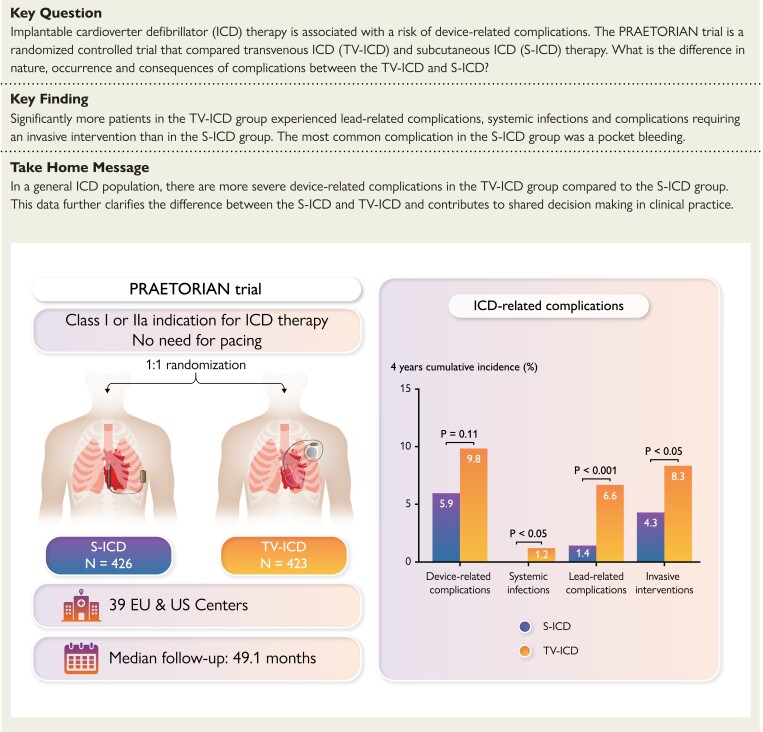
Structured Graphical Abstract
Structured Graphical Abstract.
Shown is the summary of the main endpoints of the manuscript: device-related complications in the subcutaneous and transvenous ICD: a secondary analysis of the PRAETORIAN trial. ICD, implantable cardioverter-defibrillator.
Keywords: Subcutaneous ICD, Transvenous ICD, Complications, Lead-related complications, Infections, Invasive interventions
Abstract
Background
The subcutaneous implantable cardioverter-defibrillator (S-ICD) is developed to overcome lead-related complications and systemic infections, inherent to transvenous ICD (TV-ICD) therapy. The PRAETORIAN trial demonstrated that the S-ICD is non-inferior to the TV-ICD with regard to the combined primary endpoint of inappropriate shocks and complications. This prespecified secondary analysis evaluates all complications in the PRAETORIAN trial.
Methods and results
The PRAETORIAN trial is an international, multicentre, randomized trial in which 849 patients with an indication for ICD therapy were randomized to receive an S- ICD (N = 426) or TV-ICD (N = 423) and followed for a median of 49 months. Endpoints were device-related complications, lead-related complications, systemic infections, and the need for invasive interventions. Thirty-six device-related complications occurred in 31 patients in the S-ICD group of which bleedings were the most frequent. In the TV-ICD group, 49 complications occurred in 44 patients of which lead dysfunction was most frequent (HR: 0.69; P = 0.11). In both groups, half of all complications were within 30 days after implantation. Lead-related complications and systemic infections occurred significantly less in the S-ICD group compared with the TV-ICD group (P < 0.001, P = 0.03, respectively). Significantly more complications required invasive interventions in the TV-ICD group compared with the S-ICD group (8.3% vs. 4.3%, HR: 0.59; P = 0.047).
Conclusion
This secondary analysis shows that lead-related complications and systemic infections are more prevalent in the TV-ICD group compared with the S-ICD group. In addition, complications in the TV-ICD group were more severe as they required significantly more invasive interventions. This data contributes to shared decision-making in clinical practice.
See the editorial comment for this article ‘Subcutaneous implantable cardioverter defibrillator for the prevention of sudden cardiac death: ready for prime-time?’, by llan Goldenberg and David T. Huang, https://doi.org/10.1093/eurheartj/ehac652.
Introduction
Implantable cardioverter-defibrillator (ICD) therapy is an effective therapy for the prevention of sudden cardiac death but comes with the risk of device-related complications.1–4 At 10 years, post-implant, the risk of lead failure can be as high as 25% in patients with a transvenous ICD (TV-ICD).5 The subcutaneous ICD (S-ICD) is a completely extravascular ICD and was developed to overcome lead-related complications.6 Currently, the S-ICD is considered a safe and viable alternative for TV-ICD therapy.7–9 Registries have shown that S-ICD therapy is associated with a complication rate of up to 15% in 5-year follow-up compared with 14% in the TV-ICD.4,9–12 However, the morbidity and clinical relevance of complications in S-ICD patients differ compared with complications related to TV-ICD patients.13
The PRAETORIAN trial is the first randomized controlled trial comparing the S-ICD and TV-ICD and demonstrated that the S-ICD is non-inferior to the TV-ICD with regard to the composite endpoint of device-related complications and inappropriate shocks in a general ICD population.14 In this primary analysis, device-related complications alone were shown to be not statistically different between S-ICD and TV-ICD. Since device-related complications after device implantation vary greatly in morbidity and clinical relevance, this prespecified secondary analysis of the PRAETORIAN trial analyses the type, nature and timing of complications and evaluates the clinical consequences in patients implanted with an S-ICD or TV-ICD.
Methods
Design and population of the PRAETORIAN trial
The PRAETORIAN trial is an international, multicentre, randomized controlled trial in which 849 patients were randomized 1:1 to receive an S-ICD or TV-ICD. Rationale and design of the PRAETORIAN trial were described in length elsewhere.15 In brief, patients with a Class I or IIa indication for ICD therapy and without the need for bradycardia pacing or cardiac resynchronisation therapy were included from March 2011 until January 2017 and randomized to S-ICD or TV-ICD therapy. Key exclusion criteria were a known ventricular tachycardia (VT) below 170 bpm or therapy refractory monomorphic VT. A dual-chamber device that is specifically deemed necessary for arrhythmia discrimination was allowed per protocol. The primary endpoint was the composite of inappropriate shocks and device-related complications. Patients were followed for a median of 49 months. ICD programming was mandated by protocol in both arms. All complications were collected, monitored, and adjudicated by a clinical events committee (CEC). All participating centres were required to have ample experience with implanting both devices. Implant procedure and follow-up were per local routine. The PRAETORIAN study protocol was approved by the institutional medical ethics committees and all patients provided written informed consent.
