Abstract
Increased recognition of the prevalence of human babesiosis in the United States, together with rising concern about the potential for transmission of this infection by blood transfusion, has provided motivation to develop definitive serologic and molecular tests for the causative agent, Babesia microti. To develop more sensitive and specific assays for B. microti, we screened a genomic expression library with patient serum pools. This screening resulted in the identification of three classes of novel genes and an additional two novel, unrelated genes, which together encode a total of 17 unique B. microti antigens. The first class (BMN1-2 family) of genes encodes seven closely related antigens with a degenerate six-amino-acid repeat that shows limited homology to Plasmodium sp. merozoite and sporozoite surface antigens. A second class (BMN1-8 family) of genes encodes six related antigens, and the third class (BMN1-17 family) of genes encodes two related antigens. The two remaining genes code for novel and unrelated sequences. Among the three classes of antigens and remaining novel sequences, five were chosen to code for the most immunodominant antigens (BMN1-2, -9, -15, and -17 and MN-10). Western blot analysis with the resulting recombinant proteins indicated that these antigens were targets of humoral immune responses during B. microti infection in humans.
Human babesiosis is a malaria-like illness that is most frequently caused by tick-transmitted intraerythrocytic parasites of the genus Babesia. In the eastern and upper Midwestern United States, Babesia microti is the most common zoonotic agent (8, 9, 20, 26, 32), whereas other species and types of Babesia (WA1, CA1, and MO1) predominate in Europe and areas of the United States where B. microti is not endemic (2, 11, 14, 29, 33). B. microti is transmitted to humans by the same tick (Ixodes scapularis) that is responsible for the transmission of Lyme disease (Borrelia burgdorferi infection) and human granulocytic ehrlichiosis (HGE) (31). The common reservoir for these organisms is the white-footed deer mouse (Peromyscus leucopus) (25), which ranges throughout North America. Deer mice are the preferred host for larval I. scapularis, while the adult tick prefers white-tailed deer as a host. The nymph stage is more indiscriminate and may feed on humans (11). With improved surveillance in the United States, and due to an expanding population of wild deer and increased encroachment of humans on rural areas, the number of reported cases of babesiosis appears to be increasing (19, 35).
Babesial parasitemia may persist in asymptomatic hosts for several months to years after initial infection, and this has led to concerns about blood safety in areas of endemicity (18). Although the risk of transmitting babesiosis through blood transfusions appears to be low, there are now many documented cases in which it has occurred and blood banks are becoming increasingly aware of this potential concern (5, 6, 7, 10). At least 26 cases of transfusion-transmitted babesiosis have been reported in the United States to date (6), and some investigators have suggested that its frequency is likely to rise in the future (18).
Currently, clinical diagnosis of babesiosis is performed in combination with other test procedures. These include (a) blood smear analysis for direct observation of intraerythrocytic parasites (22), (b) inoculation of small mammals with patient blood, and (c) the use of an indirect immunofluorescent antibody (IFA) test using fixed organisms (26). These methods are relatively insensitive, expensive to perform, and/or labor-intensive. Blood smears are frequently used but often lead to false-negative results due to the low parasite burden often seen for B. microti infections, as well as the small size of the parasite. Injection of hamsters or gerbils with patient blood is sensitive, but it is a slow and expensive process (21). A PCR test is available for diagnosis of B. microti infections based on the use of ribosomal DNA probes for B. microti (3, 30). This test, while highly sensitive and specific, is not currently applicable either to the doctor's office or for high-throughput testing. Therefore, there is a need to identify new B. microti antigens for use in developing an inexpensive, rapid-format diagnostic test for early diagnosis and potentially for use in vaccine development. In this paper, we describe the serological expression cloning of several novel genes for B. microti. Diagnostic tests based on recombinant antigens expressed by these genes could be useful in detecting carriers of B. microti and would enable development of tests that could potentially be used for blood screening.
MATERIALS AND METHODS
Genomic expression library construction.
B. microti genomic DNA (MN1 strain; Mayo Clinic, Rochester, Minn.) was isolated from infected hamster blood with an ion-exchange column (Qiagen Inc., Valencia, Calif.). Twenty micrograms of total genomic DNA was sonicated (B. Braun Biotech, Inc., Allentown, Pa.) to generate fragments of approximately 0.5 to 5.0 kbp. DNA fragments were blunted with T4 DNA polymerase (Gibco BRL, Grand Island, N.Y.) and ligated to EcoRI adapters (Stratagene, La Jolla, Calif.). Adapted inserts were then phosphorylated with T4 polynucleotide kinase (Stratagene) and size selected with a Sephacryl S-400-HR column (Sigma Chemical Co., St. Louis, Mo.). Insert DNA was ligated to Lambda ZAP II, an EcoRI-calf intestinal alkaline phosphatase-treated vector (Stratagene), and the ligation mix was packaged with Gigapack II Gold packaging extract (Stratagene).
Expression screening.
Immunoreactive proteins were screened from approximately 3 × 105 PFU with nitrocellulose filters (Schleicher and Schuell, Keene, N.H.). Reactive plaques were assessed with Escherichia coli-adsorbed B. microti-infected patient serum pools (a pool of five high-titer patients for BMN1 clones and a pool of four low-titer patients for MN clones). Positive plaques were visualized with 125I-conjugated protein A (NEN Life Science Products, Boston, Mass.) or with an alkaline phosphatase-conjugated goat anti-human immunoglobulin G (heavy and light) secondary antibody (Zymed Laboratories Inc., South San Francisco, Calif.), developed with nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (Gibco BRL). Excision of phagemid followed the Lambda ZAP II protocol (Stratagene), and resulting plasmid DNA was sequenced with an automated sequencer, model 377 (Perkin-Elmer/ABI, Foster City, Calif.) using M13 forward, reverse, and internal DNA sequencing primers. Nucleic acid and protein homology searches were performed with DNAStar (Madison, Wis.) against the EMBL-GenBank release 99 and the Swiss, PIR, and Translated release 97. Predicted protein translocation sites were analyzed with the PSORT program (National Institute for Basic Biology, Okazaki, Japan).
