Abstract
Background
Respiratory syncytial virus (RSV) is an important cause of disease in older adults. We evaluated the safety and immunogenicity of a stabilized RSV prefusion F subunit (RSVpreF) vaccine candidate with/without adjuvant in adults aged 65–85 years.
Methods
Primary cohort participants were equally randomized to 1 of 7 RSVpreF formulations: 60 µg with either Al(OH)3 or CpG/Al(OH)3, 120 µg with either Al(OH)3 or CpG/Al(OH)3, 240 µg with either Al(OH)3 or CpG/Al(OH)3, 240 µg unadjuvanted, or placebo, administered concomitantly with high-dose seasonal inactivated influenza vaccine (SIIV). Participants in the month 0,2 cohort were randomized to RSVpreF 240 µg with CpG/Al(OH)3 or placebo, administered at months 0 and 2.
Results
All RSVpreF vaccine candidates elicited robust and persistent serum neutralizing responses when administered alone or with SIIV. There was no notable difference in neutralizing response between the formulations, including those containing CpG. In the month 0,2 cohort, there was no booster effect of dose 2. SIIV responses were similar or slightly lower with concomitant administration of RSVpreF. Most systemic and local reactions were mild and more frequent after RSVpreF than placebo.
Conclusions
RSVpreF formulations were well tolerated and elicited robust neutralizing responses in older adults; however, CpG/Al(OH)3 did not further enhance responses.
Clinical Trials Registration. NCT03572062.
Keywords: RSV, respiratory syncytial virus, vaccine, prefusion F subunit
A stabilized RSV prefusion F subunit (RSVpreF) vaccine candidate with/without adjuvant was evaluated in adults aged 65–85 years. RSVpreF vaccine candidates were well tolerated and elicited robust and persistent serum neutralizing responses; adjuvanted formulations did not further enhance responses.
Human respiratory syncytial virus (RSV) is the most common cause of severe acute lower respiratory tract illness in infants and an important cause of disease in older adults [1]. Although typically associated with mild symptoms in young adults, RSV can lead to more severe complications, including lower respiratory tract illness, in older adults and those with underlying medical conditions, such as chronic kidney disease, congestive heart failure, chronic obstructive pulmonary disease, stroke, solid organ cancer, and hematologic malignancy [2, 3]. In US adults >65 years old, RSV leads to approximately 177 000 hospitalizations and 14 000 deaths annually [4, 5]. Low serum neutralizing titers are associated with increased risk of severe RSV disease, suggesting that increasing serum neutralizing titers by immunization may reduce disease severity [6]. Although other RSV candidate vaccines have been evaluated [7, 8], none have been successful in preventing RSV disease, and current treatment consists of supportive care [9]. An RSV subunit vaccine (RSVpreF) that contains prefusion stabilized F immunogens from the 2 RSV antigenic subgroups (A and B) is currently in development. In a first-in-human study in healthy adults 18–85 years old, RSVpreF was safe, well tolerated, and highly immunogenic [10, 11]. Because immunosenescence can reduce vaccine responses in the elderly, the addition of adjuvants to vaccine formulations may enhance immune responses in older adults [12, 13].
The first-in-human phase 1/2 study evaluated RSVpreF formulations with and without Al(OH)3, and no benefit of the addition of Al(OH)3 was observed. Here, we report the results of a study of RSVpreF in healthy adults 65–85 years old in which RSVpreF formulations with the oligodeoxynucleotide adjuvant CpG, a toll-like receptor 9 agonist, bound to Al(OH)3 were investigated to determine whether the addition of CpG further improves humoral and cellular responses in elderly participants.
METHODS
Study Design
This phase 1/2 randomized, placebo-controlled, observer-blind, dose-finding study (NCT03572062) was conducted at 12 sites in Australia. Participants were enrolled into 2 cohorts (Supplementary Figure 1). The primary cohort evaluated several RSV vaccine dose levels and formulations, which were administered as a single dose concomitantly with seasonal inactivated influenza vaccine (SIIV). An additional cohort evaluated a 2-dose regimen of RSV vaccine (240 µg dose level with CpG/Al(OH)3) administered without SIIV in a month 0, month 2 schedule (month 0,2 cohort). Participant enrollment commenced at the beginning of the southern hemisphere influenza season; however, a delay in SIIV availability prevented completion of enrollment into the primary cohort. The protocol was amended in August 2019 to include the month 0,2 cohort, to supplement enrollment. Because participants were enrolled into the month 0,2 cohort before the influenza season, concomitant SIIV was not administered to this cohort. Twelve months of follow-up after completion of vaccination were planned for both cohorts, but the sponsor terminated the study early on 10 August 2020. The planned 12-month follow-up of the primary cohort was completed. Follow-up of the month 0,2 cohort was shortened to 6 months after dose 2.