Endpoints and definitions
In this prespecified secondary analysis, all complications adjudicated by the CEC as device-related were analysed. Device-related complications included the following: device-related infections that resulted in a lead or generator extraction; pocket bleedings—also called pocket haematoma—that required drain insertion, blood transfusion or prolonged hospitalization; device-related thrombotic events; pneumothorax or haemothorax that led to intervention or prolongation of hospitalization; lead perforation; tamponade; lead repositioning and other complications related to the lead or generator that led to medical or surgical intervention. Generator replacements due to normal battery depletion were not included as device-related complications.
Lead-related complications were defined as complications directly caused by insertion or chronic placement of the ICD lead or leading to extraction, repositioning, or replacement of the ICD lead. Systemic infections were defined as infections with positive blood cultures and sepsis was defined as a dysregulated host response to infection causing life-threatening organ dysfunction.16 Acute complications were defined as device-related complications occurring within ≤ 30 days, and late complications were defined as complications occurring more than 30 days after device implantation. Procedure-related complications were defined as complications within 30 days including device-related bleeding, thrombotic event, defibrillation test failure, perforation, tamponade, pneumothorax, and implantation failure. Lead and/or device repositioning, lead and/or device replacements, device extractions, pocket explorations, or drain insertions after initial implantation or implantation attempt were considered invasive interventions.
Statistical analysis
All data were analysed using an intention-to-treat analysis. An as-treated analysis was also performed for lead-related complications. Descriptive statistics are reported as mean ± standard deviation or median with interquartile range (IQR) for continuous variables and numbers and percentages for categorical variables. Baseline variables were compared using the Fisher exact test, χ2 test, Student t-test, or Mann–Whitney U test, as appropriate. For time-to event variables, Kaplan–Meier curves displaying the pattern of events were constructed and 48-month Kaplan–Meier estimates of the event rate are reported for both study groups and compared using log-rank tests. Participants without events were censored at their last known event-free time point. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated by Cox proportional hazard models. The proportional hazard assumptions were assessed by scaled Schoenfeld residuals and visually comparing the plot of the log of cumulative hazard between treatments. Univariable and multivariable Cox proportional hazard models were performed to find predictors for device-related complications and device-related complications requiring invasive interventions. Two-sided P-values <0.05 were considered statistically significant. P-values were not adjusted for multiplicity. All statistical analyses were performed using R software version 4.0.3 (RStudio PBC).
Results
Patient characteristics
A total of 849 patients were randomized to an S-ICD (N = 426) or TV-ICD (N = 423). Details and primary results of the PRAETORIAN trial are published elsewhere.14 Baseline characteristics of the total cohort are indicated in Supplementary material online, Table S1. In short, median age was 63 years (IQR: 54–69 years) in the S-ICD group and 64 years (IQR, 56–70 years) in the TV-ICD group, 20.9% were female in the S-ICD group and 18.4% in the TV-ICD group, 67.8% had an ischaemic cardiomyopathy in the S-ICD group and 70.4% in the TV-ICD group. In the S-ICD group, 81.2% and in the TV-ICD group 80.1% received the ICD for primary prevention. The median follow-up time was 48.0 months in the S-ICD group and 50.6 months in the TV-ICD group. Implant experience per implanter, median implantation duration, incision technique, use of prophylactic antibiotics, use of general anaesthesia, and defibrillation test (DFT) performance are given in Supplementary material online, Table S2.
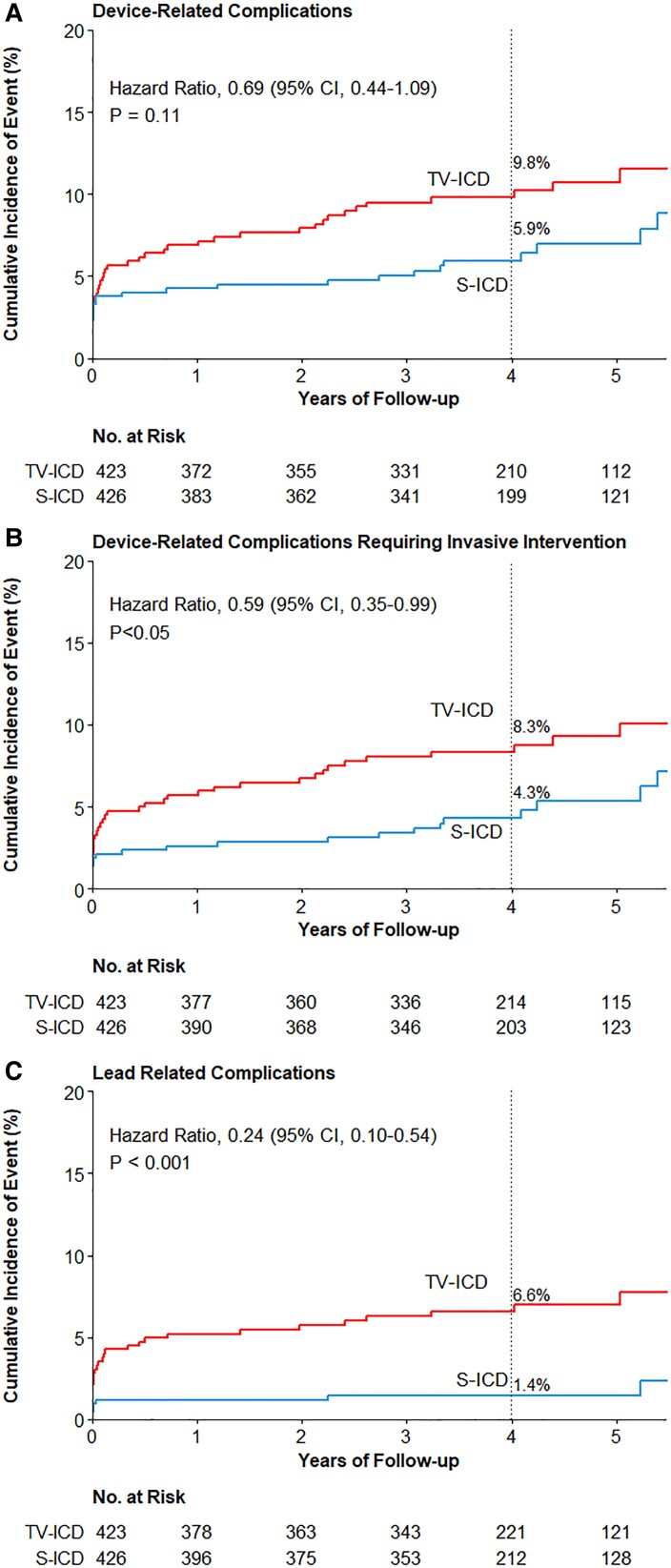
Thirty-one patients in S-ICD group experienced a total of 36 device-related complications and 44 patients in the TV-ICD group had a total of 49 device-related complications. There was no statistical difference in the total number of patients experiencing a device-related complication in the S-ICD group and TV-ICD group (48-month Kaplan–Meier estimated cumulative incidence, 5.9% and 9.8%, respectively; HR, 0.69 (95% CI: 0.44–1.09); P = 0.11, Figure 1). In the TV-ICD group, three (6.8%) patients had a dual-chamber ICD implanted and two (4.5%) patients had a CRT-D implanted. In the S-ICD group, one patient had a dual-chamber TV-ICD (3.2%) at the time of experiencing a device-related complication. Four patients in the S-ICD group and five patients in the TV-ICD group experienced more than one device-related complication during follow-up (see Supplementary material online, Table S3). Median time from initial implant to first device-related complication was 11 days (IQR: 1–1218 days) in the S-ICD group and 41 days (IQR: 2–736 days) in the TV-ICD group (P = 0.78). The median age of patients experiencing a complication in the S-ICD group was 65 years (IQR: 58–69 years) and 62 years (IQR: 56–70) in the TV-ICD group. The characteristics of patients with device-related complications were similar in both arms except for the history of atrial fibrillation (AF) which was significantly higher in the S-ICD group (41.9% vs. 6.8%, P = <0.001, Table 1). A comparison of the baseline characteristics of patients with and without device-related complications in both groups is shown in Supplementary material online, Table S4. A history of AF was a positive predictor for device-related complications in the S-ICD group and a negative predictor in the TV-ICD group (see Supplementary material online, Table S5). Body mass index (BMI) in the S-ICD group and a history of AF in the TV-ICD group were independent predictors for device-related complications requiring an invasive intervention (see Supplementary material online, Table S6).
