Abstract
One of the essential features of all pathogenic strains of Salmonella enterica is the ability to enter into nonphagocytic cells. This pathogenic property is mediated by the Salmonella pathogenicity island 1 (SPI-1)-encoded type III secretion system. Expression of components and substrates of this system is subject to complex regulatory mechanisms. These mechanisms include a number of specific and global transcriptional regulatory proteins. In this study we have compared in S. enterica serovars Typhimurium and Typhi the effect of mutations in flagellar genes on the phenotypes associated with the SPI-1 type III protein secretion system. We found that serovar Typhi strains carrying a null mutation in either of the flagellar regulatory genes flhDC or fliA were severely deficient in entry into cultured epithelial cells and macrophage cytotoxicity. This defect could not be reversed by applying a mild centrifugal force, suggesting that the effects of the mutations were not due to the absence of motility. In contrast, the same mutations had no significant effect on the ability of serovar Typhimurium to enter into cultured Henle-407 cells or to induce macrophage cell death. Consistent with these observations, we found that the mutations in the flagellar regulatory proteins significantly reduced the expression of components of the SPI-1-encoded type III system in serovar Typhi but had a marginal effect in serovar Typhimurium. Our results therefore indicate that there is an overlap between regulatory mechanisms that control flagellar and type III secretion gene expression in Salmonella serovar Typhi.
Salmonella enterica serovar Typhi is the cause of typhoid fever in humans, which remains a global health problem. According to the World Health Organization, there are an estimated 16.6 million cases and 600,000 deaths per year due to typhoid fever, predominantly in Asia and Africa (37).
One of the essential features of all pathogenic Salmonella strains is their ability to enter epithelial cells, a phenotype that is mediated by the type III secretion system encoded at centisome 63 of their chromosome within Salmonella pathogenicity island 1 (SPI-1) (12). This system enables the translocation of a battery of effector proteins into the host cell cytosol, thereby stimulating a number of cellular responses. These responses include the production of proinflammatory cytokines and the stimulation of the reorganization of the actin cytoskeleton, leading to bacterial uptake into intestinal epithelial cells. In macrophages the signaling events stimulated by Salmonella lead to the initiation of programmed cell death.
Previous studies have indicated the importance of motility for Salmonella invasion of cultured cells (25, 26, 30, 33, 45). However, it is unclear whether there is a direct requirement for motility in order for Salmonella to enter nonphagocytic cells or whether mutations in flagellum-associated genes have an indirect effect on the expression of the invasion phenotype. Mutations in chemotaxis genes such as cheA, cheR, cheW, and cheY, which confer a smooth-swimming phenotype, rendered S. enterica serovar Typhimurium more invasive than wild-type strains (25). In contrast, mutations in cheB, which result in a “tumbly” phenotype, rendered these bacteria deficient in entry (25, 30). Serovar Typhimurium nonmotile strains can regain wild-type levels of entry if a mild centrifugal force is applied during the internalization process. In contrast, Liu et al. reported that centrifugation cannot reverse the entry deficiency of serovar Typhi Fla−, Mot−, and Che− mutants, suggesting that unlike serovar Typhimurium, serovar Typhi requires intrinsic, intact motility for host cell invasion (33). These findings also suggest the existence of differences in the entry mechanisms between serovar Typhimurium and the host-adapted serovar Typhi. The present study was designed to investigate the role of motility in entry of serovar Typhi into cultured epithelial cells. We found an overlap between the regulatory mechanisms that control flagellar and invasion gene expression.
MATERIALS AND METHODS
Bacterial strains, bacteriophages, and growth conditions.
The bacterial strains used in this study are listed in Table 1. Bacteria were grown in L broth or in high-osmolarity L broth (with 0.3 M NaCl, pH 7.0), and when required, the following antibiotics were added at the concentrations indicated: ampicillin, 100 μg/ml; chloramphenicol, 30 μg/ml; kanamycin, 50 μg/ml; streptomycin, 100 μg/ml; and tetracycline, 12.5 μg/ml. Bacteriophage P22HTint-mediated transduction was used for transduction of markers into Salmonella strains (40). Conjugations were carried out by filter mating as described elsewhere (27).
TABLE 1.
Bacterial strains used in these studies
| S. enterica serovar and strain | Relevant genotype | Reference or source |
|---|---|---|
| Dublin SL5928 | fliC::Tn10 | Bruce Stocker, Stanford University |
| Typhimurium | ||
| SL1344 derivatives | ||
| SL1344 | Wild type | 21 |
| χ3420 | fliGHI::Tn10 | 34 |
| SB164 | invF::xylE | 27 |
| SB165 | invA::xylE | 27 |
| SL5928 | fliC::Tn10 | |
| SB200 | fliC::Tn10 | P22HTint [SL5928]→SL1344 (this study) |
| SB205 | invF::xylE fliA::Tn10 | P22HTint [SJW80]→SB164 (this study) |
| SB206 | invA::xylE fliA::Tn10 | P22HTint [SJW80]→SB165 (this study) |
