ABSTRACT
Zinc is an important trace element, and its importance for male infertility has been reported. The aim of the study was to assess whether the serum zinc concentrations were related to semen quality in male infertility patients. In 2010 subjects who consulted at our male infertility clinic between November 2018 and May 2021, serum zinc concentrations were assessed along with age, sperm concentration, sperm motility, endocrine panel, and body mass index (BMI). A normal zinc concentration was observed in 1069 (53.2%), subclinical deficiency in 845 (42.0%), and deficiency in 79 subjects (3.9%). On the other hand, high a zinc level was observed in only 17 subjects (0.9%). The serum zinc concentration did not relate with age, sperm concentration, sperm motility, luteinizing hormone (LH), follicle stimulating hormone (FSH), testosterone, and body mass index (BMI). However, normozoospermic subjects showed significantly higher zinc concentrations than among azoospermic (included non-obstructive; NOA and obstructive; OA) and cryptozoospermic patients. Furthermore, the zinc concentration was lower in NOA subjects when comparing to oligozoospermia and/or asthenozoospermia. An association between zinc concentration and semen analysis remained unclear. This study was cross-sectional and retrospective, however, this is a largest investigation of the zinc concentration during reproductive life span in Japan. Further accumulation of cases are required to further examine the potential relationship between zinc concentration and semen quality.
Key Words: serum zinc concentration, male infertility, semen quality, azoospermia
INTRODUCTION
Zinc is a the second most abundant trace element in the human body which cannot be stored, and plays an important role in over 300 enzymes as well as the onset of action of over 1000 transcription factors in vivo. The distribution of zinc in the human body is mainly in bone, eyeball, liver, muscle, kidney, prostate, spleen, and minimally observed in blood.1 Zinc plays unique roles in males and acts as a hormone balancer for testosterone, prostate in sexual health and functions as well as an antibacterial agent in the male reproductive system. Zinc deficiency impedes spermatogenesis and sperm quality, and has a negative effect on serum testosterone concentrations.2 A symptom of the zinc deficiency is widely documented such as lack of taste, stomatitis, and the developmental disability of children. Recently zinc deficiency may relate with low birth weight.3 Whilst the importance of zinc has been recognized, its evaluation in male infertility is limited. We therefore assessed the serum zinc concentration in subjects who visited our male infertility division and investigated whether zinc concentration related to semen quality.
MATERIALS AND METHODS
In total, 2378 subjects were visited to our male infertility unit between November 2018 and May 2021. Of these, 2010 cases were included in this study. Serum zinc concentration was assessed along with age, sperm concentration, sperm motility, luteinizing hormone (LH), follicle stimulating hormone (FSH), testosterone, and body mass index (BMI). According to the 2021 WHO criteria,4 oligozoospermia is defined as less than 15 million/mL of sperm concentration. Asthenozoospermia is defined as <42% sperm motility or less than 30% with progressive motility. On the other hand, cryptozoospermia is defined as a type of low sperm count where ejaculated semen contains less than 100,000 spermatozoa per ml. With cryptozoospermia, the sperm count may fluctuate and a zero sperm count in the ejaculate may be initially measured. Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics of 2010 subjects
| 5% | median (interquartile range) | 95% | |
| Age (year) | 28.0 | 36.0 (32–40) | 48.0 |
| Spouse age (year) | 27.0 | 35.0 (31–38) | 43.0 |
| Duration of infertility (year) | 0.5 | 2.0 (1–4) | 8.0 |
| Testicular volume (right/left) mL | 5.0/4.0 | 16 (12–20) / 14 (10–18) | 24.0 / 22.0 |
| LH mIU/mL | 1.9 | 4.50 (3.23–6.10) | 9.9 |
| FSH mIU/mL | 2.1 | 4.70 (3.40–6.90) | 20.2 |
| Testosterone ng/mL | 2.1 | 4.50 (3.40–5.79) | 7.9 |
| BMI kg/m2 | 18.9 | 23.0 (21.2–25.2) | 29.4 |
Each data was expressed as median (interquartile range), and 5 and 95 percentiles representing the tails of the distribution were installed.
