Keywords: Drosophila melanogaster, amino acid, dietary preconditioning, stress tolerance, mTORC1, GCN2
Abstract
Dietary interventions that restrict protein intake have repeatedly been shown to offer beneficial health outcomes to the consumer. Benefits such as increased stress tolerance can be observed when individual amino acids are restricted, thus mimicking dietary protein restriction. Here, we sought to further understand the relationship between dietary amino acids and stress tolerance using Drosophila melanogaster. Using a chemically defined medium for Drosophila, we found that transiently restricting adult flies of a single essential amino acid generally protects against a lethal dose of the naturally occurring insecticide, nicotine. This protection varied with the identity of the focal amino acid and depended on the duration and intensity of its restriction. To understand the molecular basis of these effects, we modified the signalling of two cellular sensors of amino acids, GCN2 and mTORC1, in combination with amino acid restriction. We found that GCN2 was necessary for diets to protect against nicotine, whereas the suppression of mTORC1 was sufficient to induce nicotine resistance. This finding implies that amino acid restriction acts via amino acid signalling to cross-protect against seemingly unrelated stressors. Altogether, our study offers new insights into the physiological responses to restriction of individual amino acids that confer stress tolerance.
1. Introduction
Nutrition is a powerful regulator of health, and manipulating the quantity and quality of diet affects fitness traits such as longevity, reproduction and stress resistance [1–3]. Transient dietary restriction, which is defined as reduced nutrient intake without malnutrition, increases stress resistance, improves metabolic health and extends lifespan across a broad range of organisms [1,4–6]. Furthermore, restricting dietary protein alone is sufficient to mimic the benefits of restricting food intake [5,7–9]. These effects can also be mimicked by restricting or removing single dietary amino acids. For instance, methionine restriction enhances tolerance to chemical and thermal stress in yeast, mice and human cells [10–12], and transient deprivation of tryptophan has also conferred protection against a model of surgical stress in mice [13]. These effects of diet have attracted interest for their potential to enhance lifelong human health. Current data indicate an important role for the evolutionarily conserved amino acid-sensing pathways in mediating these effects [7,13], though little is known about the downstream steps that are required to confer protection or the breadth of stress resistance they afford.
The presence or absence of dietary amino acids is detected and signalled by the complementary effects of the intracellular kinases, GCN2 (General Control Non-derepressible 2) and mTORC1 (mechanistic Target Of Rapamycin Complex 1), which are highly conserved and well characterized for their role in growth, metabolism and lifespan [8]. In the presence of amino acids GCN2 is inactive, but mTORC1 is activated and signals to a cascade of downstream effectors that promote protein translation and growth [14]. By contrast, when amino acids are absent, GCN2 is activated and mTORC1 is suppressed, ultimately resulting in reduced global translation and the promotion of autophagy; a process whereby cellular components are engulfed and broken down to be recycled for use in the synthesis of selected proteins important for resisting stress [15,16]. As amino acids are recycled via autophagy, their presence has the potential to reactivate mTORC1 despite the ongoing nutrient shortage. To ensure that mTORC1 remains inactive during nutrient scarcity, GCN2 also sustains mTORC1 suppression during prolonged amino acid deprivation [17]. These responses to amino acid shortage are important for prioritizing survival over growth when nutrients are scarce.
The full extent of these signalling interactions and the specific molecules by which each of the individual amino acids are detected are still being uncovered. Some essential amino acids, such as methionine, leucine and arginine, have been directly linked to molecular sensors upstream of mTORC1 [18–20]. However, it hasn't yet been determined whether there are other amino acids that regulate mTORC1 activity in vivo. By contrast, GCN2 is thought to detect intracellular depletion of any amino acid by binding uncharged tRNAs, which accumulate when there are insufficient free cognate amino acids [21]. GCN2 can also suppress mTORC1 activity through multiple effectors [17,22], meaning that ultimately, the balance of activity of both of these kinases is sensitive to the levels of any single amino acid.
As the mechanisms for amino acid detection are highly conserved across eukaryotes. This makes animals like the fruit fly Drosophila melanogaster, with their short generation times and abundance of genetic tools, excellent models to understand the relationship between dietary amino acids, amino acid signalling and their roles in stress tolerance. Particularly for fruit flies, extensive genetic and pharmacological tools can be coupled with a chemically defined (holidic) medium to investigate how restricting individual dietary amino acids confers tolerance to stressors [23].
A group of ecologically relevant stressors for flies are toxic plant-derived insecticides. These molecules are metabolized and excreted by the drug detoxification system, which consists of three evolutionarily conserved phases [24]. Phase I and II metabolism catalyse modifications of toxins into more hydrophilic molecules that are more easily excreted in phase III metabolism [25]. The superfamilies of genes involved in these phases of detoxification include CYP450 (Cytochrome P450) monooxygenases, GSTs (Glutathione S-transferases), UGTs (UDP-Glucuronyl transferases) and ABC (ATP-binding cassette) transporters [25,26]. Since xenobiotic toxins are ingested as part of regular food consumption, it is reasonable to anticipate that there is a link between nutrition and the need to detoxify these compounds. We therefore hypothesized that food composition, in particular amino acid restriction, could be an effective tool to modify resistance to chemical stressors and that this may be mediated by evolutionarily conserved nutrient sensors. If so, understanding how dietary interventions protect organisms against endobiotic and xenobiotic toxins would be useful in understanding the role of diet in animal, and therefore human, health.
Here we found that transient deprivation of almost any essential amino acid increased nicotine resistance in Drosophila. However, the magnitude of this dietary protection depended on the duration of exposure to the amino acid-depleted diet, the identity of the amino acid being manipulated, and to what extent the amino acid was being restricted. We also found that this protection was driven by interactions between GCN2 and mTORC1. These data highlight a new role for dietary amino acids in modifying the stress tolerance of Drosophila and reveals new insights into how this protection is affected.
2. Results
2.1. Removing an essential amino acid from the diet can protect flies against future toxin stress
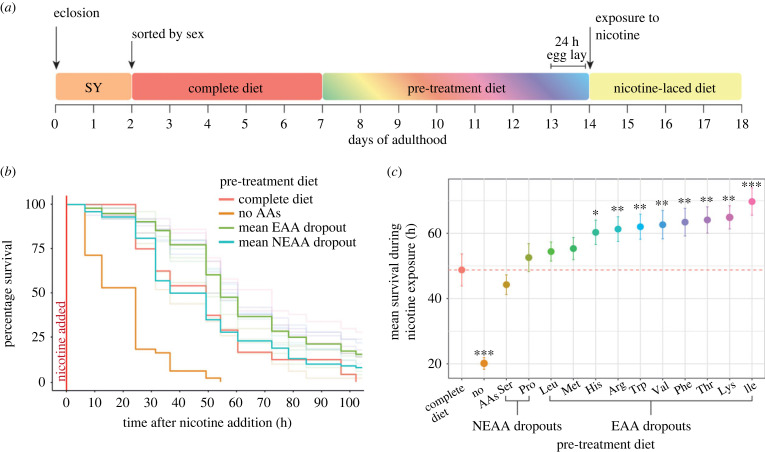
In previous studies, restriction of dietary protein or individual amino acids has conferred stress tolerance in mice [5,7,13]. To identify the mechanisms through which amino acids confer protection, we first sought to examine if this phenomenon also occurred in flies. To do this, we tested if short-term exposure to individual amino acid dropout diets could protect flies against exposure to nicotine, a chemical stressor that is detoxified by the evolutionarily conserved drug detoxification system [27] (figure 1a).
