Abstract
The evolving field of the microbiome and microbiota has become a popular research topic. The human microbiome is defined as a new organ and is considered a living community of commensal, symbiotic and pathogenic microorganisms within a certain body space. The term ‘microbiome’ is used to define the entire genome of the microbiota. Bacteria, archaea, fungi, algae and small protists are all members of the microbiota, followed by phages, viruses, plasmids and mobile genetic elements. The composition, heterogeneity and dynamics of microbiomes in time and space, their stability and resistance, essential characteristics and key participants, as well as interactions within the microbiome and with the host, are crucial lines of investigation for the development of successful future diagnostics and therapies. Standardization of microbiome studies and harmonized comparable methodologies are required for the transfer of knowledge from fundamental science into the clinic. Human health is dependent on microbiomes and achieved by nurturing beneficial resident microorganisms and their interplay with the host. The present study reviewed scientific knowledge on the major components of the human respiratory microbiome, i.e. bacteria, viruses and fungi, their symbiotic and parasitic roles, and, also, major diseases of the human respiratory tract and their microbial etiology. Bidirectional relationships regulate microbial ecosystems and host susceptibility. Moreover, environmental insults render host tissues and microbiota disease-prone. The human respiratory microbiome reflects the ambient air microbiome. By understanding the human respiratory microbiome, potential therapeutic strategies may be proposed.
Keywords: respiratory microbiome, respiratory mycobiome, respiratory virome, respiratory bacteriome
1. Introduction
A human microbiome is a group of communities of symbiotic and pathogenic microorganisms' genomes within certain body regions. Bacteria, archaea, fungi, algae, small protists, phages, viruses, and some genetic elements (plasmids) should be considered a part of it, i.e. all non-human material. The term ‘microbiota’ defines living microorganisms at a certain location. There are many distinct microbial ecosystems residing outside and inside the human body. Gastrointestinal microbiota is the most complex and most populated microbiota containing 100 trillion microorganisms (1). The Human Microbiome Project in 2007 revealed new data that was not accessible by older methods. DNA sequencing methods, including 16S ribosomal RNA sequencing, metagenomic sequencing, and microbial metatranscriptomics changed our understanding of human microbial communities entirely. The dogma of sterile lungs was deconstructed and the microbiome in the lungs was revealed (2). The maintenance of microbial homeostasis is recognized as an important health factor and its susceptibility to environmental factors, like antibiotics, diet, alcohol, and smoking, is intensively studied (3). We will hereby summarize the current understanding of the microbiome-environment interactions, and the involvement of microbiota in the pathogenesis of respiratory diseases. We will also underscore some important future directions. The field of human microbiomes and their clinical significance is developing swiftly. An increasingly growing toolkit to investigate microbiomes and their relationships with host systems delivers new data continuously which needs to be reviewed again and again.
Microbial factors play a crucial role in the development of human airways and lungs. Microorganisms determine immune tolerance by recruiting immune cells with a regulatory capacity and directly modulating the structural integrity of the respiratory tissues and remodeling in the diseased lungs (4,5). Furthermore, the development of immune tissues, like mucosa-associated lymphoid tissue in the nose 55 and nasopharynx, is orchestrated by microbial ecosystems (6). Viruses, bacteria, fungi, and protozoa co-reside within specific niches created by structural and immune cells, microbial symbionts, and spatial and biochemical factors, where microbial cooperation is balanced by host systems. Symbiosis may manifest in mutualistic, commensal, or antagonistic relationships.
Already present prenatally respiratory microbiome is further affected by birthing style, breastfeeding, childhood antibiotic and antipyretic treatments, lifestyle, and many other key factors, like living and crowding conditions, presence of siblings, owning furry pets, etc.
With the age, the upper respiratory tract is gradually and transiently colonized primarily by micro-aspiration of typical resident bacterial, viral and fungal assemblages with their distinct topography. Some direct dispersal via mucosa was also demonstrated (7). In healthy subjects, microbial ecosystems limit pathogens preventing their overgrowth and spread (8). Microbial ecosystems might be defined by characteristics, such as healthy, infection-prone, pro-inflammatory, or others. The knowledge behind these definitions is not yet sufficient.
The upper respiratory tract is the primary source of the lower tract microbiome, i.e. lower airways and lungs are seeded via the aspiration (9). The upper respiratory tract microbiome is replenished from the environmental microorganisms' populations and oral microflora (10). The microbiome in the lower airways and lungs consists of transient microorganisms and its composition is determined by the balance between microbial immigration and elimination. New-generation sequencing enables detailed characterization of previously unknown microbiomes, i.e., their bacterial, viral, and fungal content (11). The size of the respiratory fungal community, i.e. mycobiome, may comprise several percent of the total microbiome as it does in the skin and gut (12). A bacterial portion of the respiratory microbiome is the overwhelmingly dominating (13) and consists of varying proportions of Firmicutes, Actinobacteria, Bacteroidetes, Proteobacteria, and Fusobacteria representatives. Virome represents a relatively small portion of the microbiome and differs in healthy and inflammatory states. Bacteriophages comprise the majority of the virome and modulate human respiratory health via bacterial hosts. Complex inter-microbial and microbial-host interactions ensure competence and maintenance clues to the immune and respiratory systems. Accumulating data demonstrate a possibility to improve that early in life when microbiota signaling is essential for immune maturation and further respiratory health. Ways to improve human respiratory microbiota and enhance respiratory health are among the major interests of scientists and clinicians worldwide.
Environmental factors and respiratory microbiomes
Environmental elements conditioning human microbiota, include air pollution, tobacco smoking, exposure to secondhand smoke, and others. Smoking modifies human microbiomes paving the way for diseases, like periodontitis, asthma, chronic obstructive pulmonary disease (COPD), cancer, etc. Effects produced by smoking include a direct influx of bacteria from the cigarettes, impairment of the host's immune responses, and changes in oxidative and proteolytic balance in the airways and alveoli. Indeed, oxygen deprivation is an important factor for microaerophilic and anaerobic bacteria enabling their dominance in the airways of smokers (14). Moreover, the smoking-induced effects might be correlated to COPD patients' microbiota (2). Cigarettes harbor microorganisms ranging from soil microorganisms and commensals to potential human pathogens, capable of causing pneumonia, bacteremia, and other infections (15).
