Abstract
An important measure of brain health is the integrity of white matter connectivity structures that link brain regions. Studies have found an association between poorer sleep quality and decreased white matter integrity. Stress is among the strongest predictors of sleep quality. This study aimed to evaluate the association between sleep quality and white matter and test if the relationship persisted after accounting for stress.
White matter microstructures were measured by diffusion tensor imaging in a population of Old Order Amish/Mennonite (N=240). Sleep quality was determined by the Pittsburgh Sleep Quality Index. Current stress levels were measured by the perceived stress scale. Exposure to lifetime stress was measured by the lifetime stressor inventory.
Microstructures of 4 white matter tracts: left and right anterior limbs of internal capsule, left anterior corona radiata, and genu of corpus callosum were significantly correlated with sleep quality (all p≤0.001). Current stress level was a significant predictor of sleep quality (p ≤ 0.001) while lifetime stress was not. PSQI remained significantly associated with white matter integrity in these frontal tracts (all p<0.01) after accounting for current stress and lifetime stress while current and lifetime stress were not significant predictors of white matter in any of the four models. Sleep quality did not have any substantial mediation role between stress and white matter integrity.
Sleep quality was significantly associated with several frontal white matter tracts that connect brain structures important for sleep regulation regardless of current or past stress levels.
Keywords: white matter, stress, fractional anisotropy, sleep quality
Introduction
Sleep is an integral part of physiological health. Sleep influences the body’s immune system and its diseases (Savard, Laroche, Simard, Ivers, & Morin, 2003) and has a strong relationship with psychological functions (Zielinski, McKenna, & McCarley, 2016). Shorter sleep duration has been associated with risk of dementia and stroke (Leng et al., 2015), cardiovascular disease (Ayas, White, Manson, et al., 2003) and metabolic disorders (Ayas, White, Al-Delaimy, et al., 2003). The mechanisms of sleep have been clarified in many respects, although how sleep is related with neurological health especially the cerebral white matter microstructures remains unclear.
Brain white matter contains myelinated axonal fibers that facilitate electrical signal transmission between neurons. The fractional anisotropy (FA) of white matter tracts as derived from brain imaging is a measure of white matter microstructure integrity. Studies have found an association between poor sleep and lower white matter integrity (Khalsa et al., 2017). Participants with insomnia have reduced connectivity of the insula and the connections between frontal and subcortical regions (Jespersen et al., 2020) and reduced FA in the anterior internal capsule (Spiegelhalder et al., 2014). Frontal- subcortical tracts run through both the anterior internal capsule, which plays a part in the regulation of sleep, and the anterior corona radiata which is important for cognitive function; both regions were among tracts showing reduced FA in participants with insomnia (S. Li et al., 2016). Consistent with this, the anterior internal capsule was also found to have reduced FA in study using phenotypes of insomnia disorder diagnosis and insomnia severity (Bresser et al., 2020). A prospective study across 16 years also found diminished FA within the frontal lobe, including the anterior and superior corona radiata, anterior internal capsule and genu and body of the corpus callosum, to be associated with current poor sleep (Sexton et al., 2017) though not with poor sleep reported across time. Reduced structural connectivity has been repeatedly found in participants with poor sleep although there is variability in which tracts are affected across studies and some studies have not found an association between sleep quality and any DTI metrics (C. Li et al., 2020).
There are multiple possible reasons for the inconsistencies between study findings; one being inclusion or exclusion of different confounders. For instance, stress is one of the most salient factors affecting sleep quality, and most sleep and white matter studies in the past have not looked at the impact of stress on the relationship between sleep quality and white matter. Stress has a strong relationship with subjective reports of sleep quality(Kashani, Eliasson, & Vernalis, 2012) as well as objective changes in sleep characteristics such as electroencephalographic (EEG) changes (Hall et al., 2015) and rapid eye movement sleep in humans (Petersen, Kecklund, D’Onofrio, Nilsson, & Akerstedt, 2013). Similar effects have been shown in mouse models in which sleep is measured before and after exposure to adverse environmental stimuli (Sanford, Fang, & Tang, 2003).
In addition to current stress, studies indicate that early life stress may influence sleep in adulthood through a long term epigenetic mechanism (Lo Martire, Caruso, Palagini, Zoccoli, & Bastianini, 2019). Furthermore there is evidence for a bidirectional relationship between sleep deprivation and elevated cortisol levels, a marker of biological stress (Leproult, Copinschi, Buxton, & Van Cauter, 1997). Thus, some of the relationship between white matter tracts and sleep reported in the literature may be attributed to current and lifetime stress that was not controlled for.
