Abstract
To determine the naturally occurring immunological responses to the Schistosoma mansoni antigens paramyosin, IrV-5, Sm-23 (MAP-3), and triose phosphate isomerase (MAP-4), a total of 119 subjects from an area of endemicity for schistosomiasis, including “resistant” subjects (n = 17) were evaluated. Specific immunoglobulin G1 (IgG1), IgG2, IgG3, IgG4, and IgA levels for each of the antigens and the cytokine profile in culture supernatants from antigen-stimulated peripheral blood mononuclear cells (PBMC) were determined. Although all the subjects had a high degree of contaminated water exposure, their infection levels were variable (0 to 1,128 eggs/g of stool). There were direct correlations between infection levels and levels of SWAP- and paramyosin-specific IgG1 and IgG4 (P < 0.05). However, an inverse correlation between infection levels and specific IgG2 to IrV-5 (P < 0.01) was observed. The evaluation of the cytokine profile (interleukin 5 [IL-5], IL-10, gamma interferon [IFN-γ], and tumor necrosis factor alpha) in response to these antigens showed inverse correlations between the degree of infection and IFN-γ levels in PBMC supernatants stimulated with paramyosin (P < 0.05) and IrV-5 (P < 0.01). Additionally, inverse correlations between the degree of infection and IL-5 levels in MAP-3- and MAP-4-stimulated PBMC supernatants (P < 0.01) were found. Logistic regression analysis was performed to adjust the results of cytokine profile by age. IL-5 production in MAP-3-stimulated PBMC supernatants was associated with lower infection levels (odds ratio = 11.2 [95% confidence interval, 2.7 to 45.8]).
Schistosomiasis is a chronic parasitic infection that affects 200 million people in Africa, South America, and Asia (35). Although treatment of infected people with schistosomicidal drugs has in part controlled the morbidity of the disease, transmission is largely unaltered (3, 5, 24). The possibility of a schistosomiasis vaccine as an additional measure to control the disease arose from the fact that the parasite does not multiply in human beings and that reduction of infection levels by schistosomicidal drugs reduces the prevalence of severe forms of the disease. Moreover, in experimental models, partial immunity can be induced by vaccination with irradiated cercariae or specific antigens (2, 11, 14, 26, 36, 37, 58, 59). Immunological studies of subjects from areas of endemicity have demonstrated a naturally occurring resistance to reinfection (4, 19, 22–25, 27, 28). Both high levels of specific immunoglobulin E (IgE) in sera and gamma interferon (IFN-γ) in antigen-stimulated peripheral blood mononuclear cell (PBMC) cultures were associated with resistance to reinfection (1, 22, 24, 25, 27, 28, 56, 57). These data suggest the participation of immunological mechanisms in human resistance to Schistosoma mansoni infection, with mixed cellular and humoral responses.
Several S. mansoni antigens have been identified and tested in experimental models, with the induction of variable levels of protection against infection (11, 32, 49, 59, 61, 68–70, 74). The World Health Organization (WHO) has selected six of these antigens for further in vitro studies with PBMC from subjects in areas of endemicity for schistosomiasis. The present study shows the immunological responses of subjects from an area where schistosomiasis is endemic to four of these antigens: paramyosin (49), irradiation-associated vaccine antigen (IrV-5) (68), triose phosphate isomerase (TPI) (32, 61), and S. mansoni 23-kDa antigen (Sm-23) (31). The immunological responses of subjects who, though exposed to contaminated water, appear on the basis of negative stool examinations to be uninfected were compared to those of infected patients with similar degrees of contaminated water exposure.
MATERIALS AND METHODS
Area of endemicity.
Caatinga do Moura is a village of 3,913 inhabitants located on the banks of a river in Bahia, a northeastern state of Brazil. Agriculture is the main economic activity of the village, and irrigation is carried out by a primitive system of canals. The river and canals are populated by snails infected by S. mansoni. The population is infected during irrigation and through the domestic use of water. The prevalence of disease is about 40%, according to a parasitological evaluation performed in 1995. Active transmission of the parasite is still present in this area.
Study subjects.
A sample of 1,064 subjects, residents of 208 houses located at about 500 meters from the river, have been monitored with parasitological examinations since 1992. In 1993 this population was treated with oxamniquine (25 to 30 mg/kg of body weight). In 1995, another parasitological evaluation was performed and water contact levels were determined by interview. The water contact levels were classified according to previous publications (19, 23), and a sample of those subjects with a high level of water contact (1 to 3 h per day) was selected to participate in the present study. This group's water contact was confirmed by a health post agent from the village through direct daily observations of activities in the river. The selection of subjects with similar water contact levels was done in an attempt to standardize both the opportunity to be infected and the natural exposure to parasite antigens. This study was approved by the Ethical Committee of the Hospital Universitário Prof. Edgard Santos. Informed consent, following the guidelines of the Brazilian Ministry of Health for research with human subjects, was obtained from all patients. The inclusion criteria were age between 5 and 60 years, high water contact levels (1 to 3 h/day), male and female representation, and at least two parasitological examinations performed on different days. The exclusion criteria were age less than 5 years and greater than 60 years, absence of water contact or doubt about water contact levels, pregnancy, and immunological disorders that may interfere with the results of immunological tests. A group (n = 119) was selected to participate in the present study and was divided into two groups. Group 1 was composed of subjects (n = 17) with negative examinations (three to six samples) in 1992 and 1995 but high exposure to contaminated water. All but two members of this group, who were uncertain, were treated with schistosomicidal drugs more than 10 years ago. The mean age of this group was 43 ± 13 years (range, 13 to 60 years), with 9 males and 8 females. There was no difference in the conditions (type of activity and time of day) under which these subjects and the infected subjects were exposed to contaminated water. In addition to having no eggs in their stools, individuals in this group were all negative for serum schistosome circulating cathodic antigen (CCA) (data not shown). Group 2 was composed of subjects with positive parasitological examinations (n = 102) with different levels of infection, including 47 with more than 200 eggs/g of stool. The mean age of this group of patients was 23 ± 15 years (range, 5 to 50 years), and their infection levels ranged from ≥24 to 1,128 eggs/g of stool. They had intestinal or hepatointestinal forms of schistosomiasis but were otherwise apparently healthy without signs of malnutrition. In both groups there were subjects infected with other intestinal parasites, such as Ascaris lumbricoides, Entamoeba histolytica, and Trichuris trichiura, without significant differences between the groups. A control group was formed from students and hospital employees who were free of S. mansoni infection but who may have been also infected with other parasites such as A. lumbricoides, E. histolytica, and T. trichiura.
Parasitological methods.
Parasitological examinations were performed periodically using Kato-Katz's method (44–46). Two to six stool samples were collected from each subject on different days. The results were shown as the arithmetic means of the numbers of eggs obtained at different days. A negative examination by Kato-Katz's method represents <24 eggs/g of stool. Kato-Katz's method is the quantitative method of choice to measure infection level and has been used extensively in epidemiological studies due to its simplicity and reproducibility when three to five parasitological examinations are performed on different days during 2 to 3 weeks (44, 45, 47). Measurements of CCA levels were performed with serum samples from a group of 52 subjects, which includes the group with negative parasitological examinations and others with different infection levels, according to a previously described technique (18, 20, 21).