Figure 1.
Removing an essential amino acid from the diet can protect flies against future toxin stress. (a) Flies were pre-treated with one of 14 diets for 7 days before chronic exposure to food containing 1 mg ml−1 nicotine. Diets included a complete diet, a diet lacking all amino acids, a serine or proline dropout and dropouts for each essential amino acid. (b) Survival curves of pre-treated flies once exposed to nicotine; bold lines represent the average survival of the transparent lines, which represent individual dropout diets. (c) Mean survival ± s.e.m. of pre-treatment groups; groups were compared to the complete diet (red). n = 50 flies that were pre-treated with a complete diet and n = 100 for all other pre-treatment groups.
First, to assess the effect of our pre-treatment diets on fly physiology, we measured the flies' fecundity immediately prior to nicotine exposure and found that diet significantly modified egg production (F13 = 358, p < 0.0001). Flies fed a complete diet or either one of two non-essential amino acid dropouts had equally high levels of egg laying (p > 0.98; electronic supplementary material, table S1 and figure S1A), whereas flies fed any one of the 10 essential amino acid dropouts displayed severely reduced fecundity (p < 0.001) that was no different from flies fed a diet that lacked all amino acids (p > 0.5). These data confirm previous observations on the requirement for these amino acids for egg production [23,28].
Following 7 days of pre-treatment on our dropout diets, flies were exposed to food containing 1 mg ml−1 nicotine and their survival was recorded. This dose of nicotine caused most flies to die within 5 days, whereas flies that were maintained without nicotine exhibited no mortality during this time (electronic supplementary material, figure S1B, , p < 0.0001), indicating that the pre-treatment diets were not causing death. We found that diet significantly affected survival under nicotine stress (, p < 0.0001), and interestingly, eight of the 10 essential amino acid dropout diets conferred protection against nicotine treatment when compared to the complete diet control (p < 0.02; figure 1b,c; electronic supplementary material, table S2). By contrast, flies fed either of the non-essential amino acid dropout diets or a diet lacking either of the essential amino acids leucine or methionine were no different from those on a complete diet (p > 0.1). Finally, flies pre-treated with food lacking all amino acids had worse survival upon nicotine exposure than complete diet controls (p < 0.001). These results indicate that some single amino acid dropouts can protect flies against subsequent toxin stress and that the degree of protection is specific to the amino acid dropout they experience.
2.2. Pre-treatment modifies responses to high doses of nicotine
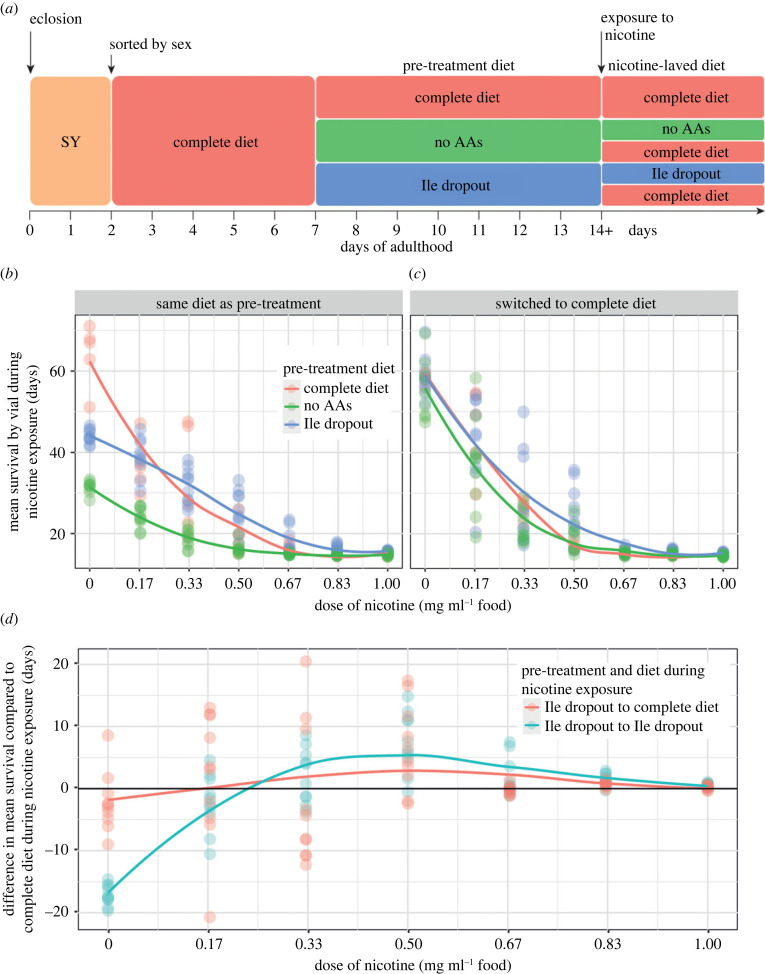
To understand the range of nicotine protection conferred by amino acid dropout diets, we pre-treated flies with either a complete diet, an isoleucine dropout, or a diet lacking all amino acids, and subsequently exposed them to nicotine concentrations between 0 and 1 mg ml−1 (figure 2a). We chose the isoleucine dropout diet for this experiment as it previously showed the greatest protection against nicotine.
Figure 2.
Pre-treatment modifies responses to higher doses of nicotine. (a) Flies were pre-treated with one of three diets (nutritionally complete, lacking all amino acids or lacking only isoleucine) for 7 days prior to chronic exposure of various nicotine concentrations. During nicotine exposure, flies were either (b) maintained on their pre-treatment diet or (c) switched to a nutritionally complete diet. (d) Difference between the mean survival of flies pre-treated with a complete diet and the survival of flies that were pre-treated with an isoleucine dropout and were either maintained on the same diet or switched to a complete diet at the time of nicotine treatment. n = 50 flies for each combination of diet and dose of nicotine.
Flies that were fed an isoleucine dropout diet showed resistance to doses of nicotine between 0.33 mg ml−1 – 1 mg ml−1 when compared to complete diet controls. When the nicotine concentration fell to 0.33 mg ml−1, isoleucine deprivation did not extend life beyond that of the complete diet controls (approx. 30d mean, p = 0.75), and at lower doses (0.17 mg ml−1 and 0 mg ml−1) adult fly survival was compromised by dietary isoleucine omission, showing that the benefits of this diet for survival are only apparent when the flies are challenged with stronger nicotine stress.
