Abstract
Background
Hyperbaric oxygen therapy (HBOT) is an effective primary and adjunctive treatment for a wide spectrum of conditions, ranging from carbon monoxide poisoning to nonhealing wounds. Although HBOT has been shown to improve morbidity and mortality rates when used as adjunctive therapy for compromised skin wounds, the strategy is still underutilized in practice, especially in the field of cosmetic and plastic reconstructive surgery.
Methods
Here we present 4 cases in which adjunctive HBOT was used to treat ischemic soft tissue wounds following facial fillers injectables, abdominoplasty, and compromise cutaneous flap after Mohs surgery reconstruction.
Results
In this report, we highlight the utility and implications of HBOT in the management of adverse outcomes following medical interventions.
Conclusions
The purpose of this case series is to add to the current existing literature examining the expanding role of HBOT as an adjunctive treatment for compromised skin and subcutaneous tissue wounds.
Keywords: hyperbaric oxygen therapy, compromised skin wounds, facial injectables, abdominoplasty, Mohs surgery
Introduction
Introduction of hyperbaric oxygen therapy (HBOT) dates back to the late 1600s when Dr Nathaniel Henshaw built the first hyperbaric chamber called a “Domicilium.”1 Despite its demonstrated efficacy in wound therapy, HBOT is frequently underutilized.2 Here we present 4 cases of compromised skin wounds secondary to filler injections, abdominoplasty, and Mohs surgery that were treated adjunctively with HBOT and demonstrated remarkable improvements in healing.
Methods
Case 1
A 53-year-old woman received 1 mL of hyaluronic acid dermal filler (Juvéderm; Allergan Aesthetics) for nasal tip refinement and presented to our clinic 10 days later with nasal tip skin necrosis (Figure 1A). The patient did not receive any hyaluronidase following the injection because she did not seek any medical attention until advanced necrosis was already present. HBOT was initiated at 2.5 atmosphere absolute (ATA) and completed 25 dives. Wound care included daily dressing changes with topical antibiotic. Following HBOT, patient had great improvement of the wound after 3 weeks with only minor pigmentation issues easily covered by makeup (Figure 1B).
FIGURE 1.
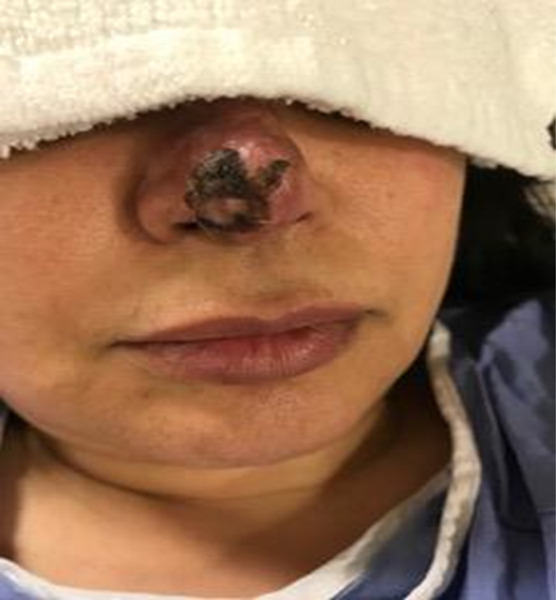
A) Necrotic tissue over nasal tip 10 days following injection of hyaluronic acid dermal filler. B) Wound improvement following HBOT, 25 dives at 2.5 ATA. ATA, atmosphere absolute; HBOT, hyperbaric oxygen therapy.