| SB208 | fliA::Tn10 | P22HTint [SJW80]→SL1344 (this study) |
| SB227 | sipC::xylE | 9 |
| SB233 | invJ::xylE | 9 |
| SB556 | flhC::Tn10 | P22HTint [SJW98]→SL1344 (this study) |
| SB557 | flhD::Tn10 | P22HTint [SJW93]→SL1344 (this study) |
| SB558 | fliD::Tn10 | P22HTint [SJW68]→SL1344 (this study) |
| SB559 | flgK::Tn10 | P22HTint [SJW563]→SL1344 (this study) |
| SB566 | invC::aphT | 10 |
| SB574 | invA::xylE flgK::Tn10 | P22HTint [SJW563]→SB165 (this study) |
| SB575 | invF::xylE flgK::Tn10 | P22HTint [SJW563]→SB164 (this study) |
| SB576 | invJ::xylE fliA::Tn10 | P22HTint [SJW80]→SB233 (this study) |
| SB577 | invJ::xylE flgM::MudJ-CM | P22HTint [TH2791]→SB233 (this study) |
| SB578 | invJ::xylE flgK::Tn10 | P22HTint [SJW563]→SB233 (this study) |
| SB579 | sipC::xylE fliA::Tn10 | P22HTint [SJW80]→SB227 (this study) |
| SB580 | sipC::xylE flgM::MudJ-CM | P22HTint [TH2791]→SB227 (this study) |
| SB581 | sipC::xylE flgK::Tn10 | P22HTint [SJW563]→SB552 (this study) |
| SB627 | flgM::MudJ-CM | P22HTint [TH2791]→SL1344 (this study) |
| SB632 | invA::xylE flgM::MudJ-CM | P22HTint [TH2791]→SB165 (this study) |
| SB634 | invF::xylE flgM::MudJ-CM | P22HTint [TH2791]→SB164 (this study) |
| LT-2 derivatives | ||
| SJW68 | fliD::Tn10 | Salmonella Genetics Stock Center |
| SJW80 | fliA::Tn10 | Salmonella Genetics Stock Center |
| SJW93 | flhD::Tn10 | Salmonella Genetics Stock Center |
| SJW98 | flhC::Tn10 | Salmonella Genetics Stock Center |
| SJW563 | flgK::Tn10 | Salmonella Genetics Stock Center |
| TH2791 | fgM::MudJ-CM | Salmonella Genetics Stock Center |
| Typhi | ||
| ISP1820 | Wild type | M. M. Levine, University of Maryland |
| SB552 | sipC::xylE | This study |
| SB554 | invJ::xylE | This study |
| SB563 | sipC::xylE fliA::Tn10 | P22HTint [SJW80]→SB552 (this study) |
| SB564 | sipC::xylE flgM::MudJ-CM | P22HTint [TH2791]→SB552 (this study) |
| SB567 | invJ::xylE fliA::Tn10 | P22HTint [SJW80]→SB554 (this study) |
| SB568 | invJ::xylE flgM::MudJ-CM | P22HTint [TH2791]→SB554 (this study) |
| SB570 | invJ::xylE flgK::Tn10 | P22HTint [SJW563]→SB554 (this study) |
| SB571 | sipC::xylE flgK::Tn10 | P22HTint [SJW563]→SB552 (this study) |
| SB572 | invA::xylE flgK::Tn10 | P22HTint [SJW563]→SB611 (this study) |
| SB573 | invF::xylE flgK::Tn10 | P22HTint [SJW563]→SB613 (this study) |
| SB600 | fliC::Tn10 | P22HTint [SL5928]→ISP1820 (this study) |
| SB604 | invC::aphT | P22HTint [SB566]→ISP1820 (this study) |
| SB606 | fli-8007::Tn10 | P22HTint [χ3420]→ISP1820 (this study) |
| SB611 | invA::xylE | This study |
| SB613 | invF::xylE | This study |
| SB616 | fliA::Tn10 | P22HTint [SJW80]→ISP1820 (this study) |
| SB617 | invA::xylE fliA::Tn10 | P22HTint [SJW80]→SB611 (this study) |
| SB619 | invF::xylE fliA::Tn10 | P22HTint [SJW80]→SB613 (this study) |
| SB620 | invA::xylE flgM::MudJ-CM | P22HTint [TH2791]→SB611 (this study) |
| SB622 | invF::xylE flgM::MudJ-CM | P22HTint [TH2791]→SB613 (this study) |
| SB624 | flgM::MudJ-CM | P22HTint [TH2791]→ISP1820 (this study) |
| SB644 | fliD::Tn10 | P22HTint [SJW68]→ISP1820 (this study) |
| SB645 | flhD::Tn10 | P22HTint [SJW93]→ISP1820 (this study) |
| SB646 | flhC::Tn10 | P22HTint [SJW98]→ISP1820 (this study) |
| SB648 | flgK::Tn10 | P22HTint [SJW563]→ISP1820 (this study) |
Motility assay.
Motility was assayed by using semisolid agar plates (containing 10 g of tryptone, 5 g of NaCl, and 3 g of agar per liter of medium) and incubation conditions of 37°C for 18 h.
Invasion assay.
Entry of Salmonella strains into cultured Henle-407 cells was assayed in 24-well tissue culture plates as described previously (14). Bacterial cultures were grown to an optical density at 600 nm of 1.1, and experiments were carried out with a multiplicity of infection of 10. When indicated, a mild centrifugal force (500 × g for 10 min) was applied to the 24-well tissue culture plates at the start of the 2-h infection period.
Macrophage cytotoxicity assay.
Macrophage cytotoxicity was assayed by ethidium homodimer-1 staining as previously described (7) with minor modifications. J774.A1 cells were grown to a confluency of about 80 to 90% on glass coverslips in Dulbecco modified Eagle medium containing 10% fetal calf serum and 1 mM sodium pyruvate. Cells were infected at a multiplicity of infection of 5, with the different bacterial strains grown to an invasion-competent state as indicated above. During the first 10 min of the 2-h invasion process, bacteria were spun onto the macrophages at 500 × g. Subsequently, cells were washed twice with Hanks balanced salt solution and incubated in 250 μl of Dulbecco modified Eagle medium–10% fetal calf serum containing 100 μg of gentamicin per ml for 1 h at 37°C. Infected macrophages were stained with 250 μl of medium containing 4 μM ethidium homodimer-1 for 20 min at 37°C. Ethidium homodimer-1 is a high-affinity, membrane-impermeant dye that can only stain DNA of nuclei of dead cells (17). Cells were washed twice with Hanks balanced salt solution, and the coverslips were mounted and sealed onto glass slides and immediately visualized by fluorescence microscopy. The number of macrophages killed by Salmonella was determined by determining the proportion of cells exhibiting fluorescence-stained nuclei. A minimum of 500 cells were examined for each bacterial strain, and the experiments were repeated at least three times.
C2,3O assay to measure gene expression with xylE gene fusions.
