Abstract
Porcine cytomegalovirus (PCMV), that is actually a porcine roseolovirus (PRV), is a common herpesvirus in domestic pigs and wild boars. In xenotransplantation, PCMV/PRV has been shown to significantly reduce the survival time of pig kidneys and hearts in preclinical trials with different non-human primates. Furthermore, PCMV/PRV has been transmitted in the first pig to human heart xenotransplantation and contributed to the death of the patient. Although transmitted to the recipient, there is no evidence that PCMV/PRV can infect primate cells including human cells. PCMV/PRV is closely related to the human herpesviruses 6 and 7, and only distantly related to the human CMV (HCMV). Antiviral drugs used for the treatment of HCMV are less effective against PCMV/PRV. However, there are well described strategies to eliminate the virus from pig facilities. In order to detect the virus and to eliminate it, highly sensitive detection methods and the knowledge of how, where and when to screen the donor pigs is required. Here, a comparative testing of organs from pigs of different ages using polymerase chain reaction (PCR)-based and immunological methods was performed. Testing young piglets, PCMV/PRV was detected effectively by PCR in blood, bronchoalveolar lavage fluid, tonsils and heart. In adult animals, detection by PCR was not successful in most cases, because the virus load was below the detection limit or the virus was in its latent stage. Therefore, detection of antibodies against selected recombinant proteins corresponding to epitopes detected by nearly all infected animals in a Western blot assay is advantageous. By contrast, immunological testing is not beneficial in young animals as piglets might have PCMV/PRV-specific antibodies obtained from their infected mother via the colostrum. Using a thoughtful combination of PCR-based and immunological methods, detection of PCMV/PRV in donor pigs for xenotransplantation is feasible and a controlled elimination of the virus by early weaning or other methods is possible.
Subject terms: Biological techniques, Immunology, Microbiology, Immunopathogenesis, Infection, Inflammation
Introduction
During the first pig heart xenotransplantation into a human patient at the University of Maryland in Baltimore, the porcine cytomegalovirus (PCMV) that is actually a porcine roseolovirus (PRV) (PCMV/PRV) was transmitted to the patient1. This virus is known to significantly reduce the survival time of pig xenotransplants in non-human primates. In the very first preclinical xenotransplantations of pig thymokidneys into baboons an activation of the PCMV/PRV was observed2. The animals showed a consumptive coagulopathy, a combination of thrombocytopenia, decreasing fibrinogen levels and uncontrollable bleeding3. These symptoms also had been seen in the Baltimore patient. A significant reduction of the survival time of pig kidneys in baboons4 and cynomolgus monkeys5 was reported. The same was demonstrated for pig hearts orthotopically transplanted into baboons: hearts from α1,3-galactosyltransferase-knockout (GTKO) pigs that express human membrane cofactor protein (CD46) and human thrombomodulin (hTM) survived no longer than 30 days when infected with PCMV/PRV, whereas baboons that received PCMV/PRV-free organs survived for 182 and 195 days6.
PCMV/PRV belongs to the genus Roseolovirus, and it is closely related to the human herpes viruses (HHV)-6A, -6B and -77. It is only distantly related to the human cytomegalovirus (HCMV), which belongs to the genus Cytomegalovirus. This data and further details on PCMV/PRV have been summarized in several reviews8–10.
PCMV/PRV is widely distributed in pig populations world-wide11–13. In a random sampling from a German slaughterhouse nearly all animals were tested positive14. PCMV/PRV was also found in wild boars15–17. PCMV/PVR is mostly acquired early in life and infection results in seroconversion and life-long latent infection11. An active infection with PCMV/PRV causes fatal systemic failure in piglets less than 3 weeks of age. The clinical symptoms of infected piglets include pneumonia, inclusion body rhinitis, and a high mortality rate. PCMV/PRV-infected sows are prone to abortion with pathological changes including edema in the heart, lungs, lymph nodes, and mesocolon11. Viral excretions were observed in young infected animals, mainly by nasal secretions. Virus excretion usually began when piglets were between 3 and 6 weeks of age and reached a maximum between 5 and 8 weeks; it was usually no longer detectable at 11-12 weeks18. Antibodies of maternal origin declined in titre between 2–3 and 5–6 weeks. Later the majority of piglets showed seroconversion as a result of virus infection17. In pigs, the epitheliochorial placenta does not allow the passage of both antibodies and immune cells from sow to fetus, so the newborn pig survival depends on the intake of maternal derived antibodies within colostrum and milk. The colostrum contains maternal IgG, IgM, and IgA, which pass through the enterocytes into the bloodstream due to a complete bowel permeability. Within 24 h, IgGs reach a serum concentration comparable to its mother19. The colostrum also contains T and B lymphocytes, which can also pass into neonate’s blood.
Based on all available data, PCMV transmission happens from mother sows to their piglets after birth and transmission via placenta was observed only under experimental settings20, but not under natural conditions18,21,22. When adult animals from a high-hygienic herd were evaluated, older animals in particular showed positivity, and within a given litter not all animals were PCMV positive. These data fit with spread through the herd by horizontal transmission, not in utero infection23. One study demonstrating generation of PCMV/PRV-negative piglets from virus positive mothers by early weaning24 shows in addition that the risk of virus transmission by the colostrum, which was still given to these animals, was zero.
In the blood of the baboons that received an orthotopic PCMV/PRV-positive heart high virus loads were measured6. The virus, obviously, replicated unrestrained in the transplanted pig heart, which was not any more under the control of the immune system of the pig. Cells producing PCMV/PRV proteins or possibly infectious virus were found in many organs of the baboon recipient25. These cells were thought to be disseminated pig cells derived from the transplant. Until now, there is no evidence that PCMV/PRV is able to infect human cells and cells of other primates26. It is still unknown how the virus contributes to the symptoms observed in non-human primates and in the Baltimore patient. An examination of the baboons after the orthotopic transplantation of PCMV/PRV-positive pig hearts showed increased levels of interleukin 6 (IL-6), tumour necrosis factor α (TNFα) and high levels of tissue plasminogen activator-plasminogen-activator-inhibitor (tPA-PAI-1) complexes6. These findings suggest a complete loss of the pro-fibrinolytic properties of the endothelial cells. It seems that viral proteins directly interact with endothelial and immune cells of the recipient eventually causing multiorgan failure.
Like all herpes viruses, PCMV/PRV is able to establish latency, which makes it difficult to detect the virus. During latency the viral DNA is stably maintained in the nucleus of the infected cells and the protein expression in latently infected cells is decreased27,28. In this situation, highly sensitive detection methods are needed and the knowledge what and how to test. The absence of a reliable method and test strategy was the reason why PCMV/PRV was not detected in the donor pig used for the Baltimore patient. Different PCR-based detection methods have been developed in order to detect the viral DNA genome2,23,29. In latency, however, the virus load is often under the detection limit of the applied PCR method. Hence, immunological assays have been developed to screen for antibodies against PCMV as indirect sign of previous virus infection. These assays were based on immunofluorescence using virus-infected cells18 or Western blot assays using recombinant fragments of the viral glycoprotein B (gB), which effectively identified PCMV-positive animals even in the case of a negative PCR result14.
