Abstract
Plastic fibers are ubiquitous in daily life with additives incorporated to improve their performance. Only a few restrictions exist for a paucity of common additives, while most of the additives used in textile industry have not been clearly regulated with threshold limits. The production of synthetic fibers, which can shed fibrous microplastics easily (< 5 mm) through mechanical abrasion and weathering, is increasing annually. These fibrous microplastics have become the main composition of microplastics in the environment. This review focuses on additives on synthetic fibers; we summarized the detection methods of additives, compared concentrations of different additive types (plasticizers, flame retardants, antioxidants, and surfactants) on (micro)plastic fibers, and analyzed their release and exposure pathways to environment and human beings. Our prediction shows that the amounts of predominant additives (phthalates, organophosphate esters, bisphenols, per- and polyfluoroalkyl substances, and nonylphenol ethoxylates) released from clothing microplastic fibers (MFs) are estimated to reach 35, 10, 553, 0.4, and 568 ton/year to water worldwide, respectively; and 119, 35, 1911, 1.4, and 1965 ton/year to air, respectively. Human exposure to MF additives via inhalation is estimated to be up to 4.5–6440 µg/person annually for the above five additives, and via ingestion 0.1–204 µg/person. Notably, the release of additives from face masks is nonnegligible that annual human exposure to phthalates, organophosphate esters, per- and polyfluoroalkyl substances from masks via inhalation is approximately 491–1820 µg/person. This review helps understand the environmental fate and potential risks of released additives from (micro)plastic fibers, with a view to providing a basis for future research and policy designation of textile additives.
Graphical Abstract
Introduction
Fibers are ubiquitous polymers in daily life. Common fiber products include clothes, carpets, face masks, etc. There are mainly three types of fibers: natural fibers (cotton, wool), synthetic fibers (polyethylene terephthalate (polyester), polyamide (nylon), acrylic, polyurethane (spandex), polypropylene, polyvinyl chloride, etc.), and artificial fibers (rayon, viscose fiber, cellulose acetate, etc.). Global fiber production was about 109 million tons in 2020, of which synthetic fibers, natural fibers, and artificial fibers account for about 62%, 32%, and 6%, respectively, and the synthetic fiber production is estimated to approach 100 million t/y by 2030 (Pepper 2021). During manufacturing, different additives are incorporated into these textiles to improve their performance for different applications.
The definition of “microplastic” refers to plastic particles < 5 mm in size. The shape of microplastics includes fragments, fibers, and beads (Zhao et al. 2022). Fiber is the predominant shape of microplastics detected in both the atmospheric and aquatic environment (Lin et al. 2018; Liu et al. 2019b; Su et al. 2018). Fibrous microplastics, i.e., microplastic fibers (MFs), refer to synthetic fibers, including polyethylene terephthalate, polyamide, acrylic, polypropylene, etc. MFs are mainly released during use and wear of synthetic textile products and also become the main source of secondary microplastics in the environment. Microfibers have a broader definition than MFs, which contain both natural and synthetic fibers smaller than 5 mm. Microfibers in the air mainly originate from drying of textiles, daily wear and tear, and solid waste incineration (De Falco et al. 2020; Dris et al. 2016; Liu et al. 2019a), while those in aquatic environment mainly originate from activities such as the washing of textiles and use of fishing nets (Napper and Thompson 2016; Xue et al. 2020).
The most common additives are dyes, flame retardants, plasticizers, antibacterial agents, antistatic agents, antioxidants, etc. (Rovira and Domingo 2019). Some chemicals on textiles have been restricted or banned according to the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals), including phthalates (bis (2–ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), restricted concentration < 0.1%), bisphenol A (BPA, restricted concentration < 0.02% for thermal paper), nonylphenol ethoxylates (NPE, restricted concentration < 0.01%), flame retardants (tris (2,3-dibromopropyl) phosphate (TRIS)), and polybromobiphenyls (PBB), which should not be used in textiles contacting with the skin (Schäfer and Herter 2021). However, there is a wide variety of additives up to more than twenty thousand (https://polymer-additives.specialchem.com/). With the emergence of more and more novel additives (e.g., synthetic phenolic chemicals) (Tan et al. 2021; Wu et al. 2019), most of the additives have not been reasonably controlled and studied. In most cases, additives are physically rather than chemically bound to the plastic polymer (Hahladakis et al. 2018). Thus, MFs can release additives to the surrounding environment easily during the process of laundry, abrasion, and transport (Akhbarizadeh et al. 2021; Hahladakis et al. 2018). When MFs enter organisms, additives will be released and migrate out. In such cases, the bioaccumulation of pollutants can be altered with the presence of MFs especially in above-fugacity scenario (Li et al. 2022).
Many studies have focused on the release of MFs from synthetic textile products; however, little attention has been paid to the “trojan horse” effects of MFs for additives. The objectives of this paper are to (1) overview the extraction and quantification methods of additives on textiles and MFs; (2) summarize the types and concentrations of additives on both traditional (i.e., clothes) and emerging MFs contributors (i.e., face masks); (3) analyze the migration and release capability of these additives; (4) and finally, estimate the annual release of additives together with MFs into aquatic and atmospheric environment, and the mass of additives inhaled and ingested into human body through the carrier of MFs.
Analytical Methods for Additives on (Microplastic) Fibers
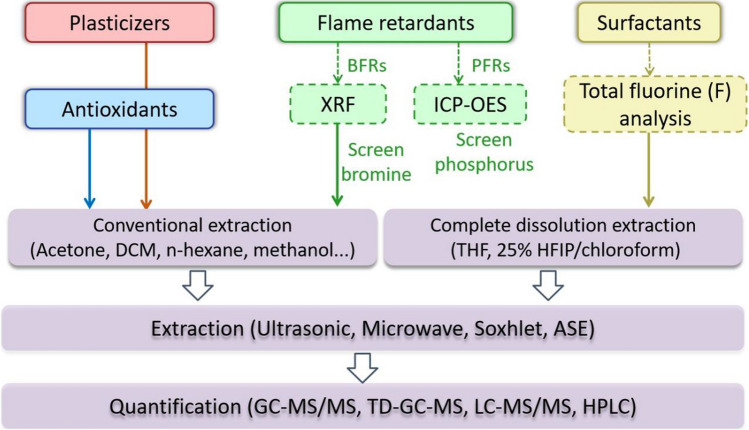
In this section, we mainly introduce the pretreatment methods (especially additive extraction methods) and analytical techniques of the predominant plastic additives, including plasticizers, antioxidants, flame retardants, and surfactants (Fig. 1).
Fig. 1.
Analytical methods of additives on synthetic textiles. (BFRs: brominated flame retardants; PFRs: phosphorus flame retardants; DCM: dichloromethane; THF: C; HFIP: 1,1,1,3,3,3-hexafluoro-2-propanol. ASE: accelerated solvent extraction; XRF: X-ray fluorescence; ICP-OES: inductively coupled plasma-optical emission spectrometry; GC–MS/MS: Gas chromatography-tandem mass spectrometry; TD-GC–MS: thermal desorption-gas chromatography-mass spectrometry; LC–MS: liquid chromatography-mass spectrometry; HPLC: high-performance liquid chromatography)
Sample Pretreatment and Extraction
Pretreatment of Fiber Products
For the extraction of plasticizers, antioxidants, and surfactants on synthetic textile products, solvent extraction is the most common method (Kim et al. 2016; Wang et al. 2019a). Ultrasonic extraction (USE) and microwave-assisted extraction (MAE) have the advantages of high extraction efficiency, short time consumption, low solvent amount, and extensive adaptability (Khan and Jahangir 2020; Kim et al. 2016; La Nasa et al. 2021; Llompart et al. 2019). For instance, USE can be effectively applied in the extraction of phthalates (PAEs) from polyethylene films and synthetic antioxidants from disposable face masks, with recovery rates of 83.2–116.9% and 51–113%, respectively (Kim et al. 2016; Liu and Mabury 2021). Accelerated solvent extraction (ASE) is another prominent method for organic pollutants extraction from sediment, which has not been widely used for textiles yet (Giergielewicz-Możajska et al. 2001; Hu et al. 2020). Additionally, some conventional solvent extraction methods, such as Soxhlet extraction, can also be used with recovery rates of up to 90% or more; however, it is often time-consuming (more than four hours) (Kim et al. 2016; Li et al. 2015).
In addition to the solvent extraction methods, direct qualitative determination techniques are emerging in recent years, such as X-ray fluorescence (XRF), total fluorine (F) analysis technique, and inductively coupled plasma-optical emission spectrometry (ICP-OES). For instance, bromine (Br) and phosphorus (P) contents can be screened in fiber products with XRF and ICP-OES (Negev et al. 2018; Petreas et al. 2016; Young et al. 2021). The total fluorine (F) analysis technique can be conducted before the extraction of per- and polyfluoroalkyl substances (PFAS) to screen samples containing F quickly (Muensterman et al. 2022; Schellenberger et al. 2022). After the USE step of PFAS, some researches also apply solid phase extraction (SPE) to eliminate matrix compound interference and further concentrate samples (Gremmel et al. 2016; Muensterman et al. 2022).
Pretreatment of Microplastic Fibers
Currently, there are limited methods that specifically target additives extraction from MFs. The bottleneck of additives’ extraction from MFs is mainly because of the mass of fiber samples collected from the natural environment is often too low to meet the detection limits of instruments. Recently, Sorensen et al. (2021) proved that when the collected MFs were heavier than 0.1 g, the additives on MFs can be successfully extracted by the USE and quantified.
The pretreatment methods of microplastic particles can provide referential experiences for MFs. Some pretreatment methods, i.e., Soxhlet extraction and USE methods for microplastics can be applied for MF additives extraction. For instance, Zhang et al. (2018) extracted PAEs and organophosphorus esters (OPEs) from microplastic particles (0.01–0.5 g) by the Soxhlet extraction method with dichloromethane (DCM). Besides, Rani et al. (2017) extracted the antioxidants (Irganox 1010, Irganox 1076, 2,6-di-tert-butyl-4-methylphenol (BHT)) from plastic powders by the USE method with DCM. In addition to conventional extraction methods, direct analysis in real-time high-resolution mass spectrometry (DART-MS) can be used as a rapid fingerprinting method to screen microplastic additives. The complex mixture of polymer degradation products (i.e., “chemical fingerprints” of environmental microplastics) resulted from thermal desorption and pyrolysis can reflect the composition of both the polymers and the additives, which has been successfully used to detect plasticizers and antioxidants in microplastics (Zhang et al. 2020d). Of note, this method can preliminarily identify the presence of some additives, but it cannot be used for accurate quantification. In the future, more studies should be carried out on developing sensitive novel extraction or determination methods for trace contaminants in MFs.
Extraction Solutions Selection
For extraction of plasticizers, antioxidants, and flame retardants on fibers, traditional extractants include DCM, acetone, ethyl ether, acetonitrile, n-hexane, and methanol. (Abdallah et al. 2017; Freire et al. 2019; Fu et al. 2012; Hajiouni et al. 2022; Negev et al. 2018; Wang et al. 2011). The mixture of hexane and acetone is the most common extraction solution. For example, n-hexane/acetone (1:1) was used to extract 15 PAEs from children’s clothes, resulting in high recovery rates ranging from 81.9 to 107%; and this recipe has also been successfully applied to extract 39 BFRs and 16 OPEs from children’s sleeping nap mats (made of polyurethane) (Stubbings et al. 2018; Tang et al. 2020). For extraction of surfactants like per- and polyfluoroalkyl substances (PFAS), methanol is commonly used (Muensterman et al. 2022; Schellenberger et al. 2022; Zheng and Salamova 2020). Since PFAS consists of a large number of substances, there are also different extraction solutions and analytical methods for volatile and nonvolatile PFAS, respectively (Table 2). For volatile PFAS (fluorotelomer alcohols (FTOHs)), ethyl acetate and n-hexane can be used as extraction solutions, while methanol and acetone/acetonitrile can be a choice for nonvolatile PFAS, such as perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkyl sulfonic acids (PFSAs) (Gremmel et al. 2016; Vestergren et al. 2015).
Table 2.
