Abstract
Extra-skeletal Ewing sarcoma (EES) is a rare sarcoma composed primarily of small round cells, capable of metastasizing and relapsing. Few cases of EES originating from the larynx have been reported, and no publications regarding laryngeal EES treated with dendritic cells-cytotoxic T lymphocytes (DC-CTL) immunotherapy have been found. We described a 29-year-old woman with a mass found in the larynx. Diffuse small round cells with scanty cytoplasm shown by histology test and extremely positive staining of CD99 revealed by immunohistochemistry helped determine the diagnosis of laryngeal EES. The patient survived for seven years with no signs of recurrence or metastasis after six cycles of DC-CTL immunotherapy based on traditional treatments. This case indicates that DC-CTL immunotherapy could be considered a new option for treating EES.
Keywords: larynx, surgery, prognosis, immunotherapy, extra-skeletal Ewing sarcoma
Introduction
Extra-skeletal Ewing sarcoma (EES) is a rare malignancy primarily composed of small round cells (1). A majority of cases are found in adolescents and young adults (2, 3). Most commonly EES affects the soft tissues in the paravertebral region, lungs, kidneys and bladder, with the head and neck region being a rare origin site (1, 4). Patients with EES commonly complain of local masses with or without regional swelling pain, increased skin temperature, and restricted movement of limbs due to nerve invasion (4). However, these symptoms are atypical and difficult to distinguish from other types of malignancies at first consultations. Generally, patients with EES have a poor prognosis and a high risk of metastasis or recurrence after traditional implementation of surgical resection, and postoperative radiotherapy and chemotherapy (5–7). A previous study involving 18 patients found that the 1-year, 3-year, and 5-year survival rates of EES after surgery combined with other treatment modalities were 82.4%, 64.2%, and 32.1%, respectively (8). In order to reach a 5-year survival rate of 60-70%, a combined treatment strategy, including surgery, radiotherapy, and high-dose chemotherapy, is required as soon as the sarcoma manifests itself (4, 9).
Head and neck tumors can initiate from the oropharynx, nasopharynx, laryngopharynx, thyroid gland, cervical trachea and cervical esophagus. According to the WHO Pathological Classification (2017), common tumors in the head and neck can be divided into malignant epithelial tumors, neuroendocrine tumors, and benign epithelial tumors, soft tissue tumors, heamatolymphoid tumors, tumors of bone and cartilage, mucosal malignant melanoma, secondary tumors (10). Patients with head and neck tumors suffer from a wide range of symptoms including a lump in the neck, a sore throat that is difficult to relieve, difficulty swallowing, and hoarseness of voice (11). Some patients similar to this case have only local masses. The late stages of carcinomas or other aggressive malignancies in the head and neck can also result in swelling metastatic lymph nodes (12). As a result, it may be difficult to determine the origin of the mass and its symptoms. In this study, we reported a case of EES with a local mass in the neck as the primary reason for consultation. Because of a lack of specificity in clinical manifestations and subsequent delayed in diagnosis and treatment, patients with EES are likely to have a poor prognosis because the sarcoma has commonly advanced to a late stage (13–15). Here, we described a case of EES which developed from the larynx and was treated with a newly developed biological immunological therapy based on surgery combined with radiotherapy and chemotherapy treatment, with a survival period of more than 5 years.
Case presentation
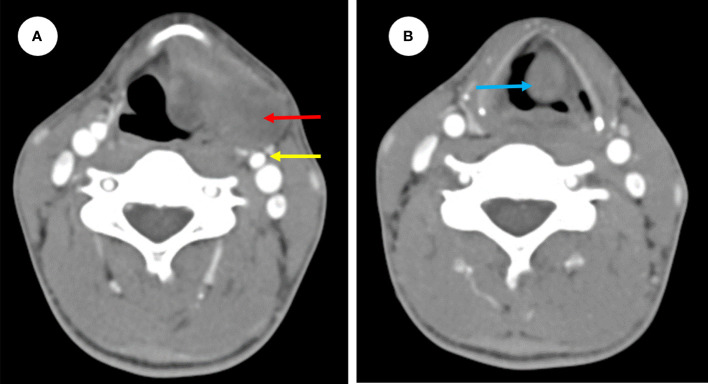
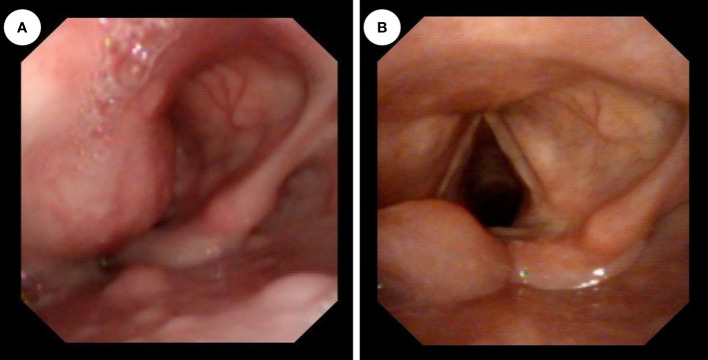
In a 29-year-old female, a painless mass in the left neck region had slowly grown over the past two months. The patient denied experiencing dyspnea, dysphagia, laryngalgia, hoarseness, or any other oppression sensations. Furthermore, she had no family history of malignant tumors. Upon physical examination a hard mass in the left submandibular region, approximately 3 cm by 2 cm in size, with normal skin temperature and color and good mobility. There were no enlarged lymph node or thyroid mass found. Laboratory examinations revealed no abnormality. An ultrasound-guided pathological biopsy of the tumor reveal that it was malignant. Afterward, enhanced computed tomography (CT) showed a mass of irregular density located anterior to the carotid and extending into both the laryngeal cavity and the left piriform fossa ( Figure 1 ). The mass was found to be lobulated, with a maximum cross-sectional area of 3.8 cm by 2.0 cm. A smooth protrusion in the laryngeal cavity was seen under fibro-laryngoscope ( Figure 2A ). Additionally, a PET-CT scan indicated no distant metastasis.