Bacterial strains were grown overnight in high-osmolarity L broth for 12 to 14 h and diluted 1:20 in a total volume of 20 ml. The cultures were grown for 4 h under mild shaking conditions to an optical density at 600 nm of approximately 1.1. Cells were lysed by sonication, and the levels of catechol 2,3-dioxygenase (C2,3O) activity were determined as described elsewhere (38). Briefly, bacterial cells were washed with 5 ml of cold 20 mM potassium phosphate buffer (pH 7.2). The bacterial pellets were resuspended in 1.5 ml of cold APB (10% acetone, 100 mM potassium phosphate buffer, pH 7.5) and sonicated on ice for 1 min to disrupt cells. Extracts were centrifuged at maximum speed in a microcentrifuge for 10 min at 4°C to remove cellular debris. The total protein concentration was determined with the BCA Protein Assay Reagent, and known concentrations of bovine serum albumin were used as standards as indicated by the manufacturer (Pierce Chemical Co., Rockford, Ill.). C2,3O activity was determined by monitoring the increase in absorbance at 375 nm at room temperature due to accumulation of 2-hydroxymuconic semialdehyde, in 3-ml polypropylene reaction cuvettes. Briefly, 2.5 ml of 100 μM potassium phosphate buffer (pH 8.0), 0.45 ml of APB, 50 μl of extract, and 10 μl of 100 mM catechol were mixed, normalized against a blank containing all of these ingredients except extract, and immediately read at a wavelength of 375 nm. The extract concentration was adjusted to obtain a reaction rate where product formation increased the optical density by no more than 0.005 per s. One milliunit corresponds to the formation at room temperature of 1 nmol of 2-hydroxymuconic semialdehyde per min per mg of protein. The molar absorption coefficient e was 42,000. Calculations were performed with the following formula: milliunits = 7.1 × 104 × (VBCA/VC2,3O) × (A375/T) × (DC2,3O/Y) × (1/DBCA), where VBCA is the volume of extract used to determine total protein concentration, VC2,3O is the volume of extract used in the C2,3O assay, A375 is the absorbance at end of time T, T is the time required to reach the A375, Y is the amount (micrograms) of protein in the VBCA as calculated from the linear quadratic equation of the protein standard curve, and D is the dilution factor, i.e., Vfinal/Vsample.
Analysis of Salmonella whole-cell lysates and culture supernatant proteins.
Overnight cultures of the different Salmonella strains were diluted 1:20 and grown in 100-mm tubes on a rotating wheel at about 30 rpm in 2.5 ml of high-osmolarity L broth containing 0.3 M sodium chloride to an optical density at 600 nm of 1.1. Cultures (1.5 ml) were then centrifuged at 14,000 × g for 30 min at 4°C. One-milliliter portions of the supernatants were collected for further analysis, and the remaining medium over the cell pellet was carefully removed without disturbing the pellet and discarded. The bacterial pellets were resuspended in 300 μl of Laemmli buffer. Seventy-five microliters of culture supernatant and 30 μl of whole-cell lysate preparations were loaded onto polyacrylamide gels and transferred to nitrocellulose membranes for Western blot (immunoblot) analysis with a monoclonal antibody directed to SipC. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis were carried out by standard protocols (39).
RESULTS
Characterization of the S. enterica serovar Typhi ISP1820 wild-type strain.
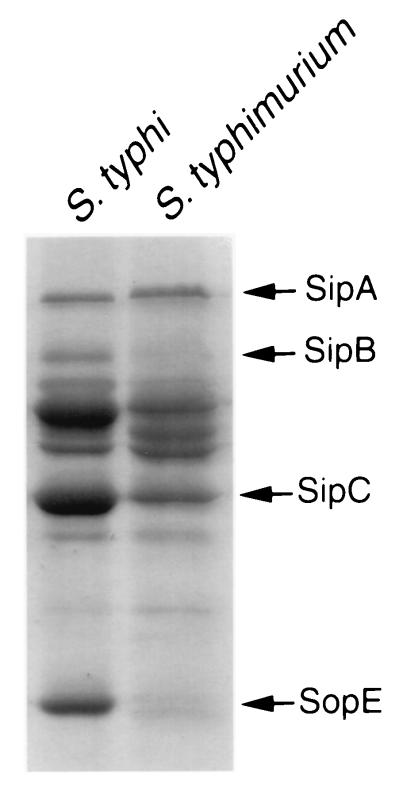
S. enterica serovar Typhi strain ISP1820, originally isolated from an outbreak of typhoid fever in Chile (kindly provided by M. M. Levine, University of Maryland), was utilized in these studies. Phenotypes in this strain associated with the centisome 63 type III secretion system, such as bacterial entry into cells and macrophage cytotoxicity, were examined as indicated in Materials and Methods. In agreement with previous reports, we observed a strong correlation between the bacterial culture conditions and the ability of strain ISP1820 to stimulate host cell responses (15, 32, 43). When grown under culture conditions which maximally induce the expression of genes associated with SPI-1 (0.3 M NaCl L broth, low oxygen tension, and late logarithmic growth phase), serovar Typhi strain ISP1820 was capable of entering into Henle-407 cells and inducing macrophage cytotoxicity in a manner that was roughly equivalent to that of S. enterica serovar Typhimurium (data not shown; see Fig. 2B and 3B). We also compared the protein profiles of culture supernatants of serovar Typhi ISP1820 and serovar Typhimurium SB300 by SDS-PAGE and Coomassie blue staining. The two strains exhibited similar (although not identical) supernatant protein profiles (Fig. 1), demonstrating the conservation of at least some of the secreted effector proteins in these two microorganisms. These results indicate that the serovar Typhi strain ISP1820 harbors a functional SPI-1 type III secretion system which, like that of serovar Typhimurium, mediates the stimulation of several host cell responses, such as membrane ruffling, leading to bacterial internalization into nonphagocytic cells and cytotoxicity towards macrophages (see below).
