Abstract
Cardiovascular risk factors (CVRF) are very prevalent in the elderly population and in addition to predisposing to cardiovascular disease they are related to functional decline, which limits the quality of life in this population. The objective of this work is to offer a review of the current evidence in the management of CVRF in the elderly population. The search strategy was executed in PubMed, Clinicalstrials.org and Embase, to search for clinical trials, observational cohort or cross-sectional studies, reviews, and clinical practice guidelines focused or including elderly population. The results provided were refined after reading the title and abstract, as well as elimination of duplicates, and were finally identified and assessed following the GRADE methodology. A total of 136 studies were obtained for all predefined risk factors, such as sedentary lifestyle, smoking, obesity and metabolic syndrome, hypertension, diabetes mellitus, dyslipidemia and alcohol. We described the results of the studies identified and assessed according to their methodological quality in different recommendation sections: diagnostic and prevention, intervention, or treatment in the elderly population. As the main limitation to the results of this review, there is the lack of quality studies whose target population is elderly patients. This issue limits the recommendations that can be made in this population. Due to this reason, comprehensive geriatric assessment seems the best tool currently available to implement the most appropriate treatment plans based on the baseline situation and comorbidity of each elderly patient.
Cardiovascular risk factors (CVRF) are highly prevalent among older adults, with hypertension being present in around 70%, obesity in 20%−40%, diabetes in 15% and dyslipidaemia in 35%.[1] Cardiovascular disease (CVD) causes 39% of deaths in octogenarians.[1] Moreover, its presence in this population is linked to cognitive decline, functional deterioration and dependency, increasing healthcare costs.[1,2,3]
Despite the importance of CVRF, there is still a wide margin for improvement in the management of CVRF in the older population. This is partly because the main risk factor for CVD is age,[3] which cannot be modified, but also because diagnostic criteria are not adjusted to this age group and their treatment goals do not always include maintaining quality of life and functional status, in addition to survival. There are numerous standards of care and clinical practice guidelines for the management of CVRF aimed at the general population, but recommendations for older adults are scarce and are too general to be easily and safely applied in clinical practice.
Moreover, frailty, a particular feature in this age group, is not usually included in guidelines on the management of CVRF and should be included in routine clinical practice since it has a demonstrated relationship with CVD in older adults.[4] This is particularly important, given the reverse epidemiology of CVRF in older adults, which refers to the loss of predictive power of classical CVRF in persons older than 70 years. Consequently, there is a need to identify other, non-traditional risk factors.
The aim of this study was to summarize the current evidence on the management of CVRF in older adults, focusing on the treatment goals specific to this age group, such as functional independence, quality of life, and the management of patients with frailty.
METHODS
The working group consisted of specialists from different fields related to the care of older adults (geriatricians, cardiologists, pharmacologists, and nurses). For each of the CVRF (sedentariness, smoking, obesity, metabolic syndrome, hypertension, type 2 diabetes mellitus, dyslipidemia and alcohol intake), a series of clinical questions was formulated on management specifically in older adults (Table 1)
Table 1. Clinical questions on the management of cardiovascular risk factors in the older population.
| Sedentariness | Alcohol |
| What are sedentariness and physical activity and how are they measured? | What are safe alcohol limits in older people? |
| Are older people more sedentary? | How can the cardioprotective effect of alcohol be explained? |
| Is sedentariness compensated by physical activity? | What are safe alcohol limits in older people with ischemic heart disease? |
| What is the importance of sedentariness in cardiovascular risk in older adults? | How much does alcohol consumption contribute to heart disease in older people? |
| How much does sedentariness contribute to all-cause mortality in older adults? | How does alcohol consumption affect peripheral vascular disease in older adults? |
| How much does sedentariness influence cardiovascular mortality in older adults? | Does alcohol affect the risk of AF in older adults? |
| How much does exercise reduce cardiovascular risk in older adults? | How does alcohol consumption influence the risk of stroke in older adults? |
| How do frailty or functional status worsen prognosis of cvrf in older adults? | Does alcohol consumption influence the prognosis of AF in older adults? |
| Do CVRF worsen prognosis in elderly patients with frailty or functional decline? | What is the relationship between alcohol consumption and hypertension in older adults? |
| Is it worth reducing sedentariness in older adults? | What is the relationship between alcohol consumption and dm in older adults? |
| How can sedentariness be decreased in older adults? | How much does alcohol consumption influence mortality in older adults with heart failure? |
| What physical activity is recommended in older adults and how should it be prescribed? | How does alcohol interact with drug therapy for CVRF? |
| Hypertension | Obesity and metabolic syndrome |
| What are the SBP and DPB targets in older patients with hypertension? | How much/how do obesity and MS influence cardiovascular risk in older adults? |
| How does frailty influence the management of hypertension in older adults? | What is the contribution of obesity/MS to cardiovascular mortality in older adults? |
| How does frailty or functional status worsen prognosis of hypertension in older adults? | What is the contribution of obesity/MS in cardiovascular morbidity in older adults? |
| Does frailty or functional decline worsen prognosis of hypertension in older patients? | Does frailty or functional decline worsen outcome of obesity in older patients? |
| What effect does exercise have in older patients with hypertension? | What is the non-pharmacological treatment of older people with obesity and/or MS? |
| Do comorbidity and other factors influence the management of hypertension? | What are the particular features of the management of older people with obesity and frailty or functional decline? |
| What is the most effective drug therapy for hypertension in older adults? | Are treatment goals the same as in young adults? |
| How do persons older than 80 years respond to antihypertensive treatment? | How can a healthy diet be encouraged in older adults? |
| What are the specific therapeutic options for the treatment of hypertension in older adults? | |
| What are the adverse effects of drug therapy for hypertension in older adults? | Dyslipidemia |
| What are the most common events in frail or dependent older adults with hypertension? | What are the considerations of the management of dislipidemia in older patients? |
| Are high cholesterol values a sign of healthy aging? | |
| Type 2 diabetes mellitus | Are treatment targets the same in older and younger adults? |
| What are the therapeutic targets in older people with DM2? | How does the management of dyslipidemia in older adults differ from that in younger adults? |
| What is the therapeutic target in older adults with frailty or functional decline and DM2? | What are the specific considerations of the management of dyslipidemia in older adults with frailty and functional decline? |
| Does frailty or functional decline worsen prognosis of DM2 in older patients? |
What adverse effects can be expected with statin therapy in older adults? |
| How does frailty or functional status worsen prognosis of DM2 in older adults? | Is statin therapy indicated for cardiovascular disease prevention in older adults? |
| Review of pharmacological recommendations: metformin | Does statin therapy prevent cardiovascular risk and mortality in older adults with or without DM2? |
| Review of pharmacological recommendations in older people with DM2: pioglitazone | What is the effect of pravastatin use versus routine care in older adults? |
| Review of pharmacological recommendations in older people with DM2: sulfonylureas | |
| Review of pharmacological recommendations in older people with DM2: meglitinides | Smoking |
| Review of pharmacological recommendations in older people with DM2: glp-1 receptor antagonist | Does smoking affect quality of life in older adults? |
| Review of pharmacological recommendations in older people with DM2: dpp4 inhibitors | Does smoking affect cardiovascular disease in older adults? |
| Review of pharmacological recommendations in older people with DM2: alpha-glucosidase inhibitors | Does smoking influence cognition in older adults? |
| Review of pharmacological recommendations in older people with DM2: insulin | Does smoking affect mortality in older adults? |
| What is the risk of overtreatment in older people with DM2? | How do frailty or functional decline worsen outcome of smoking in older patients? |
| How do these therapeutic options modify comorbidities (CKD, HF)? | Do frailty or functional decline worsen outcome of smoking in older patients? |
| What differences are there between intensive versus standard glycemic control in older adults? | Therefore, is smoking cessation beneficial in older age? |
| Are smoking cessation interventions effective in older adults? |
We reviewed the literature using the GRADE (Grading of Recommendations of Assessment Development and Evaluations) system.[5] We analysed study design and quality, consistency, and direct and indirect evidence (Tables 2-8, citations are listed in Supplementary 1). Overall quality was defined as follows: high (very unlikely that new studies will change confidence in the results); moderate (likely that new studies will change confidence in the results); low (likely that new studies will affect confidence in the quality of the results); and very low (any result is highly doubtful). We assessed only full-text publications.
Table 2. Review of studies about sedentarism in older people.
| Studies | Quality assessment | Summary of findings | Quality | |||||||||
| Ref | N | Study design | Limitations | Inconsistency | Indirectness | Imprecision | Imprecision | Relative effect estimates | Absolute effect estimates | |||
| BMI: body mass index; CS: Cohort study; CONS: consensus; CV: cardiovascular; CVRF: cardiovascular risk factors; h: hours; HDL: High density lipoproteins; HR: Hazard ratio; I: Important; m: men; min: minute; N: Number of participants in analysis; NA: Not Applicable; OBS: observational study; OR: odds ratio; PC: use of pc computer; Quality: quality assessed according to GRADE methodology; RCT: random clinical trial; Ref: bibliography reference annex 1; RR: relative risk; SBP: systolic blood pressure; SR: systematic review; TW: televisión; U: Undetected; UV: unavailable; VI: Very Important; w: women. | ||||||||||||
| What are and how to measure sedentarism and physical activity? | ||||||||||||
| 1 | NA | SR | I | U | U | U | U | NA | NA | Low | ||
| 2 | NA | CONS | I | U | NA | NA | I | NA | NA | Low | ||
| Are older people more sedentary? | ||||||||||||
| 3 | 1 216 | CS | I | U | I | U | U | UV | For every additional 1000 steps/day decrease 0.038 meter/second in pulse wave, 0.095 Increase in compliance coefficient, 0.26% Decrease in aortic increase index and 0.005mm Decrease in carotid thickness | Low | ||
| 4 | 1 367 | CS | I | U | U | U | U | UV | UV | Very low | ||
| 5 | 2 918 | CS | I | U | I | U | U | UV | HR 1.51 (longer sedentarism lifestyle) | Moderate | ||
| 6 | NA | SR | I | I | U | U | I | UV | UV | Very low | ||
| Is a sedentarism lifestyle compensated by physical activity? | ||||||||||||
| 7 | 1 47 | OBS | VI | U | U | I | U | UV | The beneficial effects of exercise (30-150 min a week) on cardiovascular health are only maintained if the sedentarism lifestyle is less than 10h a day | Low | ||
| 8 | 1 914 | OBS | I | I | U | I | U | UV | Physical activity favors lower weight + BMI+ waist circumference + blood pressure | Low | ||
| 9 | 71 018 | OBS | I | U | I | U | U | RR 1.18 (> 10 h/day vs <5 h/day seated) | UV | Moderate | ||
| 10 | 2 765 | OBS | I | U | U | U | U | UV | Each extra minute of sedentarism: RR 0.008 BMI RR 0.234 waist circunference/ RR 0.0018 cholesterol TT-HDL / RR 1.059 diabetes |
Very low | ||
| Which is the impact of sedentarism in the cardiovascular disease in the elderly? | ||||||||||||
| 11 | 5 638 | OBS | I | U | I | U | U | HR 1.62 (> 11h /day vs < 9h /day) HR 1.54 (sedentarism periods) |
UV | Low | ||
| 12 | 495 | OBS | I | U | I | U | U | UV | Each hour of sedentarism is associated with OR 1.22 (w) and 1.57 (m) of having CVRF. A greater respiratory capacity attenuates the adverse effect of sedentarism lifestyle on CVRF | Low | ||
| 13 | 92 234 | OBS | I | U | I | U | U | HR 1.12 (1.05-1.21) (total mortality) HR 1.13 (0.99-1.29) (CV mortality) HR 1.27 (1.04-1.55) (acute coronary mortality) HR 1.21 (1.07-1.37) (cancer mortality) |
UV | Low | ||
| 14 | 425 | OBS | VI | U | I | I | U | UV | Sedentarism = lower HTA and HDL physical activity/minute: lower waist perimeter + SBP + glycemia + HDL elevation |
Low | ||
| 15 | 2 657 | OBS | I | U | U | U | U | HR -0.67 mortality CV (non-sedentarism) 6.4% CV mortality risk (1h/day of sedentarism vs non-sedentarism) |
UV | Low | ||
| How to act in the frail sedentary elderly? | ||||||||||||
| 16 | 1 635 | OBS | I | U | I | MI | U | RR 0.04% CV (1 MIN/day /10 years non CV mortality) RR 0.03% non CV mortality) |
Greater physical activity: lower risk in a population with cardiovascular risk and without it | Low | ||
| Is it worth reducing sedentarism lifestyle in the elderly? | ||||||||||||
| 17 | 2 635 | CS | I | U | U | U | U | Mortality: HR 0.91 (0.76-1.10) (new sedentarism) HR 0.86 (0.7-1.05) (chronic sedentarism) HR 0.75 (0.62-0.90) (non-sedentarism) |
UV | Moderate | ||
| 18 | 287 | OBS | VI | U | U | U | U | +10min Tw=+2.9 min sedentarism +10min PC=+2.2 min sedentarism |
UV | Low | ||
| What physical activity is rcsommended in the elderly and how to prescribe it? | ||||||||||||
| 19 | 120 | RCT | U | U | U | U | U | +1% activity (17min) -1.21% sedentarism (25 min) |
NA | High | ||
| 20 | 136 | OBS | I | U | I | VI | U | NA | NA | Low | ||
Table 8. Review of studies on alcohol intake in the elderly.
