Abstract
Lipomembranous fat necrosis (LFN) is an uncommon but distinct form of fat necrosis, which is characterized by eosinophilic, crenulated and/or serpiginous membranes. LFN exhibits macrocystic, microcystic and/or crushed features. LFN is routinely detectable on hematoxylin and eosin (H&E)-stained sections, and is present both in the acute phase and in the later or fibrous stage of necrotic fatty lesions. Smaller crushed LFN embedded within fibrous tissues may be difficult to recognize on H&E-stained sections, but can be highlighted by some staining techniques, including Masson trichrome, periodic acid-Schiff, orcein, long Ziehl-Neelsen stain, silver impregnation, phosphotungstic acid-hematoxylin and luxol fast blue staining. LFN was initially considered a specific feature of Nasu-Hakola disease, but has since been identified in various subcutaneous or intraabdominal lesions related to ischemic conditions or venous insufficiency. In addition, LFN is detectable in intra-articular loose bodies and aortic valves with or without dysfunction, suggesting that LFN is also associated with ischemia-like hypoxic conditions or malnutrition. LFN is considered to be a histological hallmark of hidden ischemic or hypoxic/malnourished conditions in various diseases; however, the exact mechanisms of LFN remain poorly understood. The present review described the clinicopathological features of this interesting, but poorly characterized, condition.
Keywords: aortic valve, intraarticular loose body, ischemia, LFN, lipomembranous changes, membranocystic changes, membranous fat necrosis, Nasu-Hakola disease, soft tissue tumor
1. Introduction
White fat cells or adipocytes are distributed throughout the human body (1,2), and damage to or necrotic changes in these cells are frequently encountered in various diseases. In pathology, the term ‘fat necrosis’ refers to intra-abdominal lesions composed of ghostly, slightly basophilic, non-nucleated fat cells, with or without calcification, associated with extravasated, activated pancreatic juice (1,3). The intra-abdominal presence of this fat necrosis, also called enzymic fat necrosis, suggests acute pancreatitis (3-6). Similar enzymic fat necrosis is occasionally observed in the subcutaneous tissues of patients with pancreatic disorders, indicating a possible diagnosis of pancreatic panniculitis (7). Nonspecific necrosis of generalized fat cells evokes an inflammatory reaction, accompanied by epithelioid cells, multinucleated histiocytic giant cells, and lymphocytes, a condition called fat granuloma (1,7). Such necrotic fat cells or lipogranulomatous lesions can be resolved during the relatively early stages of the disease. However, another unique form of white fat necrosis, designated lipomembranous fat necrosis (LFN) (7-15), also called lipomembranous changes (8,15-21), membranous fat necrosis (1,15,22-29), membranocystic changes or fat necrosis (7,8,11,14-17,28-34), membranous lipodystrophy-like changes (30,31), and pseudomembranous fat necrosis (7,35), is found in fibrotic tissues or later stages of various diseases (9,12,14-16,18,19,21,35). In this review, we describe clinicopathological features of this interesting, but poorly characterized condition.
2. Morphological characteristics of LFN
LFN is microscopically characterized by eosinophilic or hyaline, convoluted, crenulated, scalloped, and/or serpiginous membrane formations on hematoxylin and eosin (H&E)-stained sections (Fig. 1A) (7,8,11,15,17,19,22-28,30,31,35). Some authors have designated LFN as membranes with an ‘arabesque’ (7-9,11,16,33) or ‘frost on a windowpane’ (34) appearance. LFN is scattered singly or in some clusters within fatty and/or fibrous tissues and shows microcystic, macrocystic, and/or crushed features (7-12,14-16,19,21-26,28). Scattered LFN within fatty tissues represents early changes of LFN, and LFN within fibrous tissues may represent chronic phase lesions (9,12,14-16,18,19,21,35). Microcystic LFN corresponds to necrotic changes in fat cells, whereas macrocystic LFN may be composed of cohesive microcystic LFNs (Fig. 2A-C) (23). Macrocystic LFN can present as a pea-sized or 2-cm cyst (8,22,23), mimicking an epidermal cyst (22).
Figure 1.
LFN. (A-C) Microcystic LFNs are characterized by (A) eosinophilic, crenulated membranes on H&E staining, stained (B) red by Masson trichrome stain, and (C) enhanced by the periodic acid-Schiff reaction (A, bar=50 µm; B and C, bar=10 µm). (D and E) Crushed LFNs (D, arrows) within fibrous tissues adjacent to microcystic LFN (D, asterisk) on H&E-stained sections are challenging to detect, but Masson trichrome stain highlights LFNs in red (E, arrows and an asterisk) on a bluish fibrous background (D and E, bar=50 µm). Insets in (D and E) represent high-power views of crushed LFN (bar=10 µm). LFN, lipomembranous fat necrosis; H&E, hematoxylin and eosin.