In fact the direction of the relationship between sleep and stress has been difficult to determine across multiple studies in both humans and animal models (Nollet, Wisden, & Franks, 2020). High stress levels interfere with sleep but in addition, sleep deprivation leads to increased subjective reports of stress. Sleep deprivation can also increase biomarkers of stress including allostatic load such as increases in blood pressure as well as oxidative stress (McEwen & Karatsoreos, 2015). Therefore we explored mediation models with paths between sleep and stress in both directions although the ability to determine directionality is limited with our cross-sectional data.
In this study, we tested whether the association between sleep quality and white matter was present and remained after accounting for current and lifetime stress. We performed brain imaging and measured sleep quality and stress levels in 240 members of the Old Order Amish and Mennonite (OOA/M) community in the area of Lancaster, Pennsylvania. Characteristic of these communities, these OOA/M participants live a rural lifestyle. The OOA/M communities in the Lancaster area share a common ancestry and emphasize family life and helping others, thus there is less disparity in wealth across families. Amish life is greatly influenced by their religion that has sets of rules outlining behaviors permitted in the Amish communities. Within the Amish communities, for example, school is finished after the completion of 8th grade; consequently, there are minimal differences in education level. These communities also experience minimal substance use and limited exposure to modern technological devices compared to the general population. Therefore, this is a relatively homogeneous demographic and socioeconomic population without many of the factors that might confound the relationship between sleep and brain structures in other populations. Participants with psychiatric disorders, such as a mood disorder that would markedly confound measures of stress and sleep, were excluded.
Methods
The study included 240 OOA/M participants [103 (M)/ 137 (F), age 37.94 ±17.89 years (mean ± s.d.)] from Pennsylvania and Maryland. Exclusion criteria included major medical and neurological conditions as well as current or lifetime psychiatric illness. The Structured Clinical Interview for DSM-5 Axis I Disorders (SCID-5) was performed to confirm lack of psychiatric diagnoses such as mood disorders, psychotic disorders, anxiety disorders, or substance use disorders. However, insomnia disorder diagnosis was not excluded as it is not part of the SCID, although other psychiatric conditions diagnosed by SCID were excluded, and therefore this sample was generally a healthy community sample. Study participants gave written informed consent as approved by the University of Maryland IRB. Clinical interviews and sleep and stress assessments were conducted on the same day. Brain Imaging was completed on a separate day from the clinical assessment but typically acquired on back to back days for a given participant.
Sleep measures
Sleep quality was ascertained by the self-reported Pittsburgh Sleep Quality Index (PSQI) which assesses sleep quality in the previous month. The PSQI summary score ranges from 1 to 21 and a global PSQI score > 5 is indicative of poor sleepers (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). A larger PSQI score indicates greater impairment of sleep quality such as shorter duration, longer time to fall asleep, greater amount of sleep disturbance and more daytime sleepiness
Stress measures
To assess lifetime stress we used the Amish Life Stressor Inventory (Bruce et al., 2021; Chiappelli et al., 2021; Kvarta et al., 2021). The inventory adapts items from the Life Stressor Checklist–Revised (Wolfe, 1997), which is a self-reported questionnaire that asks about the occurrence of stressful events over the lifetime, for example, experiences of abuse, neglect, or violence. We added questions tailored to the Amish population including questions such as “Has a family member or close friend ever left the Amish community?” or “Have you ever separated from your church community?” This Amish Life Stressor Inventory (LSI) scores the total number of events the participants report with 14 being the maximum score. To assess current subjective stress level we used the Perceived Stress Scale (PSS) (Cohen & Williamson, 1988). The PSS is a self-report questionnaire, with 40 being the maximum score, that asks the participant to rate the frequency of certain stress-related feelings over the last month, such as feeling overwhelmed versus feeling confident in the ability to cope with problems. Scores of 0–13 would be considered low; 14–26 moderate; and 27–40 high perceived stress.
Imaging
The imaging data were collected at Maryland Psychiatric Research Center using a Siemens Prisma 3 Tesla scanner using a 64-channel coil. Diffusion weighted imaging (DWI) data were collected using an expansion of the HCP protocol that consisted of 6 shells of b-values (b=600, 900, 1200, 1500, 1800, and 3000 s/mm2) with 98 isotropically distributed diffusion-weighted directions per shell collected twice with the reversal of the phase encoding and readout gradients (anterior-to-posterior AP and posterior-to-anterior PA) to correct for spatial distortions, including twenty b=0 images interleaved within the acquisition. The data was collected using a multiband, echo-planar, spin-echo, T2-weighted sequence (TE/TR/Multiband Factor=97/4000ms/4 with the FOV=200 mm) with an isotropic spatial resolution of 1.6 mm. Diffusion data were preprocessed using HCP Diffusion pipeline (Glasser et al., 2013; Sotiropoulos et al., 2013) that was combined with DESIGNER diffusion preprocessing tools including advance denoising, Gibbs ringing correction, and correction of the EPI distortions (Ades-Aron et al., 2018). Fractional anisotrophy (FA) maps were obtained by fitting the diffusion tensor model using the FSL-FDT toolkit (Behrens et al., 2003). The population-based diffusion tensor imaging (DTI) cerebral white matter tract atlas developed at John Hopkins University and distributed with the FSL package (Wakana, Jiang, Nagae-Poetscher, van Zijl, & Mori, 2004) was used to calculate average FA values along the spatial course of major white matter tracts. The applied FA threshold was 0.2 and the FSL version number was 6.0. The data were processed using FSL’s tract-based spatial statistics (TBSS; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS) analytic method modified to project individual FA values onto the ENIGMA-DTI skeleton. The protocol, ENIGMA-DTI template FA image along with its skeleton and mask, source code and executables, are all publicly available. (http://www.nitrc.org/projects/enigma_dti).