| Studies | Quality assessment | Summary of findings | Quality | |||||||||
| Ref | N | Study design | Limitations | Inconsistency | Indirectness | Imprecision | Imprecision | Relative effect estimates | Absolute effect estimates | |||
| CS: Cohort study; HR: Hazard ratio; I: Important; NA: not applicable; OBS: observational study; OR: odds ratio; Re: bibliography reference annex 1; RR: relative risk; sig: significant; RV: review; U: undetected; UV: Unavailable; VI: Very Important; Quality: quality assessed according to GRADE methodology. | ||||||||||||
| How is alcohol intake evaluated in the elderly? | ||||||||||||
| 117 | 3 058 | OBS | I | U | U | U | U | NA | NA | Low | ||
| 118 | 3 815 | OBS | I | U | I | U | U | Increased likelihood of medication-alcohol intake | UV | Low | ||
| 119 | 1 500 | RV | I | ND | NA | NA | U | NA | NA | High | ||
| Is it safe to consume alcohol in the elderly? | ||||||||||||
| 120 | 542 | OBS | VI | U | U | U | U | RR -13% Hypertension (alcohol intake vs abstinence) | UV | Very low | ||
| 121 | NA | RV | VI | ND | NA | ND | SI | NA | NA | Very low | ||
| How does alcohol intake influence cardiovascular risk in the elderly? | ||||||||||||
| 122 | NA | RV | VI | U | NA | NA | U | NA | NA | Very low | ||
| 123 | 1 896 | OBS | I | U | U | U | U | Alcohol intake vs abstinence: healthy values (fibrinogen, HDL cholesterol, apo A-liporotein, insulin) and unhealthy (LDL cholesterol. blood pressure) | UV | Low | ||
| 124 | 4 655 | OBS | I | U | U | U | U | HR 0.5 (0.3-0.9) (h) HR 0.7 (0.4-1.1) (m) |
UV | Very low | ||
| 125 | 983 | OBS | I | U | U | U | U | RR 0.69 (former consumers) RR 0.54 (mild consumer) RR 0.44 (moderate consumer) RR 0.21 (high consumer) |
UV | Low | ||
| 126 | 4 410 | OBS | I | U | U | I | U | RR 0.90 (<1 alcohol intake/week) RR 0.93 (1-6 alcohol intake/week) RR 0.76 (7-14 alcohol intake/week) RR 0.58 (>14.alcohol intake/week) |
UV | Low | ||
| HOW COULD THE CARDIOPROTECTIVE EFFECT OF ALCOHOL be justified? | ||||||||||||
| 127 | 553 | OBS | VI | U | U | U | U | No significant differences alcohol-Systolic blood pressure significant differences alcohol-Dyastolic blood pressure |
UV | Low | ||
| 128 | 4 247 | OBS | I | U | U | U | U | Mild consumer: < intimate-medium thickness High consumer: > intimate-medium thickness |
UV | Low | ||
| How does alcohol influence heart failure? | ||||||||||||
| 129 | 1 332 | OBS | I | MI | U | U | U | Consumptium vs no consumptium: HR +1.19 (mortality including heart failure) HR -0.79 (mortality without including heart failure) |
UV | Low | ||
| 130 | 2 235 | OBS | I | U | U | U | U | Mortality in consumers vs abstinents: HR 0.79 mild consumer / HR 0.53 moderate consumer and risk of heart failure: HR 0.81 mild consumer / HR 0.75 moderate consumer |
UV | Low | ||
| 131 | 6 083 | OBS | I | U | U | I | U | No relation between consume and heart failure | UV | Low | ||
| 132 | 4 490 | OBS | I | U | U | U | U | HR 0.77 lower risk in mild consume | UV | Low | ||
| 133 | 5 595 | OBS | I | U | U | U | U | Risk for heart faliure: HR 0.82 (mild consumers vs abstinents) HR 0.66 (moderate consumers vs abstinents) HR 1.15 (former consumers vs abstinents) |
UV | Low | ||
| 134 | 5 888 | OBS | I | U | U | I | U | Average survival: + 383 days (moderate consumers vs abstinents) |
UV | Low | ||
| Do the elderly benefit from alcohol cessation treatments? | ||||||||||||
| 135 | NA | RV | VI | U | ND | U | U | UV | NA | Very low | ||
| 136 | 51 | OBS | I | U | U | U | U | UV | UV | Very low | ||
| 137 | 925 | CS | VI | U | I | I | U | Abstinence 5 years: 52% (> 50 years) 40% (<50 years) | NA | Very low | ||
Table 3. Review of studies on smoking in the elderly.
| Studies | Quality assessment | Summary of findings | Quality | |||||||||
| Ref | N | Study design |
Limitations | Inconsistency | Indirectness | Imprecision | Publication bias |
Relative effect estimates | Absolute effect estimates |
|||
| CI: confidence Interval; COGTEL: Cognitive Telephone Screening Instrument; COPD: chronic obstructive pulmonary disease; CS: cohort study; CV: cardiovascular; CVD: cardiovascular disease; COGTEL: Cognitive Telephone Screening Instrument; COPD: chronic obstructive pulmonary disease; EXS: exsmokers; HR: Hazard ratio; I: Important; m: men;NA: no applicable; NS: non smoking; OBS: observational study; OR: odds ratio; RCT: random clinical trial; Ref: bibliography reference annex 1; RR: relative risk; RV: Review; S: smokers; SR: systematic review; U: Undetected; UV: unavailable; VI: very important; QALY: quality-adjusted life year; Quality: quality assessed according to GRADE methodology; w: women. | ||||||||||||
| Does smoking affect cardiovascular disease in the elderly? | ||||||||||||
| 21 | 1 412 749 | CS | U | I | NA | I | U |
HR 1.48 (95% CI. 1.15-1.90) (angina and acute coronary syndrome) |
UV | Low | ||
| 22 | 8 807 | CS | U | I | NA | I | U | HR 2.25 (CI 95%; 1.05-4.81) (NS. 50-59years) HR 0.9 (0.63-1.29) (NS. 60-74years) |
UV | Low | ||
| Does smoking affect quality of life in the elderly? | ||||||||||||
| 23 | 3 652 | CS | U | I | NA | U | U | -3.5 QALY (EXS) vs -8.8 QALY (NS) | UV | Low | ||
| 24 |
382 |
CS |
I |
U |
NA |
U |
U |
Physical problems (carrying, climbing stairs, and walking) OR (3.71-6.6-2.34) (NS) OR (7.32-3.39-3.74) (NS <10years) OR (4.53-3.35-2.66) (NS >11years) |
UV | Very low | ||
| Does smoking affect cognition in the elderly? | ||||||||||||
| 25 | UV | RS | U | I | NA | U | U | S vs EXS-NS: Alzheimer 1.59 (1.15-2.2) vs 0.99 (0.81-1.23) Vascular dementia 1.35 (0.9-2.02) vs 1.05 (0.72-1.54) Inespecific dementia 1.16 (0.9-1.5) vs 0.9 (0.75-1.07) Cognitive decline 1.2 (0.9-1.59) vs 0.9 (0.74-1.1) |
UV | High |
||
| 26 | 1 697 | CS | I | U | NA | U | U | COGTEL -0.31 pts (-0.51.-0.11) (20 pack/year) COGTEL -2 pts (-3.59; -0.4) (S vs NS) |
UV | Low | ||
| Does smoking affect mortality in the elderly? | ||||||||||||
| 27 | UV | SR | I | I | NA | U | U | Relative mortality: 1.83 (95% CI 1.65-2.03) (S vs NS) vs 1.34 (1.28-1.34) (EXS vs NS) |
UV | Low | ||
| 28 | 13 030 | CS | U | U | NA | I | U | Relative mortality: 2.61 (95%CI 2.15-3.18) (S vs Ns) Relative mortality CVC: 2.67 (1.92-2.67) and 1.95 (1.33-2.86) (m 45-64 and 65-74 years) vs 4.28 (2.29-7.99) and 2.67 (1.28-5.58) (w 45-64 and 65-74) Cancer: 3 (2.15-4.18) and 4.5 (2.82-6.10) (NS m 45-64 and 65-74 years) vs 2.27 (1.64-3.44) and 4.45 (2.5-7.93) (NS. w. 45-64 and 65-74 years) |
UV | High | ||
| 29 | 478 248 | CS | U | I | NA | U | U | CVD mortality: HR 1.82 (1.45-2.28) (NS 40 years) HR 0.96 (0.67-1.38) (NS 80 years) Non-CVD mortality: HR 1.69 (1.57-1.82) (NS 50 years) HR 1.4 (1.17-1.69) (NS 80 years) |
UV | Low | ||
| 30 | 503 905 | CS | I | VI | NA | U | U | CVD mortality: HR 2.07 (CI 95% 1.38-2.39) (NS) Acuye coronary syndrome: HR 1.98 (1.75-2.25) (NS) vs HR 1.18(1.06-1.32) (NS/ EXS) stroke: HR 1.58 (1.4-1.78) (NS) vs HR 1.17 (1.07-1.26) (NS/ EXS) |
UV | High | ||
| Is it beneficial to quit smoking in older people? | ||||||||||||
| 31 | 267 010 | OBS | I | U | NA | U | U | COPD risk hospitalization: HR 6.81 (CI95%: 5.87-7.89) (NS/ EXS) General risk hospitalization: HR 0.84 (95% CI. 0.82-0.86) 16% (c/10 years smoking cessation) |
UV | Low | ||
| 32 | 95 683 | CS | I | I | NA | I | U | Stroke (NS/ EXS): RR 1.39 (95%CI 1.13-1.70) (m) vs RR 1.65 (1.21-2.25) (w) Acute coronary syndrome (NS/ EXS): RR 1.6 (1.39-1.84) (m) vs RR 3.35 (2.23-5-02) (w) CVD total (NS/ EXS) RR 1.60 (1.39-1.84) (m) vs RR 2.06 (1.69-2.51) (w) |
UV | Low | ||
| 33 | 877 243 | CS | I | VI | NA | U | U | Mortality (NS): RR 2.43 (2.21-2.48) (m) vs RR 1.68 (1.56-1.8) (w) Mortality (NS 3-5 years): RR 1.29 (0.88-1.69) (m) vs RR 1.55 (1.17-1.93)(w) |
UV | High | ||
| Are smoking cessation interventions effective in the elderly? | ||||||||||||
| 34 | 470 | RCT | I |
U | U | VI | U |
88% vs 82% (try to smoking cessation 6 months) 40% vs 33 % (p<0.05) (smoking cessation 6 months) 88% vs 82% (p<0.05) (try to smoking cessation 12 months) 33% vs 31% (smoking cessation 12 months) |
UV | Low | ||
| 35 | 177 92 (in treatment group) |
RCT | I | U | U | I | U | OR 2.24 (CI 95%; 1.07-4.69) (w) | UV | Low | ||
| 36 | 1 553 1 016 (in treatment group) |
RCT | I | U | U | VI | U |
OR 1.1 (0.7-1.8) (control vs therapy 1) vs OR 1.7 (1.1-2.6) (control vs therapy 2) |
UV | Low | ||
| 37 | 315 (in treatment group) | CS | U | U | NA | VI | U | HR 0.74 (CI 95%; 0.6-0.92) | UV | Low | ||
Table 4. Review of studies on obesity and metabolic syndrome in the elderly.
| Studies | Quality assessment | Summary of findings | Quality | |||||||||
| Ref | N | Study design | Limitations | Inconsistency | Indirectness | Imprecision | Publication bias |
Relative effect estimates |
Absolute effect estimates |
|||
| CVR: cardiovascular risk; HR: Hazard ratio; I: Important; NA: not applicable; RCT: random clinical trial; Ref: bibliography reference annex 1; SR: systematic review; UV: Unavailable; U: Undetected ; Quality: quality assessed according to GRADE methodology; VI: Very Important ; w: women. | ||||||||||||
| How does obesity influence cardiovascular risk in the elderly? | ||||||||||||
| 38 | 79 | RCT | U | U | I | U | Low | UV | UV | Moderate | ||
| 39 | 1 588 | Post hoc Analysis |
U | U | I | U | Low | NA | NA | Low | ||
| 40 | 792 | Transversal study | U | U | I | I | Low | NA | NA | Moderate | ||
| 41 | NA | Review | I | VI | VI | VI | High | NA | NA | Very low | ||
| 42 |
221 270 |
SR | I | U | I | I | Low | NA | NA | Moderate | ||
| 43 | NA | Review | I | UV | UV | UV | Uv | NA | NA | Low | ||
| What is the non-pharmacological management of the elderly patient with obesity and/or metabolic syndrome: exercise and diet? | ||||||||||||
| 44 | 1 868 | RCT | U | U | I | U | U | Recent metabolic syndrome/consumption of cheese: HR 1.31 (1.10. 1.56) (53.8% w) | UV | High | ||
| 45 | 74 | RCT | U | I | I | I | Low | Decrease of metabolic syndrome from 100% to 51.9% (6months of treatment) 100% a 16.2% (12 months of treatment) |
100% Low-moderate CVR-moderate (treatment) vs 13.5% very high CVR and 27% high risk (p < 0.001) |
High | ||
| 46 | 260 | RCT | UV | UV | UV | UV | Uv | NA | NA | Moderate | ||
| 47 | 1 533 | RCT | U | U | U | I | High | UV | UV | High | ||
Literature Search
We searched for clinical trials, observational cohort studies, cross sectional studies, reviews and clinical practice guidelines in Pubmed, Clinicaltrials.org and Embase.
The search terms used and cross-referenced for each of the clinical questions established were as follows: obesity AND elderly AND “cardiovascular risk”; obesity AND “oldest old” AND “cardiovascular risk”; metabolic syndrome AND elderly AND “cardiovascular risk”; “Metabolic syndrome” AND oldest old AND “cardiovascular risk”; sedentary AND “older adults” AND cardiovascular AND “risk factor”; alcohol AND “cardiovascular risk factors” AND older adults. We searched for literature published up to August 1, 2020. There were no strict criteria for age, to allow inclusion of all publications focused on older ages.
Evaluation and Synthesis of the Literature
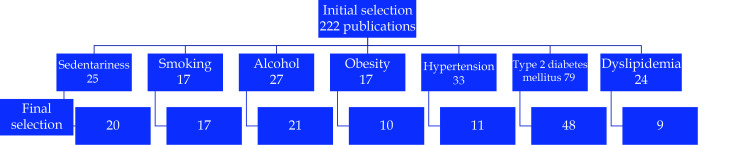
The search results were screened, with elimination of duplicates and irrelevant studies. Among relevant studies, 136 were identified and assessed (Figure 1).
Figure 1.
Flowchart of the screening process for the selection of eligible articles.
RESULTS
The evidence was synthesized in terms of diagnosis, prevention and treatment (Table 9).
Table 9. Overall results on cardiovascular risk factors in the older population. Diagnosis, prevention and treatment.
