Abstract
Cesarean section via a transverse uterine fundal incision is performed in patients with placenta previa to reduce blood loss. We describe a case of uterine rupture in a pregnant woman who previously underwent a cesarean section and recovered from cardiac arrest by multidisciplinary management.
Keywords: cardiac arrest, cardiopulmonary resuscitation, cesarean section, perimortem cesarean delivery, uterine rupture, uterine transverse incision
A transverse uterine fundal incision scar causes uterine rupture in subsequent pregnancies. It is difficult to evaluate the risk in the third trimester, when the gravid uterus is enlarged. Perimortem cesarean delivery is difficult to initiate when massive hemorrhage and cardiac arrest occur.

1. INTRODUCTION
A defective cesarean section scar is a well‐known risk factor for uterine rupture in subsequent pregnancies. 1 Uterine rupture during pregnancy may result in progressive intra‐abdominal bleeding, causing maternal shock and disseminated intravascular coagulation (DIC). When a patient develops cardiac arrest, prompt cardiopulmonary resuscitation (CPR) and an emergency procedure called perimortem cesarean delivery (PMCD) are urgently needed when resuscitation does not result in the return of spontaneous circulation (ROSC). 2 For the early detection of uterine rupture during pregnancy, transabdominal ultrasonography of a previous cesarean section scar is considered useful to evaluate the risk in most cases. However, it may become difficult to observe the scar in cases where the previous incision was made on the uterine fundus, as the gravid uterus was enlarged. Perinatal management and outcomes in similar cases have rarely been reported. 3 , 4 In the present case report, we describe a case of uterine rupture in a pregnant woman with a previous cesarean section by a transverse uterine fundal incision who recovered from cardiac arrest by CPR and PMCD and survived by multidisciplinary management. We also reviewed cases of uterine rupture in pregnant women who underwent a previous cesarean section via a transverse uterine fundal incision.
2. CASE PRESENTATION
The patient's first pregnancy occurred at the age of 33 years, and a cesarean section was performed at a previous hospital by a transverse uterine fundal incision with double‐layer closure of the uterine muscle due to placenta previa, in which the placenta covered the anterior uterine wall. Sagittal T1‐weighted magnetic resonance imaging (MRI) with contrast revealed a cesarean section scar defect at the uterine fundus 12 months postpartum (Figure 1). Considering the risk of uterine rupture, it was recommended that the patient did not become pregnant in future. The couple wished for a second child, and the patient conceived by in vitro fertilization at another clinic at 38 years.
FIGURE 1.
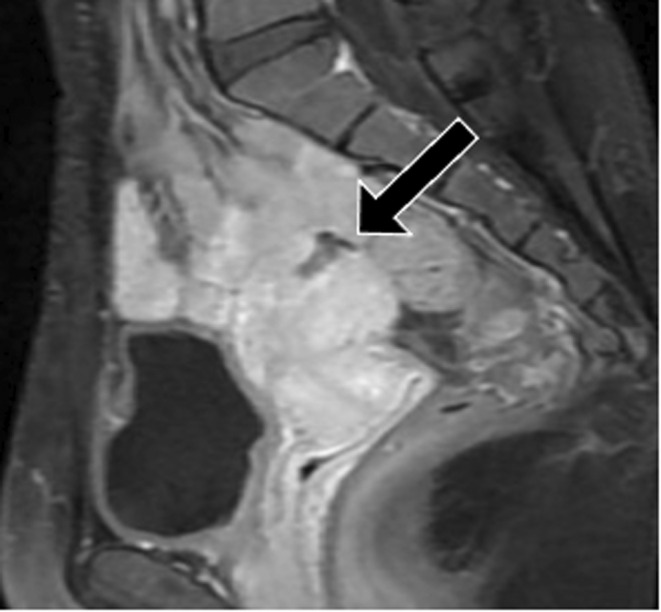
Sagittal T1‐weighted magnetic resonance imaging with contrast at 12 months postpartum showed a cesarean section scar defect at the uterine fundus (arrow)
The patient received prenatal care (PNC) at our hospital at 9 weeks of gestation. During the first visit, a previous scar was observed. Transvaginal ultrasonography revealed that a part of the muscle layer in the uterine fundus was very thin or interrupted (Figure 2). Despite being informed of the risk of maternal and fetal morbidity due to uterine rupture, the couple decided to continue with the pregnancy.
FIGURE 2.

