Abstract
Purpose
To investigate the added value of right down decubitus (RDD) CT when determining adjacent organ invasion in cases of advanced gastric cancer (AGC).
Materials and Methods
A total of 728 patients with pathologically confirmed T4a (pT4a), surgically confirmed T4b (sT4b), or pathologically confirmed T4b (pT4b) AGCs who underwent dedicated stomach-protocol CT, including imaging of the left posterior oblique (LPO) and RDD positions, were included in this study. Two radiologists scored the T stage of AGCs using a 5-point scale on LPO CT with and without RDD CT at 2-week intervals and recorded the presence of “sliding sign” in the tumors and adjacent organs and compared its incidence of appearance.
Results
A total of 564 patients (77.4%) were diagnosed with pT4a, whereas 65 (8.9%) and 99 (13.6%) patients were diagnosed with pT4b and sT4b, respectively. When RDD CT was performed additionally, both reviewers deemed that the area under the curve (AUC) for differentiating T4b from T4a increased (p < 0.001). According to both reviewers, the AUC for differentiating T4b with pancreatic invasion from T4a increased in the subgroup analysis (p < 0.050). Interobserver agreement improved from fair to moderate (weighted kappa value, 0.296–0.444).
Conclusion
RDD CT provides additional value compared to LPO CT images alone for determining adjacent organ invasion in patients with AGC due to their increased AUC values and improved interobserver agreement.
Keywords: Stomach Neoplasms, Multidetector Computed Tomography, Neoplasm Invasiveness, Pancreas, Task Performance and Analysis
Abstract
목적
진행성 위암의 인접 장기 침범을 결정함에 있어 우측와위 CT의 추가적 가치를 살펴보았다.
대상과 방법
병리학적으로 입증된 T4a (p4a), 외과적 그리고 병리학적으로 입증된 T4b (sT4b, pT4b) 위암 환자 중 좌후사위 및 우측와위 자세가 포함된 프로토콜의 CT를 촬영한 환자 총 728명이 포함되었다. 2명의 영상의학과 전문의가 2주 간격으로 각각 우측와위 CT 없이, 우측와위 CT와 함께 좌후사위 CT를 분석하여 5점 척도를 사용하여 T 병기를 평가하고 종양과 인접 장기 사이의 “미끄러짐 징후”의 존재를 기록했다.
결과
564명의 환자(77.4%)가 pT4a로 진단되었다. 65명(8.9%)과 99명(13.6%)의 환자가 각각 pT4b, sT4b로 진단되었다. 좌후사위 CT 단독 분석에 비하여 우측와위 CT가 추가되었을 때, T4b와 T4a를 구별하기 위한 곡선 아래 면적(area under the curve; 이하 AUC) 값이 두 검토자 모두에서 유의하게 증가했다(Ps < 0.001). 하위집단분석에서 T4a와 췌장을 침범한 T4b 위암을 구별하기 위한 AUC 값 역시 두 검토자 모두에서 증가했다(Ps < 0.050). 관찰자 간 일치도 역시 향상되었다(가중 카파 계수, 0.296–0.444).
결론
진행위암에서 인접 장기 침범을 판단함에 있어, 우측와위 CT가 추가되었을 때 좌후사위 CT 단독 분석에 비해 더 높은 AUC 값과 관찰자 간 일치도를 보임으로써 추가적 가치가 있었다.
INTRODUCTION
Although its incidence is declining worldwide, gastric cancer is still a common malignancy, which ranks as the sixth most frequently diagnosed cancer and the fourth leading cause of cancer-related deaths worldwide (1,2). Even though its prevalence varies according to geographic regions, gastric cancer is more common in Asian countries and is the most commonly diagnosed cancer and the fourth leading cause of cancer death in South Korea (3,4). Complete surgical resection is the only curative treatment for locoregional gastric cancer (5,6,7); however, gastric cancers are often diagnosed at an advanced stage (8,9). The term “locally advanced gastric cancer (AGC)” refers to tumors infiltrating or adherent to adjacent organs or structures with or without lymph node involvement in patients without distant metastasis (10). Depending on the location where the tumor has invaded adjacent organs, different treatment strategies are required in patients with AGC. More specifically, in cases of T4a or lower tumors, curative surgical resection is recommended, whereas, in T4b tumors that invade adjacent organs, the tumors are considered unresectable (11). Therefore, accurate staging of gastric cancer, in particular, ≤ T4a versus T4b, is critical for planning optimal treatment (12,13,14).
CT is a standard imaging modality widely used for preoperative TNM staging of gastric cancer (15,16,17). By the introduction of multidetector CT (MDCT), which facilitates multiplanar reformation images and various three-dimensional images, including surface-rendered images and virtual gastroscopy images, the diagnostic accuracy of MDCT for the detection and staging of gastric cancers has been improved thus far (18,19,20,21,22). By virtue of technical improvements in CT, there has been considerable advancement in optimizing CT protocol for gastric diseases, also known as CT gastrography. For current CT gastrography technique, we have introduced 30° left posterior oblique (LPO) position CT to maximize distention and minimize residual fluid in the distal part of the stomach since 2004 (21). Furthermore, an effervescent agent is given to patients to minimize false-negative and false-positive calls and to make it easier to reconstruct three-dimensional images (20). Nevertheless, the differentiation of T4b tumors from ≤ T4a tumors remains challenging (23,24).