| Sedentariness |
| BMI: body mass index; COPD: chronic obstructive pulmonary disease; CV: cardiovascular; CVR: cardiovascular risk; DM: diabetes mellitu; DBP: diastolic blood pressure; GLP-1 receptor antagonists: glucagon-like peptide 1 receptor antagonist; HDL-C: high-density lipoprotein colesterol; LDL-C: low-density lipoprotein colesterol; MS: metabolic syndrome; NPH insulin: Neutral Protamine Hagedorn; SBP: systolic blood pressure; SGLT-2: sodium-glucose type 2 cotransporter. |
| Persons older than 60 years spend 60%-80% of their time in sedentary activities; sedentariness is even greater in persons with cardiovascular disease. |
| Some secondary activities (eg, reading, using the computer, arts and crafts) are associated with a lower risk of dementia and may enhance social interaction. |
| Prolonged sedentary time (more common in men) is more harmful than shorter episodes (more common in women). |
| Sedentariness can negatively impact cardiovascular health despite moderate physical activity. The more sedentary a person, the higher the BMI, blood glucose level and functional limitations after adjustment for physical activity and vice versa. |
| The relationship between sedentariness and cardiovascular disease is linear (each additional hour of sedentariness is associated with a 22%-27% risk of having cardiovascular risk factors), while more physical activity decreases the probability of cardiovascular risk. |
| Moderate-intense physical activity (measured with accelerometers) positively impacts cardiovascular risk factors in older people. The benefit of mild physical activity is more controversial varies among studies. |
| Cardiovascular mortality is 33% lower in non-sedentary versus sedentary individuals older than 60 years and increases by 6.4% for each additional hour per day of sedentary behavior. |
| In older adults with frailty (a high risk of gait disability according to measurements of functional limitations in the lower extremities, ability to walk 400 meters in ≤15 minutes, take < 20 minutes of moderate-intense physical activity/week), there is a demonstrated linear relationship between sedentariness and cardiovascular risk. |
| Mortality decreases in formerly sedentary older people, but remains higher than in never sedentary individuals. Equally, becoming newly sedentary increases mortality although the risk is still lower than in consistently sedentary persons. |
| There are contradictory results on whether programs to increase physical activity in older people also reduce sedentariness and consequently these two behaviors should be approached separately. |
| There are no strategies that approach sedentariness specifically in older adults. |
| Clinical practice guidelines should stress both increasing physical activity and reducing sedentariness among older adults. |
| The recommendations of the World Health Organization on physical activity in persons aged ≥ 65 years consist of a minimum of 150 minutes per week of moderate aerobic activity or 75 minutes of vigorous aerobic activity, or an equivalent combination of moderate and vigorous activity. |
| Persons not following physical activity recommendations should attempt to increase the duration, frequency and, finally, intensity of exercise as a goal until they reach recommended levels. |
| Prescription of physical exercise should be progressive, with an individualized plan, and have the same precision as any other medical treatment and should set clear goals. The exercise program should include aerobic exercise, strength training, flexibility and balance, as well as recommendations on health education. |
| Exercise programs for patients with sarcopenia or frailty should include the specificity, frequency and duration required to achieve improvement. |
| In older patients with cardiovascular risk factors, cardiovascular conditioning is essential as it attenuates the adverse effect of sedentariness, even in patients not adhering to exercise recommendations. |
| Perhaps the simplest way to increase aerobic physical activity in robust older adults is to set a goal of exceeding 10,000 steps per day. |
| Smoking habit |
| Smoking is a major risk factor for cardiovascular disease and an independent risk factor for stroke and acute myocardial infarction, as well as cardiovascular mortality, independently of age. |
| The risk is 2-fold higher in smokers than in never-smokers and increases and occurs earlier in heavy smokers. |
| The risk of all cardiovascular events decreases by 46% in ex-smokers 5-10 years after quitting. |
| Given the reduction in cardiovascular risk after smoking cessation, it should be recommended even in very old adults. |
| In peripheral arterial disease, there is evidence that the risk is higher in older smokers than in younger smokers; moreover, the smoking-related risk of abdominal aneurysm remains high irrespective of age. |
| Smoking is associated with a higher risk of Alzheimer disease and worse cognitive function. However, cognitive decline can be reversed 5 years after smoking cessation and maximum recovery is achieved 30 years after quitting. |
| Quality of life is impaired by as much as 55% in older smokers, depending on the number of cigarettes smoked and the number of years since starting. Eleven years after quitting, quality of life can be enhanced in older people, reaching the same levels as in never-smokers. |
| Non-cardiac mortality and lung cancer increases in both smokers and ex-smokers throughout life, reaching a peak at 60-70 years. |
| In older adults, the number of pack/years also influences the risk of mortality. The longer the time since quitting, the greater the reduction in excess mortality. |
| Reducing smoking leads to a 10% reduction in preventable admissions and hospital costs associated with smoking-related chronic diseases-common in older people-such as COPD (5 years after quitting), angina, complications of diabetes, and congestive heart failure (between 5 and 14 years after quitting). |
| Quitting smoking for 2 years reduces the risk of coronary disease and, after 2-4 years, the risk of stroke; maximum reduction is achieved 10-14 years after cessation. |
| Older people who quit smoking at the age of 65 years gain up to 2 years or life in men and up to 3.7 years in women. In contrast, smokers bring forward the risk of cardiovascular mortality by more than 5 years. |
| The benefits of smoking cessation are maintained even in persons older than 70 years, indicating that it is never too late to quit. |
| Recommendations for smoking cessation in older adults include setting a quit date, with a prior preparation period, the use of nicotine replacement therapy, and follow-up to ensure treatment adherence and reduce the risk of relapse. |
| Older smokers hospitalized for an acute myocardial infarction should more frequently be counseled to quit, as only 40% are currently advised to do so. |
| Personalized smoking cessation interventions should be considered in older adults rather than routine clinical practice or the use of clinical practice guidelines to improve the results of serious quit attempts and prolong periods of abstinence. |
| Obesity and metabolic syndrome |
| Patients with MS have twice the risk of cardiovascular disease than those without MS. |
| In the Spanish population, the risk of cardiovascular disease in patients with MS is more than 22% higher than in the general adult population; cardiovascular disease predominates in men until the age of 65 years and increases in women after this age. |
| A strong association has been demonstrated between cardiovascular disease and high blood pressure, cardiovascular events and mortality, type 2 diabetes and MS. To detect these risk factors, the waist-to-height ratio is considered a more effective anthropometric marker than BMI or waist circumference |
| Results on moderate red wine consumption indicate a relationship with a lower prevalence of MS in an older Mediterranean population with high cardiovascular risk compared with an older Spanish population not consuming red wine. |
| The results found on higher consumption of low-fat dairy products indicate an association with a lower risk of metabolic syndrome in persons at high risk of cardiovascular disease in a Mediterranean population. In contrast, higher cheese intake was related to a higher risk of MS. |
| In older patients with a high risk of cardiovascular disease, a Mediterranean diet supplemented with extra virgin olive oil or nuts is not associated with the development of MS. However, these Mediterranean diets are appropriate to cause reversion of metabolic syndrome. |
| Exercise capacity is inversely related to arterial stiffness and age in persons with MS. |
| Decreasing arterial stiffness at any age through physical exercise, measured through pulse wave analysis, provides clearer evidence on the effect of physical exercise in reducing cardiovascular risk in patients with MS. |
| No studies were found on the particular features of the management of obesity and MS in older patients with frailty and functional decline. |
| No studies were found on the particular features of drug therapy in the treatment of obesity and MS specifically in older patients. |
| Hypertension |
| Antihypertensive therapy is effective in the population older than 65 years, demonstrated by a significant reduction in all-cause and cardiovascular mortality, as well as by a decrease in cardiovascular events as a composite outcome or individually (fatal and non-fatal stroke or acute myocardial infarction). |
| The aim of antihypertensive therapy, in terms of blood pressure targets, is not specifically defined, with a SBP cut-off value of 140 mmHg. Greater reductions in all-cause and cardiovascular mortality have been observed with SBP targets of < 140 mmHg and DBP < 90 mmHg. |
| Intensive antihypertensive therapy (target SBP value < 120 mmHg) versus standard therapy (target SBP value < 140 mmHg) is more effective in significantly reducing severe composite cardiovascular events (cerebrovascular events such as stroke and coronary events such as acute myocardial infarction) as well as in decreasing all-cause mortality. |
| Patients without chronic kidney disease at treatment initiation may have a higher risk of developing renal insufficiency (reduced glomerular filtration rate) with intensive therapy. |
| Se ha de tener en cuenta que cifras tensionales < 140 mmgHg, podrían relacionarse con una mayor tasa de efectos adversos graves. SBP values < 140 mmgHg may be related to a higher rate of severe adverse effects. |
| Intensive therapy in persons older than 80 years (maintaining SBP values < 120 mmHg) has been shown to produce a significantly greater reduction in all-cause mortality and major cardiovascular events (composite variable) than standard therapy (SBP < 140 mmHg); clinicians should be alert to a possible clinically significant reduction in kidney function (> 30% decrease in glomerular filtration rate) and a higher rate of acute renal insufficiency with intensive therapy. |
| Treatments aiming at SBP values < 140 mmHg have not shown a significant reduction in mortality in older patients with frailty in studies where frailty is defined as baseline vulnerability (gait speed, grip strength, Fried model, Groeningen frailty index, osteoporotic fracture index). |
| The risk of cardiovascular events has been demonstrated to be higher in patients older than 75 years with frailty than in those without frailty, independently of the intensity of antihypertensive treatment. |
| Intensive therapy that achieves sustained SBP values < 120 mmHg in older patients decreases the number of major cardiovascular events and all-cause mortality in absolute terms compared with standard therapy (sustained SBP values < 120 mmHg). |
| Antihypertensive treatments with diuretics in general (both potassium-sparing and thiazide diuretics) have favorable results versus placebo or other treatments in reducing cardiovascular risk, both in terms of cardiovascular and all-cause mortality. The results are more consistent in terms of their ability to reduce serious cardiovascular events overall and particularly in decreasing cerebrovascular morbidity and mortality (stroke). These antihypertensive treatments with diuretics show contradictory results in terms of the rate of adverse events but the rate is not higher than with other antihypertensive therapies. |
| Antihypertensive therapy with beta-adrenergic blockers has not been demonstrated to significantly reduce cardiovascular risk versus placebo or other treatments; this treatment has only been shown to significantly decrease cerebrovascular morbidity and mortality versus placebo, but has a higher rate of adverse events than other antihypertensive therapies. |
| Antihypertensive treatment with calcium channel blockers versus placebo has shown good results in reducing cardiovascular risk, cardiovascular and all-cause mortality, and cardiovascular morbidity and mortality overall and especially in decreasing cerebrovascular events, with a lower rate of adverse events versus other antihypertensive therapies. |
| Antihypertensive drug therapy with angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are less well studied in the older population; only one meta-analysis has demonstrated a reduction in cardiovascular morbidity and mortality measured as a composite outcome but not different events separately. |
| In the population older than 55 years, simple exercises such as handgrip exercise training for more than 4 weeks produce a clinically significant reduction in SBP and DBP, although the benefit on cardiovascular risk has not been assessed. |
| Type 2 diabetes mellitus |
| The retrieved studies assessing intensive vs conventional glucose control in patients ≥ 65 years do not demonstrate reductions in mortality, major cardiovascular events (fatal and non-fatal), or micro- and macrovascular events, but do report a higher yearly rate of severe hypoglycemic episodes. In other studies, strict glucose control in patients ≥ 65 years has shown a reduction in the composite outcome (major macrovascular events and microvascular events), only in terms of a lower incidence of kidney disease. |
| Studies conducted in patients aged ≥ 80 years with type 2 diabetes have shown that very low (< 6%) or very high (> 8.5%) glycosylated hemoglobin levels were associated with higher mortality compared with intermediate levels (7.0%-7.4% or 8%-8.4%). |
| Studies have demonstrated a “U”-shaped association between blood glucose levels and frailty in older people with type 2 diabetes: the risk of frailty increases with extremely high, low or elevated blood glucose levels. |
| Treatments based on strict blood glucose control in older patients with type 2 diabetes have an increased risk of falls, functional deterioration and some geriatric syndromes. |
| Low glycosylated hemoglobin levels (7%-7.9%) have been associated with worse functional status than higher levels (8%-8.9%) in patients with type 2 diabetes and a mean age of 80 years. |
| There are no results from clinical trials evaluating the effect of intensive glucose control on cardiovascular events in older patients with frailty and type 2 diabetes. |
| In older patients, glycemic control targets should be adapted to functional and cognitive status, comorbidities and life expectancy. |
| In older patients with type 2 diabetes and good functional and cognitive status, virtually no comorbidity and good life expectancy, it is recommended to maintain targets similar to those of young adults with diabetes (HbA1c 7%-7.5%). |
| In patients with functional disability, frailty, high comorbidity burden, or dementia, treatment for glycemic control should avoid symptomatic hyperglycemia and hypoglycemia. The aim of treatment is to maintain HbA1c targets of 7.5%-8.5%; targets are around 7.5%-8% in patients with mild or initial-phase disability or around 8%-8.5% if disability is moderate or severe. |
| In patients receiving palliative care, it is recommended to avoid symptomatic hyperglycemia and hypoglycemic episodes, not perform HbA1c monitoring and maintain glycemia < 200 mg/dL. |
| In patients with type 2 diabetes ≥ 65 years, metformin is recommended as the first-line oral glucose-lowering agent due to its ability to reduce mortality and other cardiovascular events and because it does not cause hypoglycemia or weight gain; it can also reduce age-related comorbidities. Metformin is contraindicated in patients with a glomerular filtration rate < 30 mL/min per 1.73 m2 due to its tendency to accumulate in blood and the increased risk of lactic acidosis. |
