Abstract
The gut microbiota comprises hundreds of species with a composition shaped by the available glycans. The well-studied starch utilization system (Sus) is a prototype for glycan uptake in the human gut bacterium Bacteroides thetaiotaomicron (Bt). Each Sus-like system includes outer-membrane proteins, which translocate glycan into the periplasm, and one or more cell-surface glycoside hydrolases, which break down a specific (cognate) polymer substrate. Although the molecular mechanisms of the Sus system are known, how the Sus and Sus-like proteins cooperate remains elusive. Previously, we used single-molecule and super-resolution fluorescence microscopy to show that SusG is mobile on the outer membrane and slows down in the presence of starch. Here, we compare the dynamics of three glycoside hydrolases: SusG, Bt4668, and Bt1760, which target starch, galactan, and levan, respectively. We characterized the diffusion of each surface hydrolase in the presence of its cognate glycan and found that all three enzymes are mostly immobile in the presence of the polysaccharide, consistent with carbohydrate binding. Moreover, experiments in glucose versus oligosaccharides suggest that the enzyme dynamics depend on their expression level. Furthermore, we characterized enzyme diffusion in a mixture of glycans and found that noncognate polysaccharides modify the dynamics of SusG and Bt1760 but not Bt4668. We investigated these systems with polysaccharide mixtures and genetic knockouts and found that noncognate polysaccharides modify hydrolase dynamics through some combination of nonspecific protein interactions and downregulation of the hydrolase. Overall, these experiments extend our understanding of how Sus-like lipoprotein dynamics can be modified by changing carbohydrate conditions and the expression level of the enzyme.
Significance
The gut microbiota comprises hundreds of species with a composition shaped by the available nutrients. Although the prominent gut microbe Bacteroides thetaiotaomicron (Bt) can express a wide range of carbohydrate-active enzymes to grow on a diversity of substrates, how these different enzymes respond to their specific polymer substrates and to one another has remained unknown. Here, we measure the dynamics of three glycoside hydrolases on the surface of living Bt cells to find that, while each is immobilized in the presence of its cognate polysaccharide, noncognate polysaccharides can also modify the movement of these enzymes on the Bt cell surface. This mechanistic investigation of glycan breakdown is of particular importance to the design of prebiotic therapeutics that benefit human health.
Introduction
The gut microbiome plays key roles in human health. In addition to providing pathogen resistance and establishing and maintaining proper immune function, the gut microbiota captures and catabolizes carbohydrates that escape host digestive enzymes (1,2,3). The nature and complexity of the carbohydrate landscape in the intestine shapes the composition of this microbial community (4). Bacteria are the most numerically dominant group of microbes, and the most represented phyla are the Firmicutes and Bacteroidetes (5,6,7). Together, these species metabolize available carbohydrates, including host-derived glycans and dietary (plant-, animal-, and microbial-derived) glycans (3). This variety is immense and some complex glycans comprise numerous distinct monosaccharides and glycosidic linkages (3,8). Carbohydrate catabolism by these bacteria influences the pool of metabolites available to drive physiological changes (9). For example, short-chain fatty acids generated by carbohydrate fermentation support intestinal and systemic health (8). More generally, there is evidence that the gut microbiota members, and Bacteroidetes in particular, are associated with several metabolic diseases (10). Therefore, investigating the mechanisms of glycan breakdown is of particular importance to the design of prebiotic therapeutics that benefit human health (11).
Human gut Bacteroidetes contain a multitude of carbohydrate-active enzymes (CAZymes) (12). These CAZymes are encoded within gene clusters termed polysaccharide utilization loci (PULs), and most PULs encode a combination of periplasmic and outer-membrane enzymes, outer-membrane substrate-binding proteins, and an outer-membrane TonB-dependent transporter that facilitate the capture, degradation, and import of individual glycans (13). This repertoire of CAZymes allows Bacteroidetes to grow on a large range of substrates, making them carbohydrate generalists (14). PULs represent about 18% of the Bacteroides thetaiotaomicron (Bt) genome (15), and many Bt PUL-encoded proteins have been extensively investigated (8,16,17). The prototypical PUL is the Bt starch utilization system (Sus), which encodes five cell-surface exposed outer-membrane proteins, SusCDEFG (18,19). SusG is an α-amylase in the glycoside hydrolase family 13 that hydrolyzes α-1,4 glycosidic bonds and is required for Bt growth on starch polymers like amylopectin (20). SusG also tolerates α-1,6 bonds in the active site, thus allowing Bt to grow on amylopectin and the fungal polysaccharide pullulan (21,22). SusD is a starch-binding protein (23) that allows Bt to sense low concentrations of starch to activate sus transcription (24). Independent of its ability to bind starch, SusD is required for growth because it works with SusC, the putative TonB-dependent beta barrel transporter, to usher oligosaccharides into the periplasm (18,24,25,26). SusE and SusF have two and three carbohydrate-binding sites, respectively, and lack enzymatic activity (27). They work together with the SusG surface binding site and the SusG carbohydrate binding module 58 to efficiently take up starch (24). Independent of its ability to bind starch, SusE also supports growth on medium-length maltooligosaccharides in the absence of SusF and binding by SusD, suggesting that Sus protein assembly is a critical feature of the system (28).
Besides Sus, other PULs are organized similarly and encode SusC- and SusD-like proteins, surface glycan binding proteins, and one or more outer-membrane glycoside hydrolases (13). Moreover, each PUL is upregulated in the presence of its cognate glycan or an inducer, such as the small oligosaccharides maltose, fructose, and Gal4; the simultaneous expression of several PULs is also possible (29,30). However, a hierarchy exists in the utilization of different carbohydrates, in particular, the dietary glycans (30,31,32). The prioritization order depends on the species and seems to rely on the ability of that organism to selectively turn on a given utilization pathway (33).
Microscopy is an approach to directly characterize the organization and dynamics of PUL-encoded proteins, and single-molecule, super-resolution fluorescence microscopy has been used previously to study Sus. Sus proteins were fluorescently labeled, imaged, and tracked one molecule at a time in living Bt cells (34,35). Single-molecule tracking of fluorescently labeled SusG revealed that a significant percentage of the SusG molecules in each cell are mobile on the cell surface in glucose; in contrast, the motion of the majority of SusG proteins is confined when the medium contains starch (34). Furthermore, this SusG confinement in the presence of starch is stabilized by SusD, SusE, and SusF, supporting a model in which the Sus proteins assemble to process starch (34). Finally, unlike SusG, single-molecule tracking found that SusE and SusF are predominantly immobile on the Bt cell surface whether in the presence or absence of starch (35). Super-resolution experiments have therefore provided significant insight into the dynamics and cooperativity of the Sus proteins, and the mobility of these molecules on the cell surface provides a real-time readout of Sus activity in living cells. However, little is known about the dynamics of other PUL-encoded enzymes and binding proteins, nor is it clear whether the single-molecule dynamics of SusG are representative of outer membrane glycoside hydrolases in other PULs. Furthermore, differences between the dynamics of Sus-like proteins from different PULs could reveal how lipoprotein organization is influenced by varied environmental conditions, such as a mixture of differentially prioritized carbohydrates.