White matter tracts used in analysis were Anterior Corona Radiata (ACR), Anterior Limb of the Internal Capsule (ALIC), Body of Corpus Callosum (BCC), Cingulum (cingulate gyrus) CGC, Cingulum (hippocampus) CGH, Corticospinal Tracts (CST), External Capsule (EC), Fornix (FX), Fornix/Stria Terminalis (FX/ST), Genu of Corpus Callosum (GCC), Inferior Fronto-Occipital Fasciculus (IFO), Posterior Corona Radiata (PCR), Posterior Limb of the Internal Capsule (PLIC), Posterior Thalamic Radiation (PTR), Retrolenticular Limb of the Internal Capsule (RLIC), Sagittal stratum (SS), Splenium of Corpus Callosum (SCC), Superior Corona Radiata (SCR), Superior Fronto-Occipital Fasciculus (SFO), Superior Longitudinal Fasciculus (SLF), Uncinate Fasciculus (UNC).
Statistics
We estimated partial correlations between each white matter tract and sleep quality while controlling for age and sex. We considered 0.05/38 = 0.0013 to be a threshold for statistical significance for these correlations following Bonferroni correction for multiple comparisons (38 tracts). We initially performed partial correlations between 38 white matter tracts and sleep quality controlling for age and sex as an initial screen to evaluate the relationships. Stress was not considered in this analysis. Once we found that frontal white matter tracts had significant correlations with sleep quality, we added multiple linear regression to evaluate sleep quality as a predictor of these white matter tracts independently or not of current or lifetime stress.
To explore the presence of a putative indirect effect of current stress on white matter integrity through sleep quality we performed a mediation analysis with current stress as predictor variable (X), sleep quality as mediator (M) and FA of the 4 tracts that were significantly associated with sleep quality (ACR-L, ALIC-L, ALIC-R, and GCC) as the outcome variable (Y) with age and sex as covariates. We also explored the alternative model where sleep quality was the predictor variable (X) and current stress as mediator (M). We used the current stress measure as in our study, this rather than lifetime stress had a significant association with sleep. We repeated these 2 mediation analysis separately for each of the four tracts. We used the IBM SPSS version 23.0 (IBM Corp. Armonk, NY) macro PROCESS (http://www.processmacro.org) PROCESS estimates both indirect and indirect effects between variables using a least squares path analysis. Indirect (mediated) effects are assessed through bootstrap confidence intervals. Significance of an indirect effects is indicated if the 95% confidence interval (95% CI) does not include zero. The number of bootstrap samples was set to 5000. As this was a cross-sectional data, the mediation analyses were exploratory. P values are not corrected for multiple comparisons.
Results
In this study population the perceived stress scale had a mean ± s.d. score of 14.04 ± 5.59. Lifetime stress inventory had a mean ± s.d. score of 2.6 ± 1.7. PSQI had a mean ± s.d. score of 3.29 ± 2.43. See Table 1 for more data on sleep characteristics of the population. The ranges of the scores were 0 to 28 for the perceived stress scale, 0 to 8 for lifetime stress scale, and 0 to 13 for PSQI in this sample.
Table 1. Sleep Characteristics of Amish Study Population.
Measures were obtained from participants answers to Pittsburgh Sleep Quality Index questions.
| Measure | Mean | SD |
|---|---|---|
| Bedtime | 9:24 PM | 48 min |
| Latency | 15 min | 15 min |
| Wake time | 5:30 AM | 54 min |
| Time in bed | 8 h 6 min | 60 min |
| Duration of sleep | 7 h 36 min | 66 min |
| Sleep Efficiency | 93% | 1% |
SD: standard deviation.
After adjustment for age and sex, poorer sleep quality was significantly correlated with lower FA of four white matter tracts: left anterior corona radiata (ACR-L) (r=−0.21, p= 0.001), left and right anterior limb of internal capsule (ALIC-L and ALIC-R) (r= −0.24, p<0.001 and r= −0.21, p< 0.001) and genu of the corpus callosum (GCC) (r=−0.23, p=0.001) (Figure 1a and b). Participants’ PSQI scores and FA values for these four tracts are shown in supplemental Figure S1.
