biology, Bacterial genetics
ComEA is a DNA-binding protein required for DNA uptake during bacterial transformation. Here, Ahmed et al. determine X-ray crystal structures of ComEA from Gram-positive bacteria, identifying a domain that is absent in Gram-negative bacteria and drives ComEA oligomerization, which is required for transformation.
Introduction
Natural competence for transformation is a mechanism of horizontal gene transfer widespread in both Gram-positive and Gram-negative bacteria, as well as in some archaea (for reviews see1,2). The transformation process consists of two major steps: uptake and transport (Fig. 1A). Uptake is the movement of environmental transforming DNA (tDNA) into the periplasm, across the thick peptidoglycan cell wall of Gram-positive bacteria or the outer membrane and thin peptidoglycan layer of Gram-negative bacteria. Transport is the subsequent translocation of single-stranded tDNA from the periplasm to the cytoplasm where it can recombine with the chromosome to generate a transformant. The structure-function studies presented here focus on the first step, DNA uptake.
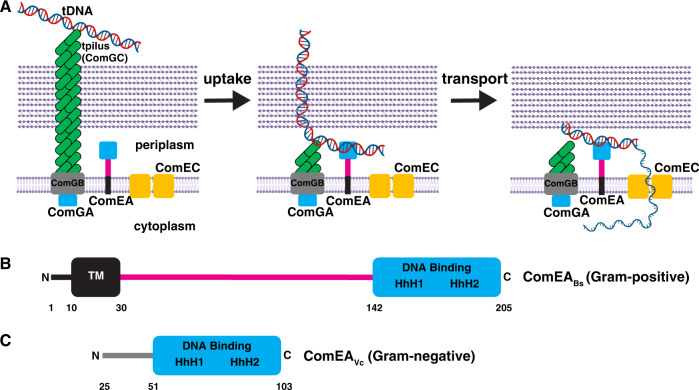
Fig. 1. Existing model of genetic transformation in Gram-positive bacteria, and ComEA domain architecture in Gram-positive and Gram-negative bacteria.
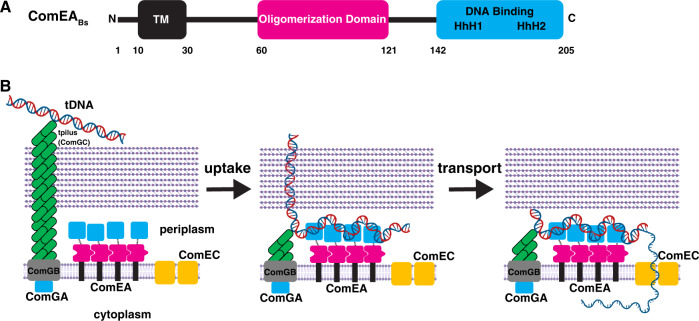
A The tpilus is composed of ComGC, whose assembly requires the ATPase ComGA. The tpilus is believed to be anchored to the membrane protein ComGB. The tpilus binds weakly to DNA and retracts to pull it into the periplasm. Here, the DNA encounters ComEA, which stabilizes binding to the cell and propels continued uptake of the DNA. ComEC is proposed to degrade one strand of DNA and provide the channel for transport of DNA into the cytoplasm. B Domain architecture of ComEA from a representative Gram-positive bacterium, B. subtilis. C Domain architecture of ComEA from a representative Gram-negative bacterium, V. cholerae. Residues 1–24 are not shown in order to highlight the fact that they comprise a predicted secretion signal that is cleaved to generate mature ComEAVc, which diffuses freely in the periplasm. TM, predicted transmembrane region. The magenta line denotes a region of unknown function, which is addressed in this study. HhH, helix-hairpin-helix motifs. Residue numbering corresponds to ComEABs or ComEAVc. Elements of the figure were created with BioRender.com.
Uptake is initiated at the cell surface when tDNA interacts with a transformation pilus (tpilus). Following this contact with the tpilus, the movement of tDNA into the periplasm is driven by its interaction with the widely conserved periplasmic protein ComEA (Fig. 1A). ComEA, was discovered in genetic screens for transformation deficiency in Bacillus subtilis. It was subsequently shown to be a non-specific DNA-binding protein required for both stable binding of tDNA to the cell and for tDNA uptake3,4. In B. subtilis and other Gram-positive bacteria, ComEA is bound to the membrane by an N-terminal transmembrane region4. This membrane anchor and a C-terminal DNA binding domain are separated by a region of unknown function consisting of ~110 amino acids (Fig. 1B). In sharp contrast, ComEA in the Gram-negatives contains only a single (stand-alone) DNA-binding domain which is free to diffuse in the periplasm5–7 (Fig. 1C).
In both Gram-negative and positive bacteria, except for Helicobacter pylori8, uptake is initiated by type 4 pili that bind DNA and retract, pulling a segment of transforming DNA into the periplasmic compartment9,10. It has been proposed that in the Gram-negative Vibrio cholerae and Neisseria gonorrhoeae, the single-domain ComEA then binds to this introduced tDNA segment, preventing loss of tDNA by backward diffusion across the outer membrane6,11. Thus, the pilus initiates uptake while ComEA operates as a Brownian ratchet12, providing the driving force for uptake of the bulk of the tDNA into the periplasm. In all bacteria, following uptake to the periplasm, one strand of tDNA is degraded and the remaining strand is transported to the cytoplasm through the ComEC membrane channel (reviewed in1,2). In Gram-positives, the membrane-associated ATPase ComFA and its partner protein ComFC, appear to provide the energy for transport. Equivalent Gram-negative proteins have not been identified.
The complex structure and membrane anchoring of ComEA in the Gram-positives suggests that it may act differently than the simple Brownian ratchet mechanism proposed for V. cholerae and N. gonorrhoeae. To investigate this complex structure and to gain insight into its mode of action, we conducted an in vitro and in vivo structure-function investigation, centered on the B. subtilis protein. Here we present X-ray crystal structures of ComEA from B. subtilis (ComEABs) and Geobacillus stearothermophilus (ComEAGs) together with complementary genetic and biophysical analyses. These studies reveal that the DNA binding domain is an atypical helix-hairpin-helix domain, and, most importantly, that a previously unidentified domain lies within the Gram-positive ComEA region of unknown function. We show that this domain drives ComEA oligomerization, and that oligomerization is required for genetic transformation. We postulate that the unexpected role of ComEA oligomerization in DNA uptake is explained by DNA-protein condensation providing a driving force for tDNA uptake across the thick cell wall of Gram-positive bacteria.
Results
X-ray crystal structures of ComEABs and ComEAGs
To gain mechanistic insight into the function of ComEA, we cloned, overexpressed, purified, and determined the X-ray crystal structures of ComEABs and ComEAGs to 3.20 Å and 3.05 Å resolution, respectively (Figs. 2A, 2B, and Table S1). No electron density was evident for the ComEABs DNA-binding domain or linker region, but there was clear density corresponding to a previously undescribed domain (amino acids 60–122) (Fig. 2A). There are seven of these domains arranged head-to-tail in the ComEABs crystallographic asymmetric unit, and we named this region the oligomerization domain (OD) (Fig. 2A).
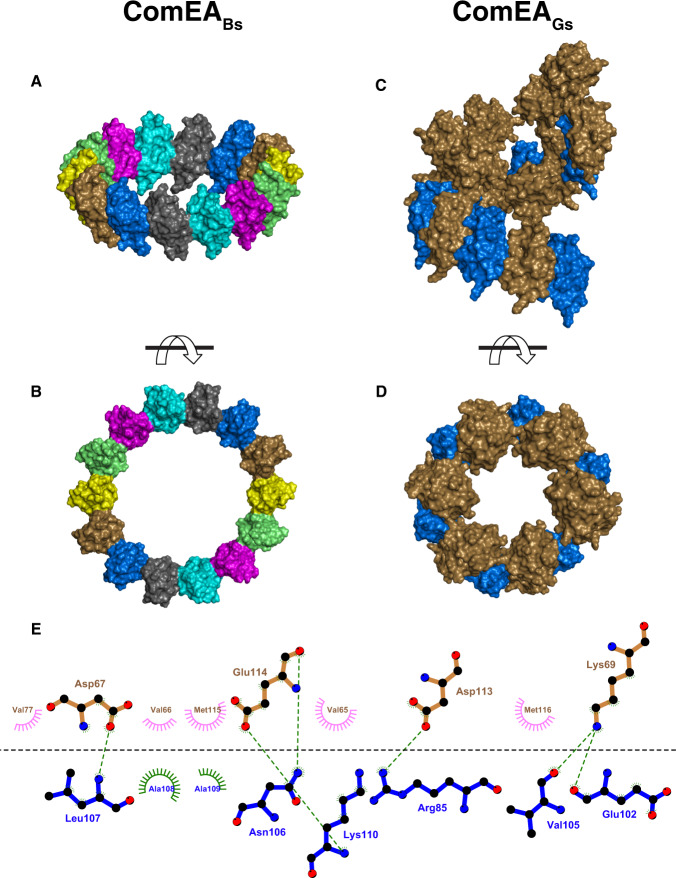
Fig. 2. ComEA X-ray crystal structures.