Expression and purification of recombinant protein.
Expression of recombinant protein was accomplished by amplifying the plasmid insert with Pfu polymerase (Stratagene) and clone-specific primers (25 to 30 nucleotides [nt]) which included a 5′ NdeI restriction site (italics), an ATG initiation codon (underlined), and a nucleotide sequence coding for six histidines (boldface) (CAATTACATATGCATCACCATCACCATCAC---) and a gene-specific 3′ primer with a stop codon and an EcoRI restriction site. The amplification product was digested with the restriction enzymes NdeI and EcoRI (Gibco BRL), gel isolated, and ligated to a pET-17b plasmid vector (Novagen, Madison, Wis.) previously digested with NdeI and EcoRI and dephosphorylated. The ligation mix was transformed into XL1 Blue competent cells (Stratagene) and plasmid DNA was prepared for sequencing (Qiagen Inc.). Methods for recombinant protein expression and purification have been previously described (34). Recombinant protein was quality checked for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by staining with Coomassie blue stain and by N-terminal protein sequencing (27) and quantified with a Micro BCA assay (Pierce, Rockford, Ill.). Recombinants were also assayed for endotoxin contamination with the Limulus assay (Bio Whittaker, Walkersville, Md.).
Study population.
Serum samples from individuals infected with B. microti, B. burgdorferi, and the agent responsible for HGE (verified by IFA test and/or PCR) were obtained from David Persing (Mayo Clinic) or from Peter J. Krause (University of Connecticut School of Medicine, Farmington). These serum samples represent a broad geographic range of B. microti infection including the upper Midwest and East Coast of the United States. Random donor sera were obtained from Boston Biomedica Inc. (West Bridgewater, Mass.).
ELISA.
Ninety-six-well microtiter plates (Corning Costar, Cambridge, Mass.) were coated overnight at 4°C with recombinant proteins (200 ng/well). Plates were then aspirated and blocked with phosphate-buffered saline (PBS) containing 1% (wt/vol) bovine serum albumin for 2 h at room temperature. This was followed by washing in PBS containing 0.1% Tween 20 (PBST). Serum (1/50) diluted in PBS containing 0.1% bovine serum albumin was added to wells and incubated for 30 min at room temperature, followed by washing six times with PBST and then incubating with protein A-horseradish peroxidase conjugate (1/20,000 dilution; Sigma Chemical Co.) for a further 30 min. Plates were then washed six times in PBST and then incubated with tetramethylbenzidine substrate (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) for an additional 15 min. The reaction was stopped by the addition of 1 N sulfuric acid, and plates were read at 450 nm using an enzyme-linked immunosorbent assay (ELISA) plate reader (Biotek Instrument EL311; Hyland Park, Va.). The cutoff for assays was determined from the mean of the negative population plus 3 standard deviations of the mean.
Western blot analysis.
Recombinant antigens (200 ng/lane) were subjected to SDS-PAGE using 15% polyacrylamide minigels. The antigens were transferred to nitrocellulose BA-85 (Schleicher and Schuell) and blocked for 1 h at room temperature with PBST. Blots were then washed three times for 10 min each in PBST and 0.5 M sodium chloride (wash buffer). Next, blots were probed for 1 h at room temperature with serum diluted 1:500 in wash buffer followed by three washes of 10 min each in wash buffer. Blots were then incubated for 45 min at room temperature with protein A-horseradish peroxidase diluted 1:20,000 in wash buffer and again washed three times for 10 min each in wash buffer. Finally, blots were incubated in chemiluminescent substrate (ECL kit; Amersham Plc, Little Charlton, United Kingdom) for ∼1 min and then exposed to X-ray film (XAR5) for 10 to 60 s as required.
RESULTS
Expression cloning and molecular characterization of B. microti antigens.