Figure 1a.
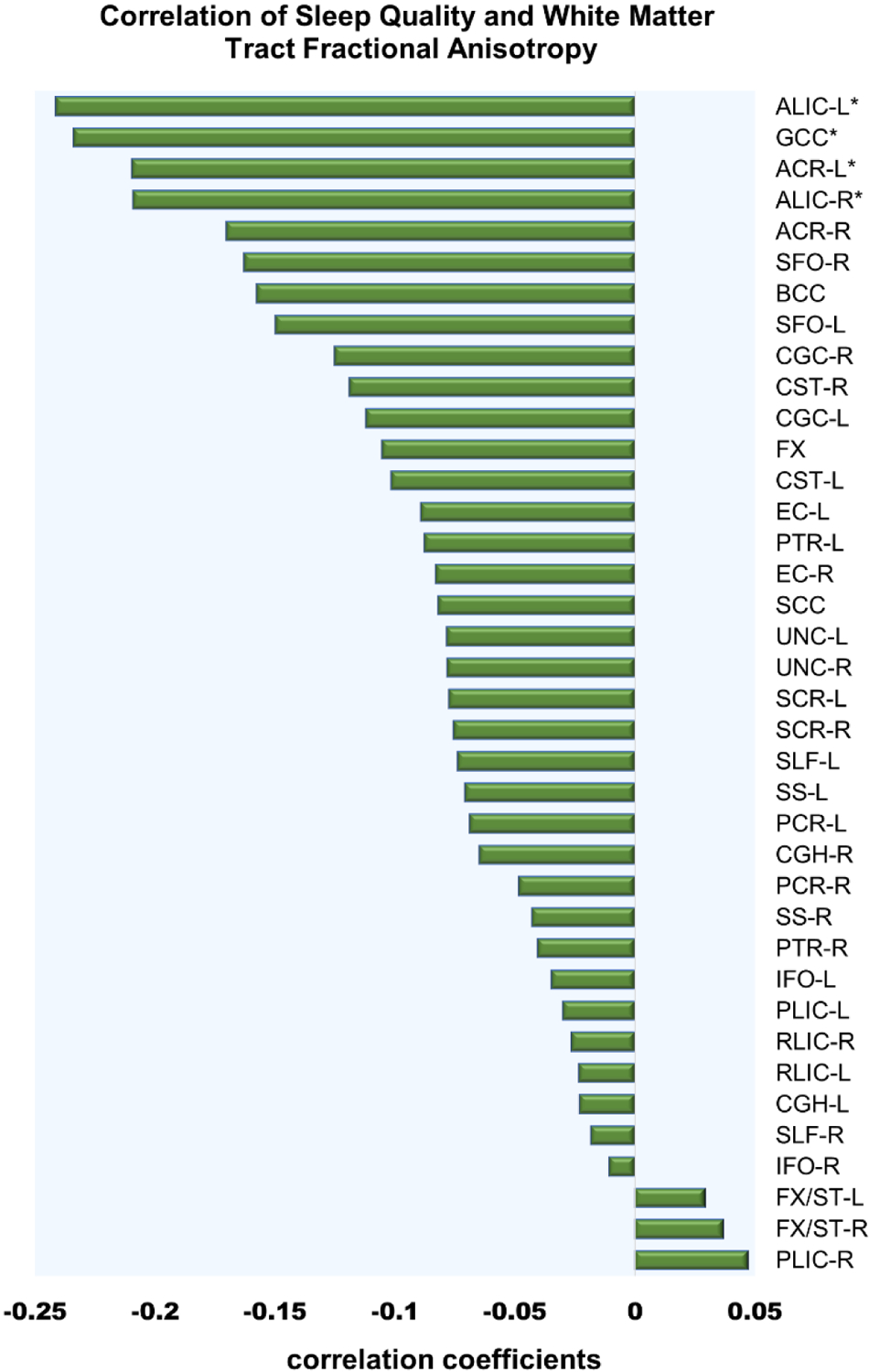
Correlation coefficients for white matter tract fractional anisotropy as related to Pittsburg sleep quality index (PSQI). Age and sex were covaried out. Negative r values indicate lower fractional anisotropy is related to higher (worse) PSQI. Asterisks indicates significant p- value after bonferroni correction for multiple comparisons.
Figure 1b.

Axial brain slice with white matter tracts labeled and color representing r values for the partial correlation between sleep quality and fractional anisotropy. In the color bar legend, the black line marks statistical significance where color to left indicates correlations with frontal tracts passed Bonferroni corrected p- value. Statistically significnt white matter tracts are marked with asterisk. Figure shows the results projected back on the original white matter tracts. Anterior Corona Radiata (ACR), Anterior Limb of the Internal Capsule (ALIC), External Capsule (EC), Fornix (FX), Fornix/Stria Terminalis (FX/ST), Genu of Corpus Callosum (GCC), Posterior Limb of the Internal Capsule (PLIC), Posterior Thalamic Radiation (PTR), Retrolenticular Limb of the Internal Capsule (RLIC), Splenium of Corpus Callosum (SCC), Superior Longitudinal Fasciculus (SLF).