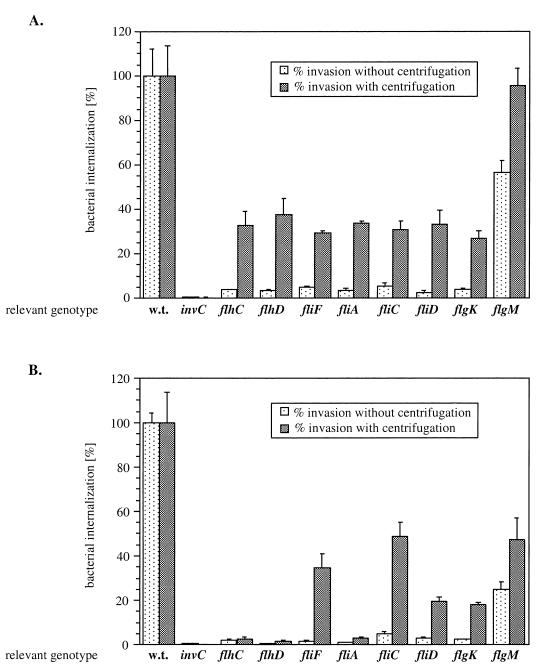
FIG. 2.
Effect of flagellar gene mutations on the ability of Salmonella strains to enter into Henle-407 cells in the presence or absence of a mild centrifugal force. Transposon insertions in the three regulatory classes of flagellar genes were introduced into S. enterica serovars Typhimurium (A) and Typhi (B), and the strains were tested for their ability to enter cultured epithelial cells in the presence or absence of a mild centrifugal force. Values represent the percentage of the bacterial inoculum that survived the gentamicin treatment and have been standardized to the level of internalization of the wild-type (w.t.) strain in each category, which was considered 100%. The values represent the means ± standard deviations from one representative experiment performed with triplicate samples. Equivalent results were obtained in several repetitions of this experiment. The actual percentage of the wild-type serovar Typhimurium and Typhi inocula that resisted the gentamicin treatment under these assay conditions ranged between 40 and 60% in different experiments.
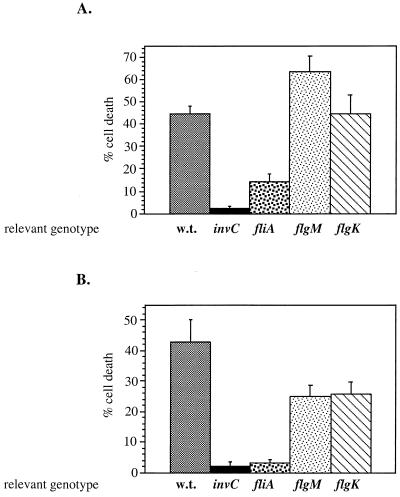
FIG. 3.
Effect of flagellar mutations on Salmonella macrophage cytotoxicity. Transposon insertions were introduced in three flagellar genes (fliA, flgM, and flgK) in S. enterica serovars Typhimurium (A) and Typhi (B) and tested for cytotoxicity in cultured J774.A1 macrophages as described in Materials and Methods. The percentage of dead cells was determined after 3 h of infection by staining with the membrane-impermeant dye ethidium homodimer-1. During the first 10 min of infection, bacteria were spun onto the macrophages at 500 × g. The values represent the average values obtained from three independent experiments, including the standard deviation between these measurements. For each sample a minimum of 500 cells were examined. w.t., wild type.
FIG. 1.
Culture supernatant protein profiles of wild-type S. enterica serovars Typhi and Typhimurium. Culture supernatant proteins from wild-type serovar Typhi (ISP1820) and serovar Typhimurium (SL1344) were obtained as indicated in Materials and Methods, separated on an SDS–10% polyacrylamide gel, and stained with Coomassie blue. The arrows on the right indicate the positions of a subset of secreted proteins.
Effect of flagellar mutations in serovars Typhi and Typhimurium on phenotypes associated with the centisome 63 type III system.
The assembly and regulation of the flagellar structure in S. enterica serovar Typhimurium is complex and has been studied extensively by several laboratories (reviewed in reference 35). Flagellar genes are grouped in operons that are clustered in five regions (I, II, IIIa, IIIb, and H2) distributed throughout the bacterial chromosome. The transcription of these genes is organized into a regulatory hierarchy of three classes (early, middle, and late genes). Expression of each class is a prerequisite for the expression of the following class in the cascade, thereby allowing coordinated expression. At the top of the hierarchy is the flhDC master regulatory operon (early genes), which is essential for the direct control of the class II (middle) genes, which encode proteins of the hook-basal body complex and FliA (or ς28), a flagellum-specific sigma factor. FliA, by itself or together with the master regulator FlhD-FlhC, activates the transcription of class III operons coding for the filament, proteins required for chemotaxis and rotation of the filament, and the anti-ς28 factor FlgM. The anti-sigma factor inhibits the transcription of class III genes indirectly by binding to FliA and preventing it from directing the RNA polymerase to recognize FliA-specific consensus sequences. Following assembly of the hook-basal body complex, the FlgM protein is secreted by the flagellum-specific export apparatus, effectively coupling flagellar assembly with transcriptional regulation (22, 31).
In order to investigate systematically the role of motility in Salmonella invasion into cultured epithelial cells, mutations in the three regulatory classes of flagellar genes were introduced by P22HTint transduction into the chromosomes of serovars Typhimurium and Typhi (Table 2). Except for the flgM strains, all flagellar mutant strains were nonmotile (data not shown).
TABLE 2.
Flagellar genes examined in this study
| Flagellar gene | Proposed function | Class in flagellar regulatory hierarchy |
|---|---|---|
| flhD | Flagellar master regulator | I |
| flhC | Flagellar master regulator | I |
| fliA | Flagellar sigma factor | II |
| fliC | Flagellin | III |
| fliD | Filament cap | III |
| flgK | Hook-filament junction | III |
| flgM | Anti-FliA (ς28) factor | III |
Strains of serovars Typhimurium and Typhi carrying mutations in the different flagellar genes were tested for the ability to enter cultured Henle-407 cells in the absence or presence of a mild centrifugal force (see Materials and Methods). As previously described (25, 26, 30, 33, 45), in the absence of centrifugation the nonmotile Salmonella strains tested were significantly deficient in their ability to gain access to cultured host cells (Fig. 2). Both serovar Typhimurium and serovar Typhi flgM strains displayed a slight decrease in the ability to invade cultured epithelial cells (60 and 25% of wild-type levels, respectively), which may be due to sterical hindrance caused by the overproduction of flagella in these strains (31).