Interestingly, although isoleucine is an essential amino acid, the flies on isoleucine dropout food always lived longer than those on diets lacking all amino acids (figure 2b; electronic supplementary material, table S3), indicating again that eliminating single amino acids has different effects on the flies’ physiology than eliminating all amino acids. These data also indicate that any protective effects of isoleucine dropout are counteracted in the longer term by the negative effects of isoleucine omission. Thus, it is possible that fly survival may be further enhanced if flies were switched from the dropout pre-treatment diet to a complete diet at the point of nicotine exposure.
Switching flies from amino acid dropout food to complete food significantly improved the long-term survival of flies that were temporarily deprived of all amino acids or of isoleucine but not exposed to nicotine (figure 2c; p < 0.001; electronic supplementary material, table S3). However, for flies exposed to nicotine, there was no difference in protection when comparing flies that were maintained on an isoleucine dropout diet against those that were switched to a complete diet during the phase of nicotine exposure (figure 2d; p = 0.63; electronic supplementary material, table S4). These results suggest that it is the diet during pre-treatment that confers the benefit for survival during nicotine exposure. Once again, we observed no beneficial effect on nicotine resistance for flies exposed to diets lacking all amino acids, whether they were switched to a complete diet during nicotine treatment or not (electronic supplementary material, table S3). Thus, the protective effect of isoleucine deprivation is confined to the pre-treatment period, during which time the flies require the presence of other dietary amino acids.
2.3. Pre-treatment duration as well as amino acid identity and availability all modify protection against nicotine stress
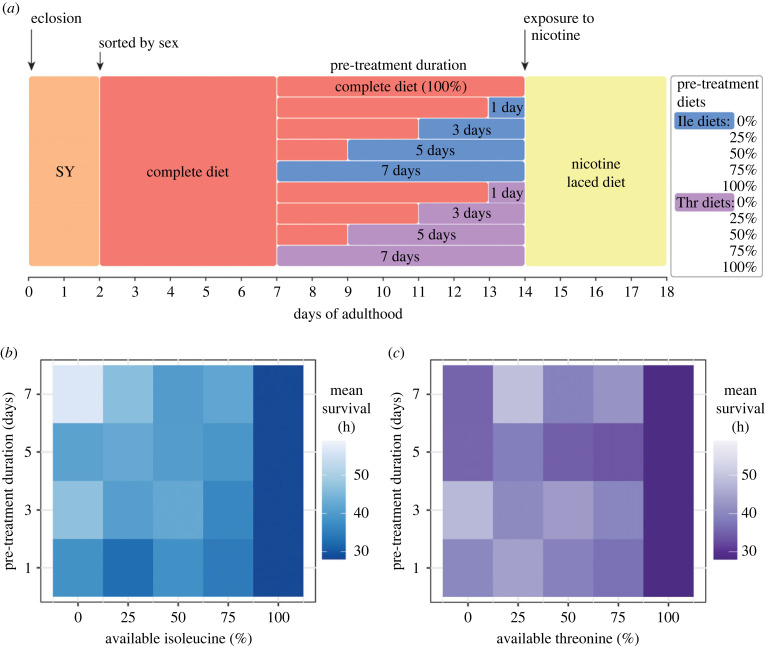
Our previous data show that only specific single amino acid deprivations can enhance fly resistance to nicotine, and that protection is gained during the pre-treatment phase. To explore whether graded restriction of an amino acid was sufficient for protection, we restricted either threonine or isoleucine to 75%, 50%, 25% or 0% of the amount in the complete diet. We opted to manipulate threonine in this assay as its removal previously showed a moderate protection (figure 1c), and we wanted to explore whether this could be further increased. As we restricted the levels of these amino acids, we simultaneously investigated the relationship that pre-treatment duration had on protection by modifying the duration of dietary pre-treatment to 7, 5, 3 or 1 day prior to nicotine exposure (figure 3a).
Figure 3.
Pre-treatment duration as well as amino acid identity and availability all modify protection against nicotine stress. (a) Flies were pre-treated with diets containing one of five amounts of either isoleucine or threonine (0%, 25%, 50%, 75% and 100%) for either 7, 5, 3 or 1 day before chronic exposure to 0.83 mg ml−1 nicotine. Mean survival of (b) isoleucine and (c) threonine modification depicted as heatmaps. n = 50 flies for each combination of pre-treatment duration and available amino acid. Pre-treatment with 100% of each amino acid (i.e. the complete diet) could not differ in duration time and n = 50 flies for this group in total.
Compared to the complete diet, we found that both the amount of available isoleucine and the pre-treatment duration modified protection during nicotine exposure (figure 3b; p < 0.001; electronic supplementary material, table S5). In general, survival improved in response to longer and more intense isoleucine dilutions such that the longest surviving flies were those pre-treated with 0% isoleucine for 7 days. However, when threonine was manipulated, we found that only the availability of amino acid (p < 0.001) and not pre-treatment duration (p = 0.67) modified survival during nicotine exposure (figure 3c; electronic supplementary material, table S6). Interestingly, pre-treating flies with a diet containing 25% threonine was the most protective. Thus, the identity of the focal amino acid, the degree to which it is diluted, and, for some amino acids, the duration of exposure can all modify the way in which protection against nicotine is acquired. These data again show that while varying the concentration of different amino acids in the diet can elicit a similar phenotype, the dynamics of the protective effects are amino acid specific.
2.4. Differences in survival during nicotine exposure between pre-treatment groups is not explained by whole-body fat/protein composition or feeding behaviour
A potential explanation for the way amino acid manipulations protect flies against nicotine exposure is that a given pre-treatment diet could prime flies to simply consume less nicotine and therefore live longer. Using a capillary feeder (CaFe) assay [29] (electronic supplementary material, figure S2A), we found that the volume of pre-treatment medium consumed was indistinguishable between diets, except for increased consumption for flies on a diet lacking amino acids (electronic supplementary material, figure S2B). Importantly, compared to flies that were pre-treated with a complete diet, flies that were fed a diet lacking isoleucine ate as much nicotine-laced medium (electronic supplementary material, figure S2C; p = 0.4; electronic supplementary material, table S7) but were longer lived (p = 0.006; electronic supplementary material, table S8). When analysing all diets together, we found that survival was modified by the amount of nicotine consumed (electronic supplementary material, figure S2D; , p < 0.001), but the relationship was positive, meaning that consuming more nicotine-containing food correlated with surviving longer. This shows that it was beneficial for the flies to continue to eat food during nicotine exposure and that the protective effects of our pre-treatment diets were not because the flies simply ate less toxin.