The term “sliding sign” has been initially proposed in ultrasonography (US), in which a dynamic motion of the tumor was seen against adjacent organs during respiratory motion or extrinsic pressure (25). Furthermore, the diagnostic benefit of sliding sign between gastric cancer and the pancreas on US has been reported in predicting pancreas invasion by gastric cancer (26). However, the diagnostic value of sliding sign on CT has not yet been investigated.
Accordingly, we hypothesized that the application of the right down decubitus (RDD) position during CT acquisition may be helpful for the identification of T4b AGCs that invade adjacent organs from ≤ T4a AGCs; if the tumors invade adjacent organs such as the pancreas, there might not be any locational change between the tumors and the pancreas on RDD CT images. On the contrary, if the tumors do not invade adjacent organs, the relative relationship between tumors and adjacent organs can be changed on RDD CT images compared with supine or LPO CT images. We designated this locational change on RDD images as a “sliding sign.”
Hence, we performed this retrospective study to investigate whether there is an added value of “sliding sign” on RDD CT for the determination of adjacent organ invasion in patients with AGC.
MATERIALS AND METHODS
This retrospective study was approved by the Institutional Review Board of our institution and informed written consent was waived due to the retrospective nature of this study (IRB No. H-1908-045-1053).
PATIENT SELECTION
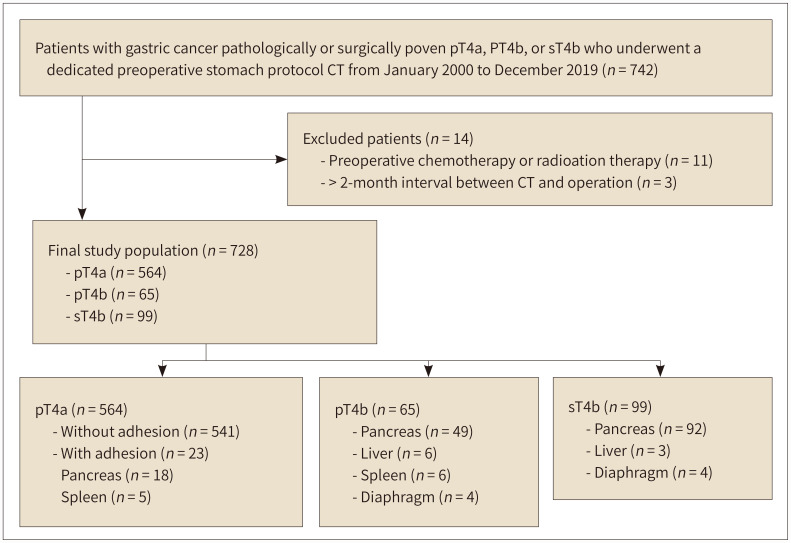
From the pathologic and surgical databases between January 2000 and December 2019, we searched 13944 patients with pathologically confirmed gastric cancer. From this cohort, the inclusion criteria for this study were as follows: 1) patients who underwent surgery for gastric cancers with pathologically proven T stage of T4a, 2) those with gastric cancer in whom adjacent organ invasion was reported on surgical (sT4b) or pathologic (pT4b) reports, 3) those who received dedicated stomach protocol contrast-enhanced CT examination including RDD position CT, and 4) the interval between CT and surgery within 2 months. Patients who received chemotherapy or radiation therapy before surgery were excluded from the study population. Finally, 728 patients were included in the study. A total of 434 male and 294 female with a mean age ± standard deviation (SD) of 59 ± 12 years (age range, 17–95 years). A flow chart of patient enrollment is provided in Fig. 1.
Fig. 1. The flow chart for patients’ enrollment.
pT4a = pathologically confirmed T4a, pT4b = pathologically confirmed T4b with adjacent organ invasion, sT4b = surgically confirmed T4b with adjacent organ invasion in the surgical field
CT TECHNIQUES
PATIENT PREPARATION
Patients were asked to fast for at least 6 hours of fasting time to ensure gastric emptying. Five minutes before CT acquisition, 10 mg of butyl scopolamine (Buscopan; Boehringer Ingelheim; Ingelheim, Germany) was intravenously administered to minimize bowel peristalsis and to facilitate hypotonia of the stomach, unless contraindicated.
ORAL CONTRAST AGENT
As optimal gastric distention is essential to minimize false-negative and false-positive calls, all patients received one pack of 4 gm effervescent granules as a negative oral contrast agent just before CT image acquisition and were instructed not to belch. As 1 gm effervescent granules produce 130 mL of CO2 gas, the patient’s stomach can be inflated by approximately 500 mL of CO2 gas.