Here, to understand how Bt surface enzymes encoded by different PULs interact with their respective substrates, we studied the galactan, levan, and starch utilization systems. These PULs were chosen because they are similar to Sus in that each encodes a single outer membrane glycoside hydrolase that is required for growth on the cognate glycan. Furthermore, the three glycoside hydrolases can be coexpressed in Bt, and their substrates have a hierarchy: galactan is prioritized over levan which is prioritized over starch (30,32). SusG is required for Bt growth on amylopectin and starch. Galactan, a homopolymer of β-1,4 galactose and a component of pectic glycans, is hydrolyzed at the cell surface by the glycoside hydrolase family 53 protein Bt4668 (16,36,37,38). Levan is a homopolymer of β-2,6 fructose that is hydrolyzed by the glycoside hydrolase family 32 protein Bt1760 at the cell surface (39,40). We describe the single-molecule dynamics of the SusG, Bt4668, and Bt1760 glycoside hydrolases, and we quantify these dynamics for cells grown in the respective cognate carbohydrates. We find that the three glycoside hydrolases have similar dynamics, and we describe the dynamics of the three glycoside hydrolases in a mixture of a cognate and a noncognate carbohydrate. We find that noncognate polysaccharides interfere with the dynamics of SusG and Bt1760, although not Bt4668. Moreover we find that noncognate polysaccharides still interfere with the dynamics of SusG after deletion of the noncognate PUL. In addition, we measure by quantitative reverse transcription polymerase chain reaction (qRT-PCR) that all three PULs are upregulated by their cognate carbohydrate but downregulated in the presence of noncognate carbohydrates. These data inform how bacteria respond to the carbohydrate environment and have implications for the design of new prebiotics for promoting health and treating disease.
Materials and methods
Carbohydrate preparation and purification
All carbohydrates were prepared from desiccated powder (30): maize amylopectin (10120-250G, Sigma-Aldrich, St. Louis, MO, USA), potato galactan (P-GALPOT, Megazyme, Bray, Ireland), levan from Erwinia herbicola (L8647, Sigma-Aldrich), barley β-glucan (low viscosity 11cSt, Megazyme), maltose, fructose, and glucose (Sigma-Aldrich). Gal3/4/5 was produced via enzymatic hydrolysis of potato galactan (37). See supporting materials and methods for detailed protocols.
Bacterial strains and growth
The Bacteroides thetaiotaomicron (VPI-5482) (Bt) strains used are listed in Table S1 and the list of primers used to construct variant strains is in Table S2. Bt strains unique to this study were constructed using allelic exchange, as first described in (23) and in supporting materials and methods. Strains were inoculated into TYG medium from freezer stocks and grown overnight at 37°C in a Coy anaerobic chamber (85% N2, 10% H2, and 5% CO2 atmosphere). Cells were back diluted the next day into minimal medium (MM) + 0.5% glucose. The next day, cells were washed with 2× MM and back diluted 1:100 in MM + 0.5 or 0.25% of single carbohydrates of interest or in 0.5% mixtures containing 0.25% of each carbohydrate.
Live-cell single-molecule imaging
Cells were imaged on an Olympus IX71 inverted epifluorescence microscope with laser photoactivation and excitation as described previously (35). The collected movies were processed with the SMALL-LABS algorithm (41) to detect and localize single molecules frame-by-frame. Localizations were time stamped and sequential localizations were assembled into a collection of all the possible trajectories. Each trajectory was analyzed individually to extract a trajectory-average apparent diffusion coefficient, Dapp. In addition, all the squared displacements from all the trajectories of each data set were pulled together and the cumulative probability distributions (CPDs) were built for 20, 40, and 60 ms time lags. See supporting materials and methods for details of the analysis.
Results and discussion
Characterization of the dynamics of glycoside hydrolases with their cognate glycans
The SusG-HT dynamics depend on interactions with amylopectin and on the protein expression level
Live Bt cells expressing the SusG-HaloTag (HT) fusion (42) at the native SusG promoter were labeled with the PA-JF549 photoactivatable dye (43) (Fig. 1 a) and imaged in the single-molecule regime (Fig. 1 b). Fluorescently labeled SusG supports bacterial growth on all carbohydrates tested (Figs. 2 b and S1). Fluorophore locations were determined frame-by-frame at 50 frames/s with typically 30-nm localization uncertainty. Sequential localizations were linked to form individual trajectories (Fig. 1 b). We imaged over 100 cells and attained over 1000 SusG-HT trajectories in each growth condition: 0.25% (w/v) of the SusG cognate polysaccharide amylopectin, the SusG inducer maltose, or glucose, respectively. The polysaccharide concentration was kept constant in all experiments to enable comparison. Representative phase-contrast images of Bt cells grown in each growth condition are displayed overlaid with the SusG-HT trajectories imaged in each cell (Fig. 1 c; Videos S1, S2, and S3). Each trajectory is colored according to its apparent diffusion coefficient (Dapp). The distribution of the Dapp values for all the individual trajectories, each weighted by the trajectory length (≥5), is shown for each growth condition (Fig. 1 d; the number of experiments, trajectories, and single-molecule displacements are given in Fig. S2). Fig. 1 c and d shows mostly immobile SusG-HT trajectories in 0.25% amylopectin. In maltose, we observed two distinct types of SusG-HT trajectories: immobile trajectories and fast-diffusing trajectories (Dapp ∼ 1 μm2/s). In glucose, most SusG-HT trajectories had intermediate diffusion coefficients Dapp ∼ 0.01 μm2/s.
Figure 1.
Single-molecule tracking of SusG-HT in living B. thetaiotaomicron (Bt) cells. (a) The native copy of a glycoside hydrolase (SusG, Bt4668, or Bt1760) is genetically fused to a HaloTag (HT) protein and labeled exogenously with PA-JF549. (b) Phase-contrast image of a Bt cell and representative fluorescence images at successive time points. The single-molecule localization fits (pink circles) and corresponding trajectories (pink lines) are overlaid. (c) Representative phase-contrast images of a Bt cell grown in 0.25% glucose, maltose, and amylopectin, respectively. The trajectories (solid lines) of representative SusG-HT molecules in each cell are overlaid. Each trajectory is color coded according to its apparent diffusion coefficient (color scale). (d) Probability density distribution of the apparent diffusion coefficients for the SusG-HT trajectories collected in cells grown in 0.25% glucose (green), maltose (orange), and amylopectin (blue), respectively. The solid lines and shaded areas represent the mean and the standard deviation of replicate experiments, respectively. (e) Cumulative probability distributions (CPDs) of squared displacements, r2 (each trajectory in (d) is a sequence of such displacements) collected in SusG-HT cells grown in 0.25% glucose (green), maltose (orange), and amylopectin (blue), respectively, plotted for the 40-ms time lag. The black curves show the fit of each CPD to a three-state simple diffusion model (Supporting Materials and Methods Eq. 2). The squared displacement values are normalized by the corresponding squared localization uncertainty, σ2. (f–h) Fits of CPD curves as in (e) as a function of time lag indicate three diffusive states for each growth condition: immobile (cyan), slow diffusion (blue-purple), and fast diffusion (pink). The percentage (weight fraction) and diffusion coefficient (color scale) of SusG-HT in each diffusion state is indicated for trajectories collected in cells grown in 0.25% (f) glucose, (g) maltose, and (h) amylopectin. Apparent diffusion coefficient values are color coded according to the color scale in (c). To see this figure in color, go online.