Figure 1.
Time-to-first-event curves for total device-related complications, device-related complications requiring invasive intervention and lead-related complications. Shown are the cumulative event rates of the first occurrence of device-related complications (A), device-related complication requiring invasive intervention (B), and lead-related complications (C). Hazard ratios are derived from Cox regressions and indicate the relative risk (subcutaneous ICD vs. transvenous ICD) of the endpoint. P-values were not adjusted for multiple comparisons. ICD, implantable cardioverter-defibrillator; S-ICD, subcutaneous ICD; TV-ICD, transvenous ICD.
Table 1.
Baseline characteristics of patients with device-related complications
| Patients with a device-related complication (N = 75) | |||
|---|---|---|---|
| Subcutaneous ICD (N = 31) | Transvenous ICD (N = 44) | P-value | |
| Age, years, median (IQR) | 65 (58–69) | 62 (56–70) | 0.47 |
| Female sex, n (%) | 6 (19.4) | 11 (25.0) | 0.78 |
| Diagnosis, n (%) | 0.64 | ||
| Ischaemic cardiomyopathy | 22 (71) | 35 (79.5) | |
| Non-ischaemic cardiomyopathy | 7 (22.6) | 7 (15.9) | |
| Inherited cardiac disease | 2 (6.5) | 1 (2.3) | |
| Hypertrophic cardiomyopathy | 1 (3.2) | 1 (2.3) | |
| Hypertrophic cardiomyopathy and Brugada | 1 (3.2) | 0 | |
| Idiopathic ventricular fibrillation | 0 | 1 (2.3) | |
| Secondary prevention, n (%) | 3 (9.7) | 4 (9.1) | 1.0 |
| Ejection fraction, %, median (IQR) | 28 (23–32)a | 29 (20–30)b | 0.99 |
| NYHA class, n (%) | 0.15 | ||
| I | 14 (45.2) | 12 (27.3) | |
| II | 12 (38.7) | 27 (61.4) | |
| III/IV | 5 (16.1) | 5 (11.4) | |
| Body mass index, kg/m2, median (IQR) | 27.4 (25.0–31.0) | 27.1 (23.9–30.8) | 0.46 |
| History of atrial fibrillation, n (%) | 13 (41.9) | 3 (6.8) | <0.001 |
| History of diabetes, n (%) | 11 (35.5) | 15 (34.1) | 1.0 |
ICD, implantable cardioverter-defibrillator, IQR, interquartile range, NYHA, New York Heart Association.
missing in one patient.
missing in four patients.
Type of device-related complications
The most common device-related complications in the S-ICD group were pocket bleedings (8/36), whereas lead dysfunctions (9/49) and infections (8/49) were most prominent in the TV-ICD group (Table 2). In the S-ICD group, 3 of 8 patients with a pocket bleeding had a history of AF. In 5 of 8 patients (62.5%) in the TV-ICD group, the infection was systemic with severe morbidity, with three patients having a sepsis, one critically ill patient with a concomitant pulmonary embolism, and one patient with a multifocal pneumonia with acute respiratory failure and need for mechanical ventilation. One of the patients in the TV-ICD group with a pocket infection without systemic involvement did not receive prophylactic antibiotics. Two patients with a systemic infection in the TV-ICD group died within 12 months after device infection. In the S-ICD group, four patients (12.9%) experienced a device-related infection, of whom none had a systemic infection (P = 0.03, Tables 2 and 3), and none died within 12 months after device infection.
Table 2.
Type of device-related complications in the S-ICD and TV-ICD
| S-ICD (N = 36) | TV-ICD (N = 49) | |
|---|---|---|
| Infection, n (%) | 4 (11.1) | 8 (16.3) |
| Pocket/pulse generator | 3 | 3 |
| Lead | 1a | 5a |
| Bleeding, n (%) | 8 (22.2) | 2 (4.1) |
| Thrombotic event, n (%) | 1 (2.8) | 2 (4.1)a |
| Pneumothorax, n (%) | 0 | 4 (8.2)a |
| Lead perforation, n (%) | 0 | 2 (4.1)a |
| Tamponade, n (%) | 0 | 2 (4.1)a |
| Lead repositioning, n (%) | 2 (5.6) | 7 (14.3)a |
| DFT failure | 1 | 0 |
| Lead dislocation | 1a | 5 |
| Lead dysfunction | 0 | 2b |
| Other lead or device complications, n (%) | 21 (58.3) | 22 (44.9) |
| Lead replacement, n (%) | 3 (8.3)a | 9 (18.4)a |
| Lead dysfunction | 0 | 7b |
| Lead dislocation | 2c | 1 |
| Lead perforation | 0 | 1 |
| Inappropriate therapy | 1 | 0 |
| Device malfunction, n (%) | 4 (11.1) | 6 (12.2) |
| Early battery depletion | 3 | 2 |
| Interrogation problem | 1 | 0 |
| Long charging time | 0 | 1 |
| Automatic ICD reset | 0 | 1 |
| Other device malfunction | 0 | 2d |
| Sensing issues, n (%) | 4 (11.1)a,c | 0 |
| Pacing indication, n (%) | 5 (13.9) | 1 (2.0)c |
| Implantation failure, n (%) | 0 | 3 (6.1)a,e |
| DFT failure with subsequent action, n (%) | 3 (8.3)a | 0 |
| Pain or discomfort, n (%) | 2 (5.6)b,c | 3 (6.1) |
DFT, defibrillator threshold testing, ICD, implantable cardioverter-defibrillator, S-ICD, subcutaneous ICD, TV-ICD, transvenous ICD.