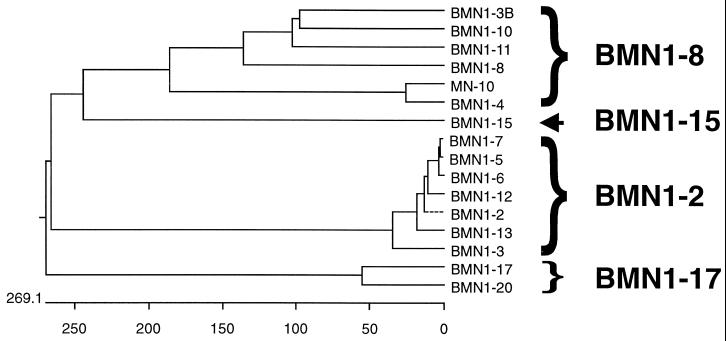
A randomly sheared B. microti genomic expression library was screened with pooled sera from infected individuals. Seventeen clones were recovered from this screening (bmn1-1 to -13, -15 to -17, and -20), and a subsequent screening with a single patient serum resulted in an additional clone (mn-10). Serum from this patient, although B. microti positive by IFA test, was not reactive with our recombinant Babesia proteins. DNA sequences and predicted protein sequences were obtained and used to determine the novelty of the clones through database searches and to direct the synthesis of recombinant polypeptides where appropriate (Fig. 1 shows relationships among predicted protein sequences). Predicted protein sequences from 9 of the 18 clones (bmn1-1, -2, -3, -5, -6, -7, -12, -13, and -16) show a high degree of homology with each other (83 to 98%); two of these clones (bmn1-1 and -16) are truncations of bmn1-3. The molecular masses of the predicted full-length proteins for bmn1-2, -3, -6, -7, and -13 are 35.1, 40.7, 33.3, 31.6, and 30.1 kDa, respectively, and GenBank accession numbers are AF206244, AF206245, AF206249, AF206250, and AF206524, respectively. Accession numbers for partial clones bmn1-5 and bmn1-12 are AF206248 and AF206523, respectively. These clones apparently represent a family of genes that are highly similar at the amino termini (N) and carboxy regions (C) (over 90% identity) and differ at the extreme C termini and midsections, which contain degenerate, six-amino-acid (6-aa) repeats (Fig. 2). This region contains from 6 to at least 22 repeats and shows limited homology to the repeat region of the Plasmodium sp. circumsporozoite and merozoite surface antigens (approximately 35 and 38% identity and 61 and 85% conservation, respectively) and to the Plasmodium falciparum soluble S antigen (45% identity and 64% conservation) (accession no. M11031, L22688, and P09593, respectively). In addition, the repeat regions show some similarity to mammalian neurofilament triplet H protein (46% identity and 75% conservation with accession no. A43778). These antigens appear to have a cleavable N-terminal signal sequence and could represent cell surface or secreted proteins, as antigen BMN1-3 contains a transmembrane domain at the carboxy terminus and is predicted to be a type Ia plasma membrane protein (Fig. 3). The genomic location of these sequences is predicted to be telomere proximal, as five of the nine genomic clones contain telomeric repeat sequence (TAAACCC) 210 nt upstream from the open reading frames (ORFs). Two clones (bmn1-2 and -6) contain telomeric repeats of at least 760 and 165 nt, respectively, at their 5′ ends, while three clones (bmn1-5, -7, and -16) contain internal telomeric repeats of approximately 100 to 170 nt.
FIG. 1.
Relationships of protein sequences predicted from B. microti genomic clones. Clones were screened from a B. microti genomic expression library with sera from patients with babesiosis. Braces indicate groupings of related antigens that are referred to as the BMN1-2, BMN1-8, and BMN1-17 families. Predicted proteins from clones bmn1-9 (not shown) and bmn1-15 (arrow) were novel and not related to the indicated groups. Relationships were determined with the MegAlign (Clustal) program from DNAStar Inc. Branch length represents the average distance between sequence pairs, while units at the bottom indicate the numbers of substitution events. A dotted line indicates a negative branch length.
FIG. 2.
Alignment of the predicted amino acid sequence of representative members of the bmn1-2-like antigen family. Partial ORFs from clones bmn1-1, -5, -12, and -16 were not included in the analysis. Alignments were constructed with the MegAlign (Clustal) program. Identities among sequences are shown in black, while conservative replacements, using the PAM250 table, are indicated by gray shading. Dashed lines indicate gaps in the sequences. The predicted signal sequence and cleavage site (↓) are indicated at the top. The location of the first 6-aa repeat is also shown. A predicted transmembrane domain is identified at the carboxy terminus by a thick line.
FIG. 3.
Alignment of the predicted amino acid sequence of representative members of the bmn1-8-like antigen family. Partial ORFs from clones bmn1-3B and -4 and mn-10 were not included in the analysis. Partial clone bmn1-11 is missing the N-terminal conserved region. Alignments were constructed with the MegAlign (Clustal) program. Identities among sequences are shown in black, while conservative replacements, using the PAM250 table, are indicated by gray shading. Dashed lines indicate gaps in the sequences. The predicted cytoplasmic, transmembrane, and extracellular domains are indicated at the top.
A second potential family of genes includes predicted protein sequences from clones (bmn1-3B, bmn1-4/mn-10, and bmn1-8, -10, and -11) that show high similarity at the N and C termini with a less conserved middle region (Fig. 3 shows three representative sequences; accession no. are AF206245, AF206246/AF206247, AF206251, AF206253, and AF206522, respectively). Protein database searches showed very weak homology (20%) to P. falciparum predicted proteins (accession no. AE001407 and AE001385). Clones bmn1-3B, -4, and -11 and mn-10 represent partial genes, and clones bmn1-4 and mn-10 are overlapping segments of the same gene. bmn1-4/mn-10 has high similarity to other family members only in the N-terminal region of homology and also differs from other family members in having a short degenerate repeat at the carboxy terminus (four repeats of 14 to 19 aa). Protein analysis of full-length clones bmn1-8 and -10 (with predicted molecular masses of 70.7 and 54.3 kDa, respectively) indicated that these predicted polypeptides have an uncleavable N-terminal signal sequence and are type II membrane proteins with a transmembrane domain from aa 8 to 24 and 9 to 25 and a cytoplasmic tail from aa 1 to 7 and 1 to 8, respectively. Clone bmn1-3 contains a full-length copy of a bmn1-2-like gene (bmn1-3) and a partial copy of a bmn1-8-like gene (bmn1-3B) 604 nt downstream and on the opposite strand.
Two clones, bmn1-17 and -20 (accession no. AF206526 and AF206527, respectively), contain partial ORFs with highly conserved predicted protein sequences (98%) and may represent a third group or family of proteins (Fig. 1). bmn1-17 contains a 445-aa ORF with a C-terminal truncation, and protein sequence analysis indicates that it could be a type II membrane protein. bmn1-20 contains the N-terminal 275 aa of an ORF, but the predicted protein sequence is extended farther 5′ than bmn1-17. This sequence contains a cleavable N-terminal signal sequence and is predicted to be extracellular. Database searches indicate a weak homology (20%) to a P. falciparum predicted secreted protein (accession no. AE001373). The remaining clones, including bmn1-9 and -15 (accession no. AF206252 and AF206525, respectively), represent partial ORFs and contained novel unrelated sequences.