Current stress was significantly associated with poorer sleep quality (r= 0.21, p=0.001) as expected, while lifetime stress was not (r= 0.13, p=0.052). In multiple regression analyses, models showed PSQI remained a significant predictor of white matter integrity of these four tracts even after additional adjustment for current stress and lifetime stress. ACR-L: t=−2.6 (5, 220), p<0.01; ALIC-L: t =−3.4(5, 220), p<0.01; ALIC- R: t=−3.1 (5, 220), p<0.01; GCC: t=−3.0 (5, 220) p<0.01. Current and lifetime stress were not significantly associated with white matter integrity in these four models.
Sleep quality had a nominally significant but small mediation role in the relationship between current stress and white matter FA in three tracts (GCC, ALIC-L, and ALIC-R); none of which is significant if corrected for multiple comparisons. Statistics for mediation paths in this model are presented in supplemental data Figure S2. Current stress had no significant mediation effect in a relationship between sleep and white matter integrity of any tracts.
Discussion
We found that sleep quality was significantly associated with white matter microstructure in 3 projecting tracts in the frontal lobe (left and right anterior limb of internal capsule, left anterior corona radiata) and 1 commissural tract (genu of corpus callosum). Current stress level was associated with sleep. Further investigation showed that sleep quality remained significantly associated with white matter microstructure in these four frontal tracts after accounting for current and lifetime stress measures, while current and lifetime stress did not significantly predict white matter integrity in the four models. There was also no evidence that they have a significant mediation role in the relationship between sleep and white matter. There was a small indirect effect of sleep quality on the relationship between current stress and white matter (GCC, ALIC-L, and ALIC-R) though this would not be significant if corrected for multiple comparisons. Therefore, these white matter tracts are associated with sleep quality largely independent of current or lifetime experience of stress.
The identified white matter tracts connect brain regions important in the regulation of sleep. The anterior internal capsule contains fibers connecting subcortical nuclei and the prefrontal cortex. Similarly, the anterior corona radiata contains fibers connecting the thalamus and frontal cortex. The pons, hypothalamus, thalamus and prefrontal cortex play a part in the regulation of sleep and wakefulness (Saper, Scammell, & Lu, 2005). Arousal depends on the thalamic radiation for integrating sensory-motor information. Aside from projecting tracts, sleep quality was associated with commissural tracts in the genu of the corpus callosum which mediates connectivity between bilateral prefrontal regions. A study of post callosotomy patients shows the integrity of the corpus callosum is crucial for cross-hemispheric traveling of slow wave sleep (Avvenuti et al., 2020).
The physiological mechanism underlying the relationship between sleep and white matter microstructures has not been definitively proven however evidence points to myelination having an important role. Sleep studies in rodents and humans have investigated the relationship between sleep and white matter on structural, electrophysiological, and genetic levels. Several studies have shown increase in expression of myelin related genes during sleep. For instance myelin structural proteins including myelin-associated oligodendrocyte basic protein, myelin associated glycoprotein, and plasmolipin (Cirelli, Gutierrez, & Tononi, 2004) are upregulated during sleep. Another study found that in oligodendrocytes, the expression levels of ≥2% of all expressed genes were associated with the sleep/wake cycle (Bellesi et al., 2013), many involved in plasma membrane maintenance and support of myelinated axons. The association of sleep and expression of oligodendrocytic myelin paranodal and inner loop protein gene and the plasmolipin gene have been replicated in other studies (Mongrain et al., 2010). Studies in mice that investigated structural change have found decreased myelin thickness after sleep deprivation in the corpus callosum and lateral olfactory tract (Bellesi et al., 2018) and an increase in oligodendrocyte precursor cell production during sleep (Bellesi et al., 2013).
The complexity of the relationship between white matter and sleep has led to attempts to unravel the causal direction, or to determine if there is a bidirectional relationship by using a longitudinal design. However, assessing sleep quality and white matter over several years yielded no conclusive results (Kocevska et al., 2019).There is evidence of a bidirectional relationship between sleep and other brain pathology such as amyloid-β (Aβ) peptide (Ju, Lucey, & Holtzman, 2014).
In our study we considered whether stress may contribute to the relationship of sleep quality and white matter, but found neither current nor lifetime stress was associated with white matter microstructures that were significantly associated with sleep quality.
In this sample of healthy participants, current stress was related to sleep quality which replicates other studies (Akerstedt et al., 2002) and supports the validity of using this stress assessment in this population to test if stress contributes to the sleep quality and white matter integrity relationship.