Analytical methods for additives on fiber products
| Additives | Polymer composition | Fiber products | Extraction | Extractants | Time | Determination equipment | Recovery | Concentration | References |
|---|---|---|---|---|---|---|---|---|---|
| Plasticizers | |||||||||
| PAEs | Polyester, nylon, spandex, cotton | Children clothes | USE | n-hexane/acetone (1:1) | 50 min (30 + 20) | GC–MS | 84.0–103% | 2.92–223 μg/g | Tang et al. (2020) |
| PAEs | Polypropylene | Face masks | USE | DCM/ethyl acetate (1:1) | 30 min × 2 | GC–MS | 79.3–113.2% | 115 –37,700 ng/g | Xie et al. (2022) |
| PAEs | Polyethylene | Polymer (polyethylene films) | / | / | / | TD-GC–MS | 92–103% | / | Kim et al. (2016) |
| USE | Tetrahydrofuran | 30 min | GC–MS | 83.2–116.9% | |||||
| Soxhlet | n-hexane | 6 h | 101–104% | ||||||
| PAEs | / | Childcare items, toys, textiles | USE | Tetrahydrofuran | 60 min | GC–MS | 100 ± 15% | 5.18–1798.14 mg/l | Khan and Jahangir (2020) |
| PAEs | / | Infant fabrics, printed textiles | Soxhlet | n-hexane | 4 h | GC–MS | 96.2–100.9% |
Infant fabrics:33.40 ± 2.29 mg/g Printed textiles:51.60 ± 0.65 mg/g |
Li et al. (2015) |
| Chlorinated Paraffins | / | Hand wipes | USE | n-hexane/acetone (1:1) | 20 min × 3 | Orbitrap-HRMS | 48–103% | 43–18,000 ng/ participant | Yuan et al. (2020) |
| Flame Retardants | |||||||||
| BFRs, OPFRs | Polyester, polypropylene, PVC/glass fiber | Carpet, curtain | / | / | / | direct probe-TOF–MS | / | / | Ionas et al. (2015) |
| BFRs, OPFRs | Polyester, acryl | Curtain | USE | 25% HFIP/ chloroform | 30 min (20 + 10) | LC–MS/MS | BFRs: 91–121%; PFRs: 82–122% | n.d. –11,500 μg/g | Miyake et al. (2017) |
| PAEs, BFRs, OPEs | Polyester, cotton | Fabrics | ASE | n-hexane/DCM/ acetone (2:1:1) | / | GC–MS | 58–130% | / | Saini et al. (2016b) |
| OPEs | Polyester, nylon, vinyl, cotton | Infant clothing and raw textiles | Solvent extraction | Methanol | 2 h | HPLC–MS/MS | 63–136% | 4.85–1.18 × 106 ng/g | Zhu et al. (2020) |
| OPEs | Polypropylene | Face masks | USE | n-hexane/acetone (1:1) | 15 min × 2 | LC–MS/MS | 47–115% | 9.71–5835 ng/g | Fernandez–Arribas et al. (2021) |
| OPEs | / | Hand wipes | USE | n-hexane/DCM (1:1) | 5 min × 3 | HPLC | 76.0–89.5% |
children’s hand wipe: 6.5–304 ng/g adult’s hand wipe: 16.1–346 ng/g |
Tan et al. (2018) |
| Antioxidants | |||||||||
| Synthetic phenolic antioxidants (SPAs), Organophosphite antioxidants (OPAs) | Polypropylene | Face masks | USE | Methanol | 60 min | LC–MS/MS | 51–113% for the 1000 ng/g spiking level |
∑SPAs: 4.44 × 103–9.15 × 104 ng/g ∑OPAs: 1.55 × 104–5.13 × 105 ng/g |
Liu and Mabury (2021) |
| Bisphenols (BPA, BPS, etc.) | Polyester, nylon, cotton | Infant clothes | USE | Acetone/DCM (1:4) | 20 min × 2 | HPLC | 60–140% |
BPA:366 ng/g BPS:15 ng/g |
Xue et al. (2017) |
| BPA BPS | Polyester, spandex, nylon, cotton | Clothes | USE | Ethyl acetate | 30 min | HPLC–MS/MS |
BPA: 81.48 ± 19.7% BPS:109.71 ± 6.56% |
BPA: < 3.30 − 1823 ng/g BPS: < 0.53 − 536 ng/g |
Wang et al. (2019a) |
| Bisphenols | Polyester, polyamide, polyacrylonitrile, wool | Raw fibers | USE | DCM or Ethyl acetate | 30 min | GC–MS | 98.7–107.4% |
Polyester: 17.78–243.35 ng/g Polyamide: 67.86–128.42 ng/g Polyacrylonitrile: 75.20–246.48 ng/g |
Sait et al. (2021) |
| Surfactants | |||||||||
| PFAS* | Polypropylene | Face masks | USE | Methanol | 30 min |
LC-qTOF (nonvolatile PFAS) GC–MS (volatile PFAS) |
nonvolatile PFAS:89–90% Volatile PFAS: 99–200% |
15–46 µg/m2 | Muensterman et al. (2022) |
| PFAS | Polyester, nylon, cotton | Furniture textiles, carpets | USE |
Nonvolatile PFAS: methanol Volatile PFAS: ethyl acetate |
15 min × 2 |
UPLC-MS/MS (nonvolatile PFAS) GC–MS (volatile PFAS) |
nonvolatile PFAS:46–108% Volatile PFAS: 62–143% |
n.d. –374 µg/m2 | Vestergren et al. (2015) |
| PFAS | Polyester, nylon, polyamide | Jackets | USE |
Nonvolatile PFAS: acetone/acetonitrile (4:1) Volatile PFAS: n-hexane |
60 min | LC–MS/MS |
nonvolatile PFAS:40–120% Volatile PFAS:100–200% |
0.03–719 μg/m2 | Gremmel et al. (2016) |
| NPE | Polyester, nylon, polyamide,etc. | Clothes | Not mentioned | Acetonitrile: water (7:3) | / | LC–MS/MS | / | 1–45,000 mg/kg | Brigden et al. (2012) |
/: not mentioned
*Nonvolatile PFAS (i.e., ionic PFAS): Perfluorinated alkyl acids (PFAAs, represented by perfluorooctane sulfonate (PFOS)), perfluorinated alkyl sulfonic acids/perfluorinated alkyl sulfonates (PFSAs, represented by perfluorooctanoate carboxylate (PFOA)), perfluoroalkyl carboxylates (PFCAs), and perfluorohexanesulfonic acid (PFHxS); volatile PFAS: more volatile substances such as fluorotelomer alcohol, fluorotelomer acrylate, etc.)
In addition to the traditional extractants mentioned above, some unconventional extraction solutions are also gradually applied. For extraction of phthalate plasticizers, tetrahydrofuran (THF) is recommended by the ISO 14389: 2014 (Wang et al. 2019b) and the Chinese national standard (Textile—Determination of the phthalate content—Tetrahydrofuran method (in Chinese), GB/T 20388–2016). Some studies prove that the THF extraction for PAEs usually exhibits better performance than other solvents, with higher recovery rate of 96.7–110.5% than that of methyl tert–butyl ether (MTBE) (38.3–58.0% recovery) or toluene (62.0–83.8%) (Al–Natsheh et al. 2015; Khan and Jahangir 2020).
Moreover, extractants that enable fibers to be “dissolved” exhibit better extraction efficiency than traditional extractants. Miyake et al. (2017) developed a novel complete dissolution extraction method, i.e., using 25% 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP)/chloroform as extractant to extract 18 brominated flame retardants (BFRs) and 15 phosphorus flame retardants (PFRs) in polyester curtains. By applying the complete dissolution method, more flame retardants were extracted than that via the conventional USE method using toluene or acetone (only 0.5–10% of those measured by the complete dissolution method). Similarly, Li and Kannan (2018) compared two extraction solutions of 25% HFIP/chloroform and acetone/DCM (v/v, 1:4); the former one showed up to 286 times higher extraction efficiency than the latter one. The two examples above confirm that HFIP can well dissolve fibers, such as polyester, nylon, and spandex. Future experiments should focus more on this solvent to gain a better extraction effect.
Extraction Time Selection
The extraction time varies largely among different extraction methods. USE uses small amounts of solvents and allows batch processing of multiple samples. When the additives on fibers are extracted by ultrasonication, the extraction process is usually repeated at least twice, with extraction time ranging from 30 to 60 min (Abdallah et al. 2017; Khan and Jahangir 2020; Wang et al. 2019a). It has been found that for polybrominated diphenyl ethers (PBDEs) extraction from textiles, 30 min is the optimal extraction time, since there is no significant change in the recovery rate beyond 30 min (Abdallah et al. 2017). MAE also has a relatively shorter extraction time of about 15–30 min (Sanchez–Prado et al. 2010). In contrast, Soxhlet extraction method requires longer time, usually at least 4 h for each extraction, making it more costly (Kim et al. 2016; Li et al. 2015; Xu 2021). ASE owns the advantage of high efficiency and automation, with the extraction time of about 15–20 min per sample (Giergielewicz-Możajska et al., 2001). However, ASE cannot be used for batch extraction and may take longer time in case of large number of samples. We summarize the appropriate extraction methods for four additives (Table 1).
Table 1.
Recommended extraction methods for four types of additives on plastic (micro)fibers
| Additives | Pretreatment | Extraction solution | Extraction time | |
|---|---|---|---|---|
| Conventional | Novel | |||
| Plasticizers | USE | Tetrahydrofuran, n-hexane, acetone | HFIP / chloroform (completed dissolved) | 20 min × (2–3 times) |
| Antioxidants | USE | Acetone, DCM, ethyl acetate | ||
| Flame retardants | USE | Acetone, n-hexane, DCM | ||
| Surfactants | USE | Methanol | ||
USE ultrasonic extraction
Instrumental Analysis
Gas chromatography-mass spectrometry (GC–MS) and liquid chromatography-mass spectrometry (LC–MS) are widely used for quantification of additives extracted from synthetic textiles. GC–MS is especially suitable for additives with low boiling point and good thermal stability. Bernard et al. (2017) compared eight different analytical methods for determination of plasticizers and found that GC–MS possessed higher sensitivity (LOD values ranging from 0.03–0.5 µg/ml) than other methods. LC–MS determination of pollutants is not limited by boiling point. Thus, it can be used to analyze large molecule substances with poor thermal stability and weak-volatilization ability, such as flame retardants like tri(2-ethylhexyl) phosphate (TEHP), triphenyl phosphate (TPHP), trimethylphenyl phosphate (TMPP), nonvolatile PFAS, etc. (Lorenzo et al. 2016; Muensterman et al. 2022). Tandem mass spectrometry (MS/MS) realizes selective reaction monitoring (SRM), which greatly reduces the noise level and improves selectivity in the analysis of complex sample matrices (Wang et al. 2020). Currently, high-performance liquid chromatography-tandem mass spectrometry (HPLC–MS/MS), and ultrahigh-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) have become very important techniques for the analysis of flame retardants, novel synthetic antioxidants, and surfactants, because of their good selectivity and sensitivity, high precision, and low detection limits (Abdallah 2016; Bastiaensen et al. 2018; Gremmel et al. 2016; Guo et al. 2016; Vestergren et al. 2015; Wang et al. 2019a; Wu et al. 2019). The ionization of molecules has an important influence on the final quantification. Electron ionization (EI) is very suitable for polar chemicals (Bourdeaux et al. 2016; Stubbings et al. 2019). However, EI is not suitable for high molecular weight chemicals due to the fragments’ difficulty for volatilization and poor thermal stability after ionization. Some soft ionization techniques such as electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) interfaces can effectively solve this limitation (Wang et al. 2020). For example, Halloum et al. (2017) found that the detection limits of GC-APCI-MS/MS were 2.5–25 and 50–100 times lower than those of GC-EI-MS/MS, respectively, for the quantification of non-brominated OPEs and brominated OPEs.
In recent years, emerging quantification techniques that do not require pretreatment have become increasingly popular (Anuar et al. 2022; Jin et al. 2022; Xu et al. 2022). Thermal desorption-gas chromatography-mass spectrometry (TD-GC–MS) and pyrolysis gas chromatography-mass spectrometry (Py-GC–MS) exhibit the advantages of high sensitivity, automation, and solvent interferences-free (Humbert et al. 2022). TD-GC–MS has been found to be effective for the quantification of brominated flame retardants (especially for BDE-209) in curtains and car interiors (Shin and Baek 2012); meanwhile, Py-GC–MS has been increasingly used for the detection of PAEs, flame retardants, ultraviolet stabilizers, and bisphenols. (Akoueson et al. 2022; Deng et al. 2022).
There are also some new analytical methods, such as time of flight mass spectrometry (TOF–MS), electron probe, and environmental forensic microscopy. The principle of TOF–MS is to measure the time for ions to reach the detector from the ion source. The heavier the ion mass, the longer the time to reach the receiver; and vice versa. As a result, ions of different masses can be separated according to their specific m/z. The advantage of TOF–MS is the fast scan speed and high sensitivity. Ionas et al. (2015) have used the ambient high-resolution mass spectrometry (direct probe-TOF–MS) to qualitatively screen flame retardants in textiles (curtains and carpets). TOF–MS was capable of quickly screening BFRs and PFRs in positive and negative ion APCI modes with [M + H] and [M–Br + O]+–, respectively. Moreover, the environmental forensic microscopy is also suitable for investigation of Br distribution (originated from BFRs) on textile surface (Ionas et al. 2015). Nevertheless, environmental forensic microscopy is only recommended for the surface distribution analysis of additives with relatively high concentration. There are also techniques that can quickly screen out samples containing F. Some studies conducted the total F analysis by combustion ion chromatography (CIC) before the extraction of PFAS (Rodgers et al. 2022; Schellenberger et al. 2022). Total F concentration can also be measured by the particle-induced gamma emission (PIGE) technique (Muensterman et al. 2022; Xia et al. 2022). The advantage of CIC or PIGE technique is that total F concentration can be quickly obtained. However, these fast-screening techniques cannot avoid the interference of substances containing fluorine; therefore, they are used as preliminary screening methods. The commonly used extraction methods, solvents, time, and quantification equipment, as well as chemical recoveries for additives in fiber products are summarized (Table 2).
Occurrence of Additives on Plastic Fibers
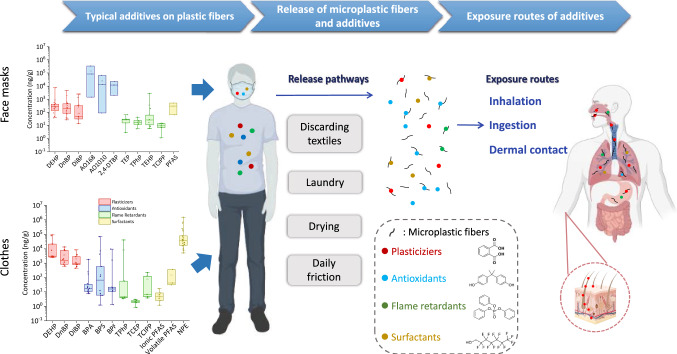
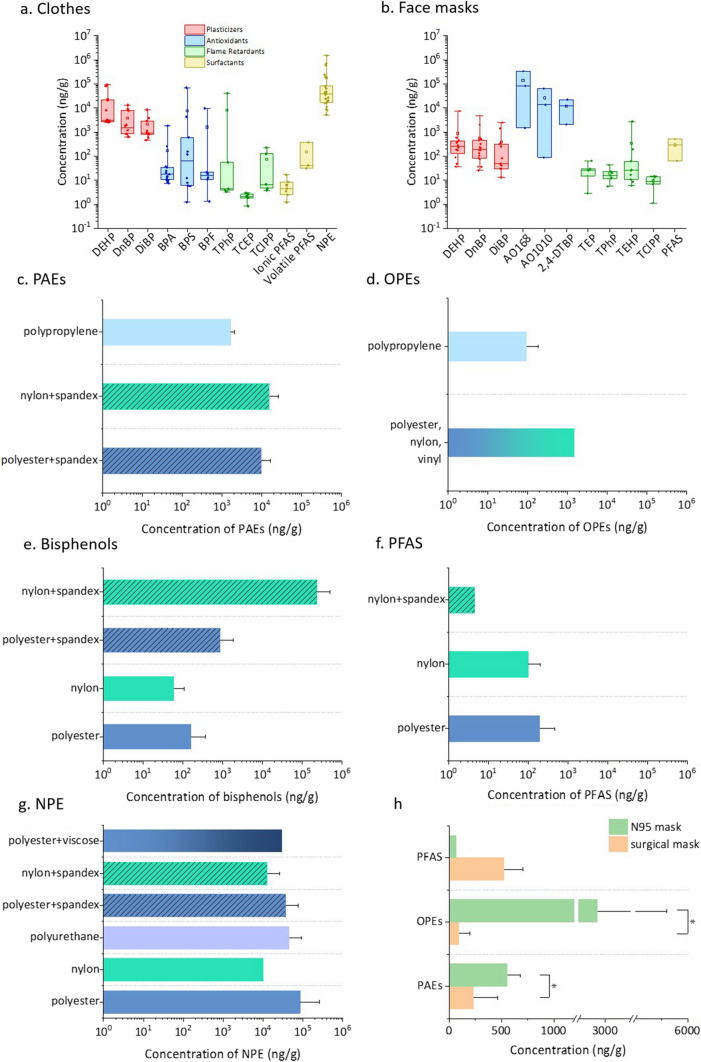
The main processes in the textile production include sizing (improving the abrasion resistance of fibers), desizing (removing sizing chemicals from textiles), scouring (removing impurities from fibers), bleaching (removing unwanted colored matters), mercerizing (improving the strength and luster of textiles), dyeing & printing (adding colors or patterns to textiles) (Athira et al. 2018). To improve the softness, flame resistance, and stability of textiles, various additives and aids are incorporated. As a consequence, some of them, such as aromatic amines, plasticizers, flame retardants, phenolic antioxidants, surfactants, antimicrobial agents, ultraviolet stabilizers (benzotriazole), anti-wrinkling resins, heavy metals, etc., may remain in the clothes (Licina et al. 2019). PAEs, bisphenols, and OPEs have been widely detected in synthetic fibers (Fig. 2a) (Tang et al. 2020; Wang et al. 2019a; Xue et al. 2017).