Recombinant protein expression and purification.
Five clones were chosen for high-level protein expression with the pET-17b vector (Novagen) (bmn1-2, -9, -15, and -17 and mn-10 [carboxy terminus]). These expression constructs were engineered to include an N-terminal six-histidine tag for ease of purification with an Ni-nitrilotriacetic acid agarose column. Recombinant constructs were 948, 900, 1,416, 1,311, and 705 nt in length, and expressed recombinant proteins were 33.4, 32.7, 51.8, 49.4, and 23.2 kDa, with pIs of 5.0, 6.0, 5.3, 4.8, and 6.2 for clones bmn1-2, -9, -15, and -17 and mn-10, respectively. Purification of rBMN1-9 and -17 and rMN-10 yielded 0.3, 0.4, and 0.25 mg/ml with endotoxin levels of 367, 250, and 90 endotoxin units per mg, respectively. Two recombinants, rBMN1-2 and -15, were gel isolated with a Bio-Rad Whole Gel Eluter which yielded 0.2 and 0.17 mg/ml with endotoxin levels of 0.0 and 123 endotoxin units per mg of protein, respectively. Recombinant proteins were visualized by SDS-PAGE and Coomassie blue staining and estimated to be over 90% pure.
Western blot analysis of recombinant antigens.
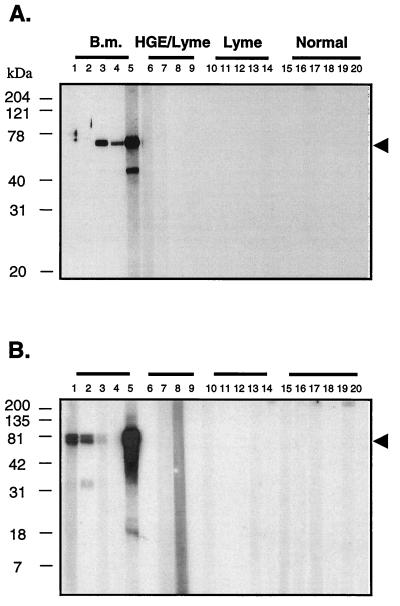
Western blot analyses were performed with four of the recombinant proteins to further characterize their reactivities and to identify the molecular size of the expressed proteins that were immunologically reactive. Examples of the Western blot analysis of the antigens rBMN1-15 and -17 are shown in Fig. 4 and with Babesia, Ehrlichia, and Lyme disease agent-infected patient sera as well as sera from uninfected individuals. Western blot data for rBMN1-2, -15, and -17 and rMN-10 are summarized from duplicate experiments in Table 1. Briefly, rBMN1-2, -15, and -17 and rMN-10 were reactive with three, four, five, and four of five sera from Babesia-infected patients, respectively, and not reactive with Ehrlichia infection (3) or Lyme disease (6) sera or with noninfection sera (6). Also, patterns of reactivity were different among recombinants such that a combination of two recombinant proteins was reactive with all Babesia infection sera (Fig. 4). In all cases, the species reactive with the B. microti-positive sera in the Western blot analysis corresponded to both the predicted mass and the Coomassie blue-stained SDS-PAGE gel of the respective recombinant proteins.
FIG. 4.
Western blot analysis for the recombinants rBMN1-15 (A) and rBMN1-17 (B) with infection and normal sera. Lanes 1 to 5 are B. microti-positive sera (titers for B. microti are 1,024, 4,096, 256, 64, and 1,024, respectively; samples 3, 4, and 5 are Lyme disease agent coinfections); lanes 6 to 9 are Ehrlichia-Lyme disease agent-positive sera, with the exception of sample 8, which is predominantly Lyme disease; lanes 10 to 14 are Lyme disease sera; and lanes 15 to 20 are normal control sera. Each lane contains ∼200 ng of recombinant protein. Protein A-horseradish peroxidase was used as conjugate, and blots were developed with ECL chemiluminescent substrate and Hyperfilm ECL. Arrowheads indicate the migration of the recombinant proteins. B.M., B. microti.
TABLE 1.
Summary of Western blot reactivity for immunodominant recombinant antigens rBMN1-2, -15, and -17 and rMN10
| Sample | Status | Babesia IFA titer | Result of Western blot analysisa
|
|||
|---|---|---|---|---|---|---|
| BMN1-2 | BMN1-15 | BMN1-17 | MN-10 | |||
| Bm8 | Babesia | 1,024 | ++ | + | +++++ | + |
| Bm21 | Babesia | 4,096 | ++ | − | ++++ | ++++ |
| Corixa 4 | Babesia | 256 | ± | ++++ | +++ | + |
| Corixa 5 | Babesia | 64 | ± | ++ | + | − |
| 252 | Babesia | >1,024 | ++++ | ++++++ | ++++++ | +++ |
| DB | HGE | <64 | − | − | − | − |
| GW | HGE | <64 | − | − | − | − |
| Corixa 34 | HGE | <64 | − | − | − | − |
| MAD | HGE | <64 | − | − | − | − |
| AC | Lyme | <64 | − | − | − | − |
| DW | Lyme | <64 | − | − | − | − |
| TA | Lyme | <64 | − | − | − | − |
| TO | Lyme | <64 | − | − | − | − |
| DZ | Lyme | <64 | − | − | − | − |
| A6-1 | Donor | NDc | − | − | − | − |
| A6-2 | Donor | ND | − | − | − | − |
| A6-3 | Donor | ND | − | − | − | − |
| A6-4 | Donor | ND | − | − | − | − |
| A6-5 | Donor | ND | − | − | − | − |
| A6-6 | Donor | ND | − | − | − | − |
−, undetectable reactivity; ±, equivocal reactivity; + to ++++++, increasing reactivity (determined empirically).