Here, we investigated blood and different tissues from juvenile and adult pigs in order to identify material with the highest probability to detect viral DNA. Additionally, the PCMV/PRV load in the pig heart as a relevant xenotransplant was analysed. In parallel, we screened for antibodies using a Western blot assay based on a recombinant fragment of the gB of PCMV/PRV.
Results
Detection of PCMV/PRV in the blood and different tissues of juvenile pigs
Ten 9-weeks old piglets were tested for PCMV/PRV by real-time PCR. The following tissues were analysed: Peripheral blood mononuclear cells (PBMCs), purified from blood, PBMCs cultivated in culture medium for 5 days, cells from bronchoalveolar lavage fluid (BALF), soft palate tonsils, heart and bone marrow. In addition, full blood was tested fresh and after one freeze-thawing process. DNA was isolated and a real-time PCR (named PCMV 1, Table 1) was performed as described in Methods. In fresh blood samples seven out of eight animals (blood samples from two animals were not available) showed a cycle threshold (Ct) value between 35 and 38 (Ct cut-off 40); in frozen blood six out of eight piglets were PCMV/PRV-positive (Fig. 1, Supplementary Table 1). Isolated fresh PBMCs were positive in five out of ten animals. When the PBMCs were cultured for 5 days in fetal calf serum-containing culture medium, only one out of ten samples was positive. BALF cells from eight out of ten animals were tested positive for PCMV/PRV, seven of the positive ones showed a low Ct value (< 31), indicating a high virus load. Tonsil tissue revealed six out of ten positive animals, two of the positive had also a low Ct value. In heart samples nine out of ten piglets were positive; bone marrow showed four out of ten positive animals.
Table 1.
Primers and probes used in this study.
| PCR assay | Primer/probe | Sequence 5ʹ-3ʹ | Amplicon (base pair) | References |
|---|---|---|---|---|
| PCMV 1 | PCMV-Fwd | ACT TCG TCG CAG CTC ATC TGA | 63 | 2,17,30* |
| PCMV -Rev | GTT CTG GGA TTC CGA GGT TG | |||
| PCMV -Probe | 6FAM-CAG GGC GGC GGT CGA GCT C-BHQ | |||
| PCMV 2 |
Fr-t (nt279-299)** Rr-t (nt373-354)** Probe (nt331-350)** |
AAT GCG TTT TAC AAC TTC ACG CTG AGC ATG TCC CGC CCT AT 6FAM-CTCTAGCGGCGTCCATCACC-BHQ |
31 | |
| pGAPDH | pGAPDH-Fwd | ACA TGG CCT CCA AGG AGT AAG A | 106 | 32 |
| pGAPDH-Rev | GAT CGA GTT GGG GCT GTG ACT | |||
| pGAPDH-Probe | HEX-CCA CCA ACC CCA GCA AGA GCA CGC-BHQ1 |
*The PCMV real-time PCR was modified and performed as duplex PCR.
**nt, nucleotide, GenBank AJ222640.
PCR polymerase chain reaction, PCMV porcine cytomegalovirus, pGAPDH porcine glyceraldehyde-3-phosphate dehydrogenase, Fwd forward primer, Rev reverse primer, nt nucleotide, 6FAM 6-Carboxyfluorescein, BHQ black hole quencher, HEX Hexachloro-fluorescein.
Figure 1.
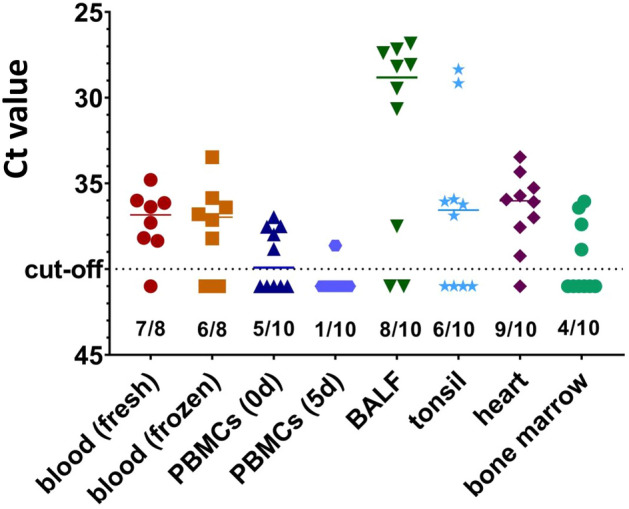
Detection of PCMV/PRV in the blood and different tissues of ten 9 weeks old piglets by real-time PCR. The number of positive/tested animals is indicated. PBMCs, peripheral blood mononuclear cells; PBMCs were tested at day 0 (0d) and after 5 days (5d) cultivation, BALF, bronchoalveolar lavage fluid.
This data show that in BALF cells the highest virus load was found. However, obtaining BALF from a pig designated for xenotransplantation is very laborious and may be associated with certain risks for the donor animal and the transplant, in this case anesthesia and short term neutropenia. The same would be true for the tonsil samples, heart biopsies (especially in the case of a heart transplantation) and bone marrow. To note, all ten animals are infected, as at least three samples of each animal were positive for PCMV/PRV (Supplementary Table 1).
Detection of PCMV/PRV in blood and PBMCs of adult pigs
Three animals were monitored for PCMV/PRV from week 12 (or 14) until week 41 (or 43) using the same real-time PCR method as used for the tissues and blood from the piglets (PCMV 1, Table 2). Fresh blood samples were positive until week 17 (pig U) or week 12 (pig V); later both animals showed no Ct values as pig W (Table 2). Fresh blood, frozen blood, fresh PBMCs, PBMCs after five days cultivation with or without PHA, from 41 or 43 weeks old animals, showed negative results for PCMV/PRV (no Ct) (Supplementary Table 2), suggesting that there is no replicating virus, replicating virus below the detection limit or the virus went to latency. Increasing the number of cells used for DNA isolation from 1 × 106 to 3 × 106 in order to increase the probability that an infected cell is among the tested ones or using an DNA isolation kit from another company which eluted the DNA at a higher concentration, did not change the result (Supplementary Table 2).
Table 2.
