Abstract
Objective
To present updated evidence on the safety, efficacy and acceptability of a direct-to-patient telemedicine abortion service and describe how the service functioned during the COVID-19 pandemic.
Study Design
We offered the study at 10 sites that provided the service in 13 states and Washington DC. Interested individuals obtained any needed preabortion tests locally and had a videoconference with a study clinician. Sites sent study packages containing mifepristone and misoprostol by mail and had remote follow-up consultations within one month by telephone (or by online survey, if the participant could not be reached) to evaluate abortion completeness. The analysis was descriptive.
Results
We mailed 1390 packages between May 2016 and September 2020. Of the 83% (1157/1390) of abortions for which we obtained outcome information, 95% (1103/1157) were completed without a procedure. Participants made 70 unplanned visits to emergency rooms or urgent care centers for reasons related to the abortion (6%), and 10 serious adverse events occurred, including 5 transfusions (0.4%). Enrollment increased substantially with the onset of COVID-19. Although a screening ultrasound was required, sites determined in 52% (346/669) of abortions that occurred during COVID that those participants should not get the test to protect their health. Use of urine pregnancy test to confirm abortion completion increased from 67% (144/214) in the 6 months prior to COVID to 90% (602/669) in the 6 months during COVID. Nearly all satisfaction questionnaires (99%, 1013/1022) recorded that participants were satisfied with the service.
Conclusions
This direct-to-patient telemedicine service was safe, effective, and acceptable, and supports the claim that there is no medical reason for mifepristone to be dispensed in clinics as required by the Food and Drug Administration. In some cases, participants did not need to visit any facilities to obtain the service, which was critical to protecting patient safety during the COVID-19 pandemic.
Implications
Medical abortion using telemedicine and mail is effective and can be safely provided without a pretreatment ultrasound. This method of service delivery has the potential to greatly improve access to abortion care in the United States.
Keywords: COVID-19, Mail, Medical abortion, Telemedicine, United States
Introduction
Telemedicine abortion is a broad term that describes the use of telecommunications (phone, videoconference, texting, email) to provide one or more aspects of abortion care such as counseling, eligibility assessment, medication provision, guidance through the process, and follow-up assessment. These services may be provided as part of or independent from the formal healthcare system and may involve some degree of in-person contact for parts of the process.
A substantial body of literature from around the globe provides evidence that telemedicine models of abortion provision are highly acceptable to clients and providers, and success rates and safety outcomes are similar to those reported for in-person care [1], [2], [3], [4], [5], [6]. Furthermore, a growing amount of data from the United States suggests that telemedicine allows people to obtain abortions at an earlier gestational age, improves access to care for rural patients, and may be associated with decreases in time to schedule an appointment and distance traveled [7,8].
Having the option to receive abortion care via telemedicine is critical, as accessing in-person care has become increasingly challenging in certain regions of the country. In 2017, 95% and 94% of counties in the Midwest and the South, respectively, did not have a facility that provided abortion care [9]. Individuals who can get to a clinic find an increasingly hostile environment outside; the National Abortion Federation's 2019 annual report on violence and disruption statistics documented 3387 incidents of obstructing facilities (up from 3038 in 2018), and 123,228 incidents of picketing (up from 99,409 in 2018) [10].
The COVID-19 pandemic has exacerbated barriers to accessing abortion care by hindering people's ability to pay for the service (due to loss of income) and limiting mobility because of childcare needs, stay-at-home orders, and the imperative to limit in-person interactions [11,12]. To mitigate some of these effects, many abortion providers have modified their clinical protocols and incorporated telemedicine to varying degrees [13]. Experts have advocated for adoption of “no-test medication abortion,” which, by not mandating screening ultrasound, blood tests, or follow-up tests unless clinically warranted, would allow the treatment to be provided without an in-person encounter [14].
