Abstract
Macrophages are important mediators of skeletal muscle function in both healthy and diseased states. In vivo specific depletion of macrophages provides an experimental method to understand physiological and patho-physiological effects of macrophages. Systemic depletion of macrophages can deplete skeletal muscle macrophages but also alters systemic inflammatory responses and metabolism, which confounds the muscle specific effects of macrophage depletion. The primary aim of this manuscript is to evaluate two methods of murine intramuscular macrophage depletion in an acute lung injury-associated indirect skeletal muscle wasting mouse model. Adult C57BL/6 (WT) and Macrophage Fas-Induced Apoptosis (MaFIA, C57BL/6-Tg) mice received clodronate liposomes or the dimerization drug AP20187 through intramuscular injection of the tibialis anterior muscle compartment, respectively. Vehicle control was injected in the contralateral muscle. We demonstrate intramuscular AP20187 in the MaFIA mouse depletes macrophages but causes an infiltration of CD45 intermediate neutrophils. In contrast, intramuscular clodronate liposomes successfully depletes macrophages without an associated increase in CD45 intermediate cells. In conclusion, intramuscular clodronate is effective for selective depletion of muscle macrophages without eliciting acute inflammation seen with AP20187 in MaFIA mice. This technique is an important tool to study the functional roles of macrophages in skeletal muscle.
Keywords: Conditional macrophage depletion, Clodronate liposomes, Transgenic mouse model, Inflammation, Skeletal muscle
1. Introduction
Macrophages are important mediators of skeletal muscle function and health in both adaptive and pathologic states (Ziemkiewicz et al., 2021). Key roles for skeletal muscle macrophages have been identified in traumatic injury and repair, exercise adaptation, and inflammatory myopathies (Saclier et al., 2013). While much has been discovered about the macrophage role in these conditions, the interplay of systemic and tissue-resident macrophages makes mechanistic understanding of muscle macrophage function challenging.
Specific depletion of macrophages provides an experimental method to further define the functional role of macrophages. One such depleting agent is clodronate. Clodronate encapsulated in liposomes specifically targets macrophage uptake by phagocytosis (Van Rooijen and Sanders, 1994). The clodronate is then released intracellularly, and clodronate is used to produce a non-hydrolyzable ATP analogue which alters mitochondrial function and induces apoptosis (Moreno, 2018). Another method utilizes a transgenic mouse, the macrophage Fas-induced apoptosis (MaFIA) mouse, in which macrophage and macrophage-related cells express an inducible suicide gene (Burnett et al., 2004). The transgene was constructed by placing enhanced green fluorescent protein (GFP) and human FK506 binding protein 1A–Fas suicide construct under control of macrophage colony stimulating factor-1 (CSF-1) receptor (c-fms) promoter. The c-fms promoter promotes expression of the transgene predominantly on cells of the mononuclear phagocyte system in mice, which have been described to include monocytes and macrophages. When the drug AP20187 is administered to MaFIA mice, it dimerizes with the suicide protein to activate Fas and induce apoptosis of cells expressing the transgene.
Intravenous and intraperitoneal routes of administration of depleting agents can deplete skeletal muscle macrophages (Summan et al., 2006). However, this comes at the expense of macrophage ablation in other locations such as blood, bone marrow, liver, and spleen (Moreno, 2018; Burnett et al., 2004). Systemic macrophage ablation using systemic clodronate or a transgenic model such as lysozyme M promoter-directed Cre (LysMCre) diphtheria toxin model have reported that macrophage depletion can lead to neutrophilia, impaired metabolism, decreased energy intake, and reduced body weight (Bader et al., 2019; Lee et al., 2014). These effects can confound macrophage depletion in skeletal muscle. Therefore, researchers interested in determining the functional role of muscle macrophages need tools to selectively deplete muscle macrophages.
In this manuscript, we describe and compare two methods of murine intramuscular macrophage depletion. These methods were evaluated in a mouse model of indirect skeletal muscle wasting induced by instillation of lipopolysaccharides into the lungs.
