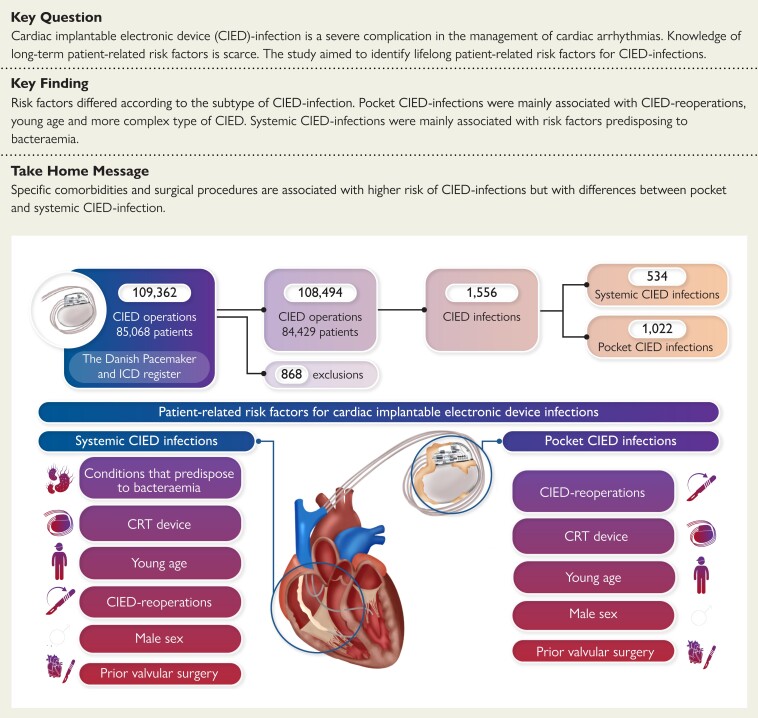
Abstract
Aims
Cardiac implantable electronic device (CIED) infection is a severe complication to modern management of cardiac arrhythmias. The CIED type and the type of surgery are recognized as risk factors for CIED infections, but knowledge of patient-related risk factors is scarce. This study aimed to identify lifelong patient-related risk factors for CIED infections.
Methods and results
Consecutive Danish patients undergoing a CIED implantation or reoperation between January 1996 and April 2018 were included. The cohort consisted of 84 429 patients undergoing 108 494 CIED surgeries with a combined follow-up of 458 257 CIED-years. A total of 1556 CIED explantations were classified as either pocket (n = 1022) or systemic CIED infection (n = 534). Data were cross-linked with records from the Danish National Patient Registry and the Danish National Prescription Registry. Using multiple-record and multiple-event per subject proportional hazard analysis, specific patient-related risk factors were identified but with several variations amongst the subtypes of CIED infection. CIED reoperations were associated with the highest risk of pocket CIED infection but also CIED type, young age, and prior valvular surgery [hazard ratio (HR): 1.62, 95% confidence interval (CI): 1.29–2.04]. Severe renal insufficiency/dialysis (HR: 2.40, 95% CI: 1.65–3.49), dermatitis (HR: 2.80, 95% CI: 1.92–4.05), and prior valvular surgery (HR: 2.09, 95% CI: 1.59–2.75) were associated with the highest risk of systemic CIED infections. Congestive heart failure, ischaemic heart disease, malignancy, chronic obstructive pulmonary disease, and temporary pacing were not significant at multivariate analysis.
Conclusion
Specific comorbidities and surgical procedures were associated with a higher risk of CIED infections but with variations amongst pocket and systemic CIED infection. Pocket CIED infections were associated with CIED reoperations, young age and more complex type of CIED, whereas systemic CIED infections were associated with risk factors predisposing to bacteraemia.
Keywords: CIED, Infection, Pacemaker, ICD, CRT
Structured Graphical Abstract
Structured Graphical Abstract.
See the editorial comment for this article ‘Big data from the Danish Pacemaker and ICD Register’, by Haran Burri, https://doi.org/10.1093/eurheartj/ehac574.
Permissions information.
The authors do hereby declare that all illustrations and figures in the manuscript are original and not require reprint permission
Introduction
Cardiac implantable electronic devices (CIEDs) are a cornerstone in modern management of cardiac arrhythmias, increasing both survival and quality of life.1–3 Infection in CIED systems is a rare,4–8 but severe complication whose clinical impact has increased during recent years.7,9 The pathogenesis is thought to be either surgery-related contamination, or haematogenous seeding.10 Infections manifest as either localized pocket CIED infection or systemic CIED infection with or without lead-associated endocarditis.10 In either cases, bacterial colonization and migration along the leads might proliferate and overlap this traditional classification. Systemic CIED infection can arise from infected heart valves but may also precipitate infective valvular endocarditis. Optimal treatment necessitates total CIED system explantation due to biofilm formation and bacterial migration along the leads.10 Most prior studies have concentrated on the early post-operative period (mainly pocket CIED infection),11 whereas knowledge about the lifelong risk is scarce, especially regarding systemic CIED infections. Several risk factors (CIED type, sex, age, CIED reoperations, temporary pacing, abstaining from prophylactic antibiotics and fever) have been linked with increased risk of CIED infections,4–7,12–14 while knowledge about most patient-related risk factors is inadequate. Aiming for a better understanding of which of the CIED recipients have the highest lifelong risk for CIED infections, we investigated the association between patient-related risk factors in addition to previously identified risk factors.4
Methods
Data sources
Data were collected from three nationwide registries: the Danish National Patient Registry (DNPR), the Danish National Prescription Registry (NPR), and the Danish Pacemaker and International Classification of Disease (ICD) register (DPIR). Data were cross-linked, using the unique personal identifier, Central Person Register (CPR) number, provided to all Danish citizens either at birth or when achieving permanent residency.15
The DPIR was founded in 1982 by physicians from all the CIED implanting centres in Denmark. It contains detailed clinical and technical information on all CIED surgeries in Denmark, including reasons for hardware explants.16 Patients are followed prospectively with regular follow-up until death or emigration. The DNPR has collected administrative and clinical data on all hospital admission in Denmark since 1977, using the ICD system, 10th revision (ICD-10) since 1995, and the Nordic Medico-Statistical Committee classification of surgical procedures (NCSPs).17,18 The NPR is an administrative register based on the Anatomical Therapeutic Chemical (ATC) classification system and containing individual-level information on all prescription-sold medication in Denmark, making it possible to track prescription history over time.19,20 Data quality of the DNPR and NPR is secured by a combination of legislation and a reimbursement driven motivation.
Study population and event definition
In a nationwide setting, all patients undergoing CIED surgery in Denmark between January 1996 and April 2018 were included. Patients were identified in DPIR and followed lifelong or right censored at the end of the study period, death, emigration, or lost to follow-up. Patients with CIED reoperations (replacement, up/downgrades, and explants) were censored at the date of surgery and re-included with a new entry date if a new CIED was implanted.
We defined CIED infections as CIED explants registered as either systemic or pocket CIED infection. Chronic diseases and prior surgery were included if occurring at any time before index CIED implantation, while temporary procedures [e.g. central venous catheters (CVCs), admission to intensive care unit and temporary transvenous cardiac pacing] were included, if occurring within 3 months prior to the index CIED implantation. Concomitant usage of pharmaceutical drugs was defined based on the principles of the waiting time distribution21 and included if prescriptions of persistent medications were as follows: (i) redeemed at any time before the index CIED implantation and (ii) redeemed continuously up till the index CIED implantation. Transient medications were included if prescriptions were redeemed <3 months prior to the index CIED implantation. ICD-10, NCSP, and ATC codes used for identification of comorbidities and concomitant medications can be found in Supplementary material online, Table S1.
The study was approved by the Danish Data Protection Agency (Jrn. 18/20048(16/39520)), the DPIR steering committee and conducted according to the Declaration of Helsinki. According to Danish law, ethics committee approval is not required for registry-based studies.
Statistical analysis
Statistical analyses were performed using Stata software package (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC). Categorical variables are presented with number and/or frequencies while continuous variables are presented as either the mean or median value with interquartile range (IQR). Charlson Comorbidity Index (CCI) were calculated for each patient and presented as mean and categorical values for each CIED type.