Fig. 2.
The concentration of typical additives in a clothes and b face masks (ng/g). The maximum, minimum, and median values were obtained from the literature. The upper and lower boundaries of each box represent the 75th and 25th percentiles, respectively. The horizontal line represents the median value. The small square represents the mean value. c–g: The concentration (ng/g) (mean ± SD) of additives on different fiber types. Data were collected from the literature and presented as average values (Brigden et al. 2013, 2012; Li and Kannan 2018; Sait et al. 2021; Tang et al. 2020; Wang et al. 2019a, 2022; Xie et al. 2022; Xue et al. 2017; Zheng and Salamova 2020). h The concentration (ng/g) (mean ± SD) of PAEs, OPEs, and PFAS in surgical and N95 face masks. Data were collected from the literature and presented as average values (Fernandez–Arribas et al. 2021; Muensterman et al. 2022; Wang et al. 2022). Of note, the original data of PFAS concentrations are 46 µg/m2 and 15 µg/m2. To match the unit of “ng/g,” we cut 10 cm2 of surgical and N95 masks, respectively, and weighed them to obtain mass average values, followed by a unit conversion to obtain the concentration of ng/g. Statistical analysis was performed using SPSS Statistics 26.0 software. Normality of the data was tested by the Shapiro–Wilk test. Difference between concentrations of additives in surgical and N95 masks was determined through Mann–Whitney U test (*p < 0.05). (DEHP: bis(2-ethylhexyl) phthalate; DnBP: dibutyl phthalate; DiBP: di-iso-butyl phthalate; BPA: bisphenol A; BPS: bisphenol S; BPF: bisphenol F; 2,4-DTBP: 2,4-di-tert-butyl-phenol; TPhP: triphenyl phosphate; TCEP: tris(2-chloroethyl) phosphate; TCIPP: tris(2-chloropropyl) phosphate; TEHP: tri(2-ethylhexyl) phosphate; TEP: triethyl phosphate; PFAS: per- and polyfluoroalkyl substances; NPE: nonylphenol ethoxylates)
The types of additives are closely related to textile material and functions. Plasticizers are one of the most widely used plastic additives; the addition amount can reach 10–70% (Hahladakis et al. 2018; Hermabessiere et al. 2017). Plasticizers are mainly used in polyurethane (PU) or PVC coating of textiles. In some cases, PVC can even contain 80% of plasticizers (Hahladakis et al. 2018). Clothing having abundant colors with rich prints and coats often exhibits higher concentrations of PAEs (Tang et al. 2020). Nylon (15,203 ± 10,382 ng/g) contains a higher PAEs concentration than polyester (9732 ± 6988 ng/g) (Fig. 2c). Tang et al. (2020) measured that total concentrations of 15 PAEs in children clothing (blends of polyester, nylon, and spandex) were 3.35–33.42 μg/g, indicating a moderate level of incorporated phthalates in plastics. REACH regulates that for toys or childcare articles, the individual or combined concentration of DEHP, DBP, BBP equal to or greater than 0.1% (by weight) (1 × 106 ng/g) should not be put on market (Negev et al. 2018). From the collected data (Fig. 2a), it can be seen that the concentration of major PAEs in the clothes does not exceed the standard (1 × 106 ng/g). Meanwhile, PAEs are widely detected in air particulate matter (Li and Wang 2015); in addition to additives remained during manufacturing, fiber fabrics may also adsorb and accumulate airborne plasticizers emitted by indoor furniture (Shi et al. 2018; Zhang et al. 2020b).
Flame retardants are added to reduce the flammability of objects; the addition amount is 3–25% for BFRs and 0.7–3% for PFRs in plastic materials (Hahladakis et al. 2018). Synthetic fibers should be treated with flame retardants, because the molten drops caused by combustion may burn the skin and lead to the burning of combustible materials around (Bourbigot 2008). Since the ban or restriction of some traditional BFRs according to the Stockholm Convention (Wu et al. 2020), the global consumption of organophosphate flame retardants in textile is increasing yearly (from 186,000 t in 2001 to 680,000 t in 2015) (Pantelaki and Voutsa 2019; Reemtsma et al. 2008). The content of flame retardants in synthetic fibers is often higher than that in cotton fabrics. The average concentration of ∑20OPEs (1.52 × 103 ng/g) in synthetic fibers (polyester, nylon, vinyl) was higher than that in cotton fabrics (442 ng/g), triphenyl phosphate (TPhP), accounting for the highest percentage (40.2% of the total concentration) (Zhu et al. 2020). It is noticed that although the presence of BFRs (e.g., PBDEs) has been detected in textiles, such as carpets, curtains, and seat leather (Abdallah et al. 2017; Portet-Koltalo et al. 2021; Shin and Baek 2012), there are no available reports about BFRs on clothing according to our best knowledge. It may be explained that organophosphorus flame retardants (OPFRs) are more multi-functional, which can act as both flame retardants and plasticizers. On the other hand, some chlorinated OPEs contain both halogens and phosphorus (e.g., tris(2-chloroethyl) phosphate (TCEP), tris(2-chloroiso-propyl) phosphate (TCIPP)), which are versatile in flame retardant action, with less odor and lower toxicity (Pandit et al. 2020). In addition to the elimination of some traditional BFRs, the flame retardants in clothing are therefore dominated by OPFRs. Moreover, fibers may also adsorb semi-volatile flame retardants from the air, since electronic products in offices are sources of flame retardants in air (Fig. 3) (Saini et al. 2016a; Saito et al. 2007).
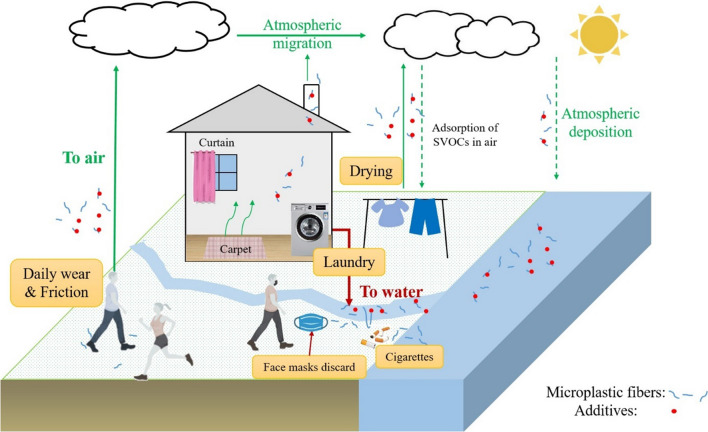
Fig. 3.
Migration and release pathways of additives from microplastic fibers. Icons are created with BioRender.com. (SVOCs: semi-volatile organic chemicals with boiling points range between 240 °C and 400 °C (Lucattini et al. 2018))
Antioxidants are used to delay the overall oxidative degradation of plastics, the addition amount of which is 0.05–3% in plastic materials (Hahladakis et al. 2018). Antioxidants include phenolic antioxidants (e.g., BPA, BPS, BHT, Irganox 1010, Irganox 1076) and organophosphite antioxidants (tris(4-nonyl-phenyl) phosphate (TNPP), tris(2,4-di-tertbutylphenyl) phosphite (AO168), etc.) (Hahladakis et al. 2018). The concentration of bisphenols is closely related to the type of the fibers; spandex exhibits higher levels of bisphenols, especially for fibers blended of nylon and spandex (Fig. 2e). Socks (blends of spandex, nylon, polyester, and cotton) are found to contain higher levels of PAEs and BPA than other clothing (Tang et al. 2020; Xue et al. 2017). Spandex is a typical elastic fiber widely used in stretchable clothing; the addition of bisphenols improves its flexibility (Bodaghi 2020). A study found that the mean concentration of ∑7 bisphenols in pantyhose made of 21–50% spandex (535,000 ng/g) was significantly higher than that in pantyhose made of 0–20% spandex (170,000 ng/g) (Li and Kannan 2018). Studies have also shown that polyester products contain more additives than cotton ones (Xue et al. 2017). Clothes made of polyester and spandex had high concentrations of bisphenols (1823 ng/g for BPA and 536 ng/g for BPS), while mean concentration of bisphenols (BPA + BPS) was only 21 ng/g in cotton clothes (Wang et al. 2019a). The use of synthetic phenolic antioxidants has gradually increased in recent years; synthetic antioxidants have been detected in disposable face masks (Liu and Mabury 2021). However, there are only few reports on synthetic antioxidants on clothing. Thus, more attention should be paid to the content of synthetic antioxidants in clothing fibers in the future.
To improve softness, smoothness, and water resistance of clothes, especially for functional garments, surfactants (e.g., per- and polyfluoroalkyl substances (PFAS), alkylphenol polyethoxylates (APEO), NPE) are often added during production process (Gremmel et al. 2016; Heydebreck et al. 2016; Holmquist et al. 2016; Licina et al. 2019; Zhang et al. 2015). The environmental hazards of PFAS have been gradually recognized due to their environmental persistence and low degradability. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) have been listed in Stockholm Convention (Groffen et al. 2021). Exposure to PFAS poses various health risks, including effects on fertility, endocrine function, obesity of children, etc. (Espartero et al., 2022). The concentration of PFAS did not show significant difference among fiber types; the mean concentration in polyester (193 ± 268 ng/g) is higher than in nylon (98 ± 100 ng/g) (Fig. 2f). PFAS has also been detected in furniture textile products (e.g., curtain, carpet, table cloth) (Vestergren et al. 2015). A study indicated that the concentrations of PFOS in two carpet samples (0.74 µg/m2 and 1.04 µg/m2) approached or even exceeded the EU regulation (1 µg/m2) (Herzke et al. 2012). PFAS function as surfactants, fabrics with fluorinated coatings may release fewer fibers after washing; however, fluorinated wastewater has a negative impact on the environment (Schellenberger et al. 2019). NPE compounds are another cheap and common surfactants. NPE and their degradation products, nonylphenol, are typical endocrine disruptors, which can affect sperm quality and lead to cancer development (Noorimotlagh et al. 2017, 2020). A survey conducted by Greenpeace International in 2012 revealed that NPE compounds were the most frequently detected substances in 20 branded textile products, with a detection rate of 63% and median concentration of 5.2–1500 mg/kg (Brigden et al. 2012). For 8 luxury brands, the detection rate of NPE was 44%, with concentrations ranging from 1.7 to 760 mg/kg (Brigden et al. 2013). It can be seen that the concentrations of NPE in some clothes exceed the REACH standard (1 × 106 ng/g) (Fig. 2a). The concentrations of NPE in polyester (84,789 ± 179,215 ng/g) are higher than that in nylon (10,000 ng/g); the presence of spandex has no effect on NPE concentration (Fig. 2g). In addition, it has been found that the mean concentration of ∑20OPEs in water-repellent fabrics made of nylon or polyester (1940 ng/g) was significantly higher than that in conventional fabrics made of cotton or polyester (313 ng/g) (Zhu et al. 2020), suggesting that functional garments may contain more additives.
With the Covid-19 pandemic, face masks made of non-woven polypropylene (PP) or polyethylene terephthalate have become emerging MFs contributors to the environment (Fadare and Okoffo 2020; Wang et al. 2022). Chemicals such as antioxidants, plasticizers, and surfactants may be added during the manufacturing of face masks (Liu and Mabury 2021; Muensterman et al. 2022; Sungur and Gulmez 2015; Xie et al. 2022). Total concentrations of PAEs and synthetic antioxidants in face masks ranged from 115 to 37,700 ng/g and from 20.0 to 575 μg/g, respectively (Liu and Mabury 2021; Xie et al. 2022). DEHP, DnBP, DiBP, 2,4-di-tert-butyl-phenol (2,4-DTBP), pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionate) (AO1010), and AO168 have been frequently detected in face masks (Liu and Mabury 2021; Xie et al. 2022). The antioxidant contents in face masks are quite high (Fig. 2b), while the types of antioxidants on face masks are different from those of clothes. Bisphenols are widely detected in clothes, while lower or undetectable levels of bisphenols are found in face masks. Only one study reported the presence of BPA in surgical masks leachates (0.8–3.2 μg/L) (Liu et al. 2022). This phenomenon may be attributed to high toxicity of bisphenols. Some other phenolic antioxidants such as BHT and butyl hydroxyanisole (BHA) may be relatively “safer,” which can even be used as food additives to extend the shelf life of fried foods (Liu and Mabury 2020; Wang et al. 2021). On the other hand, some novel antioxidants (e.g., AO168, AO1010) receive less attention and lack of effective regulatory measures.
Different types of face masks exhibit different additive concentrations (Fig. 2h). N95 masks contained more flame retardant OPEs and PAEs (OPEs:11.6 ± 10.3 µg/mask (2924.4 ± 2873.2 ng/g), PAEs: 2300 ± 150 to 5200 ± 800 ng/mask (556.0 ± 124.5 ng/g)) than surgical masks (OPEs:0.24 ± 0.27 µg/mask (93.6 ± 107.1 ng/g), PAEs: 55 ± 35–1700 ± 140 ng/mask (230.9 ± 236.6 ng/g)) (Fernandez–Arribas et al. 2021; Wang et al. 2022). However, this phenomenon has not been clearly interpreted. We speculate that this may be due to the higher filtering capacity of N95 masks for bacteria or particulate matter. The density of polypropylene in N95 masks is higher than in normal masks. Therefore, the manufacturing process is more complex, resulting in higher OPEs or PAEs levels. However, there are exceptions that not all N95 masks have higher additive levels. For instance, Muensterman et al. (2022) found that the total PFAS concentrations in surgical masks (46 µg/m2, converted to be 521.7 ng/g) were higher than that in N95 masks (15 µg/m2, converted to be 64.8 ng/g).
Compared with non-fiber plastics or microplastics, the contents of additives in plastic fibers are generally equivalent to the same order of magnitude or even higher. For example, the concentrations of 16 PAEs in PP take-out food containers were 1.62–8.62 μg/g, while the concentrations of 15 PAEs in clothing fibers were 3.35–33.42 μg/g (Han et al. 2021; Tang et al. 2020). Compared with PP fragments (Table 3), face mask fibers (made of PP) exhibit lower levels of phenolic antioxidants and higher levels of plasticizers such as PAEs.
Table 3.