2. Methods
2.1. Animals and acute lung injury model
The Wake Forest School of Medicine Institutional Animal Care and Use Committee approved all procedures. Eight- to twelve-week-old male wild-type C57BL/6 (WT) and Macrophage Fas-Induced Apoptosis (MaFIA, C57BL/6-Tg) mice (The Jackson Laboratory, Bar Harbor, ME) were anesthetized with an intraperitoneal injection of ketamine (150 mg/kg) and acetylpromazine (13.5 mg/kg), and the trachea was exposed. Escherichia coli lipopolysaccharide (LPS, O55:B5 L2880, Sigma-Aldrich) (ALI mice) at 3 μg/g mouse was instilled intratracheally using a 20-gauge catheter as previously described (Files et al., 2012).
2.2. Intramuscular macrophage depletion
AP20187 (B/B Homodimerizer, Takara Bio USA) was dissolved in 100% ethanol to form a stock solution at a concentration of 62.5 mg/ml and stored at −20 °C. A dosing solution of 10 mg/kg active drug was prepared from stock solution, PEG-400 (100%), and Tween (2%) per manufacturer instructions. A solution (vehicle control) containing of ethanol, PEG-400 (100%), and Tween (2%) was prepared similarly. AP20187 or vehicle control were dosed at 2 μl solution/g mouse per leg (5 μg/g AP20187). The dosing solutions were used within 30 min of preparation.
Liposomes were stored at 4 °C. Prior to injection, the clodronate-containing liposomes (5 mg/ml) and PBS-containing liposomes (vehicle control) were removed from the refrigerator and allowed to acclimate to room temperature (18 °C). The tubes containing liposomes were inverted 8–10 times to ensure even distribution. Liposomes were dosed at 20 μl of solution per leg (100 μg clodronate). In the ALI mouse model, mice were injected 1 day before and 1 day following lung injury.
The mouse fur was removed from bilateral lower limbs overlying the tibialis anterior (TA) muscle compartment. Skin was cleaned with 70% ethanol and the dosing solution injected in four aliquots along the body of the TA muscle compartment, as previously described (Files et al., 2012).
2.3. Flow cytometry
Following euthanasia, TA muscles were removed and cut into small pieces with scissors. The tissue was processed with a skeletal muscle dissociation kit according to manufacturer instructions (GentleMACS Dissociator, Miltenyi Biotec, Auburn, CA) to form a single-cell suspension. After viability staining (Supplementary Fig. 3A) and FcBlock (Mouse BD Fc Block, BD Biosciences, Cat#553141), the cells were stained with fluorochrome-conjugated surface antibodies (Supplementary Fig. 3A). After staining, CountBright Absolute Counting Beads were added according to manufacturer instructions (Invitrogen, Carlsbad, CA) and the cells were resuspended in 250 μl FACS to bring the total volume of the flow tube to 300 μl. Flow cytometry analysis was performed on BD LSRFortessa X-20 Analyzer and analyzed using FlowJo software (BD Biosciences). Live-dead threshold was determined by mixing heat killed PBMC 1:1 with live PBMC. After gating for single cells, forward/side scatter, and live cells, macrophages were defined as CD45+CD11b+CD11c−Ly6G−SSCloLy6Clo. Monocytes were defined as CD45+CD11b+CD11c−Ly6G−SSCloLy6Chi. Neutrophils were defined as CD45+CD11b+CD11c−F4/80−Ly6G+. Dendritic cells were defined as CD45+CD11b+CD11c+ (see gating strategy in Supplementary Fig. 3B).
2.4. Statistical analysis
Data are expressed as the mean ± SEM. To determine differences in inflammatory parameters in lung and muscle, unpaired Mann-Whitney (nonparametric) test were performed comparing the outcome variable between the 2 groups. Statistical analysis was performed using GraphPad Prism software (GraphPad Software). Graphs were generated using GraphPad Prism.