Lyme, Lyme disease.
ND, not determined.
ELISA evaluation of B. microti recombinant antigens.
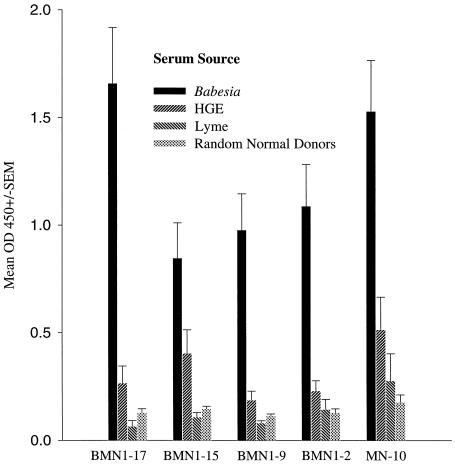
The reactivity of the recombinant proteins was compared to antibody titers obtained using the indirect fluorescence assay (IFA) typically used in diagnosing B. microti infections. Table 2 shows representative data for sera from patients with active babesiosis, Lyme disease, HGE, or coinfections and from healthy individuals (random donors) with the recombinant proteins rBMN1-2, -15, and -17 and rMN-10. Although the patterns of reactivity of the four recombinants are very different, these recombinants are capable of detecting samples that are also reactive in immunofluorescence assays, particularly if a combination of recombinants were to be used (Table 2; BMN1-17/MN-10). A sensitivity and specificity of approximately 98% each were achieved with a combination of recombinants BMN1-17 and MN-10. All Babesia infection sera were detected, except sample Bm22, a resolved, transfusion-acquired infection sample which was PCR negative at the time of collection and which had a low IFA titer (1:128). The Babesia-negative and Ehrlichia (HGE)- and Lyme disease agent-positive infection sera showed little or no reactivity with the recombinants, as did the random donor control sera. Figure 5 shows the mean optical density values for the infection sera and controls tested with the recombinant antigens listed above plus rBMN1-9. Reactivities of Babesia-positive sera are significantly higher than those of all other groups assayed. Elevated values for Ehrlichia-positive sera could be due to silent B. microti infection; low-level infections; infection with other types of Babesia, such as WA1; or nonspecific reactivity due to high serum antibody titers. When taken together, these data indicate the high specificity and sensitivity of the recombinants with B. microti infection sera versus other tick-borne infections and healthy controls.
TABLE 2.
ELISA reactivity of B. microti recombinants rBMN1-2, -15, and -17 and rMN-10 with sera from individuals with B. microti infections, Lyme disease, HGE, or coinfections as well as from random donors
| Antigen | ELISA resulta
|
||||
|---|---|---|---|---|---|
| Infection serum panel (IFA positive)
|
Controls (random normal donors) | ||||
|
Babesia positive
|
Babesia negative
|
||||
| Babesia | Babesia-Ehrlichia | Ehrlichia | Lyme disease agent | ||
| BMN1-2 | 27/40 | 2/3 | 1/4 | 0/10 | 1/73 |
| BMN1-15 | 31/41 | 3/3 | 0/4 | 1/10 | 0/40 |
| BMN1-17 | 33/40 | 3/3 | 0/4 | 0/10 | 0/86 |
| MN-10 | 31/39 | 3/3 | 0/4 | 1/10 | 1/98 |
| BMN1-17–MN-10 | 39b/40 | 3/3 | 0/4 | 1/10 | 1/98 |
Data are shown as number of ELISA-positive samples/total number of IFA-positive samples. The cutoff for the assays was determined from the mean of the normal random donor population plus 3 standard deviations of the mean.
One sample that was not detected, Bm22, is a resolved, transfusion-acquired infection sample which was PCR negative at the time of collection and had a low IFA titer (1:128).
FIG. 5.
Summary of ELISA reactivity with recombinant antigens rBMN1-2, -9, -15, and -17 and rMN-10 and sera from patients with B. burgdorferi-B. microti infection (n = 25), Ehrlichia (HGE)-B. burgdorferi infection (n = 10), or Lyme disease (B. burgdorferi) (n = 10) and control sera (n = 32 to 54) depending on specific recombinant. Bars indicate the mean optical density at 450 nm plus 1 standard error of the mean (SEM). Cutoff values for each assay were determined from the mean of the normal random donor population plus 3 standard deviations of the mean.
DISCUSSION
In contrast to the situation with B. microti, molecular biological methods have been extensively applied to other Babesia species of significance to the veterinary diagnostic market. Novel recombinant antigens for use in serodiagnostic tests for other Babesia species such as Babesia equi, Babesia canis, Babesia caballi, Babesia bigemina, and Babesia bovis have been described previously (16, 17, 28, 36). Such antigens may provide insights into immunodominant antigens that may be expected for B. microti. In particular, several recombinant merozoite antigens have been identified as immunodominant and have diagnostic significance. For instance, in B. bigemina a family of at least nine related but not identical genes were identified by expression cloning and shown to encode a family of proteins containing an internal repeating motif that was linked to a highly conserved C-terminal portion. This family of genes coded for native proteins in the 50- to 70-kDa region (35). Immunoreactive merozoite antigens of 42, 44, 60, 77, and 225 kDa have also been purified from B. bovis (16). In addition, two recombinant antigens were expression cloned from B. bovis, Bv80 (80-kDa protein) and BvVA1 (homologous to a 225-kDa protein) (4). Both of these proteins contained a repeating peptide motif with relatively conserved amino- and carboxy-terminal regions.
