Abstract
The major cytotoxic effect of the verotoxins (VTs) produced by strains of VT-producing Escherichia coli is the inhibition of host-cell protein synthesis, but VTs are also suspected to play a role in apoptotic cell signaling and cytokine release. Four differentially expressed genes, including mkp-1 (encoding mitogen-activated protein kinase phospatase 1), were detected by differential display reverse transcription-PCR (DD RT-PCR) stimulated by VT1 in Caco-2 cells. Northern blot analysis showed the induction of mkp-1 mRNA 6 h after VT1 stimulation. Neither mutant VT1 (mutVT1), harboring two mutations in the A subunit (E167Q-R170L), nor cycloheximide induced mkp-1 mRNA, but mkp-1 mRNA was detected with both wild-type VT1 (wtVT1) and anisomycin, a 28S rRNA inhibitor. Therefore, we concluded that the A subunit of VT1 was essential for mkp-1 induction. Increased amounts of phosphorylated c-Jun protein were also found with wtVT1 and anisomycin. Although the precise mechanism of induction of MKP-1 is unknown, we hypothesized that 28S rRNA not only was a sensor for ribotoxic stress, but also was involved in the signal cascade of MKP-1. This is the first report of detection by DD RT-PCR of cellular genes induced by bacterial toxins.
Strains of verotoxin (VT)-producing Escherichia coli (VTEC; or Shiga toxin-producing E. coli), major serotype O157:H7, in humans are associated with hemorrhagic colitis, hemolytic uremic syndrome, thrombotic thrombocytopenia purpura (17, 37), and encephalitis (6). Previous reports have shown that purified VTs (or Shiga toxins) damage endothelial cells in glomeruli (1, 25) and induce apoptosis in human proximal (19) and distal (18) renal tubular cells in vitro, as well as damaging neurons in the third ventricle or hippocampus in rabbits (11). These findings suggest that VTs have a pivotal role in the pathogenesis of VTEC infection. The precise mechanisms that involve the toxins in the development of colonic ulceration, hemorrhagic colitis, and systemic complications, however, remain unclear.
VTs are bipartite molecules composed of a single enzymatic A subunit and five receptor-binding B subunits (34) that bind to neutral glycolipid globotriaosylceramide (Gb3). After VTs are internalized by host cells via clathrin-coated pits (40), the A-B subunits dissociate, and the A subunit inhibits cellular protein synthesis by hydrolyzing the N-glycoside bond of adenosine at position 4324 of 28S rRNA (9, 24, 33, 36).
In recent years, VTs have also been implicated in the induction of cellular apoptosis and the release of various kinds of cytokines. In vitro, VT-induced apoptosis was observed in Vero cells (13), Burkitt's lymphoma cells (27), and an astrocytoma cell line (2). On the other hand, cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), IL-6, and IL-8, were induced in vitro by VTs in both human monocytes and human macrophages (35, 45), and IL-8 was induced in the Caco-2 cell line (47). Moreover, the induction of endothelin-1 in bovine aortic endothelial cells (4) suggests that endothelin-1 plays a role in the pathophysiology of hypertension in human VTEC infections.
This evidence led to speculation about whether VTs were involved in the signal transduction of target cells, which might lead to complications. However, which pathway is responsible for causing the transmission of the signals of VTs for apoptosis has not been clarified. One report suggested that the VT1 B subunit was responsible for inducing apoptosis in a Burkitt's lymphoma cell line (27) or in an astrocytoma cell line (2). Another report showed that the B subunit did not cause apoptosis in Vero cells (46). Concerning the release of cytokines, the VT1 B subunit showed no induction of cytokines in monocytes (45), and the VT1 A subunit was reported to be essential for IL-8 induction (47).
In this study, with the human epithelial cell line Caco-2, derived from colon cancer, we report the genes regulated by VT1 stimulation, as detected by the repeated differential display reverse transcription-PCR (DD RT-PCR) technique. Our study is the first to report on the differentially expressed genes induced by bacterial toxins; in addition, we investigated the signal cascades initiated by the differentially expressed gene. Our results showed that the DD RT-PCR technique is a useful tool for investigating the cellular signal cascade stimulated by bacterial toxins. Elucidation of mechanisms of the cytotoxicity of VTs might be clinically useful in treating their effects.
MATERIALS AND METHODS
VT1 and mutant type VT1 purification.
Wild-type VT1 (wtVT1) was purified from E. coli O157:H7 (RIMD 0509890, a VT1- and VT2-positive strain) by a modification of a method previously described (20). In brief, after polymyxin B extraction, immunoaffinity column chromatography was carried out with anti-VT1 rabbit immunoglobulin G (IgG) (19). After cloning of the PCR-amplified wtVT1 gene (primers: sense, 5′-GCGTGGAGGATGTCAAGAA-3′; antisense, 5′-GAACCGGCAACAACTGACTG-3′) into the pBluescript SK(−) vector (Stratagene, La Jolla, Calif.), recombinant mutant VT1 (mutVT1) was prepared by amino acid substitution by oligonucleotide-directed site-specific mutagenesis (QuickChange site-directed mutagenesis kit; Stratagene). Glutamic acid at position 167 was replaced by glutamine and arginine at position 170 by leucine (primers: sense, 5′-GCTCAAGCCTTACTTTTTCGGC-3′; antisense, 5′-GCCGAAAAAGTAAGGCTTGAGC-3′). mutVT1 was purified by anti-VT1 immunoaffinity column chromatography as described above.
Cell culture, cell viability, and RNA isolation.
Human Caco-2 cells (ATCC no. ECA86010202) were cultured in Dulbecco's modified Eagle medium (DMEM) (Nikken Seibutsuigaku, Kennkyuusyo, Japan) supplemented with 10% fetal bovine serum (Gibco/BRL, Gaithersburg, Md.) and 100 μg of gentamicin per ml.