Figure 1.
Preoperative enhanced CT images showing a mass of irregular density (red arrow) located anterior to the carotid (yellow arrow) extending into both the left side of preepiglottic space (red arrow) (A) and the laryngeal cavity (blue arrow) (B).
Figure 2.
(A) Preoperative fiberoptic laryngoscopy showed a smooth bulge in the left aryepiglottic fold, protruding into the laryngeal cavity, and the glottic fissure was invisible. (B) After the operation, normal laryngeal structures were observed using a fiberoptic laryngoscope, the glottic region was fully exposed, and there were no obvious abnormalities in the hypopharynx.
After the malignancy of the tumor was confirmed, surgical excision was performed. During the surgery, an arcuate incision of about 5 cm in length was made along the dermatoglyphic pattern at the bulge of the mass in the left neck. Subsequently, the skin, subcutaneous tissue, and cervical pinna muscles were incised to separate the flaps. The surgical boundary was superior to the level of the hyoid bone and inferior to the level of the left thyroid cartilage notch. After the incision of the deep cervical fascia, a mass of about 3.5cm by 3.0 cm by 2.0 cm was revealed in the left greater horn of the hyoid bone and the left superior cornu of the thyroid cartilage. The tumor was lobulated and slightly firm with intact envelope. The superior laryngeal artery was ligated to protect the superior laryngeal nerve, and the mass was carefully separated along the capsule. It was bluntly separated along the capsule on the surface, and the tumor protruded into the preepiglottic space and paraglottic space across the upper-left edge of the thyroid cartilage and laryngeal mucosa was not involved. Negative pressure drainage was placed in the surgical cavity and the incision was sutured layer by layer. No metastatic lymph node was found.
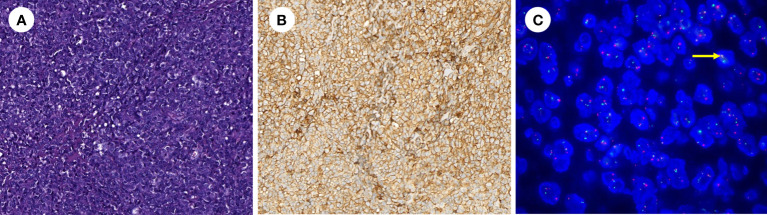
The excised mass was lobulated and hard. Microscopically, it seemed to be nodular in distribution and was composed of diffuse small round or oval cytoplasm deficient cells with marked cellular heterogeneity, evident nuclear division and scattered necrosis ( Figure 3 ). Anti-CD99 antibody (CD99) (ZA-0577, ZSGB-BIO, Wuxi, China) immunohistochemistry results showed extremely positive staining (approximately 100%). Furthermore, immunohistochemistry results revealed that calcitonin (CT) (ZA-0578, ZSGB-BIO, Wuxi, China), CD3 (ZA-0503, ZSGB-BIO, Wuxi, China), CD20 (ZM-0039, ZSGB-BIO, Wuxi, China), CD21(ZM-0040, ZSGB-BIO, Wuxi, China), leukocyte common antigen (LCA) (ZM-0183, ZSGB-BIO, Wuxi, China), Melanoma Marker (HMB45) (ZM-0187, ZSGB-BIO, Wuxi, China), S-100 (ZA-0225, ZSGB-BIO, Wuxi, China), and Myogenin (MY) (ZA-0592, ZSGB-BIO, Wuxi, China) were all negative. Moreover, the fusion of Ewing sarcoma breakpoint region 1 (EWSR1) with Friend leukaemia integration-1(FLI1) was detected using EWSR1 and FLI1 probes (F.01253-01, GZLBP, Guangzhou, China) by fluorescence in situ hybridization (FISH), and there were positive findings in the tumor cells ( Figure 3C ). Everything mentioned above led to a diagnosis of ESS originating from the larynx.
Figure 3.
(A) Hematoxylin–eosin stain showing the mass consists of small round cells (hematoxylin-eosin, original magnification ×200). (B) Immunohistochemistry revealed extremely positive CD99 staining (× 200). (C) The fusion of EWSR1 with FLI1 was positively detected by FISH in tumor cells (yellow arrow) (× 1000).