Antigens.
S. mansoni-specific antigens were provided as part of a WHO project to evaluate in vitro immune responses to S. mansoni-specific antigens. The antigens used were soluble extract of whole adult S. mansoni (SWAP), purified native paramyosin, IrV-5, and multiple antigenic peptides containing T- and B-cell epitopes designed from the antigens Sm-23 (MAP-3) and TPI (MAP-4). Each antigen was tested for cytotoxicity by measuring the inhibition of the lymphoproliferative response of healthy controls to a suboptimal concentration of phytohemagglutinin mitogen. Nonspecific contaminants, such as lipopolysaccharide, were excluded because the antigens did not induce responses in healthy control subjects (n = 10).
Immunological procedures.
The cellular immune response was evaluated from January to December 1996. Blood was heparinized, and plasma was separated and stored at −20°C for the evaluation of the humoral immune response. PBMCs were isolated from heparinized blood by density gradient centrifugation using Histopaque 1077 (Sigma Diagnostics, St. Louis, Mo.).
Humoral immune response.
Analysis of Ig isotypes (IgG1, IgG2, IgG3, IgG4, IgE, and IgA) specific to SWAP and S. mansoni-specific antigens was performed by enzyme-linked immunosorbent assay (ELISA), using a modification of a previously described technique (48). Briefly, plates were coated with each antigen at previously established concentrations (SWAP and paramyosin at 10 μg/ml and IrV-5, MAP-3, and MAP-4 at 1 μg/ml) and left overnight at 4°C. For specific IgE measurement, IgG antibodies were removed by RF absorbent (Behring Diagnostics Inc., Westwood, Mass.) following the manufacturer's instructions. The sera were diluted 1:2 in phosphate-buffered saline (PBS)–0.05% Tween (PBST) and incubated with the same volume of RF absorbent that had been resuspended in 1.5 ml of distilled water. After incubation at room temperature for 15 min, samples were centrifuged at 650 × g for 5 min. The IgG-preabsorbed plasma samples were used at a final dilution of 1:4. The plates coated with the antigens were blocked with PBS–3% bovine serum albumin, and 100-μl plasma samples were incubated overnight at 4°C at dilutions previously tested for each of the isotypes for the different antigens. Mouse anti-human antibodies against each Ig isotype were added to the plates (1:500). After incubation for 2 h at 37°C and six washes with PBST, anti-mouse Ig coupled with peroxidase was added and the plates were incubated for 1 h at 37°C (1:1,000). These antibodies were kindly provided by Victor Tsang from the Centers for Disease Control and Prevention. After six more washes with PBST, the reaction was developed by the addition of TMB substrate (tetramethylbenzidine urea peroxide-stabilized chromogen; ICN Biomedicals Inc.) and stopped with H2SO4. Plates were read in a spectrophotometer at 450 nm, and results were expressed as optical density (OD). Sera from control subjects, free of S. mansoni infection but contaminated with other intestinal parasitic infections, were used to calculate the cutoff for each of the specific isotypes (mean plus 2 standard deviations [SD] of the OD of the controls). Standardization of the serum dilutions, as well as the dilutions of the anti-isotype and the conjugate, were performed for each specific isotype using sera from two schistosomiasis subjects with high and low antibody titers to SWAP. To minimize intratest variation, the IgG isotype analyses for each patient were performed on the same plate. The brand of substrate solution TMB and the duration used for the color reaction were the same for all experiments.
Determination of cytokine profile from PBMC-stimulated cultures.
Levels of the cytokines (interleukin 5 [IL-5], IL-10, IFN-γ, and tumor necrosis factor alpha) were measured in supernatants from PBMC cultures stimulated with the different antigens. Briefly, 3 × 106 cells in 1 ml of RPMI 1640 (GIBCO-BRL) supplemented with 10% AB Rh-positive sera were stimulated with the different antigens at optimal concentrations determined previously. The optimal antigen concentrations were the ones that induced significant responses in the schistosomiasis patient PBMCs but that did not induce a response in the control PBMCs. The concentrations of the antigens were as follows: SWAP, 10 μg/ml; paramyosin, 60 μg/ml; IrV-5, 2 μg/ml; MAP-3, 100 μg/ml; MAP-4, 100 μg/ml. After a 72-h incubation, the supernatants were collected and stored at −20°C until cytokine measurement. Cytokine concentration was determined by a sandwich ELISA technique, and the results were expressed as picograms per milliliter based on a standard curve. Cytokine levels above 30 pg/ml were considered positive responses (value not seen in normal control subjects).
Statistical methods.
The humoral and cellular immune responses to each of the antigens were analyzed as continuous variables and correlated with infection levels (number of eggs per gram of stool) by the Spearman correlation test, since they do not fit a Gaussian distribution. The design of the study, taking only subjects with similar water contact levels, eliminated any influence of this variable on the differences found in infection levels. The differences in immune responses between the group of subjects with negative examinations and the group with >200 eggs/g were analyzed by the Mann-Whitney test. These statistical analyses were performed using the program InStat for Macintosh.
Because of the age dependence of the intensity of infection, logistic regression analysis was performed to adjust the immunological data by age. Cytokine data were categorized as positive (≥30 pg/ml; levels not seen in control subjects) or negative, and infection levels were categorized as either less than or greater than or equal to 200 eggs/g of stool. Odds ratios (OR) and confidence intervals (CI) were calculated. The significance in terms of the logistic regression models was tested using χ2, the difference in deviance, which was assumed to follow a χ2 distribution under the null hypothesis that the term was unimportant. The Hosmer and Lemeshow goodness-of-fit test was used in the final model. The logistic regression analysis was performed using the SAS system, version 6.12 (SAS Institute Inc., Cary, N.C.), for IBM.
RESULTS
Study subjects and infection levels.
The mean age of the 119 studied patients was 23 ± 15 years, with 73 males and 46 females. In spite of having similar contaminated water contact levels, these subjects had a high variability in infection levels. The mean (± SD) number of eggs per gram of stool for these subjects was 246 ± 361 (range, 0 to 1,128 eggs/g of stool), including 17 with no detectable eggs, 42 with 1 to 100, 15 with 101 to 200, 18 with 201 to 400, and 27 with greater than 400 eggs/g of stool. An inverse correlation between age and infection levels from 1992 (rs = −0.24; P < 0.01) and 1995 (rs = −0.22; P < 0.01) was observed (Spearman's correlation test) (data not shown). Additionally, a direct correlation between infection levels in 1992 and reinfection levels in 1995 was observed (rs = 0.38; P < 0.01; Spearman's correlation test) (data not shown). Levels of the S. mansoni CCA were below the cutoff (OD = 0.043) in all subjects with negative parasitological examinations. In subjects with positive parasitological examinations (n = 80), there was a direct correlation between the number of eggs per gram of stool and the CCA levels (rs = 0.40, P = 0.0002; Spearman's correlation test).
Humoral immune responses to SWAP- and S. mansoni-specific antigens.