Centrifugation significantly reversed the invasion defect of all serovar Typhimurium flagellar mutants (Fig. 2A). However, centrifugation compensated for the invasion deficiency of only a subset of serovar Typhi flagellar mutants (Fig. 2B), since application of a centrifugal force failed to reverse the invasion defect of strains carrying a mutation in flhDC or fliA, which encode transcriptional regulatory proteins (Fig. 2B).
Another phenotype associated with the Salmonella centisome 63 type III secretion system is the induction of apoptosis in macrophages. This prompted us to test whether strains of serovars Typhimurium and Typhi carrying mutations in flagellar genes (fliA, flgM, and flgK) are cytotoxic for macrophages (see Materials and Methods). Serovar Typhi strains carrying a mutation in the flagellar sigma factor (fliA) showed little macrophage cytotoxicity, which was analogous to that exhibited by a type III secretion-defective invC mutant strain (Fig. 3B). Serovar Typhimurium strains carrying the same mutation (fliA) also exhibited a significant decrease in their ability to kill macrophages (Fig. 3A). Mutations in the class III genes flgK and flgM in both serovars had little or no effect on their ability to kill macrophages.
In summary, only serovar Typhi strains carrying mutations in the flagellar transcription activators (FlhDC and FliA) remained unable to enter Henle-407 cells and induce macrophage cell death when a centrifugal force was applied during the infection process.
Effect of flagellar mutations on the expression of SPI-1 genes in serovars Typhi and Typhimurium.
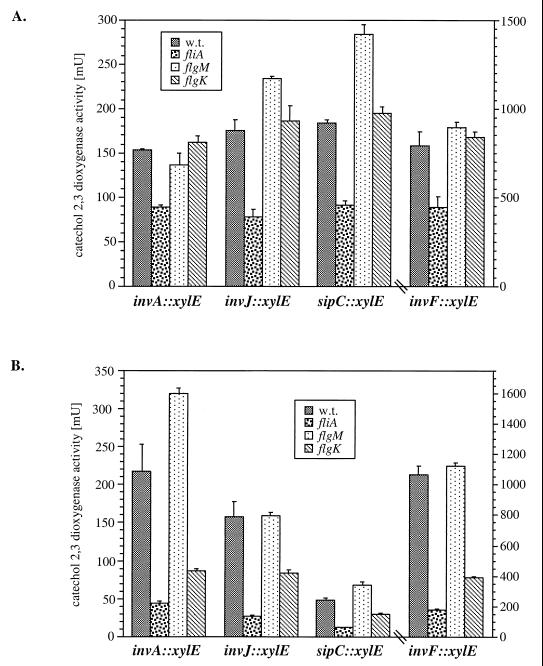
S. enterica serovar Typhi strains carrying mutations in the flagellar regulatory genes failed to enter into cultured epithelial cells or induce apoptosis in macrophages, even when a centrifugal force was applied. However, nonmotile strains carrying mutations in genes that encode flagellar structural components and belong to class III in the regulatory cascade remained invasive. These results suggested that motility per se is not required for bacterial invasion and raised the possibility that flagellar regulatory proteins may influence the expression of genes encoding the SPI-1 type III secretion system. To test this hypothesis, we examined in both serovars Typhi and Typhimurium the effect of null mutations in flagellar regulatory genes on the transcription of genes associated with the centisome 63 type III secretion system. Using reporter gene fusions, we analyzed the effect of null mutations in the positive regulator FliA and the negative regulator FlgM on the expression of invA, invF, invJ, and sipC (as described in Materials and Methods). These genes encode essential proteins of the SPI-1 encoded type III system, such as components of the type III machinery (InvA and InvJ) (8, 16), a type III secreted protein (SipC) (28), and an essential transcriptional regulator (InvF) (9, 27). Mutations in any of these genes render serovars Typhimurium and Typhi noninvasive for tissue culture cells and cause an increased 50% lethal dose in orally infected BALB/c mice (14).
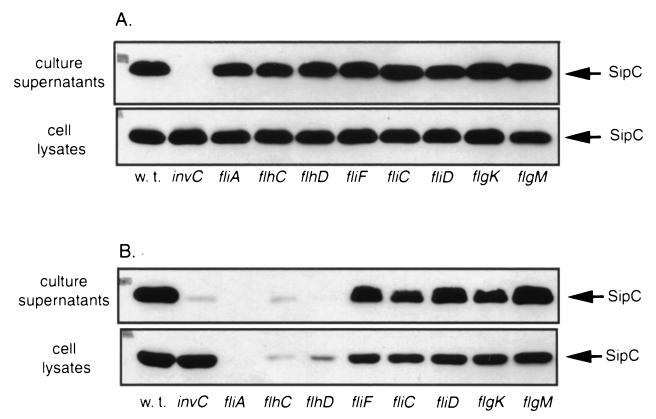
A mutation in fliA resulted in a reduction of SPI-1 gene expression that was more pronounced in serovar Typhi than in serovar Typhimurium (Fig. 4). In contrast, the transcription of SPI-1-encoded genes in serovar Typhimurium or Typhi was not affected or was slightly increased by the introduction of a loss-of-function mutation in the flagellar anti-sigma factor (flgM) (Fig. 4). In order to confirm these observations, we examined the levels of the type III secreted protein SipC in serovar Typhi and Typhimurium strains carrying mutations in the three regulatory classes of flagellar genes. The levels of SipC in serovar Typhimurium were not altered by the introduction of null mutations in genes belonging to any regulatory class (Fig. 5A). In contrast, in serovar Typhi, introduction of null mutations in either fliA or flhCD significantly reduced the levels of SipC in both culture supernatants and whole-cell lysates (Fig. 5B). Consistent with the gene expression results, introduction of mutations in flgM or in the class III flagellar gene fliC, fliD, or flgK did not affect the levels of SipC protein in either culture supernatants or whole-cell lysates. These results indicate that FlhDC and FliA play an important role in the transcriptional regulation of genes encoding the centisome 63 type III system in serovar Typhi and a less important albeit measurable role in serovar Typhimurium.