LH: luteinizing hormone
FSH: follicle stimulating hormone
BMI: body mass index
Statistical analysis
Serum zinc concentration categories were defined following criteria based on the treatment guidelines of zinc deficiency published by the Japanese Society of Clinical Nutrition: deficiency (<60 μg/dL), subclinical deficiency (≥60 to <80 μg/dL), and normal (≥80 μg/dL).1 Mann–Whitney U test was used for the comparison of the zinc concentration measured in the morning and the afternoon. On the other hand, Steel-Dwass test was employed for the comparison of the zinc concentration for every diagnosis result. The coefficient of correlation was judged by Spearman’s rank correlation coefficient. A P-value < 0.05 was considered as statistically different. All the statistical analyses were performed using statistical analysis software R (https://www.r-project.org/).
RESULTS
Semen analysis revealed that normozoospermia was apparent in 1124 (55.9%) subjects, oligozoospermia and/or asthenozoospermia in 625 (31.1%), non-obstructive azoospermia (NOA) in 109 (5.4%), cryptozoospermia in 96 (4.8%), obstructive azoospermia (OA) in 40 (2.0%), along with other categories including 16 (0.9%; ejaculatory dysfunction (EjD) n=12; spinal cord injury (3), retrograde ejaculation due to diabetes mellitus (5) and after posterior urethral valve incision (1), and unknown (3), respectively, male hypogonadotropic hypogonadism (MHH) n=4).
The 1069 subjects (53.2%) showed normal zinc concentration. Subclinical zinc deficiency was found in 845 (42.0%), and zinc deficiency was in 79 (3.9%) subjects. A high concentration of zinc was found in only 17 (0.9%) subjects. Of these who showed high zinc concentrations, only one patient was prescribed zinc containing supplement for three months. The results of zinc concentration of 2010 subjects were shown in Table 2. Overall, the mean zinc concentration value was 81 μg/dL and a histogram and box mustache of zinc is shown in Figure 1. When the blood collecting time value was compared morning or afternoon, the zinc concentration value was decreased about 20 μg/dL in the afternoon. This difference was significant. (P<0.005) (Figure 2)
Table 2.
The results of zinc concentration of 2010 subjects
| n (%) | |
| Deficiency (<60 μg/dL) | 79 (3.9) |
| Subclinical deficiency (60–79 μg/dL) | 845 (42.0) |
| Normal (80–130 μg/dL) | 1069 (53.2) |
| High value (>131 μg/dL) | 17 (0.9) |
The standard value of following zinc depends on the medical treatment guidelines 2018.1
Fig. 1.
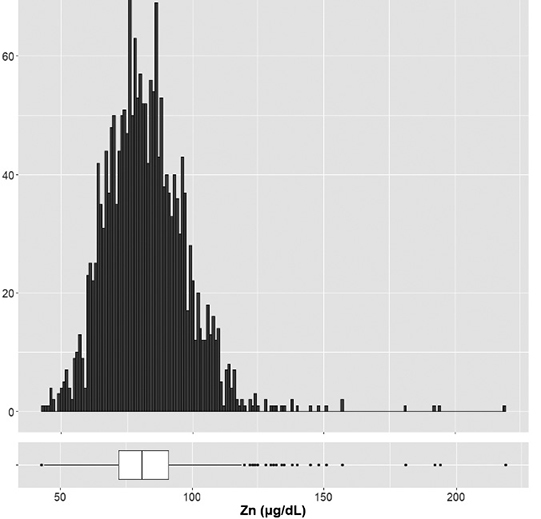
Histogram and box mustache of zinc concentrations
The box mustache of zinc concentrations was expressed median value with interquartile range, and median value was 81μ/dL.
Fig. 2.
The difference in zinc concentration by the blood drawing time
The zinc concentration value was decreased about 20μg/dL in the afternoon. This difference was statistically significant. (P<0.005)
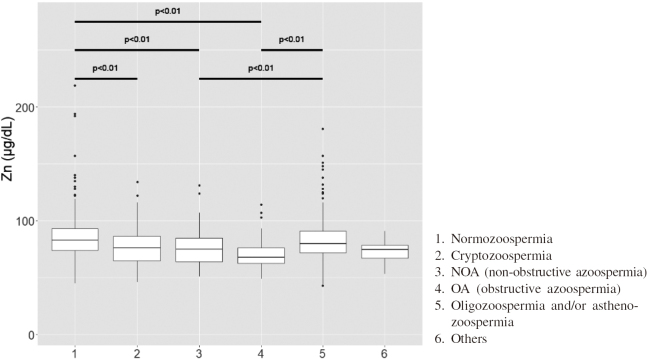
Although the zinc concentration was evaluated at the same time with sperm concentration and sperm motility, there were no relationships. (Figure 3) Furthermore, the coefficient of correlation between age, sperm concentration, sperm motility, LH, FSH, testosterone, zinc concentration, and BMI were evaluated and no significant differences were found. The zinc concentration was compared within each diagnosis. The zinc concentration was statistically lower in cryptozoospermia, NOA, and OA subjects when compared with normozoospermic subjects. (P<0.01) Oligozoospermia and/or asthenozoospermia subjects showed statistically higher value of zinc when compared NOA and OA subjects. (P<0.01) (Figure 4)
Fig. 3.