The TelAbortion Project is a direct-to-patient service model whereby participating clinics counsel and screen patients remotely, and then send mifepristone and misoprostol by mail to those who are eligible. Because of restrictions on mifepristone imposed by the Food and Drug Administration (FDA) under the drug's Risk Evaluation and Mitigation Strategy (REMS), we implemented TelAbortion as a research study conducted under an Investigational New Drug (IND) application. Specifically, the REMS for mifepristone states that the drug must be dispensed to patients only in clinics, medical offices, and hospitals, which is commonly interpreted as prohibiting the mailing of the medication. Results from the first 32 months of the project (May 2016–December 2018) in which 248 packages were sent found that the service was safe, effective, efficient and satisfactory [6]. The objective of this analysis is to present data on safety, efficacy and acceptability collected from May 2016 through September 2020 (inclusive of the previously published data), during which time the study expanded dramatically both geographically and in sample size, and describe how the service functioned amidst the numerous challenges imposed by the COVID-19 pandemic.
Methods
In the reporting period, the study was implemented at 10 institutions (4 independent clinics, 4 Planned Parenthood affiliates, and 2 academic medical centers) that provided the TelAbortion service in 13 states (CO, GA, HI, IA, IL, MD, ME, MN, MT, NM, NY, OR, WA) and Washington, DC. Five sites provided the service in states where they were not physically located because their clinicians were licensed there. One site stopped recruitment in 2017 due to slow enrollment. Sites were added on a rolling basis, with the newest site beginning enrollment in May 2020. Before adding each state to our study, we confirmed that it had no laws that prohibited the service, although some state laws constrained or complicated the way the service was offered.
Patients interested in receiving a TelAbortion underwent a prescreening process by phone that reviewed basic eligibility requirements and explained the study procedures. Those who wished to proceed obtained any necessary tests at laboratories or radiology offices and had evaluations with a study clinician via videoconference during which the clinician confirmed eligibility, obtained consent electronically, and agreed on a plan for evaluating abortion outcome using ultrasound, serum hCG tests, or urine pregnancy test (UPT). Individuals were required to obtain a pre-abortion ultrasound or pelvic exam, and were deemed eligible for TelAbortion if the study clinician determined that the patient would be able to receive and take the mifepristone at ≤70 days of gestation and had no suspicion that the pregnancy was ectopic or nonviable (See the prior paper for a full description of procedures and eligibility requirements [6].).
Sites sent participants packages containing one tablet of mifepristone 200 mg and eight tablets of misoprostol 200 mcg (one site prescribed the misoprostol instead), an instruction sheet, and a UPT if indicated. Sites advised participants to take mifepristone followed by misoprostol 800 mcg within 48 hours either vaginally or buccally, as per standard clinic practice, and to take the other 800 mcg of misoprostol if no bleeding occurred within 24 hours after the first dose. In the event of any problems, sites instructed participants to call, recommended they seek in-person care if indicated, and followed up with them until the resolution of the problem. Following standard practice at the site, study clinicians evaluated abortion outcome using patient history, ultrasound, serum HCG tests before and after mifepristone ingestion, pelvic examination, and/or urine pregnancy testing. At sites that offered more than one method, the participant and clinician agreed on which method to use. Within a month after mailing each study package, sites conducted follow-up contacts to review any test results, assess abortion outcome, and inquire about any adverse events or unplanned visits. Once the abortion was complete, sites conducted a short, structured satisfaction questionnaire by telephone.
The study team made some modifications to study procedures over time. For the first 127 participants, the study paid for care and medications provided directly by sites. We offered participant compensation of $50 until late 2018, when we stopped to better mirror standard provision of abortion care and obtain more accurate data on acceptability and satisfaction (245 participants in our sample were offered compensation). Other changes included a switch from paper data forms to a secure browser-based electronic data capture application in early 2019. If a participant was lost to follow-up and outcome and/or satisfaction data had not been collected, the sites sent via email a link to an online survey for the participant to complete. As new practice guidelines became available, sites could make corresponding changes in care for their TelAbortion patients (as permitted within the constraints of the study protocol); these included forgoing Rh typing or prophylaxis with anti-D immunoglobulin for participants under a certain gestational age and advising participants to take a second dose of misoprostol routinely in the 9th and/or 10th week of gestation [15].
