ABSTRACT
Perioperative blindness, especially posterior ischemic optic neuropathy (PION), is an uncommon but potentially devastating complication. We report a case of a 65-year-old male patient who underwent laryngopharyngectomy, bilateral neck dissection, and free jejunum flap reconstruction, but then experienced PION in his right eye following postoperative bleeding and bilateral internal jugular veins (IJVs) compression. Despite systemic corticosteroid therapy, his visual recovery prognosis was poor. The specific mechanism responsible for PION remains unclear, and no therapy has been shown to improve this condition. As such, prevention of perioperative PION remains the only available strategy. Surgeons should be aware of this rare potential complication and its risk factors and strive to avoid it. As postoperative bleeding and IJV compression are one of important risk factors for PION, avoiding these are critical.
Key Words: ischemic optic neuropathy, blindness, hematoma, postoperative bleeding, internal jugular vein
INTRODUCTION
Perioperative blindness following general anesthesia during nonocular surgery is a rare but potentially devastating complication. Lesions can occur at any portion of the visual pathways, from the corneas to the occipital cortex. The most common site of injury is the optic nerve, and the most frequently reported cause of permanent perioperative visual loss is ischemic optic neuropathy.1 The type of ischemic optic neuropathy depends on the lesion site; anterior ischemic optic neuropathy is caused by ischemia of the optic nerve head, and posterior ischemic optic neuropathy (PION) is caused by ischemia of the posterior segment of the optic nerve.2
Causal relationships have not been established for the majority of hypothesized risk factors of perioperative PION. Among these factors, however, hypotensive disorder is particularly important.2 And internal jugular vein (IJV) ligation is sometimes said to be a risk factor.1,3-5 Given its relationship with them, postoperative bleeding and IJV compression are also risk factors, but no previous reports of PION following each or both of them exist in the literature. This following is the first reported case of PION following them.
CASE REPORT
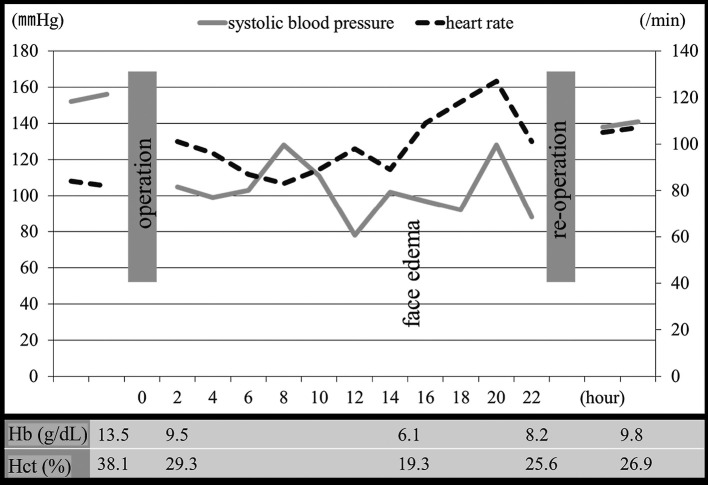
A 65-year-old man was referred to our department complaining of hoarseness. He had a history of hypertension. An examination revealed a squamous cell carcinoma of the hypopharynx, staged as T4N0M0. Chemotherapy did not affect the tumor, so he underwent surgery, which was performed under general anesthesia in the supine position with hyperextension of the cervical spine. The procedure comprised a laryngopharyngectomy, bilateral neck dissection levels II–V with preservation of the bilateral IJVs (Fig. 1), and reconstruction with the free jejunum flap. The jejunum flap was anastomosed end-to-end to the right superior thyroid artery and end-to-side to the right IJV. Surgery lasted 13.5 hours, and total blood loss was 740 g. The lost blood volume was replaced with packed red blood cells and plasma volume expanders. Preoperative systolic blood pressure was approximately 150 mmHg and the lowest was 80 mmHg for several minutes. Though three other similar hypotensive episodes of this level occurred, most of the systolic blood pressure was kept over 100 mmHg during surgery. Hematocrit dropped from 38.1% preoperatively to 29.3% postoperatively and hemoglobin from 13.5 g/dL to 9.5 g/dL.
Fig. 1.