Comparison of typical additive concentrations in plastic fibers and other shapes of plastics
| Additives | Concentration of additives in fibers | Reference | Concentration of additives in plastics | Reference |
|---|---|---|---|---|
| PP fibers (face masks) | Liu and Mabury (2021) | PP plastic fragments | Rani et al. (2017) | |
| AO1010 | 0.0898–65.4 μg/g | 17–155 μg/g | ||
| AO1076 | 0–49.9 μg/g | 0–169 μg/g | ||
| BHT | 0–2.38 μg/g | 0.02–1.0 μg/g | ||
| 2,4-DTBP | 0–22.5 μg/g | 0.64–11 μg/g | ||
| ∑ phenolic antioxidants | 4.44–91.5 μg/g | 53.2–200.3 μg/g | ||
| PP fibers (face masks) | PP flakes and fragments | Zhang et al. (2018) | ||
| PAEs | 115–37,700 ng/g | Xie et al. (2022) | 0.29–27.2 ng/g | |
| OPEs | 9.71–5835 ng/g | Fernandez–Arribas et al. (2021) | 6.38–2377.5 ng/g |
Release of Additives from (Micro)Plastic Fibers
Release to Water
Washing of synthetic textiles is one of the most important routes for the release of additives from plastic fibers (Luongo et al. 2016; Wang et al. 2019a; Zheng and Salamova 2020). Abrasion of synthetic textiles during laundry is also an important source of microplastics released to aquatic environment (Siegfried et al. 2017). About 2.1 × 105 MFs could be released from polyester clothes during a single machine wash (Sillanpaa and Sainio 2017).
The release of MFs and additives is affected by the following factors summarized in Table 4: water volume, temperature, duration, washing program, use of detergent/softener/textile finishes, fabric types, numbers of washing, fabric weave construction, and chemical properties of additives (De Falco et al. 2018; Hernandez et al. 2017; Kelly et al. 2019; Napper and Thompson 2016; Saini et al. 2016b; Wang et al. 2019a) (Table 4). The factors affecting cotton fiber release were also included, since cotton fibers and plastic fibers may have the same release pattern (such as the use of textile finishes released more fibers, regardless of the fiber type). On the other hand, different fiber types may have different release patterns during washing.
Table 4.
Factors affecting the release of plastic fibers, natural fibers, and fiber additives to water
| Target | Factors | References | ||
|---|---|---|---|---|
| MFs release | Water volume | High water volume wash caused more MFs release than lower water volume | Kelly et al. (2019) | |
| Temperature/time | Higher temperature and longer time caused more MFs release | Cotton et al. (2020); Dalla Fontana et al. (2020) | ||
| Detergent/softener | The use of detergent caused more MFs release, while the use of softener reduces the MFs release | De Falco et al. (2018); Hernandez et al. (2017) | ||
| Fiber type* | Polyester or acrylic fabrics shed more fibers than cotton blended fabric | Napper and Thompson (2016) | ||
| Polyester fabrics released fewer fibers than cotton ones | Sillanpaa and Sainio (2017) | |||
| Polypropylene and polyurethane face masks released fewer microfibers than cotton ones | De Felice et al. 2022) | |||
| Fabric weave construction | Textile with short spun-staple yarn construction shed more MFs than those with woven construction and filamentous yarns | Vassilenko et al. (2021) | ||
| Fabrics sewed with double heat-sealing released less MFs than those sewed with normal thread | Dalla Fontana et al. (2021) | |||
| Mechanical treatment (brushed, sanded or sheared) | Mechanically treated fabrics shed more MFs than untreated ones | Vassilenko et al. (2021) | ||
| Textile finishes* | Fabrics treated with finishes (dyes, durable press, and water repellent) shed more microfibers during laundering than untreated ones | Zambrano et al. (2021) | ||
| Additives release | Chemical properties of additives | Polarity (log KOW) | Polar chemicals (log KOW < 4, e.g., aliphatic OPEs: TnBP, TCEP, TCIPP) are more likely to be released to water; non-polar chemicals (log KOW > 6, e.g., DEHP, BFRs) hardly release to water | Saini et al. (2016b) |
| Hydrophilicity | Migration rate of PFAS from infant clothes reached 100% at 20 °C and 50 °C | Zheng and Salamova (2020) | ||
| Salinity | For polyamide MFs, 2 chemicals were identified in the 14–-day seawater leachates, but not in freshwater leachates | Sait et al. (2021) | ||
The release of MFs to water depends on the washing conditions, while the release of additives is related to their chemical properties. Additives with higher polarity or hydrophilicity are more prone to be released to aquatic environment (Table 4). Besides, additives can be released to the surrounding environment since most of them are not chemically bound to the polymer matrix (exception: TBBPA is chemically bounded) (Hermabessiere et al. 2017).
In addition to the washing of synthetic fiber products, discarding cigarette butts or face masks can also cause MFs or chemicals released to aquatic environment (Fig. 3). Discarded cigarette butts result in about 300,000 tons of cellulose acetate MFs entering the aquatic environment annually. What accompanied is the release of toxic chemicals such as nicotine, carcinogenic tar, polycyclic aromatic hydrocarbons, and heavy metals (cadmium, lead), which have been proven to pose toxic risk to marine organisms (Shen et al. 2021; Torkashvand et al. 2020; Wright et al. 2015). Micro and nano scale polymeric fibers and heavy metals such as cadmium, lead, and antimony have also been detected in face mask leachate. The presence of heavy metals may be attributed to the dyes used in production of colored masks (Sullivan et al. 2021; Sungur and Gulmez 2015). MFs released from face masks can also become carriers of additives and contaminants. It is estimated that approximately 3.4 billion disposable face masks are discarded globally every day, which cause complex environmental problems (Aragaw 2020; Benson et al. 2021). Moreover, there is also growing interest in novel environmental friendly face masks, such as polylactic acid (PLA) biodegradable masks (Soo et al. 2022). With the advantage of faster degradation rate, biodegradable fibers are also more likely to release additives.
Once MFs enter the aquatic environment, ultraviolet irradiation will accelerate fiber degradation and additives release. Ultraviolet exposure of two months resulted in surface degradation (holes appearance) of polyamide fibers and fragmentation (length reduction) of polyester fibers. In seawater leachates, the concentration of additives (TPhP, TCEP, etc.) released by MFs increased with increasing time (Sorensen et al. 2021). The leaching of additives caused by fragmentation or degradation of plastic fibers deserves further attention.
Release to Air
The release of MFs and additives to the air is also an important pathway. Via daily wear of polyester clothes and human activity, one person can release about 1.03 × 109 MFs to the air per year (De Falco et al. 2020). The drying process is another important source of MFs release (Kapp and Miller 2020). A household tumble dryer could release 433,128 (cotton) and 561,810 (polyester) microfibers in 15 min; the annual release of microfibers by a dryer may be even greater than the number of microfibers released through washing (Tao et al. 2022). Although many literatures reported the release of MFs to the air, little attention has been paid to the additives on MFs. Future study should focus more on additives release to the air together with MFs.
There are two main pathways for additives to be released to the air from MFs: (1) direct release by evaporation effect; (2) indirect release by the MFs generated by abrasion. The latter pathway is less studied. Schellenberger et al. (2022) explored the emission mechanism of PFAS from functional textiles (polyamide) under outdoor weathering conditions, revealing that in addition to the direct evaporation release, PFAS could also be released from abrasion and degradation of fibers. Moreover, some flame retardants (e.g., decabromodiphenylethane (DBDPE), PBDEs) released from electronic dryer may become indirect source of additives released to the air together with MFs (Saini et al. 2016b; Schecter et al. 2009).
MFs can account for up to 33% of the total microplastics in urban dust (Dehghani et al. 2017). These MFs can become carriers of additives during suspension, deposition, and migration in the air. PAEs, bisphenols, and flame retardants have widely been detected in airborne dust (Mitro et al. 2016). Zhang et al. (2020a) reported that the concentration of BPA in indoor dust samples was proportional to the concentration of polycarbonate (PC)-based microplastics, which also further confirmed that microplastic (fibers) is an important source of contaminants in dust.
After the outbreak of Covid-19 pandemic, face masks have become a contributor of polypropylene MFs. Additives like PAEs, OPEs, or synthetic antioxidants in them may be released to the air together with the use and abrasion of face masks. The exposure to MFs or additives through inhalation deserves attention. In regard to the humidity during breathing and higher temperature in summer, the release of some additives (e.g., OPEs) from face masks may increase (Fernandez–Arribas et al. 2021).
Release in Organisms
Plastics can act as a carrier of additives and transport over long distances. The disposal of face masks has become an emerging environmental problem in the last two years. For the first time, a PP face mask has even been found in the feces of a green sea turtle (Chelonia mydas) near the coast of Japan; the risk of exposure to additives through plastics ingestion is of concern (Chowdhury et al. 2021; Fukuoka et al. 2022). MFs are ubiquitous in the marine environment, which are easily ingested by organisms of all trophic levels due to their small sizes. Ingestion of MFs by aquatic organisms can lead to growth inhibition, impairment of the immune system, and disruption of the gut microbiota; MFs have higher acute toxicity for lower taxa aquatic organisms (Rebelein et al. 2021). However, many exposure studies of MFs fail to distinguish between the toxicity effects of MFs and their additives (Alnajar et al. 2021). Although some indoor exposure experiments point out that MFs can be excreted gradually by organisms from their bodies through digestion (Grigorakis et al. 2017; Song et al. 2019), the additives loaded on MFs may be desorbed under intestinal conditions.
Most current experiments only focus on the biological effects of MFs, ignoring the exposure risks caused by additives in MFs. Additives have been proven to be released in organism from plastics or microplastics. In addition to chemical property of additives (log KOW), unique gastric environment of certain organisms such as higher temperature (body temperature of seabirds ≈ 40℃), low pH value, and the occurrence of stomach oil may accelerate the leaching of additives (Andrade et al. 2021; Kühn et al. 2020; Sun et al. 2021; Tanaka et al. 2013). At present, indoor exposure experiments on biological effects of microplastics and chemicals mostly focus on granular microplastics, due to the ease of purchase or preparation of granular microplastics. However, fibrous microplastics rather than granular ones are the most common type of microplastics in actual aquatic environment. In view of this, there exists a vacancy in research on the release of additives to organisms from fibers or MFs.
Estimation of Additive Amounts Released by Microplastic Fibers
As estimated by De Falco et al. (2020), one person could release about 2.98 × 108 polyester MFs to water via laundry and 1.03 × 109 to air via wearing polyester clothes per year. We converted the MF number concentration to mass concentration referring to the formula of Leusch and Ziajahromi (2021), i.e., 129.2 g to water and 446.5 g to air.
Here, we took the most common plastic additives PAEs as an example. According to the collected data, the concentration of PAEs in clothes ranges about 3.35–33.42 μg/g (Chai et al. 2017; Li et al. 2019; Liu et al. 2020; Tang et al. 2020). Assuming that the concentration of PAEs on the MFs is the same as clothes, i.e., the additives in clothes can all be released with the fiber without loss. The mass of PAEs released per person per year:
Based on a global population of 8 billion, the global mass of PAEs released to water is 3.46–34.55 t per year via washing, and to air is 11.97–119.39 t per year via wearing polyester clothes. Similarly, the global mass of OPEs, bisphenols, PFAS, and NPE released from MFs per year is 0.0050–10.09 t, 0.0060–552.98 t, 0.0046–0.39 t, and 1.24–568.48 t to water, respectively; and 0.017–34.88 t, 0.021–1911.02 t, 0.016–1.36 t, and 4.29–1964.6 t to air, respectively (Table 5).
Table 5.
Estimation of the mass of additives released and exposure concentrations
| Additives | Concentrations of additives on fibers or MFs (μg/g) | Mass of additives released from polyester MFs by a person per year (mg/person)a | The global mass of additives released per year (t) (based on a population of 8 billion) | Exposure of additives through MFs released by clothing per year (μg/person)b | Estimated daily intakes (EDIinhalation) of additives from face masks | Exposure of additives through direct inhalation associated with face masks per year (μg/person)c | Tolerable daily intakes (TDIs) (μg/kg BW/d) d | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| To water | To air | To water | To air | Inhalation | Ingestion | Surgical masks | N95 | ||||
| PAEs | 3.35–33.42 | 0.43–4.32 | 1.50–14.92 | 3.46–34.55 | 11.97–119.39 | 0–391.40 | 0.34–12.37 |
surgical masks: 0.3–4.9 ng/kg BW/d (adults) 1.9–30 ng/kg BW/d (toddler) N95 masks: 14–27 ng/kg BW/d (adults) 85–160 ng/kg BW/d (toddler) Wang et al. (2022) |
5.46–89.18 (adults) 6.42–101.4 (toddler) |
254.8–491.4 (adults) 287.3–540.8 (toddler) |
/ |
| OPEs | 0.00485–9.764 | 0.0006–152.46 | 0.0021–526.87 | 0.0050–10.09 | 0.017–34.88 | 0–114.34 | 0.00049–3.61 |
surgical masks: 0.04–1.02 ng/kg BW/d (adults) N95 masks: 0.46–29.2 ng/kg BW/d (adults) Fernandez–Arribas et al. (2021) |
0.73–18.56 (adults) | 8.37–531.44 (adults) | / |
| Bisphenols | 0.00585–535 | 0.0008–69.12 | 0.0026–238.88 | 0.0060–552.98 | 0.021–1911.02 | 0–6264.85 | 0.00059–197.95 | / | / | / | |
| PFAS | 0.0045–0.382 | 0.0005–0.05 | 0.002–0.17 | 0.0046–0.39 | 0.016–1.36 | 0–4.48 | 0.00045–0.14 |
0.04–0.10 μg/kg BW/d (adults) 0.1–0.13 μg/kg BW/d (toddler) Muensterman et al. (2022) |
728–1820 (adults) 338–439.4 (toddler) |
/ | |
| NPE | 1.2–550 | 0.15–71.06 | 0.54–245.58 | 1.24–568.48 | 4.29–1964.6 | 0–6440.5 | 0.12–203.5 | / | / | / | |
| DEHP | 2.68–3.22 | 0.34–0.42 | 1.20–1.44 | 2.77–3.33 | 9.57–11.50 | 0–37.71 | 0.27–1.19 |
surgical masks: 0.87–1.2 ng/kg BW/d (adults) 5.3–7.5 ng/kg BW/d (toddler) N95 masks: 4.2–14 ng/kg BW/d (adults) 25–85 ng/kg BW/d (toddler) Wang et al. (2022) |
/ | 50 | |
| TCIPP | 0.0038–0.23 | 0.0005–0.03 | 0.0017–0.10 | 0.0039–0.24 | 0.013–0.82 | 0–2.69 | 0.00038–0.085 | Not mentioned | / | 10 | |
| BPA | 0.0076–1.81 | 0.001–0.23 | 0.0034–0.81 | 0.0078–1.87 | 0.027–6.46 | 0–21.20 | 0.00076–0.67 | Not mentioned | / | 50 | |
aone person could release 129.2 g MFs to water via laundry and 446.5 g MFs to air via wearing polyester clothes per year, according to the estimation byDe Falco et al. (2020)
bone person could inhale and ingest about 0–11.71 g and 0.10–0.37 g MFs per year, according to the estimation by Zhang et al. (Zhang et al. 2022, 2020c)
cAverage body weight was assumed to be 70 kg for adults and 13 kg for toddlers (Fernandez–Arribas et al. 2021; Wang et al. 2022), assuming that the mask is worn 260 days per year (the approximate number of working days per year)
dTolerable daily intake (TDI) values were obtained from the literature (Fernandez–Arribas et al. 2021; Wang et al. 2022)
Exposure and Health Risks
Dermal Exposure
Clothes cover approximately 85% of human skin and act as a barrier to block environmental pollutants. However, clothes can also be a potential exposure source of certain chemicals (Fig. 4). For textiles (especially clothes), dermal exposure is an important exposure pathway. Dermal exposure doses of PAEs and bisphenols were 11.83–950 ng/kg BW/d (302.3–24,272.5 μg /year) and 0.21–0.26 ng/kg BW/d (5.4–6.6 μg/year), respectively (Liu et al. 2020; Tang et al. 2020; Xue et al. 2017). Socks containing BPA had great effect on infant, with a maximum BPA exposure dose of 7.28 ng/kg BW/d (Xue et al. 2017). As mentioned above, PVC prints are mostly found in children’s clothing, which contain high levels of PAEs. Children and infants are the most vulnerable groups to endocrine disruptors. Sweating can increase the risk of dermal exposure to additives such as PAEs or BPA (Liu et al. 2020; Xue et al. 2017). Bad habits such as biting and sucking fingers of infants and children may also pose exposure risk of oral ingestion. According to a survey, the mean levels of PAEs (DEHP 6.74%, DINP 1.32%) in childcare products (toys, baby mattresses and textiles, baby diaper pads) exceed the 0.1% standard of the European Union, which are likely to pose high oral or dermal exposure risks (Negev et al. 2018).