Participants were allocated to vaccine groups using an interactive web-response system. Participants in the primary cohort were equally randomized to 1 of 7 dose levels and formulations (3 dose levels of RSVpreF [60 µg, 120 µg, and 240 µg], each formulated with Al[OH]3 or CpG/Al[OH]3, or 240 µg RSVpreF alone) or placebo, all administered with concomitant SIIV. Those in the month 0,2 cohort were equally randomized to receive 2 doses, 2 months apart, of 240 µg RSVpreF with CpG/Al(OH)3 or placebo without concomitant SIIV. The participants, investigators, study coordinator, and laboratory personnel were blinded to vaccine assignment throughout the study. The sponsor study team were blinded until the conduct of planned interim analyses when safety and immunogenicity data were available 1 month after vaccination (primary cohort) and 1 month after dose 2 (month 0,2 cohort). Study site vaccine dispensers and administrators were not blinded due to differences in the physical appearance of vaccine formulations and placebo.
Ethical conduct of the trial is summarized in the Supplementary material.
Participants
Study participants were healthy men and women 65–85 years old. Women were not of childbearing potential. Exclusion criteria included any previous investigational RSV vaccination; known infection with HIV, hepatitis B virus, or hepatitis C virus; severe allergic reaction to any vaccine or other substance, including documented allergy to egg proteins or chicken proteins; any autoimmune or immunodeficient conditions; treatment with immunosuppressive therapy; and receipt of blood/plasma product or immunoglobulin within 60 days of study entry or during the study. For the primary cohort only, influenza vaccination within 6 months of study entry was also an exclusion criterion. A full list of inclusion and exclusion criteria is included in the Supplementary material.
Exploratory cellular analyses were conducted on whole-blood samples from a subset of participants (the peripheral blood mononuclear cell [PBMC] subset). The PBMC subset was selected at the study site level from those sites with access to appropriate specimen processing laboratories.
Vaccination
Each RSVpreF vaccine candidate was provided as vials containing a lyophilized mixture of equal amounts of 2 stabilized prefusion F antigens, 1 from each RSV subgroup, A and B. Lyophilized RSVpreF was reconstituted to 0.5 mL with 1 of 3 diluents: Al(OH)3, CpG/Al(OH)3, or water for injection. Three dose levels of RSVpreF antigen (60, 120, or 240 μg) were each evaluated in formulations containing Al(OH)3 or CpG/Al(OH)3. The formulation without Al(OH)3 or CpG/Al(OH)3 consisted of a single 240 μg dose of RSVpreF. Placebo was a sterile 0.9% normal saline solution in a 0.5 mL dose. Vaccine candidates or placebo were injected into the left deltoid. Participants in the primary cohort also received a commercially available high-dose trivalent SIIV (Fluzone HD, Southern Hemisphere 2019), injected into the right deltoid on the same day as immunization with the RSVpreF candidates.
Safety Assessments
We assessed the safety and tolerability of adjuvanted RSV vaccine administered concomitantly with SIIV in the primary cohort. Safety and tolerability of doses 1 and 2 administered 2 months apart in the month 0,2 cohort were also evaluated.
Participants recorded information about local reactions and systemic events occurring within 14 days after vaccination using e-diaries. Local reactions and systemic events were graded according to severity scales derived from the US Food and Drug Administration Center for Biologics Evaluation and Research guidelines [14]. Grade 4 events required investigator confirmation.