Results of the real-time PCR testing of blood and the Western blot analysis or three pigs at four different time points corresponding to the age of the animals.
| Animal | Gender | Real-time PCR results (Ct) | Western blot assay | ||||
|---|---|---|---|---|---|---|---|
| 14 weeks | 17 weeks | 19 weeks | 43 weeks | 14 weeks | 43 weeks | ||
| Pig U | female | 35.3 | 39.0 | No Ct | No Ct | Positive | Positive |
| 12 weeks | 15 weeks | 17 weeks | 41 weeks | 12 weeks | 41 weeks | ||
| Pig V | female | 37.6 | No Ct | No Ct | No Ct | Positive | Positive |
| Pig W | female | n.s | n.s | n.s | No Ct | n.s | Positive |
n.s.—no sample available. PCR polymerase chain reaction, Ct cycle threshold.
Positive values are in bold and negative values (no ct) in italics.
Furthermore, another PCR system (named PCMV 2, Table 1) was used, which was developed previously and found very sensitive31. Using this PCR, pigs U, V and W were also found negative (Supplementary Table 3). A log10 dilutional series of PCMV-positive porcine DNA was comparatively tested with the PCMV 1 and the PCMV 2 PCR assays. The results showed that the PCMV 1 assay is one dilution level of a log10 dilutional series more sensitive than the PCMV 2 assay (Supplementary Table 3). The PCMV 1 also revealed lower Ct values than PCMV 2 for identical samples indicating to be more sensitive than PCMV 2 assay.
Screening for antibodies against PCMV/PRV
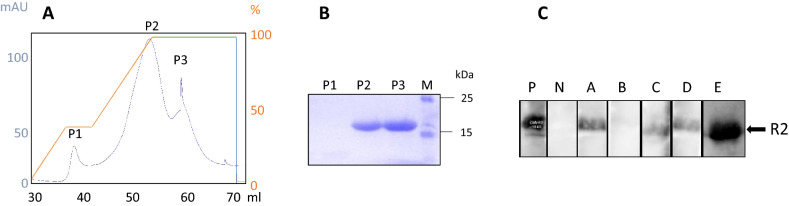
To screen for antibodies against PCMV/PVR as indirect evidence of a virus infection, a Western blot assay as described previously14 was performed. Two fragments of the glycoprotein B (gB) of PCMV/PRV, a N-terminal R1 and a C-terminal R2 (sequences 539–929 and 2771–16,663 of the gB of PCMV, Acc. No: AF268039), had been used. However, many tests in the past have shown that the vast majority of the infected animals reacted with the R2 fragment14. Therefore, we concentrated on fragment R2. The recombinant protein was produced in E. coli, purified by affinity chromatography (Fig. 2A), characterized by SDS-PAGE (Fig. 2B) and used as antigen in Western blot assays (Fig. 2C). Sera from five adult pigs (designated A to E) were examined. All animals had been tested PCMV-negative in the PCR assay (not shown). All but one of the adult animals A to E reacted with the R2 band on the blot membrane (Fig. 2C).
Figure 2.
(A) HisTrap column elution profile of the recombinant R2 fragment of gB of PCMV/PRV, (B) SDS-PAGE of the purified R2 fragment. P2 and P3 correspond to the peak fractions shown in (A), (C) Result of the Western blot analysis of sera from adult pigs A, B, C, D and E. A to E pigs were around 4 months old. P, positive control, N, negative control. Serum dilution 1:300, goat anti-pig horse reddish peroxidase was used for detection. Blots were cut prior to hybridization with antibodies during blotting, each stripe was incubated with an individual serum.
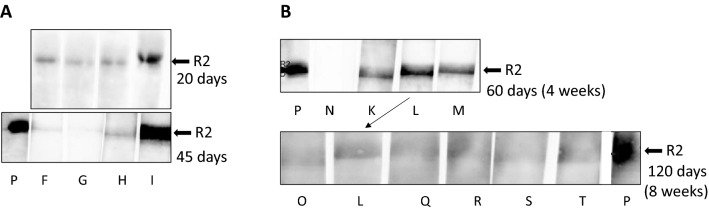
This Western blot assay was also used to screen young piglets. Sera from piglets designated F to I, which had been tested negative by PCR (not shown), gave a weak positive reaction in the Western blot assay at day 20 after birth (Fig. 3A, upper panel). We proposed that this result may be due to maternal antibodies derived from the colostrum that the animals received from their infected mother sow. When we tested the same animals again 45 days after birth, a rapid decline of the antibody concentration in two of the four animals was observed. Only in the serum of pig I the antibody titer increased, indicating that this animal is infected and reacts with own antibodies against the infection (Fig. 3A, lower panel).
Figure 3.
Detection of antibodies against the recombinant R2 fragment of the glycoprotein B in young animals. (A) Detection of antibodies against the R2 protein in the sera of four animals (F, G, H, I) after 20 days and after 45 days. (B) Detection of antibodies against the R2 fragment in the sera of eight animals (K, L, M, O, Q, R, S, T) from the same litter at day 60 and day 120. The serum from animal L was tested on both days. Animals O, L, Q, R and S, were male, T was a female. P, positive control, N, negative control. Serum dilution 1:300, goat anti-pig horse reddish peroxidase was used for detection. Blots were cut prior to hybridization with antibodies during blotting, each stripe was incubated with an individual serum.
To confirm this result, a Western blot analyses was performed with sera from eight animals from a single litter at day 60 and day 120 (Fig. 3B). A significant decline of the antibody titer was observed, indicating that the maternal antibodies transmitted by the colostrum disappeared.
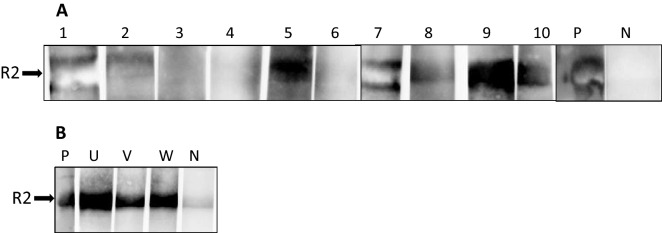
When sera from the ten nine weeks old piglets (animals 1–10), which were found positive in at least in three tissues when tested by PCR (Fig. 1, Supplementary Table 1) were analysed in the Western blot assay, seven of the animals were positive (Fig. 4A). Four of them showed a very strong reaction. Since antibodies from the colostrum disappeared at latest 8 weeks after birth (Fig. 3A,B), this strong reaction can only be explained by a de novo antibody production in response to the virus infection.
Figure 4.
Result of the Western blot analysis using the recombinant R2 fragment of the glycoprotein B of PCMV/PRV. (A) nine weeks old animals (number 1 to 10) with positive PCR results in at least one tissue (Fig. 1, Supplementary Table 1) and (B) the pigs U, V, and W, 43 or 41 weeks old and which were PCR-negative (Table 2). P, positive control, N, negative control. Serum dilution 1:300, goat anti-pig horse reddish peroxidase was used for detection. Blots were cut prior to hybridization with antibodies during blotting, each stripe was incubated with an individual serum.