Device-related complication that are combined in the endpoint lead-related complications: 1/4 sensing issues in the S-ICD group was a reduced RV sensing of the RV lead (crossover in S-ICD group); 1/3 DFT failure with subsequent action was included as this one was amongst others related to incorrect lead position; one lead repositioning after DFT failure was not included in this combined endpoint as DFT continued to fail after lead repositioning and was therefore not seen as related to the lead; 1/3 implant failures in the TV-ICD group was included in the lead-related endpoint as in this patient RV lead positioning was impossible despite multiple attempts.
Four patients with a lead dysfunction had an increased threshold of which two had also an increased impedance, two patients had an undersensing of the RV lead and three patients had a lead fracture.
Crossover prior to complication. Only 1/2 patients with a lead dislocation, 1/4 patient with sensing issues, and 1/2 patients with pain or discomfort in the S-ICD group had a crossover to TV-ICD before this complication.
One battery/voltage error, one capacitor charging error.
In 2/3 patients there was no venous access during implantation, in 1/3 patients RV lead positioning was impossible despite multiple attempts.
Table 3.
Forty-eight-month Kaplan–Meier estimated cumulative incidence of main endpoints
| S-ICD | TV-ICD | P-valuea | HR (CI) | |
|---|---|---|---|---|
| Device-related complications | 5.9% | 9.8% | 0.11 | 0.69 (0.44–1.09) |
| Invasive interventions | 4.3% | 8.3% | 0.047 | 0.59 (0.35–0.99) |
| Lead-related complications | 1.4% | 6.6% | < 0.001 | 0.24 (0.10–0.54) |
| Systemic infections | 0 | 1.2% | 0.03 | – |
| Acute complications | 3.8% | 4.7% | 0.49 | 0.79 (0.41–1.53) |
P-values were not adjusted for multiple comparisons.
A total of 2 of 36 patients in the S-ICD group underwent a pulse generator replacement before experiencing a device-related complication. In one of these patients, the device-related complication was related to the replacement procedure. Two patients in the S-ICD group had a crossover to a TV-ICD before the device-related complication occurred, due to a non-systemic infection and a sensing issue, respectively. Both patients experienced a lead-related complication after crossover (see Supplementary material online, Table S7). In the TV-ICD group, one patient underwent a pulse generator replacement and had a device-related complication related to this replacement procedure. One patient in the TV-ICD group developed a pacing indication 13 months after having a crossover to an S-ICD due to TV-ICD implant failure (see Supplementary material online, Table S7).
Lead-related complications
Significantly less patients experienced lead-related complications in the S-ICD group (48-month Kaplan–sMeier estimated cumulative incidence 1.4% and 6.6%, respectively; HR: 0.24; 95% CI: 0.10–0.54; P < 0.001, Figure 1 and Table 3). In the TV-ICD group, 29 patients had 32 lead-related complications compared with seven patients with seven lead-related complications in the S-ICD group. Three patients in the TV-ICD group with multiple leads experienced a total of four lead-related complications; two lead replacements were due to dysfunction of the right ventricular lead and one tamponade and one pneumothorax occurred in patients with a CRT-D. Supplementary material online, Table S8 shows an overview of all lead-related complications. In total, four drain insertions were performed as an intervention after a lead-related complication of which none were associated with a subsequent infection. Two of seven patients in the S-ICD group experienced a lead-related complication while having a TV-ICD, one was a sensing issue and one was an atrial lead dislocation after having a dual-chamber ICD implanted. The as-treated analysis showed five lead-related complications in five (1.0%) patients with an S-ICD and 34 lead-related complications in 31 (7.0%) patients with a TV-ICD (P < 0.001).
Acute and late device-related complications
Eighteen of 36 (50.0%) and 21 of 49 (42.9%) of the device-related complications were acute complications in the S-ICD and TV-ICD group, respectively (Table 4). In the S-ICD group, pocket bleedings (7/18) were the most frequent type of acute complication, whereas in the TV-ICD group, lead repositioning was most frequent (6/21) (Table 4). Of the acute complications, 11 (61.1%) in the S-ICD group and 13 (61.9%) in the TV-ICD group were procedure-related and four (22.2%) in the S-ICD group and 16 (76.2%) in the TV-ICD group were lead-related. The most frequent late complication in the S-ICD group was the development of a pacing indication (5/18), and in the TV-ICD group, a lead replacement (8/28) followed by a device infection (7/28) (Table 4). All five patients in the S-ICD group developed a pacing indication more than 1 year after device implantation (see Supplementary material online, Table S9). In 2 of these patients, a single-chamber pacemaker was implanted, whereas in the other three patients, the S-ICD was exchanged for a TV-ICD or CRT-D (see Supplementary material online, Table S7). Half of the total complications occurred in the first 3 months after implantation in both arms (S-ICD 50.0% and TV-ICD 51.0%, Supplementary material online, Table S9).
Table 4.
Timing of device-related complications after last implanted device
| 30 days | > 30 days | |
|---|---|---|
| S-ICD, n (%) | 18 (50.0) | 18 (50.0) |
| Infection | 1 | 3 |
| Pocket bleeding | 7 | 1a |
| Thrombotic event | 1 | 0 |
| Lead repositioning | 2 | 0 |
| Other lead or device complication | 7 | 14 |
| Lead replacement | 2 | 1 |
| Device malfunction | 1 | 3 |
| Sensing issues | 1 | 3 |
| Defibrillation test failure | 3 | 0 |
| Pain or discomfort | 0 | 2 |
| Pacing indication | 0 | 5 |
| TV-ICD, n (%) | 21 (42.9) | 28 (57.1) |
| Infection | 1 | 7 |
| Bleeding | 2 | 0 |
| Thrombotic event | 0 | 2 |
| Perforation | 2 | 0 |
| Lead repositioning | 6 | 1 |
| Pneumothorax | 4 | 0 |
| Tamponade | 2 | 0 |
| Other lead or device complication | 4 | 18 |
| Lead replacement | 1 | 8 |
| Device malfunction | 0 | 6 |
| Implantation failure | 3 | 0 |
| Pain or discomfort | 0 | 3 |
| Pacing indication | 0 | 1 |
ICD, implantable cardioverter-defibrillator, S-ICD, subcutaneous ICD, TV-ICD, transvenous ICD.