Case 2
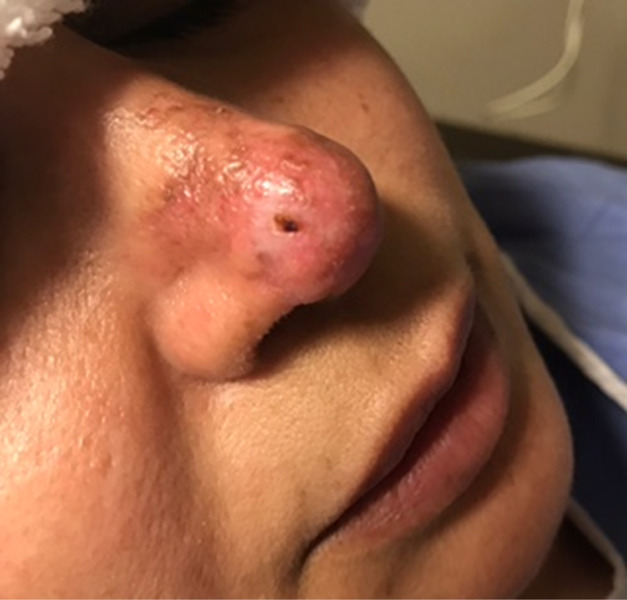
A 30-year-old woman who received hyaluronic acid dermal filler (Juvéderm) injections into the left side of her chin at an outside clinic developed complications 24 hours after treatment (Figure 2A). She was injected with hyaluronidase and referred to our wound clinic 2 days later. On examination, the patient presented with swelling and bruising on the left side of her chin; examination showed skin remodeling overlying the injection site with possible ischemia and mild smile asymmetry. HBOT was initiated, and the patient successfully completed 5 dives at 2.5 ATA before contracting COVID and discontinuing her treatments. Nonetheless, patient had complete resolution of remodeling of the skin (Figure 2B) and improvement in smile asymmetry (Figure 2C) after 3 weeks.
FIGURE 2.
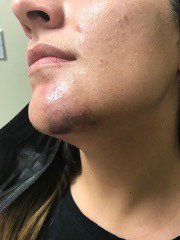
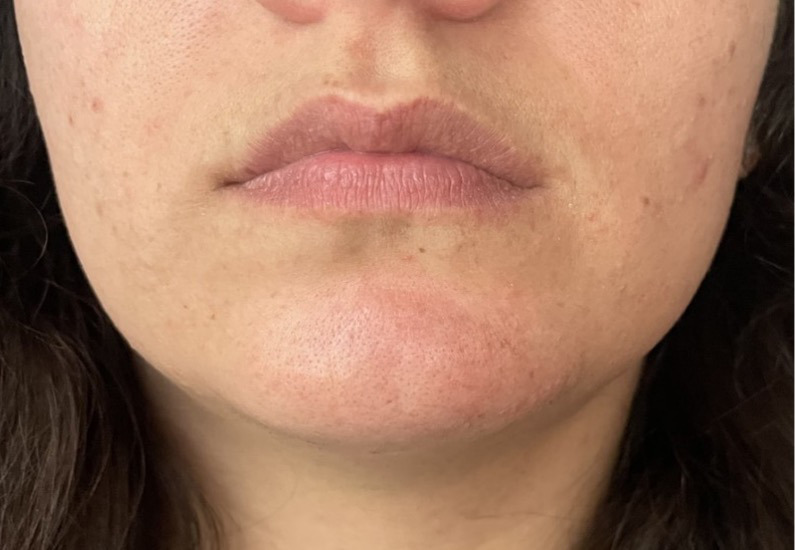

A) Swelling with skin remodeling and smile asymmetry following chin injection of hyaluronic acid dermal filler. After 5 HBOT dives at 2.5 ATA, the patient had B) complete resolution of skin remodeling and C) smile asymmetry. ATA, atmosphere absolute; HBOT, hyperbaric oxygen therapy.
Case 3
A 46-year-old woman with a history of sleeve gastrectomy with extensive adhesiolysis and open cholecystectomy underwent multiple surgeries outside of the US, including breast lift with implants, gluteal lift, torsoplasty, abdominoplasty, and liposuction. Preoperatively, she was informed about the risks involved due to the pre-existing upper abdominal incision. She accepted the risks, including suprapubic soft tissue necrosis. She presented to our office 17 days after abdominoplasty surgery for evaluation of a nonhealing postsurgical wound on the lower abdominal wall. On examination, the wound was swollen, erythematous, and indurated with scant serosanguineous drainage and tissue necrosis (Figure 3A). The patient completed 34 dives at 2.5 ATA with improvement of induration and drainage before undergoing surgical debridement of persistent necrotic tissue. Once well-demarcated, the wound was debrided in the operating room. The left side of the wound was closed primarily with sutures while a small area on the right side was left to close by secondary intention (Figure 3B). The patient completed 5 additional dives at 2.5 ATA after debridement, totaling 39 dives, and a smart negative pressure vacuum-assisted closure device was placed for 6 weeks. During the treatment, the patient was able to go back to work with limited duties until complete healing was achieved 3 months later (Figure 3C).