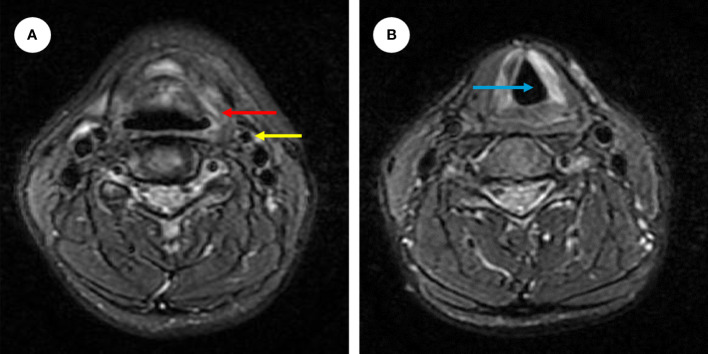
Postoperatively, after anti-inflammatory treatment and 4 days of surgical cavity drainage, the patients recovered well and a fibro-laryngoscope revealed a healed laryngopharyngeal mucosa. On the tenth day after surgery, the surgical sutures in the neck were removed. The patient did not complain of dyspnea and dysphagia. To reduce the risk of recurrence, postoperative radiotherapy and chemotherapy were performed. Forty days after the operation, the patient underwent 30 times of radiation totaling 60Gy within 6 weeks. And the chemotherapy began 16 days after the radiation, which was 4 cycles of intravenous transfusion of 2.0 grams of isosfamide from day 1 to 5 and 5 milligrams of epirubincin in day 1 or day 1 and 2, and each cycle lasted for 5 days. No adverse event was reported. In addition, the patient accepted cellular immunotherapy considering the rarity of EES and its poor prognosis. The cellular immunotherapy paradigm involved 6 cycles of autotransfusion of immune cells via intravenous transfusion, each cycle includes four cell infusions. We collected peripheral blood from patients to culture DC-CTL cells, and DC cells were stimulated with autologous tumor lysates (100 μg/ml) on the 6th day of culture. Then, the DC cells were harvested and co-cultured with CTL on the 7th day and total 14 days were cells cultured until first harvest (The protocol used for generation of DC-CTL in reference 16). The final cell suspension was mentioned as 2200 ml. We harvested 1000 ml of suspension for each day and supplemented them with 700 ml of fresh medium on the day 1 and day 2, 400 ml of fresh medium on the day 3. The proportion of CD3+/CD8+ CTL (Kit: 662967, BD Bioscience, USA) detected by flow cytometry (Canto II, BD Bioscience, USA; DIVA software, BD Bioscience, USA) and the number of cells per reinfusion are as follows: cycle 1, CD3+/CD8+ CTL accounts for 70.50%, day 1 (D1) 1.451×10^9 cells, D2 1.016×10^9 cells, D3 1.312×10^9 cells and D4 1.557×10^9 cells; cycle 2, CD3+/CD8+ CTL accounts for 73.03%, D1 0.7×10^9 cells, D2 1.432×10^9 cells, D3 0.848×10^9 cells and D4 1.336×10^9 cells; cycle 3, CD3+/CD8+ CTL accounts for 61.02%, D1 1.512×10^9 cells, D2 1.24×10^9 cells, D3 1.112×10^9 cells and D4 0.864×10^9 cells; cycle 4, CD3+/CD8+ CTL accounts for 84.51%, D1 1.272×10^9 cells, D2 0.954×10^9 cells, D3 1.16×10^9 cells and D4 0.896×10^9 cells; cycle 5, CD3+/CD8+ CTL accounts for 84.87%, D1 0.8×10^9 cells, D2 0.768×10^9 cells, D3 0.448×10^9 cells and 0.656×10^9 cells; cycle 6, CD3+/CD8+ CTL accounts for 56.50%, 1.44×10^9 cells, 0.984×10^9 cells, 0.72×10^9 cells and 1.504×10^9 cells. Additionally, 100IU of recombinant human interleukin 2 was injected subcutaneously after cell re-infusion on D2 and D4. Laboratory examinations showed no abnormality and the patient reported no discomfort following each immunotherapy session. One year after the combined treatment strategy, enhanced magnetic resonance imaging (MR) revealed no evidence of recurrence ( Figure 4 ). A postoperative fiberoptic laryngoscopy revealed normal laryngeal structures ( Figure 2B ). Seven years after surgery, the patient showed no signs of recurrence or metastasis.
Figure 4.
Enhanced MR images showing no tumor recurrence in the precarotid (yellow arrow) and preepiglottic spaces (red arrow) (A) and the normal anatomy of the laryngeal cavity (blue arrow) (B).
Discussion
EES is a rare sarcoma in the neck region, with rare laryngeal EES reported. Since the discovery of Ewing sarcoma in 1921, few cases of EES, a subset of Ewing sarcoma that developed from extraosseous tissue, have been reported (17). Generally, for patients with EES, surgery combined with radiotherapy and chemotherapy would be strongly recommended, despite a poor prognosis and extremely low 5-year survival rate (6, 18). In this study, we reported a case of EES arising from the larynx, which extended into both the laryngeal cavity and the piriform fossa, and investigated the potential of dendritic cells-cytotoxic T lymphocytes (DC-CTL), an emerging biological therapy, in the treatment of EES.
EES is a highly aggressive malignancy. However, due to the lack of specificity in clinical manifestation, this type of sarcoma is difficult to diagnose. EES, like most malignant tumors, only shows heterogeneously enhanced lesions upon medical imaging, or results in compressive symptoms when the sarcoma grows large (8). Imaging interpretation alone could not exclude a primary thyroid tumor in this patient. Fine needle aspiration pathology also only suggests its malignancy, and in order to identify the tumor surgical resection was required to determine its specific nature.
While microscopic small round cells can alert clinicians of the presence of uncommon tumor in the head and neck, it may be difficult to distinguish the EES from other small round cell tumors such as rhabdomyosarcoma (19). In such a situation, immunohistochemistry would be necessary. In this patient, negative calcitonin (CT) staining ruled out the possibility of medullary thyroid carcinoma (20), the negative staining of CD3, CD20, CD21 and LCA excluded the possibility of lymphoma (21), the HMB45 (-) and S-100 (-) eliminated the chance of melanoma (22), and the MY (-) ruled out the probability of rhabdomyosarcoma (20). Moreover, a highly positive CD99 staining aided in the diagnosis of EES (23). CD99, regulated by EWSR1–FLI1, is a cell-surface glycoprotein and a useful diagnostic marker for Ewing sarcoma (24, 25). In immunochemistry tests, diffuse membranous expression of CD99 is evident and the accuracy can reach 95% in Ewing sarcomas (26).