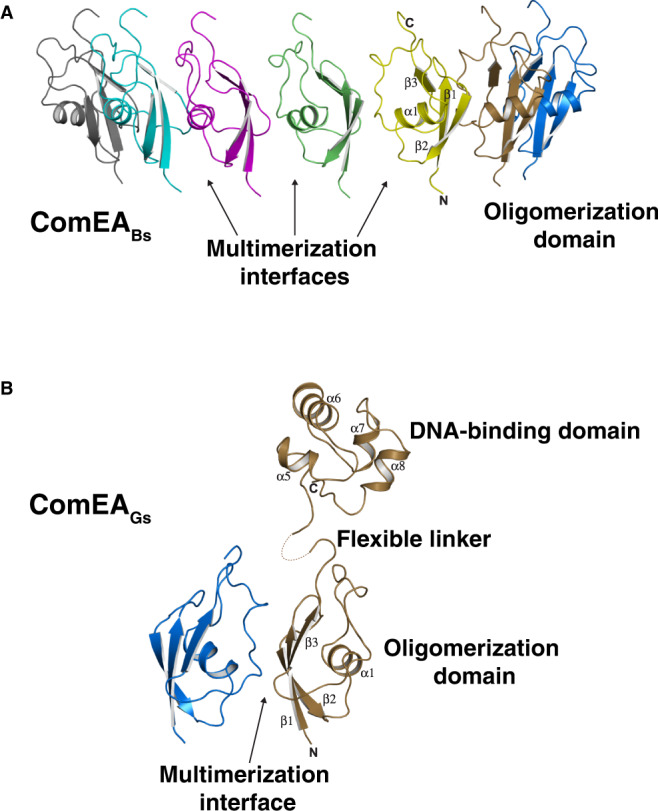
A) X-ray crystal structure of ComEABs. One asymmetric unit containing seven protomers is shown. Arrows here point to only three of the six multimerization interfaces in the asymmetric unit. B) X-ray crystal structure of ComEAGs. One asymmetric unit containing two protomers is shown.
In contrast to ComEABs, in the ComEAGs structure there was interpretable electron density corresponding not only to the OD (amino acids 62–124) but also to one DNA-binding domain (amino acids 143–207) per asymmetric unit, which contained two OD protomers again arranged head-to-tail (Fig. 2B). Because there is no interpretable electron density corresponding to the ComEAGs linker region (amino acids 125–142) of either protomer, it is unknown which of the OD monomers in the asymmetric unit OD dimer connects to the single modeled DNA-binding domain. In fact, we could model the DNA-binding domain in the ComEAGs structure only because it made fortuitous crystal contacts. We conclude from the ComEA structures that the linker domain is flexible, that there are minimal contacts between the OD and DNA-binding domains, and that the ODs have the potential to form multimers in solution.
We note that OD multimerization extends beyond the asymmetric unit (Fig. 2A, B). More specifically, in the ComEABs structure, the seven-membered asymmetric unit uses the OD multimerization interface to form a 14-membered head-to-tail ring around the crystallographic two-fold axis (Fig. 3A, B). Similarly, in the ComEAGs structure, examination of symmetry mates reveals that the two-membered asymmetric unit uses the OD multimerization interface to form a kinked-ring spiral along the crystallographic screw axis, containing 12 head-to-tail protomers per turn (Fig. 3C, D). A slight tilt between ComEAGs protomers (Fig. S1) has minimal effects on the ComEA inter-dimer bonds, but it causes ComEAGs to form a kinked-ring rather than a closed ring within the crystals.
Fig. 3. ComEA multimerization as observed in the X-ray crystal structures.
A and B Front and side views of the ComEABs crystallographic ring, respectively. C and D Front and side views of the ComEAGs crystallographic kinked ring, respectively. E Schematic representation of the ComEAGs multimerization interface. ComEA chains A and B are depicted as blue and brown bonds, respectively. Hydrogen bonds are depicted as green dashed lines. Hydrophobic contacts are depicted as lines radiating from the semicircles and spheres. The schematic was produced with LigPlot+50.
ComEA oligomerization interface amino acids are conserved (Fig. S2) and mediate conspicuous intermolecular interactions between the OD domains of ComEA protomers, e.g., the side chains of ComEAGs Arg85 and Asp113 (corresponding to ComEABs Arg83 and Asp111) which form intermolecular salt bridges, (Fig. 3E). This suggested that ComEA may oligomerize not only in the crystals but also in solution and we proceeded to explore this possibility.
ComEA oligomerizes in solution
To confirm that the OD observed in both ComEA X-ray crystal structures mediated reversible self-association in solution, we analyzed different concentrations of ComEAGs using sedimentation velocity analytical ultracentrifugation (SV-AUC)13. At 10.3 μM, the majority of ComEAGs has a sedimentation coefficient of 1.6 S, consistent with the ComEA monomer. However, at 157 μM, the majority of ComEAGs has a sedimentation coefficient of 2.2 S, suggesting reversible dimerization as a function of mass action (Fig. 4A). At an intermediate concentration of 31.3 μM we observed both monomer and dimer, and were able to fit the sedimentation velocity data to a discrete monomer-dimer equilibrium model13, resulting in a Kd of 33.8 μM (95% confidence intervals: 19.3 μM, 48.4 μM) (Table S4). The apparent ComEA dimerization affinity is likely an underestimation of its in vivo dimerization affinity, because diffusion of ComEA in vivo is limited to two dimensions in the cell membrane.
Fig. 4. AUC analysis of ComEA, ComEA-A108Y, and the ComEA oligomerization domain.
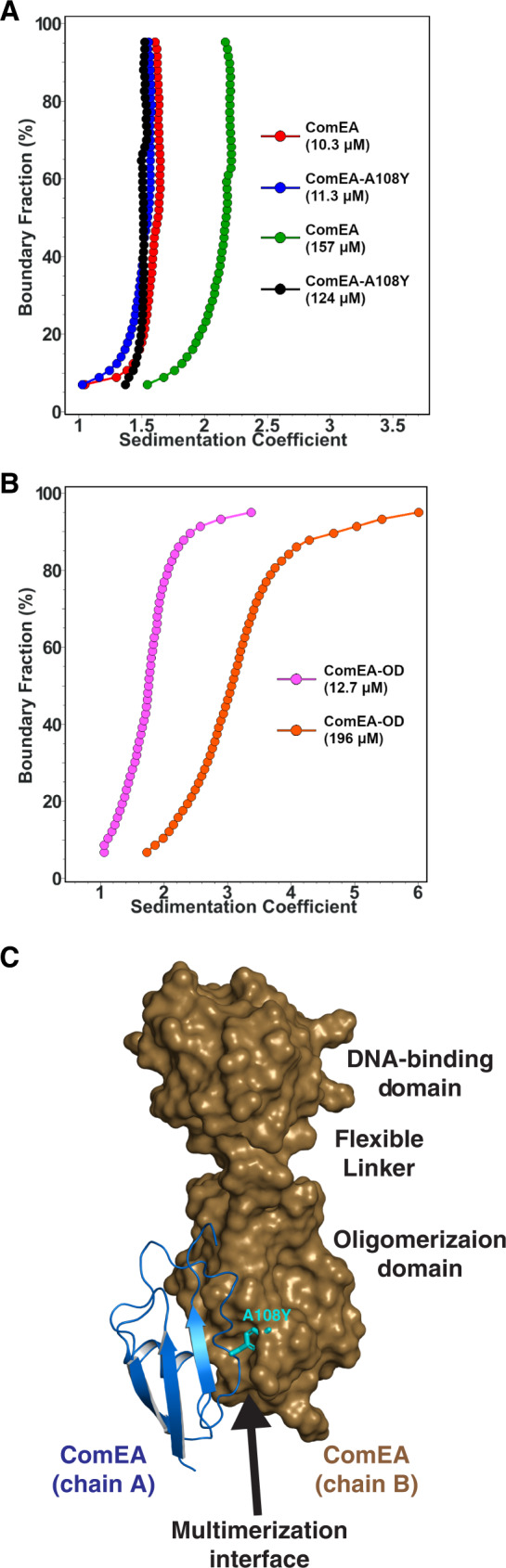
A Integral sedimentation coefficient distribution overlays comparing the dimerization potential of ComEAGs at 10.3 μM (red) and 157 μM (green) and ComEAGs-A108Y at 11.3 μM (blue) and 124 μM (black). Only ComEAGs dimerizes at higher concentration, while ComEAGs-A108Y remains monomeric. B Integral sedimentation coefficient distribution overlays of the ComEAGs OD at 12.7 μM (magenta) and 196 μM (orange), showing reversible self-association. C Structure of ComEAGs with chain A depicted as a cartoon and chain B depicted as a surface. ComEAGs chain A Ala108 was mutated to Tyr and is depicted as magenta sticks.
Consistent with the SV-AUC analysis of ComEAGs, a truncated ComEAGs protein consisting of the OD alone (ComEAGs-OD) also multimerized in solution (Fig. 4B). However, ComEAGs-OD formed primarily monomers and dimers at low concentration (12.7 μM) and larger oligomers with a maximum molar mass around 100 kDa at high concentration (196 μM) (Fig. S3). One possible kinetic explanation for the different multimeric states of ComEAGs and ComEAGs-OD at similar concentrations is that the presence of the ComEAGs DNA-binding domain slows the OD multimerization search, limiting collisions, thus slowing the OD multimerization rate (kon).
To confirm that ComEA multimerization is driven by the OD, we examined the X-ray crystal structures to identify a small amino acid buried in the OD multimerization interface that we could replace with a larger amino acid, introducing steric bulk and disrupting multimerization. We identified ComEAGs Ala108 (corresponding to ComEABs Ala106) and substituted it with tyrosine (Figs. 3E, 4A, C, and S4). Indeed, ComEAGs-A108Y was monomeric at both 11.3 μM and 124 μM (Fig. 4A). These SV-AUC experiments demonstrate that the OD drives ComEA multimerization in solution as predicted from the X-ray crystal structures.