Tobacco smoking is among the major factors modulating human airway microbiota composition and participating in disease development. Similarly, air pollution affects microbiomes via chemical and physical effects and also via the influx of airborne microorganisms. Particulate matter (PM)-associated microbiome is dominated by bacteria, belonging to Actinobacteria and Proteobacteria phyla. PM-bound viruses spike in January and February (16). The level of pollution does not correlate with the number of PM-associated microorganisms, but the ambient temperature does. Seasonal and indoor/outdoor variations are also described (17). The most prevalent microorganism is Proteobacteria, followed by Bacteroidetes, Actinobacteria, Cyanobacteria, and Firmicutes (18). Human pathogens were found in more than 10% of PM samples. In addition, PM levels might be an important factor in the spread of respiratory viruses. For instance, among PM collected in Bergamo, Italy, during the coronavirus outbreak more than half of the samples carried SARS-CoV-2(19). Seasonal PM microbial characteristics were studied by Italian scientists and winter microbiota was dominated by the spore-forming bacteria, while summer microbial composition differed and plant-associated bacteria dominated (20). In addition, the PM-associated microbiome composition correlates with the human upper airway microbiome (21). Thus, the ambient air microbiome serves as a reservoir to replenish the human airway microbiome. Importantly, direct effects of air pollution on the human airway microbiome include a decrease in Actinobacteria, which is known to be associated with a healthy microbiome, and an increase in Moraxella, which is a known respiratory pathogen (22). However, more studies are needed to elucidate the exact effects of air pollution on the human microbiomes of the exposed airways and lungs.
2. Bacteriome part of the respiratory microbiome
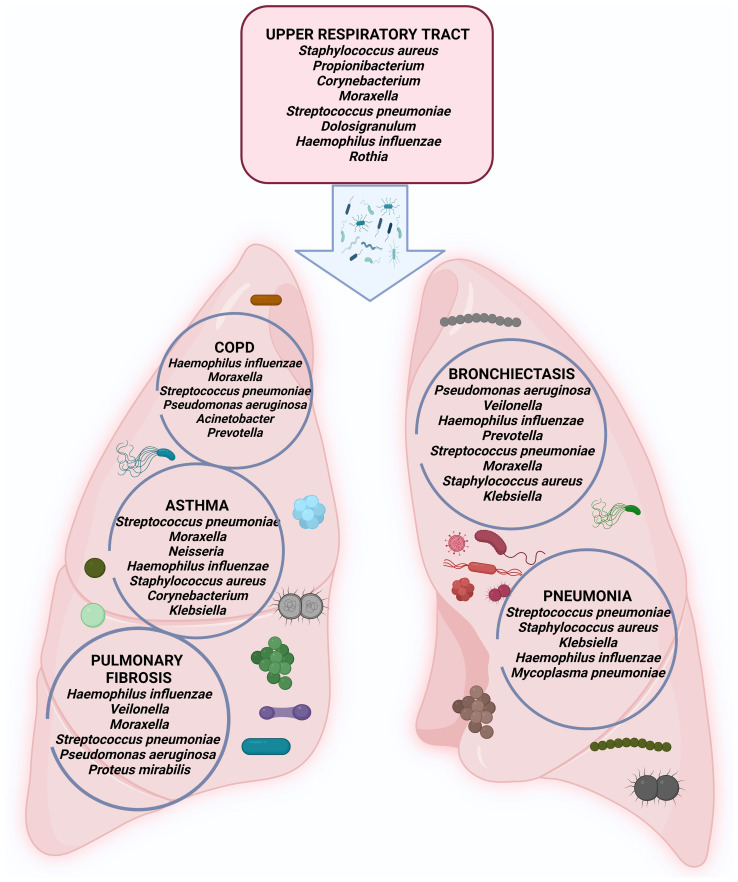
Alterations of lung bacterial communities have been associated with numerous lung diseases including COPD, bronchial asthma (BA), bronchiectasis (BE), and others. Bacteria dominate fungi and viruses with four phyla (Firmicutes, Proteobacteria, Bacteroidetes, and Actinobacteria) making 97% of all sequences. Homeostasis of respiratory tract-resident bacterial communities depends on microbial immigration, mucosal dispersion, and elimination. Patients with respiratory diseases often have defective airway clearance, bacterial biofilms formed and resistant microbial communities persisting through immune clearance and antibiotics. Major players of the human respiratory bacteriome are represented in Fig. 1.
Figure 1.
Major bacteria that cause diseases throughout the human respiratory tract.
Bacteriome and COPD
COPD is an umbrella term for several irreversible chronic inflammatory diseases affecting airways and parenchyma. Data show that the airway microbiome is implicated in COPD manifestation, severity, and long-term prognosis. The microbiome-based approaches for therapeutic interventions may be needed.
The abundance and diversity of microbiota differ in COPD and correlate with the disease severity (23). Moreover, exacerbations, persistent inflammation, and antibiotic/steroid treatments impact the composition of lung microbiota further. Multiple studies report decreased microbial diversity and colonization by Haemophilus influenzae, beta-lactamase-positive Haemophilus influenza, Moraxella catarrhalis, and Streptococcus pneumonia in COPD and Pseudomonas aeruginosa-in severe COPD (24).
Lung bacterial composition is associated with respiratory function as demonstrated in the BALF study of never-smokers and smokers with or without COPD. Severely decreased respiratory function was associated with Veillonella, Prevotella, and Streptococcus increase. However, such an association between bacterial content and respiratory function but not the disease itself supports the idea that lung bacteriome shifts are part of the pathogenesis of many diseases, not only COPD (25). In a sputum study Proteobacteria, H. influenzae, M. catarrhalis and P. aeruginosa together were named important players for COPD exacerbations, and cooperation of the resident microorganisms was demonstrated leading to enrichment for specific taxa (26). In lung explants, many separate, distinct, and individual microbial communities were mapped (27). COPD bacteriome varies not only spatially but also in time. Many studies describe transitory microbiota changes during exacerbations. For instance, stable COPD sputum microbiota differs from exacerbated COPD (28). In COPD patients on ventilatory support and antibiotics, bacterial communities differ significantly and changes are treatment duration-dependent (29). In sum, some reports support the existence of a distinct COPD microbiome while others show overlapping healthy, smokers', and COPD microbiomes. It may be explained by the heterogeneity of COPD, different study designs, and small group sizes.
Bacteriome in bronchial asthma
Bronchial asthma is a heterogeneous chronic inflammatory airway disease characterized by bronchial hyperactivity and obstruction. It is increasingly accepted that microbiota plays an important role in disease etiology and development. Decreased bacterial diversity is detected in BA and correlates with the disease severity (30). Certain bacteria might be associated with bronchial hyperactivity and obstruction, i.e., Proteobacteria, Firmicutes (Streptococci), and Gammaproteobacteria (31). Respiratory commensals Veillonella and Prevotella were less prevalent. Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis have been found in the lower and upper respiratory tract of children during asthma crises (32).