Antibodies to SWAP were detected in a high percentage of subjects, with 95% responding with total IgG (mean of OD ± standard error of the mean [SEM], 0.4 ± 0.02), 98% with IgG1 (mean of OD ± SEM, 1.4 ± 0.07), 75% with IgG4 (mean of OD ± SEM, 0.4 ± 0.06), and 58% with IgA (mean of OD ± SEM, 0.7 ± 0.04). Among the specific S. mansoni antigens, paramyosin-specific IgG1 was detected in 50% of the subjects (mean of OD ± SEM, 1.35 ± 0.07) and IgG4 was detected in 48% (mean of OD ± SEM, 0.23 ± 0.05). IrV-5-specific IgG4 was detected in 25% of subjects (mean of OD ± SEM, 0.14 ± 0.05), and higher titers of IgG3 and IgG2 were found in 22 (mean of OD ± SEM, 1.3 ± 0.08) and 12% (mean of OD ± SEM, 0.7 ± 0.08) of the subjects, respectively. MAP-3-specific IgG3 was found in 53% of the subjects (mean of OD ± SEM, 0.16 ± 0.03), but higher levels of IgA were found in 21% of them (mean of OD ± SEM, 1.3 ± 0.07). Only 22% of the subjects responded to MAP-4 with specific IgG1 (mean of OD ± SEM, 0.26 ± 0.01). Table 1 shows the results of the correlation analysis. Direct correlations between infection levels (number of eggs per gram of stool) and levels of specific IgG1 (OD) and IgG4 to SWAP and paramyosin were observed (P < 0.05; Spearman's correlation), suggesting that these isotypes are markers for high levels of infection. However, inverse correlations between infection levels and the levels of specific IgG2 to SWAP (P = 0.05) and IrV-5 (P < 0.05) were found (Spearman's correlation test). This indicates that people with lower infection levels produced higher levels of IgG2 specific for these antigens. A direct correlation between IrV-5-specific IgG2 and IFN-γ levels in response to this same antigen (P < 0.01; Spearman's correlation test) (data not shown) was observed. No correlation between infection levels and levels of SWAP-specific IgE (P > 0.05; Spearman's correlation test) was observed. However, an inverse correlation between SWAP-specific IgE/IgG4 ratio and infection levels (P < 0.05; Spearman's correlation test) was found.
TABLE 1.
Correlation between infection levels and antigen-specific isotype response in plasma and cytokine profile in antigen-stimulated PBMCs from subjects from an area where schistosomiasis is endemic
| Variable correlated with infection levelsc | Spearman's rs |
|---|---|
| IgG1 specific to SWAP | 0.31a |
| IgG1 specific to paramyosin | 0.31a |
| IgG4 specific to SWAP | 0.30a |
| IgG4 specific to paramyosin | 0.33a |
| IgG2 specific to SWAP | −0.15b |
| IgG2 specific to IrV-5 | −0.27a |
| IFN-γ to SWAP | −0.19b |
| IFN-γ to paramyosin | −0.23a |
| IFN-γ to IrV-5 | −0.24a |
| IL-5 to MAP-3 | −0.32a |
| IL-5 to MAP-4 | −0.33a |
P < 0.01.
P = 0.05.
Numbers of eggs per gram of stool.
Cytokine profile of stimulated PBMC cultures from subjects from an area of endemicity for schistosomiasis.
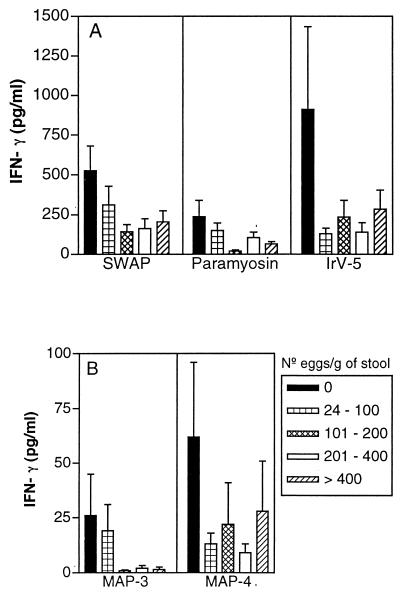
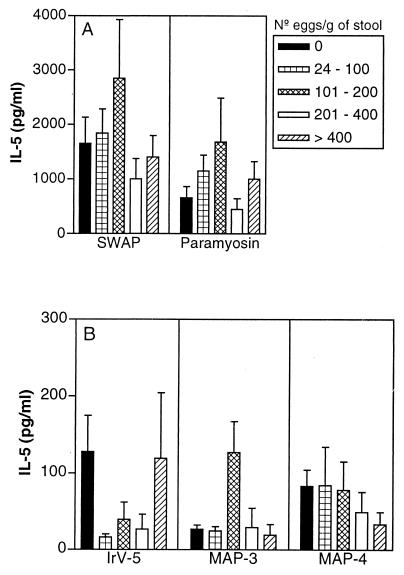
The percentages of patients who produced IL-5 and IFN-γ and the ranges and means ± SEMs of cytokine concentrations in antigen-stimulated PBMC cultures from schistosomiasis patients are shown in Table 2. Paramyosin stimulated IL-5 production by PBMC from 53% of the studied subjects. Compared to other defined antigens, paramyosin induced the highest IL-5 production (1,013 ± 170 pg/ml) but also induced IFN-γ production (126 ± 25 pg/ml). In contrast, IrV-5 induced little IL-5 production but strongly promoted IFN-γ production (284 ± 83 pg/ml) by PBMC from 61% of the subjects. MAP-4 induced more IL-5 than IFN-γ production in 40% of the subjects (67 ± 20 pg/ml), and MAP-3 induced lower levels of both IFN-γ and IL-5 in a smaller proportion of the study group (26 and 3%, respectively). Figure 1 shows IFN-γ production in response to the different antigens in subjects classified according to their degrees of infection. A higher production of IFN-γ is observed in PBMC supernatants stimulated with IrV-5, SWAP, and paramyosin in subjects with negative stool examinations but exposed to contaminated water, and decreasing levels in infected subjects are observed. There was an inverse correlation between IFN-γ in response to paramyosin and IrV-5 and infection levels (P < 0.05; Spearman's correlation test) (Table 1). Figure 2 shows IL-5 levels in PBMC supernatants stimulated with the different antigens from the study subjects classified according to their infection levels. Interestingly, higher levels of IL-5 were observed in subjects with 101 to 200 eggs/g of stool in response to the antigens SWAP, paramyosin, and MAP-3, corresponding to the subjects who produced lower levels of IFN-γ (Fig. 1). However, the subjects with negative stool samples also produced high levels of IL-5 in response to SWAP and paramyosin. Inverse correlations between IL-5 levels in PBMC supernatants stimulated with MAP-3 and MAP-4 and infection levels were also observed (P < 0.01; Spearman's correlation) (Table 1). The logistic regression analysis showed that age below 20 years was considered a risk for high infection levels (≥200 eggs/g of stool; OR, 12.5; 95% CI, 4.1 to 38.2; P < 0.0001). After adjustment by age, only the IL-5 response to MAP-3 was associated with lower infection levels (OR, 11.2; 95% CI, 2.8 to 45.8; P < 0.0007).
TABLE 2.