PATIENTS’ POSITIONING
Immediately after the administration of negative oral contrast, patients were initially positioned in the 30° LPO position by applying a pillow between their back and the CT table. Since 2004, we have introduced 30° LPO position CT to maximize distention and minimize residual fluid in the distal part of the stomach (21). In this LPO position, arterial phase (AP) and portal venous phase (PVP) CT images were obtained. After the acquisition of CT images in the LPO position, patients were promptly re-positioned in the RDD position, and then delayed phase (DP) CT images were acquired in the RDD position.
CT ACQUISITION PROTOCOL
A variety of MDCT scanners were used in our study owing to their retrospective design. All patients underwent MDCT with 4, 8, 16, 64, 128, or 320 detector rows. The acquisition CT parameters used for these MDCTs were as follows: detector configuration (0.63–1 mm), pitch (0.89–1.35), rotation time (0.5–1 seconds), tube voltage (100–120 kVp), tube current (150–250 mAs), slice thickness (2–5 mm), and reconstruction interval (2–5 mm).
Contrast-enhanced dynamic CT images were obtained after administration of an iodinated contrast agent at a concentration of 350–370 mg I/mL at a dose of 1.5 mL/kg and a rate of 3–5 mL/s using an automatic power injector. Saline chase was performed at the same rate for 10 seconds. AP images were obtained 13–17 seconds after the attenuation of the descending thoracic aorta reached 100 Hounsfield units using the bolus tracking technique. Scanning on AP was acquired from the dome of the liver to 5 cm below the lower margin of the gastric gas shadow to sufficiently include the entire stomach. PVP images were obtained using a fixed delay of 60–70 seconds after the contrast administration. Scanning was performed from the dome of the liver to the upper thigh to include the entire peritoneal cavity. After the acquisition of AP and PVP CT images in the LPO position, patients were promptly repositioned in the RDD position, and then DP CT images were acquired with the same scan range as the AP CT.
CT IMAGE ANALYSIS
Two board-certified abdominal radiologists (with 6 years and 9 years of experience in abdominal imaging) assessed the T staging of gastric cancer according to the American Joint Committee on Cancer eighth staging system. They were completely blinded to the final histopathological and surgical results. However, they were aware of this study’s aim to assess the diagnostic value of RDD position CT for predicting adjacent organ invasion in patients with AGC. For the first session, they interpreted T staging using the LPO position CT alone. Each reader scored the T stage of gastric cancer using a 5-point confidence scale: 1, definitely ≤ T4a; 2, probably ≤ T4a; 3, possibly T4b; 4, probably T4b; and 5, definitely T4b. The reviewers were asked to determine T4a versus T4b mainly according to the sliding sign demonstrated on RDD CT images. Two weeks after the first session, they interpreted CT images again using LPO position CT combined with RDD position CT. First, they recorded the presence or absence of a CT sliding sign between the tumors and adjacent organs on RDD CT images compared to conventional LPO CT images. The lesions were designated as not having a sliding sign when the transmural AGC was closely abutted to an adjacent organ without any relative locational change between tumors and organs on RDD position CT compared to LPO position CT. Illustrated images demonstrating the CT sliding sign are provided in Fig. 2. For cases in which the CT sliding sign was negative, reviewers were asked to specify the organs in which the CT sliding sign was lost. They then scored the T stage of gastric cancer using a 5-point confidence scale: 1, definitely ≤ T4a; 2, probably ≤ T4a; 3, possibly T4b; 4, probably T4b; and 5, definitely T4b. Before each assessment, the reviewers were instructed to consider a score of 3 to 5 to define T4b in a dichotomous analysis.
Fig. 2. “Sliding sign” in the right down decubitus position CT.
A, B. An illustration (A) and corresponding CT image (B) in the left posterior oblique position reveal a tumor (arrow) at the posterior wall of the gastric body is widely abutting to the pancreas body. The fat plane between the tumor and the pancreas is obliterated (arrowheads).
C. This illustration shows that the stomach and tumor, which are located in the peritoneal cavity, exhibit free movement in a right down decubitus position, whereas the pancreas is fixed as the pancreas is fixedly located at the retroperitoneum during the position change. Resultantly, there is a “sliding sign” (arrow) between the gastric tumor and the pancreas body.
D, E. An illustration (D) and corresponding CT image (E) in the right down decubitus position show that a fat plane (asterisks in D and arrowheads in E) newly appears between the tumor (arrow) and the pancreas (P), suggesting that the tumor does not invade the pancreas. The right down decubitus CT is rotated 90 degrees clockwise so as to show it in a familiar orientation (thick blue arrow in D).