Although respiratory infections may not be the crucial triggers in BA, specific bacterial-host interactions may significantly increase the risk of BA exacerbations (33). Moreover, the differences in nasal bacteriome may serve as indicators of different BA phenotypes (34). Furthermore, the risk to develop BA is microbiome-related according to the pediatric study (35). Further microbiome studies of BA patients are needed to open new insights, suggest microbiome-based biomarkers of the disease, and design better treatment options.
Bacteriome in bronchiectasis
Bronchiectasis (BE) is a disease of permanent abnormal bronchial dilation, infection, and inflammation. P. aeruginosa and H. influenzae are the most common pathogens in BE (36). Pseudomonas dominant patients have poor clinical outcomes, worse lung function, and require recurrent antibiotic treatments (37). Other important bacteria are Prevotella spp., Str. pneumoniae, nontuberculous mycobacteria, Moraxella catarrhalis, S. aureus, Escherichia, and Klebsiella spp. (38). Culture-independent methods corroborated the findings of traditional culture-based methods. Interestingly, the frequency of bacteria varies geographically, H. influenza and P. aeruginosa are more prevalent in Europe and Asia, while Mycobacterium avium, M. abscessus, M. chelonae, and P. aeruginosa are more common in the US (39,40).
It is known that BE exacerbations occur in less complex microbial co-occurrence networks, and reduced diversity, where a higher degree of antagonism exists. Microbial antagonism deepens during exacerbations and interactions, but not the abundance of certain microbial groups defines the risk of exacerbations (41).
However, BE microbiome is little understood and requires further studies to clarify the development and progression of the disease.
Bacteriome and pulmonary fibrosis
Pulmonary fibrosis (PF) is a progressive alveolar fibrosis with inflammatory infiltration. Genetics, autoimmunity, certain medicines, toxic agents, and ionizing radiation contribute to PF development. The importance of respiratory microbiome is among the risk factors for PF. Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pneumoniae, Haemophilus parainfluenza, Pseudomonas aeruginosa, and Proteus mirabilis were detected in PF patients (42). Microbiome findings are insufficient to explain the pathogenesis of the disease but might serve as a source of the disease biomarkers. When compared to other diseases, bacterial abundance in PF patients was significantly higher than in COPD or healthy controls (43). Exacerbated PF was related to a four-fold higher bacterial burden, with an increase in Campylobacter and Stenotrophomonas and a decrease in Veillonella (44).
Microbiota and fibrosis have a bidirectional relationship, i.e. fibrosis impairs microbial clearance and blunts innate immune responses (45), while bacterial products directly promote fibrosis. For instance, staphylococci release a peptide corisin and induce apoptosis of lung epithelial cells. PF is detected in the lungs of corisin-exposed mice. Moreover, lung corisin levels are significantly increased in PF patients with acute exacerbation compared to stable PF (46). Such dual interrelationships should be considered when designing treatment strategies. Equally important is to consider microbiome composition and richness as a reflection of the fibrotic state of the host lung tissue and a possibility to target the microbiome for the diagnostics and management of the disease.
Bacteriome and acute respiratory infections and pneumonia
Upper airways are the first line of defense constantly exposed to various environmental and host factors which contribute to, shape, damage, and replenish airway microbiota. Specific local conditions, like secretions, mucus flow, and air circulation, are important determinants of bacterial colonization. Bacteria reside in patch-type populations and are dependent on migration, dispersal, colonization, extinction, and transition of the incoming and resident bacteria, e.g. the ability of S. aureus to invade populations dominated by Str. pneumoniae is limited, while other species may invade (47). Disease states in the upper airways correlate with microbiome shifts, especially it's virome part. However, bacteriome remains an important factor in all infectious and noninfectious diseases of the upper airways and other organs.
Pneumonia is an acute respiratory infection of the alveoli and distal airways and remains a major cause of high morbidity and mortality worldwide. Various microorganisms can cause pneumonia, including bacteria, viruses, and fungi, also members of healthy lung microbiota. Pneumonia manifests with the rapid shift from a healthy microbiome to dysbiosis where low diversity, high pathogen burden, and inflammation of host tissues dominate. Pathogenetic mechanisms behind this shift include environmental exposure, like tobacco smoke or air pollution, mechanical ventilation, direct and indirect interactions between microorganisms, etc. Pneumonia appears as the major complication of the dysbiosis of the lungs. Culture-independent detection methods have proved that a healthy lower respiratory tract accommodates various microbes without induction of any symptoms of infection.
Nasopharyngeal samples, collected during the infection episodes, show the presence of Moraxella, Haemophilus and Streptococcus, Corynebacterium, Dolosigranulum, Staphylococcus, Acinetobacter, Pseudomonas, and Bifidobacterium (48). Bacteriome's role in disease onset, progression, and therapeutic response is undisputed although the major pathogens are viruses. New studies have shown the connection between the lung microbiome and the pathogenesis of pneumonia. Typical pneumonia pathogens include Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, Haemophilus influenzae, also Mycoplasma pneumoniae, Legionella pneumophilia, and Chlamydophila psittaci. The potential pathogens often are residents within the microbial ecosystem in the lower airways. Certain combinations might be associated with more severe pneumonia or oppositely, disease resistance. For instance, the Prevotella-rich microbiome supports inflammation and increases mortality, while Pseudomonas species support disease-resistant lung microbiome and suppression of pathogens (49).
The upper airways' microbiome replenishes the microbiome of the lower airways and has direct implications for pneumonia. Pneumonia was not only associated with dysbiosis in the nose but also with bacterial overgrowth of single species and the absence of distinct anaerobic bacteria. Also, less rich microbiomes might be associated with the susceptibility to inflammation. It is becoming increasingly clear that pneumonia is a multifactorial disease where pathogenic bacteria are only partially responsible, and the entire human microbiome and host factors are also implicated.
3. Human respiratory mycobiome
Culture-independent detection methods prove that human airways and lungs contain fungi as an integral part of their microbiomes. Mycobiome is a diverse array of fungal species residing within a specific body space. Inhalation of spores and complex interaction with the bacterial and host systems are determining factors for fungal entrance, dispersion, and growth in the airways and lungs (50). Fungi contain so-called pathogen-associated molecular patterns, like glucans, chitin, and mannans, able to trigger immune responses in the respiratory epithelium (51). Fungal overgrowth in the human airways has been linked to many chronic diseases (52). Fungal communities also have systemic effects executed via biologically active molecules (53).
