Abstract
Aims
Catheter ablation (CA) for ventricular tachycardia (VT) can improve outcomes in patients with ischemic cardiomyopathy. Data on patients with non-ischemic cardiomyopathy are scarce. The purpose of this systematic review and meta-analysis is to compare early CA for VT to deferred or no ablation in patients with ischemic or non-ischemic cardiomyopathy.
Methods and results
Studies were selected according to the following PICOS criteria: patients with structural heart disease and an implantable cardioverter-defibrillator (ICD) for VT, regardless of the antiarrhythmic drug treatment; intervention–early CA; comparison–no or deferred CA; outcomes–any appropriate ICD therapy, appropriate ICD shocks, all-cause mortality, VT storm, cardiovascular mortality, cardiovascular hospitalizations, complications, quality of life; published randomized trials with follow-up ≥12 months. Random-effect meta-analysis was performed. Outcomes were assessed using aggregate study-level data and reported as odds ratio (OR) or mean difference with 95% confidence intervals (CIs). Stratification by left ventricular ejection fraction (LVEF) was also done. Eight trials (n = 1,076) met the criteria. Early ablation was associated with reduced incidence of ICD therapy (OR 0.53, 95% CI 0.33–0.83, p = 0.005), shocks (OR 0.52, 95% CI 0.35–0.77, p = 0.001), VT storm (OR 0.58, 95% CI 0.39–0.85, p = 0.006), and cardiovascular hospitalizations (OR 0.67, 95% CI 0.49–0.92, p = 0.01). All-cause and cardiovascular mortality, complications, and quality of life were not different. Stratification by LVEF showed a reduction of ICD therapy only with higher EF (high EF OR 0.40, 95% CI 0.20–0.80, p = 0.01 vs. low EF OR 0.62, 95% CI 0.34–1.12, p = 0.11), while ICD shocks (high EF OR 0.54, 95% CI 0.25–1.15, p = 0.11 vs. low EF OR 0.50, 95% CI 0.30–0.83, p = 0.008) and hospitalizations (high EF OR 0.95, 95% CI 0.58–1.58, p = 0.85 vs. low EF OR 0.58, 95% CI 0.40–0.82, p = 0.002) were reduced only in patients with lower EF.
Conclusion
Early CA for VT in patients with structural heart disease is associated with reduced incidence of ICD therapy and shocks, VT storm, and hospitalizations. There is no impact on mortality, complications, and quality of life. (The review protocol was registered with INPLASY on June 19, 2022, #202260080).
Systematic review registration
[https://inplasy.com/], identifier [202260080].
Keywords: systematic review, ventricular tachycardia (VT), catheter ablation, all-cause mortality, quality of life
Introduction
Sustained ventricular tachycardia (VT) in patients with structural heart disease is a life-threatening condition posing the risk of syncope and sudden cardiac death, especially with reduced left ventricular ejection fraction (LVEF). Ischemic and non-ischemic cardiomyopathy are the most common conditions associated with VT (1). Most antiarrhythmic drugs are of little value and their use is restricted in patients with LV systolic dysfunction (2). Implantable cardioverter-defibrillators (ICDs) are usually considered first-line treatment in patients with structural heart disease and VT, but this therapy does not prevent VT recurrences and ICD shocks are associated with increased mortality and decreased quality of life (3–7). Several studies and a few meta-analyses in patients with previous myocardial infarction and severe/moderate LV systolic dysfunction have shown that endocardial catheter ablation (CA) can reduce VT recurrences, ICD therapy/shocks, and VT storm (8–11). However, data on patients with non-ischemic cardiomyopathy have shown that ablation more often necessitates an epicardial approach and is less effective, probably due to the more frequent presence of midmyocardial or subepicardial arrhythmogenic substrate (12–15). This additional epicardial approach in patients with diverse etiologies might influence hard clinical outcomes, including all-cause and cardiovascular mortality.
The objective of this systematic review and meta-analysis is to investigate whether early CA with either endocardial or endo-epicardial approach for scar-related monomorphic VT improves outcomes (defined as any appropriate ICD therapy, appropriate ICD shocks, all-cause mortality, VT storm, cardiovascular mortality, cardiovascular hospitalizations, complications, and quality of life) in adult patients with ischemic or non-ischemic cardiomyopathy and ICD regardless of the antiarrhythmic drug treatment, compared to deferred ablation or no ablation.
Methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16) (Supplementary File 1) and is based on a protocol agreed upon by all authors.
Eligibility criteria
The studies included were selected according to the following PICOS criteria: patients ≥18 years old with structural heart disease and an ICD implanted or planned to be implanted for VT, regardless of the antiarrhythmic drug treatment status; interventional arm with CA performed regardless of the access (endocardial or endo-epicardial) and approach (substrate-guided or electrophysiologically guided ablation); comparison arm with no ablation or deferred ablation; ≥3 of the outcomes reported (see Section “Study selection and outcomes”); published randomized studies with follow-up ≥12 months. The length of follow-up was selected with mortality outcomes in mind.
The exclusion criteria were: studies on patients with hypertrophic cardiomyopathy, myocarditis, Chagas disease, congenital heart diseases, surgical ablation, and stereotactic radioablation.
Information sources and search strategy
PubMed, Directory of Open Access Journals (DOAJs), and Cochrane Library databases were searched independently by two of the authors (TS and MS) in June 2022. The search was not restricted by language or time period. A search string with the keywords “ablation” AND “ventricular tachycardia” was used. A PubMed search within the title and abstract was done and the results were narrowed using the filter “clinical trial.” DOAJ search was done within the titles, then the search was rerun within the abstracts. The results were narrowed using the filter “medicine.” Cochrane Library was searched within the title, abstract, and keyword with word variations, and the results were filtered using the filter “trials.”
Study selection and outcomes
Automation tools were not used at any step of the selection process. Duplicate titles were removed. The remaining titles and abstracts were reviewed by all authors independently for appropriateness. All irrelevant publications were removed. The remaining titles were reviewed in full text by the authors independently. All titles that were not rejected/approved unanimously were reviewed again for appropriateness and the final decision was taken after discussion and consensus among all authors. The remaining full-text publications were included in the review and meta-analysis.
The main outcomes analyzed include any appropriate ICD therapy, appropriate ICD shocks, all-cause mortality, and VT storm. Additional outcomes include cardiovascular mortality, cardiovascular hospitalizations, and serious adverse events related to the assigned treatment. Quality of life (physical and mental components) at the last visit was added to the additional outcomes with the amendment of the review protocol.
Data extraction and risk of bias assessment
Data on the outcomes and other variables were extracted independently by two of the authors (TS and VT) from the full texts and any supplementary files available into standardized Excel tables, and verified and approved by the third author (MS). Detailed data on quality of life were requested by e-mail from the first authors of three of the trials.
Other data extracted for the systematic review included: first author, year of publication, overall sample size, sample size of the experimental and comparison arms, comparator, inclusion criteria, ablation procedure design, access to the heart, use of navigation system, procedural end-point, primary outcome, secondary outcomes, age, sex, New York Heart Association class, LVEF, hypertension, diabetes mellitus, use of beta-blockers, amiodarone, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), and mineralocorticoid receptor antagonists, percentage of cardiac resynchronization therapy system with defibrillator (CRT-D) implants, percentage of ICDs implanted before the ablation, length of follow-up, cross-over to the ablation arm, and serious adverse events related to the study procedures.
Risk of bias assessment was done using the revised Cochrane Risk of Bias 2 tool (RoB 2) for randomized trials in categories: selection (random sequence generation and allocation concealment), performance (blinding of participants and personnel), detection (measurement of the outcome), attrition (missing outcome data), reporting (selection of the reported result), and others. The assessment was done by all authors independently. Disagreements were resolved by discussion and consensus.