Figure 2.

Hematoxylin and eosin-stained macrocystic and microcystic LFN. (A) Macrocystic LFNs (asterisks) and microcystic LFNs are found in subcutaneous tissues (bar=200 µm). (B) High-power views of aggregated small LFNs localized near macrocystic LFNs (bar=50 µm). (C) High-power views of the macrocystic wall of LFN showing septa-like hyaline, crenulated membranes transformed from cohesive LFNs (bar=50 µm). LFN, lipomembranous fat necrosis.
LFN is stained red with Azan-Mallory or Masson trichrome stain (Fig. 1B) (11,28,30,31). LFN is also highlighted by periodic acid-Schiff staining with or without diastase digestion (Fig. 1C) (7-12,16,18,22-26,28-30,35), Sudan black B staining (10,11,16,22,24-27,30,31), oil red O staining (23), orcein staining (10), long Ziehl-Neelsen staining (16,23-27), silver impregnation (30,31), phosphotungstic acid-hematoxylin staining (11,16), and luxol fast blue staining (11,16,22,23,27,30,31). LFN occasionally shows noncystic, crushed features embedded within fibrous tissues and may be difficult to recognize (Fig. 1D). Accordingly, LFN can be discriminated from fibrosis using additional staining methods (Fig. 1E). A recent report (29) showed that LFN stains maroon to purple when exposed to Russell-Movat pentachrome stain. LFN is negative with alcian blue (11,22,24,30,31), elastic stain (Weigert-van Gieson, elastica van Gieson stain, and Verhoeff elastic stain) (22,28-31,35), methenamine silver (11), and Prussian blue (11,22). Immunohistochemically, LFN is positive for CD68 (11,35) and lysozyme (11,35) and negative for S-100 protein (28), CD34 (11,35), muscle specific antigen (11,35), and factor XIIIa (11,35). On ultrastructural examination, LFN is composed of poorly defined minute tubule-like structures (Fig. 3) and/or tiny vesicle-like structures (11,22,28,30,31). Unstained LFN shows yellow-green autofluorescence on fluorescent microscopy (10,11,23-27). Older LFN sometimes exhibits weak red staining with Masson trichrome stain, consistent with previous observations demonstrating that LFN is weakly fuchsinophilic (29), or may show negative results with Masson's trichrome stain (11,16). In addition, older LFN may be positive for van Gieson elastic stain (35) and may lack or exhibit weak expression of CD68 and lysozyme (35). Older LFN can also show Kossa stain-positive calcification (18,33,35).
Figure 3.
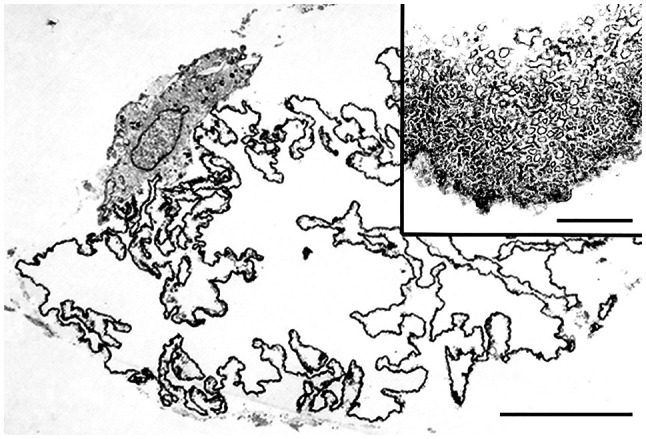
Ultrastructural features of LFN. LFN composed of serpiginous membranes and focally adherent to a mononuclear phagocyte (bar=10 µm). Inset depicting a high-power view of the microtubular structures of membranes (bar=1 µm). LFN, lipomembranous fat necrosis.
3. Historical recognition of LFN
Approximately 60 years ago, the ‘arabesque’ or ‘membranocystose-like’ features of LFN were recognized in biopsy specimens from osseous cystic lesions in a 28-year-old Japanese male (36), as reported by Terayama (37) at a Japanese orthopedic meeting in 1961. From the detailed postmortem findings of this case, Nasu et al (38) proposed the term ‘lipomembranous dystrophy’ in 1973. Järvi et al (39) independently reported two cases showing similar lipomembranous features as ‘membranous reticulin dysplasia of bones’ in 1964. Hakola et al (40) and Hakola and Partanen (41) summarized cases of Finnish families showing both progressive dementia and lipomembranous polycystic osteodysplasia in 1970. Based on these historical aspects, this autosomal recessive disorder has been designated ‘Nasu-Hakola disease’, ‘Nasu-Hakola syndrome’, or ‘Järvi-Hakola-Nasu disease’ (9,11,28,35,42,43) and is now known to be caused by loss-of-function variants in TYROBP/DAP12 or TREM2 (44-46).