Patient underwent an extraction of the S-ICD due to expired ICD indication after which a pocket bleeding occurred.
Interventions
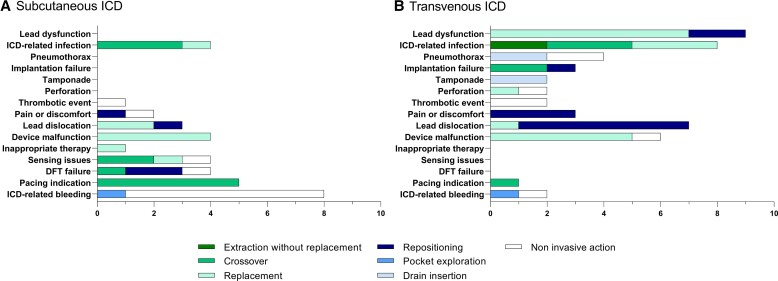
Patients in the S-ICD group underwent significantly less often invasive interventions as a result of device-related complications compared with the TV-ICD group (48-month Kaplan–Meier estimated cumulative incidence 4.3% vs. 8.3%, HR: 0.59; 95% CI: 0.35–0.99 P = 0.047, Figure 1 and Table 3). An overview of the specific interventions per type of device-related complication is shown in Figure 2. In total, 25 of 36 device-related complications in the S-ICD group and 42 of 49 device-related complications in the TV-ICD group required an invasive intervention (Table 5). The most common invasive intervention in the S-ICD group was a crossover (11/25) and in the TV-ICD group, a lead replacement (9/42). The most frequent reason for crossover in the S-ICD group was the development of a pacing indication (5/11) followed by an infection (3/11). In the TV-ICD group, the most common reason for crossover was an infection (3/6) (see Supplementary material online, Table S7).
Figure 2.
Type of intervention after device-related complications. Shown are the different interventions after a specific device-related complication in the S-ICD group (A) and the different interventions after a specific device-related complication in the TV-ICD group (B). Details on type of non-invasive interventions are presented in Table 5. DFT, defibrillator threshold testing, ICD, implantable cardioverter-defibrillator, S-ICD, subcutaneous ICD, TV-ICD, transvenous ICD.
Table 5.
Interventions after device-related complications
| S-ICD (N = 36) | TV-ICD (N = 49) | |
|---|---|---|
| Invasive interventions, n (%) | 25 (69.4) | 42 (85.7) |
| Replacement pulse generator, n (%) | 4 (11.1) | 4 (8.2) |
| Replacement lead, n (%) | 3 (8.3) | 9 (18.4) |
| Extraction with replacement pulse generator + lead, n (%) | 2 (5.6) | 4 (8.2) |
| Extraction without replacement, n (%) | 0 | 2 (4.1) |
| Crossover, n (%) | 11 (30.6) | 6 (12.2)a |
| Repositioning lead, n (%) | 2 (5.6) | 8 (16.3) |
| Repositioning pulse generator, n (%) | 1 (2.8) | 3 (6.1) |
| Reposition pulse generator + lead, n (%) | 1 (2.8) | 1 (2.0)b |
| Pocket exploration, n (%) | 1 (2.8) | 1 (2.0) |
| Drain insertion, n (%) | 0 | 4 (8.2) |
| Non-invasive interventions, n (%) | 11 (30.6) | 7 (14.3) |
| Additional DFT, n (%) | 2 (5.6) | 0 |
| Reprogramming, n (%) | 0 | 1 (2.0) |
| Blood transfusion, n (%) | 3 (8.3) | 0 |
| Thrombolysis, n (%) | 1 (2.8) | 0 |
| Change in medication, n (%) | 1 (2.8) | 2 (4.1) |
| Conservative, n (%) | 4 (11.1) | 4 (8.2) |
DFT, defibrillator threshold testing; ICD, implantable cardioverter-defibrillator; S-ICD, subcutaneous ICD; TV-ICD, transvenous ICD.
One patient in the TV-ICD group received an S-ICD due to TV-ICD implantation failure and later developed a pacing indication for which a TV-ICD was implanted and received a total of two crossovers.
One patient underwent a right-sided TV-ICD implantation.
In the TV-ICD group, 7 of 8 patients required intravenous antibiotics due to a device-related infection. In the S-ICD group all patients with an infection were treated with oral antibiotics. In 3 of 8 patients in the TV-ICD group with an infection, the infection occurred within 1 year after implantation and a lead explantation was performed. In the remaining five patients, a lead extraction was performed with and without the use of powered sheaths. After extraction of the S-ICD following infection, an S-ICD was re-implanted in one patient and in the three other patients, a TV-ICD was implanted. After extraction of the TV-ICD following infection, three patients received an S-ICD, three patients underwent a re-implantation of a TV-ICD and in two patients the device was extracted without replacement (Figure 2).
Hospitalization and healthcare burden
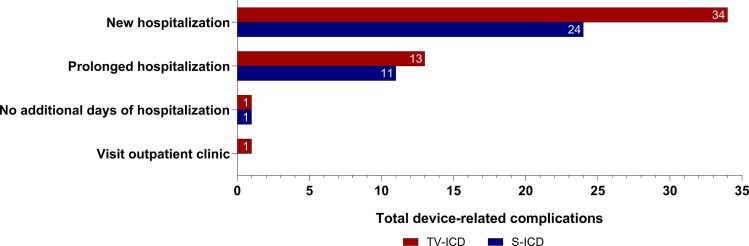
A new hospitalization was required for 24 of 36 (66.7%) device-related complications in 23 patients in the S-ICD group and for 34 of 49 (69.4%) device-related complications in 33 patients in the TV-ICD group (Figure 3). In the TV-ICD group, 13 (26.5%) complications resulted in a prolonged hospitalization compared with 11 (30.6%) in the S-ICD group. The median number of additional hospitalization days was similar in both the S-ICD and TV-ICD group (S-ICD 2.5 (IQR 1–5); TV-ICD 3.0 (IQR 1–7), P = 0.49). The total number of additional hospital days due to device-related complications was 151 days in the S-ICD group and 354 days in the TV-ICD group.
Figure 3.
Burden of device-related complications on the healthcare system. Shown is the burden of device-related complications on new hospitalizations, prolonged hospitalizations and visits to the outpatient clinic. ICD, implantable cardioverter-defibrillator, S-ICD, subcutaneous ICD, TV-ICD, transvenous ICD.
Discussion
This prespecified secondary analysis of the PRAETORIAN trial demonstrated a difference in nature and severity of device-related complications in the S-ICD and TV-ICD. Lead-related complications and systemic infections are significantly lower in the S-ICD group compared with the TV-ICD group. On the other hand, pocket bleedings are more frequent in patients implanted with an S-ICD. In the TV-ICD group, device-related complications resulted in significantly more invasive interventions (Structured Graphical Abstract).