Fig. 4.
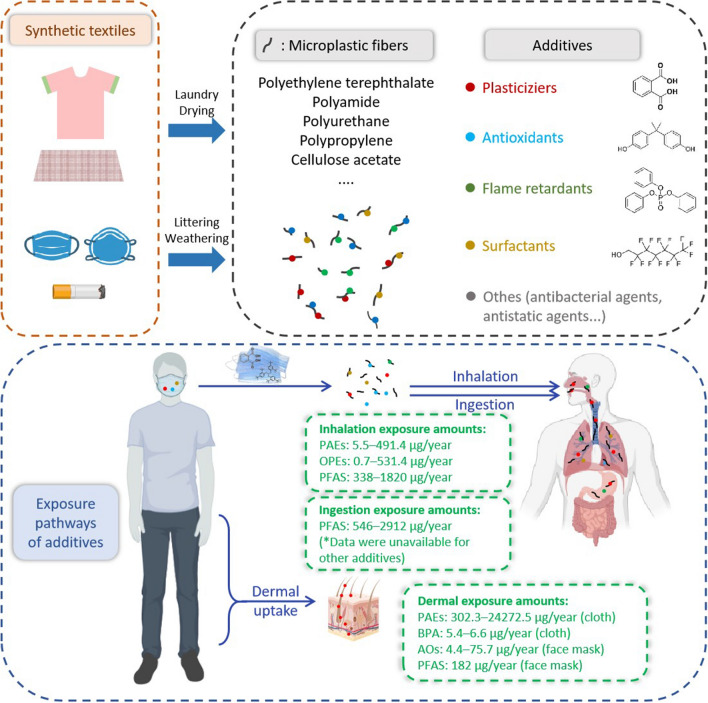
The additives in MFs and human exposure pathways of additives in textiles. The exposure amounts (i.e., estimated daily intake (EDI) values, expressed in the unit of ng/kg BW/d or μg/kg BW/d), were obtained from the literature (Fernandez–Arribas et al. 2021; Liu and Mabury 2021; Liu et al. 2020; Muensterman et al. 2022; Tang et al. 2020; Wang et al. 2022; Xue et al. 2017). Average body weight was assumed to be 70 kg for adult. We assumed that the mask is worn 260 days per year and clothes are worn 365 days per year. The unit of exposure amounts is expressed as μg/year in Fig. 4. Icons are created with BioRender.com. (MFs: microplastic fibers; PAEs: phthalates; BPA: bisphenol A; OPEs: organophosphorus esters; AOs: synthetic antioxidants, including synthetic phenolic antioxidants and organophosphite antioxidants; PFAS: per- and polyfluoroalkyl substances)
Inhalation and Ingestion (of Microplastic Fibers)
Human beings and other organisms are exposed to MFs mainly via three routes, including inhalation, ingestion, and dermal exposure. Only the former two exposure routes can cause actual MF intake. Inhalation of MFs can adversely affect the respiratory tracts (Lim et al. 2021; Moolgavkar et al. 2001), which has also been suggested to be associated with the formation of ground glass nodules in human lungs (Chen et al. 2022). Ingestion of MFs has been confirmed in various organisms, including aquatic organisms (fish, decapods, bivalves, zooplankton, etc.), terrestrial organisms (earthworm, snails), and even human beings (Lahive et al. 2022; Rebelein et al. 2021; Song et al. 2019; Zhang et al. 2022). MFs ingestion can cause oxidative stress and inflammation in fish (Zhao et al. 2021), MFs ingestion may be associated with inflammatory bowel disease in human beings (Yan et al. 2022), and even immune disorders and increased risk of neoplasia in the long run (Prata et al. 2020).
As a necessity under the Covid-19 pandemic, the additive inhalation risks caused by wearing face masks deserve attention. N95 masks may cause higher inhalation risk than general surgical masks (Table 5). According to the collected estimated daily intake (EDI) values, the exposure amounts of additives from face masks are 0.7–1820 μg/person/year through inhalation and 546–2912 μg/person/year through ingestion (Fig. 4, Table 5). Attentionally, although the EDI value of DEHP is at a safe level (not exceed the TDI value of 50,000 ng/kg BW/d, Table 5), wearing N95 masks for long time (occupational groups, such as doctors), taking high physical activity, and under higher temperature or humidity in summer may pose higher inhalation risk (Fernandez-Arribas et al., 2021; Muensterman et al. 2022). The chemicals in face masks may be inhaled or ingested orally under long time of wearing; thus, it is necessary to regulate the type and content of additives in face masks in the context that Covid-19 will possibly coexist with humans for a long time.
In addition to the risk caused by direct release of additives on fiber products, there are also effects posed by additive release from MFs, posing higher risks than plastic monomers (Rodrigues et al. 2019). MFs can enter organisms directly via inhalation through the respiratory tract or ingestion through the digestive tract. We calculated the exposure amounts of additives through microplastic fibers inhalation and ingestion and are listed in Table 5. Based on the data provided by Zhang et al. (Zhang et al. 2022, 2020c), i.e., one person could ingest approximately (2.3–8.5) × 104 microfibers via dining and inhale (0–3.0) × 107 microplastics per year. Since airborne microplastics are mainly fibrous, we assumed that 90% of the inhaled MPs are fibers. After conversion, the mass of microfibers inhaled and ingested per person per year is 0–11.71 g and 0.10–0.37 g, respectively (the conversion of MF mass and quantity refer to the formula of Leusch and Ziajahromi (2021)). We selected the three most common chemicals (DEHP, BPA, TCIPP) as an example. For instance, one person may inhale about 0–37.71 μg DEHP (the most typical PAE with high detection frequency and concentration) and ingest about 0.27–1.19 μg DEHP with MFs per year (Table 5). The tolerable daily intake (TDI) value of DEHP is 50 μg/kg BW/d. Assuming that average body weight to be 70 kg for adult, the TDI value for DEHP is 1.28 g/person per year (50 μg/kg BW/d *70 kg*365 d = 1.28 g), the mass of DEHP that one person may inhale or ingest per year does not exceed the TDI value. Similarly, the mass of BPA or TCIPP that one person may inhale or ingest per year does not exceed the TDI value. According to our estimation (Table 5), the maximum exposure amounts of additives through inhalation and ingestion of MFs released from clothing are 4.48–6440.5 µg/person/year and 0.14–203.5 µg/person/year, respectively. Such situation still cannot be ignored and deserves further attention.
Conclusions and Outlook
MFs are ubiquitous in our daily lives, since actions such as the washing, drying, and abrasion of clothes, human contact friction, and the use and discard of face masks all cause MFs release into the environment. However, there is insufficient understanding about additives on MFs. When MFs are abrased and released, the additives can also be released into the environment accordingly, posing potential ecological and health risks to organisms.
In this review, we first summarized analytical methods of additives in synthetic textiles, and recommended sample extraction and compounds quantification methods for typical additives. Second, we comprehensively analyzed the types and concentrations of additives in textile fibers and MFs. Typical additives in traditional fiber products (clothes) and emerging fiber product (face masks) include plasticizers (DEHP, DBP), flame retardants (TCEP, TPhP, TEHP), antioxidants (bisphenols, AO168, AO1010, DBP), and surfactants (PFAS, NPEs), at concentrations of 100–106 ng/g. Finally, we discussed the main release pathways of additives in MFs to the environment, i.e., release to water through washing and release to the air through abrasion or drying. Additives in fiber products pose health risks through inhalation (0.7–1820 μg/person/year), ingestion (546–2912 μg/person/year), and dermal exposure to MFs (4.4–24,272.5 μg/person/year).
Collectively, we reviewed the occurrence and abundance of additives in synthetic textiles, which can release MFs via various daily life processes, including laundry, drying, abrasion, etc. The wide occurrence and exposure amounts of additives from MFs/fibers were confirmed, indicating that MFs pollution in daily life and the potential health risks should not be underestimated.
Finally, several perspectives on the research of chemical additives in MFs were proposed: (1) There exists a vacancy in extraction or analysis methods targeting additives on MFs. Since the mass of environmental MFs collected is too low to meet the detection limits of instruments, future research could focus more on the development of equipment with high sensitivity, automation, and approaches without extraction pretreatment. (2) Current studies mainly focus on the release of additives from large plastic fibers, whereas little attention has been paid to the carrier role of MFs. Much more future work needs to be performed to understand the potential leaching of additives from MFs. (3) The chemical additives exposure risk is mainly obtained by estimating EDI values from large plastic fibers. However, humans are more easily to be exposed to additives released from MFs, which only received little attention yet and warrant further in-depth research.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (42077371), the National Key Research and Development project (2022YFC3105900), and the Research Funds of Happiness Flower of the East China Normal University (2021ST2110).
Abbreviations
- 2,4-DTBP
2,4-Di-tert-butyl-phenol
- A01010, Irganox 1010
Pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionate
- AO168
Tris(2,4-di-tertbutylphenyl) phosphite
- APEO
Alkylphenol polyethoxylates
- ASE
Accelerated solvent extraction
- BBP
Benzyl butyl phthalate
- BFR
Brominated flame retardants
- BHA
Butyl hydroxyanisole
- BHT
2,6-Di-tert-butyl-4-methyl phenol
- BPA
Bisphenol A
- BPF
Bisphenol F
- BPS
Bisphenol S
- DCM
Dichloromethane
- DEHP
Bis(2-ethylhexyl) phthalate
- DIBP
Di-iso-butyl phthalate
- DnBP
Dibutyl phthalate
- FTOHs
Fluorotelomer alcohols
- HFIP
1,1,1,3,3,3-Hexafluoro-2-propanol
- Irganox 1076
Octadecyl-3-(3,5-di-tert-buty-4-hydroxyphenyl) propionate
- MAE
Microwave-assisted extraction
- MF
Microplastic fiber
- NP
Nonylphenol
- NPE
Nonylphenol ethoxylates
- OPE
Organophosphorus esters
- OPFR
Organophosphorus flame retardants
- PAE
Phthalate
- PBB
Polybromobiphenyls
- PBDE
Polybrominated diphenyl ethers
- PFAS
Per- and polyfluoroalkyl substances
- PFCA
Perfluoroalkyl carboxylic acids
- PFOS
Perfluorooctanesulphonate
- PFSA
Perfluoroalkyl sulfonic acids
- PP
Polypropylene
- TCEP
Tris(2-chloroethyl) phosphate
- TCIPP
Tris(2-chloropropyl) phosphate
- TCPP
Tris(2-chloroisopropyl) phosphate
- TEHP
Tri(2-ethylhexyl) phosphate
- TEP
Triethyl phosphate
- THF
Tetrahydrofuran
- TMPP
Trimethylphenyl phosphate
- TnBP
Tributyl phosphate
- TNPP
Tris(4-nonyl-phenyl) phosphate
- TPhP
Triphenyl phosphate
- TRIS
Tris (2,3-dibromopropyl) phosphate
- USE
Ultrasonic extraction
- XRF
X-ray fluorescence
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuye Chen, Email: Ye13918597662@163.com.
Qiqing Chen, Email: chenqiqing@sklec.ecnu.edu.cn.
Qun Zhang, Email: 772366084@qq.com.
Chencheng Zuo, Email: 51213904041@stu.ecnu.edu.cn.
Huahong Shi, Email: hhshi@des.ecnu.edu.cn.