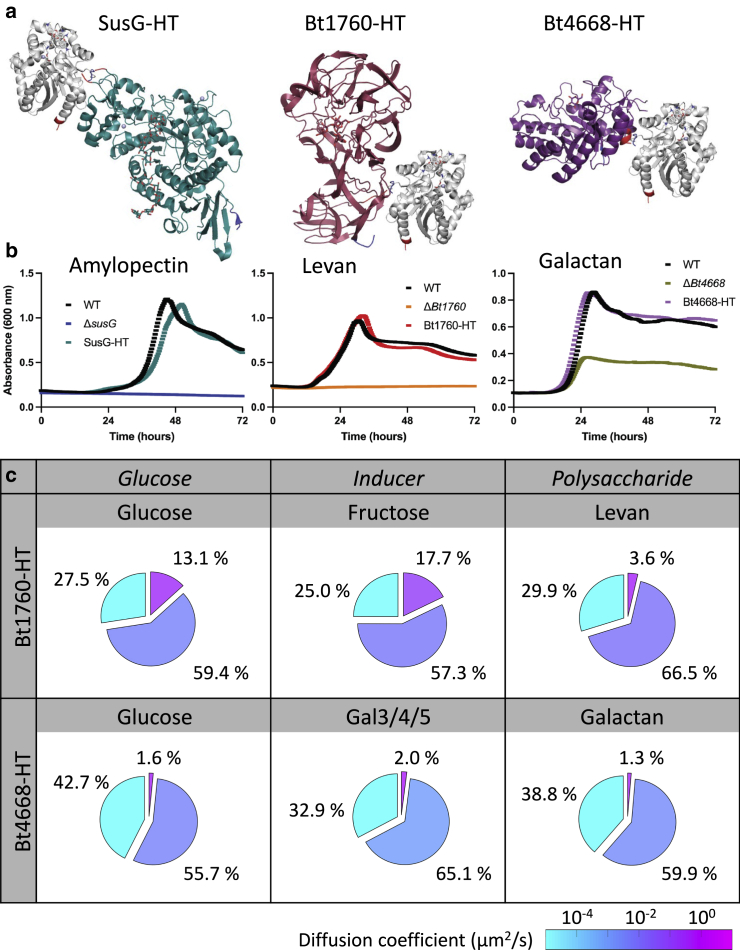
Figure 2.
SusG-HT, Bt1760-HT, and Bt4668. (a) Predicted positioning of the HaloTag (HT) protein in a fusion to SusG with bound maltoheptaose (PDB: 3K8L) (21), Bt1760 with bound levotetraose (PDB: 6R3U) (40), and Bt4668 with bound galactose (PDB: 6GPA) (38) (teal, red, and purple, respectively). Rhodococcus HaloTag with bound tetramethyl rhodamine ligand (PDB: 6U32) (42) is positioned where it was cloned into each protein (gray). In all proteins, the N-terminus is blue and the C-terminus is red, except in SusG, where the site of HaloTag incorporation is red. (b) Bt expressing SusG-HT, Bt1760-HT, and Bt4668-HT grow on 0.5% amylopectin, levan, and galactan, respectively. The corresponding gene deletion strains are also shown to demonstrate that deletion of the genes of interest completely abrogates growth on the cognate glycan. (c) Bt1760-HT and Bt4668-HT dynamics in their cognate carbohydrates. The CPDs of the squared displacements for Bt1760-HT and Bt4668-HT trajectories collected in Bt cells grown in their respective cognate carbohydrates (as indicated) were fit to a three-state log-normal model (Supporting Materials and Methods Eq. 2) to differentiate between three diffusive states for each growth condition: immobile (cyan), slow diffusion (blue-purple), and fast diffusion (pink). The percentage (weight fraction) and diffusion coefficient (color scale) of the glycoside hydrolase in each diffusion state is indicated for trajectories collected in cells grown in 0.25% (w/v) of the indicated carbohydrate. Apparent diffusion coefficient values are color coded according to the color scale. To see this figure in color, go online.
The Bt cell outlines (white) are determined from the corresponding phase-contrast image. The single-molecule localization fits (circles) and corresponding trajectories (lines) are overlaid with the same colors as in Fig. 1c. Below the scale bar is the date of the experiment, the movie number, the number of the photo-activation pulse that the sequence follows (in parentheses), and the frame number. These indicators are displayed in green during the imaging frames and in red during the photo-activation pulse. Scale bar, 1 μm; imaging rate, 20 ms/frame.
The Bt cell outlines (white) are determined from the corresponding phase-contrast image. The single-molecule localization fits (circles) and corresponding trajectories (lines) are overlaid with the same colors as in Fig. 1c. Below the scale bar is the date of the experiment, the movie number, the number of the photo-activation pulse that the sequence follows (in parentheses), and the frame number. These indicators are displayed in green during the imaging frames and in red during the photo-activation pulse. Scale bar, 1 μm; imaging rate, 20 ms/frame.
The Bt cell outlines (white) are determined from the corresponding phase-contrast image. The single-molecule localization fits (circles) and corresponding trajectories (lines) are overlaid with the same colors as in Fig. 1 c. Below the scale bar is the date of the experiment, the movie number, the number of the photo-activation pulse that the sequence follows (in parentheses), and the frame number. These indicators are displayed in green during the imaging frames and in red during the photo-activation pulse. Scale bar, 1 μm; imaging rate, 20 ms/frame.
We further quantified the SusG-HT dynamics by considering the entire collection of displacements rather than individual trajectories, which are collections of displacements. Specifically, we considered the cumulative probability distributions (CPDs) of the squared displacements at 20, 40, and 60 ms in each growth condition (Figs. 1 e and S2). Analyzing the CPDs with a global fit to the three time lags enabled us to measure heterogeneities within each trajectory (44,45) to complement the apparent diffusion coefficient analysis above, which provides a single average Dapp for each trajectory. We found that the distribution of the squared displacements can be fit with a sum of three log-normal distributions, each centered on the mean squared displacement in the absence of any anisotropy in the diffusion medium (Fig. S2). Therefore, the CPD of the squared displacements was fit with the corresponding three log-normal distributions, yielding two diffusion coefficients Dfast and Dslow (the third one is set to zero to account for displacements that are below the resolution of the experiment) for each growth condition (supporting materials and methods, Eqs. 2 and 3). Fig. 1 f–h shows the fit parameters: the fraction of immobile (cyan), slow-diffusing (blue-purple), and fast-diffusing (pink) SusG-HT molecules for each growth condition.
We measured a higher fraction (38.0%) of immobile SusG-HT molecules for cells grown in amylopectin compared with maltose (30.5%) and glucose (32.6%). Moreover, in amylopectin, 61.7% of displacements were assigned to a slow-diffusing state with Dslow = 0.001 μm2/s. This diffusion coefficient is below the ∼5 × 10−3 μm2/s resolution of the experiment, indicating that the slow-diffusing population of SusG-HT in amylopectin can also be considered immobile. We therefore conclude that the dynamics of SusG-HT in amylopectin are predominantly immobile; only 0.3% of the SusG-HT is mobile in amylopectin (Figs. 1 h and S2). On the other hand, in maltose, 7.0% of the displacements were assigned to a fast-diffusing state (Dfast = 1.10 μm2/s). In maltose, as in amylopectin, the slow-diffusing population of SusG-HT can also be considered immobile (Dslow = 0.002 μm2/s). In glucose, 3.6% of the displacements were fast diffusing. Moreover, the significant population (63.8%) of slow-diffusing SusG-HT was in fact mobile with an intermediate diffusion coefficient value of Dslow = 0.021 μm2/s (Figs. 1 f and S2).