Transvaginal ultrasonography at 9 weeks of gestation showed that a part of the muscle layer in the uterine fundus was very thin or interrupted (arrow)
The patient received PNC every 2 weeks, and an ultrasound study was performed for early detection of possible fetal membrane bulging outside of the uterus. At 28 weeks of gestation, the patient was diagnosed with total placenta previa, which was mostly located in the posterior uterine wall. At 30 weeks of gestation, the scar was no longer visible on transabdominal ultrasonography. Therefore, the patient was recommended to undergo MRI to evaluate the thickness of the uterine muscle layer. Considering the maternal and fetal risks of sudden uterine rupture, hospitalization is also recommended. However, the patient refused. At 33 weeks of gestation, the patient required hospital transfer via an ambulance at home for sudden severe abdominal pain. Unfortunately, during transport, the patient developed cardiac arrest 10 min before arriving at our hospital, and CPR was initiated by paramedics.
Upon arrival, the patient was unconscious, with a Glasgow Coma Scale (GCS) score of E1V1M1, and was asystole. Intubation was performed immediately, and a central venous catheter was inserted. CPR was continued with the administration of epinephrine. The gravid uterus was compressed to the left side to relieve aortocaval compression and make resuscitation more effective. Blood tests and arterial blood gas analysis showed the following results: fibrinogen level, 111 mg/dL; pH, 6.8; pO2 level, 16.6 mmHg, pCO2 level, 90.0 mmHg; hemoglobin level, 6.6 g/dL; lactate level, 12.7 mmol/L, and potassium level, 5.3 mEq/L. A point‐of‐care obstetric ultrasound showed a massive accumulation of free fluid in the abdominal cavity and fetal death. Therefore, the uterine rupture was strongly suspected. The patient recovered from arrest with six cycles of CPR 19 min after arrival at our emergency department. The total duration of CPR was 29 min. The decision was made to perform PMCD in the operating room. Although she had exhibited asystole twice in the operating room, PMCD was initiated 6 min after the decision.
During laparotomy, a massive intra‐abdominal hemorrhage was observed. To shorten the operative time, a low transverse incision on the uterus was first performed. A deceased male fetus weighing 1984 g was delivered; the placenta was easily removed, and bleeding from the attached uterine surface was not obvious. Meanwhile, an 8 cm‐sized uterine rupture was detected at the previous transverse uterine fundal incision site, and the amniotic membrane was intact. The rupture site was quickly repaired using double‐layer closure. It appeared that the bleeding was controlled; therefore, hysterectomy was not required. At the end of the surgery, the total volume of blood loss was 2500 ml. Blood transfusion with 14 units of packed red blood cells, 40 units of packed platelets, 10 units of packed fresh frozen plasma, 12 units of packed cryoprecipitate, and 3 g of fibrinogen concentrate was required to treat DIC caused by massive hemorrhage. Unfortunately, massive vaginal bleeding recurred postoperatively. Computed tomographic angiography showed engorged bilateral uterine arteries supplying the entire uterus. Interventional radiology (IVR) revealed extravasation in both uterine arteries (Figure 3A). Embolization of the bilateral uterine arteries was performed, and the bleeding was controlled (Figure 3B). The patient was transferred to the intensive care unit after IVR. Her vital signs gradually stabilized. Impairment of consciousness persisted (GCS score: E4, Vt, M2). On the 119th postoperative day, the patient was discharged from our hospital and was transferred to a rehabilitation facility.
FIGURE 3.

(A) Angiography prior to interventional radiology (IVR) revealed extravasations (arrow) in both uterine arteries. (B) Angiography after interventional radiology (IVR) showed obliteration of the bilateral uterine arteries (arrow)
3. DISCUSSION
In the current case, the uterine rupture occurred on the scar of a prior cesarean section performed on the uterine fundus. We can conclude that: (a) evaluating the thickness of the previous scar at the fundus by ultrasonography becomes more difficult as the gravid uterus is enlarged. (b) Performing PMCD with uterine rupture following cardiac arrest and with progressive DIC is difficult.
Although the incidence of uterine rupture after cesarean section is as low as 0.3%, 5 it can occur suddenly even in the absence of labor, resulting in maternal and fetal morbidity and mortality. 6 To reduce the risk of uterine rupture and preterm birth in the next pregnancy, in most cases a low transverse uterine incision is generally chosen rather than a vertical incision. 7 Another benefit of this choice is that early detection of muscle layer changes on the scar by ultrasonography in subsequent pregnancies is much more likely. However, when the previous scar is on the uterine fundus, it becomes more difficult to evaluate the thickness of the scar using ultrasonography as the gravid uterus is enlarged. MRI is an effective method for determining uterine wall thickness during pregnancy. 8 However, it is uncertain when or how many MRIs should be performed during pregnancy, and when admission or termination should be recommended due to the findings. There are limited reports of uterine rupture caused by prior transverse uterine fundal incision. 3 , 4 Previous case reports and the current case are summarized in Table 1. Two patients, including the current patient, underwent MRI at 12 months postpartum, which revealed cesarean section scar thinning and a defect at the uterine fundus. Uterine rupture was diagnosed at 21, 30, and 33 weeks of gestation. Only one patient without symptoms was diagnosed with uterine rupture by MRI and delivered a live neonate. In this case, the diameter of the rupture site was 5–7 mm. In the other two patients, the ruptured sites were large enough to expel their fetuses outside the uterus, causing severe abdominal pain. Thus, the safety and strategies of perinatal management have not been established for subsequent pregnancy following transverse uterine fundal incision, although PMCD was reported to be a possible procedure to reduce blood loss in patients with placenta previa in some cases.