Effect measures and synthesis methods
Outcomes were assessed using aggregate study-level data. Dichotomous outcomes are reported as odds ratio (OR) with 95% confidence intervals (CIs). The difference in the quality of-life scores between the experimental and control arms at the last visit is reported as a mean difference (MD) with 95% CI. A p-value < 0.05 for the overall effect was considered significant.
Studies were deemed eligible for each synthesis if the specific outcome was reported.
Data for dichotomous outcomes and quality-of-life scores were extracted directly from the study report and/or the supplementary files.
The results of the syntheses are presented as forest plots with studies arranged in descending order by publication date. Plots include the number of events (or the mean difference), total number of patients in each arm of each study, the overall number of events and patients, and the weight of the study. Statistical tests for heterogeneity and for overall effect are also shown in the forest plots. The main characteristics of the studies are shown in Tables 1, 2 arranged by publication date.
TABLE 1.
Main characteristics of the studies.
| Study | SMASH-VT | VTACH | VANISH | SMS | BERLIN VT | PARTITA | SURVIVE-VT | PAUSE-SCD |
| Author | Reddy | Kuck | Sapp | Kuck | Willems | Della Bella | Arenal | Tung |
| Year | 2007 | 2010 | 2016 | 2017 | 2020 | 2022 | 2022 | 2022 |
| Sample size (n) | 128 | 107 | 259 | 111 | 159 | 47 | 144 | 121 |
| Sample size experimental/ comparator (n) |
64/64 | 52/55 | 132/127 | 54/57 | 76/83 | 23/24 | 71/73 | 60/61 |
| Experimental arm | ICD + pre- or post-ICD ablation | Ablation followed by ICD implantation | ICD + ablation | Ablation followed by ICD implantation | Ablation followed by ICD implantation | ICD + ablation | ICD + ablation | ICD + pre- or post-ICD ablation |
| Comparator arm | ICD alone, no ablation | ICD alone, no ablation | ICD + AAD escalation, no ablation | ICD alone, no ablation | ICD implantation + deferred ablation (after 3 ICD shocks) | ICD alone, no ablation | ICD + AAD, no ablation | ICD alone, no ablation |
| Inclusion criteria | Age >18 years, history of MI (documented by ECG or cardiac imaging) and a planned or a recent (within 6 month) implantation of an ICD for VF, hemodynamically unstable VT, or syncope with inducible VT during invasive EPS; patients with an ICD for primary prevention and with subsequent appropriate ICD therapy for a single event | Age 18–80 years, indication for a secondary prevention ICD after documented stable clinical VT without reversible cause, CAD, previous MI, and reduced LVEF ≤50% | Previous MI, an ICD implanted, and an episode of VT during treatment with amiodarone or another class I or class III AAD within the previous 6 months | Age 18–80 years, CAD, LVEF ≤40% and clinically unstable spontaneous VT, or cardiac arrest, or syncope with unstable VT inducible at the baseline EPS | Previous MI (>4 weeks), LVEF 30–50%), life-threatening ventricular arrhythmias necessitating ICD implantation, including documented sustained VT | ICM or NICM and primary or secondary prevention indication for ICD, after having received 1st appropriate shock for VT after the observational phase | Previous MI (>6 weeks), optimal medical treatment (if ventricular dysfunction), and an episode of very symptomatic VT defined as: (1) sustained VT treated using ICD shock (<6 months); and (2) sustained VTs with syncope, even if terminated with ATP; monomorphic VT necessitating ICD; not receiving AAD (amio + bb or sotalol + bb or amio) | Age >18 years, ICM, DCM, ARVC, EF <50% and an indication for an ICD for secondary prevention of monomorphic VT or criteria for primary prevention ICD with inducible monomorphic VT during EPS |
| Procedure design | Prophylactic substrate ablation in sinus rhythm–linear lesions at good pace-mapping sites, ablation of late/fractionated potentials, encirclement of small infarcts | Prophylactic ablation in stable VT; substrate modification in non-inducible or unstable VT by pace-mapping based linear lesions | Activation mapping and ablation of all inducible VTs; if not tolerated–substrate ablation guided by pace-mapping and late potentials. | Prophylactic ablation of all inducible VTs; substrate modification. | Preventive ablation of late potentials | Late potential ablation in sinus rhythm followed (if necessary) by activation mapping and ablation in VT | Substrate ablation in sinus rhythm–late potentials, conducting channels, pace-mapping; AAD not allowed in the ablation arm | Substrate ablation in sinus rhythm–late/abnormal potentials within the scar, pace-mapping; activation mapping in stable VT |
| Access | Endocardial | Endocardial | Endocardial | Endocardial | NR | Endocardial/endo-epicardial | Endocardial | Endocardial/ endo-epicardial |
| EAM | CARTO | CARTO/EnSite | Yes (NS) | CARTO/EnSite | Yes (NS) | CARTO/EnSite | CARTO/EnSite | EnSite |
| Procedure end-point | NR (non-inducibility?) | Non-inducibility or abolition of all channels within the scar | Termination and non-inducibility of VT | Non-inducibility | Abolition of all late potentials and non-inducibility | Abolition of late potentials and non-inducibility | Elimination of all arrhythmogenic substrate (post-ablation induction of VT avoided) | Non-inducibility of targeted VT and elimination of abnormal EGMs; complete non-inducibility not mandatory |
| Primary outcome | Survival free from any appropriate ICD therapy (shocks or ATP) | Time from ICD implantation to recurrence of any sustained VT or VF | Composite of death at any time after randomization or VT storm or appropriate ICD shock after a 30-day treatment period | Time to first recurrence of VT/VF | Composite of all-cause death and unplanned hospitalization for either symptomatic ventricular tachyarrhythmia or worsening HF | Composite of death from any cause or worsening HF that led to hospitalization | Composite of CV death, appropriate ICD shock, unplanned hospitalization for worsening HF, or severe treatment-related complications | Composite of VT recurrence (any appropriate ICD therapy or documented SMVT), CV hospitalization (for HF, complications or arrhythmia), or death |
| Secondary outcomes | Freedom from any appropriate ICD shock, death, and ICD storm | Survival free from death, syncope, hospital admission for a cardiac reason, and VT storm | Each of the primary outcome components and adverse effects | Appropriate ICD therapies, QoL, hospital readmissions because of a cardiac indication, and severe clinical events (death, number of syncope and of electrical storm episodes) | Sustained VT or VF, appropriate ICD therapy, inappropriate ICD therapy, death from any cause, death from cardiac causes, unplanned hospitalization for any cause, unplanned hospitalization for cardiac reasons, change in QoL, short-term success and complications of ablation, all serious adverse events | Death from cardiac causes, recurrences of sustained VT or VF, appropriate ICD therapy, electrical storm | Each of the primary outcome components as well as sustained VT or VF, appropriate and inappropriate ICD therapies, death from any cause, unplanned hospitalization for ventricular arrhythmias and cardiac events, change in LVEF, and QoL | Each of the primary outcome components |
AAD, antiarrhythmic drug; amio, amiodarone; ARVC, arrhythmogenic right ventricular cardiomyopathy; ATP, antitachycardia pacing; bb, beta-blocker; CAD, coronary artery disease; CV, cardiovascular; DCM, dilated cardiomyopathy; EAM, electroanatomic mapping; EF, ejection fraction; EGM, electrogram; EPS, electrophysiologic study; HF, heart failure; ICD, implantable cardioverter-defibrillator; ICM, ischemic cardiomyopathy; MI, myocardial infarction; NA, not available/applicable; NICM, non-ischemic cardiomyopathy; NR, not reported; NS, not specified; QoL, quality of life; SMVT, sustained monomorphic VT; VT, ventricular tachycardia; VF, ventricular fibrillation.
TABLE 2.