Sera from adult pigs U, V, and W, which were older than 41 weeks, and which were tested negative for PCMV/PVR in real-time PCR from week 17 on (Table 2) were all strongly positive in the Western blot assay (Fig. 4B). Hence, the animals U, V, and W were infected with PCMV/PVR despite the negative PCR result.
Discussion
In the first transplantation of a pig heart into a human patient a pig virus, PCMV/PRV, was transmitted to the patient1. This underlines the requirement to pay more attention to the aspects of virus safety in xenotransplantation. Here, we demonstrate how difficult it is to detect this virus, especially in his latent state. But we also demonstrate that using a combination of PCR-based and immunological assays, it is possible to detect the virus and using these methods also to eliminate PCMV/PRV.
There are three main aspects of testing: how, when and where. First, how to test: The modified real-time PCR developed by Mueller et al.2 is a sensitive and specific tool to detect the virus. We modified the method performing a duplex real-time PCR that detect the PCMV/PRV DNA and simultaneously an internal control, the cellular gene porcine glycerinaldehyd-3-phosphat-dehydrogenase (pGAPDH)30. The pGAPDH indicates a successful extraction of DNA and a correct performance of the PCR assay. In addition, we developed several new real-time PCRs and nested PCRs and analyzed Göttingen minipgs (GöMP) which are potential donor pigs for clinical pig islet cell transplantations in Germany31,33. The sensitivity of these tests was down to 2–5 DNA copies. We previously compared a real-time PCR, which was newly developed (here called PCMV 2), with a nested PCR and a real-time PCR performed in an American commercial laboratory23. Testing 10 GöMP, our real-time PCR detected PCMV/PRV in 4 sera, while our nested PCR detected the virus in 3 sera, and the commercial real-time PCR detected no virus in all pigs. Hence, it is not only important to have a PCR or a real-time PCR method that detects the virus in principle but also to know its sensitivity, e.g., how many virus particles can be detected by this approach. When we compared the modified duplex real-time PCR using primers and probe developed by Mueller et al.2 (here called PCMV 1), with the duplex real-time PCR PCMV 2, we found that their sensitivity is approximately the same (Supplementary Fig. 3).
Second, when to test: Our results show that in young piglets the virus is detected by real-time PCR easily, but not by antibody detection: If the mother sow is infected there are maternal antibodies from colostrum in the blood (Fig. 3). Approximately after 45 days the titer started to decline and after 120 days the maternal antibodies almost completely disappeared. This result correlate well with other findings18. In adult pigs, it is nearly impossible to detect the virus in blood samples by PCR, nested PCR or real-time PCR if the virus is in its latent state. However, our Western blot assay demonstrated that the pigs had developed antibodies against PCMV/PRV indicating an infection (Table 2, Fig. 4B). Due to the latent state of the virus a once infected pig has to be considered PCMV/PRV-positive for its life time.
Third, where to test: For diagnostic purposes blood is often used. In the present study, PCMV/PRV was detected by real-time PCR in the blood from seven out of eight young animals (Fig. 1), but in none of the adult animals (Table 2), similar to former results33. It is important to note, that all ten animals tested were PCMV/PRV-positive (Supplementary Table 1). However, the tissues, which were found positive differed from animal to animal (Fig. 1 and Supplementary Table 1). This shows that the distribution of the virus in different animals (which were housed together) varied. In a previous study PCMV/PRV was found mainly in the kidney, but also in the pancreas, liver and spleen of a genetically modified pig25. In parallel, the organs from three related pigs of the same age were screened: In two animals the highest virus load was found in the nose, in one animal in the spleen. Lower amounts were found in the heart and kidney. Altogether, this data shows, that the distribution of PCMV/PRV is very complex and difficult to predict25. When tissue samples of these animals were tested by immunohistochemistry using specific antibodies no PCMV/PRV was detected in the liver, spleen, kidney, heart, lung and lymph nodes. This indicates the low sensitivity of immunohistochemistry and the low or more likely the absent expression of viral proteins in the latent state of virus infection25.
In previous publications PCMV/PRV was found in infected embryos mainly in leptomeningeal cells, hepatic sinusoidal cells, peritoneal macrophages, periosteal cells and occasional alveolar cells, but the placenta did not appear to be a primary site of viral replication21. If seropositive sows were infected again with the virus a superinfection occurred that in a few cases resulted in fetal deaths and congenitally infected pigs. However, a superinfection cannot be categorically distinguished from a reactivation of the virus. PCMV/PRV was found in the capillary endothelium and alveolar macrophages from the lungs of some fetuses22. Virus was also isolated from the nasal mucosa. Some fetuses yielded virus from the spleen and central nervous system, the endothelial cells of the cerebrum showed positive immunofluorescence22.
Other authors investigated human decay accelerating factor (CD55) transgenic pig herds being bred for xenotransplantation, but kept under conventional farm conditions. They identified PCMV/PRV in a wide range of organs including potential xenotransplants (liver, kidney and heart). The spleen was PCMV/PRV DNA positive in all infected pigs34. In another study five pig tissues (lung, liver, salivary gland, kidney, and gut) derived from multiple animals from a well-characterized herd of inbred miniature swine were tested for PCMV/PRV using a PCR. Lung, liver, salivary gland, and kidney were always positive, the gut tissue was consistently PCR negative35.
In order to test whether low-invasive or non-invasive methods can be applied which would allow performing regular follow-ups and further breeding of the animals, we tested sera, ear biopsies, as well as oral and anal swabs collected from ten 10-day-old Aachen minipigs (AaMP) by a sensitive nested PCR as well as by uniplex and duplex real-time PCR36. PCMV/PRV DNA was detected most frequently in oral and anal swabs. To note, comparison of duplex and uniplex real-time PCR systems demonstrated a higher sensitivity of uniplex real-time PCR when the copy numbers of the target genes were low (less 200). Please note, the piglets were 10 days old.
We were surprised that only one out of ten animals was identified PCMV/PRV-positive when gradient-purified PBMCs were cultured for 5 days (Fig. 1A). In contrast, screening of fresh PBMCs of these animals yielded five out of ten animals positive. In a previous study cultivation for five or seven days even resulted in an activation of the virus and a higher probability to detect the virus25. Obviously, in the experiment here the virus-positive cells died during cultivation or adherent PCMV/PRV-positive macrophages were lost when testing.
When pigs from a multiplier unit operated by Spring Point Project were screened for PCMV/PRV, the virus was detected in the spleen of all tested six animals, isolated islet cells from three of the six animals were positive and two of these three cases were even positive in cultured islets23.