IL-5 and IFN-γ responses from PBMCs stimulated with S. mansoni vaccine candidate antigens for subjects from an area where schistosomiasis is endemic
| Antigen | Cytokine | No. of patients | % Respondersa | Cytokine level (pg/ml)
|
|
|---|---|---|---|---|---|
| Range | Mean ± SEM | ||||
| Paramyosin | IL-5 | 116 | 53 | 0–9,713 | 1,013 ± 170 |
| IFN-γ | 119 | 50 | 0–1,937 | 126 ± 25 | |
| IrV-5 | IL-5 | 116 | 29 | 0–2,388 | 57 ± 82 |
| IFN-γ | 119 | 61 | 0–9,027 | 284 ± 83 | |
| MAP-3 | IL-5 | 111 | 26 | 0–399 | 37 ± 8 |
| IFN-γ | 115 | 3 | 0–516 | 11 ± 5 | |
| MAP-4 | IL-5 | 116 | 40 | 0–2,208 | 67 ± 20 |
| IFN-γ | 118 | 14 | 0–629 | 24 ± 8 | |
Responder level, ≥30 pg/ml.
FIG. 1.
IFN-γ levels (mean ± standard error) in PBMC supernatants stimulated with SWAP, paramyosin, and IrV-5 (A) and MAP-3 and MAP-4 (B) from subjects from an area of endemicity for schistosomiasis with negative parasitological examinations or with different infection levels (numbers of eggs per gram of stool).
FIG. 2.
IL-5 levels (mean ± standard error) in PBMC supernatants stimulated with SWAP and paramyosin (A) and IrV-5, MAP-3, and MAP-4 (B) from subjects from an area of endemicity for schistosomiasis with negative parasitological examinations or with different infection levels (numbers of eggs per gram of stool).
Comparison of humoral and cellular immune responses between subjects with “resistant” phenotype and subjects infected with ≥200 eggs/g of stool.
Immunological responses of resistant subjects (n = 17) with negative examinations in 1992 and 1995 (3 to 6 samples) but highly exposed to contaminated water were compared with those of the infected subjects with more than 200 eggs/g of stool (n = 47). The mean age (± SD) of these resistant subjects (42 ± 13 years; range, 13 to 60 years) was significantly higher than that of the group with ≥200 eggs/g of stool (mean ± SD, 16 ± 8 years; range, 8 to 40 years) (P < 0.01; Student's t test). Similar percentages of males and females were found in both groups.
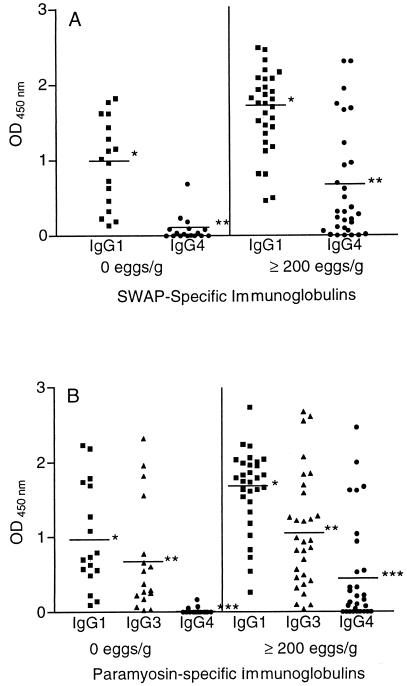
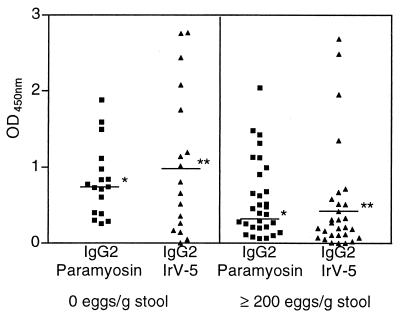
The comparison of specific isotype levels between subjects with the resistant phenotype and the ones infected with ≥200 eggs/g confirmed the data shown in the correlation analysis. SWAP-specific IgG1 and IgG4 levels were higher in the infected group (P < 0.01; Mann-Whitney test), as shown in Fig. 3A. Moreover levels of paramyosin-specific IgG1, IgG3, and IgG4 were also higher in the infected group (P < 0.05; Mann-Whitney test) (Fig. 3B). Although there were no differences in SWAP-specific IgE levels between the groups, the IgE-to-IgG4 ratio was higher in the group with the resistant phenotype (P < 0.05; Mann-Whitney test) (data not shown). Paramyosin-specific IgE was detected in only five people, one from the resistant group and four from the infected group (OD variation, 0.031 to 0.101). IrV-5-specific IgE was also detected in five people, two from the resistant group and three from the infected group (OD variation, 0.132 to 0.789). Specific IgE to MAP-3 and MAP-4 was not detected in any of the subjects tested. Additionally, levels of IgG2 specific to paramyosin and IrV-5 were higher in the resistant group (P < 0.05; Mann-Whitney test), as shown in Fig. 4. No differences in the other antigen-specific isotypes between these two groups of patients were found.
FIG. 3.
Levels of SWAP-specific IgG1 and IgG4 (A) and paramyosin-specific IgG1, IgG3, and IgG4 (B) in subjects from an area where schistosomiasis is endemic with negative parasitological examinations and, in infected subjects with ≥200 eggs/g of stool. The Mann-Whitney test was used. ∗ and ∗∗∗, P < 0.01; ∗∗, P < 0.05.
FIG. 4.
Levels of paramyosin- and IrV-5-specific IgG2 in subjects from an area where schistosomiasis is endemic with negative parasitological examinations and in infected subjects with ≥200 eggs/g of stool. ∗, P < 0.05; ∗∗, P < 0.01 (Mann-Whitney test).
The levels of production of cytokines in supernatants of antigen-stimulated PBMCs in these groups of resistant and infected (≥200 eggs/g of stool) patients were compared. Higher levels of IFN-γ were seen in PBMCs from resistant subjects stimulated with SWAP, IrV-5, and MAP-3 (mean ± SEM, 527 ± 154, 911 ± 523, and 25 ± 19 pg/ml, respectively) than in PBMCs from highly infected subjects (mean ± SEM, 188 ± 46, 217 ± 72, and 2 ± 1 pg/ml, respectively; P < 0.05; Mann-Whitney test). IL-5 levels were higher in PBMCs from resistant subjects stimulated with IrV-5, MAP-3, and MAP-4 (mean ± SEM, 128 ± 47, 26 ± 5, and 83 ± 21 pg/ml, respectively) than in PBMCs from highly infected subjects (mean ± SEM, 85 ± 55, 22 ± 13, and 38 ± 14 pg/ml, respectively; P < 0.05; Mann-Whitney test). No differences in IL-10 and TNF-α levels between the groups (P > 0.05; Mann-Whitney test) were found (not shown).