Main characteristics of the patient population of the studies analyzed.
| Study | SMASH-VT | VTACH | VANISH | SMS | BERLIN VT | PARTITA | SURVIVE-VT | PAUSE-SCD |
| Author | Reddy | Kuck | Sapp | Kuck | Willems | Della Bella | Arenal | Tung |
| Year | 2007 | 2010 | 2016 | 2017 | 2020 | 2022 | 2022 | 2022 |
| Sample size (n) | 128 | 107 | 259 | 111 | 159 | 47 | 144 | 121 |
| Intervention/control sample size (n) | 64/64 | 52/55 | 132/127 | 54/57 | 76/83 | 23/24 | 71/73 | 60/61 |
| Age (years) | 67 ± 9/66 ± 10 | 67.7 ± 8.3/64.4 ± 8.2 | 70.3 ± 7.3/67.0 ± 8.6 | 68.4 ± 7.7/65.9 ± 8.4 | 66 ± 10/66 ± 9 | 71.2 ± 8.1/65.6 ± 9.6 | 70 (63–75)/71 (64–76) | 51 (45.5–65)/57 (47–63) |
| Males (%) | 92/81 | 96/91 | 92.9/93.2 | 87/81 | 88.2/86.7 | 83/88 | 98.6/93.2 | 73.3/88.5 (p = 0.03) |
| NYHA class (%) (ablation/control | 84/77% in I-II; 16/23% in III-IV | NR (class IV excluded) | 77.3/75.5% in I-II; 22.7/24.4% in III | NR (class IV excluded) | 77.6/78.3% in I-II; 22.4/21.7% in III | 87/81% in I-II; 13/19% in III | 91.4/93.2% in I-II; 8.6/6.8% in III | 76.7/83.6% in I-II; 20.3/13.1% in III; 3.3/3.3% in IV |
| LVEF (%) | 30.7 ± 9.5/32.9 ± 8.5 | 34 ± 9.6/34.1 ± 8.8 | 31.2 ± 10.7/31.1 ± 10.4 | 32 ± 6.9/30.4 ± 7.3 | 41 ± 6/41 ± 6 | 31.9 ± 9/32.4 ± 8.3 | 35 (26–41)/33 (25–40) | 41 (31–60)/40 (30–48) |
| HTN (%) | 73/67 | NR | 69.3/69.7 | NR | 81.6/79.5 | 81/68 | 78.9/64.4 | 31.7/34.4 |
| DM (%) | 38/50 | NR | 31.5/28.0 | NR | 30.3/26.5 | 19/41 | 29.6/20.5 | 13.3/24.6 |
| b-blocker (%) | 94/98 | 75/75 | 93.9/96.1 | 91/91 | 76.3/71.1 | 100/100 | 97.2/86.1 | 78.3/86.9 |
| ACEI/ARB (%) | 92/92 | NR | 87.9/87.4 | 90/100 | 61.8/71.1 | 87/92 | 98.6/90.3 | 43.3/50.8 |
| MRA (%) | NR | NR | NR | NR | 23.7/25.3 | NR | 55.7/60.9 | NR |
| Amiodarone (%) | 0/0 (exclusion criterion) | 35/35 | 64.4/66.1 | 30/35 | 40.8/26.5 | 5/21 | 0/87.1 | 33.3/37.7 |
| CRT-D (%) | 0/0 | 0/0 | 22/17.3 | 9.3/10.5 | 11/3.6 | 30/33 | 15.5/18.1 | 6.7/6.6 |
| ICD implanted before the ablation | 87% | 7.7% | 100% | 11% | 0% | 100% | 95.8% | NR |
| Follow-up (months) | 22.5 ± 5.5 | 22.5 ± 9 | 27.9 ± 17.1 | 27.6 ± 13.2 | 13.2 ± 9.5 | 24.2 (8.5–24.4) | 23.8 (16.6–24)/23.3 (9.4–23.9) | 31.3 (20.1–40) |
| Cross-over to ablation (n) | NR | 12 | 11 | 1 | 8 | 1 | 18 | NR |
| Severe complications related to the study procedures(n) | 3/0 | 6/9 | 20/39 | 13/9 | 14/8 | 0/0 | 7/21 | 5/0 |
Cells with two numbers present intervention/control arms. The central tendency is presented as mean ± SD or median (IQR 25–75%). ACEI, Angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CRT-D, cardiac resynchronization therapy system with defibrillator; DM, diabetes mellitus; HTN, hypertension; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonist; NA, not available/applicable; NYHA, New York Heart Association; NR, not reported.
We conducted a random-effect meta-analysis using the inverse-variance DerSimonian–Laird model estimator. An outcome less frequently present in the experimental intervention arm has an OR < 1. A positive value of MD favors the experimental arm. Heterogeneity between studies was assessed with the Cochrane Q statistic and the I2 statistic. Values of the I2 statistic ≤50% were considered to show low/moderate heterogeneity, while those >50% were accepted as designating substantial/considerable heterogeneity. Review Manager 5.4 (17) was used for writing the review and performing the statistics.
Sensitivity analysis was done using the leave-one-out approach by excluding consecutively one study at a time.
Funnel plots for publication bias were constructed. Funnel plot asymmetry was assessed by the R package metafor for jamovi 2.3 (18) using rank correlation and regression tests. p-value < 0.05 was considered significant.
Certainty assessment
Certainty assessment was done using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group tool (19). All authors had access to the tool and worked independently. Outcomes were assessed in the following domains: risk of bias, inconsistency, indirectness, and imprecision. The results are presented in a summary-of-findings table including absolute and relative measures of effect with CIs and the certainty of evidence.
Results
Search results and study selection
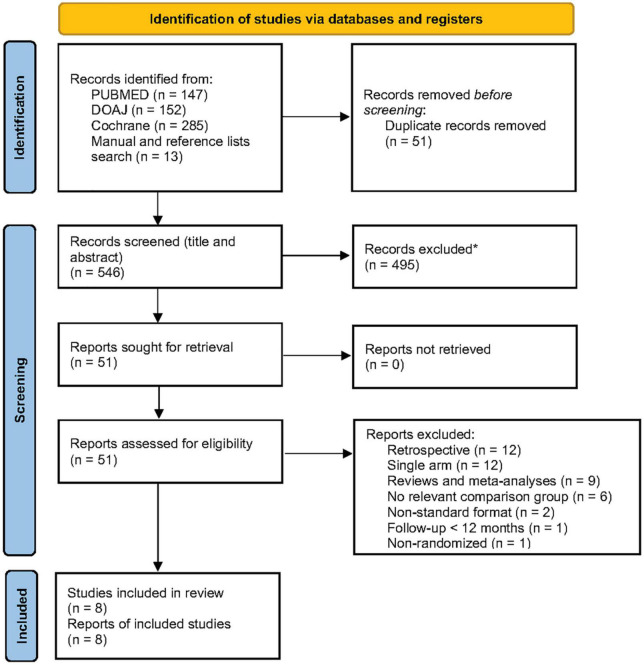
Overall, 597 records were identified. After the removal of duplicates and irrelevant studies, 51 reports were assessed for eligibility. Forty-three reports were excluded with reasons, and the remaining eight studies were included in the review (Figure 1). The search was repeated using the same criteria on August 7, 2022 without providing any new studies fulfilling the eligibility criteria.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart for study identification and screening. *Automation tools were not used. From: Page et al. (16). For more information, visit: http://www.prisma-statement.org/.
Several studies that were deemed unsuitable for this review deserve to be mentioned. The short follow-up of only 6 months was the single reason to exclude the small randomized feasibility CALYPSO pilot trial (20). With the two mortality outcomes in mind, we had decided beforehand to include studies with longer follow-ups thus allowing for more events. Another large prospective study on 206 patients (non-ischemic cardiomyopathy in 43%) by Acosta et al. (21) fulfilled almost all inclusion criteria except for its non-randomized design and only a few outcomes were reported. Two Japanese studies were also excluded (22, 23). Both together had almost 190 patients with different etiologies of structural heart disease, but were retrospective and single-arm, and reported only a limited number of outcomes. Finally, a recent study by Yadav with 72 patients had to be removed from further review as well due to its non-randomized nature and only a few outcomes reported (24).