3. Results
3.1. Intramuscular administration of AP20187 in the MaFIA mouse causes leg swelling and increased TA muscle weight
We first tested AP20187 intramuscular injection in the MaFIA mouse model. The mice underwent intratracheal LPS instillation and at day 3 after LPS, we administered intramuscular AP20187 in one TA muscle and vehicle control in the contralateral leg (Supplementary Fig. 1A). Body weight changes were similar between ALI mice that received the intramuscular injections (−18.8 ± 3.5% at day 5 post ALI) and mice that did not receive intramuscular injections (−18.2 ± 3.7% at day 5 post ALI) (Supplementary Fig. 1B). The leg that received AP20187 was visibly swollen compared to the contralateral leg (Supplementary Fig. 1C) and, at harvest, the AP20187-treated TA muscle wet weight was increased 74.0% (77.6 ± 4.5 mg) compared to the vehicle TA (44.6 ± 3.4 mg) (p = 0.03 by Mann-Whitney test) (Supplementary Fig. 1D).
3.2. Intramuscular administration of AP20187 in the MaFIA mouse achieves ablation of cells expressing the target transgene but causes an infiltration of neutrophils
Flow cytometry analysis of CD45+ cells isolated from AP20187 treated muscle showed a significant decrease in GFP geometric mean fluorescence intensity index compared to vehicle control, consistent with successful macrophage depletion (Fig. 1A). All samples from all mice in the AP20187 experiment were analyzed under the CD45hi and CD45intermediate subsets as CD11b+CD11c−Ly6G−SSCloLy6Clo macrophages, CD11b+CD11c−Ly6G−SSCloLy6Chi monocytes, CD11b+CD11c−F4/80−Ly6G+ neutrophils, CD11b+CD11c+ dendritic cells. The percent of total CD45+ classified as CD45hi in vehicle control treated muscle is significantly higher than in AP20187 treated muscle (36.8 ± 4.0% versus 2.4 ± 0.7%, p = 0.03 by Mann-Whitney test). The percent of total CD45+ classified as CD45intermediate in vehicle control treated muscle is significantly lower than in AP20187 treated muscle (63.2 ± 4.0% versus 