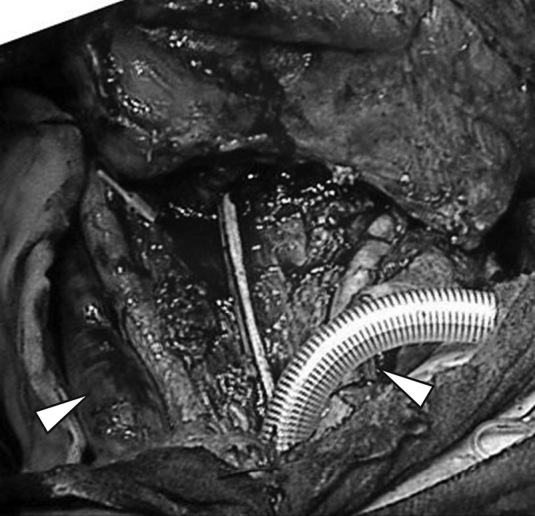
Operative findings
Laryngopharyngectomy and bilateral neck dissection levels 2–5 with preservation of bilateral IJVs (white arrows) were performed.
The patient received intensive postoperative care, sedation, and artificial respiration. Postoperative systolic blood pressure was maintained around 100 mmHg, but dropped to 60 mmHg, and patient pulse rate increased in the afternoon of postoperative day 1. They were controlled by reducing sedative drug and infusion. Severe generalized facial and neck edema followed several hours later. Complete blood count confirmed decreases in hematocrit (19.3%) and hemoglobin (6.1 g/dL). Though the postoperative bleeding was supposed, examination of the neck and the finding of drain output were negative around this time. We diagnosed some were effect of blood dilution caused by infusion. As the time went by, vital sign became unstable and the blood transfusion was needed. However, as hypouresis and hyperkalemia made blood transfusion difficult, hemodialysis was planned. Around this time, though examination of patient was still the same, the possibility of bleeding somewhere was supposed and whole body contrast enhanced computed tomography was checked. It revealed a large hematoma in the bilateral neck and the preserved bilateral IJVs were not being able to be identified at the hematoma existing level (Fig. 2). Release of the hematoma at bedside and reoperation was performed immediately. The large hematoma and bleeding from the anastomosed artery obstructed the bilateral IJVs. The removal of the hematoma decompressed the IJVs, and hemostasis was achieved with one stitch of 10-0 nylon. The free jejunum showed adequate perfusion throughout the reoperation probably because the right IJV might be partially occluded by the hematoma, and its removal improved perfusion in the right IJV. During the reoperation the new blood loss was little and vital signs were stable. Postoperatively facial edema was relieved. Perioperative time-related events, vital signs, hemoglobin, and hematocrit levels are displayed in Fig. 3.
Fig. 2.
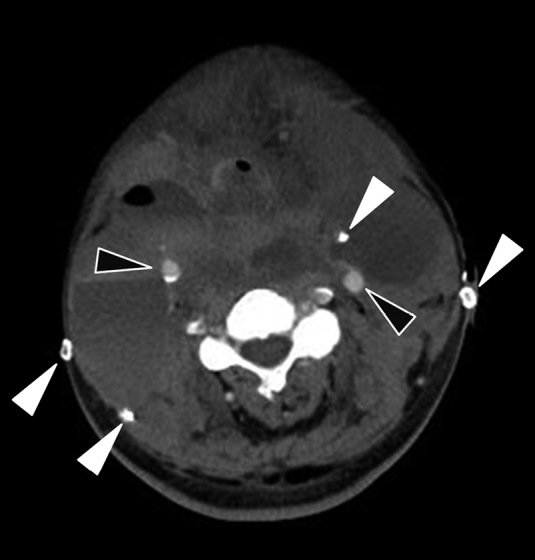
Contrast enhanced computed tomography of the neck hematoma
Axial contrast enhanced computed tomography of the neck showing a large hematoma in the bilateral neck. Although bilateral common carotid arteries (black arrows) and drain tubes (white arrows) can be identified, the preserved bilateral IJVs cannot be identified at this level.
Fig. 3.