FIGURE 3.
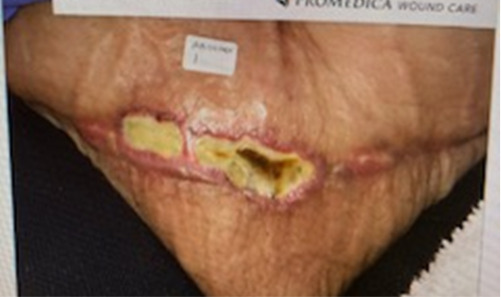
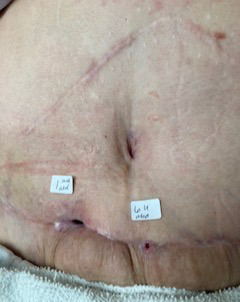
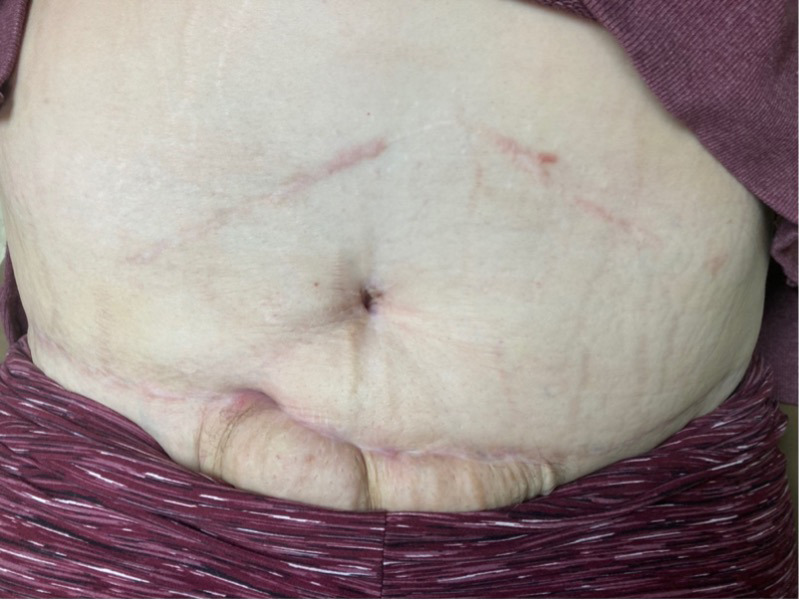
A) Nonhealing surgical wound in patient with history of multiple cosmetic surgeries following sleeve gastrectomy. B) Primary closure of wound with sutures with small area on right side left to close by secondary intention. C) Complete healing at 3 months following 39 HBOT dives at 2.5 ATA and 6 weeks of smart negative pressure vacuum-assisted closure. ATA, atmosphere absolute; HBOT, hyperbaric oxygen therapy.
Case 4
A 73-year-old woman who was a current cigarette smoker underwent Mohs surgery for distal nasal dorsum basal cell carcinoma with closure using advancement of rectangular nasal dorsum cutaneous flap. (Figure 4A). Postoperatively, she developed flap tip necrosis (Figure 4B), and the decision was made to proceed with wound care and HBOT. Wound care included daily cleansing with soap and water followed by application of topical antibiotic ointment and dressing. The patient completed 20 dives at 2.5 ATA and experienced complete resolution of nasal tip necrosis after 5 weeks (Figure 4C).
FIGURE 4.