Traditionally, surgical excision remains the mainstay of EES treatment, with radiotherapy and chemotherapy used as adjuvant empirical therapies (19). To gain a better understanding of laryngeal and cervical EES, previous studies were reviewed and listed in Table 1 (27–53). The studies are limited to English language literature published between 1982–2021. In the previous 32 cases, 3 tumors originated from the larynx and composed approximately 9.1% cervical EES. We found that 21 of these patients had clear documentation of treatment modalities and prognoses. Further, we compared the effectiveness of different treatment modalities on prognosis finding that the average survival period of patients who underwent surgical excision (n = 15) was 25 months and that those without surgery (n = 6) survived 41.33 months on average. Patients who received radiotherapy (n=15) survived 36.20 months on average, while those who did not receive radiotherapy (n = 6) survived 13.33 months. The survival period of chemotherapy-treated patients (n = 19) was 32.05 months vs. 7.00 months for those who did not receive chemotherapy (n = 2). It is noteworthy that each of these patients accepted at least one kind of conventional modality of treatment. The study indicates that radiotherapy and chemotherapy play a better role in treating cervical EES. However, the assumption is based on a small sample size, which may lead to biased speculation. Furthermore, despite the fact that most patients with cervical EES were treated with combined treatment modalities, only three patients survival more than 5 years. Therefore, the effectiveness of different modalities of EES treatment should be further evaluated through more studies. In addition, patients with cervical EES have a poor prognosis, a high recurrence rate ranging from 15 to 30%, and a low 5-year survival rate (54). Therefore, a more effective treatment to improve EES prognosis is required.
Table 1.
Summary of previous published articles on cervical EES.
| References | No. | Age (years) | Gender | Lesion site | Tumor size | Clinical manifestation | Treatment | Follow-up (months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Ansari MH et al. (2019) (27) | 1 | 3 | F | Right-sided neck | 4×3×1cm | Right-sided neck swelling for 2 months | S | Unknown | NED |
| Whaley JT, et al. (2010) (28) | 2 | 19 | F | Post neck muscle | Diameter 4cm | Unknown | CT+XRT | 156 | NED |
| Abdel Rahman H, et al. (2010) (29) | 3-6 | Unknown | Unknown | Neck | Unknown | Unknown | Unknown | Unknown | Unknown |
| Yao-jie FENG, et al. (2020) (30) | 7 | Unknown | Unknown | Root of neck | Unknown | Unknown | Unknown | Unknown | Unknown |
| Yao-jie FENG, et al. (2020) (30) | 8 | Unknown | Unknown | Submandibular | Unknown | Unknown | Unknown | Unknown | Unknown |
| Yi-liang HOU, et al. (2002) (31) | 9 | 18 | M | Root of right-sided neck | 1.4×1.0×0.8cm | Movable neck mass with slight pain | S + CT+XRT | 36 | NED |
| Ying CHEN, et al. (2002) (32) | 10 | 27 | F | Unknown | 12×10×8cm | Notable neck mass with tenderness | S + CT+XRT | 24 | NED |
| Xiao-long LIN, et al. (2021) (33) | 11 | 30 | F | Left-sided neck | 8×5cm | Notable mass in the left neck with swelling pain in the throat | S+ CT | 9 | Lost to follow-up |
| Shuang Wang, MD, et al. (2021) (34) | 12 | 36 | F | Left-sided neck | 9×8×6cm | Notable painless mass with dysphagia | S+ CT+XRT | 19 | NED |
| Gazula S, et al. (2019) (35) | 13 | 4-month-old | F | Left submandibular | 2.9×2.8×2.0cm | Notable swelling for 3 weeks, with the mass increasing gradually | S + CT | 24 | NED |
| Ali S, et al. (2008) (36) | 14 | 14 | M | Right-sided neck | 12×5cm | A rapidly extending mass for 1 month | CT+XRT | Unknown | Unknown |
| Van Der Meer G, et al. (2017) (37) | 15 | 12 | M | Posterior to tracheal and anterior to vertebra | Diameter 3.5cm | Sleep disorder, short of breathing, sore throat and stridor | CT+XRT | 18 | Radiotherapy causing an edematous larynx and mucositis |
| Maroun CA, et al. (2019) (38) | 16 | 54 | M | Right paraglottic space | 5.0×3.8×3.8cm | Notable mass with hoarseness | CT+XRT | 12 | NED |
| Yang YS, et al. (2004) (39) | 17 | 74 | M | Larynx | 3.5×2.0cm | Acute aggravate dyspnea | S +XRT | 6 | NED |
| Lynch MC, et al. (2014) (40) | 18 | 45 | F | Larynx | Diameter 2.9cm | A rapidly growing lump in the right side of neck with hoarseness. | CT+XRT | Unknown | NED |
| Wygoda A, et al. (2013) (41) | 19 | 68 | M | Larynx | 2.0×1.9×1.7cm | Hoarseness and occasional aphonia | CT+XRT | 30 | NED |
| Khosla D, et al. (2019) (42) | 20 | 8 | F | Parapharyngeal space | Unknown | Difficulty in breathing and swallowing, earache and bleeding from the mouth | CT+XRT | 12 | Died of distant metastasis |