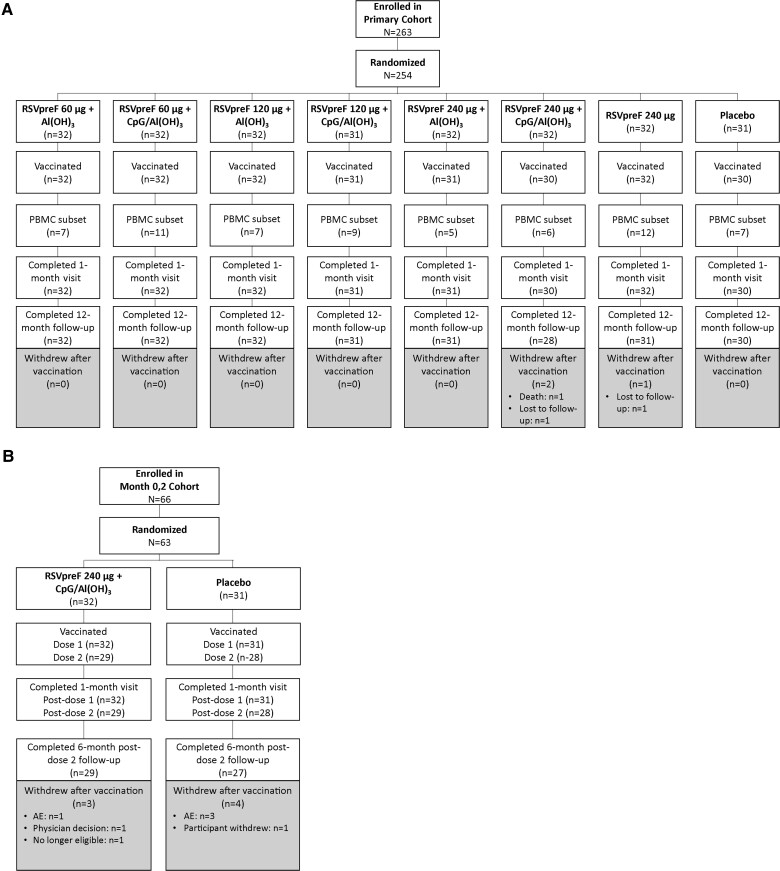
Adverse events (AEs) occurring within 1 month after vaccination 1 in the primary cohort and through 1 month after vaccination 2 in the month 0,2 cohort were collected. In addition, medically attended AEs (MAEs), AEs of specific interest (autoimmune and neuroinflammatory conditions), and serious AEs (SAEs) occurring within 12 months after vaccination (primary cohort) or 6 months after vaccination 2 (month 0,2 cohort) were collected at scheduled study follow-up visits (Figure 1).
Figure 1.
Participant disposition in the (A) primary cohort and (B) month 0,2 cohort. Abbreviations: AE, adverse event; PBMC, peripheral blood mononuclear cell; RSVpreF 60, 120, and 240, stabilized respiratory syncytial virus prefusion F subunit vaccine (dose levels 60, 120, and 240 µg, respectively).
Immunogenicity Assessments
Immune responses elicited by RSVpreF administered concomitantly with SIIV, based on RSV A and RSV B serum neutralizing titers, and those elicited by SIIV given alone or with RSVpreF vaccine, based on hemagglutination inhibition (HAI) titers for all SIIV strains, were measured before and 1 month after single-dose administration in the primary cohort. In the primary cohort, RSV A and RSV B neutralizing titers were measured at additional time points (1 week [PBMC subset of participants] and 3, 6, and 12 months after vaccination) and prefusion F-binding immunoglobulin G (IgG) and levels of Ig binding nonvaccine RSV antigens (matrix, nucleoprotein, and/or G [Ga or Gb]), were measured before vaccination and 1, 3, 6, and 12 months after vaccination. H3N2-neutralizing titers were measured before vaccination and 1 month after vaccination. The RSV neutralization assay and RSV Luminex immunoassays have been described previously [10].
In the month 0,2 cohort, the immune responses were measured before and 1 month after the first dose, before the second dose, and 1 and 6 months after the second dose. RSV A and B neutralizing titers, prefusion F-binding IgG, and nonvaccine RSV antigen-binding Ig levels were measured. An additional immunogenicity assessment planned at 12 months after vaccination 2 was not done.
Additional exploratory analyses of cellular immune responses to RSV vaccine formulations, including characterization of RSV F-specific memory B-cell frequencies (FluoroSpot) and T-cell phenotypes, and cytokine secretion profiles (ELISpot and intracellular cytokine staining) were conducted using PBMCs isolated before vaccination and 1 week and 1 month after vaccination from a subset of participants in the primary cohort (Figure 1A). These assays are described in the Supplementary material.