Recently, we demonstrated that there is a cross-reactivity between antibodies directed against PCMV/PRV and HHV-6 and that sera from HHV-6-positive humans react in our Western blot assay37. It will be interesting to learn, whether the antibodies against HHV-6 present nearly in each human individual will have an impact on PCMV/PRV transmission, either reducing the virus transmission or even enhancing it for example by enhancing antibodies, i.e., antibody-dependent enhancement (ADE) or by harmful antibodies directed against the PCMV antigens expressed in the pig transplant. At the moment there is no information about the presence of antibodies against HHV-6 and PCMV in the Baltimore patient, but it was shown that the patient was HHV-6 positive1. It is important to note that our Western blot assay using the two recombinant protein fragments of the gB of PCMV/PRV can only be used for the detection of an PCMV/PRV transmission when the patient is HHV-6 negative. However, if PCMV/PVR replicates well and the virus load is high, the viral DNA can be detected easily by PCR as was seen in the Baltimore patient.
There is another interesting aspect learned from the transmission of PCMV/PRV into non-human primate recipients and into the Baltimore patient. Numerous publications described a systemic inflammation in xenotransplant recipients (SIXR)38–40 and a detrimental role of inflammation in pig orthotopic heart xenotransplantation41. SIXR is characterized by activated innate immune cells expressing tissue factor (TF). C-reactive protein, fibrinogen and interleukin 6 (IL-6) are extremely increased. The increased C-reactive protein levels are observed prior the development of consumptive coagulopathy38–41. Some of these features were also found in the patient in Baltimore and in baboons which received a PCMV-positive pig transplant, for example increased levels of IL-6 and TNFα and disruption of coagulation2–6. In vitro, an activation of the porcine TF in porcine aortic endothelial cells by PCMV/PRV infection was observed3. Therefore, it will be of great importance to dissect the effects of undetected PCMV/PRV infection (not tested or tested with the suboptimal method) and reaction against the xenotransplant.
An excellent testing is also important for monitoring antiviral treatment. Antiviral drugs which are successfully used to treat HCMV infections in humans such as ganciclovir, valacyclovir, cidofovir have also been show the be to some extend effective against PCMV/PRV in vitro42. However, the situation in vivo is different43. PCMV viral loads were unaltered by ganciclovir treatment. Only cidofovir and foscarnet might have therapeutic efficacy for PCMV in vivo in achievable concentrations, although these agents often carry significant toxicity in transplant recipients. Ganciclovir, valacyclovir and cidofovir were used to treat the Baltimore patient1. After the first application of cidofovir at day 43, the PCMV/PRV load in the patient continued to increase, but after the second application (day 49) it seemed to decrease. However, at day 43 the patient received also an injection of human IgG. This preparation contained most likely antibodies against HHV-6 due to its high seroprevalence of > 90% in the human population44. We have recently shown37 that antibodies against HHV-6 are cross-reactive against PCMV/PRV. Therefore, these antibodies could be the reason for the decrease in virus load.
Although high PCMV/PRV virus load was detected in the patient and some of the clinical features resemble the ones observed in the baboons with PCMV/PRV-positive hearts1,6, it remains unclear as to which part the PCMV/PRV contributed to the death of the patient, who was already very ill before transplantation45. One of the factors, which may have been also contributed is the application of human IgG46. As was found later, this preparation contained antibodies against pig tissues.
It would be useful to have an effective drug against PCMV or even a vaccine able to prevent virus transmission. But the virus transmission can be prevented by easy methods such as early weaning24. Since under natural conditions no transmission of the virus via the placenta was observed18,23, cesarean delivery34 or embryo transfer are not necessary to remove the virus. Even colostrum deprivation does not seem to be required, since early weaning alone was successful24. However, isolated breeding and housing of the animals + is required to avoid a re-entry of the virus into a PCMV/PRV-free facility. A re-entry of the virus may also happen by cloning or somatic cell nuclear transfer SCNT using oocytes from slaughterhouses, which normally are PCMV/PRV-positive47.
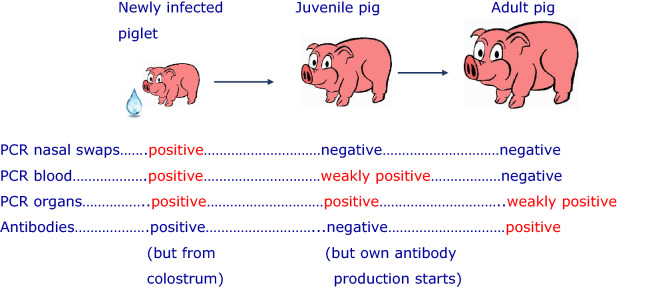
Taken together, it is possible to detect and eliminate PCMV to guaranty safe future clinical xenotransplantations using effective methods (Fig. 5). There is no need to re-open the discussion that xenotransplantation may introduce new zoonotic viruses into the human population43.
Figure 5.
Detection strategies for PCMV/PRV in pigs of different age and infection status. The results of a given method marked in red represent a reliable result when used at the correct time with the correct test material. If possible, PCR and antibody testing should be performed simultaneously and the tests should be repeated, especially in the case of newborn and juvenile animals.
Methods
Animals, blood and other tissues
For the molecular biology detection of PCMV, samples from ten Large White x Landrace crossbred pigs (9 males, 1 female) were repurposed from an unrelated animal experiment. Piglets were purchased from a commercial provider with the age of 5 weeks and maintained in two separated groups at the animal facility at the Free University Berlin. At the age of nine weeks, lithium-heparin (LH) blood and serum were sampled by heart puncture of sedated animals (Ursotamin; Serumwerk Bernburg AF 20 mg/kg body weight (BW), Azaperon; Janssen-Cilag GmbH 2 mg/kg BW and Xylavet; CP-Pharma Handelsgesellschaft mbH 36 mg/kg BW) before euthanizing the pigs by intracardial injection of T61 (10 mg/kg BW; Intervet, Unterschleißheim, Germany). A portion of LH blood was processed immediately, while the other portion was frozen away at − 20 °C. Serum samples were stored at − 20 °C.
Bronchoalveolar lavage (BAL) was performed on isolated stanched lungs by inserting a tracheal tube through an incision in the right bronchus and flushing the lung lobe with 250 mL phosphate buffered saline (PBS) containing 2 mM EDTA. BAL fluid (BALF) was filtered (70 µm cell strainer) and BALF cells were pelleted and counted before being stored at − 20 °C. A sample of heart muscle tissue was taken from the cardiac apex. Tonsils of the soft palate were sampled as major pharyngeal mucosal-associated lymphoid tissue. Tonsillar tissue and heart tissue samples were shock-frozen in liquid nitrogen after their removal and stored at − 80 °C until further processing. For isolating bone marrow cells five posterior ribs were extracted per pig and stored over night at 4 °C. The following day, muscular and connective tissues were removed and the outer surface was treated with 70% ethanol before cutting the ribs in 2 cm bone fragments and flushing out the bone marrow with RPMI-1640 (PAN-Biotech GmbH) supplemented with 2 mM EDTA. Bone marrow cells were pelleted by centrifugation and resuspended with ice cold ammonium-chloride-potassium lysing buffer for 2 min at room temperature to lyse red blood cells before cell numbers were determined. Bone marrow cells were frozen at − 80 °C.