| Cho SI, et al. (2007) (43) | 21 | 49 | M | The parapharynx with pulmonary metastasis. | Unknown | Diplopia and mild headache | CT | 20 | No improvement of metastatic lesions in rectum and blindness. |
| Rama-López J, et al. (2017) (44) | 22 | 70 | M | Left supraclavicular fossa | 6.1×6.7×7.1cm | A rapidly growing mass | NC+ S + CT+XRT | 24 | NED |
| Gustafson RO, et al. (1982) (45) | 23 | 18 | M | Right-side neck | 14×10×7cm | Notable growing mass | S + CT+XRT | 28 | NED |
| Schmidt S, et al. (2010) (46) | 24 | 16 | M | Neck | Unknown | Painful lump | S+ CT+XRT | 7 | NED |
| Chirila M, et al. (2013) (47) | 25 | 48 | M | Thyroid | 10×10cm | Thyroid recurrent episodes of acute obstructive respiratory distress | S + CT | 1 | Died |
| Adapa P, et al. (2009) (48) | 26 | 9 | F | Thyroid | 4.0×4.5×6.0cm | Painless swelling | S + CT+XRT | 72 | NED |
| Wei-yu ZHU, et al. (2021) (49) | 27 | 30 | F | Left supraclavicular region | 6×5×3cm | Progressive enlarged mass with feeling of swallowing obstruction | S + CT+XRT | 36 | NED |
| Kabata P, et al. (2017) (50) | 28 | 34 | M | In the lower left neck | 5.8×5.8×6.0cm | Upper respiratory tract infection, sore throat, and difficulties in swallowing | NC + S | 18 | NED |
| Bishop JA, et al. (2015) (51) | 29 | 19 | M | Thyroid | Unknown | Neck mass | S | Unknown | NED |
| Bishop JA, et al. (2015) (51) | 30 | 36 | F | Thyroid | Unknown | Goiter | S | Unknown | Unknown |
| Maldi E, et al. (2012) (52) | 31 | 66 | M | Thyroid | Diameter 4.5cm | A single nodule on the left lobe of the thyroid gland | S | 8 | Metastatic disease has been discovered |
| Seipel AH, et al. (2021) (53) | 32 | 54 | F | Thyroid | 3.7×3.1×2.1cm | Unknown | S + CT+XRT | 15 | NED |
F, female; M, male; S, Surgery; CT, chemotherapy; NC, Neoadjuvant chemotherapy; XRT, radiotherapy; NED, no evidence of disease; DC-CTL, dendritic cells-cytotoxic T lymphocyte immunotherapy.
Currently, cellular immunotherapy plays an increasingly important role in the treatment of malignancies (55, 56). It could kill tumor cells by extracting immune cells from patients’ peripheral blood and reinfusing them after activation and expansion (57). Dendritic cells (DC), natural killer cells (NK), and cytotoxic T lymphocytes (CTL) could be the cell types extracted and expanded (57–59). Physicians should choose an immune cell type appropriate for the specific carcinoma or sarcoma when using cellular immunotherapy (57). Until now, studies have shown that DC-CTL immunotherapy can improve the prognosis and quality of life of patients with liver cancer, small cell lung cancer, prostate cancer, and other cancers (60, 61). There are also studies showing that hematopoietic stem cell transplantation or immune cell biotherapy can extend the life expectancy of patients with EES to some extent when combined with traditional treatments (62, 63). The patient who we studied received six cycles of dendritic cells-cytotoxic T lymphocytes (DC-CTL) immunotherapy after surgery, as well as radiotherapy and chemotherapy. In the process of DC-CTL treatment, DC could promote the proliferation of CTL and induce tumor-specificity CTL, and the expanded CTL will then promote its binding capacity to the targeted tumor cells, causing tumor cells to dissolve and die (64). However, due to the low incidence of EES, the effectiveness of cellular immunotherapy is not representative or convincing. Furthermore, there is possibility of immune rejection complications which should be monitored. Fortunately, the patient studied survived more than 5 years without recurrences or complications. We have reasons to believe that DC-CTL therapy could be considered an adjuvant therapy for malignancies. To the best of our knowledge, this was the first case of EES arising from the larynx treated with DC-CTL therapy. However, the true contribution of DC-CTL to curing EES remains unclear, due to the traditional treatment that preceded its use. Furthermore, because of our lack of experience, the efficacy of DC-CTL therapy should be evaluated in more malignancies, and the side effects should be appraised simultaneously.
Conclusions
We described a rare case of laryngeal EES that was successfully treated with traditional surgery, radiotherapy and chemotherapy, as well as newly developed DC-CTL therapy. It provides us with a new treatment option for EES or other malignancies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical Committee of Yantai Yuhuangding Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XS and CJ made the diagnosis; HW and JW wrote the initial draft of the manuscript; XS, QW, and YM provided clinical data; QW, CR, and JW proofread manuscript; HW, JG, and YY provided follow-up information; CJ and CR reviewed the literature and previous research; all the authors took part in the revision of the manuscript and approved the submission.