LFN was initially considered a specific morphology of Nasu-Hakola disease (35,38,39). However, subcutaneous LFN has occasionally been discovered in patients without this hereditary disease (8-33,35,36,47,48). Machinami (31) reported subcutaneous LFN within necrotic legs caused by impaired arterial blood supply, such as thromboangiitis obliterans, arteriosclerotic obliterans, and progressive systemic sclerosis (LFN incidence rates: 38, 75, and 50%, respectively). Poppiti et al (22) observed LFN in the thoracic subcutaneous tissues of a 66-year-old man without Nasu-Hakola disease or other underlying diseases and identified LFN in 7 (21%) of 33 consecutive cases of subcutaneous fat necrosis. Furthermore, Coyne et al (23) found LFN in 11 (44%) of 25 irradiated breast tissues and in 13 (31%) of 42 nonirradiated necrotic fat tissues of the breast. Therefore, LFN is not rare and not specific to Nasu-Hakola disease. The most recent version of the dermatopathology textbook Lever's Histopathology of the Skin, 11th Edition (7) has designated LFN as a distinct type of adipocyte necrosis in panniculitis, although the previous versions (49,50) had described LFN as a condition that was relatively specific to lipodermatosclerosis.
4. LFN in various locations and diseases
Non-neoplastic subcutaneous lesions and breast tissues
Subcutaneous LFN has been reported in venous insufficiency diseases (including hypodermatitis sclerodermiformis, stasis dermatitis, deep venous thrombosis, thrombophlebitis, varicose veins, and lipodermatosclerosis) (11,14-16,19,30-32,34,35,48), erythema nodosum (9,11,14,35), erythema induratum (11,35), traumatic panniculitis (11,35), pancreatic panniculitis (11,35), necrobiosis lipoidica (9,11,35), nodular cystic fat necrosis (8,18), sclerosing lipogranuloma (35), morphea or scleroderma (9,11,18,35), lupus panniculitis or discoid lupus erythematosus (9,11,17,20,21,33,35), Behçet disease (11), Sjögren syndrome (18), mixed connective tissue disease (12), polyarteritis nodosa or vasculitis (9,11,35), lichen amyloidosis (47), erysipelas (9), atypical mycobacteria or miliary tuberculosis (11,13,35), diabetes mellitus (11,16,35), and subcutaneous sarcoidosis (35).
Abdominal lesions
Ramdial and Singh (25) reported that microcytic LFN was found in 10 cases of appendix epiploica. Appendix epiploica is characterized by calcified fibrous nodules protruding from the colonic serosal surface or isolated as free bodies in the abdominal cavity (25,51). Ramdial and Bagratee (26) found LFN in 9 (4%) of 217 ovarian mature cystic teratomas. Nistal et al (10) identified LFN in 3 torn testes accompanied by thrombosed veins.
Intra-articular loose bodies
Intra-articular loose bodies are caused by osteochondral fracture, joint surface integration, torn meniscus, fibrinous synovitis, and primary synovial chondromatosis (43,52-55). LFN is found in necrotic bone marrow within intra-articular loose bodies related to osteochondritis dissecans (43). Matsukuma et al (56) reported LFN in 7 (13%) of 55 intra-articular loose bodies; 4 were found in necrotic bone marrow derived from osteochondral fracture, and the other 3 were associated with viable fat cells without bone marrow structures.
Cardiac valves and LFN
White fat cells can be found in cardiac valves, possibly representing fatty metaplasia (1). Matsukuma et al (28) reported concomitant age-dependent fatty metaplasia of the aortic valves and LFN in 52 (63%) of 82 nondysfunctional aortic valves and in 58 (83%) of 70 dysfunctional aortic valves (Fig. 4A-D). Sekulic SP and Sekulic M (29) found LFN with viable fat cells in 129 (18%) of 719 aortic valves, in 26 (9%) of 284 mitral valves, but did not find LFN in 24 tricuspid valves or 15 pulmonary valves.
Figure 4.

LFN in aortic valves embedded within aortic valves. (A and C) Low-power views (bar=200 µm) of (A) hematoxylin and eosin and (C) Masson trichrome staining demonstrating calcified and fibrously thickened aortic valves. (B) High-power views of the area indicated by the arrow in (A), showing an eosinophilic, crenulated LFN (bar=10 µm). (D) High-power views of the area indicated by the arrow in (C), clearly demonstrating reddish LFN by Masson trichrome staining (bar=10 µm). LFN, lipomembranous fat necrosis.