The zinc concentration compared with sperm concentration and sperm motility
There was no difference between the zinc concentration and semen quality.
Fig. 4.
Zinc concentrations compared among diagnoses
The zinc concentration was significantly lower value in NOA and OA when compared with normozoospermia. (P<0.01) Furthermore, NOA compared with oligozoospermia and/or asthenozoospermia, the zinc concentration was statistically decreased in NOA. (P<0.01)
DISCUSSION
Zinc is an essential trace element, next to iron in the human body and biological half-life is about 280 days. As for the subject who did not eat meat and fish proteins enough, zinc deficiency can easily happen.5 In one study, a comparison of zinc levels was done in Japanese faculty of liberal arts students (with less knowledge about dietary needs) and medical department students (higher knowledge), medical department students had higher serum zinc concentration. The mean value of serum zinc was 67 μg/dL for the liberal arts student, and 103 μg/dL for the medical department student.6 Reduced absorption of zinc from the bowels may be caused by excessive coffee, orange juice, calcium, and alcohol intake.1 Excretion of zinc may increase in subjects having with liver disease (chronic hepatitis, cirrhosis), diabetes, kidney disease, dialysis from dialytic fluid, leading to zinc deficiency. In addition, medicine for rheumatoid arthritis, Parkinson’s disease, gout, diabetes, insomnia, depression, epilepsy may also accelerate excretion of zinc. According to a large Japanese study, the proportions of subjects with zinc deficiency and marginal deficiency were 0.4–0.6% and 38.4–46.0%.7 The deficiency/ marginal deficiency group had significantly lower lipid profiles and nutritional status. They concluded that these findings suggest a possible association between serum zinc levels and nutritional status and health behaviors.7 Malnutrition may occur in elderly people. A cross-sectional, population-based survey revealed that about 10% subjects aged 65–87 years showed zinc deficiency.8 Deficiency of zinc may cause infertility during reproductive age, even in a physically unimpaired subject without any symptoms.9 Thus, several possible factors are related to zinc deficiency, including malnutrition, alcohol consumption, and zinc supplementation status.10
Human semen contains several trace elements such as calcium, copper, manganese, magnesium, selenium, and zinc which are necessary for reproductive health. Zinc is essential for the maintenance of germ cells, the progression of spermatogenesis, and the regulation of sperm motility.10 Zinc accumulates in germ cells, particularly in the mitochondria of spermatogonia and spermatozoa. The negative correlation between seminal plasma zinc and sperm viability is a clear indicator of the importance of zinc in spermatogenesis.11 Using an in vitro testicular organ culture system from the Japanese eel, zinc deficiency was induced apoptosis of the germ cells. This cell death was rescued by the addition of zinc to the cultures. The zinc concentration in human seminal plasma is higher than in other tissues.12 It has been suggested that zinc acts as an important anti-inflammatory factor and that it is involved in oxidative metabolism of sperm. At the end of spermatogenesis, zinc is highly concentrated in the tail of mature spermatozoa and involved in sperm motility.12
Zinc is also known to be essential for sexual maturity and onset of estrus. Zinc is needed for the normal functioning of the hypothalamus-pituitary-gonadal axis. Low zinc levels have a negative effect on serum testosterone concentration.13 Zinc has many important functions in the spermatozoa physiology, including effects on lipid flexibility and sperm membrane stabilization.14 It also has a regulated role in capacitation and the acrosome reaction of sperm and is essential for conception and embryonic implantation.15 Poor zinc nutrition may be a risk factor for low quality of sperm in idiopathic male infertility. Thus, zinc is important trace element for the male infertility in several different reason. Mirnamniha et al emphasized that measurement of trace elements in men with idiopathic infertility is necessary.16
Previous reports of serum zinc concentrations, in Japanese men younger than 40 years are limited number (n=82), with a mean of zinc level of about 85 μg/dL.7 In 217 cases of Japanese men during their reproductive age, median serum zinc was 86.3 μg/dL.17 These values were higher than our 2010 cases including 1069 normozoospermia. The reason may be related to the fact that our subjects were all suffering from male infertility. A systematic review and meta-analysis revealed that age was statistically significantly associated with declines in semen quality, including semen volume and sperm motility.9 On the other hand, Tsujimura et al reported that the correlation between zinc concentration and sperm count, and motility was not significant.17 Their subjects excluded azoospermia. In our study, the zinc concentration was statistically lower in cryptozoospermia, NOA, and OA subjects when compared with normozoospermic subjects. Oligozoospermia and/or asthenozoospermia subjects showed statistically higher values of zinc when compared with NOA and OA. OA is usually a reversible pathophysiology and many sperm exist in epididymis and testis. NOA is progressive and an irreversible pathophysiology. Curiously, both pathophysiology showed decreased zinc concentrations when compared with normozoospermia. The reason remains unclear. Thus, correlation serum zinc concentration and semen quality is still controversial.