Funding
Taishan Scholars Project (ts20190991); Yantai Science and Technology Program (2022YD006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Agir H, Brasch HD, Tan ST. Extra-skeletal ewing's sarcoma of the submandibular gland. J Plast Reconstr Aesthet Surg (2007) 60(12):1345–8. doi: 10.1016/j.bjps.2006.01.046 [DOI] [PubMed] [Google Scholar]
- 2. Rud NP, Reiman HM, Pritchard DJ, Frassica FJ, Smithson WA. Extraosseous ewing’s sarcoma. A study 42 cases. Cancer (1989) 64:1548.e53. doi: [DOI] [PubMed] [Google Scholar]
- 3. Chao TK, Chang YL, Sheen TS. Extraskeletal ewing’s sarcoma of the scalp. J Laryngol Otol (2000) 114:73.e5. doi: 10.1258/0022215001903744 [DOI] [PubMed] [Google Scholar]
- 4. Riggi N, Suvà ML, Stamenkovic I. Ewing's sarcoma. N Engl J Med (2021) 384(2):154–64. doi: 10.1056/NEJMra2028910 [DOI] [PubMed] [Google Scholar]
- 5. Grünewald TGP, Cidre-Aranaz F, Surdez D, Tomazou EM, de Álava E, Kovar H, et al. Ewing Sarcoma. Nat Rev Dis Primers. (2018) 4(1):5. doi: 10.1038/s41572-018-0003-x [DOI] [PubMed] [Google Scholar]
- 6. Gaspar N, Hawkins DS, Dirksen U, Lewis IJ, Ferrari S, Le Deley MC, et al. Ewing Sarcoma: Current management and future approaches through collaboration. J Clin Oncol (2015) 33(27):3036–46. doi: 10.1200/JCO.2014.59.5256 [DOI] [PubMed] [Google Scholar]
- 7. Biermann JS, Chow W, Reed DR, Lucas D, Adkins DR, Agulnik M, et al. NCCN guidelines insights: Bone cancer, version 2.2017. J Natl Compr Canc Netw (2017) 15(2):155–67. doi: 10.6004/jnccn.2017.0017 [DOI] [PubMed] [Google Scholar]
- 8. Xie CF, Liu MZ, Xi M. Extraskeletal ewing's sarcoma: a report of 18 cases and literature review. Chin J Cancer. (2010) 29(4):420–4. doi: 10.5732/cjc.009.10402 [DOI] [PubMed] [Google Scholar]
- 9. Stahl M, Ranft A, Paulussen M, Bölling T, Vieth V, Bielack S, et al. Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer. (2011) 57(4):549–53. doi: 10.1002/pbc.23040 [DOI] [PubMed] [Google Scholar]
- 10. El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumors. 4th ed. Lyon, France: IARC; (2017). [Google Scholar]
- 11. Crozier E, Sumer BD. Head and neck cancer. Med Clin North Am (2010) 94(5):1031–46. doi: 10.1016/j.mcna.2010.05.014 [DOI] [PubMed] [Google Scholar]
- 12. Jereczek-Fossa BA, Jassem J, Orecchia R. Cervical lymph node metastases of squamous cell carcinoma from an unknown primary. Cancer Treat Rev (2004) 30(2):153–64. doi: 10.1016/j.ctrv.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 13. Luksch R, Tienghi A, Hall KS, Fagioli F, Picci P, Barbieri E, et al. Primary metastatic ewing's family tumors: results of the Italian sarcoma group and Scandinavian sarcoma group ISG/SSG IV study including myeloablative chemotherapy and total-lung irradiation. Ann Oncol (2012) 23(11):2970–6. doi: 10.1093/annonc/mds117 [DOI] [PubMed] [Google Scholar]
- 14. Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the children's oncology group. J Clin Oncol (2012) 30(33):4148–54. doi: 10.1200/JCO.2011.41.5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ozerlat I. Chemotherapy. intensified therapy for Ewing sarcoma. Nat Rev Clin Oncol (2012) 9(12):671. doi: 10.1038/nrclinonc.2012.200 [DOI] [PubMed] [Google Scholar]
- 16. Yang L, Ren B, Li H, Yu J, Cao S, Hao X, et al. Enhanced antitumor effects of DC-activated CIKs to chemotherapy treatment in a single cohort of advanced non-small-cell lung cancer patients. Cancer Immunol Immunother. (2013) 62(1):65–73. doi: 10.1007/s00262-012-1311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ewing J. The classic: Diffuse endothelioma of bone. Proc New York Pathological Society. (1921) 12:17. doi: 10.1097/01.blo.0000229311.36007.c7 [DOI] [PubMed] [Google Scholar]
- 18. Ginsberg JP, Goodman P, Leisenring W, Ness KK, Meyers PA, Wolden SL, et al. Long-term survivors of childhood Ewing sarcoma: report from the childhood cancer survivor study. J Natl Cancer Inst (2010) 102(16):1272–83. doi: 10.1093/jnci/djq278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pappo AS, Dirksen U. Rhabdomyosarcoma, Ewing sarcoma, and other round cell sarcomas. J Clin Oncol (2018) 36(2):168–79. doi: 10.1200/JCO.2017.74.7402 [DOI] [PubMed] [Google Scholar]
- 20. Wells SA, Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. American Thyroid association guidelines task force on medullary thyroid carcinoma. revised American thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid. (2015) 25(6):567–610. doi: 10.1089/thy.2014.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou X. Antibody selection in pathological diagnosis of lymphomas. Chin J Diagn Pathol (2010) 17(1):4–6. doi: 10.3969/j.issn.1007-8096.2010.01.002 [DOI] [Google Scholar]
- 22. Perniciaro C. Dermatopathologic variants of malignant melanoma. Mayo Clin Proc (1997) 72(3):273–9. doi: 10.4065/72.3.273 [DOI] [PubMed] [Google Scholar]
- 23. Riggi N, Stamenkovic I. The biology of Ewing sarcoma. Cancer Lett (2007) 254(1):1–10. doi: 10.1016/j.canlet.2006.12.009 [DOI] [PubMed] [Google Scholar]