REFERENCE STANDARD
The patients’ medical records were thoroughly reviewed by one radiologist (with 4 years of experience in radiologic imaging). After an extensive review of surgical and pathologic findings, patients were divided into four groups: 1) pT4a without any invasion or adhesion to adjacent organs (pT4a group), 2) pT4a with adhesion to adjacent organs on surgical findings (adhesion group), 3) sT4b with adjacent organ invasion on surgical findings without pathologic proof (sT4b group), and 4) pT4b with pathologically proven adjacent organ invasion (pT4b group). Because gastric cancers could have invaded or adhered to multiple organs simultaneously, patients were classified and analyzed based on the dominantly adherent or invaded organ for per-patient analysis. In addition, each organ-based analysis was performed for per-organ analysis. According to the dominantly invaded or adherent adjacent organs, patients were further divided into two groups: pancreas versus another organ (spleen, liver, or diaphragm). For the cases in the adhesion group, the presence of adhesion was considered when gastric cancer was directly adherent to adjacent organs, not indicating the presence of adhesion in the abdominal cavity by other causes including previous surgery.
STATISTICAL ANALYSIS
Descriptive statistics were described as means and SD or medians and ranges for continuous variables. Categorical variables are reported as frequencies and percentages.
Receiver operating characteristic (ROC) curve analysis was performed to compare the diagnostic performances of both independent reviewers between LPO position CT with and without RDD position CT for differentiating T4b from ≤ T4a in patients with AGC. The differences between the areas under the curves (AUCs) were determined using a univariate z-score test. This comparison indicated the effect of adding RDD position CT to LPO position CT on the radiologists’ performance. Sensitivity and specificity for diagnosing adjacent organ involvement were also estimated using surgical or pathologic reports as the reference standard.
Weighted kappa (κ) statistics were used to assess the interobserver agreement between the two radiologists. Weighted κ values were interpreted as follows: < 0.2, poor agreement; 0.2–0.4, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; and 0.81–1.0, almost perfect agreement (27).
All statistical analyses were performed using SPSS statistics (version 25.0, IBM Corp., Armonk, NY, USA) and MedCalc Statistical Software version 18.9.1 (MedCalc Software bv, Ostend, Belgium; https://www.medcalc.org). p values of less than 0.05 were considered statistically significant.
RESULTS
CLINICAL CHARACTERISTICS
The patients’ clinical characteristics are shown in Table 1. Out of a total of 728 patients, 564 (77.4%) were pathologically diagnosed with pT4a cancer. Of these, 23 patients were diagnosed with adhesions to adjacent organs (18 patients with the pancreas and five with the spleen) on the surgical field and were designated as the adhesion group. In the remaining 164 patients, 65 were histopathologically confirmed as pT4b (pathologic T4b group) and 99 were surgically diagnosed with sT4b (surgical T4b group). In the pathologic T4b group, 75.3% (49/65) of the tumors invaded the pancreas, and the remaining 16 tumors invaded the liver (n = 6), spleen (n = 6), and diaphragm (n = 4). In 99 tumors with surgically T4b (sT4b), most of the tumors (92.9%, 92/99) invaded the pancreas, and the remaining seven tumors invaded the liver (n = 3), and the diaphragm (n = 4). Figs. 3, 4, 5 show the examples of pT4a cancer, pT4a cancer with adhesion to pancreas, and pT4b cancer with pancreas invasion.
Table 1. Clinical Characteristics of the 728 Patients.
| Sex, M:F | 434:294 | ||||
| Age, years, median ± SD (range) | 59 ± 12 (17–95) | ||||
| Final T staging | |||||
| pT4a | |||||
| Without adhesion | 541 | ||||
| With adhesion | |||||
| Pancreas | 18 | ||||
| Non-pancreas | |||||
| Liver | 0 | ||||
| Spleen | 5 | ||||
| pT4b | |||||
| Pancreas | 49 | ||||
| Non-pancreas | |||||
| Liver | 6 | ||||
| Spleen | 6 | ||||
| Diaphragm | 4 | ||||
| sT4b | |||||
| Pancreas | 92 | ||||
| Non-pancreas | |||||
| Liver | 3 | ||||
| Spleen | 0 | ||||
| Diaphragm | 4 | ||||
Numbers represent the number of patients.
pT4a = pathologically confirmed T4a, pT4b = pathologically confirmed T4b with adjacent organ invasion, SD = standard deviation, sT4b = surgically confirmed T4b with adjacent organ invasion in the surgical field
Fig. 3. A 64-year-old female with advanced gastric cancer with a pathologically proven T4a stage.
A. Contrast-enhanced axial CT obtained in a left posterior oblique position reveals a diffuse low-attenuating wall thickening (arrows) at the gastric antrum. There is a broad attachment (arrowheads) between the tumor and pancreas neck at the far distal portion of the tumor, and a flat plane (arrowheads) between them was obliterated. Therefore, both radiologists scored 4 (probably T4b) with pancreas invasion.
B. CT perfomed in a right down debucitus reveals a sliding sign between the gastric tumor (arrow) and pancreas neck (P). A linear fat plane (arrowheads) between the tumor (arrow) and pancreas neck (P) is newly visualized. Both radiologists recorded the presence of sliding signs and scored 1 (definitely T4a) without adjacent organ invasion. The patient subsequently underwent a subtotal gastrectomy. There is no evidence of adjacent organ invasion in the surgical field. Moderately differentiated adenocarcinoma with pT4aN0 stage is finally diagnosed via histopathology.