In this paper, we describe the expression cloning of novel, seroreactive B. microti antigens that fall into two and possibly three families (BMN1-2, -8, and -17) and two additional antigens, BMN1-9 and -15. Antigens in the BMN1-2 family show a high degree of homology but differ at the extreme carboxy termini and in the 6-aa repeat region where they differ in type and number of repeats (6 to at least 22 repeats) (15). This repeat region shows limited homology to P. falciparum circumsporozoite and merozoite surface antigens and the soluble S antigens (accession no. M11031, L22688, and P09593, respectively). Although antigens in the BMN1-2 family have a predicted cleavable N-terminal signal sequence and no predicted transmembrane regions, except antigen BMN1-3, which is predicted to be a type 1a plasma membrane protein, they do have hydrophobic regions near the C terminus and thus could represent secreted or membrane-bound proteins. Examples of similar proteins include the type Ia Plasmodium circumsporozoite proteins, which also have a cleavable signal sequence and a C-terminal transmembrane domain (accession no. J02695). Insight into the genomic location and a possible mechanism for antigenic variation within the BMN1-2 gene family comes from the proximity of telomeric repeats to these genes. Five of nine independent genomic clones contain a telomeric repeat sequence 210 nt upstream from the ORFs. Two of these clones contain longer stretches of terminal repeat sequence (at least 760 nt for bmn1-2), while three contain internal short regions of telomeric repeat sequence (100 to 170 nt). These internal telomeric repeat sequences could represent chromosome breakage near the genes and healing by telomere addition or rearrangement and deletion events involving subtelomeric regions (23, 24). Among parasitic protozoans, chromosomes are divided into conserved central domains and polymorphic chromosome ends, which contain many antigen-encoding genes. This compartmentalization of antigens in chromosome extremities in P. falciparum may have led to the scattering of gene families on several chromosome ends (12). For example, the var genes of P. falciparum, which encode the variant erythrocyte surface antigens (PfEMP1), are clustered both in the conserved central region and among repetitive subtelomeric sequences on several chromosomes (13). Thus, the genomic location of at least some members of the B. microti BMN1-2 family is proposed to be telomere proximal, a location which could account for variation seen within this family through rearrangement events within and among chromosomes (1).
A second possible family of antigens is predicted from clones bmn1-3(B), -4, -8, -10, and -11 and mn-10 (BMN1-8 family). The predicted proteins from these clones share highly similar N and C termini and diverge in the middle region with the exception of clone bmn1-4/mn-10, which is similar only at the N terminus. Although these polypeptides share very weak homology with P. falciparum hypothetical proteins, functional information can be obtained by analysis of predicted protein translocation sites. Predicted full-length polypeptides from bmn1-8 and -10 (70.7 and 54.3 kDa, respectively) appear to have uncleavable N-terminal signal sequences and are predicted to be type II plasma membrane proteins with short cytoplasmic tails (7 to 8 aa) followed by transmembrane domains of 17 aa. Thus, these proteins could represent surface antigens with large extracellular domains. Genomic location and mechanism for antigenic variation, as with the BMN1-2 family, can be extrapolated from clone sequence information. Genomic clone bmn1-3 contains a full-length copy of a bmn1-2-like gene, referred to as bmn1-3, and, on the opposite strand, a partial copy of a bmn1-8-like gene (bmn1-3B) approximately 600 nt downstream. Because the BMN1-2 family is associated with telomeric repeats, at least some members can be predicted to have a subtelomeric genomic location. The proximity of the bmn1-2-like and bmn1-8-like genes could indicate that these genes are compartmentalized to the same chromosomal regions. If so, the same mechanisms for antigenic variation, such as chromosomal rearrangement and recombination, could be occurring within both the BMN1-2 and the BMN1-8 antigen families. However, one cannot exclude the possibility that variation among family members is a result of cloning artifacts or is due to the source of genomic DNA being a B. microti isolate.
A third likely antigen family is represented by partial clones bmn1-17 and -20 (both contain C-terminal truncations) which show 98% aa identity. bmn1-17 contains a 445-aa ORF with six internal repeats of 32 aa and is predicted to be a type II membrane protein. bmn1-20 contains only a partial ORF (N-terminal 275 aa), but the predicted protein sequence is extended farther 5′, contains a cleavable N-terminal signal sequence, and is predicted to be extracellular. Taken together, these data could represent an additional family of surface or secreted antigens. Two additional clones, bmn1-9 and -15, contain novel ORFs with N-terminal truncations and are unrelated to the previous clones. These clones, in addition to bmn1-2, mn-10, and bmn1-17, were reconstructed for protein overexpression and purification because of their predicted high antigenic indices and assayed in both ELISA and Western blot formats for reactivity in patient serum samples.
With this work, we identify 18 clones derived from expression screening, and from these, we describe five immunodominant antigens from B. microti. Western blot and ELISA analyses demonstrate that a combination of two or more of these recombinant antigens might be sufficient to detect infection in most patients. Each recombinant shows a different pattern of reactivity by both Western blot analysis and ELISA with a panel of Babesia infection sera, and values correspond to IFA titers. One infection serum sample was equivocal by ELISA; however, this sample was a transfusion-acquired, PCR-negative infection sample with a low IFA titer (1:128). With a combination of these recombinant antigens, it should be possible to develop a B. microti-specific ELISA to replace IFA assays and a rapid diagnostic test to replace current microscopy and PCR. These tests would allow for the screening of silently infected individuals who are a risk to those receiving blood transfusions and to assess patients with Lyme disease who might also be infected with babesiosis. Detection of individuals with subclinical infection or coinfection might potentially allow for early treatment that would reduce the duration of parasitemia and would help eliminate contaminated blood from the blood supply. Current work is focused on the development of diagnostic assays that will utilize these novel recombinant antigens.