ComEA oligomerizes on DNA
We further speculated that ComEA multimerization plays an important role in its interaction with DNA. To test this, we used multi-wavelength analytical ultracentrifugation (MW-AUC)14–17 to measure the binding of wild-type ComEAGs and ComEAGs-A108Y to DNA. MW-AUC is a novel technique that permits the spectral separation of protein and DNA species based on their unique absorbance spectra. Consequently, it is possible to measure the molar stoichiometry of the complexes formed and identify the type of macromolecule(s) forming each hydrodynamic species. The hydrodynamic measurements depend on each species’ molar mass and hydrodynamic radius.
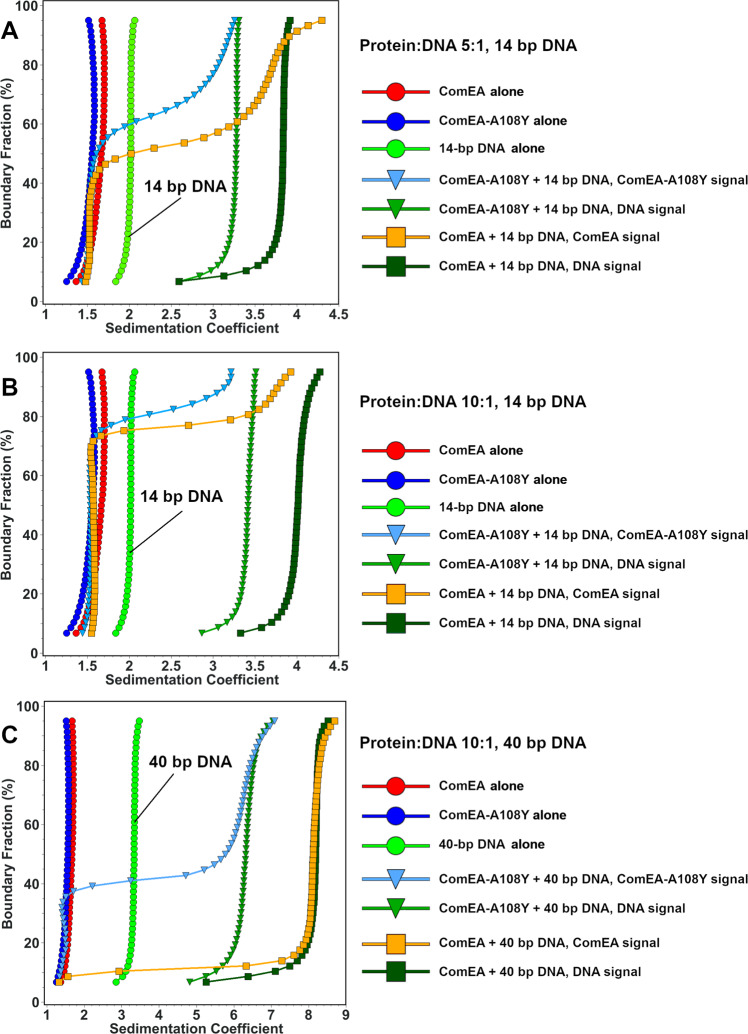
To elucidate the interactions between ComEA and double-stranded DNA, we mixed wild-type and mutant ComEA with a 14-bp double-stranded DNA duplex at a 5:1 and a 10:1 molar protein excess and a 10:1 protein excess over a 40-bp double-stranded DNA duplex (Fig. 5A–C and Table S2). In all cases, the DNA concentration was held constant at 1.5 μM, and a well-defined complex was formed, with less than 10% of the DNA remaining free in the solution. The deconvoluted DNA sedimentation pattern suggests the presence of a saturated, homogeneous complex being formed in all cases. As expected, the DNA sequestered excess protein until it was fully occupied, and the 14-bp sequence accommodated fewer ComEA molecules than the 40-bp sequence.
Fig. 5. AUC analysis of ComEA and ComEA-A108Y, and their interactions with 1.5 μM DNA.
A and B Mixtures 5:1 and 10:1 molar ratios of wild-type ComEAGs and ComEAGs-A108Y with the 14-bp DNA molecule, respectively. Here, the DNA signals still suggest full saturation with protein, but the protein signals show more heterogeneous sedimentation coefficient distributions, consistent with more rapid exchange with the protein-DNA complex, which suggests a faster koff rate. More than 90% of the DNA is complexed with protein, shifting the 14 bp DNA distribution from 2.0 S for the control by itself to 3.3 S for the mutant, and 3.9 S for the wild-type in panel A. In panel B, increasing the protein concentration to 10:1, marginally shifts the DNA sedimentation for the wild-type further to about 4.0 S, while barely affecting the DNA sedimentation when mixed with the mutant. C Integral sedimentation coefficient distribution overlays for the deconvoluted protein and DNA signals from the 10:1 mixture of ComEAGs and ComEAGs-A108Y with 1.5 μM of the 40-bp DNA molecule. Unbound ComEA in the presence of DNA co-sediments with ComA in the absence of DNA. Again, more than 90% of the DNA signal shifts from the position of free 40 bp DNA at 3.3 s to a homogeneous composition at 6.3 S for the mutant, and 8.1 S for the wild-type, suggesting saturation of the DNA with ComEA. The ComEA signal closely tracks the DNA signal, suggesting a tight complex formation with a slow koff rate. For all plots, reference controls of each molecule by itself are shown as circles (ComEA: red circles, ComEA-A108Y: blue circles, 14 or 40 bp DNA, as indicated: green circles), symbols for interactions between DNA and wildtype protein are shown as squares (ComEA signal: orange squares, DNA signal: dark green squares) and interactions between ComEA-A108Y and DNA are shown in triangles (ComEA-A108Y signal: olive triangles, DNA signal: light blue triangles).
The sedimentation coefficient of the complex differed for wild-type and mutant and depended on the length of the DNA. The 14-bp double-stranded DNA duplex sedimented at 2.0 S in isolation but formed a 3.3 S complex with ComEAGs-A108Y, and a 3.9 S complex with wild-type ComEAGs in the 5:1 mixture (Fig. 5A). For this mixture, we measured a ~3:1 protein:DNA ratio for the complex formed with ComEAGs, and a ~2:1 ratio for the complex formed with the ComEAGs-A108Y protein. For the 10:1 protein:DNA mixture, a slightly larger 3.5 S complex was formed with ComEAGs-A108Y, a 4.0 S complex was formed with wild-type ComEAGs, and the protein:DNA ratios did not change significantly (Fig. 5B).
The 40-bp DNA sediments at 3.3 S when examined by itself, but, when mixed in a 10:1 ratio with ComEAGs-A108Y resulted in a 6.3 S complex, while the same mixture with wild-type ComEAGs resulted in a 8.1 S complex (Fig. 5C). For the 40-bp DNA, the difference between protein:DNA molar ratios of the formed complexes was more pronounced between wild-type and mutant. The wild-type ComEAGs displayed an approximately 7:1 ratio, but the complex between DNA and ComEAGs-A108Y suggested the presence of about a 4.7:1 ratio. While the accuracy of these ratios depends on the estimated molar extinction coefficients in the formed complexes, there is a clear difference in the amount of ComEA bound to DNA for the wild-type and mutant proteins.
In sum, these results suggest that wild-type ComEA, capable of oligomerizing, forms larger complexes with DNA than the A108Y mutant. Oligomerization facilitates efficient and cooperative packing of ComEA on DNA, which is further supported by gel shift analysis (detailed below).
ComEA oligomerization is required for transformation
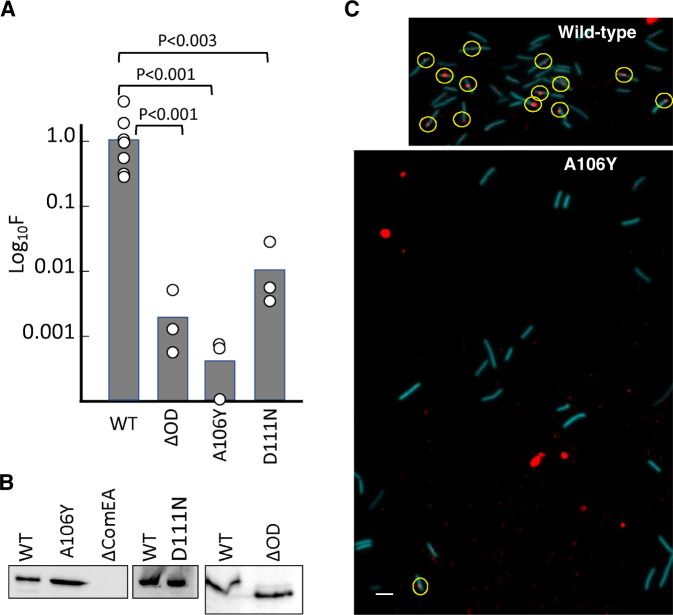
The conservation in Gram-positive bacteria of both the ComEA OD domain itself and, significantly, the residues buried in the multimerization interface (Figs. S2 and 3E), led us to hypothesize that the OD is important for transformation. We measured this in vivo by comparing B. subtilis transformation in strains containing wild-type ComEABs; ComEABs-D111N, which disrupts a conserved intermolecular salt-bridge between D111 and R83 (corresponding to ComEAGs Asp113 and Arg85, Fig. 3E); ComEABs-A106Y (corresponding to ComEAGs-A108Y), which, as shown above, sterically blocks OD multimerization (Fig. 4A, C); and ComEA-ΔOD, containing a deletion of the entire OD, while leaving intact both the transmembrane region and the DNA-binding domain. In comparison to wild-type ComEABs, ComEABs-D111N reduced B. subtilis transformation nearly 100-fold (Fig. 6A). Even more striking were the ComEABs-A106Y and ComEA-ΔOD mutations that reduced B. subtilis transformation about 1,000-fold, a phenotype equivalent to that of a complete comEA deletion. Western blot analysis showed that the wild-type and mutant ComEA proteins were similarly expressed in B. subtilis (Fig. 6B). To determine whether the A106Y oligomerization deficient mutant exerts its effect on transformation by preventing DNA uptake to the periplasm, we used rhodamine-labeled tDNA, which can be taken up into the periplasm but cannot cross the cell membrane18. As shown in (Fig. 6C), the A106Y mutation prevents stable DNA association with competence-expressing cells, which we have shown previously required uptake to the periplasm18. We conclude that ComEA oligomerization plays an unexpected and necessary role in the transformation of B. subtilis and likely of other Gram-positive bacteria as well.