We conducted a multiple-record and multiple-event per subject proportional hazard analysis to identify independent risk factors. Cardiac comorbidities, medical conditions associated with secondary immunodeficiencies,22 and their related medications were evaluated for an association with CIED infection. Likewise, were surgical and invasive procedures assessed along with covariates previously associated with increased risk of CIED infection. Each covariate was first entered in the model alone, giving the unadjusted strength of association between the covariate and the risk of CIED infection, presented as hazard ratio (HR) with 95% confidence intervals (CIs). Subsequently, the covariates were individually evaluated in a reduced multivariate analysis along with previously identified patient-, CIED- and surgery-related risk factors.4 A few variables were excluded from the final multivariate model due to collinearity or very few cases of CIED infections. All clinical or statistically significant covariates were then evaluated for their strength of association when adjusted for all the other covariates in a so-called full long multivariate model. By removing the covariate, causing the least change in significance, evaluated by a likelihood-ratio test, the model was gradually reduced to a final multivariate model consisting of statistically significant independent risk factors along with clinical pre-determined risk factors [temporary pacing, ischaemic heart disease (IHD), congestive heart failure (CHF), and chronic obstructive pulmonary disease (COPD)]. The final model was tested for effect modification by testing interaction terms between sex, age, CIED type, and type of CIED surgery. Lastly, we applied the final multivariate model to the two different types of CIED infections, i.e. pocket and systemic CIED infections. The regression coefficients were illustrated as HRs with 95% CI in a rope ladder plot.23 All statistical analysis were two-sided and P-values of <0.05 were considered significant.
Results
Study population
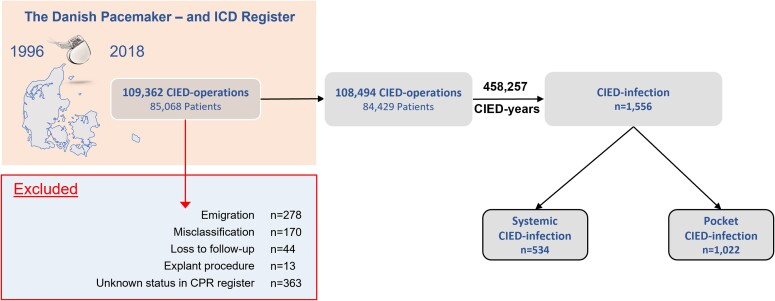
During the study period, 109 362 CIED surgeries were performed in 85 068 patients. A total of 868 CIED surgeries in 639 patients were excluded primarily due to misclassification and loss to follow-up (Figure 1), thus resulting in 84 429 patients undergoing a total 108 494 CIED surgeries, (80 430 de novo implantations, 21 565 replacements and 6499 up/downgrades). De novo CIED implantations consisted primarily of pacemakers (PMs) (77%), followed by implantable cardioverter defibrillators (ICDs) (15%) and cardiac resynchronization therapy-PM/defibrillator (CRT-P/D) systems (8%). The PM patients had a median age of 77 (IQR: 69–84) and were nearly equally split between sexes (56% males), whereas ICD and CRT-P/D patients were younger and with a higher proportion of males. Pacemaker patients had fewest comorbidities, but amongst all CIED patients, only a minority had very high CCI scores (>5). IHD was previously diagnosed in 75% of ICD and 78% of CRT-D patients, while 89% of the CRT-P/D patients suffered from CHF (Table 1). Median follow-up was 3.76 (IQR: 1.60–6.48) years per CIED unit with a combined follow-up of 458 257 CIED-years. This resulted in a mean follow-up of 5.43 (95% CI: 5.40–5.47) years per patient.
Figure 1.
Consort diagram of the study population. Between January 1996 and April 2018, 109 362 cardiac implantable electronic device surgeries were performed in Denmark. A total of 868 surgeries were excluded from the analysis due to misclassification, loss to follow-up, and emigration resulting in 108 494 surgeries. ICD, implantable cardioverter-defibrillator; CIED, cardiac implantable electronic device.
Table 1.
Patient characteristics at first cardiac implantable electronic device implant (n = 80 430)
| Variable | Pacemaker | Implantable cardioverter-defibrillator | Cardiac resynchronization therapy-pacemaker | Cardiac resynchronization therapy-defibrillator |
|---|---|---|---|---|
| n (%) | 62 213 (77.4) | 11 841 (14.7) | 2990 (3.7) | 3386 (4.2) |
| Age at implant, median (IQR) | 77 (69–84) | 64 (54–71) | 71 (61–78) | 68 (60–74) |
| Sex, n (%) | ||||
| Female | 27 319 (43.9) | 2344 (19.8) | 905 (30.3) | 679 (20.1) |
| Male | 34 894 (56.1) | 9497 (80.2) | 2085 (69.7) | 2707 (79.9) |
| Indication, n (%) | ||||
| AV-block/CHF and BBB | 28 799 (46.3) | — | 2442 (81.7) | — |
| Sinus node dysfunction | 22 465 (36.1) | — | 76 (2.5) | — |
| Atrial fibrillation | 9125 (14.7) | — | 117 (3.9) | — |
| Primary prophylactic ICD | — | 3812 (32.2) | - | 2141 (63.2) |
| Secondary prophylactic ICD | — | 7562 (63.9) | — | 1006 (29.7) |
| Other | 1824 (2.9) | 467 (3.9) | 355 (11.9) | 239 (7.1) |
| Comorbidities | ||||
| Diabetes mellitus Type 1, n (%) | 3083(5.0) | 676 (5.7) | 207 (6.9) | 319 (9.4) |
| Diabetes mellitus Type 2, n (%) | 8189 (13.2) | 1851 (15.6) | 576 (19.3) | 820 (24.2) |
| Ischaemic heart disease, n (%) | 20 572 (33.1) | 8819 (74.5) | 1634 (54.7) | 2646 (78.2) |
| CABG, n (%) | 2532 (4.1) | 2321 (19.6) | 210 (7.0) | 661 (19.5) |
| Congestive heart failure, n (%) | 11 086 (17.8) | 6478 (54.7) | 2656 (88.8) | 3027 (89.4) |
| Atrial fibrillation/flutter, n (%) | 24 195 (38.9) | 2983 (25.2) | 1060 (35.5) | 1025 (30.3) |
| Supraventricular tachycardia, n (%) | 4325 (7.0) | 764 (6.5) | 187 (6.3) | 199 (5.9) |
| Hypertension, n (%) | 24 555 (39.5) | 4252 (35.9) | 1206 (40.3) | 1487 (43.9) |
| COPD, n (%) | 6285 (10.1) | 1232 (10.4) | 496 (16.6) | 536 (15.8) |
| Cardiomyopathy, n (%) | 1683 (2.7) | 2387 (20.2) | 1451 (48.5) | 1274 (37.6) |
| Valvular heart disease, n (%) | 8274 (13.3) | 1113 (9.4) | 644 (21.5) | 492 (14.5) |
| Renal insufficiency, any n (%) | 2829 (4.5) | 632 (5.3) | 273 (9.1) | 258 (7.6) |
| Cerebrovascular disease, n (%) | 10 854 (17.5) | 1477 (12.5) | 404 (13.5) | 543 (16.0) |
| Dementia, n (%) | 1555 (2.5) | 35 (0.3) | 14 (0.5) | 15 (0.4) |
| Cancer, any type, n (%) | 6940 (11.2) | 776 (6.6) | 363 (12.1) | 303 (8.9) |
| CCI (2011), mean | 0.95 | 1.65 | 2.55 | 2.49 |
| CCI (2011): 0–1, n (%) | 42 598 (68.5) | 4264 (36.0) | 193 (6.5) | 183 (5.4) |
| CCI (2011): 2–3, n (%) | 16 120 (25.9) | 6607 (55.8) | 2266 (75.8) | 2691 (79.5) |
| CCI (2011): ≥ 4, n (%) | 3495 (5.6) | 970 (8.2) | 531 (17.7) | 512 (15.1) |
AV, atrioventricular; BBB, bundle branch block; CABG, coronary artery bypass graft; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; CHF, heart failure; ICD, implantable cardioverter-defibrillator; IQR, interquartile range.
We identified 1556 explants, registered as either pocket (n = 1022) or systemic (n = 534) CIED infection. Pocket CIED infection was the most common type of CIED infection irrespective of CIED type, accounting for about two thirds of all CIED infections and only slightly less in ICD patients (57%). When stratified by the type of CIED infection, median time to CIED infection differed considerably with 249 (IQR: 62–769) and 468 (IQR: 77–1385) CIED days for pocket and systemic CIED infection, respectively. The median time to pocket CIED infection differed only slightly between CIED types, whereas the median time to systemic CIED infection differed considerably, between 640 (IQR: 102–1480) and 197 (IQR: 58–616) CIED days for PM and CRT-D patients, respectively (Table 2).
Table 2.
Time to CIED infections, by cardiac implantable electronic device type
| Variable | Pacemaker | Implantable cardioverter-defibrillator | Cardiac resynchronization therapy-pacemaker | Cardiac resynchronization therapy-defibrillator | Total |
|---|---|---|---|---|---|
| CIED infections (total), n | 927 | 316 | 101 | 212 | 1556 |
| Pocket CIED infections, n (%) | 638 (69) | 179 (57) | 66 (65) | 139 (66) | 1022 (66) |
| Systemic CIED infections, n (%) | 289 (31) | 137 (43) | 35 (35) | 73 (34) | 534 (34) |
| Median time to CIED infection (combined), days (IQR) | 350 (64–1043) | 311 (74–1050) | 243 (85–823) | 227 (63–633) | 296 (68–946) |
| Median time to pocket CIED infection, days (IQR) | 263 (60–816) | 246 (72–874) | 205 (80–742) | 239 (68–633) | 249 (62–769) |
| Median time to systemic CIED infection, days (IQR) | 640 (102–1480) | 419 (76–1278) | 301 (85–1311) | 197 (58–616) | 468 (77–1385) |
CIED, cardiac implantable electronic device; IQR, interquartile range.