Here we present descriptive analyses of our service delivery data. For data on participant characteristics (Table 1 ) our unit of analysis was the individual so that participants who had multiple abortions were not counted more than once. For abortion outcome, unplanned encounters, and satisfaction data (Tables 2 and 3 ), we utilized the abortion as the unit of analysis as we did not want to undercount any of these outcomes. We defined an adverse event as serious if it was fatal, life-threatening or resulted in hospitalization, transfusion, or significant disability. An unplanned clinical encounter was any visit to a clinician after the study package was mailed, except visits to obtain anti-D immunoglobulin, contraception, or routine ultrasound or lab tests to evaluate abortion outcome in the absence of concerning symptoms. The study team evaluated the reason for each unplanned encounter to determine whether or not it was abortion-related. We used 3/13/20 as the start date of the COVID crisis as that was the date the federal government declared COVID-19 to be a “national emergency” [16].
Table 1.
Characteristics of TelAbortion study participants who were sent a medical abortion package: n (%) or median (range)a
| N = 1356 | |
|---|---|
| Age 15–24 years 25–34 years 35–47 years |
346 (25.5) 735 (54.2) 275 (20.3) |
| Highest level of education completed Less than HS HS/GED More than HS |
65/1305 (5.0) 357/1305 (27.4) 883/1305 (67.7) |
| Number of previous pregnancies 0 1 ≥ 2 |
344 (25.4) 291 (21.5) 721 (53.2) |
| Number of previous medical abortions 0 1 ≥2 |
1086/1348 (80.6) 210/1348 (15.6) 52/1348 (3.9) |
| Gestational age at prescreenb 18–35 days 36–63 days 64–68 days Median (range) |
280 (20.6) 1053 (77.7) 23 (1.7) 42 (18-68) |
| Distance of residence from provider (Continental US)c 1–9.9 mi 10–49.9 mi 50–99.9 mi 100–149.9 mi ≥ 150 mi |
80/1075 (7.4) 347/1075 (32.3) 169/1075 (15.7) 92/1075 (8.6) 387/1075 (36.0) |
| Hawaiian island of residence Oahu Other island |
55/281 (19.6) 226/281 (80.4) |
| Race/ethnicity (more than 1 category allowed)c White Black Hispanic Asian/Pacific Islander Native American Multi-racial, not specified |
750/1073 (69.9) 163/1073 (15.2) 87/1073 (8.1) 93/1073 (8.7) 42/1073 (3.9) 8/1073 (0.7) |
| Classification of participant's current addressc Urban Rural |
826/1031 (80.1) 205/1031 (19.9) |
| Payment method for care provided by site (more than 1 method allowed)d Private/public insurance Self-pay Abortion fund |
470/1228 (38.3) 899/1228 (73.2) 175/1228 (14.3) |
| Actual/planned payment method for pre-abortion tests obtained elsewhere (more than 1 method allowed)d Private/public insurance Self-pay Abortion fund None; did not have any preabortion tests |
606/919 (65.9) 332/919 (36.1) 26/919 (2.8) 327 |
Does not include 32 second abortions and 2 third abortions during the study period. For participants with multiple abortions, we included only the first.
Using clinician's estimate of gestational age at time package sent and then backdated.
Question not asked in first version of study forms.
Does not include 127 participants who had study site services paid for by study.
Table 2.