The agreement between the distribution of the apparent diffusion coefficients measured from individual trajectories (Fig. 1 d) and the distribution of the squared displacements (Fig. 1 e–h) suggests that the SusG-HT molecules seldom transition between diffusion states over the course of a trajectory, and this stability is also observed in the representative displayed tracks (Fig. 1 c; Videos S1, S2, and S3). Moreover, these results are in good agreement with previously published literature on SusG dynamics (34,35), although the current work detects and characterizes a novel fast-diffusing population at ∼1 μm2/s, i.e., an order of magnitude faster than the previously characterized fast state. Here, the use of the bright and photostable PA-JF549 dye allowed us to image this very fast-diffusing population, and the photoactivation enabled us to perform experiments in the context of high SusG-HT expression levels. Indeed, the fast-diffusing population identified in this study is prevalent mainly in maltose, i.e., in conditions where the sus PUL is most upregulated.
Finally, we observed different SusG-HT dynamics in glucose and maltose, i.e., at low and high SusG-HT expression levels. This difference indicates that we must distinguish between the effects of the upregulation of the sus PUL and the effects of interactions between the glycoside hydrolase and the polysaccharide substrate to interpret the dynamics of SusG-HT in its cognate polysaccharide amylopectin. Consequently, our results show that 1) interactions between SusG-HT and amylopectin decrease the fast-diffusing population of SusG-HT (comparing cells grown in maltose with those grown in amylopectin); and 2) upregulation of the sus PUL changes the SusG-HT dynamics from a prevalent intermediate-diffusing population (for cells grown in glucose) to separate fast-diffusing and immobile populations (for cells grown in maltose).
The SusG model can be extended to the Bt1760 and Bt4668 glycoside hydrolases
We investigated the dynamics of the Bt1760-HT and Bt4668-HT glycoside hydrolases with the same imaging methodology. HT was appended to the C-terminus of Bt1760 and Bt4668 with a three-alanine linker (Fig. 2 a). Bt1760-HT and Bt4668-HT support growth on levan and potato galactan, respectively (Fig. 2 b). Deletion of Bt1760 precludes growth on levan. The ΔBt4668 strain is not fully attenuated for growth on potato galactan, likely because 10% of this carbohydrate is made up of pectin and not galactan, the former of which can be utilized via a different PUL (38). Bt1760-HT and Bt4668-HT support growth in all conditions described (Figs. S3 and S4). Fitting the CPDs of the squared displacements of Bt1760-HT and Bt4668-HT in their cognate polysaccharides (levan and galactan, respectively; Figs. 2 and S5) indicated a high fraction of immobile glycoside hydrolase (29.9 and 38.8%, respectively), a slow-diffusing state with Dslow close to or below the resolution of the experiment, respectively, and a low fraction of fast-diffusing molecules (3.6 and 1.3%, respectively). In comparison, in their respective cognate inducers (fructose and Gal3/4/5, respectively) (37,39), there was a substantial increase in the fast-diffusing Bt1760-HT population (17.7%), and a more modest increase in the fast-diffusing population for Bt4668-HT (2.0%) (Figs. 2 and S5). It was not feasible to purify a single galactan oligosaccharide in sufficient quantities for these studies; because Gal3, Gal4, and Gal5 have similar affinities to the regulatory protein in the galactan PUL (37), a Gal3/4/5 combination was prepared instead. Finally, Bt4668-HT had an intermediate diffusion coefficient for the slow-diffusing state: Dslow = 0.004 μm2/s in glucose (Figs. 2, S5, and S6).
These results indicate that Bt1760-HT and Bt4668-HT exhibit similar dynamics to SusG-HT in their respective cognate carbohydrates. However, we note that the fastest population of Bt1760-HT molecules in fructose diffuses significantly slower (Dfast = 0.126 μm2/s) than the fastest population of SusG-HT molecules in maltose (Dfast = 1.10 μm2/s). This result is consistent with the greater overlap between the two apparent diffusion modes of Bt1760-HT in fructose (Fig. S5) compared with those of SusG-HT in maltose (Fig. 1 d). Taken together, the results from Figs. 1 and 2 support the following working model for the dynamics of the three glycoside hydrolases SusG-HT, Bt1760-HT, and Bt4668-HT (Fig. 3):
-
1)
In the presence of the respective inducer for each glycoside hydrolase, the glycoside hydrolase can exist in two different dynamical states: one immobile and one freely diffusing. We hypothesize that the immobile population results from the transient interactions between the glycoside hydrolase and other outer-membrane proteins regulated on the same PUL. For instance, single-molecule imaging has previously revealed interactions between SusG and the outer-membrane SusCDEF proteins based on the dynamics of SusG-HT in cells knocked out for SusC, SusD, and SusEF (34,35). These transient outer-membrane interactions will be favored at high glycoside hydrolase expression levels because the inducer (i.e., maltose for SusG) also induces expression of these other outer-membrane proteins in its PUL, so the induction produces a high density of outer-membrane proteins. Our measurements of immobile trajectories for SusG-HT, Bt1760-HT, and Bt4668-HT in maltose, fructose, and Gal3/4/5, respectively, suggest that, in this high-density condition, a series of local interactions can cooperate to result in long-lasting, apparently immobile trajectories, even in the absence of the polysaccharide.
-
2)
In the presence of the respective cognate polysaccharide for each glycoside hydrolase, the decrease of the freely diffusing population fraction of glycoside hydrolase in our measurements suggests the formation of a tight complex between the glycoside hydrolase and the other outer-membrane proteins of the PUL. In this case, we hypothesize that the complex is strongly stabilized by direct interactions between the polysaccharide and the outer-membrane proteins. Our previous work demonstrates that recombinant SusE, SusF, and SusG bind to soluble and insoluble corn starch (21,27). That these proteins recognize larger α-glucan structures is supported by isothermal titration calorimetry, and coimmunoprecipitation and cross-linking experiments have demonstrated the presence of a SusCDE complex in the outer membrane (35,46). That SusF and SusG were not captured in cross-linking experiments when cells were grown in maltose suggests that the polysaccharide is required for SusF and SusG to transiently interact with outer-membrane Sus components. Indeed, SusG diffuses faster in the presence of amylopectin in a ΔSusEF or ΔSusD background compared with a wild-type background, suggesting Sus outer-membrane complex formation (34).
-
3)
In glucose, our measurements of a fast-diffusing population and an intermediate-diffusing population suggest a rapid transition between freely diffusing glycoside hydrolases and immobile glycoside hydrolases that are interacting with other outer-membrane proteins in the PUL. This transition must be faster than the acquisition time (20 ms) of our experiment since we observe an apparent intermediate diffusion coefficient: we interpret the intermediate state as an average of the fast-diffusing and the transient immobile state.
Figure 3.
Working model of SusG-HT, Bt1760-HT, and Bt4668-HT glycoside hydrolase dynamics in their cognate carbohydrates. (a) In glucose, the glycoside hydrolase transitions from a freely diffusing (F) state to an immobile (I) state where it interacts transiently with one or few outer-membrane proteins. (b) In the presence of the inducer, the glycoside hydrolase interacts with many outer-membrane proteins due to the high level of expression of the PUL. These cooperative protein interactions stabilize the complexed glycoside hydrolase (by ΔEC) and subtly increase the activation barrier (ΔEA) compared with glucose, thus reducing the transition rate between I and F. (c) The polysaccharide provides an extra stabilization of the complexed glycoside hydrolase (ΔEP), dramatically shifting the equilibrium toward the I state. To see this figure in color, go online.