The change of zinc concentration in the day is remarkable, and its level gradually decreases from the morning through the afternoon. Even the stress such as an operation may greatly decrease its value. It rises when hungry, with about 20% decrease after a meal 2–3 hours after meal. It is desirable therefore to collect blood on an empty stomach and early in the morning to avoid a risk of reporting a lower zinc concentration level.1 Zinc concentration was obviously high about 20 μg/dL in the morning than in the afternoon in our study.
There are some limitations in this study. Recently it was found that results showing normal or abnormal findings were different between the first and second semen tests in approximately one-quarter of men,18 however, we evaluated the semen analysis only once. Furthermore, about 30% of subjects received blood sampling during the afternoon. Although absorption of zinc from the bowels is disturbed by an excessive intake of such as coffee or orange juice, we did not examine the intake situation of the meal and drinks. These could influence the difference of zinc concentration. Although this study was cross-sectional and retrospective, serum zinc was assessed for the 2,010 male infertility patients. This is a largest investigation of zinc concentrations during reproductive age in Japanese men. Further follow up of zinc concentrations taken at similar times and conditions are required.
CONCLUSIONS
Zinc deficiency/ marginal deficiency were observed in 3.9%/42.0% in our male infertility unit. Although zinc concentrations did not have any relation to age, endocrine panel, and BMI, correlation between zinc concentration and deteriorated sperm count and motility was statistically significant. This study was cross-sectional and retrospective, however, this is a largest investigation of zinc concentration for reproductive age in Japan. It is necessary to continue the evaluation of zinc sequentially during a male’s reproductive age.
ACKNOWLEDGEMENT
The authors thank all of the members of the IVF Laboratory team of the Asada Ladies Clinic and the staff of the Asada Fertility Research Center, Nagoya, Japan.
DISCLOSURES
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines, and written informed consent was obtained from all participants.
ETHICAL APPROVAL
The protocol for this research project, including its use of human subjects, was approved by a suitably constituted Ethics Committee. (Our approval number: 2021-01, Date of approval by the Ethical Review Committee: 2021/2/19)
CONFLICT OF INTEREST
Each author has no COI with regard to this manuscript.
PREVIOUS PRESENTATION
This paper was presented at 66th Meeting of Japan Society for Reproductive Medicine held at Yonago, November 11–12, 2021.
Abbreviations
- NOA
non-obstructive azoospermia
- OA
obstructive azoospermia
- EjD
ejaculatory dysfunction
- MHH
male hypogonadotropic hypogonadism
REFERENCES
- 1.Kodama H, Itakura H, Ohmori H, et al. Practice guideline for zinc deficiency 2018 [in Japanese]. J Jpn Soc Clin Nutr. 2018;40(2):120–167.