Studies of the inter-kingdom relationships between bacteriomes and mycobiomes show that Aspergillus and Malassezia might be associated with exacerbations of cystic fibrosis (CF), while Scedosporium and Pseudomonas- with the decline of lung function (50). In addition, simultaneous assessment of mycobiome and bacteriome revealed fungus-to-bacteria diversity 10 times higher in lungs in comparison to the gut, supporting the notion that lung mycobiome is replenished differently, most likely via spore inhalation. The complex interaction between members of different kingdoms is realized via cross-feeding, specialized metabolites, and other mechanisms.
In CF Aspergillus fumigatus colonization correlates with lower lung capacity, more frequent hospitalizations, and more prominent radiological abnormalities (54). Indeed, fungal complications in CF patients are mostly caused by filamentous fungi. Mycobiome is an important etiological component not only in CF but also in other pulmonary diseases, like COPD. Implications of the fungi in COPD development were studied in patients from Singapore, Malaysia, and Scotland (55). The airway mycobiome in stable COPD was diverse and dependent on geography. Distinct genera, i.e. Alternaria, Aspergillus, Cladosporium, Cryptococcus, Mycosphaerella, Penicillium, Trametes, and Wickerhamomyces, were found in COPD lungs but not in the healthy lungs. Importantly, no differences between mycobiome profiles were detected between patients with COPD and patients with BE and COPD overlap. Clustering analysis revealed that increased exacerbation rate and higher mortality might be characterized by microbiome enrichment with Aspergillus, Penicillium, and Curvularia genera. In these patients systemic specific-IgE responses to the fungi were detectable. In addition, loss of fungal diversity was associated with increased two-year mortality in COPD.
Inter-microbiome relationships define fungal presence, e.g., human intestinal microbiome data demonstrate that affecting bacterial communities will significantly impact fungal species and vice versa (56). Similar relationships were detected in other microbiomes suggesting that respiratory microbiomes are not an exception. Disruption of airway bacterial-fungal balance, characterized by the loss of commensal bacterial taxa and by the enrichment of pathogenic fungal taxa, is implicated in COPD, i.e., Prevotella and Veillonella exhibit inverse relationships with pathogenic fungal taxa such as Candida palmioleophila and Aspergillus spp. (57). In a respiratory tract mycobiome study of HIV patients with and without COPD, oral washes, sputum, and BALF were analyzed, and 39 fungal species were more abundant in the BALF and 203 species-in the sputum, proving species-specific distribution. The primary fungus enriched in the lungs of individuals with HIV and COPD was Pneumocystis.
The role of the mycobiome in BE was investigated and Aspergillus, Cryptococcus, Clavispora, Botrytis, and Alternaria genera were identified (58). In the other study, healthy individuals had no detectable airway Aspergillus, while high proportions of BE patients had detectable A. fumigatus and/or A. terreus. Indeed, A. fumigatus followed by Aspergillus niger, Aspergillus terreus, and Aspergillus flavus is the most common in BE (59).
In sum, fungal genera within respiratory microbiomes are low in diversity, individually shaped, linked to gut mycobiome, and play a significant role in the decline of lung functions and disease progression.
4. Human respiratory virome
The previous view of viruses being obligate pathogens is changed. The human virome is a part of the microbiome and includes pathogenic viruses, resident viruses, bacteriophages, and retroviral elements. Novel detection methods revealed viral genetic diversity and new viruses present in humans. The prevalence of respiratory viruses among asymptomatic children is documented, including rhinoviruses, bocaviruses, and coronaviruses (60). However, the roles of these viruses in asymptomatic individuals are still unclear. Bacteriophages make a major part of the virome. They intervene in human health by regulating the prevalence of bacteria.
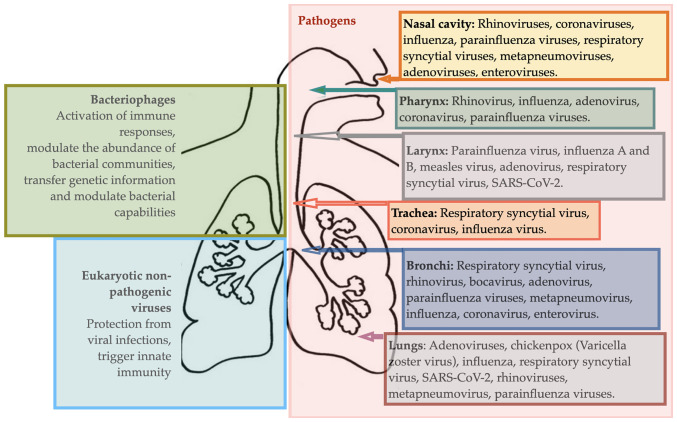
Human airways and lungs are the major sources of viral infection-related mortality worldwide. The recent coronavirus-caused disease 2019 (COVID-19) outbreak was a unique pandemic due to the combination of a high reproducibility, super-spreading, and global immunologically naïve population. It has led to the highest global number of deaths in the past 20 decades compared to any other pandemic. An overview of respiratory viral diseases is provided in Fig. 2.
Figure 2.
Viral life forms of the human respiratory tract.
Viral infections of upper airways
High morbidity and mortality associated with viral infections are partially related to complicated diagnostics since viral infections often do not produce detectable lesions. Novel detection techniques are needed. Moreover, some viral infections can cause a cascade of destructive processes and swiftly disappear, like influenza. At the host level, viral infections affect the epithelium, phagocytes, T lymphocytes, and macrophages, support adherence of bacteria to the epithelium, up-regulate inflammatory mediators and change the extracellular environment and microbiota. The impact of viruses on the development of noninfectious, chronic pulmonary diseases such as BA and COPD is also well-known (61).
Viral infections of the upper respiratory tract are a group of diseases mainly caused by viruses entering the organism via inhalation of droplets, later invading the mucosa, and damaging epithelium in the upper airways.