Glycoside hydrolase dynamics in a mixture of cognate and noncognate glycans
Noncognate glycans also affect the dynamics of SusG and Bt1760
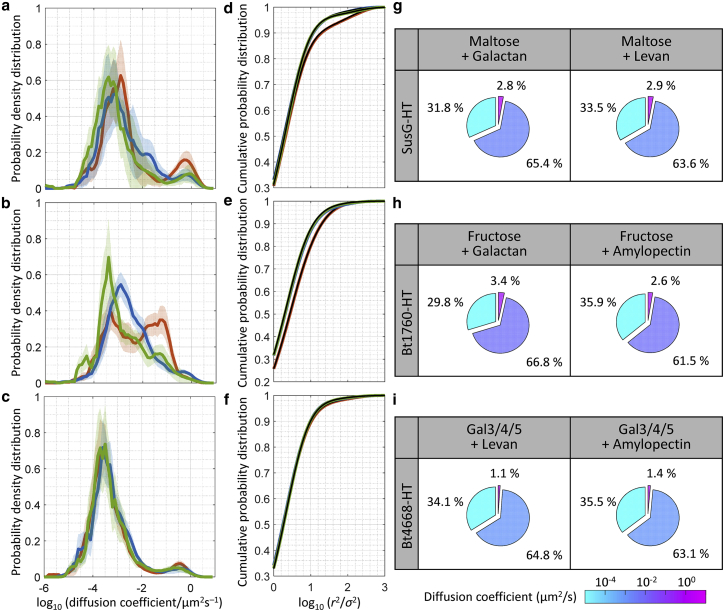
First, we measured the dynamics of each of the SusG-HT, Bt1760-HT, and Bt4668-HT glycoside hydrolases in a mixture of the cognate inducer and a noncognate polysaccharide. All three HT-tagged proteins support growth on each carbohydrate and carbohydrate mixture assessed here (Figs. S1, S3, and S4). In addition, the HT does not affect growth in glucose in any of the strains (Fig. S7). No in vitro evidence exists to support stable interactions between the glycoside hydrolases and their noncognate polysaccharides. Still, we observed that the populations of fast-diffusing SusG-HT and Bt1760-HT are reduced in the presence of a noncognate polysaccharide (Figs. 4 a–h and S9). The effect of the noncognate polysaccharides is especially important for Bt1760-HT: although 17.7% of Bt1760-HT molecules diffuse rapidly in fructose (Fig. 2), analysis of the CPD of the squared displacements of Bt1760-HT in a mixture of fructose and galactan or fructose and amylopectin indicated that this fast-diffusing population drops significantly to 3.4 and 2.6%, respectively (Figs. 4 h and S9). Similarly, we measured 2.8 and 2.9% populations of the fast-diffusing state of SusG-HT in a mixture of maltose and galactan or maltose and levan, respectively (Figs. 4 g and S9), compared with 7.0% in maltose (Fig. 1 g). On the other hand, the population of fast-diffusing SusG-HT is much less reduced in a mixture of maltose and β-glucan, a carbohydrate that cannot be catabolized by Bt: we measured 4.7% fast-diffusing displacements (Fig. S9). Although the SusG and Bt1760 dynamics are strongly affected by noncognate glycans, the addition of the noncognate levan or amylopectin to the Gal3/4/5 inducer does not significantly change the dynamics of Bt4668-HT (Fig. 4): we measured 1.1 and 1.4% populations of fast-diffusing Bt4668-HT molecules for cells grown in a mixture of Gal3/4/5 and levan or Gal3/4/5 and amylopectin, respectively. These values are similar to the 2.0% fast-diffusing population of Bt4668-HT measured in Gal3/4/5 alone (Fig. 2).
Figure 4.
SusG-HT, Bt1760-HT and Bt4668-HT dynamics in the presence of a noncognate carbohydrate. (a–c) Probability density distribution of the apparent diffusion coefficients and (d–f) cumulative probability distributions of squared displacements, r2, plotted for the 40-ms time lag, for (a, d) SusG-HT in maltose (0.25%, orange), maltose and galactan (0.25% each, blue), maltose and levan (0.25% each, green); (b, e) Bt1760-HT in fructose (0.25%, orange), fructose and galactan (0.25% each, blue), fructose and amylopectin (0.25% each, green); (c, f) Bt4668-HT in Gal3/4/5 (0.25%, orange), Gal3/4/5 and levan (0.25% each, blue), Gal3/4/5 and amylopectin (0.25% each, green). The solid lines and shaded areas in (a–c) represent the mean and the standard deviation of replicate experiments, respectively. The black curves in (d–f) show the fit of each CPD to a three-state simple diffusion model (Supporting Materials and Methods Eq. 2). Corresponding percentage (weight fraction) and diffusion coefficient (color scale) of (g) SusG-HT, (h) Bt1760-HT, and (i) Bt4668-HT in each diffusion state. To see this figure in color, go online.
Second, we measured the dynamics of SusG-HT, Bt1760-HT, and Bt4668-HT in cells grown in a mixture of their respective cognate polysaccharide and a noncognate polysaccharide. The distributions of the apparent diffusion coefficient of SusG-HT, Bt1760-HT, and Bt4668-HT are very similar in these polysaccharide mixtures compared with cells grown in the cognate polysaccharide alone and the analysis of the CPD of the squared displacements also indicated no significant differences (Fig. S10). Moreover, we did not measure significantly different dynamics of SusG-HT in a mixture of amylopectin and fructose (Fig. S10) compared with amylopectin alone.
Thus, we conclude that, in the absence of the cognate polysaccharide, a noncognate polysaccharide can interfere with the dynamics of SusG and Bt1760. Interestingly, the interference is characterized by a decrease of the fraction of the fast-diffusing state, mimicking the action of the cognate polysaccharide. Based on the known structures and carbohydrate specificities of these enzymes, a direct interaction with the noncognate polysaccharide is highly unlikely. Via isothermal titration calorimetry (Fig. S8), SusG-HT showed no detectable affinity for levan; the small amount of heat released upon mixing SusG-HT with galactan is not enough to quantify in this assay. This finding coupled with the fact that SusG-HT does not break down either polysaccharide (Fig. S8), suggests that noncognate polysaccharide binding to SusG-HT is not a primary driver of the effects of the polysaccharide on protein dynamics. Moreover, if nonspecific interactions were a driving factor here, we would expect all polysaccharides, including β-glucan, to have this effect, which is not the case: the β-glucan polysaccharide that is not recognized by Bt does not influence lipoprotein mobility. Therefore, we believe this change in dynamics likely reflects that polysaccharide binding to cognate receptors on the cell surface has a global effect on the diffusion of all cell surface proteins. For instance, polysaccharide bound to one or more cell surface proteins may act as a net, not only trapping its target proteins but also restricting the fluidity of the membrane and thus the free diffusion of other cell surface proteins. Accordingly, we observed no significant effect for β-glucan, a carbohydrate that is not catabolized by Bt, suggesting that the interference is directly related to the ability of Bt to bind or catabolize the noncognate polysaccharide (Fig. S9).