Since our protective pre-treatment diets significantly reduced the egg production of flies without significantly reducing feeding behaviour, we anticipated that they would be under a positive energy balance and would therefore accumulate more body fat than controls. To assess if this were the case and if it contributed to nicotine resistance, we compared the amounts of triacylglycerol (TAG) and protein found in our flies on the same four dietary pre-treatments as we had used previously (electronic supplementary material, figure S3A). While flies on the amino acid-free or the isoleucine dropout diets had less whole-body protein than flies on the complete or the 25% threonine diet (electronic supplementary material, figure S3B; p < 0.03; electronic supplementary material, table S9), neither whole-body fat content nor the ratio of fat : protein in these flies was significantly different across treatments (electronic supplementary material, figure S3C). These data indicate that body composition cannot explain the nicotine resistance phenotype.
2.5. GCN2 and mTORC1 signalling confer protection against nicotine stress
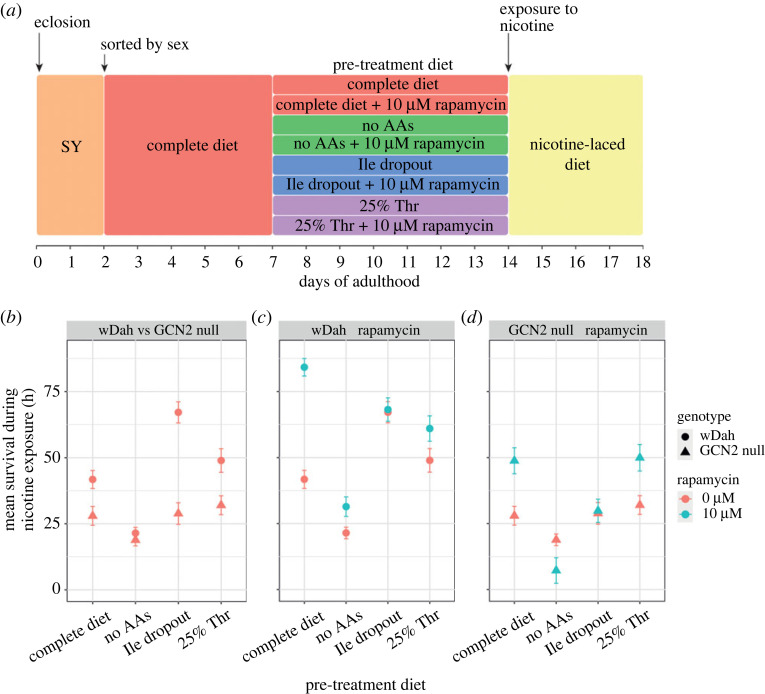
Flies signal amino acid status via two evolutionarily conserved intracellular kinases: GCN2 and mTORC1. These act in a complementary fashion, with mTORC1 activated by the presence of amino acids, whereas GCN2 is activated by the absence of an amino acid. Thus, we would expect our pre-treatment diets to activate GCN2 and perhaps cause suppression of mTORC1. Although, to what extent mTORC1 is suppressed is not clear given that our single amino acid dilution diets still contained the amino acids that are known to be activators of mTORC1, including leucine, methionine and arginine. To understand how changes in GCN2 and mTORC1 activity may be involved in protection against nicotine, we used genetic and pharmacological tools to suppress the activities of both kinases, individually and simultaneously, while also manipulating the diet composition (figure 4a).
Figure 4.
GCN2 is necessary for dietary protection against future nicotine stress and suppression of mTOR is sufficient to induce protection. (a) Prior to chronic exposure to 0.83 mg ml nicotine, GCN2 null and wDah control flies were pre-treated for 7 days with one of four diets that either contained 0 µM or 10 µM of the mTOR suppressor rapamycin. (b–d) Mean survival ± s.e.m. of (b) wDah and GCN2 nulls, (c) wDah pre-treated with food containing either rapamycin- or vehicle (ethanol) and (d) GCN2 nulls pre-treated with rapamycin- or ethanol-laced food. n = 50 flies for each combination of genotype, rapamycin concentration and pre-treatment diet.
Overall, GCN2 null flies were less resistant to nicotine than wild-type flies (figure 4b; electronic supplementary material, table S10; , p = 0.028) and in contrast with wild-type flies, neither isoleucine dropout nor threonine dilution induced protection against nicotine (electronic supplementary material, table S11). By contrast, when wild-type flies were pre-treated with the mTORC1 suppressor rapamycin, which doesn't alter consumption of pre-treatment (F1 = 0.03, p = 0.84) or nicotine-containing food (F1 = 0.31, p = 0.58), nicotine resistance was elevated for all pre-treatment groups (figure 4c; p < 0.02; electronic supplementary material, table S11) except for those on the isoleucine dropout diet (p = 0.36), which were already protected when compared to flies on the complete diet (p < 0.001). Treatment with rapamycin also improved nicotine resistance in GCN2 null flies that were pre-treated with either a complete diet or a 25% threonine diet (figure 4d; p < 0.002; electronic supplementary material, table S11), but not for GCN2 null flies that were pre-treated with an amino acid-free diet or an isoleucine dropout diet (p > 0.2). Together these data indicate that intact GCN2 is required for dietary protection against nicotine stress and that mTORC1 suppression is sufficient for protection. They also indicate that the isoleucine dropout may act via mTORC1 suppression, but only when GCN2 activity is intact. By contrast, the more subtle protection offered by threonine dilution can be augmented by mTORC1 suppression, whether or not GCN2 is intact. These data point to a complex interaction whereby the dilution of individual amino acids confers protection against nicotine by tuning GCN2 activation and mTORC1 suppression.
3. Discussion
Treating organisms with nutritionally modified diets has been shown to increase stress tolerance in different model systems [10–12], although the exact mechanisms by which this occurs remain elusive. In this paper, we expand these findings by establishing that briefly exposing Drosophila to amino acid-manipulated diets can enhance their tolerance of a chemical stressor. This type of protection is an example of cross-modal hormesis whereby a mild dietary stress can elicit protection against another, apparently unrelated, stressor [30]. Importantly, we found that the magnitude of protection depends on several factors, including the identity of the manipulated amino acid, the intensity of deprivation and the duration of pre-treatment. We also found that this protection is mediated by interactions between the nutrient-sensing kinases GCN2 and mTORC1. These data provide insights into the way diet and nutrient signalling can be harnessed to manipulate an evolutionarily conserved aspect of stress protection. This has important implications for pest control in agriculture as well as for animal and human health.
Amino acids are both building blocks for protein biosynthesis and required to trigger signalling to regulate proteostasis and anabolism so that physiology is tuned to match the supply of available nutrients [31]. Changes in the amount and relative abundance of dietary amino acids can modify organismal behaviour, growth, reproduction and lifespan, but which amino acids are key, and for what reason, is not known. One of the more well-studied amino acid manipulations is methionine restriction, which can enhance stress resistance and lifespan in rodents [11,12,32,33], and can extend lifespan in flies [34,35]. But methionine restriction may not be special in this regard since further work in flies has since shown that lifespan can also be enhanced by simultaneously restricting the three branched chain amino acids (BCAA; isoleucine, leucine and valine) or by restricting another set of three essential amino acids (threonine, histidine and lysine) [36]. Together, these data show that manipulations of various dietary amino acids can modify the consumer's physiology and fitness, but because we do not fully understand the biological roles of each amino acid, we cannot deduce the logic by which each may be important for a particular phenotype. In our study, transiently depriving flies of each one of the essential amino acids revealed a protective effect against nicotine that ranged from approximately 40 to 100% longer life following isoleucine deprivation to no protection at all following methionine or leucine deprivation. It will be interesting in future studies to determine the breadth of this protection as additional phenotypes may provide useful in understanding the metabolic physiology of amino acids.