De novo implantation was associated with the lowest risk of CIED infection, for both subtypes of CIED infections, and increased considerably after each reoperation, especially amongst pocket CIED infections (Table 3). The risk of CIED infection was highest in the early post-implantation period and gradually declining, resulting in a cumulative incidence of 0.38% and 0.21% for pocket and systemic CIED infection, respectively, 12 months after a de novo implantation (Table 4). Similar results were found following reoperations, but with a substantially higher risk of pocket than systemic CIED infection after 12 months, at 1.10 vs. 0.33% (Table 4).
Table 3.
Risk of cardiac implantable electronic device CIED infection, by accumulated cardiac implantable electronic device interventions
| Pocket CIED infections | Systemic CIED infections | Total CIED infections | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall Incidence | Incidence rate/1000 CIED-years | Overall incidence | Incidence rate rate/1000 CIED-years | Overall incidence | Incidence rate/1000 CIED-years | ||||||
| CIED implants | CIED-years | n (%) | 95% CI | (95% CI) | n (%) | 95% CI | (95% CI) | n (%) | 95% CI | (95% CI) | |
| 1st operation De novo implantation | 80 430 | 344 165 | 525 (0.65) | 0.59–0.71 | 1.53 (1.40–1.67) | 344 (0.43) | 0.38–0.48 | 1.00 (0.90–1.11) | 869 (1.08) | 1.01–1.15 | 2.52 (2.36–2.70) |
| 2nd operation (1st reoperation) | 20 759 | 86 079 | 295 (1.42) | 1.26–1.59 | 3.43 (3.06–3.84) | 112 (0.54) | 0.44–0.65 | 1.30 (1.08–1.57) | 407 (1.96) | 1.78–2.16 | 4.73 (4.29–5.21) |
| 3rd operation (2nd reoperation) | 5292 | 20 361 | 127 (2.40) | 2.00–2.85 | 6.24 (5.24–7.42) | 43 (0.81) | 0.59–1.09 | 2.01 (1.56–2.84) | 170 (3.21) | 2.75–3.72 | 8.35 (7.18–9.70) |
| 4th operation (3rd + reoperation) | 2013 | 7651 | 75 (3.73) | 2.94–4.64 | 9.80 (7.81–12.29) | 35 (1.74) | 1.21–2.41 | 4.57 (3.28–6.37) | 110 (5.47) | 4.51–6.55 | 14.38 (11.93–17.33) |
| Total | 108 494 | 458 257 | 1022 (0.94) | 0.89–1.00 | 2.23 (2.10–2.37) | 534 (0.49) | 0.45–0.54 | 1.17 (1.07–1.27) | 1556 (1.43) | 1.36–1.51 | 3.40 (3.23–3.57) |
CI, confidence interval; CIED, cardiac implantable electronic device.
Table 4.
Cumulative incidence and incidence rate of cardiac implantable electronic device infection over time, by type of cardiac implantable electronic device surgery
| Pocket infection | Systemic Infection | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| De novo CIED implantation | De novo CIED implantation | |||||||||
| Follow-up (months) | Infections (n) | Cumulative incidence (%) (95% CI) | Rate/1000 DY (95% CI) | Infections (n) | Cumulative incidence (%) (95% CI) | Rate/1000 DY (95% CI) | ||||
| 1 | 88 | 0.11 | 0.09–0.14 | 13.48 | 10.94–16–61 | 53 | 0.07 | 0.05–0.09 | 8.12 | 6.20–10.62 |
| 3 | 91 | 0.23 | 0.20–0.26 | 7.18 | 5.84–8.81 | 46 | 0.13 | 0.10–0.15 | 3.63 | 2.72–4.84 |
| 6 | 55 | 0.30 | 0.27–0.34 | 3.01 | 2.31–3.92 | 36 | 0.17 | 0.15–0.21 | 1.97 | 1.42–2.73 |
| 12 | 56 | 0.38 | 0.34–0.43 | 1.59 | 1.22–2.07 | 24 | 0.21 | 0.18–0.24 | 0.68 | 0.45–1.02 |
| 24 | 87 | 0.52 | 0.47–0.58 | 1.42 | 1.15–1.75 | 44 | 0.28 | 0.24–0.32 | 0.72 | 0.53–0.96 |
| 36 | 48 | 0.62 | 0.56–0.68 | 0.93 | 0.70–1.23 | 34 | 0.35 | 0.30–0.39 | 0.66 | 0.47–0.92 |
| 48 | 33 | 0.69 | 0.63–0.82 | 0.77 | 0.55–1.08 | 29 | 0.41 | 0.37–0.47 | 0.08 | 0.47–0.97 |
| 60 | 21 | 0.75 | 0.68–0.82 | 0.60 | 0.39–0.92 | 24 | 0.48 | 0.43–0.57 | 0.68 | 0.46–1.02 |
| 72 | 18 | 0.81 | 0.74–0.89 | 0.65 | 0.41–1.03 | 14 | 0.53 | 0.47–0.60 | 0.50 | 0.30–0.85 |
| 84 | 6 | 0.84 | 0.77–0.92 | 0.29 | 0.13–0.64 | 19 | 0.62 | 0.55–0.70 | 0.92 | 0.59–1.44 |
| 96 | 11 | 0.91 | 0.83–1.01 | 0.78 | 0.43–1.40 | 11 | 0.69 | 0.61–0.78 | 0.76 | 0.42–1.38 |
| 108 | 5 | 0.97 | 0.87–1.07 | 0.60 | 0.25–1.44 | 6 | 0.76 | 0.66–0.87 | 0.72 | 0.32–1.61 |
| 120 | 3 | 1.03 | 0.91–1.16 | 0.68 | 0.22–2.12 | 2 | 0.81 | 0.69–0.95 | 0.40 | 0.10–1.60 |
| Reoperation | Reoperation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up (months) | Infections (n) | Cumulative incidence (%) (95% CI) | Rate/1000 DY (95% CI) | Infections (n) | Cumulative incidence (%) (95% CI) | Rate/1000 DY (95% CI) | ||||
| 1 | 58 | 0.21 | 0.16–0.27 | 25.35 | 19.60–32.8 | 17 | 0.06 | 0.04–0.10 | 7.43 | 4.62–11.96 |
| 3 | 80 | 0.50 | 0.42–0.59 | 17.95 | 14.42–22.34 | 26 | 0.16 | 0.12–0.21 | 5.83 | 3.97–8.57 |
| 6 | 66 | 0.75 | 0.66–0.86 | 10.27 | 8.07–13.07 | 15 | 0.21 | 0.17–0.28 | 2.33 | 1.41–3.87 |
| 12 | 86 | 1.10 | 0.98–1.23 | 6.96 | 5.64–8.60 | 28 | 0.33 | 0.27–0.40 | 2.27 | 1.57–3.28 |
| 24 | 85 | 1.49 | 1.35–1.65 | 3.99 | 3.23–4.93 | 21 | 0.43 | 0.35–0.52 | 0.99 | 0.64–1.51 |
| 36 | 50 | 1.77 | 1.61–1.95 | 2.83 | 2.15–3.74 | 22 | 0.55 | 0.46–0.65 | 1.25 | 0.82–1.89 |
| 48 | 29 | 1.97 | 1.79–2.16 | 2.02 | 1.40–2.91 | 20 | 0.68 | 0.58–0.81 | 1.39 | 0.90–2.16 |
| 60 | 18 | 2.13 | 1.94–2.33 | 1.59 | 1.00–2.53 | 16 | 0.83 | 0.70–0.97 | 1.42 | 0.87–2.31 |
| 72 | 9 | 2.23 | 2.03–2.45 | 1.05 | 0.55–2.02 | 9 | 0.93 | 0.79–1.09 | 1.05 | 0.55–2.02 |
| 84 | 8 | 2.35 | 2.14–2.59 | 1.3 | 0.65–2.60 | 8 | 1.06 | 0.90–1.24 | 1.30 | 0.65–2.60 |
| 96 | 7 | 2.51 | 2.27–2.78 | 1.73 | 0.82–3.64 | 3 | 1.12 | 0.95–1.33 | 0.74 | 0.24–2.31 |
| 108 | 0 | 2.51 | 2.27–2.78 | 0 | 0 | 1 | 1.16 | 0.98–1.38 | 0.43 | 0.06–3.03 |
| 120 | 1 | 2.61 | 2.31–2.95 | 0.81 | 0.11–5.76 | 2 | 1.34 | 1.05–1.71 | 1.62 | 0.41–6.49 |
CI, confidence interval; CIED, cardiac implantable electronic device.