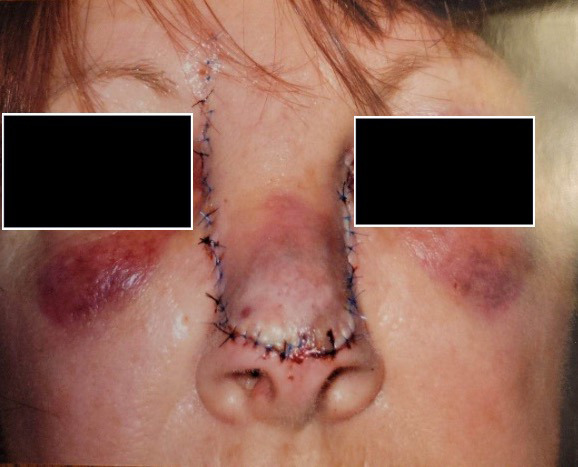
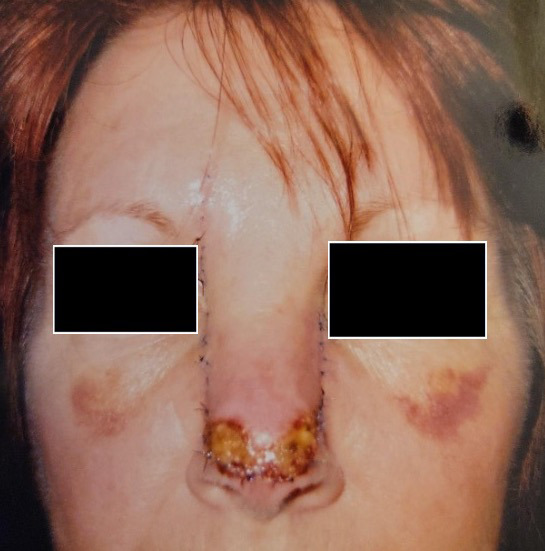
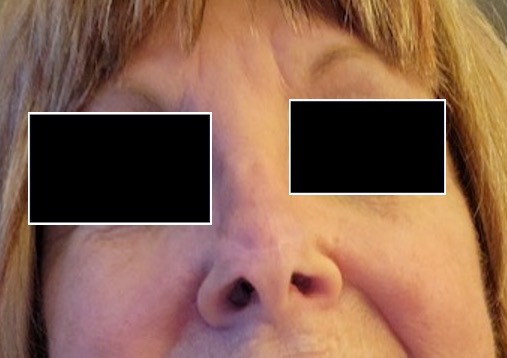
A) Postoperative photo of 73-year-old patient who underwent Mohs surgery for basal cell carcinoma. B) Necrosis involving nasal dorsum cutaneous flap tip. C) Resolution of necrosis at 5 weeks after 20 HBOT dives at 2.5 ATA as adjunct to conventional antimicrobial wound care. ATA, atmosphere absolute; HBOT, hyperbaric oxygen therapy.
Results
Common treatment strategies for filler complications include hyaluronidase, but patients can continued to have ischemia, making HBOT an effective alternative therapy.1 In case 1, the patient experienced skin necrosis likely due to artery thrombosis from direct injection into the artery or to skin ischemia from injection mass effect. In case 2, the patient most likely experienced direct ischemia of the overlying skin from the mass effect of the filler and pressure leading to weakness of the orbicularis orris muscle causing lip asymmetry.
Old surgical scars are also a risk factor for postoperative complications.2 In case 3, the patient developed complications following her abdominoplasty, likely due to the Kocher incision at the right subcostal region disrupting the lumbar and intercostal blood flow to the suprapubic area. Flaps are commonly susceptible to arterial insufficiency and venous congestion.3 When patients have other risk factors, such as older age, flap length, and tension during closure, they are at increased risk for flap compromise and ischemia, such as our patient in case 4.