| Studies with patients with type 2 diabetes discharged with a principal diagnosis of acute myocardial infarction or heart failure demonstrate that treatments with thiazolidinediones (pioglitazone) and metformin reduced all-cause mortality. These results contradict those of other studies reporting a higher risk of admission for heart failure and acute myocardial infarction (with rosiglitazone) or all-cause mortality. Thiazolidinediones increase weight, produce fluid retention and increase the risk of heart failure, loss of bone mineral density in older women with diabetes and fracture risk. |
| Drug therapy with sulfonylureas in patients with type 2 diabetes aged ≥ 65 years has not been demonstrated to reduce mortality but has been shown to increase the risk of hypoglycemia; there are no studies demonstrating a reduction in macro- or microvascular complications. |
| Glibenclamide use is not recommended in patients with glomerular filtration rate < 30 mL/min per 1.73 m2 or in those with severe liver failure or an elevated risk of hypoglycemia (a history of severe hypoglycemia, cognitive decline, major depression, advanced age with pluripathology and disability, and low BMI). |
| Meglitinides have been demonstrated to have a lower risk of hypoglycemia than sulfonylureas. There are no studies demonstrating their effect or benefit on cardiovascular events in the older population with diabetes. Repaglinide is excreted mainly through the biliary route and can be an alternative in patients with glomerular filtration rate < 30 ml/min per 1.73 m2. |
| Alpha-glucosidase inhibitors (acarbose) reduce postprandial glycemia without increasing the risk of hypoglycemia in older patients with type 2 diabetes; clinicians should bear in mind their adverse gastrointestinal effects (flatulence and diarrhea) and scarce efficacy. |
| Use of dipeptidylpeptidase-4 (DPP-4) inhibitors in older patients with type 2 diabetes has proven effectiveness in reducing glycosylated hemoglobin and glycemia and demonstrated safety even in adults of advanced age in terms of the incidence of cardiovascular events, mortality, and hospitalization for heart failure and has also been shown to be well tolerated (very low risk of hypoglycemia and neutral effect on weight). DPP-4 inhibitors can be administered in patients with renal insufficiency by adjusting the dose, except lingliptin due to its biliary excretion. |
| In patients with type 2 diabetes aged ≥ 70 years, use of lixisenatide, a glucagon-like peptide-1 antagonist, reduces glycosylated hemoglobin and postprandial glycemia versus placebo. It is also associated with greater weight loss and more frequent development of gastrointestinal symptoms such as nausea and vomiting. In two pooled analyses of patients aged ≥ 65 years in clinical trials with lixisenatide and liraglutide, no differences were found in safety or efficacy compared with younger patients. |
| There are no studies on GLP-1 receptor antagonists demonstrating their effect on cardiovascular events in older adults with type 2 diabetes, although liraglutide and semaglutide have decreased cardiovascular events in studies with younger patients with type 2 diabetes. These drugs have been associated with a risk of pancreatitis, although this finding has not been confirmed in all studies. |
| Studies of the effect of treatment with sodium-glucose type 2 cotransporter (SGLT-2) inhibitors have demonstrated that they act independently of insulin, interfering with glucose reabsorption and favoring glucosuria. |
| Empagliflozin has been demonstrated to reduce all-cause and cardiovascular mortality; empagliflozin, canagliflozin and dapagliflozin have been shown to reduce hospitalization for heart failure. |
| Data show no differences in safety and efficacy between the older and younger population; however, these drugs should be administered cautiously in the older population due to the lack of specific data for this age group. Older patients should also be well selected due to their tendency to hypertension and sensitivity to water loss, as well as their risk of fractures (canaglafloxin), toe and metatarsal amputations and possible rare cases of euglycemic ketoacidosis (entire drug group). |
| Basal insulin analogs (glargine, determir and degludec) have not been shown to improve glycemic control but have been associated with a lower frequency of hypoglycemic episodes (nocturnal). These treatments can be considered as the treatments of choice versus NPH insulin in patients with a tendency to hypoglycemia. |
| In older patients with diabetes, maintenance of combined therapy with oral antidiabetic agents is recommended to reduce insulin doses and avoid the use of complex insulin regimens. |
| In older patients, insulin therapy is associated with a higher risk of hypoglycemia, falls and fractures; therefore, decision-making on this therapy should be personalized in this age group with priority given to safety. |
| Biphasic, or premixed, forms of insulin are associated with better glycemia control (in patients poorly controlled with basal insulin); these forms are also associated with weight gain and a higher risk of hypoglycemia. |
| Simplifying and de-escalating antidiabetic treatment is recommended in older patients after comprehensive geriatric assessment. The use of more complete regimens (e.g., basal-bolus) should be reserved for patients requiring them and who maintain good quality of life, life expectancy and self-care ability. |
| A multimodal intervention consisting of physical exercise and nutritional education has been shown to be effective in improving functional status in older patients with type 2 diabetes mellitus and frailty. |
| Dyslipidemia |
| Primary prevention measures for dyslipidemia should be the same in older adults as in younger adults, including smoking cessation and avoiding sedentariness and overweight. |
| Diet is especially important in the older population, with avoidance of restrictive diets and malnutrition risk, particularly in persons with cognitive decline and nursing home residents. |
| Older patients benefit from a comprehensive approach with attention paid to comorbidity and geriatric syndromes. |
| Drug therapy used for secondary prevention should be the same in older patients as in the younger population. |
| Lipid-lowering therapy in the older population, with LDL-C reduction, prevents both individual and composite cardiovascular events (cardiovascular death, myocardial infarction, stroke, and coronary revascularization), especially in secondary prevention, with similar benefits as those in the younger population. |
| Initiating statin therapy should be considered in primary prevention in older patients at high or very high cardiovascular risk. |
| Initiating low-dose statin therapy is recommended in patients with significant renal insufficiency or a high probability of drug interactions, depending on their tolerance and target LDL-C levels, to avoid more serious secondary effects (such as myalgia or myopathy) and rare adverse effects (such as rhabdomyolysis). |
| Alcohol consumption |
| In the general population, recommended intake should not exceed 25 grams of alcohol per day, because, after this cut-off, the potential benefit of alcohol decreases. Given that older adults are more sensitive to the effects of alcohol, it has been postulated that neither sex should exceed 10 g alcohol per day. |
| The recommendation of 10 grams of alcohol per day seems to be too restrictive, based on current scientific evidence; on the other hand, older adults should never be advised to start or increase alcohol consumption to gain a cardioprotective effect. |
| Current consensus on healthy alcohol consumption in adults holds that there is a U-shaped protective effect with light-moderate intake and a harmful effect with intake of large quantities. |
| Despite the possible protective effect of light-moderate alcohol consumption in older adults, there are potential interactions between alcohol and some drugs, including antidiabetic agents, anticoagulants and antithrombotics. |
| The possible cardioprotective effect of alcohol intake seems to be influenced by sex, age, consumption pattern, genetics, and improved lipid profile (increased HDL-C and reduced LDL-C), better glycemic control (reduced blood insulin level on increasing insulin sensitivity), decreased platelet aggregability and markers of systemic inflammation (C-reactive protein, interleukin 1α and 6 and tumor necrosis factor), which seems to translate into reduced atherosclerosis. |
| Alcohol intake, even in moderate quantities (< 30 g alcohol per day), seems to increase blood pressure, although this does not increase overall cardiovascular risk or risk of heart failure. |
| Moderate intake (< 20 g per day) decreases the risk of heart failure in adults, probably through its protective effect on known risk factors for heart failure such as diabetes mellitus, hypertension, and ischemic heart disease. |
| The effect of maintaining light-moderate alcohol intake in older persons with heart failure is unclear. The data on its long-term effects on mortality are contradictory. |
| Alcohol abuse has no protective cardiovascular effect and increases the risk of heart failure and hypertension. |
| Response to rehabilitation is better among adults > 65 years than among the younger population; important factors seem to be a social and family environment favoring abstaining from alcohol and maintaining prolonged treatments. |
| It is essential to approach the specific factors associated with relapses in older adults, such as isolation and health problems, as well as the belief that it is too late to change. |
| Among older people in rehabilitation, resuming social and family relationships is considered essential to prevent recurrences. |
| Older adults should undergo alcohol detox as inpatients due to the greater complications and length of the process, partly influenced by comorbidity. |
| Benzodiazepines should be used with extreme caution, due to the higher risk of adverse effects. Evidence on other drug therapy is limited. |
| Long-term follow-up and support are essential to maintain abstinence. |
Sedentariness
The overall quality of the evidence is low. There were no specific diagnostic criteria for sedentariness in older adults.
Prevention. All-cause mortality decreased in formerly sedentary older adults but remained higher than in consistently non-sedentary individuals.[6] Moreover, mortality increased among newly sedentary adults but less so than in consistently sedentary individuals.[6]
The results on whether increasing physical activity in older adults decreases sedentariness are contradictory,[2,6] and there are no strategies that specifically target sedentariness in older adults, except the recommendation of reducing sitting time and taking more frequent breaks during sedentary behaviour.[7]
Intervention-treatment. Moderate-to-intense physical activity (measured with an accelerometer) positively impacts CVRF in older adults,[8] while the benefit of light physical activity is more controversial.[4,8,9] Prescription of physical activity should be progressive, individualized, precise and with clear objectives. The program should include aerobic exercise, strength training, flexibility and balance, as well as health education. Exercise programs for patients with sarcopenia or frailty lack the specificity, frequency and duration needed for patient improvement, [10] and it is especially important to achieve cardiovascular conditioning in patients with CVRF. Perhaps the simplest way to increase aerobic physical exercise in robust older adults is to set a target of more than 10,000 steps a day (high level of evidence).[11]
Smoking
The overall quality of studies is low. There are no specific diagnostic criteria in older adults.
Prevention. Older adults who smoke or are ex−smokers have a higher risk of all-cause mortality and mortality due to CVD and cancer,[12,13,14] as well as a higher risk of cognitive decline.[15,16] Mortality reaches a peak around the age of 60-70 years.[17,18,19] The number of pack/years also influences mortality risk and the longer the time since smoking cessation, the greater the risk reduction.[18,19] The risk of all cardiovascular events 5-10 years after quitting is reduced by 46%,[17,20] indicating that, given current life expectancy, smoking cessation should be recommended even in very old persons. Older smokers lose quality-adjusted life years, and the higher the number of cigarettes smoked and the longer they have smoked, the greater the loss (up to 55%).[21] Quality of life begins to improve 11 years after quitting in older adults and can equal that in never-smokers.[21]
Intervention-treatment. Recommendations do not differ from those in the rest of the population, focusing on the need for clear prescription by the health team, setting a date for quitting smoking with a prior preparation period, and the use of nicotine replacement therapy, which lacks specific recommendations for older adults in the product information sheet. Regarding personalized interventions for older adults, some clinical trials have reported satisfactory results versus routine clinical practice or guideline recommendations in serious attempts to quit or short periods of abstinence, but unfortunately these affects did not persist in the long term.[21,22,23]
Obesity and Metabolic Syndrome
The overall quality of the studies is moderate or high, particularly those examining treatment and prevention.
There are no specific diagnostic criteria for obesity and metabolic syndrome in older adults. However, in the last few years, knowledge has increased on sarcopenic obesity as an especially important entity in functional decline in older adults.[24]
Prevention. The prevalence of metabolic syndrome in the elderly seems to be lower in older adults with moderate red wine consumption[25] and higher intake of low-fat dairy products.[26]
Treatment-intervention. No studies were found on the management of obesity and metabolic syndrome specifically in older adults with frailty and functional decline or on drug therapy for the treatment of obesity and metabolic syndrome in older adults. Exercise capacity seems to be inversely related to arterial stiffness and age in persons with metabolic syndrome.[27] In older patients at high risk of CVD, a Mediterranean diet supplemented with extra virgin olive oil or nuts seems to reverse metabolic syndrome.[28]
Hypertension
The overall quality of studies is moderate or high. There were no specific studies on diagnosis in the older population.
Prevention. In older patients with frailty and those older than 80 years, there are doubts about the benefit of treatment,[29,30,31] but meta-analyses in this patient subgroup show a reduction in cardiovascular morbidity and mortality when expressed as a composite outcome including various cardiovascular events or cerebrovascular morbidity and mortality, or all-cause mortality.[32,33]
Intervention-treatment. In older adults, intensive targets (systolic blood pressure [SBP] < 120 mmHg or diastolic blood pressure [DBP] < 90 mmHg) seem to reduce all-cause mortality[34,35] or cardiovascular mortality as a composite,[36] or individual outcome.[34] However, achieving targets < 140 mmHg was related to more frequent severe adverse effects[33] versus standard targets (SBP < 140 mmHg). Older adults with frailty derive no mortality benefit from the application of intensive therapy.
Regarding non-pharmacological treatment such as exercise, after the age of 55 years, handgrip exercise training for more than 4 weeks may produce clinically significant reductions in SBP and DBP. [37]
Diuretics (potassium-sparing or thiazide diuretics), beta-blockers and calcium channel blockers have been shown to effectively reduce cardiovascular risk. However, calcium channel blockers seem to have a more favourable safety profile, while beta-blockers may have a higher rate of adverse events. These conclusions are drawn from clinical trials and meta-analyses of these trials, and consequently the evidence is of high quality. There is less evidence on angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers (Table 5), and its overall quality is moderate.
Table 5. Review of studies on hypertension in the elderly.
| Studies | Quality assessment | Summary of findings | Quality | |||||||||
| Ref | N | Study design | Limitations | Inconsistency | Indirectness | Imprecision | Publication bias | Relative effect estimates | Absolute effect estimates | |||