Risk factors
In the univariate analysis, several comorbidities, pharmaceutical drugs and numerous surgical and invasive procedures, were all associated with an increased risk of any type of CIED infection (see Supplementary material online, Table S2). However, most of these were non-significant in the reduced multivariate model and thus not included in the final multivariate model (see Supplementary material online, Figure S1 and Supplementary material online, Table S3).
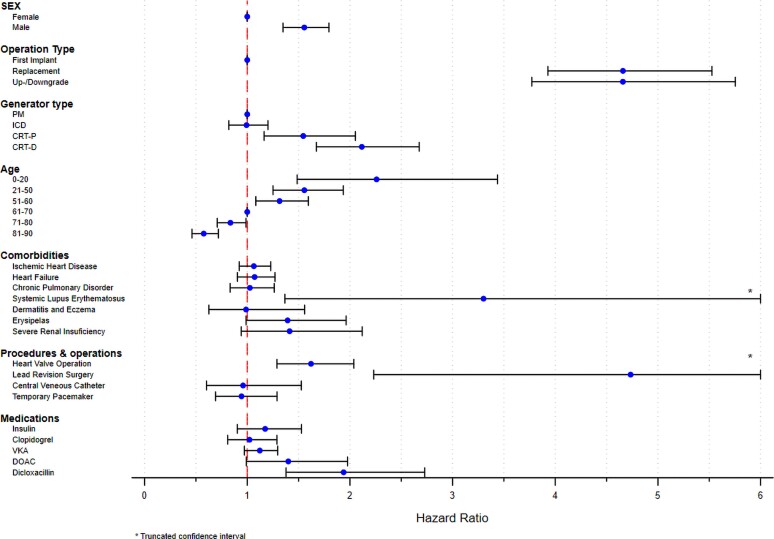
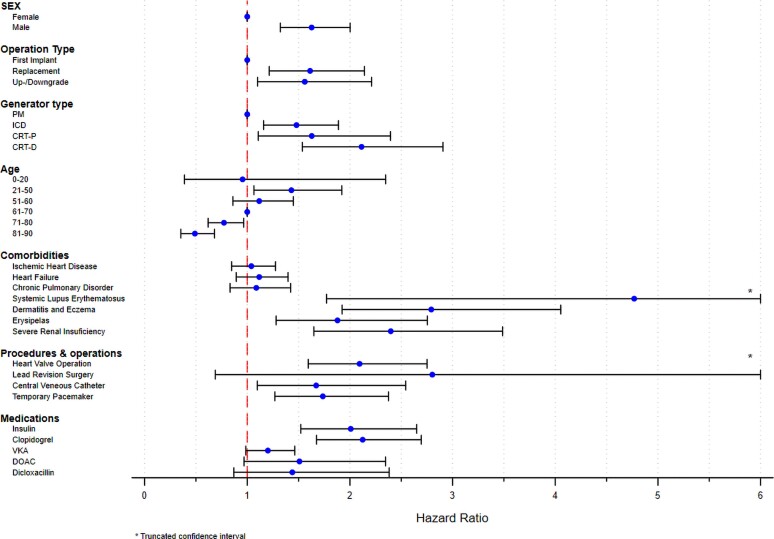
In the multivariate analysis, de novo PM implantation in female patients between 61 and 70 years was chosen as a reference. Male sex, young age, and CRT systems were independent risk factors in both subtypes of CIED infections, while IHD, CHF, and COPD did not reach statistical significance in neither (Table 5, Supplementary material online, Figure S1, Supplementary material online, Table S3). The multivariate analysis of the subtypes of CIED infections revealed important variations in HRs amongst the examined risk factors. In pocket CIED infection, CIED reoperations [replacement (HR: 4.66, 95% CI: 3.93–5.53), up-/downgrades (HR: 4.66, 95% CI: 3.77–5.75) and lead revisions (HR: 4.73, 95% CI: 2.23–10.04) were associated with the highest risk, but also systemic lupus erythematosus (SLE) (HR: 3.30, 95% CI: 1.37–7.98), prior valvular surgery (HR: 1.62, 95% CI: 1.29–2.04), and recent usage of dicloxacillin (HR: 1.94, 95% CI: 1.38–2.73) were significant (Table 5, Figure 2). In contrast, we identified SLE (HR: 4.77, 95% CI: 1.77–12.83), severe renal insufficiency (chronic kidney disease stage 4–5)/dialysis (HR: 2.40, 95% CI: 1.65–3.49), prior valvular surgery (HR: 2.09, 95% CI: 1.59–2.75), dermatitis (HR: 2.80, 95% CI: 1.92–4.05) and usage of insulin (HR: 2.01, 95% CI: 1.52–2.65), to be associated with the highest risk of systemic CIED infection (Table 5, Figure 3).
Table 5.
Multivariate Cox regression risk factor analysis of pocket and systemic cardiac implantable electronic device infections
| Variable | Pocket CIED infections | Systemic CIED infections | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | Total (n) | n | HR | 95%CI | P-value | n | HR | 95%CI | P-value | ||
| Female sex | 42 165 | 274 | REF | 125 | REF | ||||||
| Male sex | 66 329 | 748 | 1.55 | 1.35 | 1.80 | <0.001 | 409 | 1.63 | 1.32 | 2.00 | <0.001 |
| Surgery type | |||||||||||
| First Implant | 80 430 | 525 | REF | 344 | REF | ||||||
| Replacement | 21 565 | 355 | 4.66 | 3.93 | 5.53 | <0.001 | 138 | 1.61 | 1.21 | 2.14 | 0.001 |
| Up/downgrade | 6499 | 142 | 4.66 | 3.77 | 5.75 | <0.001 | 52 | 1.56 | 1.10 | 2.21 | 0.012 |
| CIED type | |||||||||||
| PM | 81 065 | 638 | REF | 289 | REF | ||||||
| ICD | 16 480 | 179 | 0.99 | 0.82 | 1.20 | 0.943 | 137 | 1.48 | 1.16 | 1.89 | 0.002 |
| CRT-P | 4628 | 66 | 1.55 | 1.16 | 2.05 | 0.003 | 35 | 1.63 | 1.11 | 2.40 | 0.013 |
| CRT-D | 6321 | 139 | 2.12 | 1.67 | 2.67 | <0.001 | 73 | 2.11 | 1.54 | 2.91 | <0.001 |
| Age group, years | |||||||||||
| 0–20 | 953 | 25 | 2.26 | 1.49 | 3.44 | <0.001 | 5 | 0.95 | 0.39 | 2.35 | 0.919 |
| 21–50 | 6912 | 129 | 1.56 | 1.25 | 1.94 | <0.001 | 68 | 1.43 | 1.06 | 1.92 | 0.018 |
| 51–60 | 10 466 | 169 | 1.31 | 1.08 | 1.60 | 0.006 | 88 | 1.12 | 0.86 | 1.45 | 0.408 |
| 61–70 | 22 948 | 269 | REF | 165 | REF | ||||||
| 71–80 | 34 865 | 296 | 0.84 | 0.71 | 0.99 | 0.035 | 156 | 0.77 | 0.62 | 0.96 | 0.023 |
| 81–90 | 27 366 | 121 | 0.58 | 0.46 | 0.72 | <0.001 | 50 | 0.49 | 0.35 | 0.68 | <0.001 |
| ≥91 | 4984 | 13 | 0.47 | 0.27 | 0.83 | 0.009 | 2 | 0.17 | 0.04 | 0.70 | 0.014 |
| Comorbidities | |||||||||||
| Severe renal insufficiency/dialysis | 1966 | 25 | 1.41 | 0.94 | 2.12 | 0.095 | 32 | 2.40 | 1.65 | 3.49 | <0.001 |
| Systemic lupus erythematosus | 150 | 5 | 3.30 | 1.37 | 7.98 | 0.008 | 4 | 4.77 | 1.77 | 12.83 | 0.002 |
| Ischaemic heart disease | 47 534 | 483 | 1.06 | 0.92 | 1.23 | 0.398 | 297 | 1.04 | 0.85 | 1.28 | 0.708 |
| Congestive heart Failure | 34 141 | 388 | 1.07 | 0.90 | 1.27 | 0.425 | 237 | 1.12 | 0.89 | 1.40 | 0.334 |
| Chronic obstructive pulmonary disease | 11 773 | 103 | 1.03 | 0.83 | 1.26 | 0.811 | 63 | 1.09 | 0.83 | 1.42 | 0.536 |
| Dermatitis and eczema | 2003 | 19 | 0.99 | 0.63 | 1.56 | 0.960 | 30 | 2.80 | 1.92 | 4.05 | <0.001 |
| Erysipelas | 2914 | 35 | 1.39 | 0.99 | 1.96 | 0.058 | 29 | 1.88 | 1.28 | 2.75 | 0.001 |
| Surgery and Procedures | |||||||||||
| Valvular surgery, any type | 5624 | 88 | 1.62 | 1.29 | 2.04 | <0.001 | 65 | 2.09 | 1.59 | 2.75 | <0.001 |
| Lead revision surgery | 78 | 7 | 4.73 | 2.23 | 10.04 | <0.001 | 2 | 2.80 | 0.69 | 11.42 | 0.150 |
| Central venous catheter | 2160 | 19 | 0.96 | 0.60 | 1.53 | 0.860 | 25 | 1.67 | 1.10 | 2.54 | 0.017 |
| Temporary pacemaker | 5735 | 43 | 0.94 | 0.69 | 1.29 | 0.718 | 47 | 1.74 | 1.27 | 2.38 | 0.001 |
| Medications | |||||||||||
| Insulin | 5828 | 62 | 1.17 | 0.90 | 1.53 | 0.231 | 61 | 2.01 | 1.52 | 2.65 | <0.001 |
| Clopidogrel | 9332 | 85 | 1.02 | 0.81 | 1.29 | 0.856 | 97 | 2.12 | 1.67 | 2.69 | <0.001 |
| Vitamin K antagonist | 25 508 | 306 | 1.12 | 0.97 | 1.30 | 0.117 | 168 | 1.20 | 0.99 | 1.46 | 0.068 |
| Direct oral anticoagulants | 4927 | 34 | 1.40 | 0.99 | 1.98 | 0.056 | 21 | 1.50 | 0.97 | 2.35 | 0.069 |
| Beta-lactamase resistant penicillins | 1827 | 35 | 1.94 | 1.38 | 2.73 | <0.001 | 16 | 1.44 | 0.87 | 2.38 | 0.157 |
Figure 2.