Statistical Analysis
The sample size for this study was not based on any formal hypothesis testing; all data were analyzed descriptively. Safety was analyzed in the safety population, which included all randomized participants who received at least 1 dose of study vaccine. Safety end points were reported by vaccination group using counts and percentages with corresponding 2-sided 95% Clopper-Pearson confidence intervals (CIs). AEs and SAEs were summarized by system organ class and preferred term. Study populations and additional statistical methods are included in the Supplementary material.
RESULTS
Participants
A total of 317 participants 65–85 years old were randomized beginning 29 April 2019 (primary cohort, n = 254; month 0,2 cohort, n = 63); the last participant visit was 19 August 2020. As shown in Figure 1, 250 primary cohort participants (98.4%) received a single administration of RSVpreF, and 247 (97.2%) completed the 12-month follow-up visit. In the month 0,2 cohort, all 63 participants received the first RSVpreF vaccination, 57 participants (90.5%) received the second RSVpreF vaccination, and 56 (88.9%) completed the 6-month post-dose 2 follow-up visit.
Participant demographic characteristics were broadly similar across vaccine groups (Supplementary Table 1). Overall, the study enrolled similar percentages of men and women (53.7% men), with a median age of 70 years. Participants were predominantly white (91.1%) and non-Hispanic/non-Latino (99.7%), and most had never smoked (52.4%) or were former (43.5%) smokers.
Safety
Reactogenicity
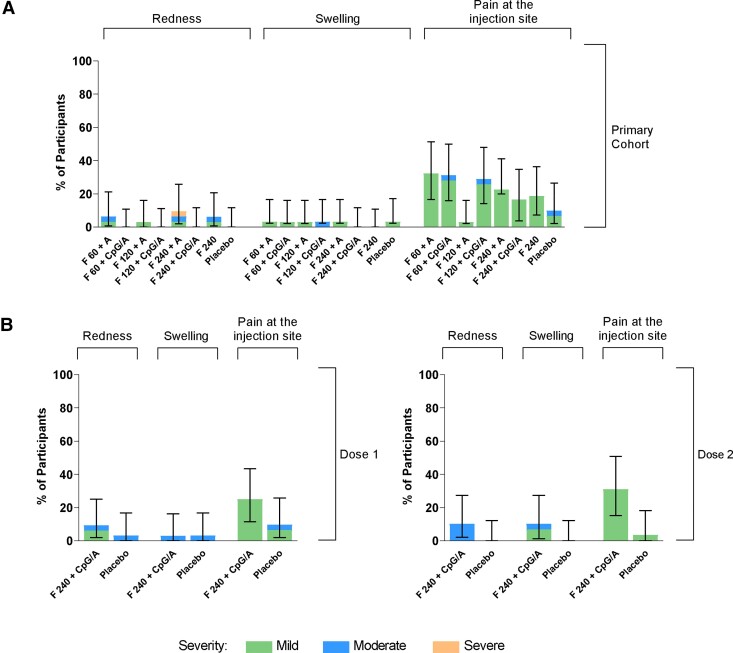
Injection-site pain was the most common local reaction in RSVpreF recipients in both cohorts (Figure 2). Most local reactions were mild and transient, with median durations of 1–2 days for injection-site pain, 1–6 days for redness, and 1–3 days for swelling across the RSVpreF groups. There were no reports of severe injection-site pain or swelling. One primary cohort participant who received RSVpreF 240 µg + Al(OH)3 reported a severe local reaction of redness with a duration of 6 days. There was no apparent trend in local reaction frequency across dose levels or formulations, and no substantial increases in frequency or severity observed with the second dose compared with the first dose in the month 0,2 cohort.
Figure 2.
Percentages of participants reporting local reactions within 14 days after vaccination in the (A) primary cohort and (B) month 0,2 cohort. Error bars are 95% confidence intervals. Abbreviations: A, Al(OH)3; CpG/A, CpG/Al(OH)3; F 60, 120, and 240, stabilized respiratory syncytial virus prefusion F subunit vaccine (dose levels 60, 120, and 240 µg, respectively).