Fig. 6. Self-association of ComEA is required for transformation.
All mutations were introduced into the chromosome of BD9007, which carries a competence-specific fusion of a sequence encoding cyan fluorescent protein (CFP) expressed from the promoter of comG, placed at the ectopic amyE locus to enable the identification of competence-expressing cells by epifluorescence microscopy. Transformation experiments were performed as biological triplicates and average frequencies, normalized to the wild-type values are plotted as bar graphs in panel A with the data points included. The larger number of data points for the wild-type control reflects the inclusion of this strain in all three biological replicates. The mean normalized values with standard deviations for the mutants were 0.0012 ± 0.00047 (ΔOD), 0.0.0005 ± 0.00024 (A106Y), 0.11 ± 0.03 (D111N). The p values were determined using two-sided t-tests. Panel B shows Western blots for the corresponding wild-type and mutant extracts using anti-GFP antiserum. ΔcomEA extracts were included as controls for the identity of the ComEA signals. Panel C shows typical epifluorescent images from the wild-type (BD9007) and its isogenic A106Y mutant equivalent, after transformation with rhodamine-labeled lambda bacteriophage DNA for 45-min. Competence-expressing cells were identified by CFP fluorescence (pseudocolored cyan) and detectable cell-associated rhodamine-tDNA signals are circled. For the wild type, 13 out of 42 cells exhibited at least one red dot, while for the 23 mutant cells only one barely detectable cell-associated dot was observed. The large red blotches in the lower panel are not associated with cells and are due to contaminating fluorescent material of unknown origin. The scale bar on the lower left of the image corresponds to 1 micron. The microscopy and Western blotting experiments in panels B and C were each repeated three times with closely similar results. Source data are provided as a Source Data file.
ComEA-DNA interactions are required for transformation
The ComEAGs X-ray crystal structure revealed not only the structure of the OD, but the three-dimensional shape of the ComEA DNA-binding domain. As predicted from sequence analysis and noted previously6, the ComEA DNA-binding domain structure contains two helix-hairpin-helix (HhH) motifs (Figs. 1B and 7A). Structures of canonical HhH-containing proteins showed that pairs of HhH motifs are typically connected by an α-helix, and these five helices together are referred to as (HhH)2 domains19. This linker helix that connects the HhH motifs is conspicuously absent in ComEA, and its two HhH motifs are instead connected by a loop (Fig. 7B). The ComEA DNA-binding domain is, therefore, an atypical HhH domain that forms a well-folded and compact DNA-binding core without the use of a connector helix.
Fig. 7. DNA binding to ComEA for transformation.
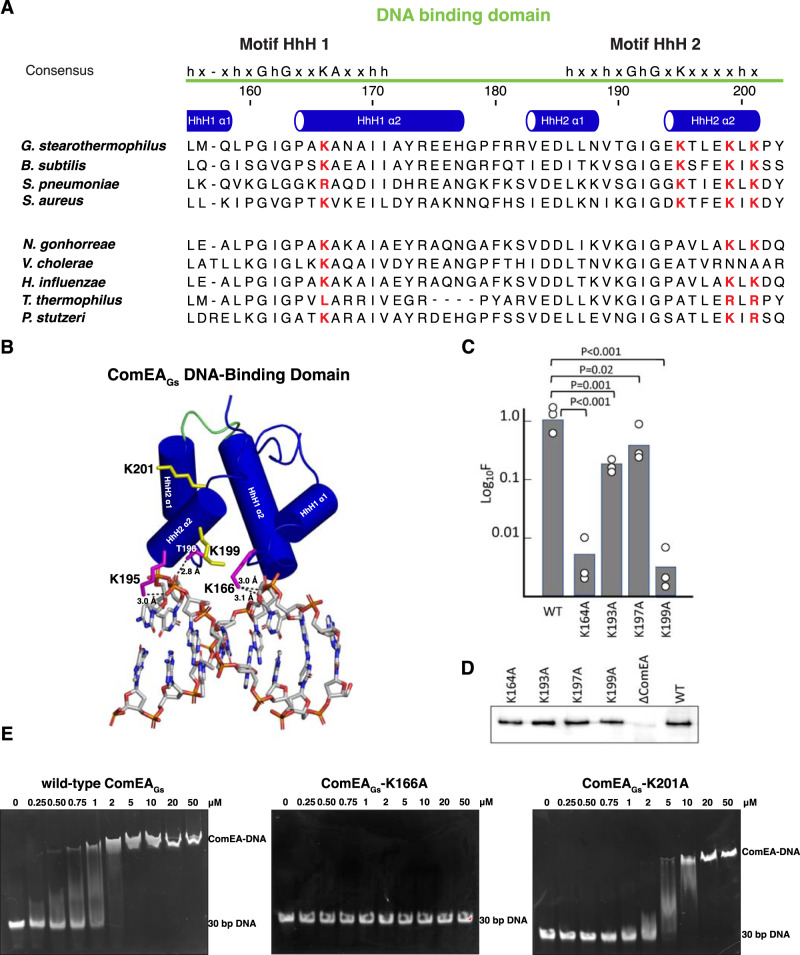
A Alignments of the HhH motifs from ComEA proteins expressed by four Gram-positive (top) and Gram-negative (bottom) bacteria that have been used for transformation studies. The four lysine residues chosen for mutagenesis are highlighted in red. Secondary structure assignments were derived from the crystal structure of ComEAGs. B Model of the ComEAGs DNA-binding domain (α-helices depicted as cylinders) in complex with dsDNA (depicted as sticks). The loop connecting the HhH1 and HhH2 is colored green. Hydrogen bonds are depicted as black dashed lines. For clarity, ComEAGs is depicted in complex with 8 bp central to the complex rather than the 15 bp used in refinement. C Transformation frequencies for the HhH motif mutants. Transformation experiments were performed as biological triplicates and average frequencies, normalized to the wild-type (BD9007) values are plotted as bar graphs with the data points included. The mean normalized values with standard deviations for the mutants were 0.0054 ± 0.004 (K164A), 0.23 ± 0.066 (K193A), 0.54 ± 0.22 (K197A), 0.0031 ± 0.0025 (K199A). The p values were determined using two-sided t-tests. D shows Western blots for the wild-type and mutant ComEA proteins obtained using anti-GFP antibody. This Western blot experiment was repeated a total of three times with nearly identical results. E EMSA analysis of wild-type ComEAGs, ComEA-K166A, and ComEA-K201A. The EMSA analysis was repeated at least two times with nearly identical results. Source data are provided as a Source Data file.
To explore ComEA-DNA interaction, we used Dali20 and HADDOCK 2.421 to generate a ComEAGs-DNA model (Fig. 7B). In addition to numerous hydrogen bonds between protein backbone nitrogens and DNA phosphate groups, which is typically how HhH domains interact with DNA19,22, we observed that the sidechains of Lys166, Lys195, and Thr196 formed hydrogen bonds with DNA phosphate groups. To begin to understand the importance of ComEA sidechain interactions to DNA binding and ComEA function, we mutated some of the corresponding interfacial residues in ComEABs (e.g., ComEABs K164 and K193) to alanine and tested their effect on transformation in B. subtilis (Fig. 7C). Furthermore, in the absence of an experimentally-determined ComEA-DNA structure, we considered the possibility that the in silico model was incomplete. For example, the ComEA-DNA model does not show possible interactions between ComEA DNA-binding domains that might occur when oligomers of ComEA bind DNA. Thus, we mutated additional ComEABs residues K197 and K199 (equivalent to ComEAGs residues K199 and K201) to alanine and tested their effects on transformation in B. subtilis.
ComEABs mutants K164A, K193A, K197A, and K199A were individually expressed from the chromosome in B. subtilis and their effects on transformation were compared to that of wild-type ComEA (Fig. 7C). Western blot analysis showed that wild-type ComEABs and the ComEABs proteins containing mutations in the HhH motifs were expressed to similar levels (Fig. 7D). These in vivo studies also showed that the K164A and K199A mutations caused more than a 2-log decrease in transformation (Fig. 7C), in contrast to the reproducible 2-4-fold effects of K193A and K197A.
In the ComEAGs-DNA model, residue K166 (equivalent to ComEABs K164) contacts DNA. Consistent with this observation and the ComEABs-K164 loss-of-function for transformation in vivo (Fig. 7C), purified ComEAGs-K166A (Fig. S4) does not bind DNA as determined using an electrophoretic mobility shift assay (EMSA) (Fig. 7E). In contrast, in the ComEAGs-DNA model, residue K201 (equivalent to ComEABs K199) does not contact DNA (Fig. 7B). ComEABs-K199A, however, displays a loss-of-function in vivo (Fig. 7C), and the purified equivalent ComEAGs-K201A (Fig. S4) displayed only about a 2-fold decrease in apparent DNA binding affinity in vitro (Fig. 7E). We suspect that ComEAGs residue K201 (ComEABs residue K199) may mediate interactions between ComEA molecules when they bind DNA or participate in DNA binding interactions not apparent in the ComEAGs-DNA model.