Pocket cardiac implantable electronic device infection. Multivariate Cox-proportional hazard analysis of risk factors for pocket cardiac implantable electronic device infections. Illustrated as a rope ladder plot. CIED, cardiac implantable electronic device; PM, pacemaker; ICD, implantable cardioverter-defibrillator; CRT-P/D, cardiac resynchronization therapy-pacemaker/defibrillator; VKA, Vitamin K antagonist; DOAC, direct oral anticoagulant.
Figure 3.
Systemic cardiac implantable electronic device infection. Multivariate Cox-proportional hazard analysis of risk factors for systemic cardiac implantable electronic device infections. Illustrated as a rope ladder plot. CIED, cardiac implantable electronic device; PM, pacemaker; ICD, implantable cardioverter-defibrillator; CRT-P/D, cardiac resynchronization therapy-pacemaker/defibrillator; VKA, vitamin K antagonist; DOAC, direct oral anticoagulant.
Discussion
In a nationwide setting with consecutive CIED patients and lifelong follow-up, we identified specific comorbidities and prior surgical procedures as associated with higher risk of CIED infections. There were differences in both risk factors and risk of CIED infection between pocket and systemic CIED infections (Structured Graphical Abstract).
Although the existing literature holds several risk factors analyses, most of these are few in numbers, single-centre cohorts, focusing on the early post-implantation period, and have not considered dissimilarities between CIED types. In our data, involving 14 CIED implantation centres, we present lifelong follow-up on the entire Danish CIED cohort, thereby giving a better estimate of the true risk of CIED infection for CIED patients. Although overall risk was low, our results reveal that the risk of CIED infection increases considerably following each reoperation. This must be acknowledged when implanting CIED systems, especially in patients with a long life expectancy and potential multiple CIED reoperations. The increased risk of CIED infections is mainly driven by an increase in pocket CIED infections and precautions aimed at minimizing these should be addressed. This includes strategies for preventing pocket haematoma, sufficient surgical skills, and considerations of prolonged regimes of antibiotics.
In our cohort, we present the largest multivariate risk factor analysis for CIED infection. The analysis includes both CIED-, surgery-, and patient-related risk factors, and were furthermore, stratified by the type of CIED infection. Previously well-described risk factors such as CIED type, CIED reoperations, sex, and age,4 remained associated with increased risk of CIED infection. We did not find any evidence of effect modification between sex, age, CIED type, and type of surgery. Although males were more prone to infections in both subgroups, there were major dissimilarities between the two common types of CIED infection, i.e. pocket and systemic CIED infection. There is no obvious reason for the diversity between sexes. However, differences in bacterial skin colonization and nasal colonization might explain some of these findings. Males are more frequently nasal carriers of Staphylococcus aureus in comparison to females.24 Furthermore, Staphylococcus epidermidis, Cutibacterium acnes, and Corynebacterium species are more abundant in males.25 These bacteria are known CIED infection pathogens10,26,27 and also complicate shoulder surgery.28,29 They naturally inhabit the axilla region28 and seem resistant to certain skin preparation regimes.30,31
It its noteworthy that we found a comparatively high fraction of systemic CIED infections, in contrast to most of the existing literature.6–8,13,14 This is likely due to differences in study design, as most prior studies focused on the early post-implantation period, in contrast to the lifelong follow-up presented in our data. This is substantiated by the differences in the median time to CIED infection, that was considerable shorter in pocket CIED infection (249 vs. 468 CIED days). This difference was expected as pocket CIED infection most likely occurs due to surgical site contamination,10,32–34 while systemic CIED infection is thought to ensue haematogenic seeding from a distant focus.
Previous valvular surgery has a 50-fold higher risk of infective endocarditis (IE)35 when compared with native heart valves. In our population, any kind of valvular heart disease was associated with an increased risk of both subtypes of CIED infections, however highest among patients with previous valvular surgery. This is in accordance with another Danish study reporting a higher risk of IE in CIED patients undergoing aortic valve replacement in comparison to non-CIED patients.36 We did, however, not find any significant association in our multivariate model with other major thoracic surgery such as CABG and aortic surgery. Therefore, it seems likely that the risk is correlated to the valvular heart disease and/or the presence of an artificial or biological valve.
Pocket CIED infections
Younger age and any type of CIED reoperations were markedly associated with an increased risk of pocket CIED infections. CIED reoperations were by far the highest risk factor and although the risk increased incrementally after each reoperation, we could not find any differences amongst the other risk factors. However, this may in part be explained by the relatively low number of patients undergoing more than two CIED reoperations. Our data show that the risk rate is highest in the early post-operative period, especially after CIED reoperations, and hereafter rapidly declining during the first 12 months, until stabilizing at a lower incidence rate. This underlines the theory of bacterial pocket contamination during CIED surgery. Asymptomatic bacterial colonization of unknown significance has been detected in patients undergoing elective CIED surgeries37,38 while others have found an association to subsequently increased risk of CIED infection.39,40 In our population, implantation of CRT-P/D-systems was associated with a significant higher risk of pocket CIED infection, whereas ICD implantation was not. These factors suggest that the longer and more challenging procedures during CRT implantation might increase the risk of pocket contamination. Furthermore, do our findings emphasize that any kind of CIED reoperation is a high-risk procedure, and thoroughness in both planning and surgical techniques is essential to avoid early CIED upgrades and CIED reoperations due to unjust CIED indications or lead displacements. Likewise, battery longevity improvements are required to reduce the number of CIED replacements.
Fever and concurrent infection6 have previously been associated with higher risk of CIED infection, but little is known about how recent infections might influence the risk of CIED infection. In our study, recently redeemed prescriptions of antibiotics were associated with a slightly increased risk of pocket CIED infection, strongest for dicloxacillin. Yet, this is likely a confounding effect, representing an underlying infection, but could also be a proxy for frailer patients, as observed in the DECODE registry, where recent hospital admission prior to CIED reoperations was associated with increased mortality.41 Nonetheless, it seems reasonable to identify these patients. Two studies have examined different approaches of enhanced antibiotic-regimes in high-risk patients.42,43 Although neither have provided unimpeachable results, it appears that an antibiotic envelope can reduce the risk of CIED infection in selected high-risk patients.42,44
Post-operative haematoma is associated with a markedly increased risk of subsequent CIED infection,7,12,45–48 therefore anticoagulants and platelet inhibitors could be expected to increase the risk of pocket CIED infection. In our analysis, neither anticoagulants nor platelet inhibitors were associated with an increased risk of pocket CIED infection. This is in line with the BRUISE-CONTROL studies,49,50 where uninterrupted coumadin or direct oral anticoagulant treatment did not increase the risk of pocket haematomas. However, contradictory to our results, additional analysis from these studies revealed an increased risk of pocket haematomas if using concomitant anticoagulants and antiplatelet drugs.51 However, our study is based on data from registries, and we did not have information about neither post-operative pocket haematoma formation nor whether the anticoagulants or antiplatelets were paused prior to CIED implantation. Yet it seems reasonable to identify these patients as the usage of antibiotic envelopes might decrease the risk of infection in these patients.42
Systemic CIED infections
Implantation of CRT-P/D but also ICD systems was associated with increased risk of systemic CIED infection. Although hypothesized, this could be due to the higher complexity in lead design,52 in addition to the additional leads. Renal insufficiency and haemodialysis are associated with increased risk of CIED infection7,13,53–56 also observed in our study, where all degrees of renal insufficiency were associated with increased risk of systemic CIED infection but with the strongest association in severe renal insufficiency/dialysis. This is probably due to the altered immunity and the frequently vascular access, thereby increasing the risk of bacteraemia.57,58
Diabetes and the usage of insulin is known to impair both cellular59 and humoral immunity,60 and correlates with our findings of increased risk of systemic CIED infections. However, usage of insulin has also been associated with increased mortality in CIED reoperations,41 and might represent diabetes in an advanced stage, with end-stage complications, favouring bacterial seeding due to skin breaches and repeated vascular entries. Atopic dermatitis and erysipelas are associated with increased risk of bacteraemia61 and also were found associated with increased risk of systemic CIED infections in our population. It could be expected that the impaired skin barrier in atopic dermatitis might lead to an increased risk of pocket CIED infection. However, this could not be confirmed in our analysis.