| ACEI: Angiotensin-converting enzyme inhibitors; ARBs: Angiotensin II receptor blockers; ARR: absolute risk reduction; BS: Blind Study; BQ: beta-blockers; CA-ANTAG: calcium antagonist; CI: confidence interval; CS: cohort study; D: diuretic; DWA: difference in weighted averages; DBP: dyastolic blood pressure;eGFR: estimated glomerular filtration rate; FI: frailty index ; HR: Hazard ratio; I: Important; m: men; NA: Not Applicable; NNTB: number needed to treat for an additional beneficial outcome; ns: not significant; OBS: observational study; OR: odds ratio; PBO: placebo group; RCT: random clinical trial; Ref: bibliography reference annex 1; RR: relative risk; s: second; SAEs: serious adverse events ;SBP: systolic blood pressure;SR: systematic review; UV: unavailable; U: Untected ; VI: very Important;vs: versus; w: women. | ||||||||||||
| Which is the impact of hypertension in the overall mortality in the elderly? | ||||||||||||
| 48 | 8 221 (4 120 with treatment) (SBP < 140 mm Hg and DBP < 90 mm Hg) |
SR | VI | I | U | I | U | RR 1.24 (CI 0.99-1.54) | 31/1000 (low SBP) 39/1000 (CI95% 31-48) |
Low | ||
| 49 | > 33 600 4 120 with treatment (SBP 130 mm Hg -140 mm Hg) |
SR |
I | I | U | U | U | RR 0.82 (CI 0.50-1.36) |
UV | Moderate | ||
| 50 | 2 636 1 317 with treatment (SBP < 12 mm Hg) 440 with treatment (FI > 0.21) 371 with treatment (walking speed < 0.8/s) |
RCT | I | I | U | U | U | HR 0.67 (0.49-0.91) (intensive treatment) HR 0.64 (0.41-1.01) p=0.05 (in frail patients) HR 0.75 (0.44-1.26) (in walking speed) |
1.78% events/year (CI95% 1.41-2.24) (intensive treatment) vs 2.63% events/year (CI 95% 2.17-3.18) (standard treatment) |
High | ||
| 51 | 96 549 18 139 (N < 140 mm Hg) 11 899 (N > 65years ≥ 140 mm Hg SBP) |
SR | I | I | U | I | U | RR 0.95 (0.81-1.11) ( > 65 years SBP≥140 mm Hg) RR 0.73 (0.52-1.02) (SBP < 140 mm Hg) |
UV | Moderate | ||
| 52 | 1167 586 with treatment (SBP < 120 mm Hg) |
RCT | VI | I | I | I | U | HR 0.67 (0.49-0.92) (intensive treatment) | Cumulative incidence events/year (3.75years): 0.11 (intensive treatment) vs 0.15 (standard treatment) | Moderate- low | ||
| 53 | 21 906 | SR OBS | VI | I | I | I | U | HR 1.02 (0.90-1.16) (SBP < 140 mm Hg /frail) HR 0.86 (0.77-0.96) (SBP < 140 mm Hg / non-frail) HR 1.01 (0.69-1.46) (DBP < 90 mm Hg /frail) HR 0.90 (0.76-1.07) (DBP < 90 mm Hg / non frail) |
UV | Moderate- low | ||
| Which is the impact of hypertension on stroke in the elderly? | ||||||||||||
| 48 | 8 221 4 120 with treatment (SBP < 140 mm Hg and DBP < 90 mm Hg) |
SR | VI | I | U | I | U | RR 1.25 (CI 0.94-1.67) | 20 /1000 (low SBP) 25 /1000 (CI95%: 19 a 33) (high SBP) |
Low | ||
| 50 | 2 636 1 317 with treatment (SBP < 120 mm Hg) 440 with treatment (FI > 0.21 mm Hg) 371 with treatment (walking speed < 0.8/s) |
RCT | I | I | U | U | U | HR 0.72 (0.43-1.21) | 0.67% events/year (CI95% 0.46-0.97) (intensive treatment) vs 0.85% events/year (CI 95% 0.61-1.19) (standard treatment) |
High | ||
| 51 | 96 549 18 139 with treatment (SBP < 140 mm Hg) 11 899 with treatment ( > 65years SBP≥140 mm Hg) |
SR | I | I | U | I | U | RR 0.70 (0.60-0.83) ( > 65 years SBP≥140 mm Hg) RR 0.65 (0.49-0.86) (SBP < 140 mm Hg) |
UV | Moderate | ||
| Which is the impact of hypertension on serious adverse events in the elderly? | ||||||||||||
| 48 | 8 221 4 120 with treatment (SBP < 140 mm Hg Y DBP < 90 mm Hg) |
SR | VI | I | U | I | U | RR 1.95 (CI 0.98-1.45) |
42/1000 (low SBP) 50/1000 (Ci95%: 41 a 61) (high SBP) |
Low | ||
| 50 | 2 636 1 317 with treatment (SBP < 120 mm Hg) 440 with treatment (FI > 0.21) 371 with treatment (walking speed < 0.8/s) |
RCT | I | I | U | U | U | HR 0.66 (0.51-0.85) (intensive treatment) HR 0.68 (0.45-1.01) p=0.06 (frail) HR 0.63 (0.40-0.99) p=0.05 (walking speed) |
2.59% events/year (CI95% 2.13-3.14) (intensive treatment) vs 3.85% events/year (CI 95% 3.28-4.53) (standard treatment) |
High | ||
| 51 | 96 549 6 779 with treatment ( > 65years SBP≥140 mm Hg) 21 042 with treatment (SBP < 140 mm Hg) |
SR | I | I | U | I | U | RR 0.78 (0.70-0.86) ( > 65 years SBP≥140 mm Hg) RR 0.75 (0.62-0.89) (SBP < 140 mm Hg) |
UV | Moderate | ||
| 52 | 1 167 586 with treatment (SBP < 120 mm Hg) |
RCT |
VI | I | I | I | U | HR 0.67 (0.50-0.90) (intensive treatment) | Cumulative incidence (3.61/year): 0.13 (intensive treatment) vs 0.18 (standard treatment) | Moderate- low | ||
| Which is the impact of hypertension on acute myocardial infarction in the elderly? | ||||||||||||
| 49 | > 33 600 4 120 with treatment (SBP 130 mm Hg -104 mm Hg) |
SR | I | I | U | U | U | RR 1.04 (CI 0.57-1.89) | UV | Moderate | ||
| 50 | 2 636 1 317 with treatment (SBP < 120 mm Hg) |
RCT | I | I | U | U | U | HR 0.69 (0.45-1.05) | 0.92% events/year (CI 95% 0.67-1.27) (intensive treatment) vs 1.34% events/year (Ci 95% 1.02-1.75) (standard treatment) |
High | ||
| 51 | 96 549 21 042 with treatment ( > 65years SBP≥140 mm Hg) 17 785 with treatment (SBP < 140 mm Hg) |
SR | I | I | U | I | U | RR 0.84 (0.73-0.99) ( > 65 years SBP≥140 mm Hg) RR 0.69 (0.47-1.02) (SBP < 140 mm Hg) |
UV | Moderate | ||
| Which is the impact of hypertension on cardiovascular mortality in the elderly? | ||||||||||||
| 48 | 8 221 4 120 with treatment (SBP < 140 mm Hg Y DBP < 90 mm Hg) |
SR | VI | I | U | I | U | RR 1.52 (CI 1.06-2.19) |
17 por 1000 (low SBP) 14 por 1000 (high SBP) |
Low | ||
| 49 | > 33 600 4 120 with treatment (SBP 130 mm Hg -140 mm Hg) |
SR | I | I | U | U | U | RR 0.75 (CI 0.41-1.39) |
UV | Moderate | ||
| 50 | 2 636 1 317 with treatment (SBP < 120 mm Hg) |
RCT | I | I | U | U | U | HR 0.60 (0.33-1.09) | 0.44% events/year (CI95% 0.28-0.70) (intensive treatment) vs 0.72% events/year (CI 95% 0.50-1.03) (standard treatment) |
High | ||
| 51 | 96 549 with treatment ( > 65years SBP ≥ 140 mm Hg) 17 785 with treatment (SBP < 140 mm Hg) |
SR | I | I | U | I | U | RR 0.84 (0.67-1.06) ( > 65 years SBP≥140 mm Hg) RR 0.62 (0.38-1.02) ns (SBP < 140 mm Hg) |
UV | Moderate | ||
| Is a higher withdrawal rate due to adverse effects in the elderly? | ||||||||||||
| 48 | 8 221 4 120 with treatment (SBP < 140 mm Hg Y DBP < 90 mm Hg) |
SR | VI | I | U | I | U | RR 0.83 (CI 0.58-1.19) | 17 por 1000 (low SBP) 14 por 1000 (CI95%: 10 a 20) (high SBP) |
Low | ||
| Which is the withdrawal rate due to adverse effects in the elderly? | ||||||||||||
| 50 | 2 636 1 317 with treatment (SBP < 120 mm Hg) |
RCT | I | I | U | U | U | HR 0.99 (0.89-1.11) | SAEs 48.4% (N 637) (intensive treatment) SAESs 48.3% (N 637) (standard treatment) |
High | ||
| 51 | 96 549 7 465 with treatment ( > 65years SBP≥140 mm Hg) 17 331 with treatment (SBP < 140 mm Hg) |
SR | I | I | U | I | U | RR 2.18 (0.73-6.54) ( > 65 years SBP≥140 mm Hg) RR 1.55 (1.21-1.95) (SBP < 140 mm Hg) |
UV | Moderate | ||
| 52 | 1 167 586 with treatment (SBP < 120 mm Hg) |
RCT | VI | I | I | I | U | HR +0.92 (0.79–1.07) (SAEs) HR 3.41 (1.92–6.06) (- 30% eGFR) HR 2.12 (95% CI 1.37–3.26) (kidney failure) |
SAES cumulative incidence (3.76 year): 0.60 (intensive treatment) vs 0.61 (standard treatment) Cumulative incidence of acute kidney failure (3.57 YEAR): 0.10 (intensive treatment) vs 0.05 (standard treatment) |
Moderate- low | ||
| What pharmarcological options for hypertension are available to improve overall mortality in the elderly? | ||||||||||||
| 54 | 26 795 13 368 with treatment |
SR | VI | U | U | U | U | RR 0.91 (0.85-0.97) ( > 60 years) RR 0.86 (0.79-0.95) 60-79 years) RR 0.87 (0.87-1.10) ( > 80 years) |
81 per 1000 (CI95% 75-90) (active) vs 95 per 1000 (control) (60-79 a) ARR 1.4%; NNTB 72 138 per 1000 (CI95% 124-157) (active) 142 per 1000 (N C) ARR ns; NNTB ns |
Moderate- High |
||
| 51 |
96 549 30 059 with treatment ( > 65years) 3 070 with treatment ( > 80years) |
SR CS | I | I | U | I | U | RR 0.87 (0.76-0.99) ( > 65 years) RR 0.98 (0.85-1.14) ( > 80 years) |
-16/1000 patientss/5 years ( > 65 a) -7/1000 patiens/5 years ( > 80 a) |
Moderate | ||
| 55 | 4 396 6 290 with treatment/year 6 330 with treatment BQ/year |
BS RCT | I | U | U | U | U | Difference (%): 3% (-14 a 18) (Active vs PBO) 16% (-5 to 33) (D vs PBO) 8% (-34 to 12) (BQ vs PBO) |
Absolute difference (1000 patients/year): 0.8 (-3.0 a 4.6) (treatment vs PBO) |
High | ||
| 56 | 3 845 1 933 with treatment |
RCT | U | U | U | U | U | HR non ajusted 0.79 (0.65-0.95) p=0.02 | SBP/1000 patients/year (no events): 47.2 (196) (treatment) vs 59.6 (235) (PBO) |
High | ||
| 57 | 55 645-94 228 19 942 with treatment D 7 937 with treatment BQ 23 362 with treatment CA-ANTAG 15 742 with treatment ACE 6 222 with treatment ARBs |
SR CS | I | I | U | I | U | RR 1.00 (0.94-1.06) (D vs others) RR 1.14 (1.04-1.25) (BQ vs others) RR 0.94 (0.91-0.98) (CA-ANTAG vs others) RR 1.04 (0.99-1.08) (ACE vs others) |
UV | High | ||
| What pharmacological options for hypertension are available to improve cardiovascular mortality in the elderly? | ||||||||||||
| 51 | 96 549 30 299 with treatment ( > 65years) 3 010 with treatment ( > 80years) |
SR CS | I | I | U | I | U | RR 0.76 (0.61-0.94) ( > 65 years) RR 0.90 (0.74-1.09) ( > 80 years) |
-16/1000 patients/5 years ( > 65 years) -19/1000 patients/5 years ns ( > 80 years) |
Moderate | ||
| 55 | 4 396 6 290 with treatment /year 6 330 with treatment BQ/year |
BS RCT | I | U | U | U | U | Difference (%): 9% (-12 to 27) ns (treatment vs PBO) 29% (4 to 48) (p=0.03) (D vs PBO) -6% (-39 to 19) ns (BQ-PBO) |
Absolute difference (1000 patients/year): 1.3 (-1.5 a 4.1) ns (treatment vs PBO) |
High | ||
| 56 | 3 845 1 933 with treatment |
RCT | U | U | U | U | U | HR non adjusted 0.77 (0.60 to 1.01) ns |
SAEs 1000 patientss/year (no events): 23.9 (99) (active) vs 30.7(121) (PBO) |
High | ||
| 57 | 55 645-94 228 19 942 with treatment D 7 937 with treatment BQ 23 362 with treatment CA-ANTAG 15 742 with treatment ACE 6 222 with treatment ARBs |
SR CS | I | I | U | I | U | RR 0.85 (0.74-0.98) (D vs PBO) RR 0.98 (0.89-1.07) (D vs others) RR 0.88 (0.62-1.29) (BQ vs PBO) RR 1.39 (1.03-1.88) (BQ vs others) RR 0.50 (0.29-0.89) (CA-ANTAG vs PBO) RR 0.95 (0.89-1.01) (CA-ANTAG vs others) RR 1.04 (0.98-1.11) (ACE vs others) RR 1.11 (0.82-1.49) (ARBs vs PBO) |
UV | High | ||
| What pharmacological options for hypertension are there to improve cardiovascular morbidity and mortality in the elderly? | ||||||||||||
| 54 | 26 795 13 368 with treatment |
SR | VI | U | U | U | U | RR 0.72 (0.68-0.77) ( > 60 years) RR 0.71 (0.65-0.77) (60-79 years) RR 0.75 (0.65-0.87) ( > 80 years) |
93 per 1000 (CI95%: 85 to 101) (treatment) vs 131 per 1000 (control) (60-79 years) ARR = 3.8%. NNTB = 27 115 per 1000 (CI95% 75 to 100) (treatment) vs 86 per 1000 (control) ( > 80 years) ARR = 2.9%. NNTB = 35 |
Moderate-high |
||
| 51 | 96 549 30 299 with treatment ( > 65years) 3 010 with treatment ( > 80years) |
SR CS | I | I | U | I | U | RR 0.72 (0.63-0.82) ( > 65 years) RR 0.75 (0.63-0.88) ( > 80 years) |
-67/1000 patients/5 years ( > 65 years) -66/1000 patients/5 years ( > 80 years) |
Moderate |
||
| 55 | 4 396 6 290 with treatment /year 6 330 with treatment BQ/year |
BS RCT | I | U | U | U | U | Difference (%): 17% (2 a 29) (p=0.03) (Active vs PBO) 35% (17 a 49) (p=0.0005) (D vs PBO) 4% (-19 a 23) (BQ vs PBO) |
Absolute difference (1000 patients/year): 4.2 (0.5 a 7.9) (treatment vs PBO) |
High | ||
| 56 | 3 845 1 933 with treatment |
RCT | U | U | U | U | U | HR non adjusted 0.66 (0.53-0.82) (p < 0.001) | SAEs (1000 patients/year): 33.7 (138) (treatment) vs 50.6 (193) (PBO) |
High | ||
| 57 | 55 645-94 228 19 942 with treatment D 7 937 with treatment BQ 23 362 with treatment CA-ANTAG 15 742 with treatment ACE 6 222 with treatment ARBs |
SR CS | I | I | U | I | U | RR 0.77 (0.69-0.87) (D vs PBO) RR 0.90 (0.82-0.98) (D vs others) RR 0.91 (0.79-1.05) (BQ VS PBO)RR 1.36 (1.11-1.77) (BQ vs others) RR 0.51 (0.31-0.85) (CA-ANTAG vs PBO) RR 1.09 (1.02-1.15) (CA-ANTAG vs others)RR 0.51 (0.39-0.66 ACE vs PBO) RR 0.96 (0.89-1.04) (ACE vs others) RR 0.84 (0.74-0.94) (ARBs vs PBO) RR 0.99 (0.92-1.07) (ARBs vs others) |
UV | High | ||
| What pharmacological options for hypertension are available to improve neurovascular morbidity and mortality in the elderly? | ||||||||||||
| 54 | 26 795 13 368 with treatment |
SR | VI | U | U | U | U | RR 0.66 (0.59-0.74) ( > 60 years) RR 0.66 (0.58-0.76) (60-79 years) RR 0.66 (0.52-0.83) ( > 80 years) |
33 per 1000 (CI95%: 29 to 38) vs 50 per 1000 (control) (60-79 years); ARR 1.7%; NNTB 59 35 per 1000 (CI95%: 27 to 43) vs 52 per 1000 (control) ( > 80 years): ARR 21.7%; NNTB 59 |
Moderate-high | ||
| 51 | 96 549 30 299 with treatment ( > 65years) 3 010 with treatment ( > 80years) |
SR CS | I | I | U | I | U | RR 0.67 (0.58-0.77) ( > 65 years) RR 0.68 (0.54-0.84) ( > 80 years) |
-28/1000 patients/5 years ( > 65 years) -29/1000 patients/5 years ( > 80 years) |
Moderate | ||
| 55 | 4 396 6 290 with treatment /year 6 330 with treatment BQ/year |
BS RCT | I | U | U | U | U | Diffence (%): 25% (3 a 42) (p=0.04) (ACTIVE vs PBO) 31% (3 a 51) (p=0.04) (D vs PBO) 18% (-14 a 40) (BQ vs PBO) |
Absolute difference (1000 patients/year): 2.7 (0.3 a 5.1) (active vs PBO) |
High | ||
| 56 | 3 845 1 933 with treatment |
RCT | U | U | U | U | U | HR non adjusted 0.70 (0.49-1.01) ns | SAEs (1000 patients/year): 12.4 (51) (treatment) vs 17.7 (69) (PBO) |
High | ||
| 57 | 55 645-94 228 19 942 with treatment D 7 937 with treatment BQ 23 362 with treatment CA-ANTAG 15 742 with treatment ACE 6 222 with treatment ARBs |
SR CS | I | I | U | I | U | RR 0.70 (0.60-0.81) (D vs PBO) RR 0.78 (0.69-0.89) (D vs others) RR 0.82 (0.69-0.89) (BQ vs PBO) RR 1.43 (1.16-1.75) (BQ vs others) RR 0.46 (0.26-0.81) (CA-ANTAG vs PBO) RR 0.96 (0.80-1.14) (CA-ANTAG vs others) RR 1.09 (0.93-1.27) (ACE vs others) RR 0.62 (0.38-1.03) (ARBS vs PBO) |
UV | High | ||
| What pharmacological options for hypertension are available to improve acute myocardial infarction in the elderly? | ||||||||||||
| 54 | 26 795 13 368 with treatment |
SR | VI | U | U | U | U | RR 0.78 (0.69-0.88) ( > 60 years) RR 0.79 (0.69-0.90) (60-79 years) RR 0.82 (0.56-1.2) ( > 80 years) |
41 per 1000 (CI95%: 36 to 47) (treatment) vs 52 per 1000 (control) (60-79 years) 17 per 1000 (CI95%: 12 to 25) (treatment) vs 21 per 1000 (control) ( > 80 years) |
Moderate-high | ||
| 51 | 96 549 30 299 with treatment ( > 65years) 3 010 with treatment ( > 80years) |
SR CS | I | I | U | I | U | RR 0.78 (0.66-0.94) ( > 65 years RR) RR 0.96 (0.57-1.63) ns ( > 80 years) |
-7/1000 patients/5 years ( > 65 years)-1/1000 patients/5 years ( > 80 years) | Moderate | ||
| 55 | 4 396 6 290 with treatment /year 6 330 with treatment BQ/year |
BS RCT | I | U | U | U | U | Difference (%): 19% (-2 a 36) (treatment Vs PBO) 44% (21 a 60) (p=0.0009) (D vs PBO) 3% (-30 a 27) ns (BQ vs PBO) |
Absolute difference (1000 P/year): 2.4 (-0.2 a 5.0) (treatment vs PBO) |
High | ||
| 56 | 3 845 1 933 with treatment |
RCT | U | U | U | U | U | HR non adjusted 0.72 (0.30-1.7) | SBP (1000 patients/years. no events): 2.2 (9) (treatment) vs 3.1 (12) (PBO) |
High | ||
| 57 | 55 645-94 228 19 942 with treatment D 7 937 with treatment BQ 23 362 with treatment CA-ANTAG 15 742 with treatment ACE 6 222 with treatment ARBs |
SR CS | I | I | U | I | U | RR 1.03 (0.89-1.20) (D vs others) RR 1.24 (0.91-1.68) (BQ vs others) RR 1.01 (0.93-1.10) (CA-ANTAG vs others) RR 0.93 (0.82-1.04) (ACE vs others) RR 0.93 (0.78-1.10) (ARBs vs others) |
UV | High | ||
| What pharmacological options for hypertension are available to improve the withdrawal due to adverse events in the elderly? | ||||||||||||
| 54 | 26 795 13 368 with treatment |
SR | VI | U | U | U | U | RR 2.91 (2.56-3.30) ( > 60 years) | UV | Moderate- low | ||
| What pharmacological options for hypertension are available to improve the adverse events in the elderly? | ||||||||||||
| 51 | 96 549 30 299 with treatment ( > 65years) 3 010 with treatment ( > 80years) |
SR CS | I | I | U | I | U | RR 1.72 (1.09-2.74) ( > 65 years) RR 4.31 (0.30-62.28) ( > 80 years) |
+100/1000 patients/5 years ( > 65 years) +27/1000 patients/5 years ( > 80 years) |
Moderate-high | ||
| 56 | 3 845 1 933 with treatment |
RCT | U | U | U | U | U | UV | Advers events: 358 (treatment group) vs 448 (PBO); p = 0.001 |
High | ||
| 57 | 55 645-94 228 19 942 with treatment D 7 937 with treatment BQ 23 362 with treatment CA-ANTAG 15742 with treatment ACE 6222 with treatment ARBs |
SR CS | I | I | U | I | U | RR 2.60 (1.38-4.88) (D vs PBO) RR 0.91 (0.27-3.08) (D vs others) RR 2.11 (0.63-7.04) (BQ vs PBO) RR 2.07 (1.74-2.45) (BQ vs others) RR 0.59 (0.45-0.78) (CA-ANTAG vs others) RR 1.17 (0.62-2.16) (ARBs vs PBO) |
UV | High | ||
| What is the effect of exercise on sbp y dbp in the elderly? | ||||||||||||
| 58 | 466 241 with treatment ( > 55years) | SR | VI | I | U | I | U | UV | SBP DWA -3.96 mmHg (N 115 > 55a) SBP DWA −4.71 mmHg (N 73 m > 55a) SBP DWA −6.52 mmHg (N 53 w > 55) DBP DWA -1.30 mmHg (N 115 > 55a) DBP DWA −2.07 mmHg (N 73 m > 55a) DBP DWA −2.40 mmHg (N 53 w > 55) |
Moderate (sbp) low (dbp) |
||
Type 2 Diabetes Mellitus
Although we retrieved a substantial number of studies, the general quality of the evidence is low, except that for dipeptidyl peptidase-4 (DPP4) inhibitors and for recommendations on multifactorial intervention in older adults with frailty[38] (Table 6).