Subconfluent cultures of 5 × 104 Caco-2 cells and 5 × 104 Vero cells in 96-well plates were precultured for 24 h and then incubated with 100 pM wtVT1 for 24, 48, and 72 h. After incubation with wtVT1, the relative cell viability was assessed with the WST-1 cell counting kit (Dojindo, Kumamoto, Japan).
Total RNA was isolated from the Caco-2 cells by a guanidine thiocyanate (GTC)-phenol-chloroform method with TRIZOL reagent (Gibco/BRL). The mRNA was prepared from Caco-2 cells, following the manufacturer's instructions, with a QuickPrep micro mRNA purification kit (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.) and with GTC and oligo(dT)-cellulose columns.
DD RT-PCR.
For DD analysis, total RNA (10 μg) was treated with 10 U of RNase-free DNase I (Gibco/BRL) for 1 h at 37°C to eliminate contaminating DNA. After the isolation of DNA-free total RNA, DD RT-PCR was carried out with a Takara fluorescence differential display kit according to the manufacturer's instruction manual (50), with a slight modification. In brief, DNase I-treated total RNA (0.5 μg/one downstream primer) was reverse transcribed with nine rhodamine-labeled downstream primers to determine the upper sequence of the poly(A)+ tail more accurately. These downstream primers incorporated oligo(dT) and an additional 2 bp, except for a thymidine residue. The RT reactions were individually performed with 50 pM downstream primers with 50 U of SuperScriptII (Gibco/BRL) in the presence of 0.1 mM deoxynucleoside triphosphates (dNTPs) for 60 min at 42°C. Each of the nine cDNA samples was amplified by PCR in combination with 24 arbitrary upstream primers and nine downstream primers, which were used in RT; consequently, a total of 216 PCR operations (24 × 9) were carried out. The PCR parameters were as follows: an initial cycle of 94°C for 2 min, 40°C for 5 min, and 72°C for 5 min; 34 cycles of 94°C for 30 s, 40°C for 2 min, and 72°C for 1 min; and one cycle of 72°C for 5 min. The amplified cDNA fragments were separated on 5% polyacrylamide gel containing 8 M urea and visualized with an FMBIO II Multi-View fluoroimage analyzer (Takara).
Reamplification, cloning, and sequencing of differentially expressed cDNAs.
Selected bands of cDNA were eluted from the polyacrylamide gel and reamplified by PCR utilizing the same primer sets used in the first RT-PCR. The reamplified products were subcloned in a pT7Blue T-vector (Novagen, Madison, Wis.) by the TA cloning method. After purification of the plasmid DNA, each clone was sequenced with a model ABI Prism 310 genetic analyzer (Perkin-Elmer, Applied Biosystems, Foster City, Calif.). We then analyzed the sequence data and performed homology searches by using BLAST software and the GenBank database.
Northern blot analysis of mkp-1 induction in Caco-2 cells.
Poly(A)+ RNA (3 μg) was used in 1% agarose-formaldehyde gels and transferred to Hybond N+ membranes (Amersham Pharmacia Biotech, Inc., Little Chalfont, United Kingdom) followed by UV cross-linking. Probes obtained from RT-PCR were labeled with [α-32P]dATP (Amersham Pharmacia Biotech, Inc.) by the PCR labeling method. The PCR primer sequences were as follows: β-actin gene (838 bp), sense, 5′-TGACGGGGTCACCCACACTGTGCC-3′; antisense, 5′-TAGAAGCATTTGCGGTGGACGATG-3′; mkp-1 (431 bp), sense, 5′-GCTGTGCAGCAAACAGTCGA-3′; antisense, 5′-CGATTAGTCCTCATAAGGTA-3′. The PCR parameters were as follows: an initial cycle of 95°C for 5 min; 25 cycles of 95°C for 1 min, 57°C for 2 min, and 72°C for 1 min; and a cycle of 72°C for 7 min. Filters were hybridized overnight at 42°C with labeled probes. After the filters had been washed, at a final stringency of 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) at 65°C, the filters were analyzed with a BAS 1500 Bioimage analyzer (Fuji film, Shizuoka, Japan).
Protein synthesis inhibition assay.
Protein synthesis was determined by assaying the incorporation of [35S]methionine. Plated in 96-well plates, samples of 5 × 104 Caco-2 cells were treated with either 100 pM wtVT1, 100 pM mutVT1, or 10 μg of cycloheximide or anisomycin per ml for various times with methionine-free DMEM. The samples were then incubated with 1 μCi of [35S]methionine per well (Amersham) at 37°C for 1 h in a 5% CO2 incubator. After the wells had been washed, 35S-labeled proteins were precipitated with ice-cold 10% trichloroacetic acid. Following this, the precipitated proteins were dissolved in 0.2 M NaOH and neutralized with 1.5 M HCl. The incorporated [35S]methionine was counted with a liquid scintillation counter (Top Count microplate scintillation counter (Packard, Meriden, Conn.) with LumaPlate (Packard, Osaka, Japan).
Western blot analysis.
For MKP-1 immunoblotting, Caco-2 cells were lysed in lysis buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 0.2 mM EGTA, 1% Triton X-100, 10% glycerol, 1 mM dithiothreitol, 20 mM sodium fluoride) that contained protease inhibitors (2 μg of aprotinin per ml, 2 μg of leupeptin per ml, 1 μg of pepstatin per ml, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride). The activity of JNK was estimated by comparing the immunoblotted c-Jun and phosphorylated c-Jun (12) with slight modifications. Caco-2 cells were lysed in 30 μl of modified radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.6], 1 mM NaF, 100 mM Na2MoO4, 1% Triton X-100) containing protease inhibitors (2 μg of aprotinin per ml, 2 μg of leupeptin per ml, 1 μg of pepstatin per ml, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride) and centrifuged at 12,500 × g for 20 min at 4°C.