Several studies have also evaluated the relationship between measurements of stress and measurements of brain structure such as gray matter volume and white matter integrity with differing findings. In an animal model, stress has been associated with reduced FA in the corpus callosum and external capsule (Nagy et al., 2020) but there were no such findings in another study (Henckens et al., 2015). Some studies in humans showed that clinically severe stress such as a diagnosis of PTSD in adolescents with history of childhood adverse events was associated with lower FA in white matter tracts (Huang, Gundapuneedi, & Rao, 2012) although whether stress experienced by an otherwise healthy population would have a substantial effect on white matter is less clear. Our analysis showed that the significant relationship between sleep quality and white matter is unlikely substantially confounded by past stressful events or current stress levels.
The choice of study participants from the Amish and Mennonite communities has both limitations and benefits. The decreased environmental heterogeneity and increased uniformity in this population can make it more difficult to detect a signal in measures of sleep and stress. It can also make it more difficult to extrapolate the findings to a larger general population. However, the mean and SD of the PSS in our sample (14.0 ± 5.6) were similar to other samples reported in larger populations, for instance 13.0 ± 6.4, n = 2,387 (Cohen & Williamson, 1988). The mean and SD of the PSQI in our sample (3.3 ±2.4) was similar to many other study populations with mean scores between 3 and 4 (Grandner, Kripke, Yoon, & Youngstedt, 2006). PSQI scores ≤ 5 are considered to represent good sleep so our findings may not generalize to populations with worse sleep quality. Data from other Amish sleep studies indicate sleep quality as well as sleep duration (Evans et al., 2011) to be similar to more general populations though the Amish had significantly earlier bed times and earlier wake times (Zhang et al., 2019). This sleep pattern is related to rural living and may be more consistent with the biological sleep cycle. An Amish population with a comparatively more homogeneous environment can limit environmental confounds such as differences in education or technology access.
Another limitation in our design is that it did not investigate the direction of the relationship of sleep and white matter; longitudinal studies are needed to fully address this limitation. A further limitation is that we did not supplement our measure of subjective sleep quality with objective sleep measures, though in a prior study of sleep in the Amish, objective actigraphy was highly correlated with results from sleep diaries (Evans et al., 2011).
In conclusion, we provide further evidence in this study of a link between sleep quality and white matter microstructures, specifically those located in frontal areas of the brain that connect regions implicated in sleep regulation. Further studies are needed to investigate whether sleep impairment has a deleterious effect on white matter integrity or degraded white matter interferes with sleep quality, or if both pathways exist.
Supplementary Material
Acknowledgements:
This study was supported by NIH Grant U01 MH108148.
Footnotes
No authors had conflicts of interests.
References
- Ades-Aron B, Veraart J, Kochunov P, McGuire S, Sherman P, Kellner E, … Fieremans E (2018). Evaluation of the accuracy and precision of the diffusion parameter EStImation with Gibbs and NoisE removal pipeline. Neuroimage, 183, 532–543. doi: 10.1016/j.neuroimage.2018.07.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerstedt T, Knutsson A, Westerholm P, Theorell T, Alfredsson L, & Kecklund G (2002). Sleep disturbances, work stress and work hours: a cross-sectional study. J Psychosom Res, 53(3), 741–748. doi: 10.1016/s0022-3999(02)00333-1 [DOI] [PubMed] [Google Scholar]
- Avvenuti G, Handjaras G, Betta M, Cataldi J, Imperatori LS, Lattanzi S, … Bernardi G (2020). Integrity of Corpus Callosum Is Essential for theCross-Hemispheric Propagation of Sleep Slow Waves:A High-Density EEG Study in Split-Brain Patients. J Neurosci, 40(29), 5589–5603. doi: 10.1523/JNEUROSCI.2571-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, … Hu FB (2003). A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care, 26(2), 380–384. doi: 10.2337/diacare.26.2.380 [DOI] [PubMed] [Google Scholar]
- Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, & Hu FB (2003). A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med, 163(2), 205–209. doi: 10.1001/archinte.163.2.205 [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, … Smith SM (2003). Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med, 50(5), 1077–1088. doi: 10.1002/mrm.10609 [DOI] [PubMed] [Google Scholar]