TABLE 1.
Summary of case reports of patients with uterine rupture who underwent previous cesarean section by transverse uterine fundal incision
| Authors [ref] | Year | CS by transverse uterine fundal incision | MRI findings after CS by transverse uterine fundal incision | Subsequent pregnancy after previous CS by transverse uterine fundal incision | ||||
|---|---|---|---|---|---|---|---|---|
| Information on CS and pregnancy outcome | Age of pregnancy | Pregnancy course before uterine rupture | Diagnosis of uterine rupture | Pregnancy outcome | ||||
| Gestational weeks | Symptoms | |||||||
| Nishida et al. [3] | 2014 |
A 29‐year‐old primigravida CS due to placenta previa at 24 weeks of gestation 〔Pregnancy outcome〕 Mother: blood transfusion, discharge without sequelae Fetus: live birth weighting 406 g, neonatal death due to chronic lung disease at postnatal days 93 |
CS scar thinness or defect at the uterine fundus: at 12 months postpartum | 31 | Hospitalization from 18 weeks of gestation until delivery, ultrasonography twice a week and a total of 4 MRI studies for evaluation of uterine muscle layer | 30 | No symptoms (interruption of muscle layer in a part of uterine funds, which had been pointed out by MRI study) |
Mother: CS and surgical repair of the ruptured site (previous transverse uterine fundal incision scar) a Fetus: live birth weighting 1832 g, discharge with normal findings at postnatal days 68 |
| Fujiwara et al. [4] | 2017 |
Maternal age and parity: data not available CS due to placenta previa accreta and large myoma 〔Pregnancy outcome〕 Mother: data not available Fetus: data not available |
Data not available | 32 | Transfer to a tertiary referral hospital due to severe abdominal pain and low back pain | 21 | Severe abdominal pain and low back pain |
Mother: cesarean hysterectomy (ruptured site: previous transverse uterine fundal incision scar) Fetus: stillbirth (evacuation into the abdominal cavity through the ruptured cite) |
| Present case | 2022 |
A 33‐year‐old primigravida CS due to placenta previa at 37 weeks of gestation 〔Pregnancy outcome〕 Mother: blood transfusion, discharge without sequelae Fetus: healthy neonate weighting 2442 g, discharge with mother at postnatal days 6 |
CS scar defect at the uterine fundus: at 12 months postpartum | 38 | PNC and ultrasonography for evaluation of uterine muscle layer every 2 weeks, refusal for hospitalization, and MRI study | 33 | Sudden severe abdominal pain |
Mother: CPR, PMCD, and surgical repair of the ruptured site (previous transverse uterine fundal incision scar), blood transfusion, IVR and intensive care, discharge with impairment of consciousness on the 119th postoperative day Fetus: stillbirth (evacuation into the abdominal cavity through the ruptured cite) |
Abbreviations: CS, cesarean section; MRI, magnetic resonance imaging; PNC, prenatal care; CPR, cardiopulmonary resuscitation; PMCD, perimortem cesarean delivery.
At cesarean section, there were two portions (looked like a well with diameter of 5–7 mm) of the uterine fundus in which there was no or only a very thin muscle layer.
Second, PMCD is performed in patients with imminent or active cardiac arrest if ROSC is not expected, with the ultimate goal of successfully resuscitating the mother and improving fetal survivability. The American Heart Association recommends that PMCD should be initiated after 4 min of failure of resuscitative efforts with a goal of delivery within 5 min (the four‐ to five‐minute rule). 2 However, cardiac arrest in pregnancy is rare, with an incidence of 2.76–7.6 per 10,000 pregnancies, and not every obstetrician has encountered this problem. Thus, when performing a PMCD, 9 , 10 the decision, timing, and location can be challenging. In the United Kingdom, a prospective, descriptive study of cardiac arrest in pregnant women revealed that maternal survival rates depended on the time from cardiac arrest to PMCD, and that cardiac arrest occurred in the hospital, not outside of the hospital. 9 This study also showed that the time from cardiac arrest to PMCD in all survivors who received CPR was within 12 min. Performing PMCD while adhering to “the four‐ to five‐minute rule” is difficult, especially for cases of cardiac arrest that occur outside of the hospital. However, the treatment may differ in various circumstances, such as hypothermia, trauma, shock, pulmonary embolism, tamponade, anaphylaxis, or even COVID‐19. In the present case, massive hemorrhage and DIC were also observed. If preoperative massive transfusions were not well prepared for coagulation disorder correction, the situation may have worsened. The time taken to perform PMCD after arrival was 25 min. The patient survived by multidisciplinary management of CPR, PMCD, surgical repair of the ruptured site, blood product transfusion, IVR, and intensive care; however, impairment of consciousness persisted.