Equivalent amounts of cell lysates were heated in 2× SDS sample buffer at 95°C for 5 min and then electrophoresed on an SDS–10% polyacrylamide gel before transfer to nitrocellulose membranes (Protran; Schleicher & Schuell, Darmstadt, Germany). The membranes were incubated with primary rabbit polyclonal antibody against MKP-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) or human c-Jun (no. 9162; New England BioLabs, Inc., Beverly, Mass.), washed, and then blotted with horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody (New England BioLabs). The membrane was developed with a Phototope-HRP Western detection kit (New England BioLabs) and exposed to X-ray film.
RESULTS
Determination of differentially expressed cDNA.
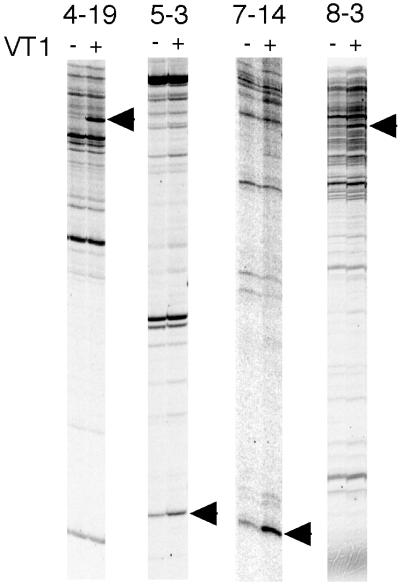
A single sample subjected to DD RT-PCR ordinarily shows 50 to 150 cDNA bands. Consequently, after one round of DD RT-PCR (216 samples), we had analyzed about 20,000 genes. As far as we know, 10,000 to 20,000 genes are expressed simultaneously in one vertebrate cell, so our DD RT-PCR protocol attempted to cover all of the genes expressed by Caco-2 cells. One serious drawback of DD RT-PCR is that nonreproducible and false-positive bands interfere with the differentially expressed bands. To counter this, we carried out three rounds of DD RT-PCR with different RNA samples. In all, 48 cDNA bands were detected as differentially expressed genes after the first round of DD RT-PCR. Among these cDNA bands, only four cDNA fragments (8.3%) were detected commonly in each of the three rounds of DD RT-PCR (Fig. 1). Sequencing and homology searching showed that clone 5-3 was identical to the gene that encodes cytochrome b561 (GenBank accession no. U06715), and that clone 4-19 was mkp-1 (also known as cl100; GenBank accession no. X68277). Although the data for the remaining two up-regulated clones had no identical genes registered in the GenBank database, clone 8-3 had zinc finger motifs (MOTIF; GenomeNet, Kyoto University), and partial homology with zinc finger proteins (GenBank accession no. X65232 or X07290). On the other hand, clone 7-14 revealed no known nucleotide motifs.
FIG. 1.
DD RT-PCR of Caco-2 cells. Caco-2 cells were treated with PBS (VT−) or 100 pM wtVT1 (VT+) for 6 h. The fluorescent DD RT-PCR products were analyzed on an FMBIO II Multi-View fluoroimage analyzer. Arrows show the differentially expressed bands. The numbers at the top show the primer sets (downstream primer-upstream primer) used in this experiment.
Expression of mkp-1 at both the mRNA and protein levels.
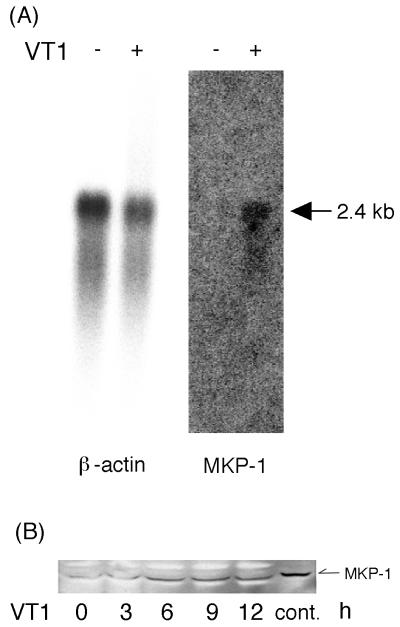
To verify that clone 4-19 truly corresponded to differentially expressed mRNAs, Northern blot analysis was performed by hybridizing specific probes for the human β-actin or mkp-1 gene. The human β-actin and mkp-1 cDNA fragments obtained from RT-PCR were labeled with [α-32P]dATP for probes. The basal expression level of mkp-1 was not detectable in Caco-2 cells before wtVT1 stimulation, but 6 h after the addition of 100 pM wtVT1, we detected 2.4 kb of mRNA, corresponding to mkp-1 (Fig. 2A). In addition, we also investigated whether MKP-1 protein was increased after treatment of Caco-2 cells with VT1. As shown in Fig. 2B, the level of MKP-1 protein was also increased by VT1 from 6 h after stimulation, although VT1 inhibits protein synthesis.
FIG. 2.
(A) Expression of mkp-1 mRNA (2.4 kb) detected by Northern blot analysis. Caco-2 cells were treated with PBS (left) or wtVT1 (right) for 6 h, and then each poly(A)+ RNA (3 μg/lane) was hybridized with mkp-1 and β-actin cDNA probes. (B) Western blot analysis of MKP-1. Caco-2 cells (5 × 106) were treated with 100 pM wtVT1 for the times indicated. HeLa cell lysates were used as a positive control (cont.).
Inhibition of 28S rRNA up-regulated the expression of MKP-1 mRNA.