- Bellesi M, Haswell JD, de Vivo L, Marshall W, Roseboom PH, Tononi G, & Cirelli C (2018). Myelin modifications after chronic sleep loss in adolescent mice. Sleep, 41(5). doi: 10.1093/sleep/zsy034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, & Cirelli C (2013). Effects of sleep and wake on oligodendrocytes and their precursors. J Neurosci, 33(36), 14288–14300. doi: 10.1523/JNEUROSCI.5102-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresser T, Foster-Dingley JC, Wassing R, Leerssen J, Ramautar JR, Stoffers D, … van Someren EJW (2020). Consistent altered internal capsule white matter microstructure in insomnia disorder. Sleep, 43(8). doi: 10.1093/sleep/zsaa031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce HA, Kochunov P, Chiappelli J, Savransky A, Carino K, Sewell J, … Hong LE (2021). Genetic versus stress and mood determinants of sleep in the Amish. Am J Med Genet B Neuropsychiatr Genet, 186(2), 113–121. doi: 10.1002/ajmg.b.32840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res, 28(2), 193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Chiappelli J, Kvarta M, Bruce H, Chen S, Kochunov P, & Hong LE (2021). Stressful life events and openness to experience: Relevance to depression. J Affect Disord, 295, 711–716. doi: 10.1016/j.jad.2021.08.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Gutierrez CM, & Tononi G (2004). Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron, 41(1), 35–43. doi: 10.1016/s0896-6273(03)00814-6 [DOI] [PubMed] [Google Scholar]
- Cohen S, & Williamson G (1988). Perceived Stress in a probability sample of the United States. Paper presented at the The social psychology of health: Claremont symposium on applied social psychology, Newbury Park, CA. [Google Scholar]
- Evans DS, Snitker S, Wu SH, Mody A, Njajou OT, Perlis ML, … Hsueh WC (2011). Habitual sleep/wake patterns in the Old Order Amish: heritability and association with non-genetic factors. Sleep, 34(5), 661–669. doi: 10.1093/sleep/34.5.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, … Consortium, W. U.-M. H. (2013). The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage, 80, 105–124. doi: 10.1016/j.neuroimage.2013.04.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Kripke DF, Yoon IY, & Youngstedt SD (2006). Criterion validity of the Pittsburgh Sleep Quality Index: Investigation in a non-clinical sample. Sleep Biol Rhythms, 4(2), 129–139. doi: 10.1111/j.1479-8425.2006.00207.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Casement MD, Troxel WM, Matthews KA, Bromberger JT, Kravitz HM, … Buysse DJ (2015). Chronic Stress is Prospectively Associated with Sleep in Midlife Women: The SWAN Sleep Study. Sleep, 38(10), 1645–1654. doi: 10.5665/sleep.5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJ, van der Marel K, van der Toorn A, Pillai AG, Fernandez G, Dijkhuizen RM, & Joels M (2015). Stress-induced alterations in large-scale functional networks of the rodent brain. Neuroimage, 105, 312–322. doi: 10.1016/j.neuroimage.2014.10.037 [DOI] [PubMed] [Google Scholar]
- Huang H, Gundapuneedi T, & Rao U (2012). White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology, 37(12), 2693–2701. doi: 10.1038/npp.2012.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen KV, Stevner A, Fernandes H, Sorensen SD, Van Someren E, Kringelbach M, & Vuust P (2020). Reduced structural connectivity in Insomnia Disorder. J Sleep Res, 29(1), e12901. doi: 10.1111/jsr.12901 [DOI] [PubMed] [Google Scholar]
- Ju YE, Lucey BP, & Holtzman DM (2014). Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol, 10(2), 115–119. doi: 10.1038/nrneurol.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani M, Eliasson A, & Vernalis M (2012). Perceived stress correlates with disturbed sleep: a link connecting stress and cardiovascular disease. Stress, 15(1), 45–51. doi: 10.3109/10253890.2011.578266 [DOI] [PubMed] [Google Scholar]
- Khalsa S, Hale JR, Goldstone A, Wilson RS, Mayhew SD, Bagary M, & Bagshaw AP (2017). Habitual sleep durations and subjective sleep quality predict white matter differences in the human brain. Neurobiol Sleep Circadian Rhythms, 3, 17–25. doi: 10.1016/j.nbscr.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocevska D, Cremers LGM, Lysen TS, Luik AI, Ikram MA, Vernooij MW, & Tiemeier H (2019). Sleep complaints and cerebral white matter: A prospective bidirectional study. J Psychiatr Res, 112, 77–82. doi: 10.1016/j.jpsychires.2019.02.002 [DOI] [PubMed] [Google Scholar]