References
- Abdallah MA-E. Environmental occurrence, analysis and human exposure to the flame retardant tetrabromobisphenol-A (TBBP-A)-a review. Environ Int. 2016;94:235–250. doi: 10.1016/j.envint.2016.05.026. [DOI] [PubMed] [Google Scholar]
- Abdallah MA-E, Drage DS, Sharkey M, Berresheim H, Harrad S. A rapid method for the determination of brominated flame retardant concentrations in plastics and textiles entering the waste stream. J Sep Sci. 2017;40:3873–3881. doi: 10.1002/jssc.201700497. [DOI] [PubMed] [Google Scholar]
- Akhbarizadeh R, Dobaradaran S, Torkmahalleh MA, Saeedi R, Aibaghi R, Ghasemi FF. Suspended fine particulate matter (PM2.5), microplastics (MPs), and polycyclic aromatic hydrocarbons (PAHs) in air: their possible relationships and health implications. Environ Res. 2021;192:110339. doi: 10.1016/j.envres.2020.110339. [DOI] [PubMed] [Google Scholar]
- Akoueson F, Chbib C, Brémard A, Monchy S, Paul-Pont I, Doyen P, Dehaut A, Duflos G. Identification of plastic additives: Py/TD–GC–HRMS method development and application on food containers. J Anal Appl Pyrolysis. 2022;168:105745. doi: 10.1016/j.jaap.2022.105745. [DOI] [Google Scholar]
- Alnajar N, Jha AN, Turner A. Impacts of microplastic fibres on the marine mussel, Mytilus galloprovinciallis. Chemosphere. 2021;262:128290. doi: 10.1016/j.chemosphere.2020.128290. [DOI] [PubMed] [Google Scholar]
- Al–Natsheh M, Alawi M, Fayyad M, Tarawneh I. Simultaneous GC-MS determination of eight phthalates in total and migrated portions of plasticized polymeric toys and childcare articles. J Chromatogr B. 2015;985:103–109. doi: 10.1016/j.jchromb.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Andrade H, Glüge J, Herzke D, Ashta NM, Nayagar SM, Scheringer M. Oceanic long–range transport of organic additives present in plastic products: an overview. Environ Sci Eur. 2021;33:85. doi: 10.1186/s12302-021-00522-x. [DOI] [Google Scholar]
- Anuar ST, Altarawnah RS, Mohd Ali AA, Lee BQ, Khalik WM, Yusof KM, Ibrahim YS. Utilizing pyrolysis–gas chromatography/mass spectrometry for monitoring and analytical characterization of microplastics in polychaete worms. Polymers. 2022;14:3054. doi: 10.3390/polym14153054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragaw TA. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar Pollut Bull. 2020;159:111517. doi: 10.1016/j.marpolbul.2020.111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athira N, Jaya DJNE, Technology P. The use of fish biomarkers for assessing textile effluent contamination of aquatic ecosystems: a review. Nat Environ Pollut Technol. 2018;17:25–34. [Google Scholar]
- Bastiaensen M, Xu F, Been F, Van den Eede N, Covaci A. Simultaneous determination of 14 urinary biomarkers of exposure to organophosphate flame retardants and plasticizers by LC-MS/MS. Anal Bioanal Chem. 2018;410:7871–7880. doi: 10.1007/s00216-018-1402-2. [DOI] [PubMed] [Google Scholar]
- Benson NU, Bassey DE, Palanisami T. COVID pollution: impact of COVID-19 pandemic on global plastic waste footprint. Heliyon. 2021;7:e06343. doi: 10.1016/j.heliyon.2021.e06343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard L, Bourdeaux D, Pereira B, Azaroual N, Barthelemy C, Breysse C, Chennell P, Cueff R, Dine T, Eljezi T, Feutry F, Genay S, Kambia N, Lecoeur M, Masse M, Odou P, Radaniel T, Simon N, Vaccher C, Verlhac C, Yessad M, Decaudin B, Sautou V. Analysis of plasticizers in PVC medical devices: Performance comparison of eight analytical methods. Talanta. 2017;162:604–611. doi: 10.1016/j.talanta.2016.10.033. [DOI] [PubMed] [Google Scholar]
- Bodaghi A. An overview on the recent developments in reactive plasticizers in polymers. Polym Adv Technol. 2020;31:355–367. doi: 10.1002/pat.4790. [DOI] [Google Scholar]
- Bourbigot S. Flame retardancy of textiles: new approaches. In: Horrocks AR, Price D, editors. Advances in fire retardant materials. Woodhead Publishing; 2008. pp. 9–40. [Google Scholar]
- Bourdeaux D, Yessaad M, Chennell P, Larbre V, Eljezi T, Bernard L, Sautou V, Grp AS. Analysis of PVC plasticizers in medical devices and infused solutions by GC-MS. J Pharm Biomed Anal. 2016;118:206–213. doi: 10.1016/j.jpba.2015.10.034. [DOI] [PubMed] [Google Scholar]
- Brigden K, Labunska I, House E, Santillo D, Johnston PJgo (2012) Hazardous chemicals in branded textile products on sale in 27 places during 2012. Greenpeace Research Laboratories Technical Report. https://www.researchgate.net/publication/263621223
- Brigden K, Hetherington S, Wang M, Santillo D, Johnston P (2013) Hazardous chemicals in branded luxury textile products on sale during 2013. Greenpeace Research Laboratories Technical Report. https://www.greenpeace.org/static/planet4-thailand-stateless/2014/02/799fe2e2-technical-report.pdf
- Chai M, Wang Y, Zhong F, Han X, Tang Z. Distribution and human risks of phthalate esters in children's clothing collected from China (in Chinese) Res Environ Sci. 2017;30:1425–1432. [Google Scholar]
- Chen Q, Gao J, Yu H, Su H, Yang Y, Cao Y, Zhang Q, Ren Y, Hollert H, Shi H, Chen C, Liu H. An emerging role of microplastics in the etiology of lung ground glass nodules. Environ Sci Eur. 2022;34:25. doi: 10.1186/s12302-022-00605-3. [DOI] [Google Scholar]
- Chowdhury H, Chowdhury T, Sait SM. Estimating marine plastic pollution from COVID-19 face masks in coastal regions. Mar Pollut Bull. 2021;168:112419. doi: 10.1016/j.marpolbul.2021.112419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton L, Hayward AS, Lant NJ, Blackburn RS. Improved garment longevity and reduced microfibre release are important sustainability benefits of laundering in colder and quicker washing machine cycles. Dyes Pigm. 2020;177:108120. doi: 10.1016/j.dyepig.2019.108120. [DOI] [Google Scholar]
- Dalla Fontana G, Mossotti R, Montarsolo A. Assessment of microplastics release from polyester fabrics: The impact of different washing conditions. Environ Pollut. 2020;264:113960. doi: 10.1016/j.envpol.2020.113960. [DOI] [PubMed] [Google Scholar]
- Dalla Fontana G, Mossotti R, Montarsolo A. Influence of sewing on microplastic release from textiles during washing. Water Air Soil Pollut. 2021;232:50. doi: 10.1007/s11270-021-04995-7. [DOI] [Google Scholar]
- De Falco F, Gullo MP, Gentile G, Di Pace E, Cocca M, Gelabert L, Brouta-Agnesa M, Rovira A, Escudero R, Villalba R, Mossotti R, Montarsolo A, Gavignano S, Tonin C, Avella M. Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ Pollut. 2018;236:916–925. doi: 10.1016/j.envpol.2017.10.057. [DOI] [PubMed] [Google Scholar]
- De Falco F, Cocca M, Avella M, Thompson RC. Microfiber release to water, via laundering, and to air, via everyday use: a comparison between polyester clothing with differing textile parameters. Environ Sci Technol. 2020;54:3288–3296. doi: 10.1021/acs.est.9b06892. [DOI] [PubMed] [Google Scholar]
- De Felice B, Antenucci S, Ortenzi MA, Parolini M. Laundering of face masks represents an additional source of synthetic and natural microfibers to aquatic ecosystems. Sci Total Environ. 2022;806:150495. doi: 10.1016/j.scitotenv.2021.150495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani S, Moore F, Akhbarizadeh R. Microplastic pollution in deposited urban dust, Tehran metropolis, Iran. Environ Sci Pollut Res. 2017;24:20360–20371. doi: 10.1007/s11356-017-9674-1. [DOI] [PubMed] [Google Scholar]
- Deng H, Su L, Zheng Y, Du F, Liu Q-X, Zheng J, Zhou Z, Shi H. Crack patterns of environmental plastic fragments. Environ Sci Technol. 2022;56:6399–6414. doi: 10.1021/acs.est.1c08100. [DOI] [PubMed] [Google Scholar]
- Dris R, Gasperi J, Saad M, Mirande C, Tassin B. Synthetic fibers in atmospheric fallout: a source of microplastics in the environment? Mar Pollut Bull. 2016;104:290–293. doi: 10.1016/j.marpolbul.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Fadare OO, Okoffo ED. Covid-19 face masks: a potential source of microplastic fibers in the environment. Sci Total Environ. 2020;737:140279. doi: 10.1016/j.scitotenv.2020.140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Arribas J, Moreno T, Bartroli R, Eljarrat E. COVID-19 face masks: a new source of human and environmental exposure to organophosphate esters. Environ Int. 2021;154:106654. doi: 10.1016/j.envint.2021.106654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C, Molina-Molina J-M, Iribarne-Duran LM, Jimenez-Diaz I, Vela-Soria F, Mustieles V, Pedro Arrebola J, Fernandez MF, Artacho-Cordon F, Olea N. Concentrations of bisphenol A and parabens in socks for infants and young children in Spain and their hormone–like activities. Environ Int. 2019;127:592–600. doi: 10.1016/j.envint.2019.04.013. [DOI] [PubMed] [Google Scholar]
- Fu KJ, Yang LS, Feng CS, Chen L. Research on detecting tris-(2, 3-dibromopropyl)-phosphate in textiles with the HPLC/DAD method. Adv Mater Res. 2012;441:640–644. doi: 10.4028/www.scientific.net/AMR.441.640. [DOI] [Google Scholar]
- Fukuoka T, Sakane F, Kinoshita C, Sato K, Mizukawa K, Takada H. Covid-19-derived plastic debris contaminating marine ecosystem: alert from a sea turtle. Mar Pollut Bull. 2022;175:113389. doi: 10.1016/j.marpolbul.2022.113389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giergielewicz-Możajska H, Dąbrowski Ł, Namieśnik J. Accelerated solvent extraction (ASE) in the analysis of environmental solid samples—some aspects of theory and practice. Crit Rev Anal Chem. 2001;31:149–165. doi: 10.1080/20014091076712. [DOI] [Google Scholar]
- Gremmel C, Froemel T, Knepper TP. Systematic determination of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in outdoor jackets. Chemosphere. 2016;160:173–180. doi: 10.1016/j.chemosphere.2016.06.043. [DOI] [PubMed] [Google Scholar]
- Grigorakis S, Mason SA, Drouillard KG. Determination of the gut retention of plastic microbeads and microfibers in goldfish (Carassius auratus) Chemosphere. 2017;169:233–238. doi: 10.1016/j.chemosphere.2016.11.055. [DOI] [PubMed] [Google Scholar]
- Groffen T, Bervoets L, Jeong Y, Willems T, Eens M, Prinsen E. A rapid method for the detection and quantification of legacy and emerging per- and polyfluoroalkyl substances (PFAS) in bird feathers using UPLC-MS/MS. J Chromatogr B. 2021;1172:122653. doi: 10.1016/j.jchromb.2021.122653. [DOI] [PubMed] [Google Scholar]
- Guo X, Mu T, Xian Y, Luo D, Wang C. Ultra–performance liquid chromatography tandem mass spec trometry for the rapid simultaneous analysis of nine organophosphate esters in milk powder. Food Chem. 2016;196:673–681. doi: 10.1016/j.foodchem.2015.09.100. [DOI] [PubMed] [Google Scholar]
- Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater. 2018;344:179–199. doi: 10.1016/j.jhazmat.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Hajiouni S, Mohammadi A, Ramavandi B, Arfaeinia H, De-la-Torre GE, Tekle-Roettering A, Dobaradaran S. Occurrence of microplastics and phthalate esters in urban runoff: a focus on the Persian Gulf coastline. Sci Total Environ. 2022;806:150559. doi: 10.1016/j.scitotenv.2021.150559. [DOI] [PubMed] [Google Scholar]
- Halloum W, Cariou R, Dervilly-Pinel G, Jaber F, Le Bizec B. APCI as an innovative ionization mode compared with EI and CI for the analysis of a large range of organophosphate esters using GC-MS/MS. J Mass Spectrom. 2017;52:54–61. doi: 10.1002/jms.3899. [DOI] [PubMed] [Google Scholar]
- Han Y, Cheng J, Tang Z, He Y, Lyu Y. Widespread occurrence of phthalates in popular take–out food containers from China and the implications for human exposure. J Clean Prod. 2021;290:125851. doi: 10.1016/j.jclepro.2021.125851. [DOI] [Google Scholar]
- Hermabessiere L, Dehaut A, Paul-Pont I, Lacroix C, Jezequel R, Soudant P, Duflos G. Occurrence and effects of plastic additives on marine environments and organisms: a review. Chemosphere. 2017;182:781–793. doi: 10.1016/j.chemosphere.2017.05.096. [DOI] [PubMed] [Google Scholar]
- Hernandez E, Nowack B, Mitrano DM. Polyester textiles as a source of microplastics from households: a mechanistic study to understand microfiber release during washing. Environ Sci Technol. 2017;51:7036–7046. doi: 10.1021/acs.est.7b01750. [DOI] [PubMed] [Google Scholar]
- Herzke D, Olsson E, Posner S. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway—a pilot study. Chemosphere. 2012;88:980–987. doi: 10.1016/j.chemosphere.2012.03.035. [DOI] [PubMed] [Google Scholar]
- Heydebreck F, Tang J, Xie Z, Ebinghaus R. Emissions of per- and polyfluoroalkyl substances in a textile manufacturing plant in China and their relevance for workers' exposure. Environ Sci Technol. 2016;50:10386–10396. doi: 10.1021/acs.est.6b03213. [DOI] [PubMed] [Google Scholar]
- Holmquist H, Schellenberger S, van der Veen I, Peters GM, Leonards PEG, Cousins IT. Properties, performance and associated hazards of state-of-the-art durable water repellent (DWR) chemistry for textile finishing. Environ Int. 2016;91:251–264. doi: 10.1016/j.envint.2016.02.035. [DOI] [PubMed] [Google Scholar]
- Hu A, Qiu M, Liu H, Xu Y, Tao Y, Yang G, He Y, Xu J, Lu Z. Simultaneous determination of phthalate diesters and monoesters in soil using accelerated solvent extraction and ultra-performance liquid chromatography coupled with tandem mass spectrometry. J Chromatogr A. 2020;1626:461347. doi: 10.1016/j.chroma.2020.461347. [DOI] [PubMed] [Google Scholar]
- Humbert K, Debret M, Morin C, Cosme J, Portet-Koltalo F. Direct thermal desorption-gas chromatography-tandem mass spectrometry versus microwave assisted extraction and GC-MS for the simultaneous analysis of polyaromatic hydrocarbons (PAHs, PCBs) from sediments. Talanta. 2022;250:123735. doi: 10.1016/j.talanta.2022.123735. [DOI] [PubMed] [Google Scholar]
- Ionas AC, Gomez AB, Uchida N, Suzuki G, Kajiwara N, Takata K, Takigami H, Leonards PEG, Covaci A. Comprehensive characterisation of flame retardants in textile furnishings by ambient high resolution mass spectrometry, gas chromatography-mass spectrometry and environmental forensic microscopy. Environ Res. 2015;142:712–719. doi: 10.1016/j.envres.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Jane L, Espartero L, Yamada M, Ford J, Owens G, Prow T, Juhasz A. Health–related toxicity of emerging per- and polyfluoroalkyl substances: comparison to legacy PFOS and PFOA. Environ Res. 2022;212:113431. doi: 10.1016/j.envres.2022.113431. [DOI] [PubMed] [Google Scholar]