97.6 ± 0.7%, p = 0.03 by Mann-Whitney test). By absolute count, AP20187 treatment resulted in a significant increase in CD45intermediate cells (1.3 × 106 ± 2.7 × 105 cells/ml/mg TA) compared to the vehicle control treated muscle (4.3 × 104 ± 1.7 × 104 cells/ml/mg TA) as well, p = 0.03 by Mann-Whitney test. (Fig. 1B, C).
Fig. 1.
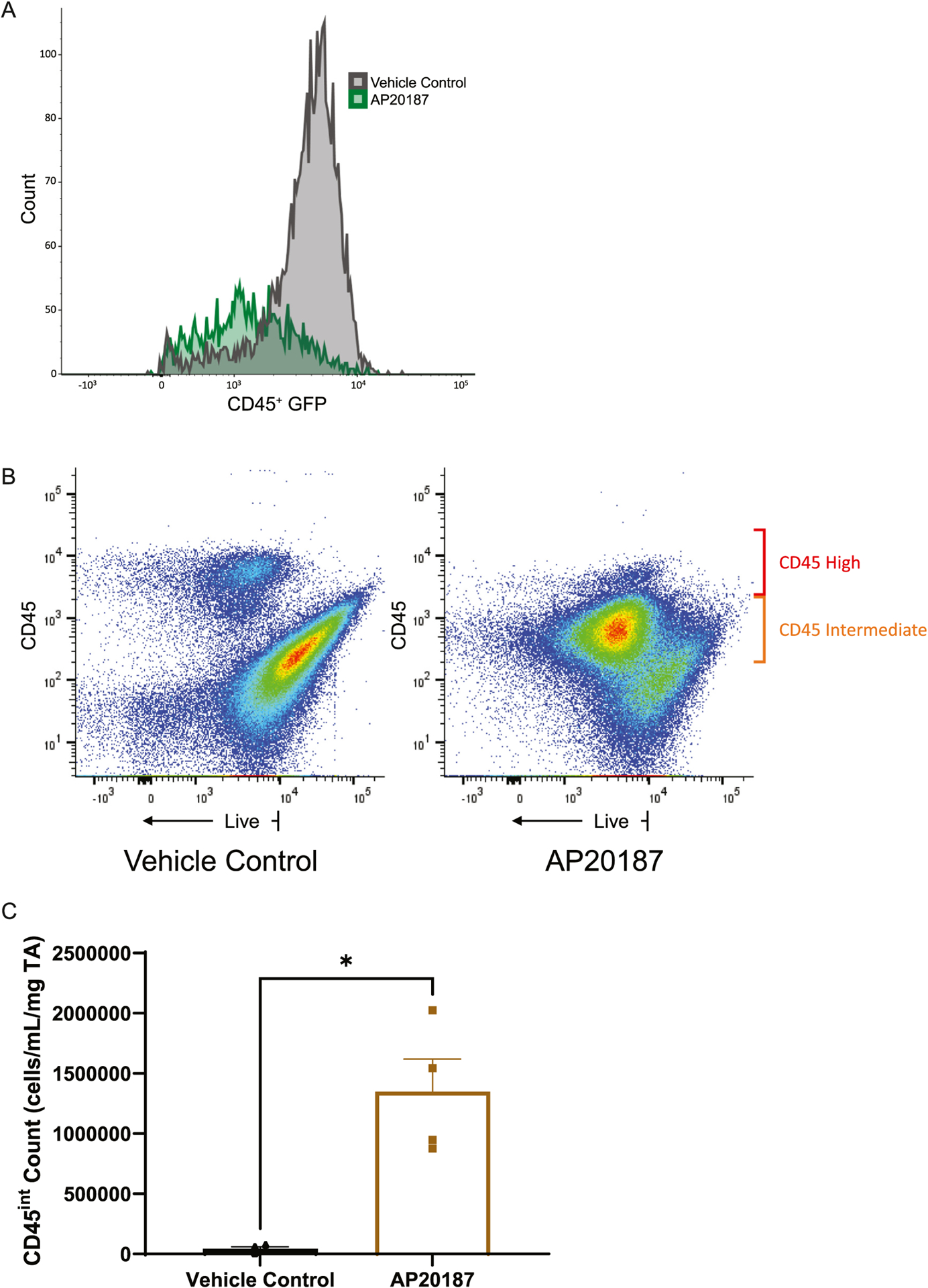
A) Representative sample (2 muscles of 1 mouse) showing flow cytometry analysis of CD45+ cells isolated from TA muscle from MaFIA mice injected with intramuscular AP20187 (green) versus intramuscular vehicle control (grey). B) Representative sample (1 mouse muscle per panel) demonstrating flow cytometry analysis demonstrating the appearance of a large population of CD45intermediate cells in AP20187 treatment compared to vehicle control treated muscle. C) Cell counts of the CD45intermediate population in AP20187 treated versus vehicle control treated muscle. This experiment is representative of two independent experiments and n = 4. *p = 0.03 by Mann-Whitney test.
In vehicle control treated muscle, the CD45hi population was composed of 5.8 ± 2.0% neutrophils, 6.8 ± 1.5% monocytes, 3.8 ± 0.5% macrophages, and 17 ± 2.3% dendritic cells. The CD45intermediate population was 22 ± 4.2% neutrophils, 3.9 ± 1.9% monocytes, 16.3 ± 3.5% macrophages, and 1.1 ± 0.1% dendritic cells. In the AP20187 treated muscle, the CD45hi population was 67.8 ± 5.6% neutrophils, 1.9 ± 0.4% monocytes, 0.9 ± 0.3% macrophages, and 7.9 ± 0.8% dendritic cells. The CD45intermediate population was 80.3 ± 1.5% neutrophils, 1.3 ± 0.2% monocytes, 13.1 ± 1.2% macrophages, and 0.3 ± 0.1% dendritic cells. A similar increase in CD45 intermediate cells compared to the vehicle control treated muscle was observed when this technique was repeated in the MaFIA mouse without ALI, suggesting that this effect is unique to AP20187 intramuscular injection independent of the systemic inflammatory response initiated by intratracheal LPS.