The common cold is the most common disease with more than 200 different viruses, differently active during summer and wintertime, as causative factors. Rhinoviruses are the most common pathogens. Other pathogens include coronaviruses, influenza, parainfluenza, respiratory syncytial viruses, and many others (62). People with weakened immune systems and respiratory or cardiac diseases are at higher risk of developing severe viral infections and complications. The role of microbiota in common cold is dual, i.e., invading viruses may initiate secondary local bacteria-caused infections, while local dysbiosis could cause impairment of epithelium and predispose an individual to the viral infection. Interventions targeting microbiota are among preventative and treatment strategies for combating common colds. Exposure to environmental (pollution, cold, dry air), microbial and other factors, such as medications (antibiotics and similar), damages airway microbiota and is associated with more prevalent and more severe common colds. It is becoming clear that viral infections may be prevented or ameliorated via supporting resilient local microbiomes (48). Knowledge of microbiome resiliency, microbial competition, and interactions within the microbiota are needed to develop probiotic solutions.
Sinusitis is an infectious inflammation within the sinuses that become inflamed and blocked by mucus. Rhinoviruses, influenza, and parainfluenza viruses are the most common causes of sinusitis (63). Microbiota's role in the onset and development of sinusitis has recently gained attention. Sinus and nasal microbiota is a source of pathogens but also serve as a protection against infection. In health, bacteria, including S. aureus, Str. epidermidis and Corynebacterium genera, reside in sinuses. Disruptions in this balance, like an increase in S. aureus concentration, may perpetuate inflammatory changes within the nasal mucosa, leading to sinusitis.
Human respiratory microbiome and Coronavirus-caused Disease 2019
In the course of COVID-19 microbiota contributes directly. It is known that dysbiosis leads to epithelial cell loss, an increase in permeability, and inflammation, thus to the increasing levels of angiotensin-converting enzyme 2 (ACE2), the target of coronavirus (64). Moreover, dysbiosis may trigger an increase in circulating inflammatory mediators (65) and self-perpetuating pro-inflammatory cytokine production, i.e. cytokine storm. Reduced microbiome diversity may be regarded as a predictive biomarker of COVID-19 severity (66). Respiratory microbiota has a distinct role in COVID-19 course and severity also due to bacterial co-infections often arising from resident bacterial communities (67). Study authors have explored the microbiome of COVID-19 patients and demonstrated gut microbiome of COVID-19 patients has a significant reduction of bacterial diversity and a significantly higher relative abundance of opportunistic pathogens and less beneficial symbionts as compared to the control group. Several gut commensals with known modulatory potential, like Faecalibacterium prausnitzii, Eubacterium rectale, and bifidobacteria, were underrepresented in COVID-19 patients and remained low up to 30 days after COVID-19 resolution. Additionally, butyrate-producing bacteria such as Faecalibacterium prausnitzii, Clostridium butyricum, Clostridium leptum, and Eubacterium were less abundant. Moreover, depletion of commensals, like Eubacterium ventriosum, Faecalibacterium prausnitzii, and others, correlated with COVID-19 severity. The respiratory microbiome of COVID-19 patients was studied in bronchoalveolar lavage fluid (BALF) and compared to pneumonia patients and healthy controls. Both, COVID-19 and community-acquired pneumonia patients had enrichment of pathogenic and commensal bacteria, indicating a significant dysbiosis (68). The post-mortem biopsies exhibited mixed bacterial and fungal infections complicating COVID-19(69).
Overall, further studies with a bigger sample size are required to clarify the composition and the role of the respiratory microbiome in the severity of coronavirus infections.
Viral infections of the lower airways
Viral infections of the lower airways include laryngotracheitis, bronchitis, bronchiolitis, and pneumonia which are all more common in children. Often infections involve both the upper and lower respiratory tract. Respiratory infections in the lower airways and lungs may be caused by more than 200 types of viruses.
Healthy lower airways are inhabited by specific microbial ecosystems. They are accessed and investigated using modern molecular techniques combined with bronchoscopy, biopsies, post-mortem tissue analysis, and some others, although precise detection techniques ensuring no compromising, overlapping, or contamination are still lacking.
Viral lower airway infections manifest with cough and difficulty breathing. Parainfluenza virus accounts for more than 75% of these infections (70) and the cause of the disease is a virus in 90% of all cases (71). In addition, distinct dysbiotic signatures are identified, viral infection-prone microbiomes are described and metatranscriptome data reveals an association between disease severity and microbiome (72).
Pneumonia is an infectious inflammatory condition affecting lung parenchyma and impairing its major function, i.e. gas exchange. The main causative agents of pneumonia vary, i.e. may include bacteria, fungi, parasites, and viruses. Community-acquired pneumonia remains a major cause of morbidity and mortality worldwide. Viral pathogens are increasingly often implicated due to pneumococcal vaccination programs, sensitive diagnostic tests, and other factors. Non-influenza viral pathogens include rhinovirus, metapneumovirus, respiratory syncytial virus, parainfluenza virus, and adenoviruses.
Chronic non-infectious respiratory diseases and viruses
In chronic inflammatory respiratory diseases, like BA, respiratory viruses are among the major pathogenetic factors of exacerbation and progression. Viral infections were often under-recognized as causes of exacerbations due to the low detection rates (73). Applying molecular detection techniques viruses were detected in nearly 40% of COPD exacerbations (74) and nearly 60% of the CF exacerbations (75). The progression of chronic pulmonary disease and it's exacerbations are increasingly linked to respiratory viruses nowadays mainly due to modern diagnostic techniques. Host responses in these diseases are imbalanced and microbiomes are often shifted. Understanding microbiota and host interplay in chronic pulmonary diseases stimulate the development of new therapies for virus-related exacerbations.
5. Concluding remarks
It is still not clear whether the respiratory disease state might be estimated based on microbiome biomarkers. However, in some diseases, like bronchiectasis, microbiome analysis may be used as a diagnostic tool. Translation of human airway microbiome-associated extensive research data and experience into the clinical practice and introduction of a diagnostic ‘microbiome test’ is imminent. We predict a substantial improvement in the diagnostics and management of certain diseases, like COPD, when airway and lung microbiome data will be made readily available to clinicians and patients.
Two directional relationships are observed between microbial ecosystems and host susceptibility to infectious and other diseases, i.e. microbiomes may serve as the source of infection-causing pathogens, and at the same time invading pathogens modulate the host's microbial communities for long periods of time.
As discussed above, the environmental insults predispose not only hosts' tissues but also human microbiota to the disease development by changing microbial composition and also a state of the host's immunity, epithelial integrity, and other factors. We believe that improvements to the human respiratory microbiome, i.e. replenishing and promoting certain species, are possible by exposing humans to rich and healthy ambient air microbiomes (e.g. forest microbiome) and may become a central therapeutic strategy for some diseases.
Although bacteriome, virome, and mycobiome are important components of respiratory microbiomes and play role in the pathogenesis of many diseases, they are differentially weighted, and certain players, like viruses or filamentous fungi, may be regarded as major pathogens in certain diseases.