Study characteristics
Eight randomized clinical trials satisfying the criteria were included in this review and meta-analysis–SMASH-VT (25), VTACH (26), VANISH (27), SMS (28), BERLIN VT (29), PARTITA (30), SURVIVE-VT (31), and PAUSE-SCD (32). These trials enrolled overall 1,076 patients–532 in the early ablation arm and 544 in the control arm. The main characteristics of the studies are shown in Tables 1, 2.
The design of the ablation procedure in all trials included some type of arrhythmogenic substrate modification. Two of the most recent studies (30, 32) allowed epicardial or endo-epicardial access. The procedural end-point was predominantly non-inducibility and abolition of the abnormal signals within the scar. PAUSE-SCD did not mandate complete non-inducibility, while in SURVIVE-VT post-ablation VT induction was even avoided. The etiology of structural heart disease was mainly ischemic. Two recent trials (30, 32) included patients with non-ischemic cardiomyopathy (pooled n = 46) and arrhythmogenic cardiomyopathy (n = 42), accounting for 8.2% of the pooled patient population. The majority of the studies (25–28, 30, 31) had a mean/median follow-up of approximately 2 years, with the shortest follow-up being 13 months (29), while the longest one reached 31 months (32).
The use of beta-blockers was around 75% in three studies (26, 29, 32) and >90% in the other five studies. ACEIs/ARBs were used in at least 87% of the patients in five studies (25, 27, 28, 30, 31). Two studies had lower use of ACEI/ARB (29, 32), while one did not report data on these drugs (26). Only one study reported the use of mineral-corticoid receptor antagonists in one-quarter of the patients (29), another one reported combined data on renin–angiotensin–aldosterone system inhibitors (31), while in the remaining six trials data were not reported. The use of amiodarone was quite variable across the trials and was an exclusion criterion in SMASH-VT and in the ablation arm of SURVIVE-VT. The overwhelming majority of the patients were men at a mean age of 65–70 years, hypertension was present in 70–80%, and diabetes mellitus was present in around 30%. Two trials did not enroll patients with NYHA class IV heart failure and did not report the NYHA class of the enrolled patients (26, 28), while in the other six trials 75–92% of the patients were in NYHA class I or II. Only two studies enrolled patients in NYHA class IV (25, 32), but only one of them reported specifically that these were 3.3% of the study population (32). In most trials, the mean LVEF was 30–35% except for BERLIN VT and PAUSE-SCD where it was 40%. Relatively more women were enrolled in PAUSE-SCD, and overall, the patients in this trial were younger, with less severe LV dysfunction, and had less hypertension and diabetes. Cross-over to ablation was reported in six studies for overall 51 patients.
Implantable cardioverter-defibrillator was implanted before the ablation in 87–100% of the patients in the ablation arm in four trials (25, 27, 30, 31), and in 8–11% in two other trials (26, 28). All patients in BERLIN VT had the ICD implanted after the ablation. In PAUSE-SCD, the “ablation was done a median 2 days before ICD implantation (IQR, 5 days before to 14 days after)” (32). CRT-D was implanted in 7–31% of the patients in six trials (27–32) and in none of the patients in the other two trials.
Quality of life was assessed in 198 patients in three studies (28, 29, 31) using the Medical Outcomes Short Form-36 general health survey (SF-36). Although SF-36 quality of life data was collected and reported also in VTACH and VANISH (33), the data were presented in a way precluding comparison and meta-analysis.
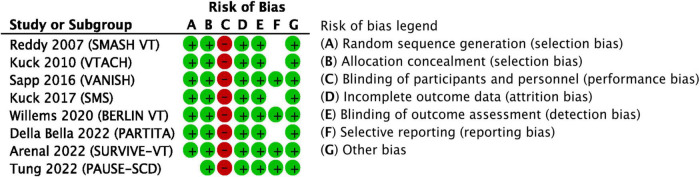
Risk of bias
The risk of bias for all domains in all studies is shown in Figure 2. One article (32) reported a switch to central randomization, while the study was ongoing and after having enrolled approximately 20% of all patients. This study showed significant male prevalence in the control arm and no differences in the other baseline characteristics. Although this difference could be due to a play of chance, we preferred to be conservative and to label the random sequence generation risk of bias as unclear. The risk of performance bias was unavoidably high in all studies given the invasive nature of the intervention and the lack of a sham control procedure. Three studies (25, 28, 32) did not report whether the outcome assessors were blinded to the intervention; however, the detection bias was deemed low. The reporting bias was deemed unclear in studies where we were not able to find a protocol published ahead of the main results article or where the protocol was significantly amended during the course of the study (25, 26, 28, 30). In all other domains, the risk of bias was deemed low. We have to note that specifically for the secondary outcome “quality of life” the risk of attrition bias and the risk of bias in the outcome measurement were high in all studies reporting this outcome (28, 29, 31).
FIGURE 2.
Results of the overall risk of bias assessment. Red circle–high risk, green circle–low risk, empty space–unclear risk.
Outcomes–Results of syntheses
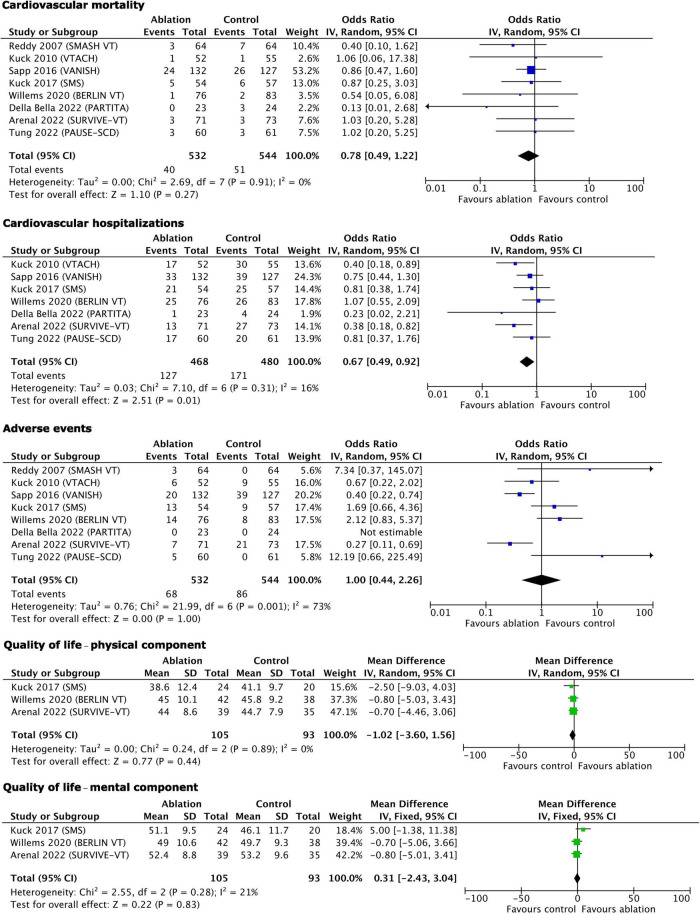
All studies reported appropriate ICD shocks, all-cause mortality, cardiovascular mortality, and adverse events related to the study procedures (no complications were reported in PARTITA and individual OR for this study was not estimable; however, this did not impact the pooled OR). Cardiovascular hospitalizations were reported by seven studies, any appropriate ICD therapy and VT storm were reported by six studies, and changes in the quality of life–by three studies.