While additional structural studies will be required to flesh out the details of the ComEA-ComEA and ComEA-DNA intermolecular interactions within a ComEA-DNA complex, ComEABs K164 appears to play a central role. Among all residues evaluated in our studies, ComEABs K164 is the most well-conserved throughout both the Gram-positives and Gram-negatives (Fig. 7A and S2). In fact, it is at least as well conserved in ComEA as the HhH motif signature residues. The equivalent V. cholerae residue, K63, was shown by the Blokesch lab to play an important role in that organism, and in silico modeling suggested it may contact DNA6, as it appears to do in our study (Fig. 7B).
In addition to pinpointing important residues for DNA binding, the EMSAs with wild-type ComEAGs and with the K201A mutant protein (Fig. 7E) exhibit evidence of cooperativity, consistent with OD-OD interactions between ComEA molecules bound to DNA. At intermediate concentrations of protein molecules, fully shifted DNA co-exists with unshifted and partially-shifted probe. A similar bimodal distribution in EMSA experiments has been reported for SSB, another non-specific DNA binding protein with documented cooperative binding behavior23.
Discussion
This study presents several notable findings. First, we have demonstrated the existence of a multimerization domain in ComEA from B. subtilis and G. stearothermophilus, also apparent in other multi-domain ComEA proteins encoded by the Gram-positives (Figs. S2 and 8A). Our AUC results confirm that the oligomerization interface deduced from the crystal structure is important for ComEA dimerization and the existence of ring structures in crystals of both ComEABs and ComEAGs, as well as an SV-AUC experiment with the isolated OD, show that ComEA may form extended multimers in vivo (Fig. 3A–D, Fig. 4B). As determined by MW-AUC, wild-type and ComEA-A108Y form DNA complexes with different molar ratios because DNA binding favors ComEA oligomerization by bringing the protein molecules into close approximation, thus providing a cooperative effect. The EMSA results displayed in Fig. 6E provide further strong support for cooperative DNA binding. While it is tempting to speculate that ComEA multimerization in vivo generates rings like those observed in the crystal structures, there is no evidence for this.
Fig. 8. ComEABs domain architecture, and proposed model of genetic transformation in Gram-positive bacteria.
A ComEABs contains an N-terminal transmembrane region, a previously unidentified OD, and a C-terminal DNA-binding domain. B We have shown that ComEA self-associates using contacts in its ODs. After retraction of the tpilus to bring a loop of DNA into the periplasm, binding of tDNA to the ComEA DNA-binding domains followed by uptake stabilize cell association, while cross-linking of distal DNA segments by binding to adjacent ComEA molecules condenses the incoming tDNA, exerting a pulling force to bring tDNA into the periplasm. Elements of the figure were created with BioRender.com.
Finally, we have shown that both oligomerization and the DNA binding HhH motifs are needed for DNA uptake to the periplasm (Figs. 6 and 7). We have shown previously that ComEA plays important roles in B. subtilis4,18. Although the initial binding of tDNA to the transformable cell takes place to a surface exposed tpilus, this attachment is labile. When a loop of tDNA is pulled into the periplasm, presumably by disassembly of the tpilus filament9, the association of tDNA with the cell becomes stable as ComEA mediates DNA uptake to the periplasm18. In Neisseria gonorrheae and V. cholerae, the equivalent role of ComEA in uptake has been convincingly ascribed to a Brownian ratchet mechanism5,6,11,12,24, in which binding to DNA prevents retrograde diffusion. Now we have shown that although the DNA binding activity of ComEA is indeed needed for transformation, consistent with a simple ratchet, so is oligomerization, hinting at a more complex process than just the rectification of diffusion. An attractive and testable possibility is that simultaneous interaction of ComEA molecules with one another and with tDNA causes condensation of the DNA-protein complexes, providing a driving force for uptake (Fig. 8B). This would resemble the condensations recently reported for the interactions of DNA with the FoxA1 transcription factor and with the FUS protein25,26, both of which bind DNA as well as exhibiting self-interaction.
Consideration of ComEA-tDNA condensation as a force-generating machine must consider the geometry of ComEA within the periplasm. The DNA binding and oligomerization domains are connected by flexible linkers of 20–25 residues and the OD is separated from the periplasmic membrane surface by another linker of about 60 residues (Fig. 8A). Thus, there are considerable degrees of freedom for the two domains within the periplasm. This mobility may be an important component of the proposed condensation mechanism, permitting adjacent ComEA molecules to contact one another through their ODs while contacting different segments of tDNA through their DNA-binding domains, thus effectively cross-linking and condensing the tDNA (Fig. 8B). We propose therefore, that ComEA in Gram-positive bacteria may not be simply a Brownian ratchet but may function as a force-generating protein for the uptake of tDNA.
A comparison of the Gram-positive and Gram-negative ComEA molecules begs two questions: why has ComEABs evolved as a membrane protein while its single-domain orthologs are free to diffuse in the periplasm, and why do only the Gram-positive ComEA proteins contain an OD? The first question may be answered by the absence of an outer membrane in Gram-positives. Membrane anchoring will prevent the diffusion and loss of small proteins through the cell wall. Indeed, some ligand-binding proteins, e.g., those that bind amino acids for uptake, are diffusible in the periplasm of Gram-negative bacteria, but are membrane-anchored in the Gram-positives27–29. The presence of an OD only in the Gram-positives may also reflect a difference in surface structure compared to the Gram-negatives. In Gram-negative bacteria, diffusion of the transforming DNA through the characteristically thin cell wall may be rapid enough for a Brownian ratchet to effect efficient entry to the periplasm. But contacts of transforming DNA with the necessary channel in the thick peptidoglycan of Gram-positives, may provide a frictional impediment to diffusion, requiring additional pulling force. This intuitive concept is supported by a study showing that the rate of diffusion through a channel is reduced as the channel is made longer30.
Methods
ComEABs production
comEA (codons 58–205) was PCR amplified from genomic DNA from B. subtilis strain 168 using Phusion High-Fidelity DNA Polymerase (New England Biolabs) and His6-BsuF and His6-BsuR primers (Table S2). The amplified insert was cloned into the NcoI and XhoI restriction sites of pET28b with a His6 tag at its C-terminal end, generating expression vector pComEABs. E. coli BL21(D3E) transformed with pComEABs was grown at 37 °C in LB media to an OD of 0.6 in the presence of 30 μg/ml kanamycin with constant shaking at 200 rpm. ComEABs expression was then induced with 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) and grown overnight at 18 °C. The cells were then pelleted and resuspended in lysis Buffer A (30 mM Tris HCl [pH 8.0], 150 mM NaCl, 10 % glycerol) supplemented with 20 μg/ml DNase. The resuspended cells were lysed using a French press, and the lysate was clarified by centrifugation at 61,000 x g for 1 h at 4 °C. The clarified lysate was loaded on His-60 resin (Takara) equilibrated with Buffer B (30 mM Tris HCl [pH 8.0], 150 mM NaCl, 20 mM imidazole). The resin was washed with Buffer C (30 mM Tris HCl [pH 8.0], 300 mM NaCl, 20 mM imidazole) and then with Buffer B. To remove residual DNA, the resin was incubated with Buffer D (30 mM Tris HCl [pH 8.0], 150 mM NaCl, 10 mM imidazole, 1 mM MgCl2, 1 mM CaCl2 and 20 μg/ml DNase) at room temperature for 30 min. The resin was washed with Buffer B and the protein was eluted with Buffer B containing 500 mM imidazole. Protein purity was analyzed using SDS-PAGE. Fractions containing ComEABs were dialyzed overnight against Buffer E (30 mM Tris HCl [pH 8.0], 50 mM NaCl) and loaded onto a Source 15Q column equilibrated with Buffer E. The protein was eluted using a linear gradient of Buffer E and Buffer F (30 mM Tris HCl [pH 8.0], 1 M NaCl) over 20 column volumes. The fractions containing purified ComEABs were pooled and concentrated using a 3 kDa MWCO centrifugal concentrator device. The concentrated protein was loaded to a Superdex200 10/300 gel filtration column (GE Healthcare), which was equilibrated with Buffer G (15 mM citrate [pH 4.5], 100 mM NaCl). The eluted protein was concentrated using a 3 kDa MWCO centrifugal filter device and stored at −80 °C.
ComEABs selenomethionine derivative production
comEA (codons 58–205) was synthesized (codon optimized for expression in E. coli (Genscript, Inc.)) and cloned into pET28b to produce pComEABs-A101M,T126M containing a C-terminal His6 tag and mutations A101M and T126M. An overnight primary culture was grown in 1X LB overnight at 37 °C in the presence of 30 μg/ml kanamycin, shaken at 200 rpm. The cells were harvested by centrifugation at 4500 x g for 15 min. The cell pellet was washed two times with SeMET base media (SelenoMethionine Medium Base Plus Nutrient Mix - Molecular Dimensions), and finally resuspended in 10 ml SeMet media containing 50 mg/L selenomethionine and a secondary SeMet medium culture was inoculated. This culture was grown at 37 °C to an OD of 0.6 after which 0.5 mM IPTG was added to induce ComEABs expression. The cells were grown for an additional 16 h at 18 °C and then pelleted by centrifugation at 4500 x g for 15 min. Selenomethionine-derivatized ComEABs was then purified using the same protocol as described for native ComEABs.
ComEAGs production
comEAGs (codons 60–207) was PCR amplified from the genomic DNA of Geobacillus stearothermophilus (ATCC 7953) using Phusion High-Fidelity DNA Polymerase (New England Biolabs) with His-Sumo-Gsth_F and His-Sumo-Gsth_R primers (Table S2). The Gibson Assembly method (New England Biolabs) was used to clone amplified PCR product into the pTB146 vector between the SapI and XhoI sites. The resulting construct pComEAGs, had a His-Sumo tag at its N-terminus. Positive clones were verified by DNA sequencing.