Fig. 4. A 21-year old female with advanced gastric cancer and pathologically proven liver invasion (pT4a) with adhesion to the pancreas.
A. This contrast-enhanced axial CT image obtained in a left posterior oblique position shows a focal low-attenuating wall thickening (arrows) at the posterior wall of the gastric high body. The tumor is broadly abutted (arrowheads) to the pancreas tail (P). Two radiologists scored the tumor as 3 (possibly T4b) with pancreas invasion.
B. A delayed phase CT image taken in a right down debucitus reveals that the gastric tumor (arrows) is still abutted (arrowheads) to the tail of the pancreas and there is no sliding sign. Therefore, both radiologists recorded the absence of sliding sign and scored 4 (probably T4b) with pancreas invasion. The patient underwent surgery. The tumor is broadly attached to the pancreas tail in the surgical field. Therefore, both distal pancreatectomy and total gastrectomy, is performed.
C. This photograph of the cut surface of the gross specimen shows focal wall thickening of the stomach (arrow). The pancreatic tail (P) is also attached to the gastric tumor. However, the microscopic image showed that tumor did not invade the pancreas capsule or parenchyma (not shown). Therefore, the final histopathologic stage is pT4a. This case shows that simple adhesion by the tumor can cause false interpretation of sliding signs.
Fig. 5. A 70-year old male with advanced gastric cancer and pathologically proven pancreas invasion (pT4b).
A. This image obtained using contrast-enhanced axial CT in a left posterior oblique position shows diffuse low-attenuating wall thickening (arrows) with punctate calcifications at the gastric angle to antrum. The posterior portion of the tumor broadly is abutted to the pancreas body. The fat plane (arrowhead) between the tumor and pancreas body is obliterated at the far distal portion of the tumor. Therefore, both radiologists scored the tumor as 3 (possibly T4b) with pancreas invasion.
B. This CT image obtained after position change to right down decubitus, shows that the gastric tumor (arrows) and pancreas body (P) are still abutted (arrowhead) and there is no sliding sign. Both radiologists recorded the absence of sliding sign and scored 5 (definitely T4b) with pancreas invasion. The patient underwent a palliative total gastrectomy. A suspicious invasion of the pancreas body is present in the surgical field. Pancreas invasion by poorly cohesive carcinoma was proven using final histopathology.
RESULTS OF ROC CURVE ANALYSIS
Table 2 lists individual performances of the two radiologists for differentiating T4b (both pT4b and sT4b) from pT4a in the two interpretation sessions. The corresponding ROC curves of the two radiologists are shown in Fig. 6. When RDD CT images were additionally provided to LPO CT images, the differential performances of the two radiologists significantly improved for all patients. For all patients, the AUC values of both radiologists were significantly increased from 0.667 to 0.917 for reviewer 1 and from 0.850 to 0.894 for reviewer 2 (p < 0.001). In a subgroup analysis, AUC values for differentiating T4b with pancreas invasion from T4a were significantly increased for both reviewers (from 0.654 to 0.893 for reviewer 1, p < 0.001; from 0.786 to 0.814 for reviewer 2, p = 0.035). For determining another organ invasion such as liver, spleen, or diaphragm, AUC values also increased for both radiologists; however, a significant difference was found only in reviewer 1 (from 0.618 to 0.725 for reviewer 1, p = 0.028; from 0.784 to 0.800 for reviewer 2, p = 0.776).
Table 2. Results of Per-Patient ROC Analysis for Differentiating T4b without or with Adhesion from T4a.
| Reference Standard | LPO CT Alone | LPO CT + RDD CT | p-Value* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC | Sensitivity (%) | Specificity (%) | AUC | Sensitivity (%) | Specificity (%) | ||||
| pT4b + sT4b | |||||||||
| Reviewer 1 | |||||||||
| All | 0.667 | 48.8 (79/162) | 84.5 (459/541) | 0.917 | 84.0 (136/162) | 97.2 (526/541) | < 0.001† | ||
| Pancreas | 0.654 | 43.3 (61/141) | 87.6 (496/566) | 0.893 | 79.4 (112/141) | 96.8 (548/566) | < 0.001† | ||
| Non-pancreas | 0.618 | 22.5 (9/40) | 96.3 (648/673) | 0.725 | 47.5 (19/40) | 99.3 (668/673) | 0.028† | ||
| Reviewer 2 | |||||||||
| All | 0.850 | 77.8 (126/162) | 86.3 (467/541) | 0.894 | 77.2 (125/162) | 94.8 (513/541) | < 0.001† | ||
| Pancreas | 0.786 | 63.8 (90/141) | 91.2 (516/566) | 0.814 | 63.8 (90/141) | 96.6 (547/566) | 0.035† | ||
| Non-pancreas | 0.784 | 72.5 (29/40) | 91.5 (616/673) | 0.800 | 67.5 (27/40) | 95.8 (645/673) | 0.776 | ||
Patients with pT4b or sT4b are designated as the T4b group.