Graph of perioperative time-related events, vital signs, hemoglobin, and hematocrit levels
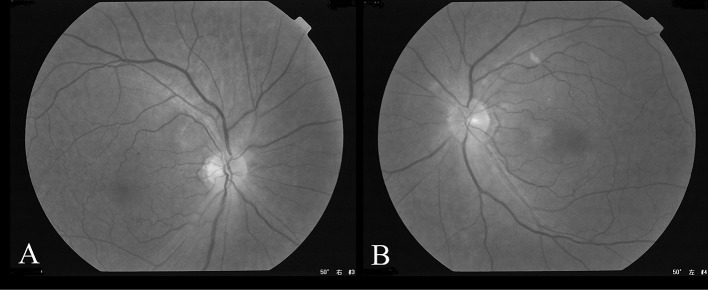
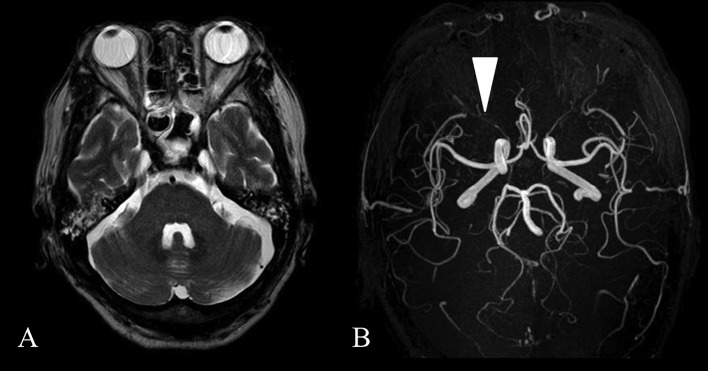
The patient received sedation and artificial respiration post-reoperation and was extubated on postoperative day 2. He was slightly drowsy on that day and next day he became more alert and complained of decreased vision in his right eye. The right eye lacked afferent pupillary reflex which was normal before that day. So we referred him for an ophthalmologic evaluation. This revealed visual acuity of light perception in his right eye and 20/20 vision in his left eye with normal intraocular pressures. The fundus examination was unremarkable and revealed a lack of optic disc edema (Fig. 4). Magnetic resonance imaging was normal and edema, occipital lobe infarction and intracranial mass were excluded. Magnetic resonance angiography showed unaltered perfusion of all major feeding arteries to the brain and the ophthalmic arteries were found to be patent (Fig. 5). Other readily identifiable causes of the visual deficit were excluded and a diagnosis of PION was made.
Fig. 4.
Fundus examination
The examination for both the right (A) and left (B) fundus showed neither optic disc edema nor any other abnormalities.
Fig. 5.
Magnetic resonance analysis
Fig. 5A: The brain magnetic resonance imaging showed no abnormalities.
Fig. 5B: Magnetic resonance angiography showed unaltered perfusion of all major feeding arteries to the brain. The right ophthalmic artery was patent (white arrow).
Despite treatment with oral prednisone 1 mg/kg/day for more than two months, visual acuity remained at the level of light perception in the right eye. One year postoperatively, no improvement was noted in his right eye. However, the free jejunum was functioning accordingly, and the patient was able to resume his normal diet.
DISCUSSION
While both anterior ischemic optic neuropathy and PION are manifestations of decreased oxygen delivery to the optic nerve, their pathophysiologic entities differ markedly. It is reasonable to assume that the pathophysiologic mechanisms, clinical settings, and presentation would differ as well.6,7
PION has three different subtypes depending on the clinical situation in which it occurs: perioperative, arteritic, and nonarteritic.3 A review of perioperative PION cases revealed that reports of PION are most common following spinal surgery (54.2%) and radical neck dissection (13.3%).1,3 Incidences after spinal surgery and neck dissection are reportedly 0.087% and 0.08%, respectively.8,9
Decreased oxygen delivery can result from poor oxygen levels in circulating blood, reduced arterial perfusion pressure, or increased resistance to blood flow.1,3 Many potential risk factors have been proposed, and include anemia due to perioperative blood loss, hemodynamic derangements including hypotension, hypotensive periods, prolonged surgical times, large fluid replacement, and use of vasoconstricting agents, among others.1,3,10-12 Systemic disorders such as hypertension, diabetes mellitus, and atherosclerotic disease are also included, as these disorders may alter proper autoregulation, leading to decreased blood flow.3,10 However, though those are not so rare among many surgical procedures, the kind of surgery PION occurs is partial to mentioned above. So some additional risk factor particular to those kinds of surgery may exist. In case of spinal surgery head-down and prone position and in patients with neck dissection conditions ligation of the IJV may be implicated in ischemic optic neuropathy.1,3-5 They may cause venous hypertension and compromise arterial perfusion. However, causal relationships have not been demonstrated, and other factors, including those described above, are required for PION to occur.1,5,11 And though most of the risk factors are systemic, 39.1% of patients were hemi-lateral involvement like ours.3
For the present case, hypertension, prolonged surgical times, large perioperative blood loss and fluid replacement were the important risk factors.
However, bilateral IJVs were preserved and vital signs or anemia was strictly controlled during surgery for micro surgical anastomosis. On the other hand, rapid postoperative bleeding caused anemia, hypotension and the hematoma that temporarily obstructed the bilateral IJVs. The delay of diagnosis and limitation of blood transfusion prolonged the risky situation. Therefore, the ischemic injury was multifactorial. However, the obstruction of the bilateral IJVs seemed to be a lethal factor. Although the detailed mechanism is unclear, we believe that the increased intracranial pressure caused by the compression of the bilateral IJV compromised the perfusion to the optic nerve. In the present case, there was an interval of 7 hours from the development of facial and neck swelling to the release of hematoma and reoperation. If exploratory incision was done immediately after neck swelling was noted, PION might have been prevented.