Abortion outcomesa and unplanned encounters, n (%)
| N = 1390 | |
|---|---|
| Neither medication taken, or medications taken after miscarriage diagnosis Lost to follow-up Known abortion outcome |
47 (3.4) 186 (13.4) 1157 (83.2) |
|
Abortion outcome at last contact Complete abortion without surgical intervention Surgical intervention Reason: ongoing pregnancy Ongoing pregnancy; carrying to term or unknownb |
n = 1157 1103 (95.3) 47 (4.1) 14 (1.2) 7 (0.6) |
|
Method used in outcome assessment among complete abortions with no surgical intervention Facility-based test (ultrasound, serum HCG, and/orpelvic exam)c No facility-based test Urine pregnancy test (UPT)d Patient history only |
n = 1103 396 (35.9) 707 (64.1) 647 (58.7) 60 (5.4) |
|
Abortion-related unplanned encounterse Emergency room (ER)/urgent care Other outpatient visit Serious Adverse Events Hospitalization Transfusionf |
n=1173 70 (6.0) 92 (7.8) 10 (0.9) 8 (0.7) 5 (0.4) |
Includes multiple abortions by same individual.
Includes one abortion where participant threw up mifepristone after 10 minutes and then decided to continue pregnancy.
Outcomes assessed with facility-based tests may also have utilized UPTs and/or patient history.
In 3 cases, the UPT result(s) were positive and the diagnosis of complete abortion was made by patient history only.
Denominator includes abortions with known outcome or any unplanned encounters that occurred after study consent was signed. Does not include encounters for lab tests, anti-D immunoglobulin, or contraception alone. Includes 1 hospitalization, 4 ER visits, and 12 other outpatient encounters that occurred prior to taking (or deciding not to take) mifepristone. Abortions may be included in more than one category.
Two of the transfusions occurred in an ER and are not included in Hospitalization.
Table 3.
Acceptability of TelAbortion to study participants at exit interview, among those with known abortion outcome: n (%)a
| Satisfaction with the serviceVery satisfactorySatisfactoryUnsatisfactory/Very unsatisfactory | n = 1022869 (85.0)144 (14.1)9 (0.9) |
| Satisfaction with speaking to provider remotely Very satisfactory Satisfactory Unsatisfactory/Very unsatisfactory |
n = 891 763 (85.6) 123 (13.8) 5 (0.6) |
| Experience getting pre-abortion testsb Easy or very easy Difficult or very difficult |
n = 693 594 (85.7) 99 (14.3) |
| Future preference TelAbortion In-person abortion No preference |
n = 886 754 (85.1) 56 (6.3) 76 (8.6) |
| Would recommend TelAbortion to a friend Yes No Maybe |
n = 892 863 (96.7) 9 (1.0) 20 (2.2) |
Includes multiple abortions by same individual.
Does not include abortions where no pre-abortion tests were planned.
Advarra Institutional Review Board, the University of Hawaii's Office of Research Compliance Human Studies Program, and Oregon Health and Science University's Institutional Review Board approved the protocol. We registered the study on clinicaltrials.gov (NCT02513043).
Results
Partnering sites sent 1390 packages to 1356 participants who were prescreened between May 11, 2016 and September 11, 2020. Thirty participants received 2 abortions and 2 received 3 abortions during the reporting period. Of the 1356 individuals who received a package, 26% were under 25 years of age, and 14 were minors (Table 1). Participants tended to contact the clinics early in the first trimester; 47% were less than 42 days gestation at the time of the prescreening. In the continental U.S., 60% of participants lived 50 miles or more from their study site, and 36% lived 150 miles or farther. Thirty participants had their packages mailed to a state that was not their state of residence. While only 38% used insurance to pay for care provided by the study site (e.g., counseling, abortion medications), 66% used insurance to pay for preabortion tests.
In 47 instances (3%), neither abortion medication was taken, or the medications were taken after a diagnosis of miscarriage (Table 2). The median gestational age on the day of mifepristone ingestion was 53 days gestation (range 29–76). In eleven abortions (1% of those with outcome information), the participant took mifepristone past 70 days of gestation. Of the 76 times (7%) a participant reported taking more than 800mcg of misoprostol from the study package, 20 did so due to gestational age, as advised by the site during counseling. In 42 instances, participants took another 800 mcg of misoprostol because of little or no bleeding, or due to concerns that they did not pass the pregnancy.