Acknowledgements
Not applicable.
Funding Statement
Funding: This work was supported by a grant (grant no. #01.2.2-LMT-K-718-03-0079) from the Lithuanian Research Council.
Availability of data and materials
Not applicable.
Authors' contributions
JR drafted the bacteriome part of the review and Fig. 1. DB drafted the virome part of the review and Fig. 2. EB drafted the mycobiome part of the manuscript. IK drafted the introductory part of the review. BJ and ED edited and revised the manuscript. RA was a major contributor in compiling and editing the entire manuscript and figures. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. Human Microbiome Project Consortium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dy R, Sethi S. The lung microbiome and exacerbations of COPD. Curr Opin Pulm Med. 2016;22:196–202. doi: 10.1097/MCP.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 3.Owyang C, Wu GD. The gut microbiome in health and disease. Gastroenterology. 2014;146:1433–1436. doi: 10.1053/j.gastro.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 4.Zheng J, Wu Q, Zou Y, Wang M, He L, Guo S. Respiratory microbiota profiles associated with the progression from airway inflammation to remodeling in mice with OVA-induced asthma. Front Microbiol. 2021;12(723152) doi: 10.3389/fmicb.2021.723152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011;10:311–323. doi: 10.1016/j.chom.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuyama S, Hiroi T, Yokota Y, Rennert PD, Yanagita M, Kinoshita N, Terawaki S, Shikina T, Yamamoto M, Kurono Y, Kiyono H. Initiation of NALT organogenesis is independent of the IL-7R, LTbetaR, and NIK signaling pathways but requires the Id2 gene and CD3(-)CD4(+)CD45(+) cells. Immunity. 2002;17:31–40. doi: 10.1016/s1074-7613(02)00339-4. [DOI] [PubMed] [Google Scholar]
- 7.Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Falkowski NR, Huffnagle GB, Curtis JL. Bacterial topography of the healthy human lower respiratory tract. mBio. 2017;8:e02287–16. doi: 10.1128/mBio.02287-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Man WH, de Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat Rev Microbiol. 2017;15:259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest. 1997;111:1266–1272. doi: 10.1378/chest.111.5.1266. [DOI] [PubMed] [Google Scholar]
- 10.Segal LN, Alekseyenko AV, Clemente JC, Kulkarni R, Wu B, Gao Z, Chen H, Berger KI, Goldring RM, Rom WN, et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome. 2013;1(19) doi: 10.1186/2049-2618-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eidi S, Kamali SA, Hajari Z, Fata A, Farid Hosseini R, Naseri A, Bakhshaee M. Nasal and indoors fungal contamination in healthy subjects. Health Scope. 2016;5(e30033) [Google Scholar]
- 12.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai W, Wang H, Zhou Q, Li D, Feng X, Yang Z, Wang W, Qiu C, Lu Z, Xu X, et al. An integrated respiratory microbial gene catalogue to better understand the microbial aetiology of Mycoplasma pneumoniae pneumonia. Gigascience. 2019;8(giz093) doi: 10.1093/gigascience/giz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason MR, Preshaw PM, Nagaraja HN, Dabdoub SM, Rahman A, Kumar PS. The subgingival microbiome of clinically healthy current and never smokers. ISME J. 2015;9:268–272. doi: 10.1038/ismej.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sapkota AR, Berger S, Vogel TM. Human pathogens abundant in the bacterial metagenome of cigarettes. Environ Health Perspect. 2010;118:351–356. doi: 10.1289/ehp.0901201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin N, Liang P, Wu C, Wang G, Xu Q, Xiong X, Wang T, Zolfo M, Segata N, Qin H, et al. Longitudinal survey of microbiome associated with particulate matter in a megacity. Genome Biol. 2020;21(55) doi: 10.1186/s13059-020-01964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira EL, Madacussengua O, Baptista P, Feliciano M. Assessment of indoor air quality in geriatric environments of Southwestern Europe. Aerobiologia. 2021;37:139–153. [Google Scholar]
- 18.Dong SR, Han YJ, Wu J, Zeng CL, Zhu KH, Chen XJ, Liu YM, Zou XQ, Zheng SL, Wen ZH, et al. Distribution of microbiota in fine particulate matter particles in Guangzhou, China. Biomed Environ Sci. 2020;33:306–314. doi: 10.3967/bes2020.042. [DOI] [PubMed] [Google Scholar]
- 19.Setti L, Passarini F, De Gennaro G, Barbieri P, Perrone MG, Borelli M, Palmisani J, Di Gilio A, Torboli V, Fontana F, et al. SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: First evidence. Environ Res. 2020;188(109754) doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brodie EL, DeSantis TZ, Parker JP, Zubietta IX, Piceno YM, Andersen GL. Urban aerosols harbor diverse and dynamic bacterial populations. Proc Natl Acad Sci USA. 2007;104:299–304. doi: 10.1073/pnas.0608255104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biswas K, Hoggard M, Jain R, Taylor MW, Douglas RG. The nasal microbiota in health and disease: Variation within and between subjects. Front Microbiol. 2015;9(134) doi: 10.3389/fmicb.2015.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mariani J, Favero C, Spinazzè A, Cavallo DM, Carugno M, Motta V, Bonzini M, Cattaneo A, Pesatori AC, Bollati V. Short-term particulate matter exposure influences nasal microbiota in a population of healthy subjects. Environ Res. 2018;162:119–126. doi: 10.1016/j.envres.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Ghebre MA, Pang PH, Diver S, Desai D, Bafadhel M, Haldar K, Kebadze T, Cohen S, Newbold P, Rapley L, et al. Biological exacerbation clusters demonstrate asthma and chronic obstructive pulmonary disease overlap with distinct mediator and microbiome profiles. J Allergy Clin Immunol. 2018;141:2027–2036.e12. doi: 10.1016/j.jaci.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dima E, Kyriakoudi A, Kaponi M, Vasileiadis I, Stamou P, Koutsoukou A, Koulouris NG, Rovina N. The lung microbiome dynamics between stability and exacerbation in chronic obstructive pulmonary disease (COPD): Current perspectives. Respir Med. 2019;157:1–6. doi: 10.1016/j.rmed.