DISCUSSION
Resistance to S. mansoni infection is well demonstrated in experimental models of schistosomiasis, either after natural infection or after immunization with irradiated cercariae or certain defined S. mansoni antigens (9, 11, 26, 29, 33, 36, 40, 50, 59, 71, 73, 74). The protective immune responses differ in these experimental models, being mainly humoral (IgE and IgG2a) in rats and mixed cellular and humoral in mice (13, 14, 34, 38, 39, 41, 42, 51, 52, 59). Although human immune responses to S. mansoni have been studied extensively, the mechanism by which humans resist schistosome infection are still unclear. Data from two different research groups in areas of endemicity in Kenya and Brazil have shown an association between resistance to reinfection and high levels of specific IgE and high IgE-to-IgG4 ratios (22, 24, 25, 28, 56, 57). Other studies in two areas of endemicity in Minas Gerais, Brazil, have described subjects who exhibit complete resistance to infection (16). These individuals showed higher levels of IFN-γ in PBMC supernatants stimulated with a schistosomula membrane extract and IgG antibodies to paramyosin and higher levels of schistosome antigen-specific IgE than infected subjects (1, 72).
The defined S. mansoni antigens tested in the present study were selected as vaccine candidates by WHO because they were shown to be protective in vaccination experiments in animals. Paramyosin was first identified in sera from mice immunized with an S. mansoni adult worm antigen in association with Mycobacterium bovis bacillus Calmette-Guérin (BCG). Immunization promotes 39% protection in mice (50, 60), and this protein was recognized by sera from putative resistant subjects from areas where schistosomiasis is endemic (16). The gene encoding IrV-5 was cloned from a cDNA library using an antibody (IrV-3) from immunized mice that was not present in sera from infected animals. The IrV-5 recombinant protein induced 75% protection in mice and 25% protection in baboons (2, 65–68). TPI is an enzyme from the glycolytic pathway, identified by a monoclonal antibody that is capable of passively immunizing naive mice against infection. The enzyme itself induces 30 to 60% protection in mice (30, 53, 61). Sm-23 is an integral membrane protein, part of a superfamily of proteins which includes CD9 and TAPA-1, first described in hematopoietic cells. Sm-23 gives 40 to 50% protection in mice (2, 31). Because of the high homology of TPI and Sm-23 with mammalian proteins, epitope mapping of them was carried out, and multiple antigenic peptides containing T- and B-cell epitopes from the less-conserved regions were designed (54, 55). Aside from data for paramyosin, nothing is known about the immune responses to these antigens in S. mansoni-resistant subjects.
The present study shows the in vitro humoral and cellular immune responses of subjects from an area of endemicity with high exposure to infection but with variable degrees of infection, including complete resistance (negative parasitological examinations). An inverse correlation between levels of IFN-γ in response to paramyosin and IrV-5 and infection levels (number of eggs per gram of stool) was observed. Moreover, there is an inverse correlation also between levels of IL-5 production in response to MAP-3 and MAP-4 and infection levels. Considering that an inverse correlation between immunological parameters and infection levels is considered a sign that a given response is protective, an argument can be made that IFN-γ production in response to paramyosin and IrV-5 and IL-5 production in response to MAP-3 and MAP-4 are protective immune responses. Although the levels of IL-5 in PBMC supernatants stimulated with MAP-3 and MAP-4 were very low, these values correlate inversely with infection levels. As MAP-3 and MAP-4 are peptidic antigens, even low production of this cytokine may have biological relevance, as it might be expected that fewer specific lymphocytes are in the circulation at any given time. Moreover, the IL-5 response to MAP-3 was associated with resistance even after adjustment by age. These data suggest that both types of cellular immune responses, Th1 and Th2, are involved in the protective response to S. mansoni. Moreover, different specific antigens induce different types of protective immune responses, such that paramyosin and IrV-5 induce IFN-γ, which correlates inversely with infection levels, and MAP-3 and MAP-4 induce IL-5, which also inversely correlates with infection levels.
The present study also shows a direct correlation between the levels of IgG1 and IgG4 specific to SWAP and paramyosin and infection levels, confirming previously published data (22, 72). These data suggest that IgG1 and IgG4 are markers of higher infection levels. High levels of IgG2 specific for SWAP and IrV-5 were associated with lower infection levels, contradicting previously published experiments done with schistosomula membrane extract (22). In mice, IgG2a antibodies are induced by IFN-γ. In the present study we showed a direct correlation between IFN-γ levels in PBMC culture supernatants stimulated with IrV-5 and levels of IgG2 specific for the same antigen. This finding suggests that IFN-γ in humans also stimulates the production of IgG2. It remains to be clarified whether this isotype is involved in protective immunity or if it is only a consequence of IFN-γ production, with IFN-γ being the protective mediator.
The present study supports the idea that both cellular and humoral immune mechanisms may be important to control S. mansoni infection and that they may be active in different sites in humans. Specific IgE, interacting with eosinophils and phagocytic cells, has been demonstrated to be effective in schistosomula destruction in vitro (6–8, 10, 12, 13, 15) and may be an important immune defense mechanism in the skin, where these cell types are found at the time of cercarial penetration. The majority of studies done with subjects from areas of endemicity support a role for IgE in resistance to S. mansoni infection (22, 24, 25, 28, 56, 57). In the present study, the levels of SWAP-specific IgE were not correlated with infection levels. However, the IgE/IgG4 ratio was indeed correlated inversely with infection levels, supporting the idea that the presence of IgG4 might block a protective role of IgE in the most-infected subjects. In mice, IFN-γ-activated macrophages are important in parasite destruction in the lungs (17, 43, 62–64). The present study supports the role of IFN-γ production in the protective response in human beings by showing higher levels of this cytokine in partially or completely resistant subjects. The mechanisms involved in parasite destruction in humans are still unclear, as well as the site of parasite killing and other cytokines involved in this process.
The choice of an antigen to be further tested as a vaccine candidate for schistosomiasis is difficult. The present data evaluating the human immune response to four of these antigens suggest that all of them naturally induce an immune response that is correlated with protection against reinfection. The data also suggest that perhaps a vaccine that induces both Th1- and Th2-like responses would be necessary to achieve higher levels of protection. The choice will also depend on the facility to produce the antigen in large scale and on the possibility of achieving a good interaction between the antigens chosen, should a multicomponent vaccine be selected. An additional concern is that a vaccine should not lead to a potentiated immunopathologic response, since the granulomatous reaction and resulting hepatic fibrosis in schistosomiasis are also immunologically mediated. Fortunately, immunized animals do not present with increased hepatic fibrosis due to schistosome infection, indicating that it is possible to achieve a balanced immunological response that protects against reinfection without being deleterious to the host. Although naturally resistant subjects from areas of endemicity do not present with increased hepatic fibrosis due to natural S. mansoni infection, immunization, especially with adjuvants, has to be carefully evaluated.
ACKNOWLEDGMENTS
This study was supported by UNDP/WORLD BANK/WHO Special Programme for Research in Tropical Disease (TDR), grant 940687; by Programa de Núcleos de Excelência (PRONEX); and by TMRC, grant AI 30639. Edgar M. Carvalho is senior investigator of the Brazilian Research Council (CNPq).
We thank Antonio de Souza and Sonia de Souza for their dedicated work in the area of endemicity. We also thank Elbe Myrtes and Jackson Lemos for secretarial assistance.