Table 6. Review of studies on diabetes mellitus 2 in the elderly.
| Studies | Quality assessment | Summary of findings | Quality | |||||||||
| Ref | N | Study desing | Limitations | Inconsistency | Indirectness | Imprecision | Publication Bias |
Relative effect estimates | Absolute effect estimates | |||
| CS: cohort study; DM2: type 2 diabetes; DPP-4 (dipeptidylpeptidase-4) inhibitor; HR: Hazard ratio; I: Important; MET: metformin; NA: Not Applicable; OBS: observational study; OR: odds ratio; PBO: placebo; PIO: pioglitazone; Quality: quality assessed according to GRADE methodology; RCT: random clinical trial; Ref: bibliography reference annex 1; SR: systematic review; SPPB: short physical performance battery; SULF: sulfonylurea; TZD: Thiazolidinediones; U: Undetected; UV: unavailable; VI: very Important; vs: versus. | ||||||||||||
| What is the therapeutic target in the elderly with DM2? | ||||||||||||
| 60 | 3 475 1 732 (with treatment) |
RCT Post hoc analysis |
I | U | U | I | U | Non fatal Acute myocardial infarction + non fatal stroke+ cardiovascular mortality: HR 0.84 (0.69-1.03) |
2.76% (intensive treatment) vs 3.10% (standard treatment) |
Low | ||
| 61 | 6 611 | RCT Subgroup analysis |
I | U | U | I | U | Macro and microvascular damage: RR 0.92 (0.83-1.03) |
19.5% (intensive treatment) vs 21% (Standard treatment) |
Low | ||
| 62 | 1 173 585 (with treatment) |
RCT | I | U | U | I | U | No significant difference in fatal events Significative difference coronary revascularization |
No diference significative in mortality events Significative difference coronary revascularization |
Low | ||
| What is the therapeutic target in frail elderly with DM2? | ||||||||||||
| 63 | 25 966 (with treatment) | CS | U | U | NA | U | U | Adjusted mortality: RR-(0.80 (0.70-0.91) (Subgroup HbA1 7-7.4 vs HbA1 8-8.4%) No differences between reference group and HbA1 <6->8.5% |
80.9 por 1000 Patients/year (subgroupHbA1 7-7.4%) |
Low | ||
| 64 | 993 497 (with treatment) | RCT POST HOC ANALYSIS |
I | U | U | I | U | Higher risk of stroke in subgroup HbA1 >8.8%- 6-7.2% |
UV | Low | ||
| 65 | 232 (with treatment DM) 1 835 (with treatment Not for DM) |
CS | I | U | NA | I | U | RR 1.40 (1.12-1.76) p=0.002 Dementia (Hiperglycemia 190 mg/dl) |
UV | Low | ||
| 66 | 200 (with treatment DM) 1 648 (with treatment Not for DM) |
CS | I | U | NA | I | U | Risk of frailty (p=0.001) (Glucose < 160 - > 180 mg/dl) |
UV | Low | ||
| 67 | 446 (with treatment) | CS | I | U | NA | I | U | Risk if falls with insuline OR 4.36 (1.32-14.46) (HbA1 ≤6 vs >8%) |
UV | Low | ||
| 68 | 132 (with treatment) | CS | I | U | NA | I | U | More frailty: strict HbA1 controls |
UV | Low | ||
| 69 | 111 (with treatment) | CS | I | U | NA | I | U | Risk of falls (p=0.01) (HbA1 < 7%) | UV | Low | ||
| What is the therapeutic target in elderly people with established functional impairment with dm2? | ||||||||||||
| 70 | 367 (with treatment) | CS | I | U | NA | I | U | Lower functional decline and mortality RR 0.88 (0.79-0.99) (HbA1 8-8.9% vs HbA1 7-7.9%) | 52% vs 58% | Low | ||
| 71 | 119 (with treatment) | CS | I | U | NA | I | U | Better lower extremity functionality (SPPB test): Lower variability in glucosa control (HbA1 <7%) | UV | Low | ||
| What is the therapeutic target in the elderly with several cvrf for each of them? | ||||||||||||
| 72 | 388 (with treatment) | CS | I | U | NA | I | U | No higher mortality risk with HbA1 7%. blood pressure 145/80 mmHg and total cholesterol < 240 mg/dl Higher mortality depending on terminal kidney disease and macroangiopathy (previous stroke, obesity and hiher levels of LDL) |
19.6% Mortality (6years) | Low | ||
| Efficacy of pharmacological treatment in the elderly: metformin | ||||||||||||
| 73 | 1 273 (with treatmentMET) | CS | I | U | NA | I | U | Reduction of annual mortality risk from any cause HR 0.87 (0.78-0.97) (MET vs other treatments) |
24.7% vs 36% | Low | ||
| 74 | 1 273 (with treatment MET) | CS | I | U | NA | I | U | Annual mortality HR 0.92 (0.81-1.06) (MET VS treatment other treatments) | UV | Low | ||
| 75 | 367 (with treatment) | CS | I | U | NA | VI | U | Higher mortality risk: (MET + strict glycemia control < 6.5%) HR 2.63; 1.39-4.97 |
UV | Very low | ||
| 76 | 8 393 (with treatmenttMET) | CS | U | U | NA | U | U | Lower mortality risk MET |
UV | Low | ||
| Efficacy of pharmacological treatment in the elderly: pioglitazone | ||||||||||||
| 74 | 819 (with treatment TZD) | CS | I | U | NA | I | U | Reduction of annual mortality from any cause HR 0.87 (0.80-0.94) Higher risk with heart failure HR 1.06 (1-1.09) |
30.1% vs 36% | Low | ||
| 73 | 2 276 (with treatment TZD) | CS | I | U | NA | I | U | Annual mortality HR 0.92 (0.80-1.05) Higher risk of readmission (1.09;1-1.20) Higher risk of readmission for heart failureHR 1.17 (1.05-1.3). |
UV | Low | ||
| 77 | 69 (with treatment TZD) 30 (with treatment PIO) |
CS | I | U | NA | I | U | Increased bone loss TZD (women): HR -0.61 ( -1.02-0.21) |
UV | Low | ||
| Efficacy of pharmacological treatment in the elderly: sulfonylureas | ||||||||||||
| 73 | 12 069 (with treatment) | CS | I | U | NA | I | U | Reduction of mortality TZD/MET not with Sulfonylureas (0.99;0.91-1.08) Higher risk of stroke ( TZD) Higher risk of readmission for heart failure (1.06;1-1.09) (TZD) |
UV |
Low | ||
| 75 | 130 N TTO SLF | CS | I | U | NA | VI | U | Higher risk of mortality Sulfonylureas (strict control of HbA1 < 7%) HR 2.49 (1.14-5.44) | UV | Very low | ||
| 78 | 5 543 (with treatment SLF) | CS | I | NA | NA | I | U | Composite outcome (mortality +atrial fibrilation +stroke+heart failure+ Acute myocardial infarction): gliburide/glipizide/repaglinide HR 0.91 (0.78-1.05) |
28.1% 30.2% 23.4% |
Low | ||
| 79 | 13 963 (with treatment SLF) | CS | I | U | NA | U | U | High risk of severe hypoglycemia: gliburide HR 16.6 (13.2-19.9) Loser risk: tolbutamide and glipicide Same high risk gliburide =clorpropamide |
gliburide (16.6/1.000 patients/year; 13.2-19.9) lower rates (3.5; 1.2-5.9) (tolbutamide and glipicide) |
Low | ||
| 80 | 139 N TTO | ECA | I | I | U | I | U | UV | Glycemia control: 80.3% glubiride vs 64.4% glipizide |
Low | ||
| Efficacy of pharmacological treatment in the elderly: metiglinide | ||||||||||||
| 78 | 740 (with treatment repaglinide) | CS | I | NA | NA | I | U | Composite outcome (mortality +atrial fibrilation +stroke+heart afilure+ Acute myocardial infarction): Repaglinide HR 0.80 (0.63-1.03) no significative differences vs glipizide/gliburide |
28.1% 30.2% 23.4% |
Low | ||
| 81 | 54 30 (with treatment) |
RCT | I | U | U | I | U | UV | Reduction HbA1 (12 weeks) Treatment (7.6±0.1%) vs basal control (6.9±0.1%) Difference -0.7±0.1% (P<0.001) vs PBO (-0.5. p=0.004) |
Low | ||
| 81 | 66 33 (with treatment) |
RCT SUBANALYSIS |
I | U | U | I | U | UV | Reduction HbA1 (104 SE): nateglinide/MET (7.8±0.2%) vs basal level (6.6±0.1%) differences -1.2±0.2%. (P<0.001) Reduction HbA1 (104 weeks): gliburide/MET (7.7±0.1) vs BASAL (6.5±0.2%) differences1.2±0.1% (p<0.001) no significative difference (p=0.310) |
Low | ||
| Efficacy of pharmacological treatment in the elderly: ddp-4 inhibitors | ||||||||||||
| 82 | 241 | RCT | U | U | U | I | U | More reduction HbA1: linagliptine −0.64% (95% CI −0.81-−0.48) (p<0.0001) |
linagliptine –0.61% (0.06) vs PBO 0.04% (0.07) |
Moderate | ||
| 83 | 278 | RCT | ND | U | U | I | U | Greater % achievement of objective HbA1: Vidalgiptine OR 3.16 (1.81-5.52) |
Vidagliptine 52.6% vs 27% PBO | Moderate | ||
| 84 | 388 | RCT | I | U | U | U | U | Better control HbA1: sitagliptine and glimeridae DIF 0.19% (0.03-0.34%) | -0.32% (sitagliptine) vs -0.51% (glimeride) |
Moderate | ||
| 85 | 441 | RCT | I | U | U | U | U | Reduction HbA1: alogliptine vs tto glipicide (-0.05% -0.13%) | -0.14% (alogliptine) vs 0.09% (glipicide) |
Moderate | ||
| 86 | 720 | RCT | U | U | U | U | U | HbA1 < 7% (52 weeks): saxagliptine vs tto glimepiridE OR 0.99 (0.73-1.34) | 37.9% vs 38.2% | High | ||
| 87 | 201 | RCT | U | U | U | U | U | Higher reduction of HbA1 and postpandrial glucose (2hours): sitagliptine | sitagliptine: difference 0.7% and 61 mg/dl | High | ||
| 88 | 335 | RCT | I | U | U | U | U | Reduction HbA1: No differences with vildagliptine | (-0.64 ± 0.07% and -0.75 ± 0.07%) | Moderate | ||
| 89 | 58 485 | CS | U | U | NA | U | U | Lower mortality: DPP4 Inh Vs no treatment (HR=0.54;0.52-0.56) Treatment for myocardial infarction, cerebrovascular accident or cardiovascular death vs no treatment (HR=0.79; 0.75-0.83) |
Mortality incidence (1000/year) (DPP4 I vs no treatment): 36.01 y 66.91 / myocardial infarction, cerebrovascular accident or cardiovascular death (26.37 y 33.41) acute myocardial infaction (6.76 8.58) stroke (20.34 y 25.85) |
Moderate | ||
| 90 | 35 206 (with treatment SULF) 9 517 (with treatmentTZD) |
CS | I | U | NA | U | U | Reduction of non- mortal infarct < DPP4 I vs SULF excepting those with MTF as base treatment: (-0.92 (-1.60, -0.24). Composite outcome VS Tiazolidinedione: -0.38 (-0.71, -0.05) Global mortality: -0.44 (-0.83, -0.06) |
Non fatal infarction (100 patients/year): 0.4 (0.2 a 0.6) (DPP4 I) 1.0 (0.8 a 1.2) (SULF) Combinated outcome: 3.9 (3.5 a 4.3) (DPP4 I) 4.5 (3.8 a 5.2) (TZD) Global mortality: 2.9 (2.6 a 3.3) (DPP4 I); 3.5 (2.9 a 4.1) (TZD) |
Low | ||
| Efficacy of pharmacological treatment in the elderly: GLP-1 receptor agonist | ||||||||||||
| 91 | 350 174 (with treatment) |
RCT | I | U | U | U | U | Higher reduction HbA1: lixixenatide |
- 0.57% (lixixenatide) vs +0.06% (PBO) (p<0.0001) | Moderate | ||
| Efficacy of pharmacological treatment in the elderly: alpha glucosidase inhibitors | ||||||||||||
| 92 | 192 93 (with treatment) |
RCT | I | U | U | I | U | UV | Reduction HbA1 (1 A): TTO acarbose -0.6±1 vs PBO | Low | ||
| 93 | 45 22 (with treatment) |
RCT | MI | U | U | I | U | UV | Blood glucose reduction fasting: 0.2 ± 0.3 (treatment) vs. -0.5 ± 0.2 mmol/l (PBO) (P < 0.05) |
Low | ||
| Efficacy of pharmacological treatment in the elderly: insuline | ||||||||||||
| 73 | 12 069 (with treatment) | CS | I | U | NA | I | U | Anual mortality for any cause: HR 0.96 (0.88-1.05) |
UV | Low | ||
| 94 | 130 (with treatment) | RCT | I | U | NA | I | U | UV | Reduction HbA1 -1.9% vs 1.4% (insuline subgroup + other hypoglycemia Treatment vs regular mixte insuline+ Human protamine) higher % HbA1 ≤7% + without nocturn hypoglycemia 55.2% vs 30.2% (p=0.006). |
Moderate | ||
| What are the effects associated with overtreatment in the elderly? | ||||||||||||
| 95 | 65 (with treatment) | CS | I | U | NA | I | U | UV | Reduction HbA1 -0.52% (0.5%) (HBA1 8-9%) p<0.001) vs Increase HbA1 0.37% (0.7%) (HBA1<7%) p=0.03) |
Low | ||
| 96 | 133 (with treatment) | CS | I | U | NA | I | U | UV | 67% (HbA1 <7%) 10.5% (hypoglycemia episodes in previous year) |
Low | ||
| 97 | 15 643 (with treatment) | CS | U | U | NA | U | U | UV | 52% strict control HbA1 (<7%) Moreover in comorbid patients older and recent weight loss |
Low | ||
| 98 | 42 669 (with treatment) | CS | I | U | NA | U | U | UV | 26% strict control: Higher risk of hypoglycemia in pharmacological group |
Low | ||
| 99 | 8 (with treatment) | OBS | MI | U | NA | VI | U | UV | HbA1: with treatment (HbA1 6.2%±0.8) vs without treatment (6.5%±0.7) | Very low | ||
| 100 | 32 (with treatment) | OBS | MI | U | NA | VI | U | UV | HbA1: with treatment (HbA1 5.2%±0.4) vs without treatment (5.8%±0.9) | Very low | ||
| 101 | 4 368 (with treatment) | CS | I | U | NA | U | U | Withdrawal of treatment: 1.28 (1.22-1.33) | Withdrawal of treatment: 71.5% intervention group VS 56% no intervention group |
Low | ||
| 102 | 2 830 (with treatment) | CS | ND | U | NA | U | U | UV | Withdrawal of treatment: 9.6% (37% hypoglycemia antecedents) | Low | ||
| How does chronic kidney disease modify therapeutic options in the elderly with diabetes? | ||||||||||||
| 103 | 4 053 1 147 (N ≥ 65years) |
SR RCT | U | U | U | U | U | Reduction HbA1: (<≥ 65years) + (glomerular filtration >60 ml/min) |
Lower reduction (≥ 65Ayears and glomerular filtration 45-60 ml/min) | Low | ||
| How does chronic heart failure modify therapeutic options in the elderly with diabetes? | ||||||||||||
| 104 | 1 833 773 (with treatment SULF) 208 (with treatment MET) |
CS | U | U | NA | I | U | UV | Mortality and hospitalization: 52% y 85% SULF vs 33% 77% MET vs 31% y 80% combined |
Low | ||