His-Sumo-ComEAGs was overexpressed in E. coli BL21(DE3). Cultures were grown in LB media at 37 °C in the presence of 100 μg/ml ampicillin to an OD600 of 0.6. His-Sumo-ComEAGs expression was induced by adding 0.5 mM IPTG followed by growth overnight at 18 °C. The cells were then pelleted and resuspended in lysis Buffer A (30 mM Tris HCl [pH 8.0], 150 mM NaCl, 10% glycerol) supplemented with 20 μg/ml DNase (Goldbio). The resuspended cells were lysed in a French press, and the lysate was clarified by centrifugation at 61,000x g for 1 h at 4 °C. The clarified lysate was loaded on His-60 resin (Takara) equilibrated with Buffer B (30 mM Tris HCl [pH 8.0], 150 mM NaCl, 20 mM imidazole). The resin was washed with Buffer C (30 mM Tris HCl [pH 8.0], 300 mM NaCl, 20 mM imidazole) and then with Buffer B. To remove residual DNA, the resin was incubated with Buffer D (30 mM Tris HCl [pH 8.0], 150 mM NaCl, 10 mM imidazole, 1 mM MgCl2, 1 mM CaCl2 and 20 μg/ml DNase) at 23 °C for 30 min. The resin was washed with Buffer B and the protein was eluted with Buffer B containing 500 mM imidazole. Protein purity was analyzed using SDS-PAGE. The His-Sumo tag was then removed by the addition of 1.5 mg/ml His-Ulp1 to ComEAGs followed by dialysis against Buffer E (30 mM Tris HCl [pH 8.0], 50 mM NaCl) overnight at 4 °C. Cleaved His-Sumo and His-Ulp1 was removed by passing the sample over fresh His-60 Ni resin (Takara). The cleaved ComEAGs without a His-Sumo tag was pooled and loaded onto a Source 15Q column equilibrated with Buffer E (30 mM Tris HCl [pH 8.0], 50 mM NaCl). The protein was eluted using a linear gradient of Buffer E and Buffer F (30 mM Tris HCl [pH 8.0], 1,000 mM NaCl) over 20 column volumes. ComEAGs obtained after Source 15Q still contained impurities, which were removed using a 5 mL Hitrap Heparin HP column (Cytiva), that had been equilibrated with Buffer E (30 mM Tris HCl [pH 8.0], 50 mM NaCl). The protein was eluted with a linear gradient of 20 column volumes of Buffer E and Buffer F (30 mM Tris HCl [pH 8.0], 1 M NaCl). Fractions containing pure ComEA were pooled and then concentrated in a 3 kDa MWCO centrifugal filter device. The concentrated protein was further purified by gel filtration chromatography using a Superdex200 10/300 column (GE Healthcare), which had been equilibrated with Buffer G (10 mM Tris [pH 8], 100 mM KCl). The ComEAGs was then concentrated using a 3 kDa MWCO centrifugal filter device and stored at −80 °C.
Generation and purification of ComEAGs-OD
comEAGs-OD (codons 60–122) was PCR amplified from the genomic DNA of Geobacillus stearothermophilus (ATCC 7953) using Phusion High-Fidelity DNA Polymerase (New England Biolabs) with His-Sumo-Gsth_F and His-Sumo-OD_R primers (Table S2). The Gibson Assembly method (New England Biolabs) was used to clone the amplified PCR product into the pTB146 vector between the SapI and XhoI sites. The resulting construct pComEAGs-OD had a His-Sumo tag at its N-terminus. Positive clones were verified by DNA sequencing. ComEAGs-OD was purified using the same protocol described for wild-type ComEAGs.
Generation and purification of ComEAGs-A108Y, ComEAGs-K166A, and ComEAGs-K201A
ComEAGs-A108Y, ComEAGs-K166A, and ComEAGs-K201A were generated using a Q5 site-directed mutagenesis kit (New England Biolabs) with mutagenic oligonucleotides described in Table S2. Positive clones were confirmed by DNA sequencing. ComEAGs-A108Y, ComEAGs-K166A, and ComEAGs-K201A were purified using the same protocol described for wild-type ComEAGs, The wild-type and mutant ComEA proteins exhibited identical solubilities and behaved similarly during purification (Fig. S4). Consistent with its inability to dimerize, the A108Y mutation caused ComEAGs to elute 0.40 ml later than wild-type ComEAGs on the Superdex200 10/300 column (GE Healthcare) equilibrated with Buffer G (10 mM Tris [pH 8], 100 mM KCl) as described above.
ComEABs-A101M,T126M and ComEABs crystallization, X-ray diffraction data collection, and structure solution
Crystals of ComEABs-A101M,T126M and ComEABs, appeared within two-three days, grew to maturity within one week, and were obtained by the vapor diffusion method by mixing 1 μl of ComEABs (10 mg/ml) with an equal volume of mother liquor solution containing 22% PEG 500 and 0.1 M succinate [pH 5.5] at 20 °C. The crystals were then cryoprotected using the same mother liquor solution supplemented with 10% glycerol.
X-ray data were collected using Blu-Ice 5 at the Stanford Synchrotron Radiation Lightsource (SSRL) beamline 14–1 at cryogenic temperature using a MAR mosaic 325 CCD detector. Diffraction data for crystals of ComEABs-A101M,T126M and ComEABs were collected at 0.97900 Å and 0.97946 Å, respectively. The data were processed using the HKL software package31. Autosol32 was used to locate the position of the ComEABs-A101M,T126M Se atoms (figure of merit = 0.36) and an initial model was built. This model was used to obtain phases for the native ComEABs structure using molecular replacement in Phaser33. Subsequently, the model was built manually in COOT34 and refined against the native diffraction data using phenix.refine35. Initial rounds of refinement included simulated annealing, individual atomic coordinate, and B-factor refinement. Subsequent rounds of refinement employed individual atomic coordinate, individual B-factor, and TLS refinement. The final model contained the electron density from residues 59- or 60-122. We did not observe electron density corresponding to the engineered start Met; or ComEA residues 58, 59 (of chain G), 123–205; or the engineered C-terminal His tag. Ramachandran statistics for ComEABs were 95.84% favored, 4.16% favored, and 0.00% outliers and were calculated using Molprobity36. Structure visualization and molecular graphics were generated in PyMOL37.
ComEAGs crystallization, X-ray diffraction data collection, and structure solution
Crystals of ComEAGs typically appeared within 2–3 days, grew to maturity within one week, and were obtained by the sitting drop vapor diffusion method at 20 °C. ComEAGs at 10 mg/ml was mixed with an equal volume of mother liquor containing 30% PEG 400 and 0.1 M ammonium nitrate. X-ray data were collected in-house using StructureStudio 2.4.6 and a Rigaku Micro/Max-007HF rotating copper anode X-ray generator with a Rigaku RAXIS-IV + + detector at room temperature. Diffraction data for crystals of ComEAGs were collected at 1.5418 Å. The data were processed using the HKL software package31. Initial phases were obtained by molecular replacement in Phaser33 using the partial structure of ComEABs as a search model. The ComEAGs model was built in COOT34 and refined using phenix.refine35. Initial rounds of refinement included simulated annealing, individual atomic coordinate, and B-factor refinement. Subsequent rounds of refinement employed individual atomic coordinate, individual B-factor, and TLS refinement. The final model contained the electron density from residues 62–124 (in chains A and B) and 143–207 (in chain A). We did not observe electron density corresponding to the engineered start Met; residues 60–61, 125–142, or 143–207 (in chain B). Ramachandran statistics for ComEAGs were 96.76% favored, 2.70% favored, and 0.54% outliers and were calculated using Molbrobity36. Structure visualization and molecular graphics were generated in PyMOL37.
Analytical ultracentrifugation
Sedimentation velocity experiments (SVEs) measure the mass transport of macromolecules in a centrifugal force field in solution and observe the sedimentation and diffusion properties of all species in a mixture, and report their partial concentrations, buoyant molar masses, and shape factors. Sedimentation and diffusion transport in the ultracentrifugation cell are described by the Lamm equation, which is solved using adaptive finite element methods38,39. Whole boundary data obtained in SV experiments are fitted by linear combinations of such solutions using advanced optimization routines40–42 that are computationally intensive and are carried out on high-performance computing platforms43. SVEs were performed in a Beckman Coulter Optima AUC at the Canadian Center for Hydrodynamics at the University of Lethbridge. Data were collected using single- or multi-wavelength UV detection. 0.45 ml of sample was filled into double-sector epon-charcoal centerpieces equipped with sapphire windows and measured in intensity mode. All experiments were performed at 20 °C, and in a buffer containing 10 mM Tris [pH 8], 100 mM KCl. ComEAGs-OD was measured at 37 krpm. ComEAGs and DNA were measured at 60 krpm. MW-AUC data involving the 14-bp DNA sequence were measured at 55 krpm, while MW-AUC data involving the 40-bp DNA sequence were collected at 43 krpm. MW-AUC data were recorded in the range of 235–285 nm with 2 nm increments, providing 26 individual datasets for each sample. All data were analyzed using UltraScan 4.044. The processing of MW-AUC data is described in detailed in Henrickson et al., 202214. Briefly, data from each wavelength were analysed using the two-dimensional spectrum analysis (2DSA)40, following the workflow described in45. After generating time-synchronous SVEs, the hydrodynamic profile is spectrally deconvoluted into the molar extinction coefficient profiles of protein and DNA. Buffer density and viscosity corrections were calculated with UltraScan using the partial concentration of each buffer component. Molar extinction profiles were determined by performing separate dilution series for each protein and DNA, collecting an absorbance spectrum across the spectral range of interest (220–300 nm) using a Genesys 10 s benchtop spectrophotometer (Thermo Fisher Scientific). The dilution series of each absorbance spectrum was fitted to an intrinsic extinction profiles as described previously44. The resulting intrinsic extinction profiles were scaled to molar concentration using an extinction coefficient of 7,450 M-1 cm-1 at 280 nm for ComEAGs-A108Y and 5,960 M-1 cm-1 at 280 nm for ComEAGs (as estimated by UltraScan from protein sequence). Diffusion-corrected sedimentaton coefficient profiles were generated using the enhanced van Holde – Weischet analysis implemented in UltraScan46. Van Holde – Weischet results are shown as G(s) integral distribution plots, which display the integrated concentration of a sedimenting species on the y axis. As displayed in Figs. 4 and 5, all concentrations are normalized between 0–100% of the boundary signal. These results suggest a Kd for the monomer-dimer equilibrium between 10 and 160 μM. To determine the dissociation constant for the monomer-dimer equilibrium of ComEAGs, a sedimentation velocity experiment of an intermediate loading concentration (31.3 μM, measured at 280 nm) was fitted to a discrete monomer-dimer model using a genetic algorithm Monte Carlo analysis as described in13 and42. Detailed fitting results are shown in Table S4.