Two different mechanisms may contribute to the observed interference
Two different mechanisms may account for the noncognate polysaccharides interfering with the glycoside hydrolase dynamics. First, based on the observed interference, we hypothesized that glycoside hydrolase dynamics are indirectly affected when the noncognate polysaccharide interacts with its cognate outer-membrane proteins. Second, since we showed that the fast-diffusing population of the glycoside hydrolases was increased upon upregulation of the PUL, we hypothesized that the catabolism of a noncognate carbohydrate regulates the PUL transcription level to affect the glycoside hydrolase dynamics. Previous evidence exists for the repression of Bt PULs associated with lower priority carbohydrates (30,31).
To test our first hypothesis, we measured the dynamics of SusG-HT in a mixture of maltose and levan in Bt cells deleted for the fructan PUL. The SusG-HT Δfructan strain cannot grow on fructose or levan (Fig. S1). Interestingly, we measured a reduced fraction of SusG-HT molecules in the fast-diffusing state (2.0% in maltose and levan compared with 4.3% in maltose alone; Figs. 5 a, c, e and S11). On the other hand, we observed only a subtle reduction in this fast-diffusing population for cells grown in a mixture of maltose and β-glucan (3.4%) (Fig. S11). Independently, we deleted the galactan PUL and measured the dynamics of SusG-HT in a mixture of maltose and galactan. The SusG-HT Δgalactan strain cannot grow on Gal3/4/5 and shows minor growth on galactan—similar to the finding that the ΔBt4668 strain is not fully attenuated for growth on potato galactan, and again likely due to the fact that 10% of this carbohydrate is made up of pectin, which does not require the galactan PUL for growth (Fig. S1) (37). Again, we measured a reduced fraction of molecules in the fast-diffusing state (0.6% in maltose and galactan compared with 6.1% in maltose alone), but we found no major effect on the fast-diffusing population in a mixture of maltose and β-glucan (3.2%) (Figs. 5 b, d, f and S11). In addition, measurements performed with these deletion strains in the presence of a mixture of amylopectin and levan or galactan show the same SusG-HT dynamics as in amylopectin alone (Fig. S12).
Figure 5.
SusG-HT dynamics in the presence of a noncognate polysaccharide in Bt cells knocked out for the corresponding PUL. (a–c) Probability density distribution of the apparent diffusion coefficients and (d–f) cumulative probability distribution of squared displacements, r2, plotted for the 40-ms time lag, for (a, c) SusG-HT Δfructan in maltose (0.25%, orange), maltose and levan (0.25% each, green); (b, d) SusG-HT Δgalactan in maltose (0.25%, orange), maltose and galactan (0.25% each, purple). The solid lines and shaded areas in (a–c) represent the mean and the standard deviation of replicate experiments, respectively. The black curves in (d–f) show the fit of each CPD to a three-state simple diffusion model (Supporting Materials and Methods Eq. 2). Corresponding percentage (weight fraction) and diffusion coefficient (color scale) of (e) SusG-HT Δfructan and (h) SusG-HT Δgalactan in each diffusion state. To see this figure in color, go online.
Because we lack evidence of interactions between SusG-HT and noncognate polysaccharides, these data are likely explained by the presence of non-PUL-encoded proteins capable of binding levan and galactan at the cell surface. Ample precedence shows that, while Bt requires PUL-encoded proteins to target distinct glycans, non-PUL-encoded proteins contribute to—and are sometimes required for—this process (39,47,48). Indeed, additional noncognate PUL-encoded genes are upregulated during growth on levan or galactan (15,48), some of which may contribute to binding these carbohydrates at the cell surface. Conversely β-glucan, which is not metabolized by Bt, is unlikely to be recognized by any of the cell surface proteins. In addition, levan generally has a smaller chain length than galactan or amylopectin, so individual molecules of levan bound to the cell surface may not limit free diffusion as much as would a larger polysaccharide, accounting for the more subtle difference in mobility when levan is combined with maltose. In contrast, galactan is a larger molecule and may “capture” more proteins, thus further restricting diffusion on the cell surface. These results suggest the intriguing possibility that protein diffusion can be limited either directly by direct interactions with a substrate or indirectly by more global changes in the diffusion of other cell surface proteins.
To test our second hypothesis, we measured the relative transcription levels of genes encoding each glycoside hydrolase by qRT-PCR (Figs. 6 and S13). We also determined the relative single-cell expression levels of SusG-HT, Bt4668-HT, and Bt1760-HT via single-cell fluorescence intensity measurements in fixed cells labeled with JF549 dye (Figs. S13 and S14). We measured a downregulation of each PUL when a noncognate polysaccharide was mixed with the cognate inducer with respect to the transcription level in the cognate inducer alone (Fig. 6). Similarly, we measured this downregulation of each glycoside hydrolase even when a noncognate polysaccharide was mixed with the cognate polysaccharide (Fig. S13). Consistently, the distributions of cell intensities also showed less expression of each glycoside hydrolase in the presence of a noncognate polysaccharide in a mixture with the cognate polysaccharide (Fig. S13) or the cognate inducer (Fig. S14). Interestingly, the dynamics of the glycoside hydrolases SusG-HT and Bt1760-HT at low expression levels (in glucose) were characterized by a decrease in the fraction of the fast-diffusing state compared with their dynamics at high-expression levels (in inducer). Therefore, the observed interference of a noncognate polysaccharide on the dynamics of SusG-HT and Bt1760-HT may also be a consequence of the downregulation of the sus and fructan PULs, respectively, following the catabolism of the noncognate polysaccharide by Bt. Indeed, the influence of other polysaccharides on the regulation of the sus and fructan PULs has been demonstrated in vitro and in vivo in the mouse gut (30,49).
Figure 6.
Relative transcription levels of the sus, fructan, and galactan glycoside hydrolases by qRT-PCR. Transcription level fold change for SusG (blue), Bt4668 (orange), and Bt1760 (yellow) for Bt cells grown in (a) maltose and galactan (0.25% each), maltose and levan (0.25% each), measured relative to levels in maltose (0.25%); (b) fructose and galactan (0.25% each), fructose and amylopectin (0.25% each), measured relative to levels in fructose (0.25%); (c) Gal3/4/5 and levan (0.25% each), Gal3/4/5 and amylopectin (0.25% each), measured relative to levels in Gal3/4/5 (0.25%). To see this figure in color, go online.
Conclusions
The dynamics of the glycoside hydrolases SusG-HT, Bt4668-HT, and Bt1760-HT were characterized in the presence of their respective cognate glycans. First, the comparison of their dynamics in the polysaccharide relative to the dynamics in the inducer showed a significant decrease of the fraction of freely diffusing glycoside hydrolase upon binding to the cognate polysaccharide. Second, the comparison of their dynamics in the inducer relative to the dynamics in glucose showed an intermediate population diffusing slowly on the outer membrane of the cell in the low-expression level (glucose) case. Taken together, the results suggest a general model for the three glycoside hydrolases in which the outer-membrane proteins of a PUL interact at high expression levels. In the presence of the polysaccharide, these interactions are significantly stabilized, and the glycoside hydrolase slows (Fig. 3). Furthermore, our results show that different glycoside hydrolases exhibit similar dynamics on the outer membrane of Bt cells.