- Jin M, Liu J, Yu J, Zhou Q, Wu W, Fu L, Yin C, Fernandez C, Karimi-Maleh H. Current development and future challenges in microplastic detection techniques: a bibliometrics-based analysis and review. Sci Prog. 2022;105:00368504221132151. doi: 10.1177/00368504221132151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp KJ, Miller RZ. Electric clothes dryers: an underestimated source of microfiber pollution. PLoS ONE. 2020;15:e0239165. doi: 10.1371/journal.pone.0239165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MR, Lant NJ, Kurr M, Burgess JG. Importance of water–volume on the release of microplastic fibers from laundry. Environ Sci Technol. 2019;53:11735–11744. doi: 10.1021/acs.est.9b03022. [DOI] [PubMed] [Google Scholar]
- Khan U, Jahangir M. Development of a new gas chromatographic–mass spectrometry method for the simultaneous analysis of all regulated phthalates in consumer goods. Int J Environ Anal Chem. 2020;100:1299–1308. doi: 10.1080/03067319.2019.1651850. [DOI] [Google Scholar]
- Kim JW, Kim Y-M, Moon HM, Hosaka A, Watanabe C, Teramae N, Choe EK, Myung S-W. Comparative study of thermal desorption and solvent extraction-gas chromatography-mass spectrometric analysis for the quantification of phthalates in polymers. J Chromatogr A. 2016;1451:33–40. doi: 10.1016/j.chroma.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Kühn S, Booth AM, Sørensen L, van Oyen A, van Franeker JA. Transfer of additive chemicals from marine plastic debris to the stomach oil of northern fulmars. Front Environ Sci. 2020;8:138. doi: 10.3389/fenvs.2020.00138. [DOI] [Google Scholar]
- La Nasa J, Biale G, Mattonai M, Modugno F. Microwave–assisted solvent extraction and double–shot analytical pyrolysis for the quali–quantitation of plasticizers and microplastics in beach sand samples. J Hazard Mater. 2021;401:123287. doi: 10.1016/j.jhazmat.2020.123287. [DOI] [PubMed] [Google Scholar]
- Lahive E, Cross R, Saarloos AI, Horton AA, Svendsen C, Hufenus R, Mitrano DM. Earthworms ingest microplastic fibres and nanoplastics with effects on egestion rate and long–term retention. Sci Total Environ. 2022;807:151022. doi: 10.1016/j.scitotenv.2021.151022. [DOI] [PubMed] [Google Scholar]
- Leusch FDL, Ziajahromi S. Converting mg/L to particles/L: reconciling the occurrence and toxicity literature on microplastics. Environ Sci Technol. 2021;55:11470–11472. doi: 10.1021/acs.est.1c04093. [DOI] [PubMed] [Google Scholar]
- Li AJ, Kannan K. Elevated concentrations of bisphenols, benzophenones, and antimicrobials in pantyhose collected from six countries. Environ Sci Technol. 2018;52:10812–10819. doi: 10.1021/acs.est.8b03129. [DOI] [PubMed] [Google Scholar]
- Li J, Wang G. Airborne particulate endocrine disrupting compounds in China: compositions, size distributions and seasonal variations of phthalate esters and bisphenol A. Atmos Res. 2015;154:138–145. doi: 10.1016/j.atmosres.2014.11.013. [DOI] [Google Scholar]
- Li X, Yang Y, Cui X, Li S, Zhu X, Tang S. Determination of phthalate esters in textiles by solid phase extraction and gas chromatography-mass spectrometry. Anal Lett. 2015;48:2544–2552. doi: 10.1080/00032719.2015.1043665. [DOI] [Google Scholar]
- Li H-L, Ma W-L, Liu L-Y, Zhang Z, Sverko E, Zhang Z-F, Song W-W, Sun Y, Li Y-F. Phthalates in infant cotton clothing: occurrence and implications for human exposure. Sci Total Environ. 2019;683:109–115. doi: 10.1016/j.scitotenv.2019.05.132. [DOI] [PubMed] [Google Scholar]
- Li M, Chen Q, Ma C, Gao Z, Yu H, Xu L, Shi H. Effects of microplastics and food particles on organic pollutants bioaccumulation in equi-fugacity and above-fugacity scenarios. Sci Total Environ. 2022;812:152548. doi: 10.1016/j.scitotenv.2021.152548. [DOI] [PubMed] [Google Scholar]
- Licina D, Morrison GC, Beko G, Weschler CJ, Nazaroff WW. Clothing-mediated exposures to chemicals and particles. Environ Sci Technol. 2019;53:5559–5575. doi: 10.1021/acs.est.9b00272. [DOI] [PubMed] [Google Scholar]
- Lim D, Jeong J, Song KS, Sung JH, Oh SM, Choi J. Inhalation toxicity of polystyrene micro(nano)plastics using modified OECD TG 412. Chemosphere. 2021;262:128330. doi: 10.1016/j.chemosphere.2020.128330. [DOI] [PubMed] [Google Scholar]
- Lin L, Zuo L-Z, Peng J-P, Cai L-Q, Fok L, Yan Y, Li H-X, Xu X-R. Occurrence and distribution of microplastics in an urban river: a case study in the Pearl River along Guangzhou City, China. Sci Total Environ. 2018;644:375–381. doi: 10.1016/j.scitotenv.2018.06.327. [DOI] [PubMed] [Google Scholar]
- Liu R, Mabury SA. Synthetic phenolic antioxidants: a review of environmental occurrence, fate, human exposure, and toxicity. Environ Sci Technol. 2020;54:11706–11719. doi: 10.1021/acs.est.0c05077. [DOI] [PubMed] [Google Scholar]
- Liu R, Mabury SA. Single-use face masks as a potential source of synthetic antioxidants to the environment. Environ Sci Technol Lett. 2021;8:651–655. doi: 10.1021/acs.estlett.1c00422. [DOI] [Google Scholar]
- Liu K, Wang X, Fang T, Xu P, Zhu L, Li D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci Total Environ. 2019;675:462–471. doi: 10.1016/j.scitotenv.2019.04.110. [DOI] [PubMed] [Google Scholar]
- Liu K, Wu T, Wang X, Song Z, Zong C, Wei N, Li D. Consistent transport of terrestrial microplastics to the ocean through atmosphere. Environ Sci Technol. 2019;53:10612–10619. doi: 10.1021/acs.est.9b03427. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang Y, Li K, Wu Y, Wang L. Phthalates in daily clothes: occurrence and human exposure. Asian J Ecotoxicol. 2020;15:186–192. doi: 10.7524/aje.1673-5897.20190303001. [DOI] [Google Scholar]
- Liu Z, Wang J, Yang X, Qe H, Zhu K, Sun Y, Van Hulle S, Jia H. Generation of environmental persistent free radicals (EPFRs) enhances ecotoxicological effects of the disposable face mask waste with the COVID-19 pandemic. Environ Pollut. 2022;301:119019. doi: 10.1016/j.envpol.2022.119019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llompart M, Celeiro M, Dagnac T. Microwave-assisted extraction of pharmaceuticals, personal care products and industrial contaminants in the environment. TrAC-Trends Anal Chem. 2019;116:136–150. doi: 10.1016/j.trac.2019.04.029. [DOI] [Google Scholar]
- Lorenzo M, Campo J, Pico Y. Ultra-high-pressure liquid chromatography tandem mass spectrometry method for the determination of 9 organophosphate flame retardants in water samples. MethodsX. 2016;3:343–349. doi: 10.1016/j.mex.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucattini L, Poma G, Covaci A, de Boer J, Lamoree MH, Leonards PEG. A review of semi-volatile organic compounds (SVOCs) in the indoor environment: occurrence in consumer products, indoor air and dust. Chemosphere. 2018;201:466–482. doi: 10.1016/j.chemosphere.2018.02.161. [DOI] [PubMed] [Google Scholar]
- Luongo G, Avagyan R, Hongyu R, Östman C. The washout effect during laundry on benzothiazole, benzotriazole, quinoline, and their derivatives in clothing textiles. Environ Sci Pollut Res. 2016;23:2537–2548. doi: 10.1007/s11356-015-5405-7. [DOI] [PubMed] [Google Scholar]
- Mitro SD, Dodson RE, Singla V, Adarnkiewicz G, Elmi AF, Tilly MK, Zota AR. Consumer product chemicals in indoor dust: a quantitative meta–analysis of US studies. Environ Sci Technol. 2016;50:10661–10672. doi: 10.1021/acs.est.6b02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y, Tokumura M, Nakayama H, Wang Q, Amagai T, Ogo S, Kume K, Kobayashi T, Takasu S, Ogawa K, Kannan K. Simultaneous determination of brominated and phosphate flame retardants in flame-retarded polyester curtains by a novel extraction method. Sci Total Environ. 2017;601:1333–1339. doi: 10.1016/j.scitotenv.2017.05.249. [DOI] [PubMed] [Google Scholar]
- Moolgavkar SH, Brown RC, Turim J. Biopersistence, fiber length, and cancer risk assessment for inhaled fibers. Inhalation Toxicol. 2001;13:755–772. doi: 10.1080/089583701316941294. [DOI] [PubMed] [Google Scholar]
- Muensterman DJ, Cahuas L, Titaley IA, Schmokel C, De la Cruz FB, Barlaz MA, Carignan CC, Peaslee GF, Field JA. Per- and polyfluoroalkyl substances (PFAS) in facemasks: potential source of human exposure to PFAS with implications for disposal to landfills. Environ Sci Technol Lett. 2022;9:320–326. doi: 10.1021/acs.estlett.2c00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napper IE, Thompson RC. Release of synthetic microplastic plastic fibres from domestic washing machines: effects of fabric type and washing conditions. Mar Pollut Bull. 2016;112:39–45. doi: 10.1016/j.marpolbul.2016.09.025. [DOI] [PubMed] [Google Scholar]
- Negev M, Berman T, Reicher S, Sadeh M, Ardi R, Shammai Y. Concentrations of trace metals, phthalates, bisphenol A and flame-retardants in toys and other children's products in Israel. Chemosphere. 2018;192:217–224. doi: 10.1016/j.chemosphere.2017.10.132. [DOI] [PubMed] [Google Scholar]
- Noorimotlagh Z, Haghighi NJ, Ahmadimoghadam M, Rahim F. An updated systematic review on the possible effect of nonylphenol on male fertility. Environ Sci Pollut Res. 2017;24:3298–3314. doi: 10.1007/s11356-016-7960-y. [DOI] [PubMed] [Google Scholar]
- Noorimotlagh Z, Mirzaee SA, Martinez SS, Rachoń D, Hoseinzadeh M, Jaafarzadeh N. Environmental exposure to nonylphenol and cancer progression risk–a systematic review. Environ Res. 2020;184:109263. doi: 10.1016/j.envres.2020.109263. [DOI] [PubMed] [Google Scholar]
- Pandit P, Singha K, Kumar V, Maity S. Advanced flame–retardant agents for protective textiles and clothing. Adv Funct Protect Text. 2020 doi: 10.1016/B978-0-12-820257-9.00016-3. [DOI] [Google Scholar]
- Pantelaki I, Voutsa D. Organophosphate flame retardants (OPFRs): a review on analytical methods and occurrence in wastewater and aquatic environment. Sci Total Environ. 2019;649:247–263. doi: 10.1016/j.scitotenv.2018.08.286. [DOI] [PubMed] [Google Scholar]
- Pepper LR (2021) Preferred fiber & materials market report 2021. https://textileexchange.org/preferred-fiber-and-materials-market-report/
- Petreas M, Gill R, Takaku-Pugh S, Lytle E, Parry E, Wang M, Quinn J, Park J-S. Rapid methodology to screen flame retardants in upholstered furniture for compliance with new California labeling law (SB 1019) Chemosphere. 2016;152:353–359. doi: 10.1016/j.chemosphere.2016.02.102. [DOI] [PubMed] [Google Scholar]
- Portet-Koltalo F, Guibert N, Morin C, De Mengin-Fondragon F, Frouard A. Evaluation of polybrominated diphenyl ether (PBDE) flame retardants from various materials in professional seating furnishing wastes from French flows. Waste Manage (Oxford) 2021;131:108–116. doi: 10.1016/j.wasman.2021.05.038. [DOI] [PubMed] [Google Scholar]
- Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T. Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ. 2020;702:134455. doi: 10.1016/j.scitotenv.2019.134455. [DOI] [PubMed] [Google Scholar]
- Rani M, Shim WJ, Han GM, Jang M, Song YK, Hong SH. Benzotriazole–type ultraviolet stabilizers and antioxidants in plastic marine debris and their new products. Sci Total Environ. 2017;579:745–754. doi: 10.1016/j.scitotenv.2016.11.033. [DOI] [PubMed] [Google Scholar]
- Rebelein A, Int-Veen I, Kammann U, Scharsack JP. Microplastic fibers—underestimated threat to aquatic organisms. Sci Total Environ. 2021;777:146045. doi: 10.1016/j.scitotenv.2021.146045. [DOI] [PubMed] [Google Scholar]
- Reemtsma T, Benito Quintana J, Rodil R, Garcia-Lopez M, Rodriguez I. Organophosphorus flame retardants and plasticizers in water and air I: occurrence and fate. TrAC–Trends Analy Chem. 2008;27:727–737. doi: 10.1016/j.trac.2008.07.002. [DOI] [Google Scholar]
- Rodgers KM, Swartz CH, Occhialini J, Bassignani P, McCurdy M, Schaider LA. How well do product labels indicate the presence of PFAS in consumer items used by children and adolescents? Environ Sci Technol. 2022;56:6294–6304. doi: 10.1021/acs.est.1c05175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MO, Abrantes N, Goncalves FJM, Nogueira H, Marques JC, Goncalves AMM. Impacts of plastic products used in daily life on the environment and human health: what is known? Environ Toxicol Pharmacol. 2019;72:103239. doi: 10.1016/j.etap.2019.103239. [DOI] [PubMed] [Google Scholar]
- Rovira J, Domingo JL. Human health risks due to exposure to inorganic and organic chemicals from textiles: a review. Environ Res. 2019;168:62–69. doi: 10.1016/j.envres.2018.09.027. [DOI] [PubMed] [Google Scholar]
- Saini A, Rauert C, Simpson MJ, Harrad S, Diamond ML. Characterizing the sorption of polybrominated diphenyl ethers (PBDEs) to cotton and polyester fabrics under controlled conditions. Sci Total Environ. 2016;563:99–107. doi: 10.1016/j.scitotenv.2016.04.099. [DOI] [PubMed] [Google Scholar]
- Saini A, Thaysen C, Jantunen L, McQueen RH, Diamond ML. From clothing to laundry water: investigating the fate of phthalates, brominated flame retardants, and organophosphate esters. Environ Sci Technol. 2016;50:9289–9297. doi: 10.1021/acs.est.6b02038. [DOI] [PubMed] [Google Scholar]
- Sait STL, Sørensen L, Kubowicz S, Vike-Jonas K, Gonzalez SV, Asimakopoulos AG, Booth AM. Microplastic fibres from synthetic textiles: environmental degradation and additive chemical content. Environ Pollut. 2021;268:115745. doi: 10.1016/j.envpol.2020.115745. [DOI] [PubMed] [Google Scholar]
- Saito I, Onuki A, Seto H. Indoor organophosphate and polybrominated flame retardants in Tokyo. Indoor Air. 2007;17:28–36. doi: 10.1111/j.1600-0668.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Prado L, Garcia-Jares C, Llompart M. Microwave-assisted extraction: application to the determination of emerging pollutants in solid samples. J Chromatogr A. 2010;1217:2390–2414. doi: 10.1016/j.chroma.2009.11.080. [DOI] [PubMed] [Google Scholar]
- Schäfer T, Herter M. Input-oriented chemicals management along the textile supply chain. Cham: Springer International Publishing; 2021. [Google Scholar]