| 105 | 1 633 (with treatment) | OBS | U | U | NA | I | U | Mortality: MET vs no treatment OR 0.65 (0.48-0.87) MET/other hypoglycemic treatments Vs no hypoglycemic treatments OR 0.72 (0.59-0.90) No reduction of mortality with insuline or hypoglycemic treatments |
UV | Low | ||
| 106 | 217 (with treatment SULF) 68 (with treatment MET) |
CS | U | U | NA | I | U | Mortality MET + combined treatment vs Sulfonylureas 0.59 (0.36-0.96) At long term (0.67; 0.51-0.88) |
UV | Low | ||
| What are the therapeutic options in the elderly with diabetes and frailty? | ||||||||||||
| 107 | 451 (with treatment) | RCT | U | U | U | U | U | Improvement SPPB: 0.85 (0.44-1.26) | Improvement SPPB: 0.83 (0.58 -1.11) | High | ||
No studies were found on diagnosis or prevention with results specifically in the older population.
Intervention-treatment. In patients ≥ 65 years, intensive glucose-lowering vs standard glucose-lowering strategies did not reduce mortality or cardiovascular events but did lead to higher annualized rates of severe hypoglycaemia.[39] None of the remaining large clinical trials,[40,41] comparing the effect of intensive versus standard glucose-lowering strategies on micro- or macrovascular events provided data specifically on the older population. Among the group of “very old” adults,[42,43] very low (< 6%) or very high (> 8.5%) glycosylated hemoglobin levels were associated with higher mortality and worse functional status. Moreover, the risk of frailty increased with extreme glycemia levels,[44,45] which also increased the risk of falls.[46,47]
The decision to prescribe insulin to this age group should be individualized with priority given to safety regarding the risk of falls, fractures and hypoglycemic episodes.[48] Basal insulin analogs (glargine, detemir and degludec) do not achieve strict glycemic control but produce fewer episodes of nocturnal hypoglycemia. The MID-frail trial demonstrated the effectiveness of a multimodal intervention composed of physical and nutritional education on improving functional status in older people with frailty and type 2 diabetes mellitus.[38]
Dyslipidemia
The general quality of selected studies on this CVRF is high. There were no studies on diagnosis or prevention reporting results specifically in the older population.
Prevention. Recommendation of highly restrictive diets should be avoided due to the associated risk of malnutrition, especially in older adults with cognitive impairment or those living in nursing homes. Reducing low-density lipoprotein cholesterol (LDL-C) levels is indicated in older patients because of its prognostic benefit in primary and secondary prevention, especially in patients with the most elevated cholesterol levels and highest cardiovascular risk.[49,50]
Intervention-treatment. Current recommendations are broadly similar to those in younger patients,[51] but attention should be paid to polypharmacy, adverse effects, comorbidity and geriatric syndromes, and a comprehensive approach should be adopted that adapts treatment goals to patients´ functional prognosis and life expectancy. In older patients, very low cholesterol levels can be associated with more frequent negative events, possibly reflecting worse nutritional status and a higher prevalence of comorbidity and geriatric syndromes.
Recommendations on drug therapy do not differ from those in the younger population, but adverse events are more frequent in older adults[52] (Table 7).
Table 7. Review of studies about dyslipidemia in older people.
| Studies | Quality assessment | Summary of findings | Quality | |||||||||
| Ref | N | Study design | Limitations | Inconsistency | Indirectness | Imprecision | Publication bias | Relative effect estimates | Absolute effect estimates | |||
| CI: confidence interval; CS: Cohort study; CV: cardiovascular; CVR: cardiovascular risk; DM: patients with diabetes mellitus; HR: Hazard ratio; LDL: low density lipoprotein; NO-DM: Non-diabetic patients; RCT: random clinical trial; Ref: bibliography reference; RR: relative risk; SR: systematic review; U: undetected; UV: unavailable; Quality: quality assessed according to GRADE methodology. | ||||||||||||
| Which is the usefulness of statins for the prevention of cardiovascular disease in the elderly on overall mortality? | ||||||||||||
| 107 | 21 435 | SR | U | U | U | U | U | RR 0.94 | UV | High | ||
| 108 | 4 802 (NO-DM 75-84 years) 743 (NO-DM > 85 years) 1 756 (DM 75-84 years) 201 (DM > 85 years) |
CS | U | U | UV | U | U | RR 0.98 (NO-DM 75-84 years) RR 0.97 (NO-DM > 8 years) RR 0.84 (DM 75-8 years) RR 1.05 (DM> 85 years) |
UV | High | ||
| Which is the usefulness of statins for the prevention of cardiovascular mortality in the elderly? | ||||||||||||
| 107 | 13 914 | SR | U | U | U | U | U | RR 0.90 | UV | High | ||
| Which is the usefulness of statins for the prevention of acute myocardial infarction in the elderly? | ||||||||||||
| 107 | 15 929 | SR | U | U | U | U | U | RR 0.60 | UV | High | ||
| Which is the usefulness of statins for the prevention of stroke in the elderly? | ||||||||||||
| 107 | 16 322 | SR | U | U | U | U | U | RR 0.76 | UV | High | ||
| Which is the usefulness of statins for the prevention of atherosclerotic cardiovascular disease in the elderly? | ||||||||||||
| 107 | 11 556 | SR | U | U | U | U | U | RR 0.89 | UV | High | ||
| Which is the usefulness of statins for the prevention of cancer in the elderly? | ||||||||||||
| 108 | 4 802 (NO-DM 75-84years) 743 (NO-DM > 85years) 1 756 (DM 75-84 years) 201 (DM > 85 years) |
CS | U | U | UV | U | U | RR 0.94 (NO-DM 75-84 years) RR 0.93 (NO-DM > 85 years) RR 0.76 (DM 75-84 years) RR 0.82 (DM > 85 years) |
UV | High | ||
| Which is the usefulness of statins for the prevention of cardiovascular risk in the elderly? | ||||||||||||
| 109 | 186 854 TOTAL (756 with CV treatment/ 295 without CV treatment) |
SR | U | U | U | U | U | RR 0.87 (0.77–0.99) (total) RR 0.85 (0.73–0.98) (with CV treatment) RR 0.92 (0.73–1.16) (without CV treatment) |
UV | High | ||
| 110 | 21 492 | SR RCT | U | U | U | U | U | RR 0.74 (95% CI. 0.61–0.89) | UV | High | ||
| 111 | UV | CS | U | U | UV | U | U | NO CVR in control LDL group (≥ 75 years) | UV | High | ||
| 112 | UV | CS | U | UV | UV | U | U | Lower CVR in control LDL group |
UV | High | ||
| IS IT BETTER THE USE OF PRAVASTINA vs USUAL PRACTICE FOR THE PREVENTION OF THE CARDIOVASCULAR RISK IN THE ELDERLY? | ||||||||||||
| 113 | 1 428 with treatment (>75 years) |
RCT | U | UV | U | U | U | HR 0.80 (IC 95%. 0.70-0.90) | 8.70% | High | ||
| 114 | 1 716 with treatment | RCT | U | UV | UV | U | U | HR 0.75 | UV | Low | ||
| 115 | 4 819 | CS | U | U | UV | U | U | UV | UV | High | ||
Alcohol
The overall quality of the selected studies is low or very low.
Diagnosis. Screening questionnaires for alcohol abuse, such as CAGE,[53] are designed for the younger population, who more commonly have occupational, legal or social complications; moreover, they focus on current rather than lifelong consumption and do not include atypical manifestations (falls or confusion) or masking due to comorbidity.[54,55] Screening tests designed for the older population are the MAST-G and its brief version SMAST-G,[56] which have good sensitivity and specificity.[54]
Prevention. Given that older adults are more sensitive to the effects of alcohol, it has been postulated that neither sex should exceed intake of 10 grams of alcohol per day,[57,58] but observational studies have reported that light or moderate consumption (12-30 g) in men older than 65 years is safe, and reduces all-cause mortality and the incidence of CVRF, especially ischemic heart disease.[59,60] Older patients should never be advised to start or increase drinking alcohol to gain a cardioprotective benefit,[59] due to the interaction between alcohol and certain drugs, including antidiabetic agents, anticoagulants and antithrombotics.[59,61] The effect of maintaining light-to-moderate alcohol consumption in elderly patients with heart failure is less clear.
Intervention-treatment. Older individuals are less frequently referred to specialist treatment.[54] However, studies suggest that response to rehabilitation among persons older than 65 years is better than in younger persons. Inpatient detox is recommended because of the greater number of complications and length of the process. Benzodiazepines should be prescribed cautiously because of the risk of adverse effects. The evidence on other drug therapies is limited.[54] Long-term follow-up and support are essential to maintain abstinence.[55]
DISCUSSION
The quality of the studies reviewed was mostly low or moderate. Moreover, many studies contained no data that could help to answer the clinical questions posed in this review.
Several points should be highlighted: first, there are no specific diagnostic criteria for any CVRF in the older population and definitions are universal. However, this review reveals that the specific characteristics of older adults may bias not only therapeutic management but also diagnosis; thus; sedentariness can be confused with functional decline and, in patients who begin to be dependent or who are in chronic pain (highly frequent and disabling in older adults), the same criteria cannot be used. Similarly, because of the high rate of sarcopenic obesity in older adults, there is a need to establish specific screening criteria. The criteria for alcoholism should perhaps also be updated, given that there may be no effects on working or family life in older adults.
Second, it is essential to remember that the aim of any treatment plan in older adults is not only survival but also quality of life and, in particular, maintaining the functional independence that maintains quality of life. A major shortfall of the studies analyzed is their failure to include these factors. Although studies recommend evaluation of patients´ baseline status before the start of statin therapy in primary prevention as well as avoiding overly restrictive diets due to the risk of undernutrition in this age group, and even include events such as the risk of falls, fractures and hypoglycemic episodes when discussing management with antidiabetic agents (Tables 5 & 6), quality of life and functional independence are only analyzed in highly specific studies.[38]
Third, in the last few years, frailty syndrome has become key in the care of older patients, in all clinical settings, but especially in the approach to patients with heart disease.[62] However, there is very little literature on how to modify CVRF management in older people with frailty. At the most, studies recommend a flexible approach in the treatment of diabetes in older patients with frailty or functional dependence, but reducing frailty is not mentioned as an outcome in studies on the treatment of CVRF or as a variable that could modify treatment.
Finally, clinical trials of drugs mainly used in older patients (such as those used for CVRF control) do not include a sufficient number of older participants,[63,64] and even less so patients with frailty, functional decline, or comorbidity. This hampers safe prescription in older patients, in whom we cannot even be sure that our interventions are appropriate since clinical trials do not reflect the reality of clinical practice.
Despite the recommendations that can be made on the basis of the available evidence, in the elderly, everything should be viewed through the prism of the presence of other geriatric syndromes. For example, diuretic use can be considered effective in reducing cardiovascular risk in older patients but can also worsen urinary incontinence and encourage, among other adverse effects, functional dependence and falls. Similarly, the use of oral antidiabetic agents in older adults should always prioritize safety[45] and current recommendations recommend adapting glycated haemoglobin targets to the profile of older adults (frailty, comorbidity, life expectancy).
The main limitation of this study is the lack of high-quality studies with older adults as the target population. Study designs are often observational, with self-reported measurements, and short study duration, and often exclude nursing home residents, reducing the quality of the available evidence and their ecological validity in older adults. Moreover, there is wide heterogeneity in the definition of older adults among authors, limiting the recommendations that can be made in this population. Another limitation is the large number of studies appearing each year on CVRF, leading to the need for guidelines to be periodically updated.