Discussion
According to the Undersea and Hyperbaric Medical Society (UHMS), there are currently 14 approved indications for HBOT: gas embolism; acute carbon monoxide intoxication; gas gangrene; crush injuries/compartment syndrome/acute traumatic ischemia; decompression sickness; arterial inefficiencies, including sudden vision loss; anemia; intracranial abscesses; necrotizing soft tissue infections; osteomyelitis; radiation injury; compromised grafts and flaps; thermal burns; and idiopathic sensorineural hearing loss.4 The Centers for Medicare and Medicaid Services covers the use of HBOT (CPT code 99183 and HCPCS G0277) when the adjunctive therapy is administered for specific conditions and, most importantly to plastic surgeons, for compromised grafts and flaps. Regarding insurance coverage, most insurers reimburse the use of HBOT as adjunctive therapy given appropriate documentation. The authors recommend ICD-10 code T86.82 for compromised skin grafts, as well as T86.828 to specify such conditions as arterial insufficiency of the flap, extrinsic venous compression of the flap, flap ischemia, flap loss, flap necrosis, and mechanical complications secondary to skin graft failure and/or rejection. The ICD-10 code L76.82 is a specific code used to indicate other postprocedural complications of skin and subcutaneous tissue and may aid establishing a diagnosis for reimbursement purposes.
HBOT utilizes high concentrations of oxygen at pressures higher than sea level pressure (ie, > 1 ATA). The UHMS currently recommends pressurization of at least 1.4 ATA or higher.5 At higher pressures, more oxygen becomes dissolved in the plasma and readily diffuses to areas of ischemia, making HBOT an effective in the treatment of chronic wounds and salvage of existing grafts and flaps.6 Evidence-based reviews have demonstrated the benefit of HBOT on compromised flaps and grafts.7,8 Numerous animal models have proven greater distal capillary growth9, reduction in flap necrosis10,11, and increased survival area of flap when compared with that seen in control animals.12,13
Similarly, several human studies have reiterated the benefit of HBOT for graft and flap survival. In a randomized control trial of 48 patients by Perrins, complete survival of the graft occurred in 64% versus only 17% of patients in the control group (P < .01).14 In short, the result of HBOT is improvement in local tissue oxygenation and healing of wounds from reduced hypoxia, ischemic damage, cellular death, inflammation, and acidosis with promotion of angiogenesis and collagen synthesis.15 While initial protocol for dives called for 30 dives, the actual number of dives depends on each patient's individual rate of improvement. Greater improvement in wound healing leads to fewer planned dives, as in our patients' cases.
Despite its proven efficacy for wounds, HBOT is severely underutilized in cosmetic and plastic reconstructive surgery practice. Our analysis has a few limitations considering that all cases were also managed concurrently with basic wound care therapy. While it can be argued that these patients may have eventually healed with just proper wound care alone, it is important to identify specific underlying risk factors that heighten the propensity for these patients to develop wound complications. When approaching therapy, it is crucial to identify key risk factors for skin compromise in aesthetic procedures, such as age, sex, comorbidities (ie, smoking, diabetes), previous surgeries, and surgical technique issues in flap design and tension in closure.2,16,17 In our patients, the decision to perform HBOT was based on several factors involving potential vascular occlusion, history of prior abdominal surgery, and ischemia refractory to other therapies. We believe that use of HBOT for these indications would benefit our patient populations more than standard wound care alone.
Our findings suggest that HBOT maybe a beneficial adjunctive therapy for compromised tissues. This study adds to the current body of literature regarding the potential therapeutic effects of HBOT and, when faced with similar scenarios, plastic surgeons should have a low threshold to refer patients to HBOT to limit tissue necrosis and lessen patient morbidity. Future directions aim to compare results with a propensity matched control group.