Of the biochemically related groups of amino acids in our experiments, we thought it particularly interesting that deprivation for each one of the three BCAA elicited a different protective response that ranged from the strongest protection (isoleucine) to intermediate protection (valine) to no protection (leucine). The BCAAs are often studied together as a group because of their role in anabolism, health and signalling [37], and there has been a lot of attention on how dietary BCAA levels alter metabolic health and longevity in mammals [38]. Interestingly, Yu et al. [39] recently showed that the restriction of isoleucine or valine, but not leucine, can improve metabolic health in both normal and obese mice. These findings directly parallel the protective effects against nicotine of each of the BCAA restrictions in our flies, indicating a possible mechanistic overlap. However, we also note that in another recent study, Yap et al. [40] found that protection against obesity-related defects in mice was conferred by restriction of dietary threonine or tryptophan, but not the BCAAs. Perhaps small differences in treatment conditions, such as duration, intensity and dietary context, have implicated different amino acids as beneficial in these two mouse studies in the same way that we have observed their effects can vary for flies. As we home in on the mechanisms by which our amino acid diets afford protection against nicotine, we will gain insights into whether these same processes can also protect against the metabolic dysfunction associated with obesity.
The two major evolutionarily conserved amino acid sensors, mTORC1 and GCN2, initiate signalling in response to opposing nutritional signals. mTORC1 is activated by amino acids, while GCN2 becomes active in the absence of amino acids [41]. These complementary signals work together to maximize the use of amino acids for anabolism when they are available, and to protect cells against deficiencies for these essential nutrients. We found that pharmacological suppression of mTORC1 on a nutritionally complete diet could phenocopy the maximum protective effects of transient amino acid deprivation. We also found that when flies were null for GCN2, neither isoleucine deprivation nor threonine restriction was protective. Further, the beneficial effects of rapamycin were dampened in GCN2 null flies. These results indicate that both kinases contribute to protect flies against nicotine, and that functional GCN2 is required to promote the full protective effects of mTORC1 suppression.
Past work with bees (Apis mellifora) and the tobacco hornworm (Manduca sexta) has shown that nicotine detoxification is achieved via enzymes in the drug detoxification system that act to detoxify and excrete xenobiotic compounds [42,43]. Many of the genes important for this system are under the control of the Nrf2 (nuclear factor erythroid 2–related factor 2) transcriptional regulator, which binds to antioxidant response elements in the regulatory regions of these genes [44,45]. Our data indicate that reduced mTORC1 and activated GCN2 may work together to activate this system, potentially by activating Nrf2 [46]. If this is the case, we expect that transient deprivation for any one of the amino acids that induced nicotine protection would also induce protection against other xenobiotics that are neutralized by this system. Alternatively, dietary amino acid deprivation may protect flies by modifying the absorption and distribution of nicotine rather than its metabolism; investigating whether protection by diet is translated to toxins other than nicotine will help determine if this is the case. If the pre-treatment diets are indeed activating the drug detoxification pathways, they may also protect consumers against detrimental endobiotic toxins that arise as a by-product of metabolism. If true, this type of system could explain how low dietary amino acids can protect against acute exposure to a toxin as well as provide benefits to long-term health in the absence of toxins [47].
An interesting new finding in our study is that both dietary concentration and duration of exposure determine the efficacy for protecting the consumer against nicotine. One explanation for this is that these conditions differentially modify signalling activity of mTORC1 and GCN2, and this results in different levels of protection. This is a plausible explanation as we know that different amino acids change both mTORC1 activity and GCN2 activity to different extents [41,48]. We also note that while signalling triggered by amino acid dilution may be beneficial, the absence of an essential amino acid may have an antagonistic effect on consumer physiology and so limit protection. This could vary by amino acid and would be a function of the extent to which each amino acid is required for protection, the amount of the amino acid that can be safely scavenged from body stores, and the duration over which protective amino acid repurposing is required. Evidence for this can be found in our data: 7 day, short term, deprivations of isoleucine protected flies against nicotine, but longer term deprivation was detrimental to health and shortened lifespan, even in the absence of nicotine. A similar situation could be envisaged for the lack of protection we observed after methionine deprivation in our study, which contrasts its protective effects when restricted in other studies [11,32,49]. Methionine is both required for the synthesis of new proteins as well as being a source of cysteine to produce glutathione. Glutathione is an important peptide for conjugation to xenobiotics in phase II detoxification and its synthesis is upregulated in bees exposed to nicotine [42]. Thus, removing methionine from the diet may stimulate the signals for protection, but at the same time prevent the production of peptides and proteins that are required for resistance. A full characterization of this phenotype will therefore not only require a better understanding of the signalling involved, but also quantification of the amino acid requirements to build the protective systems being activated.
4. Conclusion
Our data reveal new insights into the way that dietary amino acid balance modifies organismal health. We found that restricting flies of an essential amino acid can protect them against subsequent chemical stress in a way that depends on interactions between the identity of the amino acid, the intensity of deprivation, and the duration of pre-treatment. This protection is mediated by interactions between GCN2 and mTORC1. This cross-modal hormesis paves the way for a better understanding of the environmental factors that alter insect susceptibility to pesticides and could open opportunities for the use of diets to help patients recover from planned treatments with toxins such as chemotherapy.
5. Methods
5.1. Fly husbandry
Experiments were conducted with adult, female, mated white-eyed Drosophila melanogaster (strain Dahomey; wDah) unless otherwise specified. wDah stocks are maintained outbred in continuous, overlapping generations that are housed in a high-density population cage at 25°C, ambient humidity and a 12:12 h light : dark cycle. The GCN2 null Drosophila melanogaster used were a gift from Dr. Sebastian Grönke and Professor Linda Partridge (Max Planck Institute for Biology of Ageing) and we introgressed the GCN2 null transgene into our wDah background. The GCN2 null stock is maintained at 18°C, 60% humidity and a 12 : 12 h light : dark cycle. All stocks are maintained on a sugar yeast (SY) diet [50].
5.2. Synthetic media
Chemically defined synthetic (holidic) media used in the experiments were prepared as described in [23] using the exome-matched (FLYaa) ratios of amino acids as described in [51]. Holidic media were prepared in advance and stored for up to four weeks at 4°C until required.
Nicotine-laced media were prepared by aliquoting 100 µl of diluted free-base nicotine (Sigma-Aldrich: N3876) in absolute ethanol onto the surface of cooled holidic media. The media were then kept at room temperature for 2 days to allow for the dispersion of nicotine before immediate use. Freshly laced media were prepared as required and were not stored.