Precisely how the dynamics of each of these proteins are influenced by interactions with their cognate Sus-like transport complex is not clear. While there is no high-resolution structure of the Bt Sus complex or the Bt4668-4671 Sus-like complex, the structure of Bt1760-1763, the Bt Sus-like complex that targets levan, has been studied in detail (25,50,51). Early structures captured the assembly of the SusCD proteins Bt1763 and Bt1762 but lacked the lipoproteins Bt1760 and Bt1761 that are coexpressed within this PUL and presumably participate in the complex. However, more recent work from this group indicates that an entire complex consisting of all four proteins can be captured using gentler extraction conditions. This complex, visualized via single-particle cryoEM, demonstrates the remarkable manner in which the SusC protein Bt1763 has dedicated docking points for all four lipoproteins (51). That the levanase Bt1760 consistently docks to the complex may be why we note a substantially lower diffusion coefficient for Bt1760-HT compared with Bt SusG-HT and Bt4668-HT. How the Bt SusC and the SusC homolog Bt4671 accommodate their respective cell surface glycoside hydrolyses remains to be determined.
To further investigate the prioritization of the PULs by Bt, we measured the dynamics of SusG-HT, Bt4668-HT, and Bt1760-HT in noncognate polysaccharide. In the presence of a noncognate polysaccharide and cognate inducer, the fraction of freely diffusing SusG-HT and Bt1760-HT decreases significantly; this decrease is not observed in a mixture of cognate and noncognate polysaccharides. Interestingly, the dynamics of Bt4668 are unaltered by the presence of a noncognate polysaccharide. Two different mechanisms were proposed to explain the effect of the noncognate polysaccharide on the dynamics of the glycoside hydrolase. First, measurements of SusG dynamics in Bt cells knocked out for the fructan or galactan PUL may be due to polysaccharide binding to non-PUL-encoded proteins on the cell surface, leading to a generalized decrease in cell surface fluidity and protein diffusion. The extent of this effect is likely related to both the number of binding sites for the polysaccharide and to the size of the polysaccharide. Second, measurements of downregulation of the sus, fructan, or galactan glycoside hydrolases in the presence of a noncognate glycan agrees with the observed decrease in freely diffusing glycoside hydrolase molecules in the presence of a noncognate polysaccharide. This proposed mechanism suffers from two limitations: it does not explain why no interference is observed in a mixture of a noncognate polysaccharide with the cognate polysaccharides and it does not explain why the dynamics of Bt4668-HT remain unchanged upon the addition of a noncognate polysaccharide, although we did measure a downregulation of the PUL in all these conditions. The galactan used in this study contains a small fraction of pectin, which upregulates other PULs; Bt4668-HT may therefore behave slightly differently in this context compared with the case where levan and amylopectin upregulate a single PUL. Future work is needed to fully explain these effects and to understand how polysaccharide binding at the cell surface affects membrane fluidity and protein diffusion.
Author contributions
L.G., H.A.B., N.M.K., and J.S.B. designed the research. L.G., H.A.B., and A.L.D. performed the research. L.G. and H.A.B. analyzed the data. L.G., H.A.B., N.M.K., and J.S.B. wrote the paper.
Acknowledgments
This work was supported by NIH grant R01-GM118475 to N.M.K. and J.S.B. Thanks to I. Weber Cravioto and N. Guibord for preliminary experiments and to E. Martens for support with qRT-PCR experiments.
Declaration of interests
The authors declare no competing interests.
Editor: Kerwyn Casey Huang.
Footnotes
Laurent Geffroy and Haley A. Brown contributed equally to this work.
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2022.10.024.
Supporting material
References
- 1.Buffie C.G., Pamer E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013;13:790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shreiner A.B., Kao J.Y., Young V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015;31:69–75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ndeh D., Gilbert H.J. Biochemistry of complex glycan depolymerisation by the human gut microbiota. FEMS Microbiol. Rev. 2018;42:146–164. doi: 10.1093/femsre/fuy002. [DOI] [PubMed] [Google Scholar]
- 4.Makki K., Deehan E.C., et al. Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Huttenhower C., Gevers D., et al. White O. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin J., Li R., et al. Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Argenio V., Salvatore F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta. 2015;451:97–102. doi: 10.1016/j.cca.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Koropatkin N.M., Cameron E.A., Martens E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012;10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krautkramer K.A., Fan J., Bäckhed F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021;19:77–94. doi: 10.1038/s41579-020-0438-4. [DOI] [PubMed] [Google Scholar]
- 10.Johnson E.L., Heaver S.L., et al. Ley R.E. Microbiome and metabolic disease: revisiting the bacterial phylum Bacteroidetes. J. Mol. Med. 2017;95:1–8. doi: 10.1007/s00109-016-1492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh C.J., Guinane C.M., et al. Cotter P.D. Beneficial modulation of the gut microbiota. FEBS Lett. 2014;588:4120–4130. doi: 10.1016/j.febslet.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 12.Lapébie P., Lombard V., et al. Henrissat B. Bacteroidetes use thousands of enzyme combinations to break down glycans. Nat. Commun. 2019;10:2043. doi: 10.1038/s41467-019-10068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grondin J.M., Tamura K., et al. Brumer H. Polysaccharide utilization loci: fueling microbial communities. J. Bacteriol. 2017;199 doi: 10.1128/JB.00860-16. e00860-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martens E.C., Kelly A.G., et al. Brumer H. The devil lies in the details: how variations in polysaccharide fine-structure impact the physiology and evolution of gut microbes. J. Mol. Biol. 2014;426:3851–3865. doi: 10.1016/j.jmb.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martens E.C., Lowe E.C., et al. Gordon J.I. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011;9:e1001221. doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ndeh D., Rogowski A., et al. Gilbert H.J. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature. 2017;544:65–70. doi: 10.1038/nature21725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salyers A.A., West S.E., et al. Wilkins T.D. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl. Environ. Microbiol. 1977;34:529–533. doi: 10.1128/aem.34.5.529-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves A.R., DElia J.N., et al. Salyers A.A. A Bacteroides thetaiotaomicron outer membrane protein that is essential for utilization of maltooligosaccharides and starch. J. Bacteriol. 1996;178:823–830. doi: 10.1128/jb.178.3.823-830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves A.R., Wang G.R., Salyers A.A. Characterization of four outer membrane proteins that play a role in utilization of starch by Bacteroides thetaiotaomicron. J. Bacteriol. 1997;179:643–649. doi: 10.1128/jb.179.3.643-649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shipman J.A., Cho K.H., et al. Salyers A.A. Physiological characterization of SusG, an outer membrane protein essential for starch utilization by Bacteroides thetaiotaomicron. J. Bacteriol. 1999;181:7206–7211. doi: 10.1128/jb.181.23.7206-7211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koropatkin N.M., Smith T.J. SusG: a unique cell-membrane-associated α-amylase from a prominent human gut symbiont targets complex starch molecules. Structure. 2010;18:200–215. doi: 10.1016/j.str.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Arnal G., Cockburn D.W., et al. Koropatkin N.M. Structural basis for the flexible recognition of α-glucan substrates by Bacteroides thetaiotaomicron SusG. Protein Sci. 2018;27:1093–1101. doi: 10.1002/pro.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koropatkin N.M., Martens E.C., et al. Smith T.J. Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices. Structure. 2008;16:1105–1115. doi: 10.1016/j.str.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron E.A., Kwiatkowski K.J., et al. Martens E.C. Multifunctional nutrient-binding proteins adapt human symbiotic bacteria for glycan competition in the gut by separately promoting enhanced sensing and catalysis. mBio. 2014;5 doi: 10.1128/mBio.01441-14. e01441-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glenwright A.J., Pothula K.R., et al. van den Berg B. Structural basis for nutrient acquisition by dominant members of the human gut microbiota. Nature. 2017;541:407–411. doi: 10.1038/nature20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollet R.M., Martin L.M., Koropatkin N.M. TonB-dependent transporters in the Bacteroidetes: unique domain structures and potential functions. Mol. Microbiol. 2021;115:490–501. doi: 10.1111/mmi.14683. [DOI] [PubMed] [Google Scholar]