Sera from young piglets at the age of 20, 45 and 60 days were obtained from a pig facility at the Chair of Livestock Biotechnology, Technical University Munich, School of Life Sciences Weihenstephan, Germany) (courtesy of Dr. Konrad Fischer). Blood and PBMCs from adult pigs at different time points from 12 to 43 weeks were obtained from the Teaching and experimental farm of the Faculty of Veterinary Medicine of the Ludwig Maximilians University Munich, Germany) (courtesy of Dr. Barbara Keßler and Prof. Eckard Wolf).
Preparation and cultivation of peripheral blood mononuclear cells (PBMCs)
To separate the PBMCs from the fresh EDTA or LH blood samples of the pigs, a density gradient medium (Pancoll human, density: 1.077 g/mL, PAN Biotech GmbH, Aidenbach, Germany) were poured in a centrifugation tube and carefully overlayed with EDTA and LH blood, respectively. The blood-medium mix was centrifugated for 25 min at 900 × g, the PBMCs were separated and washed twice with PBS by centrifugation at 300 × g for 10 min. For cultivation, 1 × 106 PBMCs per sample were seeded in 12-well plates (Sarstedt AG & Co. KG, Nümbrecht, Germany), solubilized in 2 mL Roswell Park Memorial Institute medium (RPMI-1640, PAN-Biotech GmbH, Aidenbach, Germany) and harvested after 5 days at 37 °C, 5% CO2. In a parallel experiment, phytohemagglutinin (PHA-L) (Invitrogen, Whaltham, MA, USA) was added at a concentration of 2.5 µg/mL to the PBMC culture.
DNA extraction
DNA of most samples were purified by using the DNeasy Blood & Tissue kit (Qiagen, Hilden, Germany). According to the results of pre-tests (data not shown here), a volume of 80 µL of fresh and frozen blood were used for the nucleic acid extraction and further processing was carried out according to the manufacturer's recommendations. For the nucleic acid extraction of the PBMC and BALF samples, 1 × 106 cells were used. The DNA extraction of the organs (tonsils, heart, bone marrow) was based on 20 mg of each tissue. All samples were eluted in 200 µL elution buffer. In addition, two alternative methods of DNA isolation were performed: To confirm the negative results, a higher number of PBMCs (3 × 106 cells) (modification 1, Supplementary Table 2) and the innuPREP Virus DNA/ RNA Kit (Analytik Jena, Jena, Germany) (modification 2) were used according to the manufacturer´s instructions. RNA/DNA from 3 × 106 cells was eluated in 30 µL nuclease-free water. The samples were stored at − 20 °C until further processing.
Real-time polymerase chain reaction (PCR)
The detection of PCMV/PRV was performed by two real-time PCR assays (PCMV 1 and PCMV 2) based on TaqMan technology as described previously31,30. All experiments were performed with the SensiFAST Probe No-ROX kit (Meridian Bioscience, Cincinnati, OH, USA) at the qPCR cycler qTOWER3 G (Analytik Jena, Jena, Germany). All assays were performed as duplex real-time PCR using the reference gene porcine pGAPDH and using a specific primer–probe mixture (Table 2). A reaction volume of 20 µL was prepared containing 1.8 µL of PCMV-FAM mix and 1.8 µL of pGAPDH-HEX mix as internal control and 4.0 µL of extracted DNA. The reaction for the PCMV 1 PCR was carried out for 2 min at 50 °C for activation, 10 min at 95 °C followed by 45 cycles comprising 15 s at 95 °C for denaturation and 60 s at 60 °C for annealing and elongation. For the PCMV 2 PCR the following conditions for amplification were used: denaturation at 95 °C for 5 min and 45 cycles of amplification with denaturation at 95 °C for 10 s, annealing at 59 °C for 20 s and extension at 60 °C for 25 s.
Protein expression and purification
The R2 fragment of the gB of PCMV/PRV was purified as described14. In brief, 500 mL E. coli BL21 cells containing the pET16b expression vector encoding PCMV-R2 were induced with 1 mM isopropyl-β-D-thiogalactopyranosid (IPTG) (Roth, Karlsruhe, Germany) at an OD600 of 0.7 and cultivated at 37 °C for 2 h. Cells were harvested, centrifuged, resupended in PBS and centrifuged again. 2 g wet cells were dissolved in 10 mL 8 M urea, 0.5 M NaCl, 15 mM imidazole, 20 mM Tris pH 7.5. After a 20 min spin at 50,000 × g the supernatant is applied to a 1 mL HisTrap HP column installed on a Äkta Prime Plus system (both GE Healthcare, Chicago, Illinois, USA) using the HisTrap affinity application template with the following buffers: (1) 6 M urea, 0.5 M NaCl, 15 mM imidazole, 20 mM Tris pH 7.5; (2) 6 M urea, 0.5 M NaCl, 500 mM imidazole, 20 mM Tris pH 7.5. PCMV-R2 eluted in two peaks as was shown by polyacrylamide-gel electrophoresis. Both fractions were used for PCMV antibody detection.
Western blot assay
For the detection of antibodies against PCMV/PRV, the Western Blot assay designed by Plotzki et al.14 was re-established. The purified R2 protein was characterized by a sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE). The protein was dissolved in sample buffer (375 mM Tris–HCl, 60% glycerol, 12% SDS, 0.6 M DTT, 0.06% bromophenol blue) and denatured for 5 min at 95 °C prior to electrophoresis. The SDS PAGE was run in a Mini-Protean Tetra Vertical Electrophoresis Cell (Bio-Rad Laboratories, Incs., Hercules, CA, USA) using a 12% polyacrylamide gel and the PageRuler prestained protein ladder (Thermo Fisher Scientific, Waltham, USA). The protein was transferred for 100 min to a Polyvinylidene fluoride membrane (ROTI PVDF, 8989.1, Roth, Karlsruhe, Germany) by electroblotting (100 mA) using the electroblotting device of peqlab Biotechnologie GmbH. Blots were cut prior to hybridization with antibodies during blotting, each stripe was incubated with an individual serum. After electroblotting the membrane was blocked for 1 h at 4 °C in 5% non-fat dry milk (Roth, Karlsruhe, Germany) in PBS with 0.05% Tween 20 (Roth, Karlsruhe, Germany) (blocking buffer). Membrane was cut into strips and incubated with sera diluted 1:300 in blocking buffer at 4 °C overnight. Afterwards, washing was performed with 0.05% PBS-T three times for 10 min each. Polyclonal goat anti-pig immunoglobulin G (IgG) Fc Secondary Antibody HRP (Invitrogen by Thermo Fisher Scientific, Waltham, USA) was diluted 1:20.000 in blocking buffer and the strips were incubated for 1 h at room temperature, followed by three washing steps for 10 min each. Detection of the signal following incubation with the ECL Western Blotting Substrate (Cytiva, Amersham) was done on the imaging device FUSION-SL 3500 WL (peqlab Biotechnologie GmbH).