References
- 1.Dominguez SM, Moshrefi S, Dobke M. Treatment protocol for acute arterial occlusion secondary to facial revolumization procedures. Emergency Medicine. 2017;(49):221–229. doi: 10.12788/emed.2017.0030 [Google Scholar]
- 2.Winocour J, Gupta V, Ramirez JR, Shack RB, Grotting JC, Higdon KK. Abdominoplasty: risk factors, complication rates, and safety of combined procedures. Plast Reconstr Surg. 2015;136(5):597e–606e. doi:10.1097/prs.0000000000001700 10.1097/PRS.0000000000001700 [DOI] [PubMed] [Google Scholar]
- 3.Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6(1):23–32. doi:10.1089/wound.2016.0707 10.1089/wound.2016.0707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moon RE, editor. Hyperbaric Oxygen Therapy Indications. 14th ed. Undersea and Hyperbaric Medical Society. Best Publishing Company; 2019. [Google Scholar]
- 5.Feldmeier JJ, Hopf HW, Warriner RA 3rd, Fife CE, Gesell LB, Bennett M. UHMS position statement: topical oxygen for chronic wounds. Undersea Hyperb Med. 2005;32(3):157–68. [PubMed] [Google Scholar]
- 6.Gill AL, Bell CN. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. Qjm. 2004;97(7):385–395. doi:10.1093/qjmed/hch074 10.1093/qjmed/hch074 [DOI] [PubMed] [Google Scholar]
- 7.Kleban S, Baynosa RC. The effect of hyperbaric oxygen on compromised grafts and flaps. Undersea Hyperb Med. 2020;47(4):635–648. doi:10.22462/10.12.2020.13 10.22462/10.12.2020.13 [DOI] [PubMed] [Google Scholar]
- 8.Boissiere F, Gandolfi S, Riot S, et al. Flap venous congestion and salvage techniques: a systematic literature review. Plast Reconstr Surg Glob Open. Jan 2021;9(1):e3327. doi:10.1097/gox.0000000000003327 10.1097/GOX.0000000000003327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manson PN, Im MJ, Myers RA, Hoopes HE. Improved capillaries by hyperbaric-oxygen in skin flaps. Surg Forum. 1980;31:564–566. [Google Scholar]
- 10.McFarlane RM, Wermuth RE. The use of hyperbaric oxygen to prevent necrosis in experimental pedicle flaps and composite skin grafts. Plast Reconstr Surg. 1966;37(5):422–430. doi:10.1097/00006534-196605000-00008 10.1097/00006534-196605000-00008 [DOI] [PubMed] [Google Scholar]
- 11.Zamboni WA, Wong HP, Stephenson LL. Effect of hyperbaric oxygen on neutrophil concentration and pulmonary sequestration in reperfusion injury. Arch Surg. 1996;131(7):756–760. doi:10.1001/archsurg.1996.01430190078020 10.1001/archsurg.1996.01430190078020 [DOI] [PubMed] [Google Scholar]
- 12.Richards L, Lineaweaver WC, Stile F, Zhang J, Zhang F. Effect of hyperbaric oxygen therapy on the tubed pedicle flap survival in a rat model. Ann Plast Surg. 2003;50(1):51–56. doi:10.1097/00000637-200301000-00009 10.1097/00000637-200301000-00009 [DOI] [PubMed] [Google Scholar]
- 13.Zhang F, Cheng C, Gerlach T, Kim DY, Lineaweaver WC, Buncke HJ. Effect of hyperbaric oxygen on survival of the composite ear graft in rats. Ann Plast Surg. 1998;41(5):530–534. doi:10.1097/00000637-199811000-00013 10.1097/00000637-199811000-00013 [DOI] [PubMed] [Google Scholar]
- 14.Perrins DJ. Influence of hyperbaric oxygen on the survival of split skin grafts. Lancet. 1967;1(7495):868–871. doi:10.1016/s0140-6736(67)91428-6 10.1016/S0140-6736(67)91428-6 [DOI] [PubMed] [Google Scholar]
- 15.Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127 Suppl 1(Suppl 1):131s–141s. doi:10.1097/PRS.0b013e3181fbe2bf 10.1097/PRS.0b013e3181fbe2bf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta V, Winocour J, Shi H, Shack RB, Grotting JC, Higdon KK. Preoperative risk factors and complication rates in facelift: analysis of 11,300 patients. Aesthet Surg J. Jan 2016;36(1):1–13. doi:10.1093/asj/sjv162 10.1093/asj/sjv162 [DOI] [PubMed] [Google Scholar]
- 17.Browne EZ. Complications of skin grafts and pedicle flaps. Hand Clinics. 1986;2(2):353–359. 10.1016/S0749-0712(21)00541-2 [DOI] [PubMed] [Google Scholar]