Inflammatory disorders, especially SLE, were also associated with a higher risk of systemic CIED infection. However, only 150 patients were diagnosed with SLE prior to CIED implantation, yet we found a considerably increased risk of both pocket and systemic CIED infections. Although this might be due to statistical chance, SLE is an autoimmune disorder characterized by an uncontrolled autoreactivity of B- and T-lymphocytes associated with an increased risk of severe infections,62,63 why it is a subject that needs to be investigated further.
Other potential risk factors
Infective endocarditis was associated with a considerable increased risk of all CIED infections (HR: 3.42, 95% CI: 2.69–4.35) in the univariate model. However, we did not include IE in our multivariate model, due to an inevitable confounding by indication between these two types of cardiac-related infections with mutual diagnostic criteria. A recent study described that 18% of patients with IE had a previously implanted CIED, of whom the majority had CIED-related IE.64 Therefore, CIED infections should always be suspected in CIED patients with IE, and CIED removal is usually recommended.10
IHD, CHF, COPD, cancer, and usage of glucocorticoids have all been associated with an increased risk of CIED infections. However, neither were significant in our multivariate analysis, whether analysed as subtype-stratified or as CIED infections altogether (see Supplementary material online, Figure S1 and Supplementary material online, Table S3). As shown by others,46,53,65 IHD and CHF were associated with increased risk of CIED infections in the univariate analysis. Almost 90% of the CRT-P and CRT-D recipients suffered from CHF but also 54 and 18% of the ICD and PM recipients, respectively. However, neither IHD nor CHF were statistically significant in the multivariate model, when stratified for the type of CIED, and it seems likely that the CIED type (ICD and CRT-P/D) bears most of the increased risk of CIED infection in these patients. However, as our data derive from registers, based on ICD codes, we do not have detailed information on neither the severity of CHF nor how it was diagnosed, and we cannot exclude a kind of selection bias in these patients. Yet, it is likely that both the CRT-P/D and ICD recipients suffered from CHF with a moderate to severe reduced ejection fraction substantiated by the indication for CIED implantation. Still, we cannot neglect the results found by others,46,53,65 and that there might be an association between severe CHF and increased risk of CIED infection, even though we did not find a significant association in our data.
Cancer, COPD, and usage of glucocorticoids were all thought to increase the risk of CIED infection due to the secondary immunodeficiencies associated with these diseases. However, in contrast to others,65 this could not be proven in our analysis. Usage of corticosteroids has been evaluated in several studies,12,13,45,53 and although the pooled estimates11 were significant, most studies had very few cases and like ours did not find a significant increased risk of CIED infections.
The presence of indwelling catheters prior to CIED implantation has previously been identified as a risk factor for CIED infections.7,11,13 It seems evident that permanent breaches of the skin-blood barrier, like drivelines used in left ventricular assist devices,66,67 permanent CVCs13 and haemodialysis catheters56 are associated with an increased risk of CIED infections. Temporary PMs have also been associated with an increased risk of CIED infections,6,12,56 and although the pooled estimates11 are significant, most of the studies have limited numbers of CIED infections. Opposing results were found in a recent Danish study,68 where temporary pacing had an adjusted HR for CIED infection of 0.85 (95 CI: 0.51–1.42). In our multivariate analysis, usage of CVC or temporary PM implantation prior to permanent CIED implantation were associated with an increased risk of systemic CIED infection. However, as systemic CIED infections are believed to develop due to haematogenous seeding ensuing considerably later acquired bacteraemia, it seems more likely that this represents an unknown confounding factor.
Clinicians should be aware of and minimize the different risk factors for CIED infection. Carefully weighing risk factors in a shared decision-making process with the patient regarding CIED interventions is of utmost importance. In high-risk patients, interventions associated with lower risk of CIED infection, such as antibiotic envelopes,42,44 leadless pacing69 and non-transvenous ICDs70 should be considered.
Strengths and limitations
This study is a retrospective register study, and therefore conclusions about causality cannot be established. However, this nationwide cohort contains 84 429 consecutive CIED patients with lifelong follow-up. The cohort was identified in the DPIR, where data have been entered prospectively since 1982, and although a few cases might have been misclassified it is unlikely that data should be flawed by systematic biases. Unfortunately, microbiological data were not available, and only patients with CIED removal, due to CIED infection were included. Therefore, our findings represent a lowest estimate of true CIED infection. CIED infection is likely profoundly underestimated in elderly, fragile patients as CIED infection might easier be overlooked in these patients. Furthermore, these patients might have been considered ineligible for CIED extraction due to the higher risk of complications in the early period of the study. However, the large number of consecutive patients in a nationwide setting and with lifelong follow-up is likely to minimize these potential flaws. The indication for explants due to CIED infections is registered as either systemic or pocket CIED infection, thereby allowing a CIED infection subtype-stratified risk factor analysis. Unfortunately, due to the retrospective nature of this study, we did not have the opportunity to provide a more distinctive classification of the CIED infections. This would have been preferred as recent evidence71,72 suggests variations in severity according to the scope of the CIED infections. Still, we believe this distinction between pocket and systemic CIED infections holds valuable information, as the diagnosis was registered prospectively by the treating physician.
Data were cross-linked with three high-quality national administrative registers, making it possible to perform a comprehensive risk factor analysis of the long-term risk of CIED infections. We included all comorbidities during any admission prior to the CIED removal. As the NPR does not hold information about drugs supplied directly by the hospital (e.g. biological treatments and chemotherapeutics), as well as over-the-counter drugs, these therapeutics could not be included in the analysis. We included numerous variables, thereby introducing a risk of statistical significance by chance. However, all univariate variables were individually evaluated in a reduced multivariate analysis before entering the final model. In the final model all the significant variables had very low P-values, thereby increasing the level of evidence and reducing the risk of a Type 1 error. Still, we cannot exclude that some of our findings may be due to multiple statistical testing, and we recommend that the results from our model should be tested in other CIED populations.
The nationwide setting gives a solid estimate of the risk of CIED infection in the current and future Danish CIED population. This, however, present a selection bias as the entire population is sampled, and our estimates of CIED infection and risk factors might not reflect other CIED populations. Although there are dissimilarities in infrastructure, socioeconomics and healthcare systems between Denmark and other countries, we believe that our results can be extrapolated to other CIED populations, especially in the Western world.
We believe that the quality of the registers, the nationwide setting with a high number of consecutive patients and lifelong follow-up provide a solid evaluation of the risk factors for CIED infection.
Conclusions
We identified specific comorbidities and surgical procedures as associated with higher risk of CIED infections in addition to previously identified surgery-, CIED- and patient-related risk factors. Pocket CIED infections were mainly associated with CIED reoperations, young age, and more complex type of CIED, whereas systemic CIED infections were correlated with risk factors predisposing to bacteraemia. These findings support the theory that pocket CIED infection occurs due to surgery-related factors, likely because of generator pocket contamination, while systemic CIED infection mainly occurs due to haematogenous seeding.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
OPEN, Open Patient data Explorative Network, Odense University Hospital, Region of Southern Denmark; www.sdu.dk/ki/open.
Contributor Information
Thomas Olsen, Department of Cardiology, Odense University Hospital, J. B. Winsløws Vej 4, 5000 Odense, Region of Southern Denmark, Denmark.
Ole Dan Jørgensen, Department of Heart, Lung and Vascular Surgery, Odense University Hospital, J. B. Winsløws Vej 4, 5000 Odense, Region of Southern Denmark, Denmark; Danish Pacemaker and ICD Register, Department of Cardiology, Odense University Hospital, J. B. Winsløws Vej 4, 5000 Odense, Region of Southern Denmark, Denmark.
Jens Cosedis Nielsen, Department of Cardiology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, 8200 Aarhus, Central Denmark Region, Denmark; Danish Pacemaker and ICD Register, Department of Cardiology, Odense University Hospital, J. B. Winsløws Vej 4, 5000 Odense, Region of Southern Denmark, Denmark.