It is widely accepted that the major cytotoxicity of VTs is derived from the inhibition of protein synthesis, so we also tested whether mkp-1 mRNA induction was correlated with the inhibitory effects of protein synthesis.
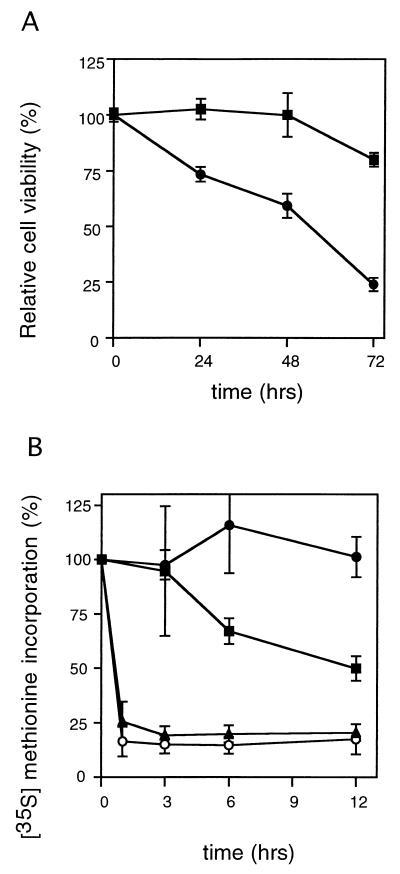
We first measured the relative cell viability of Caco-2 and Vero cells to confirm the cytotoxicity of the purified wtVT1 (Fig. 3A). Cell viabilities of Caco-2 cells after stimulation with 100 pM wtVT1 were 102.6% ± 4.7% (24 h), 99.9% ± 9.7% (48 h), and 80.0% ± 3.1% (72 h). In comparison, the cell viabilities of Vero cells after stimulation with 100 pM wtVT1 were 73.3% ± 3.3% (24 h), 59.3% ± 5.5% (48 h), and 24.0% ± 1.3% (72 h), which indicates they were much more sensitive to wtVT1 than Caco-2 cells were. Since we added VT1 at a subconfluent culture of Caco-2 cells, our Caco-2 cells showed more sensitivity to VT1 than in the other report (16).
FIG. 3.
Effect of cytotoxicity of wtVT1 on viability of Caco-2 and Vero cells. (A) Caco-2 (■) and Vero cells (●) were treated with 100 pM wtVT1 for the indicated periods. The relative cell viability of these cells was calculated. Means ± standard deviations (n = 3) are shown. (B) Caco-2 cells were incubated with 100 pM wtVT1 (■), 100 pM mutVT1 (●), 10 μg of cycloheximide per ml (▴), or 10 μg of anisomycin per ml (○) for the periods indicated. The relative incorporation of [35S]methionine compared to that in PBS-treated Caco-2 cells is shown. Means ± standard deviations (n = 3) are shown.
The inhibitory effect of protein synthesis was calculated by measuring the incorporation of the [35S]methionine after exposure of Caco-2 cells to wtVT1, mutVT1 (E167Q/R170L), cycloheximide, and anisomycin (Fig. 3B). Even after 12 h, 100 pM mutVT1 did not decrease [35S]methionine incorporation in Caco-2 cells (97.6% ± 6.8% at 1 h, 101.3% ± 24.4% at 12 h), but 100 pM wtVT1 gradually decreased protein synthesis (94.7% ± 29.8% at 1 h, 49.9% ± 5.7% at 12 h). The protein synthesis inhibitors cycloheximide and anisomycin more rapidly suppressed [35S]methionine incorporation in Caco-2 cells than wtVT1 did: 10 μg of cycloheximide per ml reduced protein synthesis to 25.6% ± 9.1% in 1 h and 20.5% ± 1.0% after 12 h of stimulation; with 10 μg of anisomycin per ml, the reductions were to 16.4% ± 6.8% in 1 h and 17.5% ± 6.9% in 12 h.
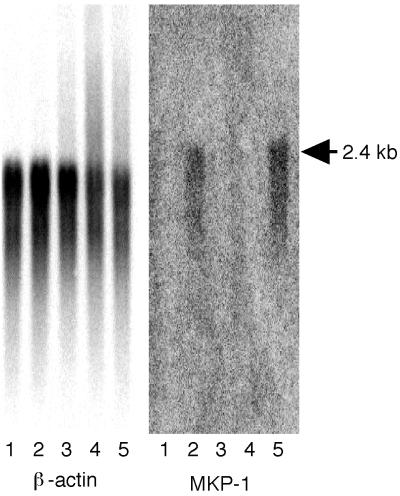
Northern blot analysis was performed to elucidate the mechanisms of and evaluate the active domain of VT1 responsible for mkp-1 mRNA induction. mkp-1 mRNA was detected after a 6-h exposure to 100 pM wtVT1 and 10 μg of anisomycin per ml, but not after exposure to mutVT1, cycloheximide, and phosphate-buffered saline (PBS) (Fig. 4).
FIG. 4.
Northern blot analysis of the β-actin and mkp-1 gene products. Caco-2 cells were treated with various stimulators for 6 h. Lanes: 1, PBS; 2, 100 pM wtVT1; 3, 100 pM mutVT1; 4, 10 μg of cycloheximide per ml; 5, 10 μg of anisomycin per ml.
c-Jun protein was phosphorylated by the inhibitors of 28S rRNA.
It has been reported that the substrates of MKP-1 are mitogen-activated protein kinases (MAPKs), including JNK (44). Anisomycin is a known agonist of JNK, so we evaluated c-Jun phosphorylation to clarify whether JNK was activated by VT1 or protein synthesis inhibitors.