- Kvarta MD, Bruce HA, Chiappelli J, Hare SM, Goldwaser EL, Sewell J, … Hong LE, (2021). Multiple dimensions of stress vs. genetic effects on depression. Transl Psychiatry, 11(1), 254. doi: 10.1038/s41398-021-01369-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Cappuccio FP, Wainwright NW, Surtees PG, Luben R, Brayne C, & Khaw KT (2015). Sleep duration and risk of fatal and nonfatal stroke: a prospective study and meta-analysis. Neurology, 84(11), 1072–1079. doi: 10.1212/WNL.0000000000001371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leproult R, Copinschi G, Buxton O, & Van Cauter E (1997). Sleep loss results in an elevation of cortisol levels the next evening. Sleep, 20(10), 865–870. [PubMed] [Google Scholar]
- Li C, Schreiber J, Bittner N, Li S, Huang R, Moebus S, … Elmenhorst D (2020). White Matter Microstructure Underlies the Effects of Sleep Quality and Life Stress on Depression Symptomatology in Older Adults. Front Aging Neurosci, 12, 578037. doi: 10.3389/fnagi.2020.578037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Tian J, Bauer A, Huang R, Wen H, Li M, … Jiang G (2016). Reduced Integrity of Right Lateralized White Matter in Patients with Primary Insomnia: A Diffusion-Tensor Imaging Study. Radiology, 280(2), 520–528. doi: 10.1148/radiol.2016152038 [DOI] [PubMed] [Google Scholar]
- Lo Martire V, Caruso D, Palagini L, Zoccoli G, & Bastianini S (2019). Stress & sleep: A relationship lasting a lifetime. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2019.08.024 [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Karatsoreos IN (2015). Sleep Deprivation and Circadian Disruption: Stress, Allostasis, and Allostatic Load. Sleep Med Clin, 10(1), 1–10. doi: 10.1016/j.jsmc.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongrain V, Hernandez SA, Pradervand S, Dorsaz S, Curie T, Hagiwara G, … Franken P (2010). Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep, 33(9), 1147–1157. doi: 10.1093/sleep/33.9.1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy SA, Vranesics A, Varga Z, Csabai D, Bruszt N, Bali ZK, … Czeh B (2020). Stress-Induced Microstructural Alterations Correlate With the Cognitive Performance of Rats: A Longitudinal in vivo Diffusion Tensor Imaging Study. Front Neurosci, 14, 474. doi: 10.3389/fnins.2020.00474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollet M, Wisden W, & Franks NP (2020). Sleep deprivation and stress: a reciprocal relationship. Interface Focus, 10(3), 20190092. doi: 10.1098/rsfs.2019.0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen H, Kecklund G, D’Onofrio P, Nilsson J, & Akerstedt T (2013). Stress vulnerability and the effects of moderate daily stress on sleep polysomnography and subjective sleepiness. J Sleep Res, 22(1), 50–57. doi: 10.1111/j.1365-2869.2012.01034.x [DOI] [PubMed] [Google Scholar]
- Sanford LD, Fang J, & Tang X (2003). Sleep after differing amounts of conditioned fear training in BALB/cJ mice. Behav Brain Res, 147(1–2), 193–202. doi: 10.1016/s0166-4328(03)00180-3 [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, & Lu J (2005). Hypothalamic regulation of sleep and circadian rhythms. Nature, 437(7063), 1257–1263. doi: 10.1038/nature04284 [DOI] [PubMed] [Google Scholar]
- Savard J, Laroche L, Simard S, Ivers H, & Morin CM (2003). Chronic insomnia and immune functioning. Psychosom Med, 65(2), 211–221. doi: 10.1097/01.psy.0000033126.22740.f3 [DOI] [PubMed] [Google Scholar]
- Sexton CE, Zsoldos E, Filippini N, Griffanti L, Winkler A, Mahmood A, … Ebmeier KP (2017). Associations between self-reported sleep quality and white matter in community-dwelling older adults: A prospective cohort study. Hum Brain Mapp, 38(11), 5465–5473. doi: 10.1002/hbm.23739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos SN, Jbabdi S, Xu J, Andersson JL, Moeller S, Auerbach EJ, … Consortium, W. U.-M. H. (2013). Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage, 80, 125–143. doi: 10.1016/j.neuroimage.2013.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalder K, Regen W, Prem M, Baglioni C, Nissen C, Feige B, … Riemann D (2014). Reduced anterior internal capsule white matter integrity in primary insomnia. Hum Brain Mapp, 35(7), 3431–3438. doi: 10.1002/hbm.22412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, & Mori S (2004). Fiber tract-based atlas of human white matter anatomy. Radiology, 230(1), 77–87. doi: 10.1148/radiol.2301021640 [DOI] [PubMed] [Google Scholar]
- Wolfe J, Kimberling R, Brown P, Chrestman K, Levin K (1997). Life Stressor Checklist-Revised (LSC-R). Paper presented at the http://www.ptsd.va.gov.
- Zhang M, Ryan KA, Wickwire E, Postolache TT, Xu H, Daue M, … Mitchell BD (2019). Self-Reported Sleep Duration and Pattern in Old Order Amish and Non-Amish Adults. J Clin Sleep Med, 15(9), 1321–1328. doi: 10.5664/jcsm.7928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski MR, McKenna JT, & McCarley RW (2016). Functions and Mechanisms of Sleep. AIMS Neurosci, 3(1), 67–104. doi: 10.3934/Neuroscience.2016.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