As conclusion, despite the large body of data on the management of CVRF in older adults, the available evidence should be applied with caution and always with a view to maintaining quality of life and functional independence. The most effective tool available is the continued use of comprehensive geriatric assessment to implement the most appropriate treatment plans according to each patient´s baseline status and comorbidity.
Grant information
Funding has been received from the Mapfre Guanarteme Foundation for the study. This funding was not used for study design or data collection, analysis and interpretation; nor in the decision to submit the article for publication. This work has received the scientific endorsement of the Spanish Society of Geriatric Medicine and the Canarian Society of Geriatrics and Gerontology.
Acknowledgements
Drs. Marta Castro Rodríguez and Almudena Areosa Sastre have carried out an external review of the results of the project and the publication, providing improvements to it in the quality of its content and its writing.
References
- 1.Robles González E What are the main causes of death of the oldest-old in Spain. Estudios Geográficos. 2009;70:567–598. [Google Scholar]
- 2.Salazar E, Lasses LA Patología cardiovascular en el anciano. Cardiovascular disease in the elderly. Arch Cardiol Mex. 2001;71:109–113. [PubMed] [Google Scholar]
- 3.Sánchez RG, Novella Arribas B, Alonso Arroyo M, et al The EPICARDIAN project, a cohort study on cardiovascular diseases and risk factors among the elderly in Spain: methodological aspects and major demographic findings. Rev Esp Salud Publica. 2004;78(2):243–255. doi: 10.1590/s1135-57272004000200010. [DOI] [PubMed] [Google Scholar]
- 4.Díez-Villanueva P, Arizá-Solé A, Vidán MT, et al Recommendations of the Geriatric Cardiology Section of the Spanish Society of Cardiology for the Assessment of Frailty in Elderly Patients With Heart Disease. Rev Esp Cardiol (Engl Ed) 2019;72:63–71. doi: 10.1016/j.recesp.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Aguayo-Albasini JL, Flores-Pastor B, Soria-Aledo V GRADE system: classification of quality of evidence and strength of recommendation. Cir Esp. 2014;92:82–88. doi: 10.1016/j.ciresp.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 6.León-Muñoz LM, Martínez-Gómez D, Balboa-Castillo T, et al Continued sedentariness, change in sitting time, and mortality in older adults. Med Sci Sports Exerc. 2013;45:1501–1507. doi: 10.1249/MSS.0b013e3182897e87. [DOI] [PubMed] [Google Scholar]
- 7.Shibata A, Oka K, Ishii K, et al Objectively assessed patterns and reported domains of sedentary behavior among japanese older adults. J Epidemiol. 2019;29:334–339. doi: 10.2188/jea.JE20180041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueiró TH, Arins GCB, Santos CESD, et al. Association of objectively measured sedentary behavior and physical activity with cardiometabolic risk markers in older adults. PLoS One. 2019; 14: e0210861.
- 9.Sandbakk SB, Nauman J, Zisko N, et al Sedentary time, cardiorespiratory fitness, and cardiovascular risk factor clustering in older adults-the generation 100 study. Mayo Clin Proc. 2016;91:1525–1534. doi: 10.1016/j.mayocp.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Witham MD, Chawner M, Biase S, et al Content of exercise programmes targeting older people with sarcopenia or frailty - findings from a UK survey. J Frailty Sarcopenia Falls. 2020;5:17–23. doi: 10.22540/JFSF-05-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swartz AM, Cho CC, Welch WA, et al Pattern analysis of sedentary behavior change after a walking intervention. Am J Health Behav. 2018;42:90–101. doi: 10.5993/AJHB.42.3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barengo NC, Antikainen R, Harald K, et al Smoking and cancer, cardiovascular and total mortality among older adults: The Finrisk Study. Prev Med Rep. 2019;14:100875. doi: 10.1016/j.pmedr.2019.100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns DM Cigarette Smoking Among the Elderly: Disease Consequences and the Benefits of Cessation. Am J Health Promot. 2000;14:357–361. doi: 10.4278/0890-1171-14.6.357. [DOI] [PubMed] [Google Scholar]
- 14.Taylor DH Jr, Hasselblad V, Henley SJ, et al Benefits of smoking cessation for longevity. Am J Public Health. 2002;92:990–996. doi: 10.2105/AJPH.92.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters R, Poulter R, Warner J, et al Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:36. doi: 10.1186/1471-2318-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mons U, Schöttker B, Müller H, et al History of lifetime smoking, smoking cessation and cognitive function in the elderly population. Eur J Epidemiol. 2013;28(10):823–831. doi: 10.1007/s10654-013-9840-9. [DOI] [PubMed] [Google Scholar]
- 17.Choi W, Kim SH, Kang SH, et al Differential impact of smoking on cardiac or non-cardiac death according to age. PLoS One. 2019;14:e0224486. doi: 10.1371/journal.pone.0224486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gellert C, Schöttker B, Brenner H Smoking and all-cause mortality in older people: systematic review and meta-analysis. Arch Intern Med. 2012;172:837–844. doi: 10.1001/archinternmed.2012.1397. [DOI] [PubMed] [Google Scholar]
- 19.Mons U, Müezzinler A, Gellert C, et al Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551. doi: 10.1136/bmj.h1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pujades-Rodriguez M, George J, Dinesh Shah A, et al Heterogeneous associations between smoking and a wide range of initial presentations of cardiovascular disease in 1 937 360 people in England: lifetime risks and implications for risk prediction. Int J Epidemiol. 2015;44:129–141. doi: 10.1093/ije/dyu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orleans CT, Boyd NR, Noll E, et al Computer tailored intervention for older smokers using transdermal nicotine. Tob Control. 2000;9(Suppl 1):I53. doi: 10.1136/tc.9.suppl_1.i53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ossip-Klein DJ, Carosella AM, Krusch DM Self-help interventions for older smokers. Tob Control. 1997;6:188–193. doi: 10.1136/tc.6.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimer BK, Orleans CT, Fleisher L, et al Does tailoring matter? The impact of a tailored guide on ratings and short-term smoking-related outcomes for older smokers. Health Educ Res. 1994;9:69–84. doi: 10.1093/her/9.1.69. [DOI] [PubMed] [Google Scholar]
- 24.Zamboni M, Rubele S, Rossi AP Sarcopenia and obesity. Curr Opin Clin Nutr Metab Care. 2019;22:13–19. doi: 10.1097/MCO.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 25.Babio N, Becerra-Tomás N, Martínez-González MÁ, et al Consumption of yogurt, low-fat milk, and other low-fat dairy products is associated with lower risk of metabolic syndrome incidence in an elderly mediterranean population. J Nutr. 2015;145:2308–2316. doi: 10.3945/jn.115.214593. [DOI] [PubMed] [Google Scholar]
- 26.Dalsgaard E, Vestergaard M, Skriver MV, et al Socioeconomic position and cardiovascular risk factors among people with screen-detected Type 2 DM: six-year follow-up of the ADDITION-Denmark trial. Prim Care Diabetes. 2014;8:322–339. doi: 10.1016/j.pcd.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Ahmadi S, Streja E, Zahmatkesh G, et al Reverse epidemiology of traditional cardiovascular risk factors in the geriatric population. J Am Med Dir Assoc. 2015;16:933–939. doi: 10.1016/j.jamda.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babio N, Toledo E, Estruch R, et al Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ. 2014;186:E649–E657. doi: 10.1503/cmaj.140764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Pinto R, Ferri C Hypertension management at older age: an update. High Blood Press Cardiovasc Prev. 2019;26:27–36. doi: 10.1007/s40292-018-0290-z. [DOI] [PubMed] [Google Scholar]
- 30.Naschitz JE Blood pressure management in older people: balancing the risks. Postgrad Med J. 2018;94:348–353. doi: 10.1136/postgradmedj-2017-135493. [DOI] [PubMed] [Google Scholar]
- 31.Aprahamian I, Sassaki E, Dos Santos MF, et al Hypertension and frailty in older adults. J Clin Hypertens. 2018;20:186–192. doi: 10.1111/jch.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musini VM, Tejani AM, Bassett K, et al Pharmacotherapy for hypertension in adults 60 years or older. Cochrane Database Syst Rev. 2019;6:CD000028. doi: 10.1002/14651858.CD000028.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomopoulos C, Parati G, Zanchetti A Effects of blood pressure-lowering treatment on cardiovascular outcomes and mortality: 13 - benefits and adverse events in older and younger patients with hypertension: overview, meta-analyses and meta-regression analyses of randomized trials. J Hypertens. 2018;36:1622–1636. doi: 10.1097/HJH.0000000000001787. [DOI] [PubMed] [Google Scholar]
- 34.Garrison SR, Kolber MR, Korownyk CS, et al Blood pressure targets for hypertension in older adults. Cochrane Database Syst Rev. 2017;8:CD011575. doi: 10.1002/14651858.CD011575.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moraes AAI, Baena CP, Muka T, et al Achieved systolic blood pressure in older people: a systematic review and meta-analysis. BMC Geriatr. 2017;17:279. doi: 10.1186/s12877-017-0672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williamson JD, Supiano MA, Applegate WB, et al Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA. 2016;315:2673–2782. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bentley DC, Nguyen CH, Thomas SG Resting blood pressure reductions following handgrip exercise training and the impact of age and sex: a systematic review and narrative synthesis. Syst Rev. 2018;7:229. doi: 10.1186/s13643-018-0876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Mañas L, Laosa O, Vellas B, et al European MID-Frail Consortium. Effectiveness of a multimodal intervention in functionally impaired older people with type 2 diabetes mellitus. J Cachexia Sarcopenia Muscle. 2019;10:721–733. doi: 10.1002/jcsm.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller ME, Williamson JD, Gerstein HC, et al. Effects of randomization to intensive glucose control on adverse events, cardiovascular disease, and mortality in older versus younger adults in the ACCORD Trial Diabetes Care 2014; 37: 634-643.
- 40.Patel A, MacMahon S, Chalmers J, et al Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 41.Araki A, Limuro S, Sakurai T, et al Long-term multiple risk factor interventions in Japanese elderly diabetic patients: the Japanese Elderly Diabetes Intervention Trial-study design, baseline characteristics and effects of intervention. Geriatr Gerontol Int. 2012;12(Suppl 1):7–17. doi: 10.1111/j.1447-0594.2011.00808.x. [DOI] [PubMed] [Google Scholar]
- 42.Hamada S, Gulliford MC Mortality in individuals aged 80 and older with type 2 diabetes mellitus in relation to glycosylated hemoglobin, blood pressure, and total cholesterol. J Am Geriatr Soc. 2016;64:1425–1431. doi: 10.1111/jgs.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yau CK, Eng C, Cenzer IS, et al Glycosylated hemoglobin and functional decline in community-dwelling nursing home-eligible elderly adults with diabetes mellitus. J Am Geriatr Soc. 2012;60:1215–1221. doi: 10.1111/j.1532-5415.2012.04041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanagita I, Fujihara Y, Eda T, et al Low glycated hemoglobin level is associated with severity of frailty in Japanese elderly diabetes patients. J Diabetes Investig. 2018;9:419–425. doi: 10.1111/jdi.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaslavsky O, Walker RL, Crane PK, et al Glucose levels and risk of frailty. J Gerontol A Biol Sci Med Sci. 2016;71:1223–1229. doi: 10.1093/gerona/glw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson JM, Dufraux K, Cook PF The relationship between glycemic control and falls in older adults. J Am Geriatr Soc. 2007;55(12):2041–2044. doi: 10.1111/j.1532-5415.2007.01430.x. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz AV, Vittinghoff E, Sellmeyer DE, et al. Health, aging, and body composition study. diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care 2008; 31(3): 391−396. Erratum in: Diabetes Care 2008; 31: 1089.
- 48.Gómez-Huelgas R, Gómez Peralta F, Rodríguez Mañas L, et al Treatment of type 2 diabetes mellitus in elderly patients. Rev Esp Geriatr Gerontol. 2018;53:89–99. doi: 10.1016/j.regg.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Robinson JG, Jayanna MB, Bairey Merz CN, Stone NJ Clinical implications of the log linear association between LDL-C lowering and cardiovascular risk reduction: Greatest benefits when LDL-C >100 mg/dl. PLoS One. 2020;15:e0240166. doi: 10.1371/journal.pone.0240166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gencer B, Marston NA, Im K, et al Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2020;396:1637–1643. doi: 10.1016/S0140-6736(20)32332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mach F, Baigent C, Catapano AL, et al 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 52.Cholesterol Treatment Trialists' Collaboration Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–415. doi: 10.1016/S0140-6736(18)31942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ewing JA Detecting alcoholism: the CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.1984.03350140051025. [DOI] [PubMed] [Google Scholar]
- 54.O'Connell H, Chin AV, Cunningham C, Lawlor B Alcohol use disorders in elderly people-redefining an age-old problem in old age. BMJ. 2003;327:664–667. doi: 10.1136/bmj.327.7416.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haighton C, Wilson G, Ling J, et al A qualitative study of service provision for alcohol related health issues in mid to later life. PLoS One. 2016;11:e0148601. doi: 10.1371/journal.pone.0148601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blow FC, Brower KJ, Schulenberg JE, et al The Michigan Alcoholism Screening Test-geriatric version (MAST-G): a new elderly-specific screening instrument. Alcohol Clin Exp Res. 1992;16:372–374. [Google Scholar]
- 57.León-Muñoz LM, Galán I, Donado-Campos J, et al Patterns of alcohol consumption in the older population of Spain, 2008-2010. J Acad Nutr Diet. 2015;115:213–224. doi: 10.1016/j.jand.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 58.Ferreira MP, Weems MK Alcohol consumption by aging adults in the United States: health benefits and detriments. J Am Diet Assoc. 2008;108:1668–1676. doi: 10.1016/j.jada.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 59.Kalla A, Figueredo VM Alcohol and cardiovascular disease in the geriatric population. Clin Cardiol. 2017;40:444–449. doi: 10.1002/clc.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perissinotto E, Buja A, Maggi S, et al Alcohol consumption and cardiovascular risk factors in older lifelong wine drinkers: the Italian Longitudinal Study on Aging. Nutr Metab Cardiovasc Dis. 2010;20:647–655. doi: 10.1016/j.numecd.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 61.Djoussé L, Biggs ML, Mukamal KJ, Siscovick DS Alcohol consumption and type 2 diabetes among older adults: the Cardiovascular Health Study. Obesity (Silver Spring) 2007;15:1758–1765. doi: 10.1038/oby.2007.209. [DOI] [PubMed] [Google Scholar]
- 62.Veronese N Frailty as Cardiovascular Risk Factor (and Vice Versa) Adv Exp Med Biol. 2020;1216:51–54. doi: 10.1007/978-3-030-33330-0_6. [DOI] [PubMed] [Google Scholar]
- 63.Crome P, Cherubini A, Oristrell J The PREDICT (increasing the participation of the elderly in clinical trials) study: the charter and beyond. Expert Rev Clin Pharmacol. 2014;7:457–468. doi: 10.1586/17512433.2014.922864. [DOI] [PubMed] [Google Scholar]
- 64.Aldea-Perona AM, García Saiz MDM, Fernandez Quintana E, et al Utilidad de la ficha técnica de los medicamentos como herramienta para mejorar la prescripción en pacientes mayores. Rev Esp Geriatr Gerontol. 2020;55:156–159. doi: 10.1016/j.regg.2020.01.002. [DOI] [PubMed] [Google Scholar]