3.3. Intramuscular administration of clodronate liposomes in the WT mouse causes minimal increase in TA muscle weight
We administered intramuscular clodronate liposomes and PBS liposome vehicle control in the contralateral TA muscle 1 day before and 1 day after intratracheal LPS instillation (Supplementary Fig. 2A). At day 3 after LPS, all mice were harvested. Body weight changes were similar between ALI mice that received the intramuscular injections (−15.6 ± 1.3%) and mice that did not receive intramuscular injections (−18.4 ± 2.1%). There was no significant difference in weight between TA muscle that was treated with clodronate liposomes (46.2 ± 1.3 mg) compared to vehicle control treatment (42.8 ± 1 mg) (p = 0.06 by Mann-Whitney test), in contrast to the 74.0% increase in TA muscle weight found with intramuscular AP20187 (Supplementary Fig. 2B).
3.4. Intramuscular administration of clodronate liposomes depletes muscle macrophage cells
Unlike AP20187, clodronate treatment was not associated with an increase CD45intermediate cells (Fig. 2A). In muscle treated with clodronate, there were 8227 ± 2090 CD45+CD11b+ cells/ml/mg TA, and in muscle treated with vehicle control, there were 13,708 ± 5664 CD45+CD11b+ cells/ml/mg TA. The CD45+CD11b+ population in clodronate treated muscle was composed of 40.1 ± 5.4% neutrophils, 11.9 ± 1.9% Ly6Clo macrophages, 8.7 ± 1.7% Ly6Chi monocytes, and 16.8 ± 4.9% dendritic cells. The CD45+CD11b+ population in vehicle control treated muscle was composed of 18 ± 4.1% neutrophils, 20 ± 1.4% Ly6Clo macrophages, 6.4 ± 1.1% Ly6Chi monocytes, and 23.9 ± 3.7% dendritic cells. There was a significantly higher percentage of neutrophils (p = 0.01 by Mann-Whitney Test) and lower percentage of macrophages (p = 0.007 by Mann-Whitney Test) in clodronate treated muscle compared to vehicle control treated muscle. By absolute count, there was no significant difference in CD45+ cells (14,479 ± 5892 versus 9097 ± 2309 cells/ml/mg TA, p = 0.2 by Mann-Whitney test), neutrophils (2662 ± 896.6 versus 3505 ± 884.1 cells/ml/mg TA, p = 0.7 by Mann-Whitney test), monocytes (1204 ± 359.9 versus 833.8 ± 312.8 cells/ml/mg TA, p = 0.6 by Mann-Whitney test), or dendritic cells (4662 ± 1691 versus 1151 ± 434.6 cells/ml/mg TA, p = 0.1 by Mann-Whitney test) between vehicle control and clodronate treatment, respectively (Fig. 2B–F). Absolute count of Ly6Clo macrophages in muscles treated with clodronate liposomes (894.6 ± 280.4 cells/ml/mg TA) are decreased significantly compared to vehicle control (3822 ± 1201 cells/ml/mg TA) (p = 0.04 by Mann-Whitney test) (Fig. 2C) consistent with successful macrophage depletion.
Fig. 2.