LFN in other tumorous lesions
LFN was also reported in subcutaneous tissues of patients with panniculitis-like T-cell lymphoma (11) and in 4 relatively large lipomas, ranging in size from 9 to 22 cm (24).
5. Pathogenesis of LFN
LFN is relatively devoid of active inflammatory reaction except for lipogranuloma or histiocytic reaction (8,9,11,16,22,24,33). Some studies (32,57) have shown that patients with subcutaneous LFN-related lesions recover after venous insufficiency treatment. Subcutaneous LFN can also be caused by ischemia or venous insufficiency, regardless of the underlying disease (8,9,11,16,17,21,30-33). Subcutaneous fatty tissues are highly vascularized (3) and would be resistant to ischemia, thereby contributing to the uncommonness of subcutaneous LFN. Appendix epiploica, torn testis, and twisted ovarian teratoma are ischemic disorders, and LFN may be present in these lesions (10,25,26). Ramdial and Bagratee (26) reported that LFN was found in only one (1.8%) of 56 mature cystic teratomas removed from patients having a history, symptoms and/or signs of teratoma torsion. In addition, they identified LFN in another 8 (5%) of 161 teratomas removed from patients without a history of teratoma torsion (26). Thus, subclinical minor torsion of ovarian teratoma occurs in approximately 5% of patients with ovarian teratoma. Coyne et al (23) suggested that LFN may be caused by a combination of factors, including prior surgery and ischemia due to radiation-related vascular changes. Lipomas are also well vascularized (3,58), but larger lipomas may also be associated with ischemia or trauma (3,24). Hence, the occurrence of LFN in larger lipomas is considered a reasonable event (24).
By contrast, intra-articular loose bodies are in an environment that is different from that of subcutaneous lesions. Articular hyaline cartilage is exposed directly to joint fluid, whereas bone and bone marrow fatty tissues are nourished by a vessel-dependent blood supply (54,55). Therefore, intra-articular loose bodies derived from articular hyaline cartilage remain alive and still grow. However, detached bone and bone marrow cannot survive and therefore may exhibit LFN through ischemic necrosis of bone marrow fat cells (43,56). Viable fat cells, which do not contain bone marrow structures, within intra-articular loose bodies are considered uncommon fatty metaplasia of detached cartilaginous cells (56). In a study by Matsukuma et al (56), LFN was observed in 3 (43%) of 7 loose bodies showing fatty metaplasia. Thus, intra-articular loose bodies may be encountered under hypoxic or malnourished conditions resembling ischemia (56). Furthermore, aortic valves are similarly avascular (59,60) and receive nutritious permeation directly from the blood flow. The presence of LFN in aortic valves indicates a morbid condition that disrupts the circulation and distribution of nutrients (28). The close relationship between the occurrence of LFN and fibrously thickened aortic valves supports that the impairment of nutrient permeation may be related to valvular fibrous thickening (28). Sekulic SP and Sekulic M (29) showed that the higher incidence of LFN in aortic valves than in mitral and tricuspid valves may be related to differences in the rheological forces present in these valves.
LFN is occasionally found in fatty tissues without characteristic clinical and histological features of ischemia. Akay et al (61) reported the presence of LFN within abdominal and femoral subcutaneous tissues without vascular changes in a patient with acute leukemia; they concluded that this case represented chemotherapy-induced LFN. Fig. 5 shows scattered LFNs within anconal subcutaneous fatty tissues adjacent to invading high-grade sarcoma in a patient receiving no chemoradiotherapy. Small arteries and veins in fatty tissues containing LFN are open and well-preserved, but small fat granulomas are also multifocally observed. We speculate that the presence of both LFN and fat granulomas in this case may be related to the presence of hypoxic or malnourished conditions.
Figure 5.

Hematoxylin and eosin-stained LFN associated with a soft tissue tumor. Scattered LFNs (arrows) in subcutaneous fatty tissues adjacent to invading high-grade sarcoma are shown in the lower field. The artery and vein in the left-sided central field are open and well-preserved. Small lipogranulomas are also multifocally present in these fatty tissues (not shown; bar=200 µm). LFN, lipomembranous fat necrosis.
Based on analysis of histochemical staining, LFN is mainly composed of ceroids (22-24,26). Some investigators have proposed several possible factors occurring after ischemic/hypoxic injury that may contribute to the formation of peculiar ceroid membranes, including anti-oxidants, reactive oxygen intermediates, released cellular enzymes, and lipoperoxidation (11,26). However, the specific mechanisms causing LFN remain poorly understood.