Rapamycin-laced media were prepared by aliquoting 30 µl rapamycin (Jomar: S1039) dissolved in absolute ethanol onto the surface of cooled holidic media. The media were then kept at room temperature for 1 day to allow for the dispersion of rapamycin before storing at 4°C for up to 7 days until required.
Liquid holidic media used in CaFe assays were prepared by omitting agar, and they were stored in falcon tubes at −20°C until required. Nicotine-laced liquid holidic medium was prepared by aliquoting 9.96 µl of 100% free-base nicotine into 12 ml liquid medium (0.83 mg ml−1).
5.3. Nicotine assays
Age-matched cohorts of flies were generated for experiments in controlled population density using the methods described by Linford et al. [52]. Briefly, adult flies were kept on apple juice agar plates with live yeast paste and allowed to lay for 24 h, after which the eggs were collected in PBS and pipetted onto SY medium. Experimental flies were reared from egg to adult using SY medium and transferred to fresh SY medium as a mixed sex group to mate for 48 h post-eclosion. Flies were mildly anaesthetized with CO2 on day 2 of adulthood to be sorted by sex onto a complete synthetic diet. Unless otherwise specified, flies were transferred to their pre-treatment diet on day 7 of adulthood and transferred to nicotine-laced medium on day 14 of adulthood. During pre-treatment, survival was recorded using the software DLife [52] as flies were transferred to fresh vials each Monday, Wednesday and Friday. When exposed to nicotine, survival was recorded three times a day for 5 days or until all flies were dead. Experiments were conducted in controlled conditions: 25°C, 70% humidity and a 12 : 12 h light : dark cycle.
5.4. Capillary feeder (CaFe) assay
Flies for the CaFe assay were generated in the same way as for nicotine assays. On day 7 of adulthood, flies were individually aspirated into vials containing 3 ml of agar (7 g l–1) and a capillary (Sigma-Aldrich: Z543241) containing 5 µl of their assigned pre-treatment diet. Consumption was measured for each fly every 24 h, and the capillaries were blotted, rinsed and refilled with fresh medium at this time. On day 14 of adulthood, all flies were presented with capillaries containing a complete medium laced with 0.83 mg ml−1 nicotine. Food consumption continued to be measured every 24 h during treatment with nicotine and survival was recorded three times a day. To control for evaporation, volume lost from capillaries kept in vials with no flies was also measured. CaFe assays were conducted in controlled conditions: 25°C, 80% humidity and a 12 : 12 h light : dark cycle.
5.5. Whole-body triglyceride and protein content assays
Flies for the body composition assays were generated in the same way as for nicotine assays. On day 14 of adulthood, after 7 days of pre-treating with diet, groups of five flies were aspirated into cryovials, frozen in liquid nitrogen and stored at −80°C until measured. Frozen flies were transferred to 1.5 ml Eppendorf tubes with 500 µl 0.05% Tween 20 in water. Using a pestle, flies were homogenized then an additional 500 µl of 0.05% Tween 20 was added and the samples vortexed for 20 s. Samples were incubated at 70°C for 5 min and then centrifuged at 5000 rpm for 1 min. The supernatant was collected and centrifuged again at 14 000 rpm for 3 min. Finally, the supernatant was again collected and used for measurements.
For TAG measurements, 50 µl of sample or a dilution series of a glycerol standard was aliquoted into a 96-well plate. Samples were aliquoted in triplicate for TAG measurements and an additional well was aliquoted as a sample blank. 200 µl of Infinity Triglycerides liquid stable reagent (ThermoFisher: TR22421) was added to each well containing sample, except blanks, to which 200 µl of water was added.
For protein measurements, 10 µl of sample or BSA standard was aliquoted in triplicate into the same 96 well plate as TAG samples. The Pierce BCA Protein Assay Kit (ThermoFisher: 23 225) was used according to the manufacturer's instructions. Samples were measured for absorbance at 540 nm for TAG and 562 nm for protein.
To calculate the whole-body composition of flies, the absorbance values of TAG blanks were subtracted from the average of each TAG triplicate. The mass of TAG per fly was then calculated from the standard curve and then normalized by dividing by the average mass of protein per fly.
5.6. Figures and data analysis
We used R v. 4.1.1 [53] to conduct all analyses and we created all plots using ggplot2 [54]. All data and scripts are publicly available at https://doi.org/10.26180/16917355.
Survival analysis was completed using Cox Proportional-Hazards modelling. We used the ‘coxph’ function from the package survival [55] to model diet as a function of time to death after initial exposure to nicotine.
To measure differences in fecundity, and also whole-body fat and protein content, we fit a regression with pre-treatment diet as the independent variable in all cases. We then modelled these data using the base R function ‘aov’. Significantly different pairs of treatments were determined by performing a post hoc Tukey's HSD test on these models using the base R ‘TukeyHSD’ function.
Nicotine dose–response curves were modelled using a self-starting exponential model from the package drLumi [56]. A limitation of this type of modelling is that only two models can be compared at once. We generated comparisons in a fully factorial manner and then adjusted p-values to account for multiple tests using a Bonferroni correction.
Linear models (base R, ‘lm’) were used to model the effect of diet on the differences in mean survival across nicotine doses. We first normalized the data relative to the complete diet, fixing the response of the complete diet to y = 0. We then compared how switching or not switching diet during nicotine exposure affected normalized survival across nicotine doses. To find parsimonious models that best fit our data, we compared two nested models at a time using base R's ‘anova’. These models were nested such that only one variable was removed from the more complex model to form the reduced model. If the models were not significantly different from each other, the model with the lowest ‘AIC’ (base R) value, typically the reduced model, was used for further analysis. We found that a second-order polynomial linear model best fit these data, and the diets were compared as a factor within the same model using ‘emtrends’ from the emmeans package [57]. We then determined whether these trends were significantly different from each other using ‘cld’ from the multcomp package [58]. To determine if these diet switch models were different from the complete diet, we used ‘emmeans’ from the emmeans package [57] and tested if the models were different from 0.
Linear models (base R, ‘lm’) were also used to model both duration of pre-treatment and dose of amino acid as a function of mean survival. When analysing these relationships, we found that second-order polynomial models best fit the data using the process outlined above. To determine whether pre-treatment duration or availability of the focal amino acid significantly impacted survival, we analysed the model using an ANOVA (type II or III) from the package car (‘Anova’; [59]).
Acknowledgements
We thank Dr Sebastian Grönke and Professor Linda Partridge (Max Planck Institute for Biology of Ageing) for allowing us to use unpublished reagents, the GCN2 null fly line, in our experiments and this manuscript. Additionally, we extend thanks to Amy Dedman, Brooke Zanco and Krithika Balagopal (Monash University) for assistance with data collection during the peak of Melbourne's COVID-19 lockdown restrictions in 2020 and 2021. We are also grateful to Joshua Johnstone (Monash University) for help with backcrossing lines and maintaining fly stocks. Finally, we would like to thank Andrea Chan (Monash University) for her involvement in the initial stages of this project.