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Opron K, Begley LA, Erb-Downward JR, Freeman C, Madapoosi S, Alexis NE, Barjaktarevic I, Graham Barr R, Bleecker ER, Bowler RP, et al. Lung microbiota associations with clinical features of COPD in the SPIROMICS cohort. NPJ Biofilms Microbiomes. 2021;7(14) doi: 10.1038/s41522-021-00185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YJ, Sethi S, Murphy T, Nariya S, Boushey HA, Lynch SV. Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol. 2014;52:2813–2823. doi: 10.1128/JCM.00035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, Young VB, Toews GB, Curtis JL, Sundaram B, et al. Analysis of the lung microbiome in the ‘healthy’ smoker and in COPD. PLoS One. 2011;6(e16384) doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tangedal S, Nielsen R, Aanerud M, Persson LJ, Wiker HG, Bakke PS, Hiemstra PS, Eagan TM. Sputum microbiota and inflammation at stable state and during exacerbations in a cohort of chronic obstructive pulmonary disease (COPD) patients. PLoS One. 2019;14(e0222449) doi: 10.1371/journal.pone.0222449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang WC, Wu MF, Huang CC, Liu SY, Chen HC, Chen YY, Hsu JY, Huang CC. Dynamics of the lung microbiome in intensive care patients with chronic obstructive pulmonary disease and community-acquired pneumonia. Sci Rep. 2020;10(11046) doi: 10.1038/s41598-020-68100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR, Boushey H. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J Allergy Clin Immunol. 2015;136:874–884. doi: 10.1016/j.jaci.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(e8578) doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, Gangnon RE, Bochkov YA, Jackson DJ, Lemanske RF Jr, Gern JE. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133:1301–1307.e3. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCauley KE, Flynn K, Calatroni A, DiMassa V, LaMere B, Fadrosh DW, Lynch KV, Gill MA, Pongracic JA, Khurana Hershey GK, et al. Seasonal airway microbiome and transcriptome interactions promote childhood asthma exacerbations. J Allergy Clin Immunol. 2022;150:204–213. doi: 10.1016/j.jaci.2022.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Losada M, Authelet KJ, Hoptay CE, Kwak C, Crandall KA, Freishtat RJ. Pediatric asthma comprises different phenotypic clusters with unique nasal microbiotas. Microbiome. 2018;6(179) doi: 10.1186/s40168-018-0564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teo SM, Tang HHF, Mok D, Judd LM, Watts SC, Pham K, Holt BJ, Kusel M, Serralha M, Troy N, et al. Airway microbiota dynamics uncover a critical window for interplay of pathogenic bacteria and allergy in childhood respiratory disease. Cell Host Microbe. 2018;24:341–352.e5. doi: 10.1016/j.chom.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers GB, Zain NM, Bruce KD, Burr LD, Chen AC, Rivett DW, McGuckin MA, Serisier DJ. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Ann Am Thorac Soc. 2014;11:496–503. doi: 10.1513/AnnalsATS.201310-335OC. [DOI] [PubMed] [Google Scholar]
- 37.Araújo D, Shteinberg M, Aliberti S, Goeminne PC, Hill AT, Fardon TC, Obradovic D, Stone G, Trautmann M, Davis A, et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J. 2018;51(1701953) doi: 10.1183/13993003.01953-2017. [DOI] [PubMed] [Google Scholar]
- 38.Amati F, Simonetta E, Gramegna A, Tarsia P, Contarini M, Blasi F, Aliberti S. The biology of pulmonary exacerbations in bronchiectasis. Eur Respir Rev. 2019;28(190055) doi: 10.1183/16000617.0055-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aksamit TR, O'Donnell AE, Barker A, Olivier KN, Winthrop KL, Daniels MLA, Johnson M, Eden E, Griffith D, Knowles M, et al. Adult patients with bronchiectasis: A first look at the US bronchiectasis research registry. Chest. 2017;151:982–992. doi: 10.1016/j.chest.2016.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan WJ, Gao YH, Xu G, Lin ZY, Tang Y, Li HM, Lin ZM, Zheng JP, Chen RC, Zhong NS. Aetiology of bronchiectasis in Guangzhou, Southern China. Respirology. 2015;20:739–748. doi: 10.1111/resp.12528. [DOI] [PubMed] [Google Scholar]
- 41.Mac Aogain M, Narayana JK, Tiew PY, Ali NABM, Yong VFL, Jaggi TK, Lim AYH, Keir HR, Dicker AJ, Thng KX, et al. Integrative microbiomics in bronchiectasis exacerbations. Nat Med. 2021;27:688–699. doi: 10.1038/s41591-021-01289-7. [DOI] [PubMed] [Google Scholar]
- 42.Richter AG, Stockley RA, Harper L, Thickett DR. Pulmonary infection in Wegener granulomatosis and idiopathic pulmonary fibrosis. Thorax. 2009;64:692–697. doi: 10.1136/thx.2008.110445. [DOI] [PubMed] [Google Scholar]
- 43.Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell AM, Murphy E, Johnston SL, Schwartz DA, Wells AU, et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190:906–913. doi: 10.1164/rccm.201403-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molyneaux PL, Cox MJ, Wells AU, Kim HC, Ji W, Cookson WO, Moffatt MF, Kim DS, Maher TM. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir Res. 2017;18(29) doi: 10.1186/s12931-017-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warheit-Niemi HI, Edwards SJ, SenGupta S, Parent CA, Zhou X, O'Dwyer DN, Moore BB. Fibrotic lung disease inhibits immune responses to staphylococcal pneumonia via impaired neutrophil and macrophage function. JCI Insight. 2022;7(e152690) doi: 10.1172/jci.insight.152690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D'Alessandro-Gabazza CN, Kobayashi T, Yasuma T, Toda M, Kim H, Fujimoto H, Hataji O, Takeshita A, Nishihama K, Okano T, et al. A Staphylococcus pro-apoptotic peptide induces acute exacerbation of pulmonary fibrosis. Nat Commun. 2020;11(1539) doi: 10.1038/s41467-020-15344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cremers AJ, Zomer AL, Gritzfeld JF, Ferwerda G, van Hijum SA, Ferreira DM, Shak JR, Klugman KP, Boekhorst J, Timmerman HM, et al. The adult nasopharyngeal microbiome as a determinant of pneumococcal acquisition. Microbiome. 2014;2(44) doi: 10.1186/2049-2618-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chonmaitree T, Jennings K, Golovko G, Khanipov K, Pimenova M, Patel JA, McCormick DP, Loeffelholz MJ, Fofanov Y. Nasopharyngeal microbiota in infants and changes during viral upper respiratory tract infection and acute otitis media. PLoS One. 2017;12(e0180630) doi: 10.1371/journal.pone.0180630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu BG, Segal LN. The lung microbiome and its role in Pneumonia. Clin Chest Med. 2018;39:677–689. doi: 10.1016/j.ccm.