6. Clinical and translational significance of pathologically detected LFN
Fat necrosis is histologically divided into lipogranuloma type fat necrosis, coagulation-like necrosis type fat necrosis, enzymic fat necrosis, and LFN (1,3,7,8-35,47-49,56,57,61). Table I summarizes these clinicopathological features. Some types of fat necrosis are occasionally intermingled and may not be specific to a disease or condition. As described above, however, LFN is closely associated with ischemia, hypoxia, or malnourishment. Therefore, when the histopathological examination detects the presence of LFN in inflamed or necrotic specimens of unknown etiology, clinicians should rule out a possible circulatory disturbance. If a distinct ischemic condition is not present clinically, a local hypoxic or malnourishment-related condition can be considered. In addition, clinicians should check the patient's history of radiation and chemotherapy because previous reports have shown that LFN may be possibly related to these modalities (23,61).
Table I.
Clinicopathological features of several types of fat necroses.
| Type of fat necrosis | Lipogranuloma type fat necrosis | Coagulation-like necrosis type fat necrosis | Enzymic fat necrosis | Lipomembranous fat necrosis |
|---|---|---|---|---|
| Favored locations | Generalized fatty tissues | Generalized fatty tissues | Distal lower extremities, buttock, abdomen, arm, elbow, scalp | Possible generalized fatty tissues; breast, lower legs, cardiac valves, abdominal cavities, testes, ovaries, intra-articular loose bodies |
| Histological features | Epithelioid and/or foamy histiocytes, giant cells, with or without scattered lipid vacuoles | Aggregated fat cells losing nuclei, usually without inflammation | Basophilic or eosinophilic liquefaction of fat cells with neutrophilia | Eosinophilic or hyaline crenulated, arabesque-like membrane formation; crushed, microcystic, and/or macrocystic |
| Pathogenesis | Nonspecific fat cell damages due to various diseases/conditions | Due to ischemia | Due to action of pancreatic lipolytic enzymes | Due to ischemia, hypoxia, or malnourishment-related conditions |
| Associated lesions | Various diseases/conditions, including inflammation, trauma, ischemia, and lipoma | Erythema induratum, calciphylaxis, appendix epiploicae, lipoma, other infarcted fatty lesions | Acute pancreatitis | Various diseases/conditions causing local ischemia, hypoxia, or malnourishment (including soft tissue tumors) |
7. Conclusions
LFN is characterized by a unique histopathology and is detectable on routine H&E staining, although its occurrence may be uncommon. We believe that LFN could be a hallmark of unexpected, hidden ischemic or ischemia-like hypoxic/malnourished conditions in various diseases. The exact pathogenesis of LFN, however, remains unknown. In addition, other possible etiologies of LFN, such as chemotherapy and radiotherapy, have not yet been evaluated. Further large-scale studies are needed to assess these factors.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
SM reviewed previous articles and drafted the manuscript. AM collected and reviewed almost all of the reference articles. OT and SO commented on the manuscript, and SO edited the manuscript. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Brooks JSJ. Adipose tissue. In: Histology of Pathologists. Mills SE (ed). 5th edition. Wolters Kluwer, Philadelphia, PA, pp133-165, 2020. [Google Scholar]
- 2.Goldblum JR, Folpe AL, Weiss SW (eds) Enzinger & Weiss's soft tissue tumors. 7th edition. Elsevier Inc., Philadelphia PA, pp225-231, 476-518, 2020. [Google Scholar]
- 3.Oakes SA. Cell injury, cell death, and adaptations. In: Robbins and cotran pathologic basis of diseases. Kumar V, Abbas AK and Aster JC (eds). 10th edition. Elsevier Inc., Philadelphia PA, pp33-69, 2021. [Google Scholar]
- 4.Klöppel G, von Gerkan R, Dreyer T. Pathomorphology of acute pancreatitis-analysis of 357 autopsy cases and 3 surgical specimens. In: Pancreatitis-concepts and classification. Gyr KE, Singer MV and Sarles H (eds). Excerpta Medica, Amsterdam, pp29-35, 1984. [Google Scholar]