* p values for the differences between the AUCs between LPO CT alone and LPO + RDD CT are determined using a univariate z-score test.
† p values with indicate statistical significance.
AUC = areas under the curve, LPO = left posterior oblique, pT4b = pathologically confirmed T4b with adjacent organ invasion, RDD = right down decubitus, ROC = receiver operating characteristic, sT4b = surgically confirmed T4b with adjacent organ invasion in the surgical field
Fig. 6. The ROC curves for two radiologists with and without RDD CT images.
A, B. These panels show the ROC curves for all the patients drafted by the reviewers. When RDD CT images are provided in addition to the LPO CT images, the AUC values obtained by both radiologists for differentiating T4b from T4a increased significantly from 0.667 to 0.917 for reviewer 1 (A) and 0.850 to 0.894 for reviewer 2 (B) (p < 0.001).
C, D. These panels show the ROC curves of reviewers for patients with pancreatic invasion. When RDD CT images are provided in addition to LPO CT images, AUC values for differentiating T4b with pancreatic invasion from T4a obtained by both reviewers increased significantly (from 0.654 to 0.893 for reviewer 1, p < 0.001; from 0.786 to 0.814 for reviewer 2, p = 0.035).
AUC = areas under the curve, LPO = left posterior oblique, RDD = right down decubitus, ROC = receiver operating characteristic
CT SLIDING SIGN
The results of the CT sliding signs are summarized in Table 3. In 23 patients with pT4a with adhesion, two reviewers recorded negative CT sliding signs in 14 patients (60%) and 10 patients (43%). In 164 patients with T4b (pT4b + sT4b), two reviewers recorded negative CT sliding signs in 121 patients (73.7%) and 135 patients (82.3%).
Table 3. Results of Sliding Sign Assessment via CT.
| CT Sliding Sign | |||
|---|---|---|---|
| Presence (+) | Absence (-) | ||
| pT4a without adhesion (n = 541) | Reviewer 1 | 509 | 32 |
| Reviewer 2 | 526 | 15 | |
| pT4a with adhesion (n = 23) | Reviewer 1 | 9 | 14 |
| Reviewer 2 | 13 | 10 | |
| T4b (pT4b + sT4b) without adhesion (n = 164) | Reviewer 1 | 41 | 123 |
| Reviewer 2 | 27 | 137 | |
| T4b (pT4b + sT4b) with adhesion (n = 187) | Reviewer 1 | 50 | 137 |
| Reviewer 2 | 40 | 147 | |
Numbers represent the number of patients.
pT4a = pathologically confirmed T4a, pT4b = pathologically confirmed T4b with adjacent organ invasion, sT4b = surgically confirmed T4b with adjacent organ invasion in the surgical field
INTEROBSERVER AGREEMENT
For the 5-point confidence score in differentiating T4b from T4a, an interobserver agreement between the two reviewers using weighted κ statistics was improved from the fair agreement (weighted κ value: 0.296, 95% confidence interval: 0.256–0.335) to the moderate agreement (weighted κ value: 0.444, 95% confidence interval: 0.404–0.484) when RDD CT images were added to LPO CT alone.
DISCUSSION
In our study, we found that the addition of RDD CT images to LPO images alone improved the radiologists’ performance in differentiating T4b from T4a. Specifically, the AUC values of both radiologists significantly increased from 0.667 to 0.917 for reviewer 1 and from 0.850 to 0.894 for reviewer 2 for all patients (p < 0.001). In a subgroup analysis, the radiologists’ performance significantly improved in differentiating T4b with pancreatic invasion from T4a for both reviewers. For the invasion of other organs such as the liver, spleen, or diaphragm, the AUC values of reviewers increased with the addition of RDD CT images. However, the difference was statistically significant only for reviewer 1. These results are consistent with our hypothesis. We initially hypothesized that the sliding sign between the pancreas and stomach cancer might be useful for differentiating gastric cancer without (T4a) and with (T4b) pancreatic invasion as the pancreas is located in the retroperitoneal space and thereby the movement during position change to the RDD position might be limited compared to the stomach, which is located within the peritoneal cavity and freely movable during a position change. On the contrary, as other adjacent organs such as the liver and spleen are also located within the peritoneal cavity, these organs might be movable along with gastric cancer regardless of tumor invasion during position change to the RDD position. In terms of diaphragm invasion, as the space between gastric cardia cancer and the diaphragm is narrower than that of other organs, the identification of the sliding sign can be more challenging.