The overall device-related complication rate of 5.9% vs. 9.8%, which was included in the primary endpoint of the trial, did not significantly differ between the S-ICD and TV-ICD. The complication rate in the TV-ICD group is comparable with earlier studies,4,12 whereas the complication rate in the S-ICD group was lower compared with earlier registries.9–11 This is most likely caused by differences in definition of device-related complications and inclusion of centres with limited S-ICD experience in the registries.
The more frequent occurrence of pocket bleedings in the S-ICD group might be related to the larger incision and larger pocket necessary for S-ICD implantation due to the larger generator size. Additionally, the majority of patients with a TV-ICD receive a pressure bandage after implantation, while after S-ICD implantation this is less often applied and can explain the more frequent pocket bleedings. Furthermore, anticoagulation strategies before TV-ICD implantation has been extensively studied, but data regarding anticoagulation strategies before S-ICD implantation are lacking and is up to the preference of the implanter.17 A retrospective study of 200 S-ICD patients showed that pocket bleedings in the S-ICD did not appear in patients with interrupted anticoagulation and suggests that interruption without bridging may reduce pocket bleedings after S-ICD implantation18 However, data on peri-procedural anticoagulation strategies within this trial were not available.
Overall, more implant experience may result in a reduction of device-related complications such as pocket bleedings in S-ICD patients.19
Device-related infections
Patients with a TV-ICD had significantly more systemic infections requiring device extraction. By its intravascular design, infections of the TV-ICD have a higher chance of developing into a systemic infection. A prospective multicentre observational registry of 1099 ICD patients showed that all infections in the TV-ICD were systemic compared with none in the S-ICD.13 In our study, all systemic infections occurred in patients implanted with a TV-ICD, no systemic infections were seen in S-ICD patients. Systemic infections usually have more serious clinical consequences, which is emphasized by the occurrence of sepsis, pulmonary embolism and respiratory insufficiency in the TV-ICD group. The 1-year mortality of a device infection triples from 7 to 24% if the transvenous lead is involved.1 In this study, two of five patients with a systemic infection died within 1 year after infection related to their TV-ICD. Additionally, if extraction is required, removal of the S-ICD pulse generator and/or leads is less complex and is associated with fewer complications and a lower mortality risk compared with the TV-ICD.20,21
Lead-related complications
As expected by the extravascular design of the S-ICD, this study showed significantly less lead-related complications in patients with an S-ICD. This finding is in line with previous meta-analyses of non-randomized studies and confirmed by the recent ATLAS trial.22–24 It was shown previously that implanting more leads results in a higher risk of lead-related complications.25 In the present study, for two lead-related complications in the TV-ICD group and one lead-related complication in the S-ICD group, it could not be excluded that the complication was caused by another than the standard right ventricular ICD lead. Patients with a TV-ICD are especially prone to lead failure after multiple years of ICD therapy because of the mechanical traction due to heart motion and arm movements. A large TV-ICD registry showed a lead failure rate of 25% after 10 years.5 A longer follow-up of patients in this study may therefore result in an even higher rate of lead-related complications in the TV-ICD group. Notwithstanding, the S-ICD received an advisory in December 2020 due to 27 reported cases of lead fractures with a calculated occurrence rate of 0.2% at 41 months.26 No lead fractures in the S-ICD were observed in this study: the extended follow-up of the PRAETORIAN trial will put this advisory on lead-related complications in S-ICD patients in further perspective.
Predictors
AF was a positive predictor for device-related complications in the S-ICD group. Although this mechanistically could be explained by a higher risk of pocket bleedings due to anticoagulation therapy, the fact that only 3 of 8 patients with a bleeding in the S-ICD group had a known history of AF suggests that also other mechanisms may play a role. Similarly, the higher number of patients with a history of AF in the S-ICD group cannot be fully explained by the higher number of pocket bleedings in this group. In the TV-ICD group, AF was a negative predictor for device-related complications which is in contrast to findings in other studies and might be caused a play of chance, since there does not seem to be a logical mechanistically explanation.27 The choice for a specific device in patients with AF remains a challenge as a history of AF is a risk factor for inappropriate therapy in patients with a TV-ICD.28
BMI was shown to be a positive independent predictor for device-related complications in the S-ICD group requiring an invasive intervention. The extravascular nature of the S-ICD and the position of the lead and generator underneath a layer of fat tissue may hamper the attachment of the lead to the fibrotic tissue in patients with a high BMI and therefore these patients may be more prone to lead dislocation. BMI could be a factor during shared decision-making when selecting device type. Specifically as a reduced BMI is associated with an increased risk on device complications in the TV-ICD.29 However, more studies with a larger volume of patients and more recent implantation techniques are necessary to further investigate this and to determine potential BMI thresholds.
Invasive interventions and hospitalization
An invasive intervention was more often needed after device-related complications in TV-ICD patients. This was mainly driven by interventions due to lead dysfunction or device-related infections and concomitantly resulted in more hospital admissions. Interventions after S-ICD implant were less often invasive, mainly driven by the non-invasive interventions after a pocket bleeding. It is unknown to what extent this was caused by physicians being more cautious due to having less experience with the S-ICD and may not be occurring with current standard of care.
Crossovers after implantation occurred more frequently from S-ICD to TV-ICD. Although development of a pacing indication was an important reason for crossover to TV-ICD, there may also have been a lower threshold for crossover from S-ICD to TV-ICD due to limited initial experience of the implanters and treating centres. An example is crossover to a TV-ICD after a failed DFT with the S-ICD. Current experience is that suboptimal implantation can result in DFT failure and can be managed with repositioning and often improve DFT outcome.30 It can therefore be speculated that, with the increased knowledge of the S-ICD, newer generation S-ICDs and current treatment of complications, the number of S-ICD complications requiring crossover may be lower if the trial would be repeated. New technology by adding a leadless pacemaker for anti-tachycardia pacing or even bradycardia pacing in the future might further reduce the need for crossover.31
The current guidelines of the European Society of Cardiology recommend S-ICD therapy, in the absence of a pacing indication, as an alternative for TV-ICD therapy.32 In this study, it was shown that even in a setting where the S-ICD had a disadvantage of limited implanter experience and therapy optimization, device-related complications in the S-ICD are less severe as they required less often an invasive intervention. Therefore, the S-ICD could become the preferred choice in patients with an ICD indication without need for pacing.