REFERENCES
- 1.Bahia-Oliveira L M, Simpson A J, Alves-Oliveira L F, Carvalho-Queiroz C, Silveira A M, Viana I R, Cunha-Melo J R, Hagan P, Gazzinelli G, Correa-Oliveira R. Evidence that cellular immune responses to soluble and membrane associated antigens are independently regulated during human schistosomiasis mansoni. Parasite Immunol. 1996;18:53–63. doi: 10.1046/j.1365-3024.1996.d01-49.x. [DOI] [PubMed] [Google Scholar]
- 2.Bergquist R. Prospects of vaccination against schistosomiasis. Scand J Infect Dis Suppl. 1990;76:60–71. [PubMed] [Google Scholar]
- 3.Bina J C. Estudo de variáveis que podem influenciar na evolução da esquistossomose mansônica: efeito da terapêutica específica e da interrupção da transmissão. Rev Patol Trop. 1997;26:69–128. [Google Scholar]
- 4.Butterworth A E, Capron M, Cordingley J S, Dalton P R, Dunne D W, Kariuki H C, Kimani G, Koech D, Mugambi M, Ouma J H, et al. Immunity after treatment of human schistosomiasis mansoni. II. Identification of resistant individuals, and analysis of their immune responses. Trans R Soc Trop Med Hyg. 1985;79:393–408. doi: 10.1016/0035-9203(85)90391-8. [DOI] [PubMed] [Google Scholar]
- 5.Butterworth A E, Curry A J, Dunne D W, Fulford A J, Kimani G, Kariuki H C, Klumpp R, Koech D, Mbugua G, Ouma J H, et al. Immunity and morbidity in human schistosomiasis mansoni. Trop Geogr Med. 1994;46:197–208. [PubMed] [Google Scholar]
- 6.Butterworth A E, Sturrock R F, Houba V. Antibody-dependent cell-mediated damage to schistosomula in vitro. Nature. 1974;252:503–505. doi: 10.1038/252503a0. [DOI] [PubMed] [Google Scholar]
- 7.Butterworth A E, Sturrock R F, Houba V, Mahmoud A A F, Sher A, Rees P H. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975;256:727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- 8.Butterworth A E, Wassom D L, Gleich G J, Loegering D A, David J R. Damage to schistosomula of schistosoma mansoni induced directly by eosinophil major basic protein. J Immunol. 1979;122:221–229. [PubMed] [Google Scholar]
- 9.Capron A, Dessaint J P, Capron M, Ouma J H, Butterworth A E. Immunity to schistosomes: progress toward vaccine. Science. 1987;238:1065–1072. doi: 10.1126/science.3317823. [DOI] [PubMed] [Google Scholar]
- 10.Capron A, Dessaint J P, Haque A, Auriault C, Joseph M. Macrophages as effector cells in helminth infections. Trans R Soc Trop Med Hyg. 1983;77:631–635. doi: 10.1016/0035-9203(83)90191-8. [DOI] [PubMed] [Google Scholar]
- 11.Capron A, Riveau G, Grzych J M, Boulanger D, Capron M, Pierce R. Development of a vaccine strategy against human and bovine schistosomiasis. Background and update. Mem Inst Oswaldo Cruz. 1995;90:235–240. doi: 10.1590/s0074-02761995000200019. [DOI] [PubMed] [Google Scholar]
- 12.Capron M, Bazin H, Joseph M, Capron A. Evidence for IgE-dependent cytotoxicity by rat eosinophils. J Immunol. 1981;126:1764–1768. [PubMed] [Google Scholar]
- 13.Capron M, Capron A. Immunoglobulin E and effector cells in schistosomiasis. Science. 1994;264:1876–1877. doi: 10.1126/science.8009216. [DOI] [PubMed] [Google Scholar]
- 14.Capron M, Capron A. Rats, mice and men—models for immune effector mechanisms against schistosomiasis. Parasitol Today. 1986;2:69–75. doi: 10.1016/0169-4758(86)90158-4. [DOI] [PubMed] [Google Scholar]
- 15.Capron M, Nogueira-Queiroz J A, Papin J P, Capron A. Interactions between eosinophils and antibodies: in vivo protective role against rat schistosomiasis. Cell Immunol. 1984;83:60–72. doi: 10.1016/0008-8749(84)90225-9. [DOI] [PubMed] [Google Scholar]
- 16.Correa-Oliveira R, Pearce E J, Oliveira G C, Golgher D B, Katz N, Bahia L G, Carvalho O S, Gazzinelli G, Sher A. The human immune response to defined immunogens of Schistosoma mansoni: elevated antibody levels to paramyosin in stool-negative individuals from two endemic areas in Brazil. Trans R Soc Trop Med Hyg. 1989;83:798–804. doi: 10.1016/0035-9203(89)90334-9. [DOI] [PubMed] [Google Scholar]
- 17.Dean D A, Mangold B L. Evidence that both normal and immune elimination of Schistosoma mansoni take place at the lung stage of migration prior to parasite death. Am J Trop Med Hyg. 1992;47:238–248. doi: 10.4269/ajtmh.1992.47.238. [DOI] [PubMed] [Google Scholar]
- 18.Deelder A M, de Jonge N, Fillie Y E, Kornelis D, Helaha D, Qian Z-L, de Caluwe P, Polderman A M. Quantitative determination of circulating antigens in human schistosomiasis mansoni using an indirect hemagglutination assay. Am J Trop Med Hyg. 1989;40:50–54. doi: 10.4269/ajtmh.1989.40.50. [DOI] [PubMed] [Google Scholar]
- 19.de Jesus A M, Almeida R P, Bacellar O, Araujo M I, Demeure C, Bina J C, Dessein A J, Carvalho E M. Correlation between cell-mediated immunity and degree of infection in subjects living in an endemic area of schistosomiasis. Eur J Immunol. 1993;23:152–158. doi: 10.1002/eji.1830230125. [DOI] [PubMed] [Google Scholar]
- 20.de Jonge N, Gryseels B, Hilberath G W, Polderman A M, Deelder A M. Detection of circulating anodic antigen by ELISA for seroepidemiology of schistosomiasis mansoni. Trans R Soc Trop Med Hyg. 1988;82:591–594. doi: 10.1016/0035-9203(88)90523-8. [DOI] [PubMed] [Google Scholar]
- 21.de Jonge N, Rabello A L, Krijger F W, Kremsner P G, Rocha R S, Katz N, Deelder A M. Levels of the schistosome circulating anodic and cathodic antigens in serum of schistosomiasis patients from Brazil. Trans R Soc Trop Med Hyg. 1991;85:756–759. doi: 10.1016/0035-9203(91)90446-6. [DOI] [PubMed] [Google Scholar]
- 22.Demeure C E, Rihet P, Abel L, Ouattara M, Bourgois A, Dessein A J. Resistance to Schistosoma mansoni in humans: influence of the IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J Infect Dis. 1993;168:1000–1008. doi: 10.1093/infdis/168.4.1000. [DOI] [PubMed] [Google Scholar]
- 23.Dessein A J, Begley M, Demeure C, Caillol D, Fueri J, dos Reis M G, Andrade Z A, Prata A, Bina J C. Human resistance to Schistosoma mansoni is associated with IgG reactivity to a 37-kDa larval surface antigen. J Immunol. 1988;140:2727–2736. [PubMed] [Google Scholar]