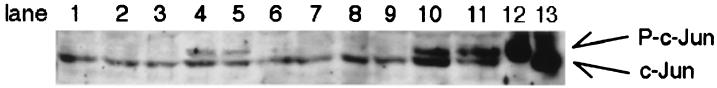
Among the substances tested, evidence of c-Jun protein phosphorylation was found 6 and 12 h after exposure to only wtVT1 and anisomycin; no similar effect was observed at any time after exposure to mutVT1, cycloheximide, and PBS (Fig. 5). In lanes 10 and 11, which were stimulated by anisomycin, we could see two intense bands. After treatment with anisomycin, the amounts of both c-Jun and phosphorylated c-Jun protein were increased. This is because anisomycin is known to be an agonist of JNK (14), and c-jun mRNA (8). Only wtVT1 and anisomycin could induce mkp-1 mRNA (Fig. 4) and phosphorylate c-Jun protein (Fig. 5).
FIG. 5.
Effect of wtVT1 and anisomycin on c-Jun protein phosphorylation. Caco-2 cells were untreated (lane 1) or treated with PBS (lane 2, 6 h; lane 3, 12 h), 100 pM wtVT1 (lane 4, 6 h; lane 5, 12 h), 100 pM mutVT1 (lane 6, 6 h; lane 7, 12 h), 10 μg of cycloheximide per ml (lane 8, 6 h; lane 9, 12 h), or 10 μg of anisomycin per ml (lane 10, 6 h; lane 11, 12 h). Lane 12, positive control (phosphorylated c-Jun, New England BioLabs); lane 13, negative control (unphosphorylated c-Jun; New England BioLabs). The top arrow shows phosphorylated (P) (activated) c-Jun, and the bottom arrow shows nonactivated c-Jun.
DISCUSSION
Early research on VTs mainly documented the role of protein synthesis inhibition (7, 9, 33); however, recent studies have been focused on other effects of VTs. VTs release proinflammatory cytokines (35, 45), activate NF-κB and AP-1 transcriptional factors (39), and induce endothelin-1 (4). VTs also induce apoptosis in several types of cells. Prior to DD RT-PCR, we tested and confirmed that only a few percentile of Caco-2 cells underwent apoptosis by the stimulation of VT1 in staining of the nucleus with 1 μg of Hoechst 33258 per ml (data not shown). Scientists are now debating whether to attribute the main responsibility for the induction of apoptosis to the A subunit, the B subunit, or both. Concerning IL-8, it has been reported that the A subunit is necessary for induction and release of IL-8 (47). However, it is unclear whether the inhibition of 28S rRNA function is required for induction and/or release of IL-8. The expression level of Gb3, the VT receptor, is reported to be important to the cytotoxicity to host cells (15). The expression levels of Gb3 in Caco-2 cells are lower than those in Vero cells, but they are still present (16).
To detect differentially expressed genes in vitro, numerous techniques have been developed, such as DD, subtractive hybridization, serial analysis of gene expression, and, more recently, cDNA array. No reports had previously appeared about the use of any of the techniques mentioned above to elucidate which cellularly responsive genes are regulated by bacterial toxins, so we applied the DD method to investigate the cytopathic effects of VT1. DD has technical limitations, because low-stringency PCR with arbitrary primers gives rise to false-positive bands, resulting in low reproducibility (29). In general, only 10 to 20% of cDNA fragments have been confirmed to be differentially expressed genes (30). Responding to this technical difficulty, we repeated the DD RT-PCR three times to eliminate false-positive bands. In the previous report, VT1 induced IL-8 mRNA in Caco-2 cells (47), but we could not detect IL-8 mRNA with DD RT-PCR in VT1-stimulated-Caco-2 cells. This may be because the primer sets used in this DD RT-PCR were not suitable for amplifying IL-8 mRNA. To answer this question, we detected the elevation of IL-8 mRNA in the same RNA sample used in DD RT-PCR, by using IL-8-specific primer sets (data not shown). Another limitation of DD RT-PCR is the quantity of mRNA under cytotoxic conditions. With VT1, a toxin that reacts slowly, it usually takes 48 to 72 h before cell death, and it is easy to adjust the dose of the toxin to extract mRNA.
Of the four cDNA clones isolated by DD RT-PCR, we focused on mkp-1, which is known as a dual-specificity MAPK phosphatase gene, because MKP-1 is responsive to growth factors or stress (41) and is related to the signal transduction pathways of the host cell. Previous reports have indicated that MKP-1 has the potential to inactivate MAPK, including JNK, extracellular signal-regulated kinase (42), and p38 MAPK in vitro and in vivo (5, 26, 32), or to inhibit TNF-α-induced apoptosis in rat mesangial cells by preventing the prolonged activation of JNK (12). In short, it seems that the induction of MKP-1 and JNK activation occurs concurrently in a feedback loop (22, 26), and the balance between induction of MKP-1 and activation of JNK may decide the fate of the cells. It is known that the induction of MKP-1 occurs in conjunction with various types of cellular stress (21, 28), but the precise mechanisms of induction are still unclear. Therefore, we examined the upregulation mechanism of mkp-1 mRNA by VT1. A mutVT1 protein, originally reported by Yamasaki et al. (48), which has two point mutations in the A subunit of VT1, was constructed. This mutVT1 protein maintains its ability to bind with Gb3 (47). From the evidence of Northern blot analysis, mkp-1 mRNA was only induced by wtVT1, but not by mutVT1; this result implied that the A subunit, which inhibits protein synthesis, was responsible for inducing mkp-1 mRNA. In human mesangial cells, mkp-1 mRNA was induced by wtVT1 (data not shown). We further compared VT1 to other protein synthesis inhibitors by using cycloheximide and anisomycin at concentrations sufficient to impair [35S]methionine incorporation by more than 80% (Fig. 3B). Our results show that only wtVT1 and anisomycin could induce mkp-1 mRNA, whereas mutVT1 and cycloheximide could not (Fig. 4). What we know of the different mechanisms of the protein synthesis inhibitors seems to explain these data. Cycloheximide blocks the translocation of peptidyl-tRNA, especially by inhibiting ribosome rolling along the mRNA (10). On the other hand, anisomycin binds to 28S rRNA at the peptidyl transferase center, and so inhibits peptidyl transferase activity (38, 43). As explained above, the A subunit possessed by VTs has N-glycosidase activity on 28S rRNA. Therefore, we speculated that the inhibition of 28S rRNA function played an important role in mkp-1 mRNA expression. Consequently, the inhibition of protein synthesis per se did not account for the induction of mkp-1 mRNA. We were led to believe that 28S rRNA not only was a sensor for ribotoxic stress, but also was involved in the signal cascade of MKP-1.