The results of the statistical syntheses of the primary and secondary outcomes are presented as forest plots in Figures 3, 4.
FIGURE 3.
Main outcomes–results of quantitative synthesis.
FIGURE 4.
Secondary outcomes–results of quantitative synthesis.
Within the primary outcomes, significant benefits from early ablation were found for any appropriate ICD therapy (OR 0.53, 95% CI 0.33–0.83, p = 0.005), appropriate ICD shocks (OR 0.52, 95% CI 0.35–0.77, p = 0.001), and VT storm (OR 0.58, 95% CI 0.39–0.85, p = 0.006). There was no effect of early ablation on all-cause mortality (OR 0.91, 95% CI 0.58–1.45, p = 0.7).
Among the secondary outcomes, only cardiovascular hospitalizations benefited from early ablation (OR 0.67, 95% CI 0.49–0.92, p = 0.01). Cardiovascular mortality (OR 0.78, 95% CI 0.49–1.22, p = 0.27), complications/serious adverse events (OR 1.00, 95% CI 0.44–2.26, p = 1.00), physical component of the quality of life (MD −1.02, 95% CI −3.60 to +1.56, p = 0.44), and mental component of quality of life (MD 0.31, 95% CI −2.43 to +3.04, p = 0.83) were not influenced by early ablation. With regard to the adverse events, it is worth emphasizing that only study procedure-related events were counted as follows: (1) Ablation-related complications and ICD implantation-related complications (in studies where ICD was implanted peri-ablation) in the early ablation group; (2) ICD implantation-related complications in the control group in trials where ICD had to be implanted peri-randomization; (3) ablation-related adverse events in the control group with deferred ablation; and (4) antiarrhythmic drug treatment-related events in the control group in trials comparing ablation to antiarrhythmic drug treatment.
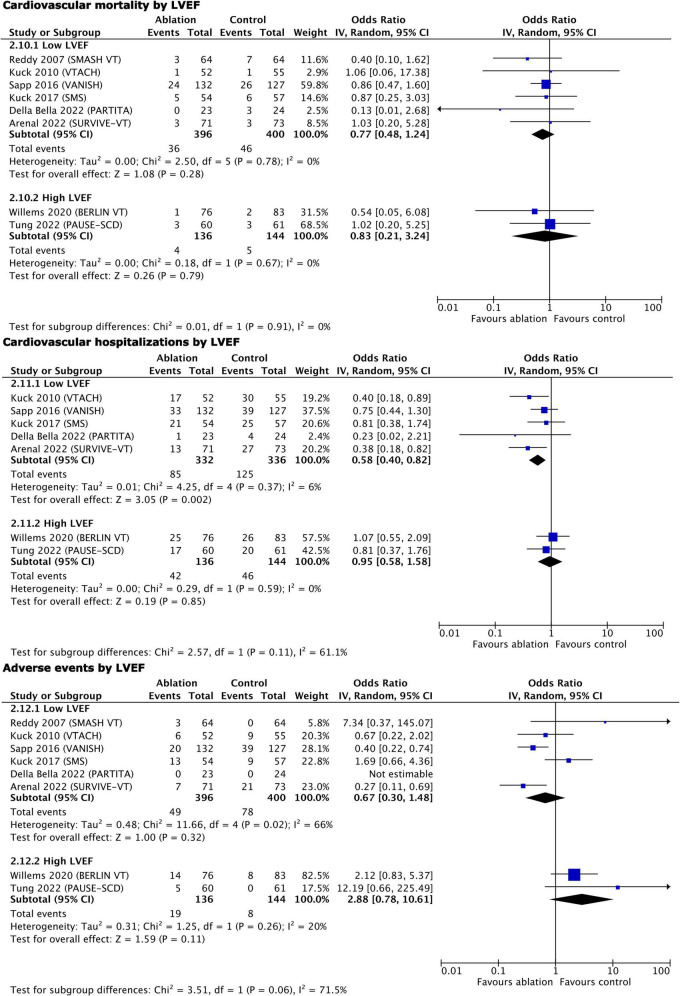
Two of the studies reported a higher mean LVEF of 40% (29, 32); thus, we performed a subgroup analysis of all primary and secondary outcomes except for VT storm and quality of life, stratified by LVEF (Figures 5, 6). Early ablation favored reduction of any appropriate ICD therapy only with higher LVEF (high LVEF OR 0.40, 95% CI 0.20–0.80, p = 0.01 vs. low LVEF OR 0.62, 95% CI 0.34–1.12, p = 0.11; p = 0.35 for subgroup differences). On the contrary, only trials with lower LVEF showed a reduction of appropriate ICD shocks (high LVEF OR 0.54, 95% CI 0.25–1.15, p = 0.11 vs. low LVEF OR 0.50, 95% CI 0.30–0.83, p = 0.008; p = 0.86 for subgroup differences), as well as of cardiovascular hospitalizations (high LVEF OR 0.95, 95% CI 0.58–1.58, p = 0.85 vs. low LVEF OR 0.58, 95% CI 0.40–0.82, p = 0.002; p = 0.11 for subgroup differences). All-cause mortality remained unaffected by LVEF stratification (high LVEF OR 1.94, 95% CI 0.68–5.54, p = 0.21 vs. low LVEF OR 0.80, 95% CI 0.50–1.27, p = 0.35; p = 0.13 for subgroup differences), as did cardiovascular mortality (high LVEF OR 0.83, 95% CI 0.21–3.24, p = 0.79 vs. low LVEF OR 0.77, 95% CI 0.48–1.24, p = 0.28; p = 0.91 for subgroup differences) and complications (high LVEF OR 2.88, 95% CI 0.78–10.61, p = 0.11 vs. low LVEF OR 0.67, 95% CI 0.30–1.48, p = 0.32; p = 0.06 for subgroup differences).
FIGURE 5.
Main outcomes, stratified by LVEF–results of quantitative synthesis.
FIGURE 6.
Secondary outcomes, stratified by LVEF–results of quantitative synthesis.
Heterogeneity and sensitivity analysis
The Q and I2 statistics showed only low to moderate heterogeneity for all primary outcomes, except for any appropriate ICD therapy where the Q statistic showed a strong tendency to significance (p = 0.06), while the I2 had a value of 52% pointing formally to substantial heterogeneity (Figure 3). Similarly, the Q and I2-tests for the secondary outcomes showed low heterogeneity, except for the complications related to the assigned treatment where it was substantial/considerable (Figure 4).
Leave-one-out sensitivity analysis was done for all primary and secondary outcomes. The removal of one study at a time resulted in no significant change in OR, MD, and CI for neither of the outcomes, demonstrating the robustness of the main analyses. The results after the removal of the study with the largest weight are shown in Table 3. In most cases, this was VANISH, being the study with the largest population enrolled and consequently with the highest number of events during the follow-up. BERLIN VT had the largest weight for the outcome “any appropriate ICD therapy,” while SURVIVE-VT had the largest weight for the two quality-of-life outcomes.
TABLE 3.