- 2.Fallah A, Mohammad-Hasani A, Colagar AH. Zinc is an essential element for male fertility: A review of Zn roles in men’s health, germination, sperm quality, and fertilization. J Reprod Infertil. 2018;19(2):69–81. [PMC free article] [PubMed]
- 3.Shankar H, Kumar N, Sandhir R, et al. Association of dietary intake below recommendations and micronutrient deficiencies during pregnancy and low birthweight. J Perinat Med. 2019;47(7):724–731. doi: 10.1515/jpm-2019-0053. [DOI] [PubMed]
- 4.Biotrelle F, Shah R, Saleh R, et al. The sixth edition of the WHO manual for human semen analysis: A critical review and SWOT analysis. Life(Basel). 2021;11(12):1368. doi: 10.3390/life11121368. [DOI] [PMC free article] [PubMed]
- 5.Wada O. What are trace elements? -Their deficiency and excess states-. JMA J. 2004;47(8):351–358.
- 6.Tomita H, Tanaka M, Ikui A. Clinical standard for diagnosis of zinc deficiency by the serum zinc value on the basis of evidence [in Japanese]. Biomed Res Trace Elem. 2007;18(1):54–62. doi: 10.11299/brte.18.54. [DOI]
- 7.Yokokawa H, Fukuda H, Saita M, et al. Serum zinc concentrations and characteristics of zinc deficiency/ marginal deficiency among Japanese subjects. J Gen Fam Med. 2020;21(6):248–255. doi: 10.1002/jgf2.377. [DOI] [PMC free article] [PubMed]
- 8.Kvamme JM, Grønli O, Jacobsen BK, Florholmen J. Risk of malnutrition and zinc deficiency in community-living elderly men and women. Public Health Nutr. 2015;18(11):1907–1913. doi: 10.1017/S1368980014002420. [DOI] [PMC free article] [PubMed]
- 9.Johnson SL, Dunleavy, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: A systematic review and meta-analysis. Ageing Res Rev. 2015;19:22–33. doi: 10.1016/j.arr.2014.10.007. [DOI] [PubMed]
- 10.Skalny AV, Skalnaya MG, Grabeklis AR, Skalnaya AA, Tinkov AA. Zinc deficiency as a mediator of toxic effects of alcohol abuse. Eur J Nutr. 2018;57(7):2313–2322. doi: 10.1007/s00394-017-1584-y. [DOI] [PubMed]
- 11.Akinloye O, Abbiyesuku FM, Oguntibeju OO, Arowojolu AO, Truter EJ. The impact of blood and seminal plasma zinc and copper concentrations on spermogram and hormonal changes in infertile Nigerian men. Reprod Biol. 2011;11(2):83–98. doi: 10.1016/s1642-431x(12)60047-3. [DOI] [PubMed]
- 12.Cheah Y, Yang W. Functions of essential nutrition for high quality spermatogenesis. Adv Biosci Biotechnol. 2011;2(4):182–197. doi: 10.4236/abb.2011.24029. [DOI]
- 13.Hunt CD, Johnson PE, Herbel J, Mullen LK. Effects of dietary zinc depletion on seminal volume and zinc loss, serum testosterone concentrations, and sperm morphology in young men. Am J Clin Nutr. 1992;56(1):148–157. doi: 10.1093/ajcn/56.1.148. [DOI] [PubMed]
- 14.Chia SE, Ong CN, Chua LH, Ho LM, Tay SK. Comparison of zinc concentrations in blood and seminal plasma and the various sperm parameters between fertile and infertile men. J Androl. 2000;21(1):53–57. [PubMed]
- 15.Eggert-Kruse W, Zwick EM, Batschulat K, et al. Are zinc levels in seminal plasma associated with seminal leukocytes and other determinants of semen quality? Fertil Steril. 2002;77(2):260–269. doi: 10.1016/s0015-0282(01)02974-0. [DOI] [PubMed]
- 16.Mirnamniha M, Faroughi F, Tahmasbpour E, Ebrahimi P, Harchegani AB. An overview on role of some trace elements in human reproductive health, sperm function and fertilization process. Rev Environ Health. 2019;34(4):339–348. doi: 10.1515/reveh-2019-0008. [DOI] [PubMed]
- 17.Tsujimura A, Hiramatsu I, Miyoshi M, et al. Relationship between serum zinc concentration and semen quality in newly-wed men. Int J Urol. 2021;28(3):289–293. doi: 10.1111/iju.14448. [DOI] [PubMed]
- 18.Blickenstorfer K, Voelkle M, Xie M, Fröhlich A, Imthurn B, Leeners B. Are WHO recommendations to perform 2 consecutive semen analyses for reliable diagnosis of male infertility still valid? J Urol. 2019;201(4):783–791. doi: 10.1016/j.juro.2018.11.001. [DOI] [PubMed]