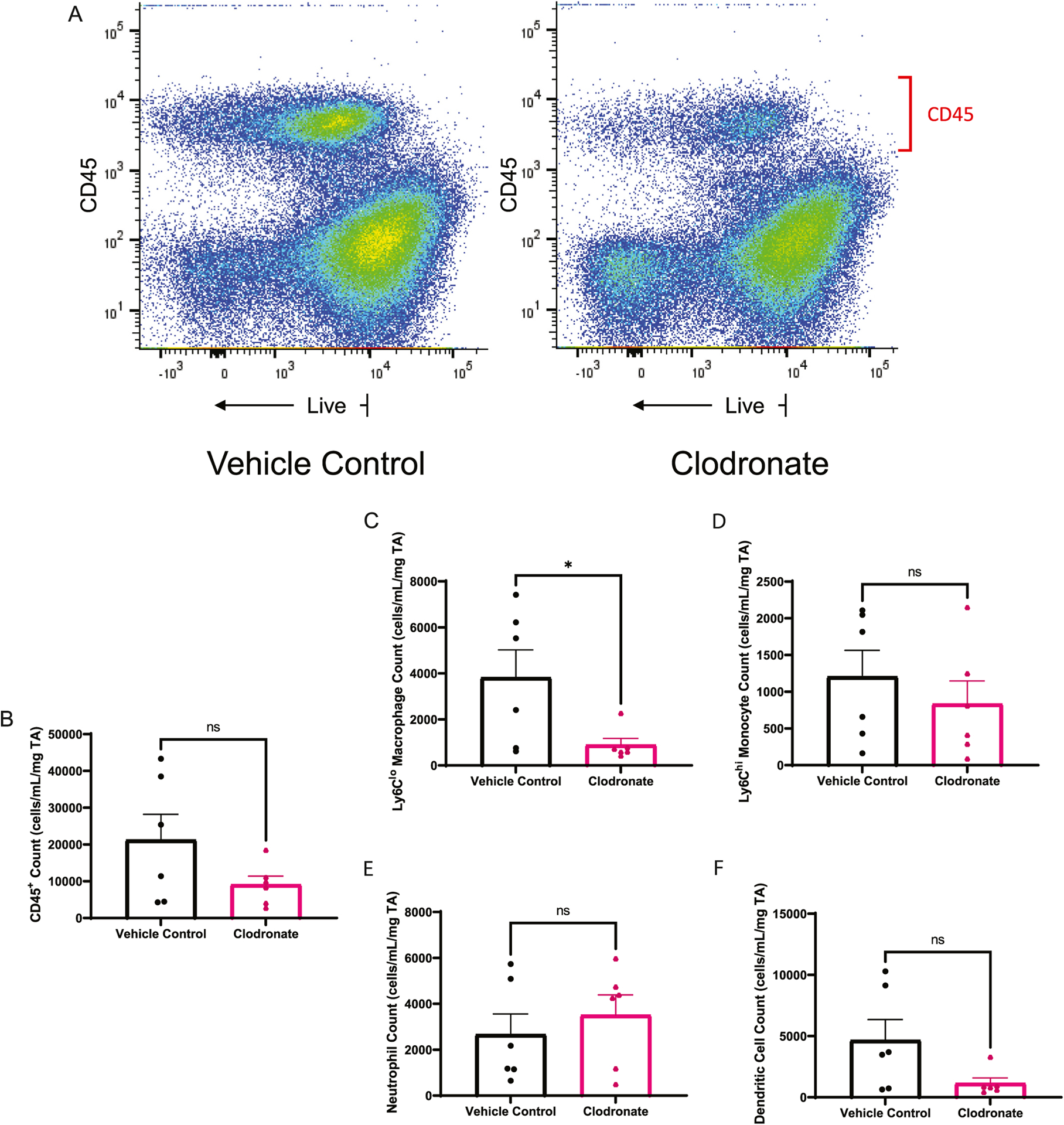
Intramuscular clodronate selectively depletes monocyte and macrophage muscle cell populations. A) Representative sample (1 mouse muscle per panel) showing flow cytometry analysis of CD45+ cells isolated from intramuscular clodronate treated compared to vehicle control mice. B) Total intramuscular CD 45+ cell populations in clodronate and vehicle treated mice. C) CD45+CD11b+CD11c−Ly6G−SSCloLy6Clo macrophages D) CD45+CD11b+CD11c−Ly6G−SSCloLy6Chi monocytes E) CD45+CD11b+CD11c−F4/80−Ly6G+ neutrophils, and F) CD45+CD11b+CD11chi dendritic cells in intramuscular clodronate or vehicle control treated mice. This experiment is representative of three independent experiments and n = 6. *p = 0.04 by Mann-Whitney test.