FIG. 4.
Effect of mutations in fliA and flgM on the transcription of components of the SPI-1 type III secretion system. The levels of transcription of the different reporter gene fusions in different S. enterica serovar Typhimurium and (A) and Typhi (B) backgrounds were measured by assaying C2,3O activity in bacterial cell lysates as indicated in Materials and Methods. The values represent the means ± standard deviations from one representative experiment performed with triplicate samples. Equivalent results were obtained in several repetitions of this experiment. w.t., wild type.
FIG. 5.
Western blot analysis of SipC production and secretion. Proteins from culture supernatants and whole-cell lysates of wild-type S. enterica serovars Typhimurium (SL1344) (A) and Typhi (ISP1820) (B) and isogenic strains carrying transposon insertions in the three regulatory classes of flagellar genes were resolved by SDS–10% PAGE, transferred to nitrocellulose, and probed with monoclonal antibodies directed to SipC as indicated in Materials and Methods.
DISCUSSION
The role of motility in Salmonella entry into host cells has been the subject of several studies (25, 26, 30, 33, 45). It has been previously shown that nonmotile mutants of Salmonella spp. are impaired in their ability to enter into cultured epithelial cells. In the case of S. enterica serovar Typhimurium, such a defect could be largely reversed by the application of a mild centrifugal force, arguing that motility per se may not be necessary for bacterial entry (25). Rather, motility may aid the entry process by facilitating the intimate contact between the bacteria and the host cell that is required for the delivery of effector proteins via the invasion-associated type III secretion system. In contrast to the case for serovar Typhimurium, the defect in invasion exhibited by nonmotile mutants of S. enterica serovar Typhi could not be reversed by the application of a centrifugal force (33). These studies suggested a more complex relationship between the flagellar and invasion-associated type III secretion systems in serovar Typhi. This is intriguing, as it is now apparent that the flagellar export and type III secretion systems are evolutionarily and functionally related (13).
Previous studies either did not take into consideration the complex regulatory cascade that controls flagellar gene expression or were carried out with poorly characterized mutants. In an attempt to clarify the role of motility and flagellum-associated genes in Salmonella entry into host cells, we introduced loss-of-function mutations in the three regulatory classes of flagellar genes in both serovars Typhimurium and Typhi and examined their effect on bacterial invasion and invasion gene expression. In agreement with previous studies, we found that introduction of mutations in flagellar genes (with the exception of flgM) impaired the ability of serovar Typhimurium to enter tissue culture cells and that this defect could be largely reversed by the application of a mild centrifugal force. These results are consistent with an indirect role for motility in the stimulation of the cellular responses leading to uptake of serovar Typhimurium.
In contrast to the case for serovar Typhimurium, serovar Typhi strains carrying loss-of-function mutations in genes encoding the flagellar transcriptional regulators flhDC or fliA remained defective for invasion into Henle-407 cells even after the application of a mild centrifugal force. However, the invasion defect resulting from a loss-of-function mutation in genes that are also required for motility but belong to a different class (class III) in the regulatory cascade could be reversed by the application of a mild centrifugal force. These results indicate that, like in serovar Typhimurium, motility per se is not required for entry of serovar Typhi into host cells. However, the failure to reverse the invasion defect of strains carrying mutations in flagellar genes belonging to class I and class II of the regulatory cascade suggested an indirect effect of these mutations on the invasion phenotype. Consistent with this hypothesis, we found that loss-of-function mutations in flhDC and fliA significantly affected the expression of invasion-associated genes in S. typhi. In contrast, the effect of flhDC and fliA mutations on the expression of invasion genes in S. typhimurium was much more reduced, consistent with a much-reduced effect of these mutations on the invasion phenotype.
These results indicate that the flagellar regulatory genes also control invasion gene expression, adding flagellar regulatory proteins to the already-extensive list of gene products reported to influence invasion gene expression. The expression of the invasion-associated type III secretion system in Salmonella is indeed subject to a remarkably complex regulation involving both specific (InvF, HilA, HilB, and SprA-HilC) and global (PhoP-PhoQ, SirA, and RcsB-RcsC) transcriptional regulatory proteins (1, 4, 5, 9, 11, 24, 27, 36, 46). How and when each one of these regulatory systems exerts its effect during the infection cycle are unknown. It is possible that the deployment of the type III secretion system may be influenced by a variety of environmental cues operating through different specific regulatory systems.
The contribution of motility to Salmonella pathogenesis has been the subject of several studies (6, 34, 41, 42). Absence of motility or flagella did not affect the oral or intraperitoneal 50% lethal dose of BALB/c mice infected with serovar Typhimurium (34). Other studies showed that flgM mutants of serovar Typhimurium are nonvirulent and showed a decreased survival in macrophages (41, 42). Mutations in the flagellar anti-sigma factor FlgM affect the growth rate of bacteria due to excess production and secretion of flagellin (31), which may diminish Salmonella's ability to survive inside the host and/or cause disease. In addition, strains carrying a flgM mutation produce twice the amount of flagella produced by wild-type strains, which may affect the ability of Salmonella to interact with host cells by steric hindrance. Introduction of a mutation in fliA into a flgM strain, resulting in a nonflagellated strain, was able to reverse the attenuating phenotype of the flgM mutation, indicating that it is the excess of flagellin production that is responsible for the attenuating effect of flgM (42). Overall, all of these studies argue for a lack of involvement of flagella in serovar Typhimurium pathogenesis. However, these experiments were carried out with BALB/c mice, which are very susceptible to serovar Typhimurium infections, therefore preventing the assessment of the impact of lesser virulence defects in vivo. In addition, the mouse model does not adequately mimic the clinical course of a nonsystemic infection.