Ethics statement
Animals were cared for in accordance with the principles outlined in the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes and the German Animal Welfare Law. All methods are reported in accordance with ARRIVE guidelines. Ethical approval was obtained from the State Office of Health and Social Affairs Berlin, Germany (Landesamt für Gesundheit und Soziales Berlin, Germany) for sampling tissues from healthy pigs (Regulation Number T0002/17) and for experimental procedures (Approval Number G0278/18). Animals shown in Fig. 1 were sedated using Ursotamin and Xylavet and euthanizing by intracardial injection of T61 as described in Material and methods. It is important to note that all materials were obtained from animals being in another experiment.
Supplementary Information
Acknowledgements
This research was supported by the Deutsche Forschungsgemeinschaft, TRR127. We would like to thank Dr. Konrad Fischer, Technical University Munich, Dr. Barbara Keßler and Prof. Eckard Wolf, Ludwig Maximilians University Munich for samples of pig sera and cells.
Author contributions
S.Hal. and S.Han. performed the PCR-based, S.Han. the Western blot detection methods, L.K. performed the expression, purification and characterization of the recombinant R2 protein, F.E. was responsible for the acquisition of the samples from the piglets, J.D. planned the study and was writing the original draft preparation, S.Hal., S.Han. L.K., F.E. and B.K. were reviewing and editing the manuscript, J.D. and B.K. were supervising the project and were responsible for funding acquisition.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The dataset generated and analyzed during the current study are all available in this manuscript including the Supplementary Tables and Supplementary Information files showing full-length gels and blots.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-25624-1.
References
- 1.Griffith BP, Goerlich CE, Singh AK, Rothblatt M, Lau CL, Shah A, Lorber M, Grazioli A, Saharia KK, Hong SN, Joseph SM, Ayares D, Mohiuddin MM. Genetically modified porcine-to-human cardiac xenotransplantation. N. Engl. J. Med. 2022;387(1):35–44. doi: 10.1056/NEJMoa2201422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller NJ, et al. Activation of cytomegalovirus in pig-to-primate organ xenotransplantation. J. Virol. 2002;76(10):4734–4740. doi: 10.1128/JVI.76.10.4734-4740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gollackner B, et al. Porcine cytomegalovirus and coagulopathy in pig-to-primate xenotransplantation. Transplantation. 2003;75(11):1841–1847. doi: 10.1097/01.TP.0000065806.90840.C1. [DOI] [PubMed] [Google Scholar]
- 4.Yamada K, et al. Porcine cytomegalovirus infection is associated with early rejection of kidney grafts in a pig to baboon xenotransplantation model. Transplantation. 2014;98(4):411–418. doi: 10.1097/TP.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sekijima M, et al. Results of life-supporting galactosyltransferase knockout kidneys in cynomolgus monkeys using two different sources of galactosyltransferase knockout Swine. Transplantation. 2014;98(4):419–426. doi: 10.1097/TP.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denner J, et al. Impact of porcine cytomegalovirus on long-term orthotopic cardiac xenotransplant survival. Sci Rep. 2020;10(1):17531. doi: 10.1038/s41598-020-73150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denner, J. et al. Comparative Analysis of Roseoloviruses in Humans, Pigs, Mice, and Other Species. Viruses.11(12), (2019). [DOI] [PMC free article] [PubMed]
- 8.Denner J. Xenotransplantation and porcine cytomegalovirus. Xenotransplantation. 2015;22(5):329–335. doi: 10.1111/xen.12180. [DOI] [PubMed] [Google Scholar]
- 9.Fishman JA. Infectious disease risks in xenotransplantation. Am. J. Transplant. 2018;18(8):1857–1864. doi: 10.1111/ajt.14725. [DOI] [PubMed] [Google Scholar]
- 10.Denner J. Reduction of the survival time of pig xenotransplants by porcine cytomegalovirus. Virol. J. 2018;15(1):171. doi: 10.1186/s12985-018-1088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edington N. Porcine cytomegalovirus. In: Straw BE, Allaire SD, Mengeling WL, Taylor DJ, editors. Diseases of Swine. Iowa State University Press; 1999. [Google Scholar]
- 12.Liu X, Liao S, Zhu L, Xu Z, Zhou Y. Molecular epidemiology of porcine cytomegalovirus (PCMV) in Sichuan province, China: 2010–2013. PLoS ONE. 2013;8:e64648. doi: 10.1371/journal.pone.0064648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutra MC, et al. Molecular survey of Cytomegalovirus shedding profile in commercial pig herds in Brazil. J. Infect. Dev. Ctries. 2016;10(11):1268–1270. doi: 10.3855/jidc.7367. [DOI] [PubMed] [Google Scholar]
- 14.Plotzki E, Keller M, Ivanusic D, Denner J. A new Western blot assay for the detection of porcine cytomegalovirus (PCMV) J. Immunol. Methods. 2016;437:37–42. doi: 10.1016/j.jim.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 15.De Maio FA, et al. Molecular detection of Porcine cytomegalovirus (PCMV) in wild boars from Northeastern Patagonia, Argentina. Rev. Argent. Microbiol. 2021;53(4):325–332. doi: 10.1016/j.ram.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Shcherbakov AV, et al. Monitoring of infectious diseases among wild boars. Vopr Virusol. 2007;52(3):29–33. [PubMed] [Google Scholar]
- 17.Hansen S, Franzo G, Menandro ML, Krabben L, Marino SF, Kaufer B, Denner J. Prevalence of the porcine cytomegalovirus virus (PCMV), a porcine roseolovirus, in wild boars in Italy and Germany. Res. Sq. 2022 doi: 10.21203/rs.3.rs-1898102/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plowright W, Edington N, Watt RG. The behaviour of porcine cytomegalovirus in commercial pig herds. J. Hyg. (Lond.) 1976;76(1):125–135. doi: 10.1017/S0022172400055017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The immune system and immunity in swine: maternal and neonatal immunity (assessed October, 3, 2022) https://www.pig333.com/articles/the-immune-system-and-immunity-in-swine-maternal-and-neonatal-immunit_13708/.