B. subtilis strains
All B. subtilis strains were ultimately derived from the laboratory strain 168. The immediate parent strain in all cases was IS75 (his leu met). All the strains were constructed by transformation and are listed in Table S3. Strains are available from the authors upon request.
B. subtilis strain construction
An E. coli plasmid (pED2232), described in18, was cut with EcoRI to liberate a 3045 bp fragment carrying a YFP-comEA construct. This fragment was isolated and cloned into pDR1664, (a thr locus insertion plasmid obtained as a kind gift from David Rudner), cut with EcoRI. The fragment contained 1,500 bp upstream from the comEA coding sequence which includes the promoter, the ribosomal binding site, and start codon of comEA. The rest of the fragment contains the YFP gene fused to the N terminus of the comEA open reading frame The resulting plasmid, pED2401, was linearized with PvuI and transformed into IS75 where it inserts into the thr locus. The native comEA open reading frame was then deleted by insertion of a kanamycin-resistance cassette-producing strain BD9007.
Mutations in ComEA were constructed using plasmid pED2401 and a Q5 Site-Directed Mutagenesis kit (New England Biolabs). The mutagenic oligonucleotides are listed in Table S2. After verification by sequencing the entire comEA reading frame, the correct plasmids were linearized with PvuI and transformed into BD5810 for insertion into thr. Finally, the native comEA reading frame was inactivated as described above.
Preparation of rhodamine-labeled DNA
0.5 μg of bacteriophage Lambda DNA (New England Biolabs) was labeled with the Mirus Label-IT rhodamine TM reagent (Mirus Bio) according to the manufacturer’s recommendations with the exception that four-fold less labeling reagent was used. The DNA was then processed through a G50 MicroSpin column (GE Healthcare) to remove excess label.
Western Blotting
Western blotting was carried out using standard methods with semidry blotting, and the nitrocellulose blots were developed using Clarity Max Western ECL substrate (Bio-Rad). The images were recorded with a Bio-Rad ChemiDoc MP imager. A 1:1000 dilution of Anti GFP Antibody (Thermofisher) was used to reveal the YFP-ComEA signals. Cultures for the preparation of extracts were grown to competence and growth was monitored in a Klett colorimeter. After collection by centrifugation, cells were resuspended in slightly different volumes to compensate for minor differences in turbidity, ensuring that equivalent total protein was loaded in each lane. Because the strains all carried an ectopic gene encoding unfused YFP, an internal loading control was present in every lane.
Transformation
All Bacillus subtilis strains were grown to competence using the 2-step method and transformed as described previously47. Transformation frequencies were determined selecting for leucine prototrophy, using biological triplicates.
Transformation with rDNA for microscopy
0.2 μg/ml rhodamine-labeled bacteriophage Lambda DNA was added to competent cultures and incubated for 45 min. 100 μl samples were washed 2X with Spizizen salts and resuspended in 100 μl Spizizen salts solution48. 1 μl of the transformed cells was mounted on a thin agarose pad for microscopy.
Microscopy
Images were acquired with Nikon Elements software and a Nikon Ti microscope, equipped with a 100× Plan Apo oil immersion objective, NA 1.40, light-emitting diode (LED) excitation sources and an Orca Flash 4.0 camera (Hamamatsu). Nikon Elements was used for data acquisition and image analysis.
Computational model of ComEAGs-DNA
The Dali server20 identified the DNA-bound structure of XPF (PDB ID 2BGW)49 as similar to the ComEAGs DNA-binding domain (residues 143–207). The ComEAGs DNA-binding domain was aligned to XPF to generate the starting model of the ComEAGs DNA-binding domain in complex with 15 bp of DNA. This model was refined in HADDOCK 2.4 using the flexible refinement in explicit solvent approach21.
Electrophoretic mobility shift assay (EMSA)
Electrophoretic mobility shift assays were performed as described previously with slight modification6. Briefly, ComEAGs at the indicated concentrations and 0.5 μM 30 bp DNA were mixed in buffer containing 10 mM Tris [pH 8], 100 mM KCl, 2.5% glycerol, 0.5 mM ethylenediaminetetraacetic acid, and 1 mM MgCl2 at room temperature for 15 min. Subsequently, 0.5 volumes of loading dye containing bromophenol blue and 2.5% glycerol was added to the samples. The samples were then immediately loaded on an 8% native polyacrylamide gel prerun at 4 °C in 0.5X TBE buffer. The gels were stained with ethidium bromide and scanned using a Bio-Rad ChemiDoc MP imager.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
X-ray diffraction data were collected at the Stanford Synchrotron Radiation Light Source. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (P41GM103393). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS, NIAID, or NIH. Support for this work was provided by National Institutes of Health Grant R01 GM057720 (M.B.N. and D.D.). Analytical ultracentrifugation experiments were supported by the Canada 150 Research Chairs program (C150-2017-00015, BD), the Canada Foundation for Innovation (CFI-37589, BD), the National Institutes of Health (1R01GM120600, BD) and the Canadian Natural Science and Engineering Research Council (DG-RGPIN-2019-05637, BD). UltraScan supercomputer calculations were supported through NSF/XSEDE grant TG-MCB070039N (BD), and University of Texas grant TG457201 (BD). The Canadian Natural Science and Engineering Research Council supports AH through a scholarship grant.
Source data
Author contributions
M.B.N and D.D. conceived the study, designed experiments, analyzed data, acquired funding, and wrote the manuscript with contributions from all authors especially B.D. and I.A. I.A. purified ComEABs and ComEAGs; designed and performed the biochemical and X-ray crystallographic studies of ComEA proteins; carried out bioinformatic analysis of ComEA; analyzed data; and generated figures and tables. J.H. performed the B. subtilis transformation studies including the Western blots. A.H. and B.D. designed and carried out the AUC and MW-AUC experiments, and generated figures and tables. F.T.K. purified ComEABs, identified ComEABs crystallization conditions, and collected native ComEABs diffraction data.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
Atomic coordinates and structure factors for ComEABs and ComEAGs have been deposited in the Protein Data Bank under accession codes 8DFK and 8DSS, respectively. Source data are provided with this paper. All AUC data (primary sedimentation velocity data, fitted model results, and reports) are stored in the UltraScan LIMS database at the Canadian Center for Hydrodynamics. Data can be shared upon request in the open source OpenAUC format supported by UltraScan (Cölfen H, Laue TM, Wohlleben W, Schilling K, Karabudak E, Langhorst BW, Brookes E, Dubbs B, Zollars D, Rocco M, Demeler B. The Open AUC Project. Eur Biophys J. 2010 Feb;39(3):347-59. 10.1007/s00249-009-0438-9. Epub 2009 Mar 19. PMID: 19296095; PMCID: PMC2812709., https://pubmed.ncbi.nlm.nih.gov/19296095/). Source data are provided with this paper.
Competing interests
The authors declare no competing interests
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
David Dubnau, Email: dubnauda@njms.rutgers.edu.
Matthew B. Neiditch, Email: matthew.neiditch@rutgers.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-35129-0.