Contributor Information
Tahlia L. Fulton, Email: tahlia.fulton@monash.edu.
Matthew D. W. Piper, Email: matthew.piper@monash.edu.
Data accessibility
All data and scripts are publicly available at https://doi.org/10.26180/16917355 [60].
The data are provided in the electronic supplementary material [61].
Authors' contributions
T.L.F.: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft and writing—review and editing; C.K.M.: conceptualization, formal analysis, funding acquisition, methodology, supervision and writing—review and editing; M.D.W.P.: conceptualization, funding acquisition, methodology, resources, supervision and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
C.K.M. was funded by Australian Research Council (grant no. FT170100259); M.D.W.P. was funded by Australian Research Council (grant no. FT150100237) and National Health and Medical Research Council (grant no. APP1182330). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
References
- 1.Fontana L, Partridge L, Longo VD. 2010. Extending healthy life span—from yeast to humans. Science 328, 321-326. ( 10.1126/science.1172539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mirth CK, Nogueira Alves A, Piper MDW. 2019. Turning food into eggs: insights from nutritional biology and developmental physiology of Drosophila. Curr. Opin. Insect Sci. 31, 49-57. ( 10.1016/j.cois.2018.08.006) [DOI] [PubMed] [Google Scholar]
- 3.Späth M, Koehler F, Hoyer-Allo K, Grundmann F, Burst V, Müller R. 2020. Preconditioning strategies to prevent acute kidney injury [version 1; peer review: 2 approved]. F1000Research 9, F1000 Faculty Rev-237. ( 10.12688/f1000research.21406.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger JMS, Hwangbo DS, Corby-Harris V, Promislow DEL. 2007. The functional costs and benefits of dietary restriction in Drosophila. Aging Cell 6, 63-71. ( 10.1111/j.1474-9726.2006.00261.x) [DOI] [PubMed] [Google Scholar]
- 5.Jongbloed F, et al. 2017. A signature of renal stress resistance induced by short-term dietary restriction, fasting, and protein restriction. Sci. Rep. 7, 40901. ( 10.1038/srep40901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell JR, et al. 2010. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell 9, 40-53. ( 10.1111/j.1474-9726.2009.00532.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harputlugil E, Hine C, Vargas D, Robertson L, Manning BD, Mitchell JR. 2014. The TSC complex is required for the benefits of dietary protein restriction on stress resistance in vivo. Cell Rep. 8, 1160-1170. ( 10.1016/j.celrep.2014.07.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirzaei H, Suarez JA, Longo VD. 2014. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol. Metab. 25, 558-566. ( 10.1016/j.tem.2014.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson LT, et al. 2015. Protein and calorie restriction contribute additively to protection from renal ischemia reperfusion injury partly via leptin reduction in male mice. J. Nutrit. 145, 1717-1727. ( 10.3945/jn.114.199380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson JE, Johnson FB. 2014. Methionine restriction activates the retrograde response and confers both stress tolerance and lifespan extension to yeast, mouse and human cells. PLoS ONE 9, e97729. ( 10.1371/journal.pone.0097729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. 2005. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4, 119-125. ( 10.1111/j.1474-9726.2005.00152.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. 1993. Low methionine ingestion by rats extends life span. J. Nutrit. 123, 269-274. ( 10.1093/jn/123.2.269) [DOI] [PubMed] [Google Scholar]
- 13.Peng W, Robertson L, Gallinetti J, Mejia P, Vose S, Charlip A, Chu T, Mitchell JR. 2012. Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Sci. Transl. Med. 4, 118ra111. ( 10.1126/scitranslmed.3002629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallinetti J, Harputlugil E, Mitchell JR. 2013. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem. J. 449, 1-10. ( 10.1042/BJ20121098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glick D, Barth S, Macleod KF. 2010. Autophagy: cellular and molecular mechanisms. J. Pathol. 221, 3-12. ( 10.1002/path.2697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meijer AJ, Codogno P. 2004. Regulation and role of autophagy in mammalian cells. Int. J. Biochem. Cell Biol. 36, 2445-2462. ( 10.1016/j.biocel.2004.02.002) [DOI] [PubMed] [Google Scholar]
- 17.Ye J, Palm W, Peng M, King B, Lindsten T, Li MO, Koumenis C, Thompson CB. 2015. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev. 29, 2331-2336. ( 10.1101/gad.269324.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu X, et al. 2017. SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway. Science 358, 813-818. ( 10.1126/science.aao3265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. 2016. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43-48. ( 10.1126/science.aab2674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chantranupong L, et al. 2016. The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell 165, 153-164. ( 10.1016/j.cell.2016.02.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wek SA, Zhu S, Wek RC. 1995. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol. Cell. Biol. 15, 4497-4506. ( 10.1128/MCB.15.8.4497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu D, Dai W, Kutzler L, Lacko HA, Jefferson LS, Dennis MD, Kimball SR. 2019. ATF4-mediated upregulation of REDD1 and sestrin2 suppresses mTORC1 activity during prolonged leucine deprivation. J. Nutrit. 150, 1022-1030. ( 10.1093/jn/nxz309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piper M, et al. 2014. A holidic medium for Drosophila melanogaster. Nat. Methods 11, 100. ( 10.1038/nmeth.2731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyanagi T. 2007. Molecular mechanism of phase I and phase II drug-metabolizing enzymes: implications for detoxification. Int. Rev. Cytol. 260, 35-112. [DOI] [PubMed] [Google Scholar]
- 25.Liska DJ. 1998. The detoxification enzyme systems. Altern. Med. Rev. 3, 187-198. [PubMed] [Google Scholar]
- 26.Jones PM, George AM. 2004. The ABC transporter structure and mechanism: perspectives on recent research. Cell. Mol. Life Sci. 61, 682-699. ( 10.1007/s00018-003-3336-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Schuler MA, Berenbaum MR. 2006. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 52, 231-253. ( 10.1146/annurev.ento.51.110104.151104) [DOI] [PubMed] [Google Scholar]
- 28.Sang JH, King RC. 1961. Nutritional requirements of axenically cultured Drosophila Melanogaster adults. J. Exp. Biol. 38, 793-809. ( 10.1242/jeb.38.4.793) [DOI] [Google Scholar]