ACKNOWLEDGMENTS
We thank Thomas Vedvick for protein sequence data and Dan Hoppe and Joe Parsons for their assistance with DNA sequencing. We also thank Jonathan Clapper for performing lipopolysaccharide assays on purified recombinants and Barbara Herwaldt for helpful discussions.
This work was partially supported by NIH grants AI42416 (M.J.L.), AI41840 (R.L.H.), AI42402 (P.J.K.), and AI41103 (D.H.P.) as well as cooperative agreement U50/CCU510528 from the Centers for Disease Control (to D.H.P.).
REFERENCES
- 1.Allred D R. Antigenic variation in Babesia bovis: how similar is it to that in Plasmodium falciparum? Ann Trop Med Parasitol. 1998;92:461–472. doi: 10.1080/00034989859438. [DOI] [PubMed] [Google Scholar]
- 2.Brasseur P, Gorenflot A. Human babesial infections in Europe. Rocz Akad Med Bialymstoku. 1996;41:117–122. [PubMed] [Google Scholar]
- 3.Conrad P A, Thomford J W, Marsh A, Telford III S R, Anderson J F, Spielman A, Sabin E A, Yamane I, Persing D H. Ribosomal DNA probe for differentiation of Babesia microti and B. gibsoni isolates. J Clin Microbiol. 1992;30:1210–1215. doi: 10.1128/jcm.30.5.1210-1215.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalrymple B P, Peters J M, Goodger B V, Bushell G R, Waltisbuhl D J, Wright I G. Cloning and characterisation of cDNA clones encoding two Babesia bovis proteins with homologous amino- and carboxy-terminal domains. Mol Biochem Parasitol. 1993;59:181–189. doi: 10.1016/0166-6851(93)90216-k. [DOI] [PubMed] [Google Scholar]
- 5.Dobroszycki J, Herwaldt B L, Boctor F, Miller J R, Linden J, Eberhard M L, Yoon J J, Ali N M, Tanowitz H B, Graham F, Weiss L M, Wittner M. A cluster of transfusion-associated babesiosis cases traced to a single asymptomatic donor. JAMA. 1999;281:927–930. doi: 10.1001/jama.281.10.927. [DOI] [PubMed] [Google Scholar]
- 6.Dodd R Y. Transmission of parasites by blood transfusion. Vox Sang. 1998;74(Suppl. 2):161–163. doi: 10.1111/j.1423-0410.1998.tb05415.x. [DOI] [PubMed] [Google Scholar]
- 7.Eberhard M L, Walker E M, Steurer F J. Survival and infectivity of Babesia in blood maintained at 25°C and 24°C. J Parasitol. 1995;81:790–792. [PubMed] [Google Scholar]
- 8.Eskow E S, Krause P J, Spielman A, Freeman K, Aslanzadeh J. Southern extension of the range of human babesiosis in the eastern United States. J Clin Microbiol. 1999;37:2051–2052. doi: 10.1128/jcm.37.6.2051-2052.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelfand J A, Callahan M V. Babesiosis. Curr Clin Top Infect Dis. 1998;18:201–216. [PubMed] [Google Scholar]
- 10.Gerber M A, Shapiro E D, Krause P J, Cable R G, Badon S J, Ryan R W. The risk of acquiring Lyme disease or babesiosis from a blood transfusion. J Infect Dis. 1994;170:231–234. doi: 10.1093/infdis/170.1.231. [DOI] [PubMed] [Google Scholar]
- 11.Gorenflot A, Moubri K, Precigout E, Carcy B, Schetters T P M. Human babesiosis. Ann Trop Med Parasitol. 1998;92:489–501. doi: 10.1080/00034989859465. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Rivas R, Hinterberg K, Scherf A. Compartmentalization of genes coding for immunodominant antigens to fragile chromosome ends leads to dispersed subtelomeric gene families and rapid gene evolution in Plasmodium falciparum. Mol Biochem Parasitol. 1996;78:137–148. doi: 10.1016/s0166-6851(96)02618-7. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Rivas R, Mattei D, Sterkers Y, Peterson D S, Wellems T E, Scherf A. Expressed var genes are found in Plasmodium falciparum subtelomeric regions. Mol Cell Biol. 1997;17:604–611. doi: 10.1128/mcb.17.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herwaldt B L, Persing D H, Precigout E A, Goff W L, Mathiesen D A, Taylor P W, Eberhard M L, Gorenflot A F. A fatal case of babesiosis in Missouri: identification of another piroplasm that infects humans. Ann Intern Med. 1996;124:643–650. doi: 10.7326/0003-4819-124-7-199604010-00004. [DOI] [PubMed] [Google Scholar]
- 15.Homer M J, Bruinsma E S, Lodes M J, Moro M H, Telford III S, Krause P J, Reynolds L D, Mohamath R, Benson D R, Houghton R L, Reed S G, Persing D H. A polymorphic multigene family encoding an immunodominant protein from Babesia microti. J Clin Microbiol. 2000;38:362–368. doi: 10.1128/jcm.38.1.362-368.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson C W, Perryman L E, Goff W L. Babesia bovis: identification of immunodominant merozoite surface proteins in soluble culture-derived exoantigen. Parasitol Res. 1997;83:776–780. doi: 10.1007/s004360050338. [DOI] [PubMed] [Google Scholar]
- 17.Knowles D P, Perryman L E, McElwain T F, Kappmeyer L S, Stiller D, Palmer G H, Visser E S, Hennager S G, Davis W C, McGuire T C. Conserved recombinant antigens of Anaplasma marginale and Babesia equi for serologic diagnosis. Vet Parasitol. 1995;57:93–96. doi: 10.1016/0304-4017(94)03113-b. [DOI] [PubMed] [Google Scholar]