Sensitivity analysis–Leave-one-out approach.
| Outcome (number of studies in the main/sensitivity analysis) | Main analysis | Sensitivity analysis |
| Any appropriate ICD therapy (6/5) | OR 0.53 (95% CI 0.33–0.83), p = 0.005 | OR 0.52 (95% CI 0.29–0.92), p = 0.02 |
| ICD shocks (8/7) | 0.52 (95% CI 0.35–0.77), p = 0.001 | 0.46 (95% CI 0.30–0.71), p = 0.0004 |
| All-cause mortality (8/7) | OR 0.91 (95% CI 0.58–1.45), p = 0.70 | OR 0.88 (95% CI 0.47–1.66), p = 0.70 |
| VT storm (6/5) | OR 0.58 (95% CI 0.39–0.85), p = 0.006 | OR 0.51 (95% CI 0.29–0.90), p = 0.02 |
| Cardiovascular mortality (8/7) | OR 0.78 (95% CI 0.49–1.22), p = 0.27 | OR 0.69 (95% CI 0.36–1.33), p = 0.27 |
| Cardiovascular hospitalizations (7/6) | OR 0.67 (95% CI 0.49–0.92), p = 0.01 | OR 0.64 (95% CI 0.43–0.95), p = 0.03 |
| Complications (8/7) | OR 1.00 (95% CI 0.44–2.27), p = 1.00 | OR 1.28 (95% CI 0.50–3.32), p = 0.61 |
| QoL physical component (3/2) | MD −1.02 (95% CI −3.60 to +1.56), p = 0.44 | MD −1.30 (95% CI −4.85 to +2.25), p = 0.47 |
| QoL mental component (3/2) | MD 0.31 (95% CI −2.43 to +3.04), p = 0.83 | MD 1.11 (95% CI −2.49 to +4.72), p = 0.54 |
CI, confidence interval; ICD, implantable cardioverter-defibrillator; MD, mean difference; OR, odds ratio; QoL, quality of life; VT, ventricular tachycardia.
Reporting biases and certainty of evidence
The rank correlation and regression tests showed potential funnel plot asymmetry only for the outcome “Appropriate ICD shocks” (p = 0.031 and p = 0.008, respectively, for the two tests). Funnel plots for all main and secondary outcomes are shown in Supplementary Figure 1.
The quality-of-life outcomes were obviously biased due to significant attrition of follow-up data–only 198 out of 414 patients had an assessment at the last visit. Besides, quality of life is a self-reported outcome (i.e., the participants are outcome assessors) and, given the fact that all studies were open-label, it is possible that the participants were biased in some way by the assigned intervention.
The summary of the findings according to the certainty of evidence (GRADE) is shown in Table 4.
TABLE 4.
Summary of findings.
| Outcomes effect measure follow-up |
Number of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects |
|
| Risk with deferred ablation or no ablation | Risk difference with early ablation* | ||||
| Any appropriate ICD therapy–assessed with: odds ratio Follow-up: mean 23.4 months |
770 (6 RCTs) |
⊕⊕⊕○ Moderatea |
OR 0.53 (0.33 to 0.83) |
440 per 1,000 | 146 fewer per 1,000 (234 fewer to 45 fewer) |
| Appropriate ICD shocks–assessed with: odds ratio Follow-up: mean 24.2 months |
1,076 (8 RCTs) |
⊕⊕⊕○ Moderatea |
OR 0.52 (0.35 to 0.77) |
313 per 1,000 | 121 fewer per 1,000 (175 fewer to 53 fewer) |
| All-cause mortality–assessed with: odds ratio Follow-up: mean 24.2 months |
1,076 (8 RCTs) |
⊕⊕⊕○ Moderateb |
OR 0.91 (0.58 to 1.45) |
145 per 1,000 | 11 fewer per 1,000 (56 fewer to 52 more) |
| VT storm–assessed with: odds ratio Follow-up: mean 24.7 months |
796 (6 RCTs) |
⊕⊕⊕⊕ High |
OR 0.58 (0.39 to 0.85) |
213 per 1,000 | 77 fewer per 1,000 (117 fewer to 26 fewer) |
| CV mortality–assessed with: odds ratio Follow-up: mean 24.2 months |
1,076 (8 RCTs) |
⊕⊕⊕○ Moderateb |
OR 0.78 (0.49 to 1.22) |
94 per 1,000 | 19 fewer per 1,000 (46 fewer to 18 more) |
| CV hospitalizations–assessed with: odds ratio Follow-up: mean 24.3 months |
948 (7 RCTs) |
⊕⊕○○ Lowb |
OR 0.67 (0.49 to 0.92) |
356 per 1,000 | 86 fewer per 1,000 (143 fewer to 19 fewer) |
| Complications–assessed with: odds ratio Follow-up: mean 24.2 months |
1,076 (8 RCTs) |
⊕○○○ Very lowc |
OR 1.00 (0.44 to 2.26) |
158 per 1,000 | 0 fewer per 1,000 (80 fewer to 149 more) |
| QoL physical component–assessed with: mean difference Scale from: 0 to 100 Follow-up: mean 20 months |
198 (3 RCTs) |
⊕○○○ Very lowb,d,e |
– | The mean QoL physical component was 0 | MD 1.02 lower (3.6 lower to 1.56 higher) |
| QoL mental component–assessed with: mean difference Scale from: 0 to 100 Follow-up: mean 20 months |
198 (3 RCTs) |
⊕○○○ Very lowb,d,e |
– | The mean QoL mental component was 0 | MD 0.31 higher (2.43 lower to 3.04 higher) |
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI, confidence interval; CV, cardiovascular; MD, mean difference; OR, odds ratio; ICD, implantable cardioverter-defibrillator; QoL, quality of life; VT, ventricular tachycardia.
Explanations:
aICD therapies as a possible surrogate measure of clinically relevant VT/FT.
bAlthough studies were conducted within a timespan of 15 years, three of them were conducted during the last 6 years and this outcome could have been modified by modern drug therapies.
cIn most studies ablation and ICD implantation were done within a narrow time window and it could be difficult to assign a potential complication to a single procedure. In the two studies comparing ablation to antiarrhythmic drugs, serious adverse events were significantly more frequent in the drug treatment arm.
dThe results were derived from approximately 48% of the pooled patient population of the three studies reporting QoL outcomes.
eHigh risk of attrition bias and high risk of bias in the measurement of the QoL outcomes for all three studies.
Discussion
This updated review with a meta-analysis of randomized trials comparing early CA for VT to deferred or no ablation in patients with structural heart disease clearly showed that appropriate ICD shocks and therapies, VT storm, and cardiovascular hospitalizations were significantly reduced with early ablation. There was no benefit from early ablation on all-cause and cardiovascular mortality, and quality of life. Reassuringly, adverse events related to the treatment were equally distributed among the intervention and control arms.
Irrespective of our better understanding of the arrhythmogenic substrate in various etiologies, technological advances, and the use of epicardial approach, the mortality outcomes in this meta-analysis, compared to previously published (10, 11), remained mostly unchanged with early CA vs. deferred of no ablation in patients with structural heart disease and an ICD implanted. The cause for such a lack of improvement might be the lack of tools providing complete single-stepped elimination of the substrate, but more probably it is the underlying disease process that progresses over time and creates new substrate or leads to pump failure and death. It could be equally possible that modern heart failure drug therapies and CRT devices actually improve survival even in patients with VT and no ablation or deferred ablation. Also, three of the trials included death as a secondary outcome and thus may have been statistically underpowered to detect small but significant differences. Nevertheless, early ablation should not be regarded as a tool to improve survival, unless new data emerge in future clinical trials.
Moreover, the ablation of non-ischemic cardiomyopathy and the use of epicardial approach in two of the included trials did not increase mortality or procedure-related complications, which is reassuring despite the small number of patients. Also, the advantages of early ablation on a significant reduction of ICD therapies, shocks, and VT storm, known from previous publications (10, 11), were preserved. In contrast to a previously published meta-analysis (10), we found a significant reduction in cardiovascular hospitalizations. All these findings may translate into reduced health-related expenditures thus potentially further reducing the burden on the healthcare systems (34).