Our studies demonstrating a close connection between the regulatory mechanisms of the flagellar and type III secretion systems indicate a need for caution in the interpretation of studies aimed at establishing a connection between flagella and virulence, particularly those studies using mutations in class I or class II genes that result in changes in gene expression. At least in the case of serovar Typhi, mutations in such genes would be expected to have a profound effect on virulence based on their effect on the expression of the invasion-associated type III secretion system. Indeed, there is epidemiological evidence suggesting that at least in some strains of serovar Typhi there is a direct correlation between bacterial motility, tissue culture cell invasion, and virulence (19). Our results also indicate that any conclusion linking the flagellar export system to other phenotypes, in particular type III secretion, should take into consideration a potential regulatory connection between these systems. For example, it has been recently proposed that the flagellar export apparatus of Yersinia enterocolitica can secrete proteins other than those associated with the flagellar system (47). Our results linking the flagellar regulatory mechanisms with the regulation of type III secretion in S. enterica coupled to the recent identification of a second type III secretion system in the Yersinia chromosome (The Sanger Center [http://www.sanger.uk.ak]) may potentially provide an alternative explanation for the results obtained in those studies.
Although coordinate expression of virulence and flagellar genes has also been reported for other microorganisms (2, 20), it is unknown whether there is a connection between the regulation of flagellar and type III secretion systems in bacteria other than Salmonella. Type III secretion-associated genes of Yersinia and Shigella spp. contain sequences in their promoter regions similar to the fliA consensus sequence (3). Although Shigella spp. are nonmotile, flagellum-related genes have been identified on their chromosomes (44) and flagellum-like structures have been observed by electron microscopy (18). In Y. enterocolitica transcription of both fliA and flgM is immediately arrested when cells are exposed to 37°C concomitant with the upregulation of type III secretion-associated genes (29). These findings suggest the possibility of a connection between the regulation of flagellar genes and genes associated with the type III secretion system in these bacteria. However, the transcription of lcrD, which encodes a component of the plasmid-encoded type III secretion machinery that is a homologue of S. enterica serovar Typhimurium InvA, was not affected in a fliA mutant (23).
Finally, it was recently demonstrated that the two-component regulatory system RcsB-RcsC of S. enterica serovar Typhi differentially modulates the expression of SPI-1-encoded genes, flagellin, and Vi antigen. Under low-osmolarity conditions the RcsB-RcsC system downregulates the expression of both flagellin and genes encoded at centisome 63, whereas the expression of Vi antigen was increased (4). In light of our studies, it is possible that the effect of RcsB-RcsC on invasion gene expression may be not direct but rather a consequence of the influence of this system on flagellar gene expression.
ACKNOWLEDGMENTS
We thank K. Hughes for useful discussions and for providing bacterial strains and members of the Galán laboratory for critical review of the manuscript.
This work was supported by Public Health Service grant AI30492 from the National Institutes of Health.
REFERENCES
- 1.Ahmer B M, van Reeuwijk J, Watson P R, Wallis T S, Heffron F. Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol Microbiol. 1999;31:971–982. doi: 10.1046/j.1365-2958.1999.01244.x. [DOI] [PubMed] [Google Scholar]
- 2.Allison C, Lai H-C, Hughes C. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol Microbiol. 1992;6:1583–1591. doi: 10.1111/j.1365-2958.1992.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 3.Andrews G P, Maurelli A T. mxiA of Shigella flexneri 2a, which facilitates export of invasion plasmid antigens, encodes a homologue of the low-calcium-response protein, LcrD, of Yersinia pestis. Infect Immun. 1992;60:3287–3295. doi: 10.1128/iai.60.8.3287-3295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arricau N, Hermant D, Waxin H, Ecobichon C, Duffey P S, Popoff M Y. The RcsB-RcsC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol Microbiol. 1998;29:835–850. doi: 10.1046/j.1365-2958.1998.00976.x. [DOI] [PubMed] [Google Scholar]
- 5.Bajaj V, Hwang C, Lee C A. hilA is a novel ompR.toxR family member that activates the expression of Salmonella typhimurium expression genes. Mol Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 6.Carsiotis M, Stocker B D, Weinstein D L, O'Brien A. A Salmonella typhimurium virulence gene linked to flg. Infect Immun. 1989;57:3276–3280. doi: 10.1128/iai.57.11.3276-3280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L M, Kaniga K, Galán J E. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 8.Collazo C M, Zierler M K, Galán J E. Functional analysis of the Salmonella typhimurium invasion genes invI and invJ and identification of a target of the protein secretion apparatus encoded in the inv locus. Mol Microbiol. 1995;15:25–38. doi: 10.1111/j.1365-2958.1995.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 9.Eichelberg K, Galán J E. Differential regulation of Salmonella typhimurium type III secreted proteins by pathogenicity island 1 (SPI-1)-encoded transcriptional activators InvF and hilA. Infect Immun. 1999;67:4099–4105. doi: 10.1128/iai.67.8.4099-4105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eichelberg K, Ginocchio C, Galán J E. Molecular and functional characterization of the Salmonella typhimurium invasion genes invB and invC: homology of InvC to the F0F1 ATPase family of proteins. J Bacteriol. 1994;176:4501–4510. doi: 10.1128/jb.176.15.4501-4510.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eichelberg K, Hardt W D, Galan J E. Characterization of SprA, an AraC-like transcriptional regulator encoded within the Salmonella typhimurium pathogenicity island 1. Mol Microbiol. 1999;33:139–152. doi: 10.1046/j.1365-2958.1999.01458.x. [DOI] [PubMed] [Google Scholar]
- 12.Galán J E. Interaction of Salmonella with host cells through the centisome 63 type III secretion system. Curr Opin Microbiol. 1999;2:46–50. doi: 10.1016/s1369-5274(99)80008-3. [DOI] [PubMed] [Google Scholar]