3.5. Intramuscular clodronate does not alter the lung injury response
To confirm that intramuscular clodronate was not altering the lung injury/inflammation response in response to intratracheal LPS, markers of lung injury were obtained in IM clodronate ALI treated mice and compared to ALI only treated. Analysis of BAL fluid leukocyte composition revealed similar cell counts and percentages of neutrophils, monocytes, and lymphocytes between ALI mice that had intramuscular treatment and ALI mice that did not receive intramuscular treatments (Supplementary Fig. 2C).
4. Discussion
Systemic methods of macrophage ablation can deplete skeletal muscle macrophages but this potentially comes at an expense of systemic effects such as neutrophilia, impaired metabolism, decreased energy intake, and reduced body weight (Summan et al., 2006; Bader et al., 2019; Lee et al., 2014). A method that permits intramuscular depletion of muscle macrophages would mitigate these confounding changes and would permit within-animal comparisons between legs by using a vehicle control in the contralateral muscle, which yields a more rigorous experimental model compared to systemic ablation. In this study, we examined the utility of two methods of murine intramuscular macrophage depletion to achieve muscle-specific macrophage ablation. Our findings show intramuscular administration of AP20187 in the MaFIA mouse depletes macrophages but causes an infiltration of CD45intermediate neutrophils, making it an ineffective agent for selective depletion. In contrast, intramuscular administration of clodronate liposomes successfully depletes macrophages without an associated increase in CD45intermediate cells. We report a ~ 80% reduction in muscle macrophages using clodronate, which is similar to Gong et al. who reported a 62% reduction in muscle macrophages using a similar technique with intramuscular liposomal clodronate (Gong et al., 2016) (See Table 1).
Table 1.
Comparison of MaFIA model and Liposomal Clodronate.
| MaFIA transgenic model | Liposomal Clodronate |
|---|---|
| Leg edema and 74% increase in TA muscle weight | No significant increase in TA muscle weight |
| Significant increase in CD45 intermediate cells | No significant increase in CD45 intermediate cells |
| Enhanced GFP labeling of cells expressing transgene | No GFP labeling |
| Limited to transgenic mouse | Can be used in different strains of mice |
Conceptually, the skeletal muscle compartment is ideal target for tissue specific genetic or pharmacologic ablation techniques, as many muscle compartments are accessible through the skin by needle puncture. Another advantage of intramuscular delivery of drugs is the ability to design experiments with within mouse controls as we have done in this experiment where one muscle receives the active drug, and the contralateral leg receives vehicle control. Tissue electroporation is another method of targeting genetic material to specific muscle, but the technique involves application of voltage pulses directly to muscle compartments to make the plasma membrane more porous and facilitate the uptake of DNA or other molecules (Files et al., 2012) and has been criticized for inducing muscle injury, as evidenced by necrosis and infiltration of macrophages (Baligand et al., 2012). Needle puncture, as used in this study, allows administration of drug while decreasing injury effects. Intramuscular administration for tissue specific genetic or pharmacologic ablation techniques is advantageous for muscle macrophage manipulation experiments.