We obtained abortion outcome information on 83% of abortions (1157/1390) and satisfaction data after 74% of abortions (1022/1390). Fourteen percent of the satisfaction questionnaires (141/1022) were completed via the online survey. Of the 1157 abortions with outcome information, 95% were complete abortions without a procedure (Table 2). Twenty-one abortions (1.8%) resulted in ongoing pregnancy. The majority of complete abortions were confirmed using a method that did not require a visit to a facility; 59% relied on UPTs, and 5% depended on patient history alone.
There were seventy unplanned visits (6%) to emergency rooms or urgent care centers for reasons related to the abortion (Table 2). Ten serious adverse events (SAEs) occurred, including five transfusions (0.4%). We determined that none of the SAEs was attributable to the telemedicine delivery of the service (e.g., they would not have been avoided if the participants had had in-person screening or picked their pills up in person).
When the COVID-19 national emergency was declared, we worked with our study sites to adapt to new challenges. The FDA required that our protocol retain the screening ultrasound requirement, but on a case-by-case basis, and following broader FDA guidance on conduct of clinical trials during the pandemic [17], sites evaluated whether forgoing the ultrasound was necessary to protect patient and provider health and safety (e.g., if the patient's locale was under stay-at-home orders, or a patient was quarantining because of COVID exposure or infection). Overall, 52% (346/669) of abortions during COVID occurred without a screening ultrasound, though this proportion varied widely by site (0%–83%). No ectopic pregnancies were reported among those who received a package during the entire analysis period (pre- and during COVID).
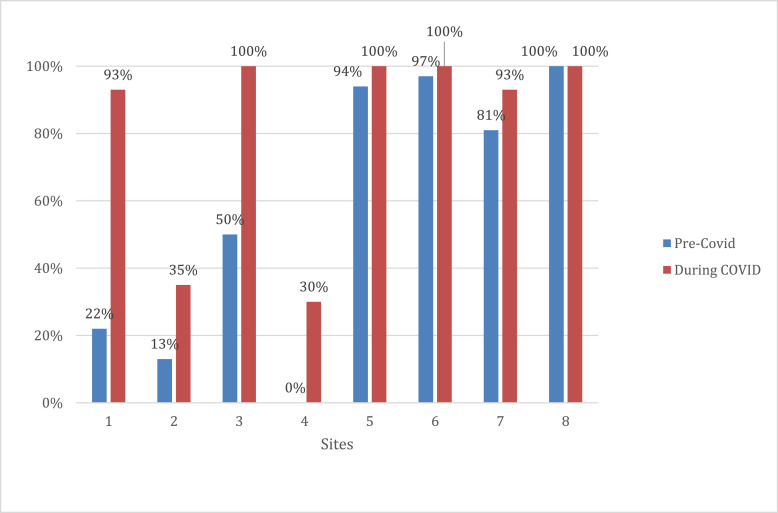
Prior to COVID, we had already started encouraging sites to preferentially offer UPT follow-up to confirm abortion outcome because participants were reporting that getting testing at facilities was burdensome. Comparing the 6 months prior to COVID to the period during COVID, selection of UPT as a follow-up method increased from 67% (144/214) to 90% (602/669). While some sites were already doing UPT follow-up for nearly all participants in the pre-COVID period, four study sites that had rarely used UPT for follow-up before COVID reported substantial increases during the COVID period (Fig. 1 ).
Fig. 1.
Percentage of abortions with urine pregnancy test selected as follow-up method before COVID and during COVID, by site.a,ba‘Pre-COVID’ period defined as 9/13/19–3/12/20; ‘During COVID’ period defined as 3/13/20–9/11/20.bDoes not include 1 site that did not enroll patients in the defined periods, and 1 site that did not enroll patients in the pre-COVID period (and enrolled 14 participants in the during COVID period).