Overall, the results of the addition of three contemporary trials with more than 300 patients in this updated review reinforce our current understanding that CA for recurrent scar-related VT should be implemented early (35, 36). Interestingly, one of those very recent trials–SURVIVE-VT (31) compared ablation to antiarrhythmic drug treatment similar to an earlier trial–VANISH (27), and in both the adverse events were more frequent in the drug treatment control arm. This further supports the notion of early CA. We also need to note that 100% of the patients in VANISH and PARTITA (n = 306 or 28.4% of the pooled patient population) and an unknown number of patients in SMASH-VT and SURVIVE-VT had received their ICD well before the ablation and the adverse events directly related to the device implantation are missed in our meta-analysis. Consequently, we could speculate that adverse events in the control arm might be even more than detected in our review, although we do not believe that the OR would be changed significantly in favor of the ablation. The inclusion of the most recent trials into the current meta-analysis might also explain the observed differences in cardiovascular hospitalization in comparison to the previously published meta-analysis. The new studies implemented newer approaches to ablation resulting in possibly better outcomes, thus ensuring less need for cardiovascular hospitalization.
To the best of our knowledge, this is the first meta-analysis including quality of life after ablation as an outcome. ICD shocks are recognized as a major cause of impaired quality of life and may translate into anxiety and depression, loss of work capacity, and sick leaves. Thus, they represent a serious problem for patients with ICD and their relatives (7). According to the results of the current meta-analysis, quality of life was not improved by early ablation which may seem counter-intuitive. This might be explained by the fact that two out of the five trials assessing this outcome reported their results in a way that was not suitable for inclusion in this meta-analysis (26, 33). In addition, only a fraction of the patients in the other three trials had a complete assessment. Because both VTACH and VANISH did not report significant changes, it seems unlikely that the results of the meta-analysis on quality of life would have been different if these trials had been included. Future studies should implement rigorous data collection on quality of life as an outcome, especially bearing in mind that hard clinical outcomes did not improve after many years of technological advances. In this regard, we need to mention that several trials on ablation for VT in structural heart disease are ongoing or are active but not yet recruiting patients. CAAD-VT (ACTRN12620000045910)1, Code STORM (See text footnote 1, ACTRN12620001176954), and EPI-VT (NCT04512911)2 will not assess the quality of life. However, this outcome is among the secondary ones in three other trials: MANTRA-VT (See text footnote 2, NCT02303639, unknown recruitment status), PREVENTIVE-VT (See text footnote 2, NCT03421834), and VANISH2 (See text footnote 2, NCT02830360). The latter two trials will evaluate also ICD shock-related anxiety and depression.
The additional quantitative synthesis stratified by LVEF showed that ICD therapies were reduced only in patients with higher LVEF, while the ICD shocks and cardiovascular hospitalizations were reduced only in patients with lower LVEF. However, the trends to benefit in the groups with non-significant change were strong, hence, we do not feel that early ablation should be applied selectively according to the baseline LVEF. Mortality outcomes were not impacted by this stratification; hence, one can speculate that VT recurrence (as reflected by appropriate ICD therapy and shocks) is only a marker for mortality risk. Moreover, there is a known association between ICD shocks and mortality (3–6), but the reduction of shocks in this and previous meta-analyses (10, 11) did not translate into mortality benefit.
Limitations of evidence
Some differences between the studies included in this meta-analysis could not be accounted for by the statistical measurement of heterogeneity. There were different enrolment criteria in terms of primary/secondary prevention and hemodynamic instability of the qualifying arrhythmia. The differences in the proportion of patients who were on baseline amiodarone therapy and the diverse protocols regarding antiarrhythmic drugs use before and after ablation might have led to some bias in the results. In addition, there was variation in the VT mapping approach as well as ablation end-point definition among the studies. Cross-over of patients who were randomized to the control arm but went on to undergo ablation during follow-up may also have introduced some heterogeneity.
Assessment of outcomes based on etiology and access to the heart was not possible in a study-level meta-analysis. Subgroup analysis based on LVEF used mean/median LVEF reported in the individual studies, hence, the data likely are overlapping and heterogenous and although we purposefully used a random effect model, it is not clear whether this was sufficient to overcome this heterogeneity.
Limitations of review processes
Assessment of risk of bias and certainty of evidence has an unavoidable element of subjectivity. However, we tried to overcome this by including all authors in the assessment and by discussing all disputed issues until reaching a uniform agreement.
Implications
The findings of our systematic review and meta-analysis of randomized studies reinforce the use of early ablation in patients with structural heart disease and recurrent VT with the purpose of reducing ICD therapies and shocks, VT storm, and hospitalizations for cardiovascular reasons, without increasing the complications related to the treatment. Unless new convincing data emerge, ablation should not be used with the purpose of reducing mortality. Future studies with rigorous data collection should shed light on the impact of ablation on health-related quality of life.
Other information
Registration and protocol
This systematic review with meta-analysis was registered with INPLASY on June 19, 2022. The protocol was amended on June 26, 2022 (quality of life added as a secondary outcome and other minor changes done). Both the original and amended versions are available at doi: 10.37766/inplasy2022.6.0080. At the time of the amendment, the search and identification of studies were closed, and the screening for eligibility had started, but the extraction of data had not been started.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TS conceived the idea, designed and wrote the protocol, took part in the study search and selection, and data extraction, and wrote the main manuscript draft. MS took part in the study search and selection and data verification. VT took part in the study selection and data extraction. MS and VT wrote parts of the manuscript. All authors independently did risk of bias assessment, participated in the certainty assessment, and critically revised the manuscript and approved it.
Footnotes
Conflict of interest
Author TS has received speaker and investigator honoraria from Bayer and AstraZeneca. VT has received speakers’ honoraria and consultancy fees from Boehringer Ingelheim, Astra Zeneca, Teva, Berlin Chemie Menarini, Pfizer, Novatis, Bayer, Merck Sharp & Dohme, and Abbott. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1063147/full#supplementary-material
References
- 1.Myerburg RJ, Reddy V, Castellanos A. Indications for implantable cardioverter-defibrillators based on evidence and judgment. J Am Coll Cardiol. (2009) 54:747–63. 10.1016/j.jacc.2009.03.078 [DOI] [PubMed] [Google Scholar]
- 2.Erath JW, Hohnloser SH. Drugs to prevent sudden cardiac death. Int J Cardiol. (2017) 237:22–4. 10.1016/j.ijcard.2017.03.066 [DOI] [PubMed] [Google Scholar]
- 3.Sweeney MO, Sherfesee L, De Groot PJ, Wathen MS, Wilkoff BL. Differences in effects of electrical therapy type for ventricular arrhythmias on mortality in implantable cardioverter-defibrillator patients. Heart Rhythm. (2010) 7:353–60. 10.1016/j.hrthm.2009.11.027 [DOI] [PubMed] [Google Scholar]