A limitation of this study is the difference in experience of the implanters and treating centres with both systems. Although centres were selected who had experience with the S-ICD, this was always less than with the TV-ICD, simply due to the shorter availability of the former in clinical practice. The difference in selection of anaesthesia during ICD implantation may also have had an unknown effect on device-related complication, for example, on post-operative pain and/or discomfort. P-values were not adjusted for multiple comparisons, and this should be taken into account when interpreting the results. Furthermore, data regarding implant techniques, such as the type of venous access, and pocket position, were lacking.
Conclusion
In the primary endpoint of the PRAETORIAN trial, there was no significant difference in overall device-related complications; however, the nature and consequences of device-related complications differ. This secondary analysis showed that lead-related complications and systemic infections were more prevalent in the TV-ICD group compared with the S-ICD group, whereas pocket bleedings were more frequently observed in patients receiving an S-ICD. Device-related complications in the TV-ICD group were considered more severe as they required significantly more often an invasive intervention. These data further clarify the difference between the S-ICD and TV-ICD and contribute to shared decision-making in clinical practice.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors thank the patients who participated in this trial, the members of the Clinical Event Committee, for adjudicating all events, the members of the data and safety monitoring board, the physicians and the research coordinators for contributions to the conduct of this trial.
Contributor Information
Reinoud E Knops, Amsterdam UMC location University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences Heart failure & Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
Shari Pepplinkhuizen, Amsterdam UMC location University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences Heart failure & Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
Peter Paul H M Delnoy, Department of Cardiology, Isala Heart Centre, Zwolle, The Netherlands.
Lucas V A Boersma, Amsterdam UMC location University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences Heart failure & Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands; Department of Cardiology, St. Antonius Hospital, Nieuwegein, The Netherlands.
Juergen Kuschyk, First Department of Medicine, University Medical Center Mannheim, Mannheim, Germany; German Center for Cardiovascular Research Partner Site Heidelberg, Mannheim, Germany.
Mikhael F El-Chami, Division of Cardiology Section of Electrophysiology, Emory University, Atlanta, GA, United States.
Hendrik Bonnemeier, Klinik für Innere Medizin III, Schwerpunkt Kardiologie und Angiologie, Universitätsklinikum Schleswig-Holstein, Campus Kiel, Kiel, Germany.
Elijah R Behr, St George’s University of London, London, United Kingdom; St George’s University hospitals NHS Foundation Trust, London, United Kingdom.
Tom F Brouwer, Amsterdam UMC location University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences Heart failure & Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
Stefan Kaab, Department of Medicine I, Ludwig-Maximillians University Hospital, München, Germany; German Center for Cardiovascular Research, Munich Heart Alliance, Munich, Germany; European Reference Network for rare, low prevalence and complex diseases of the heart: ERN GUARD-Heart.
Suneet Mittal, The Valley Health System, Ridgewood, NJ, United States.
Anne-Floor B E Quast, Amsterdam UMC location University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences Heart failure & Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
Willeke van der Stuijt, Amsterdam UMC location University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences Heart failure & Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
Lonneke Smeding, Amsterdam UMC location University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences Heart failure & Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
Jolien A de Veld, Amsterdam UMC location University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences Heart failure & Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
Jan G P Tijssen, Amsterdam UMC location University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences Heart failure & Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
Nick R Bijsterveld, Department of Cardiology, Flevoziekenhuis, Almere, the Netherlands.
Sergio Richter, Department of Electrophysiology, Heart Center at University of Leipzig, Leipzig, Germany; Heart Surgery, Heart Center Dresden, Carl Gustav Carus Medical Faculty, Dresden University of Technology, Dresden, Germany.
Marc A Brouwer, Department of Cardiology, Radboud University Medical Center, Nijmegen, the Netherlands.
Joris R de Groot, Amsterdam UMC location University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences Heart failure & Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
Kirsten M Kooiman, Amsterdam UMC location University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences Heart failure & Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
Pier D Lambiase, Office of the Director of Clinical Electrophysiology Research and Lead for Inherited Arrhythmia Specialist Services, University College London and Barts Heart Centre, London, United Kingdom.
Petr Neuzil, Department of Cardiology, Homolka Hospital, Prague, Czech Republic.
Kevin Vernooy, Department of Cardiology, Cardiovascular Research Institute Maastricht, Maastricht University Medical Center, Maastricht, the Netherlands.
Marco Alings, Department of Cardiology, Amphia Hospital, Breda, the Netherlands; Werkgroep Cardiologische Centra Nederland, Utrecht, the Netherlands.
Timothy R Betts, Oxford Biomedical Research Centre, Oxford University Hospitals NHS Trust, Oxford, United Kingdom.
Frank A L E Bracke, Department of Electrophysiology, Catharina Hospital Eindhoven, Eindhoven, the Netherlands.
Martin C Burke, CorVita Science Foundation, Chicago, IL, United States.
Jonas S S G de Jong, Department of Cardiology, OLVG, Amsterdam, Netherlands.
David J Wright, Liverpool Heart and Chest Hospital, Liverpool, United Kingdom.
Ward P J Jansen, Department of Cardiology, Tergooi MC, Blaricum, The Netherlands.
Zachary I Whinnett, National Heart and Lung Institute, Imperial College London, London, United Kingdom.
Peter Nordbeck, University and University Hospital Würzburg, Würzburg, Germany.
Michael Knaut, Heart Surgery, Heart Center Dresden, Carl Gustav Carus Medical Faculty, Dresden University of Technology, Dresden, Germany.
Berit T Philbert, Department of Cardiology, The Heart Centre, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Jurren M van Opstal, Medical Spectrum Twente, Enschede, the Netherlands.
Alexandru B Chicos, Division of Cardiology, Northwestern Memorial Hospital, Northwestern University, Chicago, IL, United States.
Cornelis P Allaart, Department of Cardiology, and Amsterdam Cardiovascular Sciences (ACS), Amsterdam UMC, Location VUMC, Amsterdam, The Netherlands.
Alida E Borger van der Burg, Medisch Centrum Leeuwarden, Leeuwarden, The Netherlands.
Jose M Dizon, Department of Medicine—Cardiology, Columbia University Irving Medical Center, New York, NY, United States.
Marc A Miller, Icahn School of Medicine at Mount Sinai, Mount Sinaï Hospital, New York, NY, United States.
Dmitry Nemirovsky, Cardiac Electrophysiology Division, Department of Medicine, Englewood Hospital and Medical Center, Englewood, NJ, United States.
Ralf Surber, Department of Internal Medicine I, Jena University Hospital, Jena, Germany.
Gaurav A Upadhyay, Center for Arrhythmia Care, Heart and Vascular Institute, University of Chicago Pritzker School of Medicine, Chicago, IL, United States.
Raul Weiss, Division of Cardiovascular Medicine, College of Medicine, The Ohio State University, Columbus, OH, United States.