- 18.Krause P J, Spielman A, Telford S R, Sikand V K, McKay K, Christianson D, Pollack R J, Brassard P, Magera J, Ryan R, Persing D H. Persistent parasitema after acute babesiosis. N Engl J Med. 1998;339:160–165. doi: 10.1056/NEJM199807163390304. [DOI] [PubMed] [Google Scholar]
- 19.Krause P J, Telford III S R, Pollack R J, Ryan R, Brassard P, Zemel L, Spielman A. Babesiosis: an underdiagnosed disease of children. Pediatrics. 1992;89:1045–1048. [PubMed] [Google Scholar]
- 20.Krause P J, Telford III S R, Ryan R, Hurta A B, Kwasnik I, Luger S, Niederman J, Gerber M, Spielman A. Geographical and temporal distribution of babesial infection in Connecticut. J Clin Microbiol. 1991;29:1–4. doi: 10.1128/jcm.29.1.1-4.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krause P J, Telford III S, Spielman A, Ryan R, Magera J, Rajan T V, Christianson D, Alberghini T V, Bow L, Persing D. Comparison of PCR with blood smear and inoculation of small animals for diagnosis of Babesia microti parasitemia. J Clin Microbiol. 1996;34:2791–2794. doi: 10.1128/jcm.34.11.2791-2794.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krause P J, Telford III S R, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing D H. Concurrent Lyme disease and babesiosis: evidence for increased severity and duration of illness. JAMA. 1996;275:1657–1660. [PubMed] [Google Scholar]
- 23.Lanzer M, deBruin D, Ravetch J V. Transcriptional differences in polymorphic and conserved domains of a complete cloned P. falciparum chromosome. Nature. 1993;361:654–657. doi: 10.1038/361654a0. [DOI] [PubMed] [Google Scholar]
- 24.Lanzer M, Fischer K, LeBlancq S M. Parasitism and chromosome dynamics in protozoan parasites: is there a connection? Mol Biochem Parasitol. 1995;70:1–8. doi: 10.1016/0166-6851(95)00021-r. [DOI] [PubMed] [Google Scholar]
- 25.Magnarelli L A, Anderson J F, Stafford III K C, Dumler J S. Antibodies to multiple tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in white-footed mice. J Wildl Dis. 1997;33:466–473. doi: 10.7589/0090-3558-33.3.466. [DOI] [PubMed] [Google Scholar]
- 26.Magnarelli L A, Ijdo J W, Anderson J F, Padula S J, Flavell R A, Fikrig E. Human exposure to a granulocytic ehrlichia and other tick-borne agents in Connecticut. J Clin Microbiol. 1998;36:2823–2827. doi: 10.1128/jcm.36.10.2823-2827.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 28.Molloy J B, Bowles P M, Jeston P J, Bruyeres A G, Bowden J M, Bock R E, Jorgenson W K, Blight G W, Dalgliesh R J. Development of an enzyme-linked immunosorbent assay for detection of antibodies to Babesia bigemina in cattle. Parasitol Res. 1998;84:651–656. doi: 10.1007/s004360050465. [DOI] [PubMed] [Google Scholar]
- 29.Persing D H, Herwaldt B L, Glaser C, Lane R S, Thomford J W, Mathiesen D, Krause P J, Phillip D F, Conrad P A. Infection with a babesia-like organism in northern California. N Engl J Med. 1995;332:298–303. doi: 10.1056/NEJM199502023320504. [DOI] [PubMed] [Google Scholar]
- 30.Persing D H, Mathiesen D, Marshall W F, Telford S R, Spielman A, Thomford J W, Conrad P A. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992;30:2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piesman J, Mather T N, Telford III S R, Spielman A. Concurrent Borrelia burgdorferi and Babesia microti infection in nymphal Ixodes dammini. J Clin Microbiol. 1986;24:446–447. doi: 10.1128/jcm.24.3.446-447.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pruthi R K, Marshall W F, Wiltsie J C, Persing D H. Human babesiosis. Mayo Clin Proc. 1995;70:853–862. doi: 10.1016/S0025-6196(11)63943-8. [DOI] [PubMed] [Google Scholar]
- 33.Quick R E, Herwaldt B L, Thomford J W, Garnett M E, Eberhard M L, Wilson M, Spach D H, Dickerson J W, Telford III S R, Steingart K. Babesiosis in Washington state: a new species of Babesia? Ann Intern Med. 1993;119:284–290. doi: 10.7326/0003-4819-119-4-199308150-00006. [DOI] [PubMed] [Google Scholar]
- 34.Skeiky Y A W, Lodes M J, Guderian J A, Mohamath R, Bement T, Alderson M R, Reed S G. Cloning, expression, and immunological evaluation of two putative secreted serine protease antigens of Mycobacterium tuberculosis. Infect Immun. 1999;67:3998–4007. doi: 10.1128/iai.67.8.3998-4007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spielman A. The emergence of Lyme disease and human babesiosis in a changing environment. Ann N Y Acad Sci. 1994;740:146–156. doi: 10.1111/j.1749-6632.1994.tb19865.x. [DOI] [PubMed] [Google Scholar]
- 36.Vidotto O, McElwain T F, Machado R Z, Perryman L E, Suarez C E, Palmer G H. Babesia bigemina: identification of B cell epitopes associated with parasitized erythrocytes. Exp Parasitol. 1995;81:491–500. doi: 10.1006/expr.1995.1142. [DOI] [PubMed] [Google Scholar]