- 13.Galán J E, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284:322–328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 14.Galán J E, Curtiss R., III Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galán J E, Curtiss R., III Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun. 1990;58:1879–1885. doi: 10.1128/iai.58.6.1879-1885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galán J E, Ginocchio C, Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992;174:4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaugain B, Barbet J, Capelle N, Roques B P, Le Pecq J B. DNA bifunctional intercalators. 2. Fluorescence properties and DNA binding interaction of an ethidium homodimer and an acridine ethidium heterodimer. Biochemistry. 1978;17:5078–5088. doi: 10.1021/bi00617a002. [DOI] [PubMed] [Google Scholar]
- 18.Giron J A. Expression of flagella and motility by Shigella. Mol Microbiol. 1995;18:63–75. doi: 10.1111/j.1365-2958.1995.mmi_18010063.x. [DOI] [PubMed] [Google Scholar]
- 19.Grossman D A, Witham N D, Burr D H, Lesmana M, Rubin F A, Schoolnik G K, Parsonnet J. Flagellar serotypes of Salmonella typhi in Indonesia: relationships among motility, invasiveness, and clinical illness. J Infect Dis. 1995;171:212–216. doi: 10.1093/infdis/171.1.212. [DOI] [PubMed] [Google Scholar]
- 20.Gyri D, Bailey M J, Allison C, Hughes C. Requirement for FlhA in flagella assembly and swarm-cell differentiation by Proteus mirabilis. Mol Microbiol. 1995;15:761–769. doi: 10.1111/j.1365-2958.1995.tb02383.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoiseth S K, Stocker B A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 22.Hughes K T, Gillen K L, Semon M J, Karlinsey J E. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- 23.Iriarte M, Stainier I, Mikulskis A V, Cornelis G R. The fliA gene encoding sigma 28 in Yersinia enterocolitica. J Bacteriol. 1995;177:2299–2304. doi: 10.1128/jb.177.9.2299-2304.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston C, Pegues D A, Hueck C J, Lee A, Miller S I. Transcriptional activation of Salmonella typhimurium invasion genes by a member of the phosphorylated response-regulator superfamily. Mol Microbiol. 1996;22:715–727. doi: 10.1046/j.1365-2958.1996.d01-1719.x. [DOI] [PubMed] [Google Scholar]
- 25.Jones B D, Lee C A, Falkow S. Invasion by Salmonella typhimurium is affected by the direction of flagellar rotation. Infect Immun. 1992;60:2475–2480. doi: 10.1128/iai.60.6.2475-2480.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones G W, Richardson L A, Uhlman D. The invasion of HeLa cells by Salmonella typhimurium: reversible and irreversible bacterial attachment and the role of bacterial motility. J Gen Microbiol. 1981;127:351–360. doi: 10.1099/00221287-127-2-351. [DOI] [PubMed] [Google Scholar]
- 27.Kaniga K, Bossio J C, Galán J E. The Salmonella typhimurium invasion genes invF and invG encode homologues to the PulD and AraC family of proteins. Mol Microbiol. 1994;13:555–568. doi: 10.1111/j.1365-2958.1994.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 28.Kaniga K, Tucker S C, Trollinger D, Galán J E. Homologues of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapatral V, Olson J, W, Pepe J C, Miller V L, Minnich S A. Temperature-dependent regulation of Yersinia enterolytica class III flagellar genes. Mol Microbiol. 1996;19:1061–1071. doi: 10.1046/j.1365-2958.1996.452978.x. [DOI] [PubMed] [Google Scholar]
- 30.Khoramian-Falsafi T, Harayama S, Kutsukake K, Pechere J C. Effect of motility and chemotaxis on the invasion of Salmonella typhimurium into HeLa cells. Microb Pathog. 1990;9:47–53. doi: 10.1016/0882-4010(90)90039-s. [DOI] [PubMed] [Google Scholar]
- 31.Kutsukake K, Ilno T. Role of the FliA-FlgM regulatory system on the transcriptional control of the flagellar regulon and flagellar formation in Salmonella typhimurium. J Bacteriol. 1994;176:3598–3605. doi: 10.1128/jb.176.12.3598-3605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee C A, Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci USA. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu S L, Ezaki T, Miura H, Matsui K, Yabuuchi E. Intact motility as a Salmonella typhi invasion-related factor. Infect Immun. 1988;56:1967–1973. doi: 10.1128/iai.56.8.1967-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lockman H A, Curtiss R., III Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect Immun. 1990;58:137–143. doi: 10.1128/iai.58.1.137-143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macnab R M. Flagella and motility. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 123–145. [Google Scholar]
- 36.Miller S I, Kukral A M, Mekalanos J J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pang T, Levine M M, Ivanoff B, Wain J, Finlay B B. Typhoid fever—important issues still remain. Trends Microbiol. 1998;6:131–133. doi: 10.1016/s0966-842x(98)01236-0. [DOI] [PubMed] [Google Scholar]
- 38.Sala-Trepat J M, Evans W C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971;11:400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 40.Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119:74–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt C K, Darnell S C, O'Brien A D. The attenuated phenotype of a Salmonella typhimurium flgM mutant is related to expression of FliC flagellin. J Bacteriol. 1996;178:2911–2915. doi: 10.1128/jb.178.10.2911-2915.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitt C K, Darnell S C, Tesh V L, Stocker B A D, O'Brien A D. Mutation of flgM attenuates virulence of Salmonella typhimurium, and mutation of fliA represses the attenuated phenotype. J Bacteriol. 1994;176:368–377. doi: 10.1128/jb.176.2.368-377.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tartera C, Metcalf E S. Osmolarity and growth phase overlap in regulation of Salmonella typhi adherence to and invasion of human intestinal cells. Infect Immun. 1993;61:3084–3089. doi: 10.1128/iai.61.7.3084-3089.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tominaga A, Mahmoud M A-H, Mukaihara T, Enomoto M. Molecular characterization of intact, but cryptic, flagellin genes in the genus Shigella. Mol Microbiol. 1994;12:277–285. doi: 10.1111/j.1365-2958.1994.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 45.Tomita T, Kanegasaki S. Enhanced phagocytic response of macrophages to bacteria by impact caused by bacterial motility or centrifugation. Infect Immun. 1982;38:865–870. doi: 10.1128/iai.38.3.865-870.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vescovi E G, Soncini F C, Groisman E A. Mg2+ as an extracellular signal—environmental regulation of salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 47.Young G M, Schmiel D H, Miller V L. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc Natl Acad Sci USA. 1999;96:6456–6461. doi: 10.1073/pnas.96.11.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]