In this study, 23 patients had pT4a tumors that adhered to adjacent organs. In these patients, two reviewers recorded that there was no sliding sign between the tumor and adjacent organ in 16 and 12 patients, respectively. Consequently, the reviewers over-staged the tumors as T4b with adjacent organ invasion in these patients. From this result, we realized that adhesion without tumor invasion could be a source of false-negative results for determining the sliding sign. Further studies to differentiate true adjacent organ invasion from simple adhesion by gastric cancer using CT imaging are warranted. Contrarily, false negatives in the diagnosis of T4b can be attributed to false positive sliding sign. In fact, reviewers have stated that it was difficult to call the presence of sliding sign when the adhesion/invasion between the tumor and the adjacent organs was minimal or focal. Therefore, we believe that such subtle invasion of the tumor to adjacent organs may lead to under-stage T4b into T4a in some cases.
We observed that interobserver agreement between the two reviewers in differentiating between T4b and T4a also improved when RDD CT images were added to the LPO CT images alone. More specifically, the weighted κ value increased from 0.296 (fair agreement) on LPO images only to 0.444 (moderate agreement) with RDD CT images. In patients with tumors attached to adjacent organs, reviewers might have some challenges in determining whether the tumor was simply located with adjacent organs side-by-side or invaded into the adjacent organs on LPO CT images. However, when RDD CT images were additionally provided, they could determine the presence or absence of adjacent organ invasion more confidently through the presence of a sliding sign. There was also a limitation due to the time delay between the two CT scans taken at two different positions. Our CT scanning protocol design involved taking an AP scan on LPO and taking a PVP scan immediately after changing the patients’ position to RDD. However, in reality, it took a variable time to change the patient’s position, depending on the patient’s physical ability and degree of cooperation. Despite the effort our radiology technicians took to reposition patients as fast as possible, in some patients it took more than 3 minutes to change the position; hence, the images were closer to the DP scan, rather than the PVP scan. In these cases, the extent of the tumor was less distinct on the image, and it was difficult to determine whether the stomach had adhered to the adjacent organs.
We acknowledge several limitations of our study. First, because this study was retrospective, there was several missing information regarding surgical and pathologic findings. In particular, pathologic confirmation in terms of adjacent organ invasion was not confirmed in all patients. Adjacent organ invasion was surgically confirmed in 99 patients. Furthermore, a variety of MDCT scanners were used in our study owing to their retrospective design. Second, we did not analyze whether there were differential CT features for tumors between T4a with adhesion and T4b. As our study primarily aimed to determine whether the absence of a sliding sign is a differential CT feature for T4b tumors, further studies investigating other useful CT features for differentiating true adjacent organ invasion from simple adhesion by gastric cancers using CT imaging should be strongly needed. Third, we recruited only two reviewers with similar expertise (6 and 9 years of experience in abdominal imaging). Therefore, a performance study recruiting more reviewers with different expertise, such as novice and experienced reviewers, should also be determined whether the sliding sign can be more helpful for novice reviewers.
In conclusion, compared to LPO CT images alone, there is an added value of “sliding sign” on RDD CT for the determination of adjacent organ invasion in patients with AGC, by showing increased AUC values and improved interobserver agreement. However, as the presence of simple adhesion between the tumor and adjacent organ can be a source of false-negative findings for a sliding sign, further studies to investigate other useful CT features for differentiating true adjacent organ invasion from simple adhesion by gastric cancers are strongly warranted.
Footnotes
- Conceptualization, K.S.H.
- data curation, J.K., K.S.H.
- formal analysis, all authors.
- funding acquisition, K.S.H.
- investigation, all authors.
- methodology, K.S.H.
- project administration, J.K., K.S.H.
- resources, K.S.H.
- software, J.K., K.S.H.
- supervision, K.S.H.
- validation, J.K., K.S.H.
- visualization, J.K., K.S.H.
- writing—original draft, J.K.