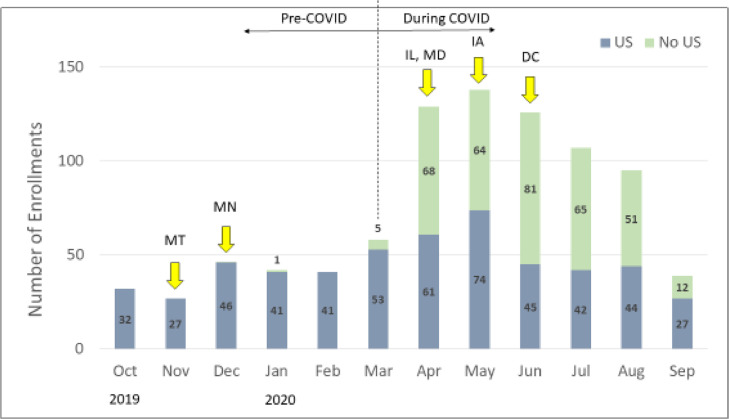
Enrollment increased dramatically in the months after March 2020; compared to January and February 2020, monthly enrollment more than tripled in April, May, and June of the same year (Fig. 2 ), Months with high enrollment were also months in which large percentages of abortions occurred without screening ultrasounds, and also in which new states were added to the project.
Fig. 2.
Enrollment and pre-abortion ultrasound (U/S) over time.aaEnrollment included from 10/1/19 through 9/11/20.*Arrows denote when new states added to the project.
Over the entire reporting period, participants were overwhelmingly satisfied with the service, and with speaking to their providers remotely (Table 3). Despite some difficulty obtaining preabortion tests reported by 14% of the sample, 85% would choose TelAbortion again, and nearly all would recommend the service to a friend.
Discussion
With a larger, more geographically diverse sample, these data confirm our earlier findings that the TelAbortion service is safe, feasible, effective, and acceptable. Mifepristone can safely be dispensed by mail, and the REMS requirement that mifepristone must be dispensed in person, instead of enhancing patient safety as it purports to do, could have the exact opposite effect, particularly during a pandemic. Our abortion success rate of 95% is comparable to rates in the literature for in-person care [18]. A substantial proportion of our participants lived significant distances from their providers (and in Hawaii, most lived on different islands), underscoring the potential of direct-to-patient services to improve access to care. Preabortion ultrasounds are usually unnecessary for safe and effective medication abortion [1,14,19,20], and we found during COVID that sites sometimes actually felt it necessary to omit the ultrasound to protect a participant's health. The finding that a higher proportion of participants used insurance to pay for preabortion tests than the abortion medications and counseling (66% vs 38%) suggests that the TelAbortion service model could lower costs for some patients. This may be true especially in states where insurance will not cover the cost of abortion but may cover the cost of tests (as there is nothing to link the tests to subsequent abortion care).
We were interested to see that only 7% of participants took a second dose of misoprostol. In light of the recent misoprostol shortage that began in September 2020 [21] (J. Price, personal communication, 2/26/21), and adhering to the general principle of conservation of resources, rather than send out a second dose to all patients as standard practice, services might consider only sending it to patients above a certain gestational age (e.g., above 64 days or 71 days depending on clinic protocols), and calling in additional doses to pharmacies as needed.
A small but noteworthy number of patients (n = 30) obtained a TelAbortion in a state other than their state of residence. As the practice of medicine occurs where the patient is physically, crossing a state border can allow a patient to access care in an environment with fewer restrictions. In these instances, participants crossed the border for their video conference and then sites mailed packages to courier or post office locations, or to friends or family members, who held the package until the participant picked it up. While this still required participants to travel, this approach may have enabled them to travel shorter distances than they would have had to in order to get to the nearest in-person appointment, and it allowed some to bypass home-state restrictions on abortions such as in-person counseling or waiting period laws. Some sites actively conducted outreach to metropolitan areas with restricted access to abortion care that were located near a border with a project state. Since 19 states prohibit the use of telemedicine for medication abortion within their borders, this may be a strategy to explore further to increase access in more restrictive states [22].