Activation of JNK occurs concurrently with the induction of mkp-1 mRNA in a feedback loop (5, 26), so we next analyzed c-Jun phosphorylation. In Caco-2 cells, JNK was activated by the exposure to wtVT1 and anisomycin, but not by exposure to mutVT1 or cycloheximide (Fig. 5). Phosphorylation of c-Jun was correlated with the expression of mkp-1 shown by Northern blot analysis (Fig. 4). From these data, we considered that VT1 transmitted the signal leading to the expression of MKP-1 and phosphorylation of c-Jun, which may result in apoptosis. In consideration of a report that ricin, a plant toxin, damages the 28S rRNA function of phosphorylated c-Jun protein in Rat-1 cells (14), we tend to think that the signals involved in the induction of MKP-1 and the phosphorylation of c-Jun both ensue from the inhibition of the 28S rRNA function. After all, VT1 not only is a protein-synthesis inhibitor in the host cells, but also is a key regulator of host-cell fate dependent on the activation of MKP-1 and JNK.
Previous reports have shown that the activation of JNK is associated with apoptosis in the hippocampus in vivo (3) under ischemic conditions (49) and in glial cells in vitro (23, 51). Accordingly, c-Jun phosphorylation caused by VT itself might play a role in the unexplained pathogenesis of the central nervous system (31) that has been found in VTEC infection, which has not been explained yet. We hope that elucidation of the molecular basis of genes differentially expressed by VT1 stimulation will provide useful information about the pathogenesis of VTEC infection that may contribute to effective clinical and therapeutic strategies.
ACKNOWLEDGMENTS
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan, a Grant for International Health Cooperation Research from the Ministry of Health and Welfare of Japan, and the “Research for the Future” program of the Japan Society for the Promotion of Sciences (JSPS-RFTF 97L00704).
REFERENCES
- 1.Adler S, Bollu R. Glomerular endothelial cell injury mediated by Shiga-like toxin-1. Kidney Blood Press Res. 1998;21:13–21. doi: 10.1159/000025838. [DOI] [PubMed] [Google Scholar]
- 2.Arab S, Murakami M, Dirks P, Boyd B, Hubbard S L, Lingwood C A, Rutka J T. Verotoxins inhibit the growth of and induce apoptosis in human astrocytoma cells. J Neurooncol. 1998;40:137–150. doi: 10.1023/a:1006010019064. [DOI] [PubMed] [Google Scholar]
- 3.Behrens A, Sibilia M, Wagner E F. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- 4.Bitzan M M, Wang Y, Lin J, Marsden P A. Verotoxin and ricin have novel effects on preproendothelin-1 expression but fail to modify nitric oxide synthase (ecNOS) expression and NO production in vascular endothelium. J Clin Investig. 1998;101:372–382. doi: 10.1172/JCI522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bokemeyer D, Sorokin A, Yan M, Ahn N G, Templeton D J, Dunn M J. Induction of mitogen-activated protein kinase phosphatase 1 by the stress-activated protein kinase signaling pathway but not by extracellular signal-regulated kinase in fibroblasts. J Biol Chem. 1996;271:639–642. doi: 10.1074/jbc.271.2.639. [DOI] [PubMed] [Google Scholar]
- 6.Cimolai N, Carter J E. Bacterial genotype and neurological complications of Escherichia coli O157:H7-associated haemolytic uraemic syndrome. Acta Paediatr. 1998;87:593–594. doi: 10.1080/08035259850158353. [DOI] [PubMed] [Google Scholar]
- 7.Donohue-Rolfe A, Jacewicz M, Keusch G T. Shiga toxin as inhibitor of protein synthesis. Methods Enzymol. 1988;165:231–235. doi: 10.1016/s0076-6879(88)65036-1. [DOI] [PubMed] [Google Scholar]
- 8.Edwards D R, Mahadevan L C. Protein synthesis inhibitors differentially superinduce c-fos and c-jun by three distinct mechanisms: lack of evidence for labile repressors. EMBO J. 1992;11:2415–2424. doi: 10.1002/j.1460-2075.1992.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endo Y, Tsurugi K, Yutsudo T, Takeda Y, Ogasawara T, Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem. 1988;171:45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- 10.Felicetti L, Colombo B, Baglioni C. Inhibition of protein synthesis in reticulocytes by antibiotics. II. The site of action of cycloheximide, streptovitacin A and pactamycin. Biochim Biophys Acta. 1966;119:120–129. [PubMed] [Google Scholar]
- 11.Fujii J, Kinoshita Y, Kita T, Higure A, Takeda T, Tanaka N, Yoshida S-I. Magnetic resonance imaging and histopathological study of brain lesions in rabbits given intravenous verotoxin 2. Infect Immun. 1996;64:5053–5060. doi: 10.1128/iai.64.12.5053-5060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y L, Kang B, Williams J R. Inhibition of the expression of mitogen-activated protein phosphatase-1 potentiates apoptosis induced by tumor necrosis factor-α in rat mesangial cells. J Biol Chem. 1998;273:10362–10366. doi: 10.1074/jbc.273.17.10362. [DOI] [PubMed] [Google Scholar]