- 24.Dunne D W, Butterworth A E, Fulford A J, Kariuki H C, Langley J G, Ouma J H, Capron A, Pierce R J, Sturrock R F. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992;22:1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- 25.Dunne D W, Butterworth A E, Fulford A J, Ouma J H, Sturrock R F. Human IgE responses to Schistosoma mansoni and resistance to reinfection. Mem Inst Oswaldo Cruz. 1992;87(Suppl. 4):99–103. doi: 10.1590/s0074-02761992000800014. [DOI] [PubMed] [Google Scholar]
- 26.Dunne D W, Hagan P, Abath F G C. Prospects for immunological control of schistosomiasis. Lancet. 1995;345:1488–1492. doi: 10.1016/s0140-6736(95)91041-7. [DOI] [PubMed] [Google Scholar]
- 27.Hagan P, Abath F G. Recent advances in immunity to human schistosomiasis. Mem Inst Oswaldo Cruz. 1992;87(Suppl. 4):95–98. doi: 10.1590/s0074-02761992000800013. [DOI] [PubMed] [Google Scholar]
- 28.Hagan P, Blumenthal U J, Dunn D, Simpson A J G, Wilkins H A. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–246. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 29.Harn D A, Cianci C M L, Caulfield J P. Research brief: Schistosoma mansoni: immunization with cercarial glycocalyx preparation increases the adult worm burden. Exp Parasitol. 1989;68:108–110. doi: 10.1016/0014-4894(89)90015-5. [DOI] [PubMed] [Google Scholar]
- 30.Harn D A, Gu W, Oligino L D, Mitsuyama M, Gebremichael A, Richter D. A protective monoclonal antibody specifically recognizes and alters the catalytic activity of schistosome triose-phosphate isomerase. J Immunol. 1992;148:562–567. [PubMed] [Google Scholar]
- 31.Harn D A, Mitsuyama M, Huguenel E D, David J R. Schistosoma mansoni: detection by monoclonal antibody of a 22,000-dalton surface membrane antigen which may be blocked by host molecules on lung stage parasites. J Immunol. 1985;135:2115–2120. [PubMed] [Google Scholar]
- 32.Harn D A, Mitsuyama M, Huguenel E D, Oligino L, David J R. Identification by monoclonal antibody of a major (28 kDa) surface membrane antigen of Schistosoma mansoni. Mol Biochem Parasitol. 1985;16:345–354. doi: 10.1016/0166-6851(85)90075-1. [DOI] [PubMed] [Google Scholar]
- 33.Harn D A, Quinn J J, Cianci C M, Ko A I. Evidence that a protective membrane epitope is involved in early but not late phase immunity in Schistosoma mansoni. J Immunol. 1987;138:1571–1580. [PubMed] [Google Scholar]
- 34.Hoffmann K F, James S L, Cheever A W, Wynn T A. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J Immunol. 1999;163:927–938. [PubMed] [Google Scholar]
- 35.Iarotski L S, Davis A. The schistosomiasis problem in the world: results of a WHO questionnaire survey. Bulletin of the W H O. 1981;59:115–127. [PMC free article] [PubMed] [Google Scholar]
- 36.James S L. A review: Schistosoma spp.: progress toward a defined vaccine. Exp Parasitol. 1987;63:247–252. doi: 10.1016/0014-4894(87)90170-6. [DOI] [PubMed] [Google Scholar]
- 37.James S L, DeBlois L A. Induction of protective immunity against Schistosoma mansoni by a nonliving vaccine. II. Response of mouse strains with selective immune defects. J Immunol. 1986;136:3864–3871. [PubMed] [Google Scholar]
- 38.James S L, Glaven J, Goldenberg S, Meltzer M S, Pearce E. Tumour necrosis factor (TNF) as a mediator of macrophage helminthotoxic activity. Parasite Immunol. 1990;12:1–13. doi: 10.1111/j.1365-3024.1990.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 39.James S L, Nacy C. Effector functions of activated macrophages against parasites. Curr Opin Immunol. 1993;5:518–523. doi: 10.1016/0952-7915(93)90032-n. [DOI] [PubMed] [Google Scholar]
- 40.James S L, Pearce E J. The influence of adjuvant on induction of protective immunity by a non-living vaccine against schistosomiasis. J Immunol. 1988;140:2753–2759. [PubMed] [Google Scholar]
- 41.James S L, Scott P. Induction of cell mediated immunity as a strategy for vaccination against parasites. New York, N.Y: Alan R. Liss; 1988. [Google Scholar]
- 42.Jankovic D, Wynn T A, Kullberg M C, Hieny S, Caspar P, James S, Cheever A W, Sher A. Optimal vaccination against Schistosoma mansoni requires the induction of both B cell- and IFN-g-dependent effector mechanisms. J Immunol. 1998;162:345–351. [PubMed] [Google Scholar]
- 43.Kassim O O, Dean D A, Mangold B L, von Lichtenberg F. Combined microautoradiographic and histopathologic analysis of the fate of challenge Schistosoma mansoni schistosomula in mice immunized with irradiated cercariae. Am J Trop Med Hyg. 1992;47:231–237. doi: 10.4269/ajtmh.1992.47.231. [DOI] [PubMed] [Google Scholar]
- 44.Katz N, Chaia G. Coprological diagnosis of schistosomiasis. I. Evaluation of quantitative techniques. Rev Inst Med Trop Sao Paulo. 1968;10:295–298. [PubMed] [Google Scholar]
- 45.Katz N, Coelho M Z, Pellegrino J. Evaluation of Kato's quantitative method through the recovery of Schistosoma mansoni eggs added to human feces. J Parasitol. 1970;56:1032–1033. [PubMed] [Google Scholar]
- 46.Katz N, Pellegrino J, Memoria J M P. Quantitative oogram method in Cebus monkeys experimentally infected with Schistosoma mansoni. J Parasitol. 1966;52:917–919. [PubMed] [Google Scholar]
- 47.Katz N, Zicker F. Correlation between symptomatology and intensity of Schistosoma mansoni infection in inhabitants from endemic areas in Minas Gerais State—Brazil. Brasilia Medica. 1975;11:55–59. [Google Scholar]
- 48.Kemeny D M. A practical guide to ELISA. New York, N.Y: Pergamon Press; 1991. [Google Scholar]
- 49.Pearce E J, James S L, Dalton J, Barrall A, Ramos C, Strand M, Sher A. Immunochemical characterization and purification of Sm-97, a Schistosoma mansoni antigen monospecifically recognized by antibodies from mice protectively immunized with a nonliving vaccine. J Immunol. 1986;137:3593–3600. [PubMed] [Google Scholar]
- 50.Pearce E J, James S L, Hieny S, Lanar D E, Sher A. Induction of protective immunity against Schistosoma mansoni by vaccination with schistosome paramyosin (Sm97), a nonsurface parasite antigen. Proc Natl Acad Sci USA. 1988;85:5678–5682. doi: 10.1073/pnas.85.15.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips S M, Bentley A G, Linette G, Doughty B, Capron M. The immunological response of congenitally athymic rats to Schistosoma mansoni infection. J Immunol. 1983;131:1466–1474. [PubMed] [Google Scholar]