- 29.Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. 2007. Prandiology of Drosophila and the CAFE assay. Proc. Natl Acad. Sci. USA 104, 8253-8256. ( 10.1073/pnas.0702726104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattson MP. 2008. Hormesis defined. Ageing Res. Rev. 7, 1-7. ( 10.1016/j.arr.2007.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Condon KJ, Sabatini DM. 2019. Nutrient regulation of mTORC1 at a glance. J. Cell Sci. 132, jcs222570. ( 10.1242/jcs.222570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trocha K, et al. 2019. Preoperative protein or methionine restriction preserves wound healing and reduces hyperglycemia. J. Surg. Res. 235, 216-222. ( 10.1016/j.jss.2018.09.071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richie JP Jr, Leutzinger Y, Parthasarathy S, Maixoy V, Orentreich N, Zimmerman JA. 1994. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 8, 1302-1307. ( 10.1096/fasebj.8.15.8001743) [DOI] [PubMed] [Google Scholar]
- 34.Grandison RC, Piper MDW, Partridge L. 2009. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462, 1061-1064. ( 10.1038/nature08619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dick KB, Ross CR, Yampolsky LY. 2011. Genetic variation of dietary restriction and the effects of nutrient-free water and amino acid supplements on lifespan and fecundity of Drosophila. Genet. Res. 93, 265-273. ( 10.1017/S001667231100019X) [DOI] [PubMed] [Google Scholar]
- 36.Juricic P, Grönke S, Partridge L. 2020. Branched-chain amino acids have equivalent effects to other essential amino acids on lifespan and aging-related traits in Drosophila. J. Gerontol. Ser A Biol. Sci. Med. Sci. 75, 24-31. ( 10.1093/gerona/glz080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nie C, He T, Zhang W, Zhang G, Ma X. 2018. Branched chain amino acids: beyond nutrition metabolism. Int. J. Mol. Sci. 19, 954. ( 10.3390/ijms19040954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Couteur DG, Solon-Biet SM, Cogger VC, Ribeiro R, de Cabo R, Raubenheimer D, Cooney GJ, Simpson SJ. 2020. Branched chain amino acids, aging and age-related health. Age. Res. Rev. 64, 101198. ( 10.1016/j.arr.2020.101198) [DOI] [PubMed] [Google Scholar]
- 39.Yu D, et al. 2021. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab. 33, 905-922.e906. ( 10.1016/j.cmet.2021.03.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yap YW, et al. 2020. Restriction of essential amino acids dictates the systemic metabolic response to dietary protein dilution. Nat. Commun. 11, 1-13. ( 10.1038/s41467-019-13993-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu X, Guo F. 2021. Amino acid sensing in metabolic homeostasis and health. Endocr. Rev. 42, 56-76. ( 10.1210/endrev/bnaa026) [DOI] [PubMed] [Google Scholar]
- 42.du Rand EE, Smit S, Beukes M, Apostolides Z, Pirk CWW, Nicolson SW. 2015. Detoxification mechanisms of honey bees (Apis mellifera) resulting in tolerance of dietary nicotine. Sci. Rep. 5, 11779. ( 10.1038/srep11779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snyder MJ, Hsu EL, Feyereisen R. 1993. Induction of cytochrome P-450 activities by nicotine in the tobacco hornworm, Manduca sexta. J. Chem. Ecol. 19, 2903-2916. ( 10.1007/BF00980591) [DOI] [PubMed] [Google Scholar]
- 44.Itoh K, et al. 1997. An Nrf2/Small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236, 313-322. ( 10.1006/bbrc.1997.6943) [DOI] [PubMed] [Google Scholar]
- 45.Kensler TW, Wakabayashi N, Biswal S. 2007. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 47, 89-116. ( 10.1146/annurev.pharmtox.46.120604.141046) [DOI] [PubMed] [Google Scholar]
- 46.Hine CM, Mitchell JR. 2012. NRF2 and the phase Ii response in acute stress resistance induced by dietary restriction. J. Clin. Exp. Pathol. S4, 7329. ( 10.4172/2161-0681.S4-004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gems D, McElwee JJ. 2005. Broad spectrum detoxification: the major longevity assurance process regulated by insulin/IGF-1 signaling? Mech. Ageing Dev. 126, 381-387. ( 10.1016/j.mad.2004.09.001) [DOI] [PubMed] [Google Scholar]
- 48.Palii S, Kays C, Deval C, Bruhat A, Fafournoux P, Kilberg M. 2009. Specificity of amino acid regulated gene expression: analysis of genes subjected to either complete or single amino acid deprivation. Amino Acids 37, 79-88. ( 10.1007/s00726-008-0199-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hine C, et al. 2015. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160, 132-144. ( 10.1016/j.cell.2014.11.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bass T, Grandison R, Wong R, Martinez P, Partridge L, Piper M. 2007. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 62, 1071-1081. ( 10.1093/gerona/62.10.1071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piper MDW, et al. 2017. Matching dietary amino acid balance to the in silico-translated exome optimizes growth and reproduction without cost to lifespan. Cell Metab. 25, 610-621. ( 10.1016/j.cmet.2017.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linford NJ, Bilgir C, Ro J, Pletcher SD. 2013. Measurement of lifespan in Drosophila melanogaster. J. Visualized Exp. 71, e50068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Team, R.C. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 54.Wickham H. 2016. Ggplot2: elegant graphics for data analysis. New York, NY: Springer. [Google Scholar]
- 55.Therneau T. 2021. A package for survival analysis in R. R package version 3.2-11. See https://CRAN.R-project.org/package=survival.
- 56.Sanz H, Aponte J, Harezlak J, Dong Y, Murawska M, Valim C. 2015. Drlumi: multiplex immunoassays data analysis. R package version 0.1.2. See https://CRAN.R-project.org/package=drLumi.
- 57.Lenth R. 2021. emmeans: estimated marginal means, aka least-squares means. R package version 1.7.0. See https://rdrr.io/cran/emmeans. [Google Scholar]
- 58.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom. J. 50, 346-363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 59.Fox J, Weisberg S. 2019. An R companion to applied regression, 3rd edition. Thousand Oaks, CA: Sage. See https://socialsciences.mcmaster.ca/jfox/Books/Companion/. [Google Scholar]
- 60.Fulton TL, Mirth CK, Piper MDW. 2022. Data from: Restricting a single amino acid cross-protects Drosophila melanogaster from nicotine poisoning through mTORC1 and GCN2 signalling. Monash University. ( 10.26180/16917355) [DOI] [PMC free article] [PubMed]
- 61.Fulton TL, Mirth CK, Piper MDW. 2022. Restricting a single amino acid cross-protects Drosophila melanogaster from nicotine poisoning through mTORC1 and GCN2 signalling. Figshare. ( 10.6084/m9.figshare.c.6328763) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Fulton TL, Mirth CK, Piper MDW. 2022. Data from: Restricting a single amino acid cross-protects Drosophila melanogaster from nicotine poisoning through mTORC1 and GCN2 signalling. Monash University. ( 10.26180/16917355) [DOI] [PMC free article] [PubMed]
- Fulton TL, Mirth CK, Piper MDW. 2022. Restricting a single amino acid cross-protects Drosophila melanogaster from nicotine poisoning through mTORC1 and GCN2 signalling. Figshare. ( 10.6084/m9.figshare.c.6328763) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data and scripts are publicly available at https://doi.org/10.26180/16917355 [60].
The data are provided in the electronic supplementary material [61].