- 13.Inward C D, Williams J, Chant I, Crocker J, Milford D V, Rose P E, Taylor C M. Verocytotoxin-1 induces apoptosis in Vero cells. J Infect. 1995;30:213–218. doi: 10.1016/s0163-4453(95)90693-2. [DOI] [PubMed] [Google Scholar]
- 14.Iordanov M S, Pribnow D, Magun J L, Dinh T-H, Pearson J A, Chen S L-Y, Magun B E. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the α-sarcin/ricin loop in the 28S rRNA. Mol Cell Biol. 1997;15:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacewicz M S, Mobassaleh M, Gross S K, Balasubramanian K A, Daniel P F, Raghavan S, McCluer R H, Keusch G T. Pathogenesis of Shigella diarrhea. XVII. A mammalian cell membrane glycolipid, Gb3, is required but not sufficient to confer sensitivity to Shiga toxin. J Infect Dis. 1994;169:538–546. doi: 10.1093/infdis/169.3.538. [DOI] [PubMed] [Google Scholar]
- 16.Jacewicz M S, Acheson D W, Mobassaleh M, Donohue-Rolfe A, Balasubramanian K A, Keusch G T. Maturational regulation of globotriaosylceramide, the Shiga-like toxin 1 receptor, in cultured human gut epithelial cells. J Clin Investig. 1995;96:1328–1335. doi: 10.1172/JCI118168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiyokawa N, Taguchi T, Mori T, Uchida H, Sato N, Takeda T, Fujimoto J. Induction of apoptosis in normal human renal tubular epithelial cells by Escherichia coli Shiga toxins 1 and 2. J Infect Dis. 1998;178:178–184. doi: 10.1086/515592. [DOI] [PubMed] [Google Scholar]
- 19.Kodama T, Nagayama K, Yamada K, Ohba Y, Akeda Y, Honda T. Induction of apoptosis in human renal proximal tubular epithelial cells by Escherichia coli verocytotoxin 1 in vitro. Microbiol Immunol. 1999;188:73–78. doi: 10.1007/s004300050107. [DOI] [PubMed] [Google Scholar]
- 20.Kongmuang U. Studies on Shiga-like toxin produced by enterohemorrhagic Escherichia coli: purification and characterization of the toxin and development of methods for identifying the toxin. Med J Osaka Univ. 1989;38:39–49. [PubMed] [Google Scholar]
- 21.Kusari A B, Byon J, Bandyopadhyay D, Kenner K A, Kusari J. Insulin-induced mitogen-activated protein (MAP) kinase phosphatase-1 (MKP-1) attenuates insulin-stimulated MAP kinase activity: a mechanism for the feedback inhibition of insulin signaling. Mol Endocrinol. 1997;11:1532–1543. doi: 10.1210/mend.11.10.9998. [DOI] [PubMed] [Google Scholar]
- 22.Landeroute K R, Mendonca H L, Calaoagan J M, Knapp M, Giaccia A J, Stork P J S. Mitogen-activated protein kinase phosphatase-1 (MKP-1) expression is induced by low oxygen conditions found in solid tumor microenvironments. J Biol Chem. 1999;274:12890–12897. doi: 10.1074/jbc.274.18.12890. [DOI] [PubMed] [Google Scholar]
- 23.Lannuzel A, Barnier J V, Hery C, Huynh V T, Guibert B, Gray F, Vincent J D, Tardieu M. Human immunodeficiency virus type I and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol. 1997;42:847–856. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- 24.Lingwood C A. Verotoxins and their glycolipid receptors. Adv Lipid Res. 1993;25:189–211. [PubMed] [Google Scholar]
- 25.Lingwood C A. Verotoxin-binding in human renal sections. Nephron. 1994;66:21–28. doi: 10.1159/000187761. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Gorospe M, Yang C, Holbrook N J. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress. J Biol Chem. 1995;270:8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- 27.Mangeney M, Lingwood C A, Taga S, Calliou B, Tursz T, Wiels J. Apoptosis induced in Burkitt's lymphoma cells via Gb3/CD77, a glycolipid antigen. Cancer Res. 1993;53:5314–5319. [PubMed] [Google Scholar]
- 28.Marquis J C, Demple B. Complex genetic response of human cells to sublethal levels of pure nitric oxide. Cancer Res. 1998;58:3435–3440. [PubMed] [Google Scholar]
- 29.Matz M V, Lukyanov S A. Different strategies of differential display: areas of application. Nucleic Acids Res. 1998;26:5537–5543. doi: 10.1093/nar/26.24.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miele G, MacRae L, McBride D, Manson J, Clinton M. Elimination of false positives generated through PCR reamplification of differential display cDNA. BioTechniques. 1998;25:138–144. doi: 10.2144/98251rr02. [DOI] [PubMed] [Google Scholar]
- 31.Mizuguchi M, Tanaka S, Fujii I, Tanizawa H, Suzuki Y, Igarashi T, Yamanaka T, Takeda T, Miwa M. Neuronal and vascular pathology produced by verocytotoxin 2 in the rabbit central nervous system. Acta Neuropathol. 1996;91:254–262. doi: 10.1007/s004010050423. [DOI] [PubMed] [Google Scholar]
- 32.Muda M, Theodosiou A, Rodrigues N, Boschert U, Camps M, Gillieron C, Davies K, Ashworth A, Arkinstall S. The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J Biol Chem. 1996;271:27205–27208. doi: 10.1074/jbc.271.44.27205. [DOI] [PubMed] [Google Scholar]