- 27.Cameron E.A., Maynard M.A., et al. Martens E.C. Multidomain carbohydrate-binding proteins involved in Bacteroides thetaiotaomicron starch metabolism. J. Biol. Chem. 2012;287:34614–34625. doi: 10.1074/jbc.M112.397380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foley M.H., Martens E.C., Koropatkin N.M. SusE facilitates starch uptake independent of starch binding in B. thetaiotaomicron. Mol. Microbiol. 2018;108:551–566. doi: 10.1111/mmi.13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martens E.C., Chiang H.C., Gordon J.I. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe. 2008;4:447–457. doi: 10.1016/j.chom.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers T.E., Pudlo N.A., et al. Martens E.C. Dynamic responses of Bacteroides thetaiotaomicron during growth on glycan mixtures. Mol. Microbiol. 2013;88:876–890. doi: 10.1111/mmi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch J.B., Sonnenburg J.L. Prioritization of a plant polysaccharide over a mucus carbohydrate is enforced by a Bacteroides hybrid two-component system. Mol. Microbiol. 2012;85:478–491. doi: 10.1111/j.1365-2958.2012.08123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwalm N.D., Townsend G.E., Groisman E.A. Prioritization of polysaccharide utilization and control of regulator activation in Bacteroides thetaiotaomicron: regulator activation controls prioritization. Mol. Microbiol. 2017;104:32–45. doi: 10.1111/mmi.13609. [DOI] [PubMed] [Google Scholar]
- 33.Pudlo N.A., Urs K., et al. Martens E.C. Symbiotic human gut bacteria with variable metabolic priorities for host mucosal glycans. mBio. 2015;6 doi: 10.1128/mBio.01282-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karunatilaka K.S., Cameron E.A., et al. Biteen J.S. Superresolution imaging captures carbohydrate utilization dynamics in human gut symbionts. mBio. 2014;5 doi: 10.1128/mBio.02172-14. 02172-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuson H.H., Foley M.H., et al. Biteen J.S. The starch utilization system Assembles around stationary starch-binding proteins. Biophys. J. 2018;114:242–250. doi: 10.1016/j.bpj.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lammerts van Bueren A., Mulder M., et al. Dijkhuizen L. Prebiotic galactooligosaccharides activate mucin and pectic galactan utilization pathways in the human gut symbiont Bacteroides thetaiotaomicron. Sci. Rep. 2017;7:40478. doi: 10.1038/srep40478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luis A.S., Briggs J., et al. Gilbert H.J. Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nat Microbiol. 2018;3:210–219. doi: 10.1038/s41564-017-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Böger M., Hekelaar J., et al. Lammerts van Bueren A. Structural and functional characterization of a family GH53 β-1,4-galactanase from Bacteroides thetaiotaomicron that facilitates degradation of prebiotic galactooligosaccharides. J. Struct. Biol. 2019;205:1–10. doi: 10.1016/j.jsb.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Sonnenburg E.D., Zheng H., et al. Sonnenburg J.L. Specificity of polysaccharide use in intestinal Bacteroides species determines diet-induced microbiota alterations. Cell. 2010;141:1241–1252. doi: 10.1016/j.cell.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ernits K., Eek P., et al. Alamäe T. First crystal structure of an endo-levanase – the BT1760 from a human gut commensal Bacteroides thetaiotaomicron. Sci. Rep. 2019;9:8443. doi: 10.1038/s41598-019-44785-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isaacoff B.P., Li Y., et al. Biteen J.S. SMALL-LABS: measuring single-molecule intensity and position in obscuring backgrounds. Biophys. J. 2019;116:975–982. doi: 10.1016/j.bpj.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deo C., Abdelfattah A.S., et al. Schreiter E.R. The HaloTag as a general scaffold for far-red tunable chemigenetic indicators. Nat. Chem. Biol. 2021;17:718–723. doi: 10.1038/s41589-021-00775-w. [DOI] [PubMed] [Google Scholar]
- 43.Grimm J.B., English B.P., et al. Lavis L.D. Bright photoactivatable fluorophores for single-molecule imaging. Nat. Methods. 2016;13:985–988. doi: 10.1038/nmeth.4034. [DOI] [PubMed] [Google Scholar]
- 44.van den Wildenberg S.M.J.L., Bollen Y.J.M., Peterman E.J.G. How to quantify protein diffusion in the bacterial membrane. Biopolymers. 2011;95:312–321. doi: 10.1002/bip.21585. [DOI] [PubMed] [Google Scholar]
- 45.Rowland D.J., Biteen J.S. Measuring molecular motions inside single cells with improved analysis of single-particle trajectories. Chem. Phys. Lett. 2017;674:173–178. [Google Scholar]
- 46.Cho K.H., Salyers A.A. Biochemical analysis of interactions between outer membrane proteins that contribute to starch utilization by Bacteroides thetaiotaomicron. 2001;183:7224–7230. doi: 10.1128/JB.183.24.7224-7230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cartmell A., Lowe E.C., et al. Bolam D.N. How members of the human gut microbiota overcome the sulfation problem posed by glycosaminoglycans. Proc. Natl. Acad. Sci. USA. 2017;114:7037–7042. doi: 10.1073/pnas.1704367114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ng K.M., Ferreyra J.A., et al. Sonnenburg J.L. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502:96–99. doi: 10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonnenburg J.L., Xu J., et al. Gordon J.I. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;25:5717. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 50.Gray D.A., White J.B.R., et al. van den Berg B. Insights into SusCD-mediated glycan import by a prominent gut symbiont. Nat. Commun. 2021;12:44. doi: 10.1038/s41467-020-20285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White J.B.R., Silale A., et al. van den Berg B. Outer membrane utilisomes mediate oligosaccharide uptake in gut bacteroidetes. bioRxiv. 2022 doi: 10.1101/2022.08.15.503959. Preprint at. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Bt cell outlines (white) are determined from the corresponding phase-contrast image. The single-molecule localization fits (circles) and corresponding trajectories (lines) are overlaid with the same colors as in Fig. 1c. Below the scale bar is the date of the experiment, the movie number, the number of the photo-activation pulse that the sequence follows (in parentheses), and the frame number. These indicators are displayed in green during the imaging frames and in red during the photo-activation pulse. Scale bar, 1 μm; imaging rate, 20 ms/frame.
The Bt cell outlines (white) are determined from the corresponding phase-contrast image. The single-molecule localization fits (circles) and corresponding trajectories (lines) are overlaid with the same colors as in Fig. 1c. Below the scale bar is the date of the experiment, the movie number, the number of the photo-activation pulse that the sequence follows (in parentheses), and the frame number. These indicators are displayed in green during the imaging frames and in red during the photo-activation pulse. Scale bar, 1 μm; imaging rate, 20 ms/frame.
The Bt cell outlines (white) are determined from the corresponding phase-contrast image. The single-molecule localization fits (circles) and corresponding trajectories (lines) are overlaid with the same colors as in Fig. 1 c. Below the scale bar is the date of the experiment, the movie number, the number of the photo-activation pulse that the sequence follows (in parentheses), and the frame number. These indicators are displayed in green during the imaging frames and in red during the photo-activation pulse. Scale bar, 1 μm; imaging rate, 20 ms/frame.