When the first surge of COVID-19 occurred in the spring, enrollment in the service soared, likely due to greater challenges in accessing in-person care, the ability for some participants to obtain the service without having to get an ultrasound (which meant they could do the entire process from home), and the addition of new states to the project. This spike in enrollment at a time when barriers to abortion care were limiting access elsewhere emphasizes the critical value of our service delivery method. Changes in practice during COVID varied widely by site, and reflected a number of factors including provider preference, institutional policies, and the provider's perception of the degree to which obtaining tests increased a patient's risk of infection or the patient's risk of infecting others.
Our data have some limitations. While we were able to improve on the follow-up rate of 77% from our first analysis (possibly due to the adoption of the online exit survey), we did not have outcome data on 13% of participants. As such, our estimates of medical abortion failure or complications may be underestimated or overestimated. As this analysis was descriptive, we are limited in the associations we can make between various aspects of the service and outcomes.
Compared to people obtaining abortions in the United States, our study population had a higher proportion of people who were older, more educated, and more likely to identify as white [23]. Telemedicine innovations need to prioritize the most disadvantaged populations so that they are not left behind. Future innovations in our project should focus on addressing this issue.
When we started the TelAbortion Project in 2016, it was the first service in the United States in which people could obtain an abortion legally without an in-person visit to an abortion provider. After a federal district court issued an injunction in July 2020 blocking the FDA from enforcing the rule that patients must pick up the abortion medication in person from their provider, several new online services launched. These promising efforts were recently threatened by a Supreme Court decision in January 2021 that reinstated the prior harmful policy. We believe our data disprove the notion that medication abortion pills must be dispensed in-person, and that direct-to-patient services that mail the pills to patients are safe, effective and feasible, even without a screening ultrasound.
Declaration of Competing Interest
None.
Funding
This work was supported by the Tara Health Foundation, the Bernard and Anne Spitzer Charitable Trust, the Lisa and Douglas Goldman Fund, and several anonymous donors. These donors had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of Planned Parenthood Federation of America, Inc.
Acknowledgments
The authors thank Fatoumata Bah and Julia Habbe for their assistance in site coordination and data cleaning.
References
- 1.Endler M, Lavelanet A, Cleeve A, Ganatra B, Gomperts R, Gemzell-Danielsson K. Telemedicine for medical abortion: a systematic review. BJOG. 2019;126(9):1094–1102. doi: 10.1111/1471-0528.15684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeNicola N, Grossman D, Marko K, Sonalkar S, Butler Tobah YS, Ganju N, et al. Telehealth interventions to improve obstetric and gynecologic health outcomes: a systematic review. Obstet Gynecol. 2020;135(2):371–382. doi: 10.1097/AOG.0000000000003646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohn JE, Snow JL, Simons HR, Seymour JW, Thompson TA, Grossman D. Medication abortion provided through telemedicine in four U.S. states. Obstet Gynecol. 2019;134(2):343–350. doi: 10.1097/AOG.0000000000003357. [DOI] [PubMed] [Google Scholar]
- 4.Fix L, Seymour JW, Sandhu MV, Melville C, Mazza D, Thompson TA. At-home telemedicine for medical abortion in Australia: a qualitative study of patient experiences and recommendations. BMJ Sex Reprod Health. 2020;46:172–176. doi: 10.1136/bmjsrh-2020-200612. [DOI] [PubMed] [Google Scholar]
- 5.Ehrenreich K, Kaller S, Raifman S, Grossman D. Women's experiences using telemedicine to attend abortion information visits in Utah: A qualitative study. Women's. Health Issues. 2019;29(5):407–413. doi: 10.1016/j.whi.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Raymond E, Chong E, Winikoff B, Platais I, Mary M, Lotarevich T, et al. TelAbortion: evaluation of a direct to patient telemedicine abortion service in the United States. Contraception. 2019;100(3):173–177. doi: 10.1016/j.contraception.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Grossman DA, Grindlay K, Buchacker T, Lane K, Blanchard K. Changes in service delivery patterns after introduction of telemedicine provision of medical abortion in Iowa. Am J Public Health. 2013;103(1):73–78. doi: 10.2105/AJPH.2012.301097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohn JE, Snow JL, Grossman D, Thompson TA, Seymour JW, Simons HR. Introduction of telemedicine for medication abortion: changes in service delivery patterns in two U.S. states. Contraception. 2020 doi: 10.1016/j.contraception.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Jones RK, Witwer E, Jerman J. Guttmacher Institute; New York: 2017. Abortion Incidence and Service Availability the United States. 2019https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. [DOI] [Google Scholar]
- 10.National Abortion Federation . 2021. NAF 2019 violence and disruption statistics. [Google Scholar]; https://5aa1b2xfmfh2e2mk03kk8rsx-wpengine.netdna-ssl.com/wp-content/uploads/NAF-2019-Violence-and-Disruption-Stats-Final.pdf [accessed 19 January].