While intramuscular AP20187 in the MaFIA mouse model successfully depletes skeletal muscle macrophages, it incites a dramatic acute inflammatory response, as seen by the leg edema and neutrophilia. This approach may have may cause systemic leak of AP20187 causing an effect similar to what has been reported in studies using systemic depleting agents or transgenic conditional ablation models (Bader et al., 2019; Lee et al., 2014). Bader et al., concluded that given similar findings were seen in various models of systemic macrophage depletion, the neutrophilia and systemic effects were likely a direct result of macrophage depletion and not of the specific depleting agent alone. In contrast, clodronate liposomes are less likely to initiate an acute systemic inflammatory response, as seen with AP20187, because liposomes have decreased ability to cross vascular barriers. While intramuscular injection of AP20187 into MaFIA mice has limitations for discerning the functional role of macrophages in the setting of a systemic initiator of muscle inflammation (eg, intratracheal LPS), its ability to incite tissue inflammation may have utility to study the role of macrophages in local, muscle-specific inflammation. Moreover, an advantage of the MaFIA model over clodronate is the enhanced GFP incorporated in the transgene which may facilitate tracking of the macrophages. Overall, intramuscular clodronate is effective for selective depletion of muscle macrophages without eliciting acute inflammation. This is an important tool to facilitate the study of macrophage function and a feature that can be used in mouse models of inflammation and indirect skeletal muscle injury.
Supplementary Material
Acknowledgment
This work was supported by National Institute of Health (NIH) [Grant number 1R01HL143452-01].
Abbreviations:
- MaFIA
Macrophage Fas-Induced Apoptosis
- WT
Wild type
- LPS
lipopolysaccharide
- ALI
acute lung injury
- GFP
green fluorescent protein
- CSF-1
colony stimulating factor-1
- c-fms
CSF-1 receptor
- LysMCre
lysozyme M promoter-directed Cre
- IM
intramuscular
- L
left
- R
right
- VC
vehicle control
- TA
tibialis anterior
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jim.2022.113329.
Declaration of Competing Interest
The authors declare no conflicts of interest in this work.
References
- Bader JE, Enos RT, Velazquez KT, Carson MS, Sougiannis AT, McGuinness OP, Robinson CM, Murphy EA, 2019. Repeated clodronate-liposome treatment results in neutrophilia and is not effective in limiting obesity-linked metabolic impairments. Am. J. Physiol. Endocrinol. Metab 316. E358–E72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baligand C, Jouvion G, Schakman O, Gilson H, Wary C, Thissen JP, Carlier PG, 2012. Multiparametric functional nuclear magnetic resonance imaging shows alterations associated with plasmid electrotransfer in mouse skeletal muscle. J. Gene. Med 14, 598–608. [DOI] [PubMed] [Google Scholar]
- Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, Cohen DA, 2004. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J. Leukoc. Biol 75, 612–623. [DOI] [PubMed] [Google Scholar]
- Files DC, D’Alessio FR, Johnston LF, Kesari P, Aggarwal NR, Garibaldi BT, Mock JR, Simmers JL, DeGorordo A, Murdoch J, Willis MS, Patterson C, Tankersley CG, Messi ML, Liu C, Delbono O, Furlow JD, Bodine SC, Cohn RD, King LS, Crow MT, 2012. A critical role for muscle ring finger-1 in acute lung injury-associated skeletal muscle wasting. Am. J. Respir. Crit. Care Med 185, 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong WY, Abdelhamid RE, Carvalho CS, Sluka KA, 2016. Resident macrophages in muscle contribute to development of hyperalgesia in a mouse model of noninflammatory muscle pain. J. Pain 17, 1081–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Qiao L, Kinney B, Feng GS, Shao J, 2014. Macrophage depletion disrupts immune balance and energy homeostasis. PLoS One 9, e99575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno SG, 2018. Depleting macrophages in vivo with Clodronate-liposomes. Methods Mol. Biol 1784, 259–262. [DOI] [PubMed] [Google Scholar]
- Saclier M, Cuvellier S, Magnan M, Mounier R, Chazaud B, 2013. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. 280, 4118–4130. [DOI] [PubMed] [Google Scholar]
- Summan M, Warren GL, Mercer RR, Chapman R, Hulderman T, Van Rooijen N, Simeonova PP, 2006. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am. J. Phys. Regul. Integr. Comp. Phys 290, R1488–R1495. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Sanders A, 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174, 83–93. [DOI] [PubMed] [Google Scholar]
- Ziemkiewicz N, Hilliard G, Pullen NA, Garg K, 2021. The role of innate and adaptive immune cells in skeletal muscle regeneration. Int. J. Mol. Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