Anouk de Weger, Amsterdam UMC location University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences Heart failure & Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
Arthur A M Wilde, Amsterdam UMC location University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences Heart failure & Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands; European Reference Network for rare, low prevalence and complex diseases of the heart: ERN GUARD-Heart.
Louise R A Olde Nordkamp, Amsterdam UMC location University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences Heart failure & Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
Funding
The PRAETORIAN trial was funded by Boston Scientific, which had no role in the design of the trial, analysis of the data, or the drafting and submission of the manuscript (grant number ISROTH20076).
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Tarakji KG, Wazni OM, Harb S, et al. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace 2014;16:1490–1495. [DOI] [PubMed] [Google Scholar]
- 2. Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol 2007;49:1851–1859. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 4. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 5. Koneru JN, Jones PW, Hammill EF, et al. Risk factors and temporal trends of complications associated with transvenous implantable cardiac defibrillator leads. J Am Heart Assoc 2018;7:e007691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bardy GH, Smith WM, Hood MA, et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med 2010;363:36–44. [DOI] [PubMed] [Google Scholar]
- 7. Weiss R, Knight BP, Gold MR, et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation 2013;128:944–953. [DOI] [PubMed] [Google Scholar]
- 8. Boersma L, Barr C, Knops R, et al. Implant and midterm outcomes of the subcutaneous implantable cardioverter-defibrillator registry: the EFFORTLESS study. J Am Coll Cardiol 2017;70:830–841. [DOI] [PubMed] [Google Scholar]
- 9. Gold MR, Lambiase PD, El-Chami MF, et al. Primary results from the understanding outcomes with the S-ICD in primary prevention patients with low ejection fraction (UNTOUCHED) trial. Circulation 2021;143:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burke MC, Aasbo JD, El-Chami MF, et al. 1-Year Prospective evaluation of clinical outcomes and shocks: the subcutaneous ICD post approval study. JACC Clin Electrophysiol 2020;6:1537–1550. [DOI] [PubMed] [Google Scholar]
- 11. Lambiase PD, Theuns DA, Murgatroyd F, et al. Subcutaneous implantable cardioverter-defibrillators: long-term results of the EFFORTLESS study. Eur Heart J 2022;43:2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ranasinghe I, Parzynski CS, Freeman JV, et al. Long-Term risk for device-related complications and reoperations after implantable cardioverter-defibrillator implantation: an observational cohort study. Ann Intern Med 2016;165:20–29. [DOI] [PubMed] [Google Scholar]
- 13. Palmisano P, Ziacchi M, Ammendola E, et al. Rate and impact on patient outcome and healthcare utilization of complications requiring surgical revision: subcutaneous versus transvenous implantable defibrillator therapy. J Cardiovasc Electrophysiol 2021;32:1712–1723. [DOI] [PubMed] [Google Scholar]
- 14. Knops RE, Nordkamp L, Delnoy P, et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med 2020;383:526–536. [DOI] [PubMed] [Google Scholar]
- 15. Olde Nordkamp LR, Knops RE, Bardy GH, et al. Rationale and design of the PRAETORIAN trial: a prospective, RAndomizEd comparison of subcuTaneOus and tRansvenous ImplANtable cardioverter-defibrillator therapy. Am Heart J 2012;163:753–760.e2. [DOI] [PubMed] [Google Scholar]
- 16. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Birnie DH, Healey JS, Wells GA, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013;368:2084–2093. [DOI] [PubMed] [Google Scholar]
- 18. Sheldon SH, Cunnane R, Lavu M, et al. Perioperative hematoma with subcutaneous ICD implantation: impact of anticoagulation and antiplatelet therapies. Pacing Clin Electrophysiol 2018;41:799–806. [DOI] [PubMed] [Google Scholar]
- 19. Knops RE, Brouwer TF, Barr CS, et al. The learning curve associated with the introduction of the subcutaneous implantable defibrillator. Europace 2016;18:1010–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Stuijt W, Quast ABE, Baalman SWE, et al. Complications related to elective generator replacement of the subcutaneous implantable defibrillator. Europace 2021;23:395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brouwer TF, Driessen AHG, Olde Nordkamp LRA, et al. Surgical management of implantation-related complications of the subcutaneous implantable cardioverter-defibrillator. JACC Clin Electrophysiol 2016;2:89–96. [DOI] [PubMed] [Google Scholar]
- 22. Rordorf R, Casula M, Pezza L, et al. Subcutaneous versus transvenous implantable defibrillator: an updated meta-analysis. Heart Rhythm 2021;18:382–391. [DOI] [PubMed] [Google Scholar]
- 23. Fong KY, Ng CJR, Wang Y, et al. Subcutaneous versus transvenous implantable defibrillator therapy: A systematic review and meta-analysis of randomized trials and propensity score-matched studies. J Am Heart Assoc 2022;11:e024756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Healey J. The ATLAS trial: avoid transvenous leads in appropriate subjects. HRS 2022; April 30, 2022; San Francisco, CA2022.
- 25. Kirkfeldt RE, Johansen JB, Nohr EA, et al. Risk factors for lead complications in cardiac pacing: a population-based cohort study of 28,860 danish patients. Heart Rhythm 2011;8:1622–1628. [DOI] [PubMed] [Google Scholar]
- 26. Boston Scientific. Important medical device advisory: Boston Scientific; Decemebr 2020 [Available from: https://www.bostonscientific.com/content/dam/bostonscientific/quality/dlt/reg-code-228/2020Dec_BSC_EmblemElectrode3501_PhysLtr_Final.pdf.
- 27. Köbe J, Wasmer K, Andresen D, et al. Impact of atrial fibrillation on early complications and one year-survival after cardioverter defibrillator implantation: results from the German DEVICE registry. Int J Cardiol 2013;168:4184–4190. [DOI] [PubMed] [Google Scholar]
- 28. Brouwer TF, Knops RE, Kutyifa V, et al. Propensity score matched comparison of subcutaneous and transvenous implantable cardioverter-defibrillator therapy in the SIMPLE and EFFORTLESS studies. Europace 2018;20:F240–F2F8. [DOI] [PubMed] [Google Scholar]
- 29. Kirkfeldt RE, Johansen JB, Nohr EA, et al. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014;35:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quast A, Baalman SWE, Brouwer TF, et al. A novel tool to evaluate the implant position and predict defibrillation success of the subcutaneous implantable cardioverter-defibrillator: the PRAETORIAN score. Heart Rhythm 2019;16:403–410. [DOI] [PubMed] [Google Scholar]
- 31. Tjong FVY, Koop BE. The modular cardiac rhythm management system: the EMPOWER leadless pacemaker and the EMBLEM subcutaneous ICD. Herzschrittmacherther Elektrophysiol 2018;29:355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC). endorsed by: association for European paediatric and congenital cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.