- 5.Schmits-Moormann P. Comparative radiological and morphological study of the human pancreas. IV. Acute necrotizing pancreatitis in man. Pathol Res Pract. 1981;171:325–335. doi: 10.1016/S0344-0338(81)80105-7. [DOI] [PubMed] [Google Scholar]
- 6.Maitra A. The pancreas. In: Robbins and cotran pathologic basis of diseases. 10th edition. Kumar V, Abbas AK and Aster JC (eds). Elsevier, Philadelphia, PA, pp881-894, 2021. [Google Scholar]
- 7.Fung MA, Requena L. Inflammatory diseases of the subcutaneous fat. In: Lever's histopathology of the skin. Elder DE, Elenitsas R, Rosenbach M, Murphy GF, Rubin AI and Xu X (eds). 11th edition. Wolters Kluwer, Philadelphia, PA, pp610-657, 2015. [Google Scholar]
- 8.Pujol RM, Wang CY, Gibson LE, Su WP. Lipomembranous changes in nodular-cystic fat necrosis. J Cutan Pathol. 1995;22:551–555. doi: 10.1111/j.1600-0560.1995.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 9.Snow JL, Su WP. Lipomembranous (membranocystic) fat necrosis. Clinicopathologic correlation of 38 cases. Am J Dermatopathol. 1999;18:151–155. doi: 10.1097/00000372-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Nistal M, González-Peramato P, Paniagua R. Lipomembranous fat necrosis in three cases of testicular torsion. Histopathology. 2001;38:443–447. doi: 10.1046/j.1365-2559.2001.01130.x. [DOI] [PubMed] [Google Scholar]
- 11.Segura S, Pujol RM. Lipomembranous fat necrosis of the subcutaneous tissue. Dermatol Clin. 2008;26:509–517. doi: 10.1016/j.det.2008.05.002. viii. [DOI] [PubMed] [Google Scholar]
- 12.Halvorson CR, Kwon SY, Kao GF, Germanas JP. Lipomembranous fat necrosis in a patient with mixed connective tissue disease. J Am Acad Dermatol. 2011;64:1010–1011. doi: 10.1016/j.jaad.2010.01.054. [DOI] [PubMed] [Google Scholar]
- 13.Yeh LJ, Shively NR, Isacke RN, Dowling CA, Stogsdill PB. Miliary tuberculosis characterised by lipomembranous fat necrosis. Lancet Infect Dis. 2015;15(1497) doi: 10.1016/S1473-3099(15)00437-5. [DOI] [PubMed] [Google Scholar]
- 14.Huang TM, Lee JYY. Lipodermatosclerosis: A clinicopathologic study of 17 cases and differential diagnosis from erythema nodosum. J Cutan Pathol. 2009;36:453–460. doi: 10.1111/j.1600-0560.2008.01049.x. [DOI] [PubMed] [Google Scholar]
- 15.Choonhakarn C, Chaowattanapanit S, Julanon N. Lipodermatosclerosis: A clinicopathologic correlation. Int J Dermatol. 2016;55:303–308. doi: 10.1111/ijd.12856. [DOI] [PubMed] [Google Scholar]
- 16.Alegre VA, Winkelmann RK, Aliaga A. Lipomembranous changes in chronic panniculitis. J Am Acad Dermatol. 1988;19:39–46. doi: 10.1016/s0190-9622(88)70149-8. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T, Furuhata Y, Tsuboi R. Lipomembranous changes and calcification associated with systemic lupus erythematosus. Clin Exp Dermatol. 2007;32:278–280. doi: 10.1111/j.1365-2230.2007.02358.x. [DOI] [PubMed] [Google Scholar]
- 18.Toritsugi M, Yamamoto T, Nishioka K. Nodular cystic fat necrosis with systemic sclerosis. Eur J Dermatol. 2004;14:353–355. [PubMed] [Google Scholar]
- 19.Walsh SN, Santa Cruz DJ. Lipodermatosclerosis: A clinicopathological study of 25 cases. J Am Acad Dermatol. 2010;62:1005–1012. doi: 10.1016/j.jaad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Khoury T, Arayssi T, Kibbi AG, Ghosn S. Extensive fat necrosis with lipomembranous changes and calcification in lupus erythematosus panniculitis is not necessarily associated with systemic lupus erythematosus. Am J Dermatopathol. 2010;32:742–743. doi: 10.1097/DAD.0b013e3181d2ce0a. [DOI] [PubMed] [Google Scholar]
- 21.Kim JS, Kim HY, Kim YG, Paek JO, Yu HJ. Lipomembranous changes associated with systemic lupus erythematosus. Clin Exp Dermatol. 2014;39:319–322. doi: 10.1111/ced.12268. [DOI] [PubMed] [Google Scholar]