- 4.Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. (2008) 359:1009–17. 10.1056/NEJMoa071098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cevik C, Perez-Verdia A, Nugent K. Implantable cardioverter defibrillators and their role in heart failure progression. Europace. (2009) 11:710–5. 10.1093/europace/eup091 [DOI] [PubMed] [Google Scholar]
- 6.van Rees JB, Borleffs CJW, de Bie MK, Stijnen T, van Erven L, Bax JJ, et al. Inappropriate implantable cardioverter-defibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol. (2011) 57:556–62. 10.1016/j.jacc.2010.06.059 [DOI] [PubMed] [Google Scholar]
- 7.Braunschweig F, Boriani G, Bauer A, Hatala R, Herrmann-Lingen C, Kautzner J, et al. Management of patients receiving implantable cardiac defibrillator shocks: recommendations for acute and long-term patient management. Europace. (2010) 12:1673–90. 10.1093/europace/euq316 [DOI] [PubMed] [Google Scholar]
- 8.Carbucicchio C, Santamaria M, Trevisi N, Maccabelli G, Giraldi F, Fassini G, et al. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long-term outcomes in a prospective single-center study. Circulation. (2008) 117:462–9. 10.1161/CIRCULATIONAHA.106.686534 [DOI] [PubMed] [Google Scholar]
- 9.Kozeluhova M, Peichl P, Cihak R, Wichterle D, Vancura V, Bytesnik J, et al. Catheter ablation of electrical storm in patients with structural heart disease. Europace. (2011) 13:109–13. 10.1093/europace/euq364 [DOI] [PubMed] [Google Scholar]
- 10.da Silva GL, Nunes-Ferreira A, Cortez-Dias N, de Sousa J, Pinto FJ, Caldeira D. Radiofrequency catheter ablation of ventricular tachycardia in ischemic heart disease in light of current practice: a systematic review and meta-analysis of randomized controlled trials. J Interv Card Electrophysiol. (2020) 59:603–16. 10.1007/s10840-020-00870-3 [DOI] [PubMed] [Google Scholar]
- 11.Kampaktsis PN, Doulamis IP, Tzani A, Cheung JW. Preventive versus deferred catheter ablation of myocardial infarct–associated ventricular tachycardia: a meta-analysis. Heart Rhythm O2. (2020) 1:275–82. 10.1016/j.hroo.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinov B, Fiedler L, Schönbauer R, Bollmann A, Rolf S, Piorkowski C, et al. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the prospective heart centre of leipzig VT (HELP-VT) study. Circulation. (2014) 129:728–36. 10.1161/CIRCULATIONAHA.113.003063 [DOI] [PubMed] [Google Scholar]
- 13.Basu-Ray I, Khanra D, Shah SK, Mukherjee A, Char SV, Jain B, et al. Meta-analysis comparing outcomes of catheter ablation for ventricular arrhythmia in ischemic versus nonischemic cardiomyopathy. Pacing Clin Electrophysiol. (2021) 44:54–62. 10.1111/pace.14129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebert M, Richter S, Dinov B, Zeppenfeld K, Hindricks G. Evaluation and management of ventricular tachycardia in patients with dilated cardiomyopathy. Heart Rhythm. (2019) 16:624–31. 10.1016/j.hrthm.2018.10.028 [DOI] [PubMed] [Google Scholar]
- 15.Sramko M, Hoogendoorn JC, Glashan CA, Zeppenfeld K. Advancement in cardiac imaging for treatment of ventricular arrhythmias in structural heart disease. Europace. (2019) 21:383–403. 10.1093/europace/euy150 [DOI] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Cochrane Collaboration. The cochrane collaboration review manager (revman). Version 5.4. Copenhagen: The Cochrane Collaboration; (2020). [Google Scholar]
- 18.The Jamovi Project. Jamovi. (Version 2.3) [computer software]. (2022). Available online at: https://www.jamovi.org (accessed June 1, 2022). [Google Scholar]
- 19.GRADEpro GDT. GRADEpro Guideline Development Tool [Software] McMaster University and Evidence Prime. (2022). Available online at: https://www.gradepro.org/ [Google Scholar]
- 20.Al-Khatib SM, Daubert JP, Anstrom KJ, Daoud EG, Gonzalez M, Saba S, et al. Catheter ablation for ventricular tachycardia in patients with an implantable cardioverter defibrillator (CALYPSO) pilot trial. J Cardiovasc Electrophysiol. (2015) 26:151–7. 10.1111/jce.12567 [DOI] [PubMed] [Google Scholar]
- 21.Acosta J, Cabanelas N, Penela D, Fernández-Armenta J, Andreu D, Borràs R, et al. Long-term benefit of first-line peri-implantable cardioverter–defibrillator implant ventricular tachycardia-substrate ablation in secondary prevention patients. Europace. (2017) 19:976–82. 10.1093/europace/euw096 [DOI] [PubMed] [Google Scholar]
- 22.Goya M, Fukunaga M, Hiroshima K, Hayashi K, Makihara Y, Nagashima M, et al. Long-term outcomes of catheter ablation of ventricular tachycardia in patients with structural heart disease. J Arrhythm. (2015) 31:22–8. 10.1016/j.joa.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki A, Yoshida A, Takei A, Fukuzawa K, Kiuchi K, Takami K, et al. Prophylactic catheter ablation of ventricular tachycardia before cardioverter-defibrillator implantation in patients with non-ischemic cardiomyopathy: clinical outcomes after a single endocardial ablation. J Arrhythm. (2015) 31:122–9. 10.1016/j.joa.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav A, Ramasamy S, Theodore J, Anantharaj A, Pillai AA, Satheesh S, et al. Catheter ablation of scar based ventricular tachycardia – procedural characteristics and outcomes. Indian Heart J. (2020) 72:563–9. 10.1016/j.ihj.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin K, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. (2007) 357:2657–65. 10.1056/NEJMoa065457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuck KH, Schaumann A, Eckardt L, Willems S, Ventura R, Delacrétaz E, et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. (2010) 375:31–40. 10.1016/S0140-673661755-4 [DOI] [PubMed] [Google Scholar]
- 27.Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. (2016) 375:111–21. 10.1056/NEJMoa1513614 [DOI] [PubMed] [Google Scholar]
- 28.Kuck KH, Tilz RR, Deneke T, Hoffmann BA, Ventura R, Hansen PS, et al. Impact of substrate modification by catheter ablation on implantable cardioverter-defibrillator interventions in patients with unstable ventricular arrhythmias and coronary artery disease: results from the multicenter randomized controlled SMS (substrate modification study). Circ Arrhythm Electrophysiol. (2017) 10:e004422. 10.1161/CIRCEP.116.004422 [DOI] [PubMed] [Google Scholar]
- 29.Willems S, Tilz RR, Steven D, Kääb S, Wegscheider K, Gellér L, et al. Preventive or deferred ablation of ventricular tachycardia in patients with ischemic cardiomyopathy and implantable defibrillator (BERLIN VT) – a multicenter randomized trial. Circulation. (2020) 141:1057–67. 10.1161/CIRCULATIONAHA.119.043400 [DOI] [PubMed] [Google Scholar]
- 30.Della Bella P, Baratto F, Vergara P, Bertocchi P, Santamaria M, Notarstefano P, et al. Does timing of ventricular tachycardia ablation affect prognosis in patients with an implantable cardioverter defibrillator? Results from the multicenter randomized PARTITA trial. Circulation. (2022) 145:1829–38. 10.1161/CIRCULATIONAHA.122.059598 [DOI] [PubMed] [Google Scholar]
- 31.Arenal A, Ávila P, Jiménez-Candil J, Tercedor L, Calvo D, Arribas F, et al. Substrate ablation vs antiarrhythmic drug therapy for symptomatic ventricular tachycardia. J Am Coll Cardiol. (2022) 79:1441–53. 10.1016/j.jacc.2022.01.050 [DOI] [PubMed] [Google Scholar]
- 32.Tung R, Xue Y, Chen M, Jiang C, Shatz DY, Besser SA, et al. First-line catheter ablation of monomorphic ventricular tachycardia in cardiomyopathy concurrent with defibrillator implantation: the PAUSE-SCD randomized trial. Circulation. (2022) 145:1839–49. 10.1161/CIRCULATIONAHA.122.060039 [DOI] [PubMed] [Google Scholar]
- 33.Gula LJ, Doucette S, Leong-Sit P, Tang ASL, Parkash R, Sarrazin JF, et al. Quality of life with ablation or medical therapy for ventricular arrhythmias: a substudy of VANISH. J Cardiovasc Electrophysiol. (2018) 29:421–34. 10.1111/jce.13419 [DOI] [PubMed] [Google Scholar]
- 34.Winterfield JR, Kent AR, Karst E, Dalal N, Mahapatra S, Jared Bunch T, et al. Impact of ventricular tachycardia ablation on health care utilization. Heart Rhythm. (2018) 15:355–62. 10.1016/j.hrthm.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 35.Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri N, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. (2019) 21:1143–4. 10.1093/europace/euz132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American college of cardiology foundation/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2018) 72:e91–220. 10.1016/j.jacc.2017.10.054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.