- Schecter A, Shah N, Colacino JA, Brummitt SI, Ramakrishnan V, Harris TR, Paepke O. PBDEs in US and German clothes dryer lint: a potential source of indoor contamination and exposure. Chemosphere. 2009;75:623–628. doi: 10.1016/j.chemosphere.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Schellenberger S, Jonsson C, Mellin P, Levenstam OA, Liagkouridis I, Ribbenstedt A, Hanning A-C, Schultes L, Plassmann MM, Persson C, Cousins IT, Benskin JP. Release of side-chain fluorinated polymer-containing microplastic fibers from functional textiles during washing and first estimates of perfluoroalkyl acid emissions. Environ Sci Technol. 2019;53:14329–14338. doi: 10.1021/acs.est.9b04165. [DOI] [PubMed] [Google Scholar]
- Schellenberger S, Liagkouridis I, Awad R, Khan S, Plassmann M, Peters G, Benskin JP, Cousins IT. An outdoor aging study to unvestigate the release of per- and polyfluoroalkyl substances (PFAS) from functional textiles. Environ Sci Technol. 2022;56:3471–3479. doi: 10.1021/acs.est.1c06812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Li Y, Song B, Zhou C, Gong J, Zeng G. Smoked cigarette butts: Unignorable source for environmental microplastic fibers. Sci Total Environ. 2021;791:148384. doi: 10.1016/j.scitotenv.2021.148384. [DOI] [PubMed] [Google Scholar]
- Shi S, Cao J, Zhang Y, Zhao B. Emissions of phthalates from indoor flat materials in Chinese residences. Environ Sci Technol. 2018;52:13166–13173. doi: 10.1021/acs.est.8b03580. [DOI] [PubMed] [Google Scholar]
- Shin JH, Baek YJ. Analysis of polybrominated diphenyl ethers in textiles treated by brominated flame retardants. Text Res J. 2012;82:1307–1316. doi: 10.1177/0040517512439943. [DOI] [Google Scholar]
- Siegfried M, Koelmans AA, Besseling E, Kroeze C. Export of microplastics from land to sea: a modelling approach. Water Res. 2017;127:249–257. doi: 10.1016/j.watres.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Sillanpaa M, Sainio P. Release of polyester and cotton fibers from textiles in machine washings. Environ Sci Pollut Res. 2017;24:19313–19321. doi: 10.1007/s11356-017-9621-1. [DOI] [PubMed] [Google Scholar]
- Song Y, Cao C, Qiu R, Hu J, Liu M, Lu S, Shi H, Raley-Susman KM, He D. Uptake and adverse effects of polyethylene terephthalate microplastics fibers on terrestrial snails (Achatina fulica) after soil exposure. Environ Pollut. 2019;250:447–455. doi: 10.1016/j.envpol.2019.04.066. [DOI] [PubMed] [Google Scholar]
- Soo XYD, Wang S, Yeo CCJ, Li J, Ni XP, Jiang L, Xue K, Li Z, Fei X, Zhu Q, Loh XJ. Polylactic acid face masks: are these the sustainable solutions in times of COVID-19 pandemic? Sci Total Environ. 2022;807:151084. doi: 10.1016/j.scitotenv.2021.151084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen L, Groven AS, Hovsbakken IA, Del Puerto O, Krause DF, Sarno A, Booth AM. UV degradation of natural and synthetic microfibers causes fragmentation and release of polymer degradation products and chemical additives. Sci Total Environ. 2021;755:143170. doi: 10.1016/j.scitotenv.2020.143170. [DOI] [PubMed] [Google Scholar]
- Stubbings WA, Schreder ED, Thomas MB, Romanak K, Venier M, Salamova A. Exposure to brominated and organophosphate ester flame retardants in US childcare environments: effect of removal of flame-retarded nap mats on indoor levels. Environ Pollut. 2018;238:1056–1068. doi: 10.1016/j.envpol.2018.03.083. [DOI] [PubMed] [Google Scholar]
- Stubbings WA, Nguyen LV, Romanak K, Jantunen L, Melymuk L, Arrandale V, Diamond ML, Venier M. Flame retardants and plasticizers in a Canadian waste electrical and electronic equipment (WEEE) dismantling facility. Sci Total Environ. 2019;675:594–603. doi: 10.1016/j.scitotenv.2019.04.265. [DOI] [PubMed] [Google Scholar]
- Su L, Cai H, Kolandhasamy P, Wu C, Rochman CM, Shi H. Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environ Pollut. 2018;234:347–355. doi: 10.1016/j.envpol.2017.11.075. [DOI] [PubMed] [Google Scholar]
- Sullivan GL, Delgado-Gallardo J, Watson TM, Sarp S. An investigation into the leaching of micro and nano particles and chemical pollutants from disposable face masks-linked to the COVID-19 pandemic. Water Res. 2021 doi: 10.1016/j.watres.2021.117033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Liu J, Zhang Y-Q, Leungb KMY, Zeng EY. Leaching of polybrominated diphenyl ethers from microplastics in fish oil: Kinetics and bioaccumulation. J Hazard Mater. 2021;406:124726. doi: 10.1016/j.jhazmat.2020.124726. [DOI] [PubMed] [Google Scholar]
- Sungur S, Gulmez F. Determination of metal contents of various fibers used in textile industry by MP-AES. J Spectrosc. 2015;2015:640271. doi: 10.1155/2015/640271. [DOI] [Google Scholar]
- Tan H, Chen D, Peng C, Liu X, Wu Y, Li X, Du R, Wang B, Guo Y, Zeng EY. Novel and traditional organophosphate esters in house dust from South China: Association with hand wipes and exposure estimation. Environ Sci Technol. 2018;52:11017–11026. doi: 10.1021/acs.est.8b02933. [DOI] [PubMed] [Google Scholar]
- Tan H, Yang L, Huang Y, Tao L, Chen D. “Novel” synthetic antioxidants in house dust from multiple locations in the Asia-Pacific region and the United States. Environ Sci Technol. 2021;55:8675–8682. doi: 10.1021/acs.est.1c00195. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Takada H, Yamashita R, Mizukawa K, Fukuwaka M-A, Watanuki Y. Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar Pollut Bull. 2013;69:219–222. doi: 10.1016/j.marpolbul.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Tang Z, Chai M, Wang Y, Cheng J. Phthalates in preschool children's clothing manufactured in seven Asian countries: occurrence, profiles and potential health risks. J Hazard Mater. 2020;387:121681. doi: 10.1016/j.jhazmat.2019.121681. [DOI] [PubMed] [Google Scholar]
- Tao D, Zhang K, Xu S, Lin H, Liu Y, Kang J, Yim T, Giesy JP, Leung KMY. Microfibers released into the air from a household tumble dryer. Environ Sci Technol Lett. 2022;9:120–126. doi: 10.1021/acs.estlett.1c00911. [DOI] [Google Scholar]
- Torkashvand J, Farzadkia M, Sobhi HR, Esrafili A. Littered cigarette butt as a well-known hazardous waste: a comprehensive systematic review. J Hazard Mater. 2020;383:121242. doi: 10.1016/j.jhazmat.2019.121242. [DOI] [PubMed] [Google Scholar]
- Vassilenko E, Watkins M, Chastain S, Mertens J, Posacka AM, Patankar S, Ross PS. Domestic laundry and microfiber pollution: exploring fiber shedding from consumer apparel textiles. PLoS ONE. 2021;16:e0250346. doi: 10.1371/journal.pone.0250346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergren R, Herzke D, Wang T, Cousins IT. Are imported consumer products an important diffuse source of PFASs to the Norwegian environment? Environ Pollut. 2015;198:223–230. doi: 10.1016/j.envpol.2014.12.034. [DOI] [PubMed] [Google Scholar]
- Wang C, Li L, Xie T, Zhang E, Shen Y, Chen H, Liu C. Simultaneous determination of six kinds of banned organophosphorous flame retardants in textiles by gas chromatography tandem mass spectrometry combined with ultrasonic extraction (in Chinese) J Instrumental Anal. 2011;30:917–921. [Google Scholar]
- Wang L, Zhang Y, Liu Y, Gong X, Zhang T, Sun H. Widespread occurrence of bisphenol A in daily clothes and its high exposure risk in humans. Environ Sci Technol. 2019;53:7095–7102. doi: 10.1021/acs.est.9b02090. [DOI] [PubMed] [Google Scholar]
- Wang X, Xue Y, Zhe D, Gao A, Guo R, Gao J. Overview of phthalate plasticizers, current regulations and standards. China Plastics. 2019;33:95–105. [Google Scholar]
- Wang X, Zhu Q, Yan X, Wang Y, Liao C, Jiang G. A review of organophosphate flame retardants and plasticizers in the environment: analysis, occurrence and risk assessment. Sci Total Environ. 2020;731:139071. doi: 10.1016/j.scitotenv.2020.139071. [DOI] [PubMed] [Google Scholar]
- Wang W, Xiong P, Zhang H, Zhu Q, Liao C, Jiang G. Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: a review. Environ Res. 2021;201:111531. doi: 10.1016/j.envres.2021.111531. [DOI] [PubMed] [Google Scholar]
- Wang X, Okoffo ED, Banks APW, Li Y, Thomas KV, Rauert C, Aylward LL, Mueller JF. Phthalate esters in face masks and associated inhalation exposure risk. J Hazard Mater. 2022;423:127001. doi: 10.1016/j.jhazmat.2021.127001. [DOI] [PubMed] [Google Scholar]
- Wright SL, Rowe D, Reid MJ, Thomas KV, Galloway TS. Bioaccumulation and biological effects of cigarette litter in marine worms. Sci Rep. 2015;5:1–10. doi: 10.1038/srep14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Venier M, Hites RA. Identification of unusual antioxidants in the natural and built environments. Environ Sci Technol Lett. 2019;6:443–447. doi: 10.1021/acs.estlett.9b00415. [DOI] [Google Scholar]
- Wu Z, He C, Han W, Song J, Li H, Zhang Y, Jing X, Wu W. Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: a review. Environ Res. 2020;187:109531. doi: 10.1016/j.envres.2020.109531. [DOI] [PubMed] [Google Scholar]
- Xia C, Diamond ML, Peaslee GF, Peng H, Blum A, Wang Z, Shalin A, Whitehead HD, Green M, Schwartz-Narbonne H, Yang D, Venier M. Per- and polyfluoroalkyl substances in North American school uniforms. Environ Sci Technol. 2022;56:13845–13857. doi: 10.1021/acs.est.2c02111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Han W, Xie Q, Xu T, Zhu M, Chen J. Face mask—a potential source of phthalate exposure for human. J Hazard Mater. 2022;422:126848. doi: 10.1016/j.jhazmat.2021.126848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. Detection of APEO in textiles by HPLC. J Text I. 2021;112:1651–1654. doi: 10.1080/00405000.2020.1835153. [DOI] [Google Scholar]
- Xu Y, Ou Q, Jiao M, Liu G, van der Hoek JP. Identification and quantification of nanoplastics in surface water and groundwater by pyrolysis gas chromatography-mass spectrometry. Environ Sci Technol. 2022;56:4988–4997. doi: 10.1021/acs.est.1c07377. [DOI] [PubMed] [Google Scholar]
- Xue J, Liu W, Kannan K. Bisphenols, benzophenones, and bisphenol A diglycidyl ethers in textiles and infant clothing. Environ Sci Technol. 2017;51:5279–5286. doi: 10.1021/acs.est.7b00701. [DOI] [PubMed] [Google Scholar]
- Xue B, Zhang L, Li R, Wang Y, Guo J, Yu K, Wang S. Underestimated microplastic pollution derived from fishery activities and “hidden” in deep sediment. Environ Sci Technol. 2020;54:2210–2217. doi: 10.1021/acs.est.9b04850. [DOI] [PubMed] [Google Scholar]
- Yan Z, Liu Y, Zhang T, Zhang F, Ren H, Zhang Y. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ Sci Technol. 2022;56:414–421. doi: 10.1021/acs.est.1c03924. [DOI] [PubMed] [Google Scholar]
- Young AS, Hauser R, James-Todd TM, Coull BA, Zhu H, Kannan K, Specht AJ, Bliss MS, Allen JG. Impact of “healthier” materials interventions on dust concentrations of per- and polyfluoroalkyl substances, polybrominated diphenyl ethers, and organophosphate esters. Environ Int. 2021;150:106151. doi: 10.1016/j.envint.2020.106151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Tay JH, Papadopoulou E, Haug LS, Padilla-Sánchez JA, de Wit CA. Complex mixtures of chlorinated paraffins found in hand wipes of a Norwegian cohort. Environ Sci Technol Lett. 2020;7:198–205. doi: 10.1021/acs.estlett.0c00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano MC, Pawlak JJ, Daystar J, Ankeny M, Venditti RA. Impact of dyes and finishes on the microfibers released on the laundering of cotton knitted fabrics. Environ Pollut. 2021;272:115998. doi: 10.1016/j.envpol.2020.115998. [DOI] [PubMed] [Google Scholar]
- Zhang S-X, Chai X-S, Huang B-X, Mai X-X. A robust method for determining water-extractable alkylphenol polyethoxylates in textile products by reaction-based headspace gas chromatography. J Chromatogr A. 2015;1406:94–98. doi: 10.1016/j.chroma.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou Q, Xie Z, Zhou Y, Tu C, Fu C, Mi W, Ebinghaus R, Christie P, Luo Y. Occurrences of organophosphorus esters and phthalates in the microplastics from the coastal beaches in north China. Sci Total Environ. 2018;616:1505–1512. doi: 10.1016/j.scitotenv.2017.10.163. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang L, Kannan K. Microplastics in house dust from 12 countries and associated human exposure. Environ Int. 2020;134:105314. doi: 10.1016/j.envint.2019.105314. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Sun Y, Zhang Q, Hou J, Wang P, Kong X, Sundell J. Phthalate exposure in Chinese homes and its association with household consumer products. Sci Total Environ. 2020;719:136965. doi: 10.1016/j.scitotenv.2020.136965. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Xu EG, Li J, Chen Q, Ma L, Zeng EY, Shi H. A review of microplastics in table salt, drinking water, and air: direct human exposure. Environ Sci Technol. 2020;54:3740–3751. doi: 10.1021/acs.est.9b04535. [DOI] [PubMed] [Google Scholar]
- Zhang X, Mell A, Li F, Thaysen C, Musselman B, Tice J, Vukovic D, Rochman C, Helm PA, Jobst KJ. Rapid fingerprinting of source and environmental microplastics using direct analysis in real time-high resolution mass spectrometry. Analy Chim Acta. 2020;1100:107–117. doi: 10.1016/j.aca.2019.12.005. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Du F, Liang W, Chen Q, Meng J, Shi H. Microfiber fallout during dining and potential human intake. J Hazard Mater. 2022;430:128477–128477. doi: 10.1016/j.jhazmat.2022.128477. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Qiao R, Zhang S, Wang G. Metabolomic profiling reveals the intestinal toxicity of different length of microplastic fibers on zebrafish (Danio rerio) J Hazard Mater. 2021;403:123663. doi: 10.1016/j.jhazmat.2020.123663. [DOI] [PubMed] [Google Scholar]
- Zhao K, Wei Y, Dong J, Zhao P, Wang Y, Pan X, Wang J. Separation and characterization of microplastic and nanoplastic particles in marine environment. Environ Pollut. 2022;297:118773. doi: 10.1016/j.envpol.2021.118773. [DOI] [PubMed] [Google Scholar]
- Zheng G, Salamova A. Are melamine and its derivatives the alternatives for per- and polyfluoroalkyl substance (PFAS) fabric treatments in infant clothes? Environ Sci Technol. 2020;54:10207–10216. doi: 10.1021/acs.est.0c03035. [DOI] [PubMed] [Google Scholar]
- Zhu H, Al-Bazi MM, Kumosani TA, Kannan K. Occurrence and profiles of organophosphate esters in infant clothing and raw textiles collected from the United States. Environ Sci Technol Lett. 2020;7:415–420. doi: 10.1021/acs.estlett.0c00221. [DOI] [Google Scholar]