Anna Margrethe Thøgersen, Department of Cardiology, Aalborg University Hospital, Hobrovej 18-22, 9000 Aalborg, North Denmark Region, Denmark.
Berit Thornvig Philbert, Department of Cardiology, Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Capital Region of Denmark, Denmark; Danish Pacemaker and ICD Register, Department of Cardiology, Odense University Hospital, J. B. Winsløws Vej 4, 5000 Odense, Region of Southern Denmark, Denmark.
Maria Hee Jung Park Frausing, Department of Cardiology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, 8200 Aarhus, Central Denmark Region, Denmark.
Niels Christian Foldager Sandgaard, Department of Cardiology, Odense University Hospital, J. B. Winsløws Vej 4, 5000 Odense, Region of Southern Denmark, Denmark.
Jens Brock Johansen, Department of Cardiology, Odense University Hospital, J. B. Winsløws Vej 4, 5000 Odense, Region of Southern Denmark, Denmark; Danish Pacemaker and ICD Register, Department of Cardiology, Odense University Hospital, J. B. Winsløws Vej 4, 5000 Odense, Region of Southern Denmark, Denmark.
Funding
The Danish Heart Association and the Region of Southern Denmark (15-R99-A5950-22895, 16/36792).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 2. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 3. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European society of cardiology (ESC). developed in collaboration with the European heart rhythm association (EHRA). Eur Heart J 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 4. Olsen T, Jørgensen OD, Nielsen JC, Thøgersen AM, Philbert BT, Johansen JB. Incidence of device-related infection in 97 750 patients: clinical data from the complete danish device-cohort (1982–2018). Eur Heart J 2019;40:1862–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johansen JB, Jørgensen OD, Møller M, Arnsbo P, Mortensen PT, Nielsen JC. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J 2011;32:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klug D, Balde M, Pavin D, Hidden-Lucet F, Clementy J, Sadoul N, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators. Circulation 2007;116:1349–1355. [DOI] [PubMed] [Google Scholar]
- 7. Prutkin JM, Reynolds MR, Bao H, Curtis JP, Al-Khatib SM, Aggarwal S, et al. Rates of and factors associated with infection in 200 909 medicare implantable cardioverter-defibrillator implants. Circulation 2014;130:1037–1043. [DOI] [PubMed] [Google Scholar]
- 8. Nery PB, Fernandes R, Nair GM, Sumner GL, Ribas CS, Menon SMD, et al. Device-Related infection among patients with pacemakers and implantable defibrillators: incidence, risk factors, and consequences. J Cardiovasc Electrophysiol 2010;21:786–790. [DOI] [PubMed] [Google Scholar]
- 9. Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol 2010;33:414–419. [DOI] [PubMed] [Google Scholar]
- 10. Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, et al. European Heart rhythm association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections—endorsed by the heart rhythm society (HRS), the Asia pacific heart rhythm society (APHRS), the latin American heart rhythm society (LAHRS), international society for cardiovascular infectious diseases (ISCVID) and the European society of clinical microbiology and infectious diseases (ESCMID) in collaboration with the European association for cardio-thoracic surgery (EACTS). Europace 2020;22:515–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015;17:767–777. [DOI] [PubMed] [Google Scholar]
- 12. Sohail MR, Hussain S, Le KY, Dib C, Lohse CM, Friedman PA, et al. Risk factors associated with early- versus late-onset implantable cardioverter-defibrillator infections. J Interv Card Electrophysiol 2011;31:171–183. [DOI] [PubMed] [Google Scholar]
- 13. Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, et al. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis 2007;45:166–173. [DOI] [PubMed] [Google Scholar]
- 14. Qintar M, Zardkoohi O, Hammadah M, Hsu A, Wazni O, Wilkoff BL, et al. The impact of changing antiseptic skin preparation agent used for cardiac implantable electronic device (CIED) procedures on the risk of infection. Pacing Clin Electrophysiol 2015;38:240–246. [DOI] [PubMed] [Google Scholar]
- 15. Pedersen CB. The danish civil registration system. Scand J Public Health 2011;39:22–25. [DOI] [PubMed] [Google Scholar]
- 16. Møller M, Arnsbo P, Asklund M, Christensen PD, Gadsbøll N, Svendsen JH, et al. Quality assessment of pacemaker implantations in Denmark. Europace 2002;4:107–112. [DOI] [PubMed] [Google Scholar]
- 17. Lynge E, Sandegaard JL, Rebolj M. The danish national patient register. Scand J Public Health 2011;39:30–33. [DOI] [PubMed] [Google Scholar]
- 18. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kildemoes HW, Sørensen HT, Hallas J. The danish national prescription registry. Scand J Public Health 2011;39:38–41. [DOI] [PubMed] [Google Scholar]
- 20. Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the danish national prescription registry. Int J Epidemiol 2017;46:798–798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pottegård A, Hallas J. Assigning exposure duration to single prescriptions by use of the waiting time distribution. Pharmacoepidemiol Drug Saf 2013;22:803–809. [DOI] [PubMed] [Google Scholar]
- 22. Chinen J, Shearer WT. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol 2010;125:S195–S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jann B. Plotting regression coefficients and other estimates. Stata J 2014;14:708–737. [Google Scholar]
- 24. Humphreys H, Fitzpatick F, Harvey BJ. Gender differences in rates of carriage and bloodstream infection caused by methicillin-resistant Staphylococcus aureus: are they real, do they matter and why? Clin Infect Dis 2015;61:1708–1714. [DOI] [PubMed] [Google Scholar]
- 25. Fierer N, Hamady M, Lauber CL, Knight R. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci U S A 2008;105:17994–17999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olsen T, Justesen US, Nielsen JC, Jørgensen OD, Sandgaard NCF, Ravn C, et al. Microbiological diagnosis in cardiac implantable electronic device infections detected by sonication and next-generation sequencing. Heart Rhythm 2022;19:901–908. [DOI] [PubMed] [Google Scholar]
- 27. Bongiorni MG, Tascini C, Tagliaferri E, Cori AD, Soldati E, Leonildi A, et al. Microbiology of cardiac implantable electronic device infections. Europace 2012;14:1334–1339. [DOI] [PubMed] [Google Scholar]
- 28. Patel A, Calfee RP, Plante M, Fischer SA, Green A. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elbow Surg 2009;18:897–902. [DOI] [PubMed] [Google Scholar]
- 29. Saper D, Capiro N, Ma R, Li X. Management of propionibacterium acnes infection after shoulder surgery. Curr Rev Musculoskelet Med 2015;8:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frausing MHJP, Kronborg MB, Johansen JB, Nielsen JC. Avoiding implant complications in cardiac implantable electronic devices: what works? Europace 2021;23:163–173. [DOI] [PubMed] [Google Scholar]
- 31. Saltzman MD, Nuber GW, Gryzlo SM, Marecek GS, Koh JL. Efficacy of surgical preparation solutions in shoulder surgery. J Bone Joint Surg Am 2009;91:1949–1953. [DOI] [PubMed] [Google Scholar]
- 32. Sandoe JAT, Barlow G, Chambers JB, Gammage M, Guleri A, Howard P, et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint working party project on behalf of the British society for antimicrobial chemotherapy (BSAC, host organization), British heart rhythm society (BHRS), British cardiovascular society (BCS), British heart valve society (BHVS) and British society for echocardiography (BSE). J Antimicrob Chemother 2015;70:325–359. [DOI] [PubMed] [Google Scholar]
- 33. Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017;14:e503–e551. [DOI] [PubMed] [Google Scholar]
- 34. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC Definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992;13:606–608. [PubMed] [Google Scholar]
- 35. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association task force on practice guidelines. J Thorac Cardiovasc Surg 2014;148:e1–e132. [DOI] [PubMed] [Google Scholar]
- 36. Østergaard L, Valeur N, Bundgaard H, Gislason G, Torp-Pedersen C, Eske Bruun N, et al. Cardiac implantable electronic device and associated risk of infective endocarditis in patients undergoing aortic valve replacement. Europace 2018;20:e164–e170. [DOI] [PubMed] [Google Scholar]
- 37. Rohacek M, Weisser M, Kobza R, Schoenenberger AW, Pfyffer GE, Frei R, et al. Bacterial colonization and infection of electrophysiological cardiac devices detected with sonication and swab culture. Circulation 2010;121:1691–1697. [DOI] [PubMed] [Google Scholar]