- 24. Rocchi A, Manara MC, Sciandra M, Zambelli D, Nardi F, Nicoletti G, et al. CD99 inhibits neural differentiation of human Ewing sarcoma cells and thereby contributes to oncogenesis. J Clin Invest. (2010) 120(3):668–80. doi: 10.1172/JCI36667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kreppel M, Aryee DN, Schaefer KL, Amann G, Kofler R, Poremba C, et al. Suppression of KCMF1 by constitutive high CD99 expression is involved in the migratory ability of ewing's sarcoma cells. Oncogene. (2006) 25(19):2795–800. doi: 10.1038/sj.onc.1209300 [DOI] [PubMed] [Google Scholar]
- 26. Ambros IM, Ambros PF, Strehl S, Kovar H, Gadner H, Salzer-Kuntschik M. MIC2 is a specific marker for ewing's sarcoma and peripheral primitive neuroectodermal tumors. evidence for a common histogenesis of ewing's sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer. (1991) 67(7):1886–93. doi: 10.1002/1097-0142(19910401)67:7 [DOI] [PubMed] [Google Scholar]
- 27. Ansari MH, Gujrathi AB, Ambulgekar V. Extraskeletal ewing's sarcoma of neck in a child- a case report. Iran J Otorhinolaryngol (2019) 31(104):173–6. [PMC free article] [PubMed] [Google Scholar]
- 28. Whaley JT, Indelicato DJ, Morris CG, Hinerman RW, Amdur RJ, Mendenhall WM, et al. Ewing Tumors of the head and neck. Am J Clin Oncol (2010) 33(4):321–6. doi: 10.1097/COC.0b013e3181aaca71 [DOI] [PubMed] [Google Scholar]
- 29. Abdel Rahman H, El-Baradie T, El-Baradie M, Bahaa S, Shalan M. Management head and neck ewing's sarcoma family of tumors: Experience of the national cancer institute, Cairo university. J Egypt Natl Canc Inst (2010) 22(1):41–7. [PubMed] [Google Scholar]
- 30. Feng Y-j, Qu J, Wei C-r, Zhang M-m, Yang Y-y, Li D-y, et al. [CT and MRI imaging features of extra-skeletal ewing's sarcoma/peripheral primitive neuroectodermal tumor]. Radiologic Practice. (2020) 35(7):900–4. doi: 10.13609/j.cnki.1000-0313.2020.07.013 [DOI] [Google Scholar]
- 31. Hou Y-l, Liu H-m, Yan H-p. [Extraosseous ewing's sarcoma: A case report]. Pract J Of Cancer. (2002) 17(4):409–9. doi: 10.3969/j.issn.1001-5930.2002.04.055 [DOI] [Google Scholar]
- 32. Chen Y, Chen Y-b, Li H-s. [Extraosseous ewing's tumor of the neck: A case report]. Chin J Of Misdiagnostics. (2002) 2(12):1786. doi: 10.3969/j.issn.1009-6647.2002.12.122 [DOI] [Google Scholar]
- 33. Lin X-l, LV H-l, Wang Y-s, Zhang J-h. [Extra-skeletal ewing's sarcoma: A case report and literature review]. Chin J Otorhinolaryngology-skull Base Surgery. (2021) 27(4):473–6. doi: 10.11798/j.issn.1007-1520.202103175 [DOI] [Google Scholar]
- 34. Wang S, Zhu W, Zhang H, Yang X. Extraosseous Ewing sarcoma of the cervical esophagus: Case report and literature review. Ear Nose Throat J (2020) 101(5):NP203-8. doi: 10.1177/0145561320953696 [DOI] [PubMed] [Google Scholar]
- 35. Gazula S, Rani VL, Jonathan GT, Kumar NN. Extraskeletal ewing's sarcoma masquerading as infantile benign neck mass. J Indian Assoc Pediatr Surg (2019) 24(3):209–11. doi: 10.4103/jiaps.JIAPS_98_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ali S, Mackenzie K, Reid R, O'Neill G, Ganly I. Cervical extraskeletal ewing's sarcoma: case report demonstrating radiological features and management. J Laryngol Otol (2008) 122(9):998–1001. doi: 10.1017/S0022215107009371 [DOI] [PubMed] [Google Scholar]
- 37. Van Der Meer G, Linkhorn H, Gruber M, Mahadevan M, Barber C. Retrotracheal extraskeletal ewing's sarcoma: Case report and discussion on airway management. Turk Arch Otorhinolaryngol (2017) 55(1):44–7. doi: 10.5152/tao.2017.1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maroun CA, Khalifeh I, Tfayli A, Moukarbel RV. Primary Ewing sarcoma of the larynx with distant metastasis: A case report and review of the literature. Curr Oncol (2019) 26(4):e574–7. doi: 10.3747/co.26.5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang YS, Hong KH. Extraskeletal ewing's sarcoma of the larynx. J Laryngol Otol (2004) 118(1):62–4. doi: 10.1258/002221504322731682 [DOI] [PubMed] [Google Scholar]
- 40. Lynch MC, Baker A, Drabick JJ, Williams N, Goldenberg D. Extraskeletal ewing's sarcoma arising in the larynx. Head Neck Pathol (2014) 8(2):225–8. doi: 10.1007/s12105-013-0492-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wygoda A, Rutkowski T, Ponikiewska D, Hejduk B, Składowski K. Ewing's sarcoma of the larynx. effective treatment with organ preservation. Strahlenther Onkol. (2013) 189(7):586–9. doi: 10.1007/s00066-013-0356-8 [DOI] [PubMed] [Google Scholar]
- 42. Khosla D, Verma S, Punia RS, Dass A, Dimri K, Kaur G, et al. Extraosseous ewing's sarcoma of the parapharyngeal space - a rare entity - with review of literature. Iran J Otorhinolaryngol (2019) 31(102):51–4. [PMC free article] [PubMed] [Google Scholar]
- 43. Cho SI, Park YH, Cho JH, Ryoo BY, Yang SH, Youn SM, et al. Extraskeletal ewing's sarcoma of the head and neck presenting as blindness. Korean J Intern Med (2007) 22(2):133–7. doi: 10.3904/kjim.2007.22.2.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rama-López J, Asensio RR, García-Garza C, Fra PL, Gassent Balaguer MD, Salva JF. Extraosseous Ewing sarcoma: Expanding the differential diagnosis of supraclavicular fossa tumors. Ear Nose Throat J (2017) 96(1):E29–32. doi: 10.1177/014556131709600104 [DOI] [PubMed] [Google Scholar]