- 11.Bateson DJ, Lohr PA, Norman WV, Moreau C, Gemzell-Danielsson K, Blumenthal PD, et al. The impact of COVID-19 on contraception and abortion care policy and practice: experiences from selected countries. BMJ Sex Reprod Health. 2020;46:241–243. doi: 10.1136/bmjsrh-2020-200709. [DOI] [PubMed] [Google Scholar]
- 12.Aiken ARA, Starling JE, Gomperts R, Tec M, Scott JG, Aiken CE. Demand for self-managed online telemedicine abortion in the United States during the Coronavirus Disease 2019 (COVID-19) pandemic. Obstet Gynecol. 2020;136(4):835–837. doi: 10.1097/AOG.0000000000004081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Upadhyay UD, Schroeder R, Roberts SCM. Adoption of no-test and telehealth medication abortion care among independent abortion providers in response to COVID-19. Contracept X 2020 Nov 21;2:100049. doi: 10.1016/j.conx.2020.100049. [DOI] [PMC free article] [PubMed]
- 14.Raymond EG, Grossman D, Mark A, Upadhyay UD, Dean Gillian, Creinin MD, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101(6):361–366. doi: 10.1016/j.contraception.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mark A, Foster AM, Grossman D, Prager SW, Reeves M, Velasquez CV, et al. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation’s Clinical Policies Committee. Contraception. 2019;99:265–266. doi: 10.1016/j.contraception.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 16.The Executive Office of the President. Declaring a National Emergency Concerning the Novel Coronavirus Disease (COVID-19) Outbreak, https://www.federalregister.gov/documents/2020/03/18/2020-05794/declaring-a-national-emergency-concerning-the-novel-coronavirus-disease-covid-19-outbreak [accessed 29 Jan 2021 ].
- 17.Food and Drug Administration. Conduct of clinical trials of medical products during the COVID-19 public health emergency, https://www.fda.gov/media/136238/download [accessed 31 Jan 2021].
- 18.Chen MJ, Creinin MD. Mifepristone with buccal misoprostol for medical abortion: a systematic review. Obstet Gynecol. 2015;126:12–21. doi: 10.1097/AOG.0000000000000897. [DOI] [PubMed] [Google Scholar]
- 19.Endler M, Beets L, Gemzell Danielsson K, Gomperts R. Safety and acceptability of medical abortion through telemedicine after 9 weeks of gestation: a population-based cohort study. BJOG. 2019;126:609–618. doi: 10.1111/1471-0528.15553. [DOI] [PubMed] [Google Scholar]
- 20.Raymond EG, Tan YL, Comendant R, Sagaidac I, Hodorogea S, Grant M, et al. Simplified medical abortion screening: a demonstration project. Contraception. 2018;97:292–296. doi: 10.1016/j.contraception.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration. Current and resolved drug shortages and discontinuations reported to FDA. https://www.accessdata.fda.gov/scripts/drugshortages/default.cfm [accessed 26 February 2021].
- 22.Guttmacher Institute. Medication abortion: state laws and policies. https://www.guttmacher.org/state-policy/explore/medication-abortion [accessed 19 January 2021 ].
- 23.Jerman J, Jones RK, Onda T. Guttmacher Institute; New York: 2016. Characteristics of U.S. abortion patients in 2014 and changes since 2008. [Google Scholar]