- 33.Obrig T G, Moran T P, Brown J E. The mode of action of Shiga toxin on peptide elongation of eukaryotic protein synthesis. Biochem J. 1987;244:287–294. doi: 10.1042/bj2440287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsnes S, Reisbig R, Eiklid K. Subunit structure of Shigella cytotoxin. J Biol Chem. 1981;256:8732–8738. [PubMed] [Google Scholar]
- 35.Ramegowda B, Tesh V L. Differentiation-associated toxin receptor modulation, cytokine production, and sensitivity to Shiga-like toxins in human monocytes and monocytic cell lines. Infect Immun. 1996;64:1173–1180. doi: 10.1128/iai.64.4.1173-1180.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reisbig R, Olsnes S, Eiklid K. The cytotoxic activity of Shigella toxin. Evidence for catalytic inactivation of the 60 S ribosomal subunit. J Biol Chem. 1981;256:8739–8744. [PubMed] [Google Scholar]
- 37.Richardson S E, Karmali M A, Becker L, Smith R. The hisopathology of the hemolytic uremic syndrome associated with verocytotoxin-producing Escherichia coli infections. Hum Pathol. 1988;19:1102–1108. doi: 10.1016/s0046-8177(88)80093-5. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Fonseca C, Amils R, Garrett R A. Fine structure of the peptidyl transferase centre on 23 S-like rRNAs deduced from chemical probing of antibiotic-ribosome complexes. J Mol Biol. 1995;247:224–235. doi: 10.1006/jmbi.1994.0135. [DOI] [PubMed] [Google Scholar]
- 39.Sakiri R, Ramegowda B, Tesh V L. Shiga toxin type 1 activates tumor necrosis factor-alpha gene transcription and nuclear translocation of the transcriptional activators nuclear factor-kappaB and activator protein-1. Blood. 1998;92:558–566. [PubMed] [Google Scholar]
- 40.Sandvig K, Olsnes S, Brown J E, Petersen O W, van Deurs B. Endocytosis from coated pits of Shiga toxin: a glycolipid-binding protein from Shigella dysenteriae 1. J Cell Biol. 1989;108:1331–1343. doi: 10.1083/jcb.108.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scimeca J C, Servant M J, Dyer J O, Meloche S. Essential role of calcium in the regulation of MAP kinase phosphatase-1 expression. Oncogene. 1997;15:717–725. doi: 10.1038/sj.onc.1201231. [DOI] [PubMed] [Google Scholar]
- 42.Simon J C, Beltman J, Cadwallader K A, McMahon M, McCormick F. Regulation of mitogen-activated protein kinase phosphatase-1 expression by extracellular signal-related kinase-dependent and Ca++-dependent signal pathways in Rat-1 cells. J Biol Chem. 1997;272:13309–13319. doi: 10.1074/jbc.272.20.13309. [DOI] [PubMed] [Google Scholar]
- 43.Sweeney R, Yao C H, Yao M C. A mutation in the large subunit ribosomal RNA gene of Tetrahymena confers anisomycin resistance and cold sensitivity. Genetics. 1991;127:327–334. doi: 10.1093/genetics/127.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tournier C, Thomas G, Pierre J, Jacquemin C, Pierre M, Saunier B. Mediation by arachidonic acid metabolites of the H2O2-induced stimulation of mitogen-activated protein kinases (extracellular-signal-regulated kinase and c-Jun NH2-terminal kinases) Eur J Biochem. 1997;244:587–595. doi: 10.1111/j.1432-1033.1997.00587.x. [DOI] [PubMed] [Google Scholar]
- 45.van Setten P A, Monnens L A, Verstraten R G, van den Heubel L P, van Hinsbergh V W. Effects of verocytotoxin-1 on nonadherent human monocytes: binding characteristics, protein synthesis, and induction of cytokine release. Blood. 1996;88:174–183. [PubMed] [Google Scholar]
- 46.Williams J M, Lea N, Roberts L M, Milford D V, Taylor C M. Comparison of ribosome-inactivating proteins in the induction of apoptosis. Toxicol Lett. 1997;91:121–127. doi: 10.1016/s0378-4274(97)03879-4. [DOI] [PubMed] [Google Scholar]
- 47.Yamasaki C, Natori Y, Zeng X T, Ohmura M, Yamasaki S, Takeda Y, Natori Y. Induction of cytokines in a human colon epithelial cell line by Shiga toxin 1 (Stx1) and Stx2 but not by non-toxic mutant Stx1 which lacks N-glycosidase activity. FEBS Lett. 1999;442:231–234. doi: 10.1016/s0014-5793(98)01667-6. [DOI] [PubMed] [Google Scholar]
- 48.Yamasaki S, Furutani M, Ito K, Igarashi K, Nishibuchi M, Takeda Y. Importance of arginine at position 170 of the A subunit of Vero toxin 1 produced by enterohemorrhagic Escherichia coli for toxin activity. Microb Pathog. 1991;11:1–9. doi: 10.1016/0882-4010(91)90088-r. [DOI] [PubMed] [Google Scholar]
- 49.Yang D D, Kuan C Y, Whitmarsh A J, Ricon M, Zheng T S, Davis R J, Rakic P, Flavell R A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 50.Yoshikawa Y, Mukai H, Asada K, Hino F, Kato I. Differential display with carboxy-X-rhodamine-labeled primers and the selection of differentially amplified cDNA fragments without cloning. Anal Biochem. 1998;256:82–91. doi: 10.1006/abio.1997.2473. [DOI] [PubMed] [Google Scholar]
- 51.Zhang P, Miller B S, Rosenzweig S A, Bhat N R. Activation of c-Jun N-terminal kinase/stress-activated protein kinase in primary glial cultures. J Neurosci Res. 1996;46:114–121. doi: 10.1002/(SICI)1097-4547(19961001)46:1<114::AID-JNR14>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]