- 22.Poppiti RJ Jr, Margulies M, Cabello B, Rywlin AM. Membranous fat necrosis. Am J Surg Pathol. 1986;10:62–69. doi: 10.1097/00000478-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Coyne JD, Parkinson D, Baildam AD. Membranous fat necrosis of the breast. Histopathology. 1996;28:61–64. doi: 10.1046/j.1365-2559.1996.252292.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramdial PK, Madaree A, Singh B. Membranous fat necrosis in lipomas. Am J Surg Pathol. 1997;21:841–846. doi: 10.1097/00000478-199707000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Ramdial PK, Singh B. Membranous fat necrosis in appendices epiploicae. A clinicopathological study. Virchows Arch. 1998;432:223–227. doi: 10.1007/s004280050159. [DOI] [PubMed] [Google Scholar]
- 26.Ramdial PK, Bagratee JS. Membranous fat necrosis in mature cystic teratomas of the ovary. Int J Gynecol Pathol. 1998;17:120–122. doi: 10.1097/00004347-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Ramdial PK, Chetty R. Vasculitis-induced membranous fat necrosis. J Cutan Pathol. 1999;26:405–410. doi: 10.1111/j.1600-0560.1999.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 28.Matsukuma S, Takeo H, Kono T, Sato K. Fat cells and membranous fat necrosis of aortic valves: A clinicopathological study. Pathol Int. 2013;63:345–352. doi: 10.1111/pin.12074. [DOI] [PubMed] [Google Scholar]
- 29.Pichler Sekulic S, Sekulic M. Adipocytes and membranous fat necrosis within native cardiac valves: Clinicopathologic characterization of histologic constituents. Cardiovasc Pathol. 2021;50(107276) doi: 10.1016/j.carpath.2020.107276. [DOI] [PubMed] [Google Scholar]
- 30.Machinami R. Membranous lipodystrophy-like changes in ischemic necrosis of the legs. Virchows Arch A Pathol Anat Histopathol. 1983;399:191–205. doi: 10.1007/BF00619579. [DOI] [PubMed] [Google Scholar]
- 31.Machinami R. Incidence of membranous lipodystrophy-like change among patients with limb necrosis caused by chronic arterial obstruction. Arch Pathol Lab Med. 1984;108:823–826. [PubMed] [Google Scholar]
- 32.Demitsu T, Okada O, Yoneda K, Manabe M. Lipodermatosclerosis-report of three cases and review of the literature. Dermatology. 1999;199:271–273. doi: 10.1159/000018264. [DOI] [PubMed] [Google Scholar]
- 33.Suda T, Hara H, Okada T, Suzuki H. Coexistence of extensive calcification and membrano-cystic changes in lupus erythematosus panniculitis associated with systemic lupus erythematosus. Eur J Dermatol. 2007;17:86–68. doi: 10.1684/ejd.2007.0194. [DOI] [PubMed] [Google Scholar]
- 34.Billings SD. Dermatosis. In: Rosai and Ackerman's surgical pathology. 11th edition. Goldblum JR, Lamps LW, McKenney JK and Myers JL (eds). Elsevier, Philadelphia, PA, pp24-25, 2018. [Google Scholar]
- 35.Diaz-Cascajo C, Borghi S. Subcutaneous pseudomembranous fat necrosis: New observations. J Cutan Pathol. 2002;29:5–10. doi: 10.1034/j.1600-0560.2002.290102.x. [DOI] [PubMed] [Google Scholar]
- 36.Fujiwara M. Histopathological and histochemical studies of membranocystic lesion (Nasu) Shinshu Med J. 1979;27:78–100. (In Japanese) [Google Scholar]
- 37.Terayama K. Two cases of cystic bone disease showing peculiar features. J Jap Orthop Ass. 1961;35(626) (In Japanese) [Google Scholar]
- 38.Nasu T, Tsukahara Y, Terayama K. A lipid metabolic disease-‘membranous lipodystrophy’-an autopsy case demonstrating numerous peculiar membrane-structures composed of compound lipid in bone and bone marrow and various adipose tissues. Acta Pathol Jpn. 1973;23:539–558. doi: 10.1111/j.1440-1827.1973.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 39.Järvi OH, Lauttamus LL, Solonen KA. Membranous reticulin dysplasia of bone. Probably a new disease entity. In: Proceedings of the 14th Scandinavian Congress of Pathology and Microbiology. Universitetsforlaget, Oslo, p51, 1964. [Google Scholar]
- 40.Hakola HP, Järvi OH, Sourander P. Osteodysplasia polycystica hereditaria combined with sclerosing leucoencephalopathy, a new entity of the dementia praesenilis group. Acta Psychiatr Scand. 1970;46 (Suppl 43):S79–S80. [PubMed] [Google Scholar]
- 41.Hakola HP, Partanen VS. Neurophysiological findings in the hereditary presenile dementia characterized by polycystic lipomembranous osteodysplasia and sclerosing leukoencephalopathy. J Neurol Neurosurg Psychiatry. 1983;46:515–520. doi: 10.1136/jnnp.46.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verloes A, Maquet P, Sadzot B, Vivario M, Thirty A, Franck G. Nasu-Hakola syndrome: Polycystic lipomembranous osteodysplasia with sclerosing leucoencephalopathy and presenile dementia. J Med Genet. 1997;34:753–757. doi: 10.1136/jmg.34.9.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machinami R (ed) Atlas of bone and joint disease. Bunkoudo, Tokyo, pp42-43, 64-65, 196-197, 1999 (In Japanese). [Google Scholar]