- 20.Edington N, Watt RG, Plowright W. Experimental transplacental transmission of porcine cytomegalovirus. J. Hyg. (Lond.) 1977;78:243–251. doi: 10.1017/S0022172400056138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edington N, Wrathall AE, Done JT. Porcine cytomegalovirus (PCMV) in early gestation. Vet. Microbiol. 1988;17(2):117–128. doi: 10.1016/0378-1135(88)90003-X. [DOI] [PubMed] [Google Scholar]
- 22.Edington N, Broad S, Wrathall AE, Done JT. Superinfection with porcine cytomegalovirus initiating transplacental infection. Vet. Microbiol. 1988;16(2):189–193. doi: 10.1016/0378-1135(88)90043-0. [DOI] [PubMed] [Google Scholar]
- 23.Abrahante JE, Martins K, Papas KK, Hering BJ, Schuurman HJ, Murtaugh MP. Microbiological safety of porcine islets: Comparison with source pig. Xenotransplantation. 2011;18(2):88–93. doi: 10.1111/j.1399-3089.2011.00632.x. [DOI] [PubMed] [Google Scholar]
- 24.Egerer S, et al. Early weaning completely eliminates porcine cytomegalovirus from a newly established pig donor facility for xenotransplantation. Xenotransplantation. 2018;25(4):e12449. doi: 10.1111/xen.12449. [DOI] [PubMed] [Google Scholar]
- 25.Fiebig U, Abicht JM, Mayr T, Längin M, Bähr A, Guethoff S, Falkenau A, Wolf E, Reichart B, Shibahara T, Denner J. Distribution of porcine cytomegalovirus in infected donor pigs and in baboon recipients of pig heart transplantation. Viruses. 2018;10(2):66. doi: 10.3390/v10020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tucker AW, Galbraith D, McEwan P, Onions D. Evaluation of porcine cytomegalovirus as a potential zoonotic agent in Xenotransplantation. Transplant. Proc. 1999;31:915. doi: 10.1016/S0041-1345(98)01833-8. [DOI] [PubMed] [Google Scholar]
- 27.Mueller NJ, Denner J. Porcine cytomegalovirus/porcine roseolovirus (PCMV/PRV): A threat for xenotransplantation? Xenotransplantation. 2022;29(5):e12775. doi: 10.1111/xen.12775. [DOI] [PubMed] [Google Scholar]
- 28.Cohen JI. Herpesvirus latency. J. Clin. Invest. 2020;130(7):3361–3369. doi: 10.1172/JCI136225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller NJ, et al. Reduction of consumptive coagulopathy using porcine cytomegalovirus-free cardiac porcine grafts in pig-to-primate xenotransplantation. Transplantation. 2004;78(10):1449–1453. doi: 10.1097/01.TP.0000141361.68446.1F. [DOI] [PubMed] [Google Scholar]
- 30.Halecker S, Metzger J, Strube C, Krabben L, Kaufer B, Denner J. Virological and parasitological characterization of mini-LEWE minipigs using improved screening methods and an overview of data on various minipig breeds. Microorganisms. 2021;9(12):2617. doi: 10.3390/microorganisms9122617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morozov VA, Morozov AV, Denner J. New PCR diagnostic systems for the detection and quantification of porcine cytomegalovirus (PCMV) Arch. Virol. 2016;161(5):1159–1168. doi: 10.1007/s00705-016-2765-3. [DOI] [PubMed] [Google Scholar]
- 32.Duvigneau JC, Hartl RT, Groiss S, Gemeiner M. Quantitative simultaneous multiplex real-time PCR for the detection of porcine cytokines. J. Immunol. Methods. 2005;306:16–27. doi: 10.1016/j.jim.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Morozov VA, Plotzki E, Rotem A, Barkai U, Denner J. Extended microbiological characterization of Göttingen minipigs: Porcine cytomegalovirus and other viruses. Xenotransplantation. 2016;23(6):490–496. doi: 10.1111/xen.12265. [DOI] [PubMed] [Google Scholar]
- 34.Clark DA, et al. Porcine cytomegalovirus in pigs being bred for xenograft organs: Progress towards control. Xenotransplantation. 2003;10:142–148. doi: 10.1034/j.1399-3089.2003.01128.x. [DOI] [PubMed] [Google Scholar]
- 35.Fryer JF, Griffiths PD, Fishman JA, Emery VC, Clark DA. Quantitation of porcine cytomegalovirus in pig tissues by PCR. J. Clin. Microbiol. 2001;39(3):1155–1156. doi: 10.1128/JCM.39.3.1155-1156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morozov VA, Heinrichs G, Denner J. Effective detection of porcine cytomegalovirus using non-invasively taken samples from piglets. Viruses. 2017;9(1):9. doi: 10.3390/v9010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiebig U, Holzer A, Ivanusic D, Plotzki E, Hengel H, Neipel F, Denner J. Antibody cross-reactivity between porcine cytomegalovirus (PCMV) and human herpesvirus-6 (HHV-6) Viruses. 2017;9(11):317. doi: 10.3390/v9110317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ezzelarab MB, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22(1):32–47. doi: 10.1111/xen.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwase H, et al. Further evidence for sustained systemic inflammation in xenograft recipients (SIXR) Xenotransplantation. 2015;22(5):399–405. doi: 10.1111/xen.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ezzelarab MB, Cooper DKC. Systemic inflammation in xenograft recipients (SIXR): A new paradigm in pig-to-primate xenotransplantation? Int. J. Surg. 2015;23(Pt B):301–305. doi: 10.1016/j.ijsu.2015.07.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson CP, et al. A perspective on the potential detrimental role of inflammation in pig orthotopic heart xenotransplantation. Xenotransplantation. 2021;28(4):e12687. doi: 10.1111/xen.12687. [DOI] [PubMed] [Google Scholar]
- 42.Fryer JF, Griffiths PD, Emery VC, Clark DA. Susceptibility of porcine cytomegalovirus to antiviral drugs. J. Antimicrob. Chemother. 2004;53(6):975–980. doi: 10.1093/jac/dkh231. [DOI] [PubMed] [Google Scholar]
- 43.Mueller NJ, et al. Reduced efficacy of ganciclovir against porcine and baboon cytomegalovirus in pig-to-baboon xenotransplantation. Am. J. Transplant. 2003;3(9):1057–1064. doi: 10.1034/j.1600-6143.2003.00192.x. [DOI] [PubMed] [Google Scholar]
- 44.Clark DA. Human herpesvirus 6. Rev. Med. Virol. 2000;10(3):155–173. doi: 10.1002/(SICI)1099-1654(200005/06)10:3<155::AID-RMV277>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Denner J. The porcine cytomegalovirus (PCMV) will not stop xenotransplantation. Xenotransplantation. 2022;29(3):e12763. doi: 10.1111/xen.12763. [DOI] [PubMed] [Google Scholar]
- 46.Cooper DKC, Yamamoto T, Hara H, Pierson RN., 3rd The first clinical pig heart transplant: Was IVIg or pig cytomegalovirus detrimental to the outcome? Xenotransplantation. 2022;29(4):e12771. doi: 10.1111/xen.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denner J. Risk of pathogenic virus transmission by somatic cell nuclear transfer: Implications for xenotransplantation. Biol. Reprod. 2022;107(3):717–722. doi: 10.1093/biolre/ioac120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset generated and analyzed during the current study are all available in this manuscript including the Supplementary Tables and Supplementary Information files showing full-length gels and blots.