- 52.Phillips S M, Linette G P, Doughty B L, Byram J E, von Lichtenberg F. In vivo T cell depletion regulates resistance and morbidity in murine schistosomiasis. J Immunol. 1987;139:919–926. [PubMed] [Google Scholar]
- 53.Reis M G, Skally P, Davis R E, Singh H, Shoemaker C. Characterization of the Schistosoma mansoni gene encoding the glycolytic enzyme, triose phosphate isomerase. Mol Biochem Parasitol. 1993;59:235–242. doi: 10.1016/0166-6851(93)90221-i. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds S R, Dahl C E, Harn D A. T and B epitope determination and analysis of multiple antigenic peptides for the Schistosoma mansoni experimental vaccine triose-phosphate isomerase. J Immunol. 1994;152:193–200. [PubMed] [Google Scholar]
- 55.Reynolds S R, Shoemaker C B, Harn D A. T and B cell epitope mapping of SM23, an integral membrane protein of Schistosoma mansoni. J Immunol. 1992;149:3995–4001. [PubMed] [Google Scholar]
- 56.Rihet P, Demeure C E, Bourgois A, Prata A, Dessein A J. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur J Immunol. 1991;21:2679–2686. doi: 10.1002/eji.1830211106. [DOI] [PubMed] [Google Scholar]
- 57.Rihet P, Demeure C E, Dessein A J, Bourgois A. Strong serum inhibition of specific IgE correlated to competing IgG4, revealed by a new methodology in subjects from a S. mansoni endemic area. Eur J Immunol. 1992;22:2063–2070. doi: 10.1002/eji.1830220816. [DOI] [PubMed] [Google Scholar]
- 58.Sher A, Hieny S, James S, Asofsky R. Mechanisms of protective immunity against Schistosoma mansoni in mice vaccinated with irradiated cercariae. II. Analysis of immunity in hosts deficient in T lymphocytes, B lymphocytes, or complement. J Immunol. 1982;128:1880–1884. [PubMed] [Google Scholar]
- 59.Sher A, James S L, Correa-Oliveira R, Hieny S, Pearce E. Schistosome vaccines: current progress and future prospects. Parasitology. 1989;98:S61–S68. doi: 10.1017/s0031182000072255. [DOI] [PubMed] [Google Scholar]
- 60.Sher A, Pearce E, Hieny S, James S. Induction of protective immunity against Schistosoma mansoni by a nonliving vaccine. IV. Fractionation and antigenic properties of a soluble adult worm immunoprophylactic activity. J Immunol. 1986;136:3878–3883. [PubMed] [Google Scholar]
- 61.Shoemaker C, Gross A, Gebremichael A, Harn D. cDNA cloning and functional expression of the Schistosoma mansoni protective antigen triose-phosphate isomerase. Proc Natl Acad Sci USA. 1992;89:1842–1846. doi: 10.1073/pnas.89.5.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smythies L E, Betts C, Coulson P S, Dowling M A, Wilson R A. Kinetics and mechanism of effector focus formation in the lungs of mice vaccinated with irradiated cercariae of Schistosoma mansoni. Parasite Immunol. 1996;18:359–369. doi: 10.1046/j.1365-3024.1996.d01-115.x. [DOI] [PubMed] [Google Scholar]
- 63.Smythies L E, Coulson P S, Wilson R A. Monoclonal antibody to IFN-gamma modifies pulmonary inflammatory responses and abrogates immunity to Schistosoma mansoni in mice vaccinated with attenuated cercariae. J Immunol. 1992;149:3654–3658. [PubMed] [Google Scholar]
- 64.Smythies L E, Pemberton R M, Coulson P S, Mountford A P, Wilson R A. T cell-derived cytokines associated with pulmonary immune mechanisms in mice vaccinated with irradiated cercariae of Schistosoma mansoni. J Immunol. 1992;148:1512–1518. [PubMed] [Google Scholar]
- 65.Soisson L A, Reid G D, Farah I O, Nyindo M, Strand M. Protective immunity in baboons vaccinated with a recombinant antigen or radiation-attenuated cercariae of Schistosoma mansoni is antibody-dependent. J Immunol. 1993;151:4782–4789. [PubMed] [Google Scholar]
- 66.Soisson L A, Strand M. Schistosoma mansoni: induction of protective immunity in rats using a recombinant fragment of a parasite surface antigen. Exp Parasitol. 1993;77:492–494. doi: 10.1006/expr.1993.1111. [DOI] [PubMed] [Google Scholar]
- 67.Soisson L M, Masterson C P, Tom T D, McNally M T, Lowell G H, Strand M. Induction of protective immunity in mice using a 62-kDa recombinant fragment of a Schistosoma mansoni surface antigen. J Immunol. 1992;149:3612–3620. [PubMed] [Google Scholar]
- 68.Strand M, Dalton J P, Tom T D. Characterization and cloning of Schistosoma mansoni immunogens recognized by protective antibodies. Acta Trop Suppl. 1987;12:75–82. [PubMed] [Google Scholar]
- 69.Tendler M. Schistosoma mansoni: protective antigens. Mem Inst Oswaldo Cruz. 1987;82(Suppl. 4):125–128. doi: 10.1590/s0074-02761987000800021. [DOI] [PubMed] [Google Scholar]
- 70.Tendler M, Brito C A, Vilar M M, Serra-Freire N, Diogo C M, Almeida M S, Delbem A C, da Silva J F, Savino W, Garratt R C, Katz N, Simpson A S. A Schistosoma mansoni fatty acid-binding protein, Sm14, is the potential basis of a dual-purpose anti-helminth vaccine. Proc Natl Acad Sci USA. 1996;93:269–273. doi: 10.1073/pnas.93.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tendler M, Vilar M M, Brito C A, Freire N M, Katz N, Simpson A. Vaccination against schistosomiasis and fascioliasis with the new recombinant antigen Sm14: potential basis of a multi-valent anti-helminth vaccine? Mem Inst Oswaldo Cruz. 1995;90:255–256. doi: 10.1590/s0074-02761995000200022. [DOI] [PubMed] [Google Scholar]
- 72.Viana I R, Correa-Oliveira R, Carvalho O S, Massara C L, Colosimo E, Colley D G, Gazzinelli G. Comparison of antibody isotype responses to Schistosoma mansoni antigens by infected and putative resistant individuals living in an endemic area. Parasite Immunol. 1995;17:297–304. doi: 10.1111/j.1365-3024.1995.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 73.Wolowczuk I, Auriault C, Gras-Masse H, Mazingue C, Vendeville C, Tartar A, Capron A. T-cell responsiveness towards various synthetic peptides of the P28 antigen in rat and mouse models during Schistosoma mansoni infection. Int Arch Allergy Appl Immunol. 1990;93:350–358. doi: 10.1159/000235265. [DOI] [PubMed] [Google Scholar]
- 74.Wolowczuk I, Auriault C, Gras-Masse H, Vendeville C, Balloul J-M, Tartar A, Capron A. Protective immunity in mice vaccinated with the Schistosoma mansoni P-28-1 antigen. J Immunol. 1989;142:1342–1350. [PubMed] [Google Scholar]