- 44.Paloneva J, Kestilä M, Wu J, Salminen A, Böhling T, Ruotsalainen V, Hakola P, Bakker ABH, Phillips JH, Pekkarinen P, et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 45.Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an incidental disease phenotype. Am J Hum Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Errichiello E, Dardiotis E, Mannino F, Paloneva J, Mattina T, Zuffardi O. Phenotypic expansion in Nasu-Hakola disease: immunological findings in three patients and proposal of a unifying pathogenetic hypothesis. Front Immunol. 2019;10(1685) doi: 10.3389/fimmu.2019.01685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee Y, Ahn SY, Ji JH, Hong SP, Bak H, Lee SH, Ahn SK. A case of membranous lipodystrophy observed in lichen amyloidosis. Ann Dermatol. 2009;21:174–177. doi: 10.5021/ad.2009.21.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ayele A, Tidman MJ, Biswas A. Pseudomembranous changes in dermis: A novel observation and potential clue for evolving lipodermatosclerosis? J Cutan Pathol. 2017;44:1070–1074. doi: 10.1111/cup.13038. [DOI] [PubMed] [Google Scholar]
- 49.McNutt NS, Moreno A, Contreras F. Inflammatory diseases of the subcutaneous fat. In: Lever's histopathology of the skin. Elder DE, Elenitsas R, Johnsons BL Jr, Murphy GF, Rubin AI and Xu X (eds). 9th edition. Lippincott Williams & Wilkins, Philadelphia, PA, pp519-549, 2005. [Google Scholar]
- 50.McNutt NS, Moreno A, Contreras F. Inflammatory diseases of the subcutaneous fat. In: Lever's histopathology of the skin. Elder DE, Elenitsas R, Johnsons BL Jr, Murphy GF, Rubin AI and Xu X (eds). 10th edition. Lippincott Williams & Wilkins, Philadelphia, PA, pp509-538, 2009. [Google Scholar]
- 51.Rosai J (ed) Rosai and Ackermans's surgical pathology. 10th edition. Mosby/Elsevier, Philadelphia, PA, pp2236, 2011. [Google Scholar]
- 52.Barrie HJ. Intra-articular loose bodies regarded as organ cultures in vivo. J Pathol. 1978;125:163–169. doi: 10.1002/path.1711250307. [DOI] [PubMed] [Google Scholar]
- 53.Milgram JW (ed) Radiologic and histologic pathology of nontumorous diseases of bones and joints. Northbook Publishing Company, Brookfield, WI, pp281-334, 1990. [Google Scholar]
- 54.Ishida T, Imamura T (eds) Surgical pathology of non-neoplastic bone and joint diseases. Bunkoudo, Tokyo, pp48-61, 226-236, 2003 (In Japanese). [Google Scholar]
- 55.O'Connell JX. Pathology of the synovium. Am J Clin Pathol. 2000;114:773–784. doi: 10.1309/LWW3-5XK0-FKG9-HDRK. [DOI] [PubMed] [Google Scholar]
- 56.Matsukuma S, Takeo H, Okada K, Sato K. Fatty lesions in intra-articular loose bodies: A histopathological study of non-primary synovial chondromatosis cases. Virchows Arch. 2012;460:103–108. doi: 10.1007/s00428-011-1172-0. [DOI] [PubMed] [Google Scholar]
- 57.Mullaaziz D, Kaptanoğlu A, Çalıkoğlu EE, Özkayalar H. A case of lipomembranous panniculitis with a dramatic response to the treatment of venous insufficiency. Dermatol Reports. 2018;10(7546) doi: 10.4081/dr.2018.7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fletcher CDM (ed) Diagnostic histopathology of tumors. 5th edition. Elsevier, Philadelphia, PA, pp1919-2001, 2021. [Google Scholar]
- 59.Schoen FJ, Sutton MS. Contemporary issues in the pathology of valvular heart disease. Hum Pathol. 1987;18:568–576. doi: 10.1016/s0046-8177(87)80356-8. [DOI] [PubMed] [Google Scholar]
- 60.Virmani R, Burke A, Farb A, Atkinson JB. Cardiovascular pathology. In: Major problems in pathology. LiVolsi VA (ed). Vol 40. 2nd edition. W.B. Saunders, Philadelphia, PA, pp231-279, 2001. [Google Scholar]
- 61.Akay OM, Urer SM, Oner U, Gulbas Z. Lipomembranous panniculitis in a patient with acute leukemia induced by chemotherapy. Leuk Res. 2008;32:669–671. doi: 10.1016/j.leukres.2007.07.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.