References
- 1.Dubnau D, Blokesch M. Mechanisms of DNA Uptake by Naturally Competent Bacteria. Annu Rev. Genet. 2019;53:217–237. doi: 10.1146/annurev-genet-112618-043641. [DOI] [PubMed] [Google Scholar]
- 2.Johnston C, Martin B, Fichant G, Polard P, Claverys JP. Bacterial transformation: distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 2014;12:181–196. doi: 10.1038/nrmicro3199. [DOI] [PubMed] [Google Scholar]
- 3.Provvedi R, Dubnau D. ComEA is a DNA receptor for transformation of competent Bacillus subtilis. Mol. Microbiol. 1999;31:271–280. doi: 10.1046/j.1365-2958.1999.01170.x. [DOI] [PubMed] [Google Scholar]
- 4.Inamine GS, Dubnau D. ComEA, a Bacillus subtilis integral membrane protein required for genetic transformation, is needed for both DNA binding and transport. J. Bacteriol. 1995;177:3045–3051. doi: 10.1128/jb.177.11.3045-3051.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gangel H, et al. Concerted spatio-temporal dynamics of imported DNA and ComE DNA uptake protein during gonococcal transformation. PLoS Pathog. 2014;10:e1004043. doi: 10.1371/journal.ppat.1004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seitz P, et al. ComEA is essential for the transfer of external DNA into the periplasm in naturally transformable Vibrio cholerae cells. PLoS Genet. 2014;10:e1004066. doi: 10.1371/journal.pgen.1004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seitz, P. & Blokesch, M. DNA transport across the outer and inner membranes of naturally transformable Vibrio cholerae is spatially but not temporally coupled. mBio5, 10.1128/mBio.01409-14 (2014). [DOI] [PMC free article] [PubMed]
- 8.Stingl K, Muller S, Scheidgen-Kleyboldt G, Clausen M, Maier B. Composite system mediates two-step DNA uptake into Helicobacter pylori. Proc. Natl Acad. Sci. USA. 2010;107:1184–1189. doi: 10.1073/pnas.0909955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellison CK, et al. Retraction of DNA-bound type IV competence pili initiates DNA uptake during natural transformation in Vibrio cholerae. Nat. Microbiol. 2018;3:773–780. doi: 10.1038/s41564-018-0174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam T, et al. Competence pili in Streptococcus pneumoniae are highly dynamic structures that retract to promote DNA uptake. Mol. Microbiol. 2021;116:381–396. doi: 10.1111/mmi.14718. [DOI] [PubMed] [Google Scholar]
- 11.Hepp C, Maier B. Kinetics of DNA uptake during transformation provide evidence for a translocation ratchet mechanism. Proc. Natl Acad. Sci. USA. 2016;113:12467–12472. doi: 10.1073/pnas.1608110113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peskin CS, Odell GM, Oster GF. Cellular motions and thermal fluctuations: the Brownian ratchet. Biophys. J. 1993;65:316–324. doi: 10.1016/S0006-3495(93)81035-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demeler B, Brookes E, Wang R, Schirf V, Kim CA. Characterization of reversible associations by sedimentation velocity with UltraScan. Macromol. Biosci. 2010;10:775–782. doi: 10.1002/mabi.200900481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henrickson A, et al. Multi-wavelength analytical ultracentrifugation of biopolymer mixtures and interactions. Anal. Biochem. 2022;652:114728. doi: 10.1016/j.ab.2022.114728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horne CR, Henrickson A, Demeler B, Dobson RCJ. Multi-wavelength analytical ultracentrifugation as a tool to characterise protein-DNA interactions in solution. Eur. Biophys. J. 2020;49:819–827. doi: 10.1007/s00249-020-01481-6. [DOI] [PubMed] [Google Scholar]
- 16.Demeler B. Measuring molecular interactions in solution using multi-wavelength analytical ultracentrifugation: combining spectral analysis with hydrodynamics. Biochem (Lond.) 2019;41:14–18. doi: 10.1042/BIO04102014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorbet GE, Pearson JZ, Demeler AK, Colfen H, Demeler B. Next-Generation AUC: Analysis of Multiwavelength Analytical Ultracentrifugation Data. Methods Enzymol. 2015;562:27–47. doi: 10.1016/bs.mie.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn J, DeSantis M, Dubnau D. Mechanisms of Transforming DNA Uptake to the Periplasm of Bacillus subtilis. mBio. 2021;12:e0106121. doi: 10.1128/mBio.01061-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao X, Grishin NV. Common fold in helix-hairpin-helix proteins. Nucleic Acids Res. 2000;28:2643–2650. doi: 10.1093/nar/28.14.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holm L. Using Dali for Protein Structure Comparison. Methods Mol. Biol. 2020;2112:29–42. doi: 10.1007/978-1-0716-0270-6_3. [DOI] [PubMed] [Google Scholar]
- 21.Honorato RV, et al. Structural Biology in the Clouds: The WeNMR-EOSC Ecosystem. Front Mol. Biosci. 2021;8:729513. doi: 10.3389/fmolb.2021.729513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doherty AJ, Serpell LC, Ponting CP. The helix-hairpin-helix DNA-binding motif: a structural basis for non-sequence-specific recognition of DNA. Nucleic Acids Res. 1996;24:2488–2497. doi: 10.1093/nar/24.13.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubiel K, et al. Structural Mechanisms of Cooperative DNA Binding by Bacterial Single-Stranded DNA-Binding Proteins. J. Mol. Biol. 2019;431:178–195. doi: 10.1016/j.jmb.2018.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hepp, C. & Maier, B. Bacterial Translocation Ratchets: Shared Physical Principles with Different Molecular Implementations: How bacterial secretion systems bias Brownian motion for efficient translocation of macromolecules. Bioessays39, 10.1002/bies.201700099 (2017). [DOI] [PubMed]
- 25.Renger R, et al. Co-condensation of proteins with single- and double-stranded DNA. Proc. Natl Acad. Sci. USA. 2022;119:e2107871119. doi: 10.1073/pnas.2107871119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quail T, et al. Force generation by protein–DNA co-condensation. Nat. Phys. 2021;17:1007–1012. doi: 10.1038/s41567-021-01285-1. [DOI] [Google Scholar]
- 27.Hediger MA. Structure, function and evolution of solute transporters in prokaryotes and eukaryotes. J. Exp. Biol. 1994;196:15–49. doi: 10.1242/jeb.196.1.15. [DOI] [PubMed] [Google Scholar]
- 28.Saurin W, Dassa E. Sequence relationships between integral inner membrane proteins of binding protein-dependent transport systems: evolution by recurrent gene duplications. Protein Sci. 1994;3:325–344. doi: 10.1002/pro.5560030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam R, Saier MH., Jr. Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol Rev. 1993;57:320–346. doi: 10.1128/mr.57.2.320-346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tottori S, Misiunas K, Tshitoyan V, Keyser UF. Channel-length dependence of particle diffusivity in confinement. Soft Matter. 2021;17:5131–5136. doi: 10.1039/D1SM00289A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otwinowski Z, Minor W. [20] Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 32.Terwilliger TC, et al. Decision-making in structure solution using Bayesian estimates of map quality: the PHENIX AutoSol wizard. Acta Crystallogr D. Biol. Crystallogr. 2009;65:582–601. doi: 10.1107/S0907444909012098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCoy AJ, et al. Phaser crystallographic software. J. Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D. Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 35.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D. Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovell SC, et al. Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 37.Schrodinger, L. L. C. The PyMOL Molecular Graphics System, Version 2.4.
- 38.Cao W, Demeler B. Modeling analytical ultracentrifugation experiments with an adaptive space-time finite element solution of the Lamm equation. Biophys. J. 2005;89:1589–1602. doi: 10.1529/biophysj.105.061135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao W, Demeler B. Modeling analytical ultracentrifugation experiments with an adaptive space-time finite element solution for multicomponent reacting systems. Biophys. J. 2008;95:54–65. doi: 10.1529/biophysj.107.123950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brookes E, Cao W, Demeler B. A two-dimensional spectrum analysis for sedimentation velocity experiments of mixtures with heterogeneity in molecular weight and shape. Eur. Biophys. J. 2010;39:405–414. doi: 10.1007/s00249-009-0413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brookes, E. H. & Demeler, B. in GECCO07: Genetic and Evolutionary Computation Conference. 361-368 (Association for Computing Machinery, New York, NY, United States, 2007).
- 42.Demeler B, Brookes E. Monte Carlo analysis of sedimentation experiments. Colloid Polym. Sci. 2007;286:129–137. doi: 10.1007/s00396-007-1699-4. [DOI] [Google Scholar]
- 43.Brookes E, Demeler B. Parallel computational techniques for the analysis of sedimentation velocity experiments in UltraScan. Colloid Polym. Sci. 2008;286:139–148. doi: 10.1007/s00396-007-1714-9. [DOI] [Google Scholar]
- 44.Demeler, B. & Gorbet, G. E. In Analytical Ultracentrifugation: Instrumentation, Software, and Applications (eds Uchiyama S., Arisaka F., Stafford W. F. & Laue T.) 119-143 (Springer Japan, 2016).
- 45.Demeler B. Methods for the design and analysis of sedimentation velocity and sedimentation equilibrium experiments with proteins. Curr. Protoc. Protein Sci. 2010;Chapter 7:Unit 7 13. doi: 10.1002/0471140864.ps0713s60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demeler B, van Holde KE. Sedimentation velocity analysis of highly heterogeneous systems. Anal. Biochem. 2004;335:279–288. doi: 10.1016/j.ab.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 47.Dubnau D, Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J. Mol. Biol. 1971;56:209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- 48.Spizizen J. Transformation of Biochemically Deficient Strains of Bacillus Subtilis by Deoxyribonucleate. Proc. Natl Acad. Sci. USA. 1958;44:1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newman M, et al. Structure of an XPF endonuclease with and without DNA suggests a model for substrate recognition. EMBO J. 2005;24:895–905. doi: 10.1038/sj.emboj.7600581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Atomic coordinates and structure factors for ComEABs and ComEAGs have been deposited in the Protein Data Bank under accession codes 8DFK and 8DSS, respectively. Source data are provided with this paper. All AUC data (primary sedimentation velocity data, fitted model results, and reports) are stored in the UltraScan LIMS database at the Canadian Center for Hydrodynamics. Data can be shared upon request in the open source OpenAUC format supported by UltraScan (Cölfen H, Laue TM, Wohlleben W, Schilling K, Karabudak E, Langhorst BW, Brookes E, Dubbs B, Zollars D, Rocco M, Demeler B. The Open AUC Project. Eur Biophys J. 2010 Feb;39(3):347-59. 10.1007/s00249-009-0438-9. Epub 2009 Mar 19. PMID: 19296095; PMCID: PMC2812709., https://pubmed.ncbi.nlm.nih.gov/19296095/). Source data are provided with this paper.