- 45. Gustafson RO, Maragos NE, Reiman HM. Extraskeletal ewing's sarcoma occurring as a mass in the neck. Otolaryngol Head Neck Surg (1982) 90(4):491–3. doi: 10.1177/019459988209000422 [DOI] [PubMed] [Google Scholar]
- 46. Schmidt S, Lackner H, Urban C. Ewing Sarcoma of the neck. Pediatr Blood Cancer. (2010) 54(2):339. doi: 10.1002/pbc.22322 [DOI] [PubMed] [Google Scholar]
- 47. Chirila M, Muresan M, Ciuleanu E, Cosgarea M. Extraosseous Ewing sarcoma and peripheral primitive neuroectodermal tumor of the thyroid gland: Case report and review. Ear Nose Throat J (2013) 92(4-5):E3–6. doi: 10.1177/014556131309200419 [DOI] [PubMed] [Google Scholar]
- 48. Adapa P, Chung TW, Popek EJ, Hunter JV. Extraosseous Ewing sarcoma of the thyroid gland. Pediatr Radiol (2009) 39(12):1365–8. doi: 10.1007/s00247-009-1388-1 [DOI] [PubMed] [Google Scholar]
- 49. Wei-yu ZHU, Yang X-m, Zeng J-j, Wei M-h, Wang S, Li W-c, et al. [Extraskeletal Ewing sarcoma of thyroid gland: A case report]. J Cent South Univ (Medical Science). (2021) 46(5):558–64. doi: 10.11817/j.issn.1672-7347.2021.200161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kabata P, Kaniuka-Jakubowska S, Kabata W, Lakomy J, Biernat W, Sworczak K, et al. Primary Ewing sarcoma of the thyroid-eight cases in a decade: A case report and literature review. Front Endocrinol (Lausanne). (2017) 8:257. doi: 10.3389/fendo.2017.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bishop JA, Alaggio R, Zhang L, Seethala RR, Antonescu CR. Adamantinoma-like Ewing family tumors of the head and neck: A pitfall in the differential diagnosis of basaloid and myoepithelial carcinomas. Am J Surg Pathol (2015) 39(9):1267–74. doi: 10.1097/PAS.0000000000000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maldi E, Monga G, Rossi D, Tosoni A, Mezzapelle R, Boldorini R. Extra-osseous Ewing sarcoma of the thyroid gland mimicking lymphoma recurrence: A case report. Pathol Res Pract (2012) 208(6):356–9. doi: 10.1016/j.prp.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 53. Seipel AH, Mechahougui H, Mach N, Triponez F, Faquin WC, De Vito C. Primary extra-osseous Ewing sarcoma of the thyroid: A case report and review of the literature. Head Neck Pathol (2021) 16(2):581–6. doi: 10.1007/s12105-021-01365-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with ewing's sarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol. (2015) 39(2):189–95. doi: 10.1016/j.canep.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 55. Wang S, Wang X, Zhou X, Lyerly HK, Morse MA, Ren J. DC-CIK as a widely applicable cancer immunotherapy. Expert Opin Biol Ther (2020) 20(6):601–7. doi: 10.1080/14712598.2020.1728250 [DOI] [PubMed] [Google Scholar]
- 56. Morales E, Olson M, Iglesias F, Dahiya S, Luetkens T, Atanackovic D. Role of immunotherapy in Ewing sarcoma. J Immunother Cancer. (2020) 8(2):e000653. doi: 10.1136/jitc-2020-000653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol (2020) 17(8):807–21. doi: 10.1038/s41423-020-0488-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hayes C. Cellular immunotherapies for cancer. Ir J Med Sci (2021) 190(1):41–57. doi: 10.1007/s11845-020-02264-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ellis GI, Sheppard NC, Riley JL. Genetic engineering of T cells for immunotherapy. Nat Rev Genet (2021) 22(7):427–47. doi: 10.1038/s41576-021-00329-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu X, Li J, Liu Y, Ding J, Tong Z, Liu Y, et al. Calreticulin acts as an adjuvant to promote dendritic cell maturation and enhances antigen-specific cytotoxic T lymphocyte responses against non-small cell lung cancer cells. Cell Immunol (2016) 300:46–53. doi: 10.1016/j.cellimm.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 61. Sui CG, Wu D, Meng FD, Yang MH, Jiang YH. Anti-prostate cancer effects of CTL cell induction in vitro by recombinant adenovirus mediated PSMA/4-1BBL dendritic cells: An immunotherapy study. Genet Mol Res (2015) 14(2):7208–17. doi: 10.4238/2015.June.29.14 [DOI] [PubMed] [Google Scholar]
- 62. Jiang T-b, Li X, Liu J, Zhong Z-q, Wang E-h, Zhang C-mææ, et al. [Extra-skeletal ewing's sarcoma treated with Large dosage chemotherapy plus ematopoietic stem cell transplantation: A case report with review]. Chin Gen Pract (2008) 11(13):1193–4. doi: 10.3969/j.issn.1007-9572.2008.13.030 [DOI] [Google Scholar]
- 63. Karadurmus N, Sahin U, Bahadir Basgoz B, Demirer T. Is there a role of high dose chemotherapy and autologous stem cell transplantation in the treatment of ewing's sarcoma and osteosarcomas? J B U. ON: Off J Balkan Union Oncol (2018) 23(5):1235–41. [PubMed] [Google Scholar]
- 64. Mami-Chouaib F, Blanc C, Corgnac S, Hans S, Malenica I, Granier C, et al. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer. (2018) 6(1):87. doi: 10.1186/s40425-018-0399-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.