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soret P, Vandenborght LE, Francis F, Coron N, Enaud R, Avalos M, Schaeverbeke T, Berger P, Fayon M, Thiebaut R, et al. Respiratory mycobiome and suggestion of inter-Kingdom network during acute pulmonary exacerbation in cystic fibrosis. Sci Rep. 2020;10(3589) doi: 10.1038/s41598-020-60015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tipton L, Ghedin E, Morris A. The lung mycobiome in the next-generation sequencing era. Virulence. 2017;8:334–341. doi: 10.1080/21505594.2016.1235671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weaver D, Gago S, Bromley M, Bowyer P. The human lung mycobiome in chronic respiratory disease: Limitations of methods and our current understanding. Curr Fungal Infect Rep. 2019;13:109–119. [Google Scholar]
- 53.Runge S, Rosshart SP. The mammalian metaorganism: A holistic view on how microbes of all Kingdoms and niches shape local and systemic immunity. Front Immunol. 2021;12(702378) doi: 10.3389/fimmu.2021.702378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Speirs JJ, van der Ent CK, Beekman JM. Effects of Aspergillus fumigatus colonization on lung function in cystic fibrosis. Curr Opin Pulm Med. 2012;18:632–638. doi: 10.1097/MCP.0b013e328358d50b. [DOI] [PubMed] [Google Scholar]
- 55.Tiew PY, Dicker AJ, Keir HR, Poh ME, Pang SL, Mac Aogáin M, Chua BQY, Tan JL, Xu H, Koh MS, et al. A high-risk airway mycobiome is associated with frequent exacerbation and mortality in COPD. Eur Respir J. 2021;57(2002050) doi: 10.1183/13993003.02050-2020. [DOI] [PubMed] [Google Scholar]
- 56.Sovran B, Planchais J, Jegou S, Straube M, Lamas B, Natividad JM, Agus A, Dupraz L, Glodt J, Da Costa G, et al. Enterobacteriaceae are essential for the modulation of colitis severity by fungi. Microbiome. 2018;6(152) doi: 10.1186/s40168-018-0538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu H, Liang Z, Cao N, Tan X, Liu Z, Wang F, Yang Y, Li C, He Y, Su J, et al. Airway bacterial and fungal microbiome in chronic obstructive pulmonary disease. bioRxiv. 2020: 2020.10.05.327536. [Google Scholar]
- 58.Mac Aogáin M, Chandrasekaran R, Lim AYH, Low TB, Tan GL, Hassan T, Ong TH, Hui Qi Ng A, Bertrand D, Koh JY, et al. Immunological corollary of the pulmonary mycobiome in bronchiectasis: The CAMEB study. Eur Respir J. 2018;52(1800766) doi: 10.1183/13993003.00766-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Máiz L, Nieto R, Cantón R, Gómez G, de la Pedrosa E, Martinez-García MÁ. Fungi in Bronchiectasis: A concise review. Int J Mol Sci. 2018;19(142) doi: 10.3390/ijms19010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Camargo CN, Carraro E, Granato CF, Bellei N. Human rhinovirus infections in symptomatic and asymptomatic subjects. Braz J Microbiol. 2012;43:1641–1645. doi: 10.1590/S1517-838220120004000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biancardi E, Fennell M, Rawlinson W, Thomas PS. Viruses are frequently present as the infecting agent in acute exacerbations of chronic obstructive pulmonary disease in patients presenting to hospital. Intern Med J. 2016;46:1160–1165. doi: 10.1111/imj.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: Advice for high-value care from the American college of physicians and the centers for disease control and prevention. Ann Intern Med. 2016;164:425–434. doi: 10.7326/M15-1840. High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. [DOI] [PubMed] [Google Scholar]
- 63.Osur SL. Viral respiratory infections in association with asthma and sinusitis: A review. Ann Allergy Asthma Immunol. 2002;89:553–560. doi: 10.1016/S1081-1206(10)62101-1. [DOI] [PubMed] [Google Scholar]
- 64.Burchill E, Lymberopoulos E, Menozzi E, Budhdeo S, McIlroy JR, Macnaughtan J, Sharma N. The unique impact of COVID-19 on human Gut microbiome research. Front Med (Lausanne) 2021;8(652464) doi: 10.3389/fmed.2021.652464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21:455–466.e4. doi: 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dhar D, Mohanty A. Gut microbiota and Covid-19-possible link and implications. Virus Res. 2020;285(198018) doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chhibber-Goel J, Gopinathan S, Sharma A. Interplay between severities of COVID-19 and the gut microbiome: Implications of bacterial co-infections? Gut Pathog. 2021;13(14) doi: 10.1186/s13099-021-00407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khatiwada S, Subedi A. Lung microbiome and coronavirus disease 2019 (COVID-19): Possible link and implications. Hum Microb J. 2020;17(100073) doi: 10.1016/j.humic.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan J, Li X, Gao Y, Zhou J, Wang S, Huang B, Wu J, Cao Q, Chen Y, Wang Z, et al. The lung tissue microbiota features of 20 deceased patients with COVID-19. J Infect. 2020;81:e64–e67. doi: 10.1016/j.jinf.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson DW. Croup. BMJ Clin Evid. 2014;2014(0321) [PMC free article] [PubMed] [Google Scholar]
- 71.Llor C, Bjerrum L. Antibiotic prescribing for acute bronchitis. Expert Rev Anti Infect Ther. 2016;14:633–642. doi: 10.1080/14787210.2016.1193435. [DOI] [PubMed] [Google Scholar]
- 72.Fujiogi M, Camargo CA Jr, Bernot JP, Freishtat RJ, Harmon B, Mansbach JM, Castro-Nallar E, Perez-Losada M, Hasegawa K. In infants with severe bronchiolitis: Dual-transcriptomic profiling of nasopharyngeal microbiome and host response. Pediatr Res. 2020;88:144–146. doi: 10.1038/s41390-019-0742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greenberg SB. Viral respiratory infections in elderly patients and patients with chronic obstructive pulmonary disease. Dis Mon. 2003;49:201–209. doi: 10.1016/s0002-9343(01)01061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, Maccallum P, Meade TW, Jeffries DJ, Johnston SL, Wedzicha JA. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 75.Wat D. Impact of respiratory viral infections on cystic fibrosis. Postgrad Med J. 2003;79:201–203. doi: 10.1136/pmj.79.930.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.