- writing—review & editing, K.S.H.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2021R1F1A1046393) and from the Seoul National University Hospital Research Fund No. 04-2020-2010.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean practice guideline for gastric cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019;19:1–48. doi: 10.5230/jgc.2019.19.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kweon SS. Updates on cancer epidemiology in Korea, 2018. Chonnam Med J. 2018;54:90–100. doi: 10.4068/cmj.2018.54.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vita F, Giuliani F, Galizia G, Belli C, Aurilio G, Santabarbara G, et al. Neo-adjuvant and adjuvant chemotherapy of gastric cancer. Ann Oncol. 2007;18 Suppl 6:vi120–vi123. doi: 10.1093/annonc/mdm239. [DOI] [PubMed] [Google Scholar]
- 6.Lee DH, Kim SH, Joo I, Hur BY, Han JK. Comparison between 18F-FDG PET/MRI and MDCT for the assessment of preoperative staging and resectability of gastric cancer. Eur J Radiol. 2016;85:1085–1091. doi: 10.1016/j.ejrad.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joo I, Kim SH, Lee DH, Han JK. Dynamic contrast-enhanced ultrasound of gastric cancer: correlation with perfusion CT and histopathology. Korean J Radiol. 2019;20:781–790. doi: 10.3348/kjr.2018.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 10.Pacelli F, Cusumano G, Rosa F, Marrelli D, Dicosmo M, Cipollari C, et al. Multivisceral resection for locally advanced gastric cancer: an Italian multicenter observational study. JAMA Surg. 2013;148:353–360. doi: 10.1001/2013.jamasurg.309. [DOI] [PubMed] [Google Scholar]
- 11.Boku T, Nakane Y, Minoura T, Takada H, Yamamura M, Hioki K, et al. Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg. 1990;77:436–439. doi: 10.1002/bjs.1800770425. [DOI] [PubMed] [Google Scholar]
- 12.Borggreve AS, Goense L, Brenkman HJF, Mook S, Meijer GJ, Wessels FJ, et al. Imaging strategies in the management of gastric cancer: current role and future potential of MRI. Br J Radiol. 2019;92:20181044. doi: 10.1259/bjr.20181044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah MA, Ajani JA. Gastric cancer--an enigmatic and heterogeneous disease. JAMA. 2010;303:1753–1754. doi: 10.1001/jama.2010.553. [DOI] [PubMed] [Google Scholar]
- 14.Kim TU, Kim S, Lee JW, Lee NK, Jeon TY, Park DY. MDCT features in the differentiation of T4a gastric cancer from less-advanced gastric cancer: significance of the hyperattenuating serosa sign. Br J Radiol. 2013;86:20130290. doi: 10.1259/bjr.20130290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi JI, Joo I, Lee JM. State-of-the-art preoperative staging of gastric cancer by MDCT and magnetic resonance imaging. World J Gastroenterol. 2014;20:4546–4557. doi: 10.3748/wjg.v20.i16.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim AY, Kim HJ, Ha HK. Gastric cancer by multidetector row CT: preoperative staging. Abdom Imaging. 2005;30:465–472. doi: 10.1007/s00261-004-0273-5. [DOI] [PubMed] [Google Scholar]
- 17.Habermann CR, Weiss F, Riecken R, Honarpisheh H, Bohnacker S, Staedtler C, et al. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology. 2004;230:465–471. doi: 10.1148/radiol.2302020828. [DOI] [PubMed] [Google Scholar]
- 18.Kim JW, Shin SS, Heo SH, Lim HS, Lim NY, Park YK, et al. The role of three-dimensional multidetector CT gastrography in the preoperative imaging of stomach cancer: emphasis on detection and localization of the tumor. Korean J Radiol. 2015;16:80–89. doi: 10.3348/kjr.2015.16.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Springer P, Dessl A, Giacomuzzi SM, Buchberger W, Stöger A, Oberwalder M, et al. Virtual computed tomography gastroscopy: a new technique. Endoscopy. 1997;29:632–634. doi: 10.1055/s-2007-1004269. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Kim AY, Lee JH, Yook JH, Yu ES, Ha HK. Positioning during CT gastrography in patients with gastric cancer: the effect on gastric distension and lesion conspicuity. Korean J Radiol. 2009;10:252–259. doi: 10.3348/kjr.2009.10.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SH, Lee JM, Han JK, Lee JY, Yang HK, Lee HJ, et al. Effect of adjusted positioning on gastric distention and fluid distribution during CT gastrography. AJR Am J Roentgenol. 2005;185:1180–1184. doi: 10.2214/AJR.04.1812. [DOI] [PubMed] [Google Scholar]
- 22.Kim YH, Lee KH, Park SH, Kim HH, Hahn S, Park DJ, et al. Staging of T3 and T4 gastric carcinoma with multidetector CT: added value of multiplanar reformations for prediction of adjacent organ invasion. Radiology. 2009;250:767–775. doi: 10.1148/radiol.2502071872. [DOI] [PubMed] [Google Scholar]
- 23.Makino T, Fujiwara Y, Takiguchi S, Tsuboyama T, Kim T, Nushijima Y, et al. Preoperative T staging of gastric cancer by multi-detector row computed tomography. Surgery. 2011;149:672–679. doi: 10.1016/j.surg.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 24.You MW, Park S, Kang HJ, Lee DH. Radiologic serosal invasion sign as a new criterion of T4a gastric cancer on computed tomography: diagnostic performance and prognostic significance in patients with advanced gastric cancer. Abdom Radiol (NY) 2020;45:2950–2959. doi: 10.1007/s00261-019-02156-3. [DOI] [PubMed] [Google Scholar]
- 25.Lim JH, Ko YT, Lee DH. Sonographic sliding sign in localization of right upper quadrant mass. J Ultrasound Med. 1990;9:455–459. doi: 10.7863/jum.1990.9.8.455. [DOI] [PubMed] [Google Scholar]
- 26.Lim HK, Kim S, Lim JH, Kim SH, Lee WJ, Chun H, et al. Assessment of pancreatic invasion in patients with advanced gastric carcinoma: usefulness of the sliding sign on sonograms. AJR Am J Roentgenol. 1999;172:615–618. doi: 10.2214/ajr.172.3.10063846. [DOI] [PubMed] [Google Scholar]
- 27.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]