- 38. Mason PK, Dimarco JP, Ferguson JD, Mahapatra S, Mangrum JM, Bilchick KC, et al. Sonication of explanted cardiac rhythm management devices for the diagnosis of pocket infections and asymptomatic bacterial colonization. Pacing Clin Electrophysiol 2011;34:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kleemann T, Becker T, Strauss M, Dyck N, Weisse U, Saggau W, et al. Prevalence of bacterial colonization of generator pockets in implantable cardioverter defibrillator patients without signs of infection undergoing generator replacement or lead revision. Europace 2010;12:58–63. [DOI] [PubMed] [Google Scholar]
- 40. Chu XM, Li B, An Y, Li XB, Guo JH. Genetic identification and risk factor analysis of asymptomatic bacterial colonization on cardiovascular implantable electronic devices. Biomed Res Int 2014;2014:725163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zoni-Berisso M, Martignani C, Ammendola E, Narducci ML, Caruso D, Miracapillo G, et al. Mortality after cardioverter-defibrillator replacement: results of the DECODE survival score index. Heart Rhythm 2021;18:411–418. [DOI] [PubMed] [Google Scholar]
- 42. Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss E, et al. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med 2019;380:1895–1905. [DOI] [PubMed] [Google Scholar]
- 43. Krahn AD, Longtin Y, Philippon F, Birnie DH, Manlucu J, Angaran P, et al. Prevention of arrhythmia device infection trial: the PADIT trial. J Am Coll Cardiol 2018;72:3098–3109. [DOI] [PubMed] [Google Scholar]
- 44. Frausing MHJP, Nielsen JC, Johansen JB, Jørgensen OD, Gerdes C, Olsen T, et al. Rate of device-related infections using an antibacterial envelope in patients undergoing cardiac resynchronization therapy reoperations. Europace 2022;24:421–429. [DOI] [PubMed] [Google Scholar]
- 45. Cengiz M, Okutucu S, Ascioglu S, Şahin A, Aksoy H, Deveci OS, et al. Permanent pacemaker and implantable cardioverter defibrillator infections: seven years of diagnostic and therapeutic experience of a single center. Clin Cardiol 2010;33:406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Joy PS, Kumar G, Poole JE, London B, Olshansky B. Cardiac implantable electronic device infections: who is at greatest risk? Heart Rhythm 2017;14:839–845. [DOI] [PubMed] [Google Scholar]
- 47. Uslan DZ, Gleva MJ, Warren DK, Mela T, Chung MK, Gottipaty V, et al. Cardiovascular implantable electronic device replacement infections and prevention: results from the REPLACE registry. Pacing Clin Electrophysiol 2012;35:81–87. [DOI] [PubMed] [Google Scholar]
- 48. Essebag V, Verma A, Healey JS, Krahn AD, Kalfon E, Coutu B, et al. Clinically significant pocket hematoma increases long-term risk of device infection. J Ame Coll Cardiol 2016;67:1300–1308. [DOI] [PubMed] [Google Scholar]
- 49. Birnie DH, Healey JS, Wells GA, Verma A, Tang AS, Krahn AD, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013;368:2084–2093. [DOI] [PubMed] [Google Scholar]
- 50. Birnie DH, Healey JS, Wells GA, Ayala-Paredes F, Coutu B, Sumner GL, et al. Continued vs. Interrupted direct oral anticoagulants at the time of device surgery, in patients with moderate to high risk of arterial thrombo-embolic events (BRUISE CONTROL-2). Eur Heart J 2018;39:3973–3979. [DOI] [PubMed] [Google Scholar]
- 51. Essebag V, Healey JS, Joza J, Nery PB, Kalfon E, Leiria TLL, et al. Effect of direct oral anticoagulants, warfarin, and antiplatelet agents on risk of device pocket hematoma. Circ Arrhythm Electrophysiol 2019;12:e007545. [DOI] [PubMed] [Google Scholar]
- 52. Swerdlow CD, Kalahasty G, Ellenbogen KA. Implantable cardiac defibrillator lead failure and management. J Am Coll Cardiol 2016;67:1358–1368. [DOI] [PubMed] [Google Scholar]
- 53. Bloom H, Heeke B, Leon A, Mera F, Delurgio D, Beshai J, et al. Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. Pacing Clin Electrophysiol 2006;29:142–145. [DOI] [PubMed] [Google Scholar]
- 54. Tompkins C, Mclean R, Cheng A, Brinker JA, Marine JE, Nazarian S, et al. End-Stage renal disease predicts complications in pacemaker and ICD implants. J Cardiovasc Electrophysiol 2011;22:1099–1104. [DOI] [PubMed] [Google Scholar]
- 55. Lekkerkerker JC, van Nieuwkoop C, Trines SA, van der Bom JG, Bernards A, van deVelde ET, et al. Risk factors and time delay associated with cardiac device infections: leiden device registry. Heart 2009;95:715–720. [DOI] [PubMed] [Google Scholar]
- 56. Bloom HL, Constantin L, Dan D, de Lurgio DB, El-Chami M, Ganz LI, et al. Implantation success and infection in cardiovascular implantable electronic device procedures utilizing an antibacterial envelope. Pacing Clin Electrophysiol 2011;34:133–142. [DOI] [PubMed] [Google Scholar]
- 57. Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I. Basic science and dialysis: disturbances of acquired immunity in hemodialysis patients. Semin Dial 2007;20:440–451. [DOI] [PubMed] [Google Scholar]
- 58. Eleftheriadis T, Liakopoulos V, Leivaditis K, Antoniadi G, Stefanidis I. Infections in hemodialysis: a concise review. Part II: blood transmitted viral infections. Hippokratia 2011;15:120–126. [PMC free article] [PubMed] [Google Scholar]
- 59. Sakowicz-Burkiewicz M, Kocbuch K, Grden M, Szutowicz A, Pawelczyk T. Diabetes-induced decrease of adenosine kinase expression impairs the proliferation potential of diabetic rat T lymphocytes. Immunology 2006;118:402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vandeligt KR, Ross SA, Matheson DS. Immunologic studies of patients with diabetes mellitus who have received long term insulin therapy: lymphocyte reactivity to insulin is correlated with impaired immunoglobulin secretion in vitro. Clin Immunol Immunopathol 1989;53:422–429. [DOI] [PubMed] [Google Scholar]
- 61. Oestergaard LB, Schmiegelow MDS, Bruun NE, Skov R, Andersen PS, Larsen AR, et al. Staphylococcus aureus bacteremia in children aged 5-18 years—risk factors in the new millennium. J Pediatr 2018;203:108–115.e3. [DOI] [PubMed] [Google Scholar]
- 62. Goldblatt F, Chambers S, Rahman A, Isenberg DA. Serious infections in British patients with systemic lupus erythematosus: hospitalisations and mortality. Lupus 2009;18:682–689. [DOI] [PubMed] [Google Scholar]
- 63. Cuchacovich R, Gedalia A. Pathophysiology and clinical Spectrum of infections in systemic lupus erythematosus. Rheum Dis Clin North Am 2009;35:75–93. [DOI] [PubMed] [Google Scholar]
- 64. Mateos Gaitán R, Boix-Palop L, Muñoz García P, Mestres CA, Marín Arriaza M, Pedraz Prieto Á, et al. Infective endocarditis in patients with cardiac implantable electronic devices: a nationwide study. Europace 2020;22:1062–1070. [DOI] [PubMed] [Google Scholar]
- 65. Birnie DH, Wang J, Alings M, Philippon F, Parkash R, Manlucu J, et al. Risk factors for infections involving cardiac implanted electronic devices. J Am Coll Cardiol 2019;74:2845–2854. [DOI] [PubMed] [Google Scholar]
- 66. Pavlovic NV, Randell T, Madeira T, Hsu S, Zinoviev R, Abshire M. Risk of left ventricular assist device driveline infection: a systematic literature review. Heart Lung 2019;48:90–104. [DOI] [PubMed] [Google Scholar]
- 67. Riaz T, Nienaber JJ, Baddour LM, Walker RC, Park SJ, Sohail MR. Cardiovascular implantable electronic device infections in left ventricular assist device recipients. Pacing Clin Electrophysiol 2014;37:225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Frausing MHJP, Nielsen JC, Johansen JB, Jørgensen OD, Olsen T, Gerdes C, et al. Rate of permanent cardiac implantable electronic device infections after active fixation temporary transvenous pacing: a nationwide danish cohort study. Heart Rhythm O2 2021;18:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. El-Chami MF, Bonner M, Holbrook R, Stromberg K, Mayotte J, Molan A, et al. Leadless pacemakers reduce risk of device-related infection: review of the potential mechanisms. Heart Rhythm 2020;17:1393–1397. [DOI] [PubMed] [Google Scholar]
- 70. Kamp NJ, Al-Khatib SM. The subcutaneous implantable cardioverter-defibrillator in review. Am Heart J 2019;217:131–139. [DOI] [PubMed] [Google Scholar]
- 71. Tarakji KG, Wazni OM, Harb S, Hsu A, Saliba W, Wilkoff BL. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace 2014;16:1490–1495. [DOI] [PubMed] [Google Scholar]
- 72. Diemberger I, Bonfiglioli R, Martignani C, Graziosi M, Biffi M, Lorenzetti S, et al. Contribution of PET imaging to mortality risk stratification in candidates to lead extraction for pacemaker or defibrillator infection: a prospective single center study. Eur J Nucl Med Mol Imaging 2019;46:194–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.