AUTHOR CONTRIBUTIONS
Kei Koshimizu: Investigation; writing – original draft. Jun Kakogawa: Investigation; supervision; writing – review and editing. Shuko Murata: Investigation; writing – original draft. Masato Suzuki: Investigation. Takashi Suzuki: Investigation. Naoki Masaoka: Investigation; supervision.
FUNDING INFORMATION
No funds, grants, or other support was received to assist with the preparation of this manuscript.
CONFLICTS OF INTEREST
The authors have no conflicts of interest or financial support for this work.
CONSENT
Informed consent was obtained from the patient's family for the publication of this case report and accompanying images in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
We would like to thank Wiley Editing Services (https://wileyeditingservices.com/en/) for English language editing.
Koshimizu K, Kakogawa J, Murata S, Suzuki M, Suzuki T, Masaoka N. Uterine rupture in the third trimester of a pregnancy subsequent to a cesarean section by transverse uterine fundal incision: A case report and literature review. Clin Case Rep. 2022;10:e06752. doi: 10.1002/ccr3.6752
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Al‐Zirqi I, Daltveit AK, Forsén L, Stray‐Pedersen B, Vangen S. Risk factors for complete uterine rupture. Am J Obstet Gynecol. 2017;216(2):165.e1‐165.e8. doi: 10.1016/j.ajog.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 2. Jeejeebhoy FM, Zelop CM, Lipman S, et al. Cardiac arrest in pregnancy: a scientific statement from the American Heart Association. Circulation. 2015;132(18):1747‐1773. doi: 10.1161/CIR.0000000000000300 [DOI] [PubMed] [Google Scholar]
- 3. Nishida R, Morikawa M, Yamada T, et al. Successful pregnancy in a woman with uterine scarring by transverse fundal cesarean section. J Obstet Gynaecol Res. 2014;40(5):1420‐1422. doi: 10.1111/jog.12361 [DOI] [PubMed] [Google Scholar]
- 4. Fujiwara‐Arikura S, Nishijima K, Tamamura C, et al. Re: transverse uterine fundal incision for placenta praevia with accreta, involving the entire anterior uterine wall: a case series. spontaneous uterine rupture during the subsequent pregnancy after transverse uterine fundal incision for placenta praevia with accreta. BJOG. 2018;125(3):389‐390. doi: 10.1111/1471-0528.14899 [DOI] [PubMed] [Google Scholar]
- 5. Hofmeyr GJ, Say L, Gülmezoglu AM. WHO systematic review of maternal mortality and morbidity: the prevalence of uterine rupture. BJOG. 2005;112(9):1221‐1228. doi: 10.1111/j.1471-0528.2005.00725.x [DOI] [PubMed] [Google Scholar]
- 6. Maymon R, Mor M, Betser M, et al. Second‐trimester and early third‐trimester spontaneous uterine rupture: a 32‐year single‐center survey. Birth. 2021;48(1):61‐65. doi: 10.1111/birt.12510 [DOI] [PubMed] [Google Scholar]
- 7. Turner MJ. Uterine rupture. Best Pract Res Clin Obstet Gynaecol. 2002;16(1):69‐79. doi: 10.1053/beog.2001.0256 [DOI] [PubMed] [Google Scholar]
- 8. Kumar I, Verma A, Matah M, Satpathy G. Utility of multiparametric MRI in caesarean section scar characterization and preoperative prediction of scar dehiscence: a prospective study. Acta Radiol. 2017;58(7):890‐896. doi: 10.1177/0284185116675659 [DOI] [PubMed] [Google Scholar]
- 9. Beckett VA, Knight M, Sharpe P. The CAPS study: incidence, management and outcomes of cardiac arrest in pregnancy in the UK: a prospective, descriptive study. BJOG. 2017;124(9):1374‐1381. doi: 10.1111/1471-0528.14521 [DOI] [PubMed] [Google Scholar]
- 10. Schaap TP, Overtoom E, van den Akker T, Zwart JJ, van Roosmalen J, Bloemenkamp KWM. Maternal cardiac arrest in The Netherlands: a nationwide surveillance study. Eur J Obstet Gynecol Reprod Biol. 2019;237:145‐150. doi: 10.1016/j.ejogrb.2019.04.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