In PION, the visual loss generally occurs immediately after surgery and progresses throughout the next few days, although it may be delayed by several days.3 It is painless and symptoms are often subtle, compared to other optical emergencies.2 Most surgeons are unfamiliar with this condition, and may either misdiagnose it as delirium upon awakening from anesthesia, or miss it in sedated patients.2 The timing of visual loss of our patient could not be accurately assessed due to his sedated state. Considering the timing of pupillary reflex was injured and the timing of risk events, we surmise that the PION must have occurred in early times following postoperative bleeding.
Diagnostic imaging shows no abnormalities, so PION diagnoses are typically made after excluding other causes of visual deficit, which takes time.3,6 In the present case, we began treatment when we suspected PION, but the final outcome was still poor. Though various therapies have been proposed, none have unequivocally shown significant promise and most reported prognoses for visual recovery are poor.1,3 A review of perioperative PION noted that in 75.8% of eyes maintained only counting fingers vision or worse.1,3
Prevention of perioperative PION should be the ultimate strategy.1 So surgeons should be aware of this rare but potential complication. Knowledge of PION pathogenesis increases awareness and identification of risk factors, which will help surgeons avoid these complications.2,12 Preventive measures include consideration of early transfusion for marked anemia, conservative fluid replacement followed by vigorous diuresis to prevent venous congestion, and strict control of blood pressure, among others.3,5,11 Postoperative bleeding and hematoma, particularly in the neck region, should be diagnosed and treated early as it highly influences the occurrence of PION.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest concerning this article.
Abbreviations
- PION
posterior ischemic optic neuropathy
- IJV
internal jugular vein
REFERENCES
- 1.Newman NJ. Perioperative visual loss after nonocular surgeries. Am J Ophthalmol. 2008;145(4):604-610. doi: 10.1016/j.ajo.2007.09.016. [DOI] [PMC free article] [PubMed]
- 2.Agostini T, Lazzeri D, Agostini V, Mani R, Shokrollahi K. Ischemic optic neuropathy and implications for plastic surgeons: report of a new case and review of the literature. Ann Plast Surg. 2011;66(4):416-420. doi: 10.1097/SAP.0b013e3181f9b25e. [DOI] [PubMed]
- 3.Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50(1):15–26. doi: 10.1016/j.survophthal.2004.10.005. [DOI] [PubMed]
- 4.Schobel GA, Schmidbauer M, Millesi W, Undt G. Posterior ischemic optic neuropathy following bilateral radical neck dissection. Int J Oral Maxillofac Surg. 1995;24(4):283–287. doi: 10.1016/s0901-5027(95)80030-1. [DOI] [PubMed]
- 5.Pazos GA, Leonard DW, Blice J, Thompson DH. Blindness after bilateral neck dissection: case report and review. Am J Otolaryngol. 1999;20(5):340–345. doi: 10.1016/s0196-0709(99)90039-x. [DOI] [PubMed]
- 6.Sadda SR, Nee M, Miller NR, Biousse V, Newman NJ, Kouzis A. Clinical spectrum of posterior ischemic optic neuropathy. Am J Ophthalmol. 2001;132(5):743–750. doi: 10.1016/s0002-9394(01)01199-0. [DOI] [PubMed]
- 7.Takayama K, Kaneko H, Kachi S, Ra E, Ito Y, Terasaki H. High-dose intravenous pulse steroid therapy for optic disc swelling and subretinal fluid in non-arteritic anterior ischemic optic neuropathy. Nagoya J Med Sci. 2017;79(1):103–108. doi: 10.18999/nagjms.79.1.103. [DOI] [PMC free article] [PubMed]
- 8.Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine(Phila Pa 1976). 1997;22(12):1319–1324. doi: 10.1097/00007632-199706150-00008. [DOI] [PubMed]
- 9.Balm AJ, Brown DH, De Vries WA, Snow GB. Blindness: a potential complication of bilateral neck dissection. J Laryngol Otol. 1990;104(2):154–156. doi: 10.1017/s0022215100112149. [DOI] [PubMed]
- 10.Dunker S, Hsu HY, Sebag J, Sadun AA. Perioperative risk factors for posterior ischemic optic neuropathy. J Am Coll Surg. 2002;194(6):705–710. doi: 10.1016/s1072-7515(02)01210-3. [DOI] [PubMed]
- 11.Roth S. Perioperative visual loss: what do we know, what can we do? Br J Anaesth. 2009;103(Suppl 1):i31-i40. doi: 10.1093/bja/aep295. [DOI] [PMC free article] [PubMed]
- 12.Rath EZ, Falick Y, Rumelt S. Posterior ischemic optic neuropathy following breast augmentation and abdominal liposuction. Can J Ophthalmol. 2009;44(3):346–347. doi: 10.3129/i09-060. [DOI] [PubMed]