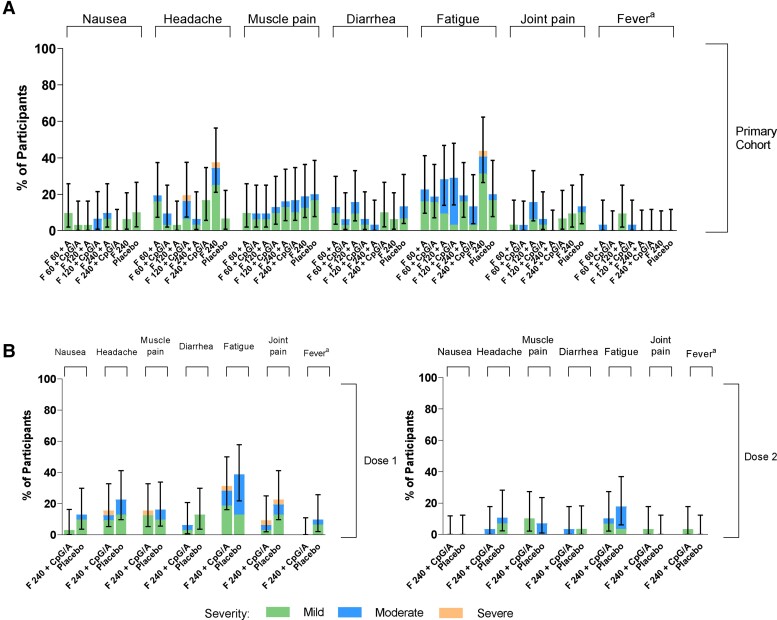
Among RSVpreF recipients in both cohorts, the most common systemic events were fatigue/tiredness, headache, and muscle pain (Figure 3). These events were reported by more participants after the first than the second dose in the month 0,2 cohort. Most events were mild in severity, with median durations of 1 day for fever, 1–9 days for fatigue/tiredness, 1–5 days for muscle pain, and 1–3 days for headache.
Figure 3.
Percentages of participants reporting systemic reactions within 14 days after vaccination in the (A) primary cohort and (B) month 0,2 cohort. aMild, moderate, severe, and grade 4 fever defined as temperatures of 38.0–38.4°C, 38.5–38.9°C, 39.0–40.0°C, and >40.0°C, respectively. There were no grade 4 reactions or events. No cases of vomiting were reported. Error bars are 95% confidence intervals. Abbreviations: A, Al(OH)3; CpG/A, CpG/Al(OH)3; F 60, 120, and 240, stabilized respiratory syncytial virus prefusion F subunit vaccine (dose level 60, 120, and 240 µg, respectively).
Two participants in the primary cohort reported severe events: 1 participant receiving RSVpreF 240 μg alone reported severe fatigue and severe headache (resolved within 1 and 2 days, respectively), and 1 participant receiving RSVpreF 120 μg + CpG/Al(OH)3 reported severe headache (resolved within 5 days). In the month 0,2 cohort, 1 participant reported severe headache, fatigue/tiredness, muscle pain, and joint pain after RSVpreF vaccination 1. No participants in either cohort experienced severe fever (≥39°C), nausea, vomiting, or diarrhea.
Adverse Events
Within the month following vaccine administration, 56 of 220 RSVpreF recipients (25.4%) and 5 of 30 placebo recipients (16.7%) in the primary cohort reported AEs, most commonly involving the system organ class of infections and infestations (n = 22 across RSVpreF groups). Similarly, in the month 0,2 cohort, 9 (28.1%) and 7 (24.1%) RSVpreF recipients and 8 (25.8%) and 3 (10.7%) placebo recipients reported AEs during the month after the first and second dose, respectively. Two AEs (mild dry skin and mild injection site reaction) were considered vaccine related in RSVpreF recipients. No AEs of specific interest were reported in either cohort.
AEs occurring during 12 months of postvaccination follow-up for the primary cohort and from the first dose through 6 months after the second dose for the month 0,2 cohort are summarized in Table 1. SAEs were reported by 30 of 250 primary cohort participants (6.3%–16.1% across RSVpreF groups; 10.0% placebo group) and 11 of 63 month 0,2 cohort participants (18.8% RSVpreF group; 16.1% placebo group). MAEs were reported at similar frequencies across the RSVpreF and placebo groups in the primary cohort (46.9%−60.0% vs 50.0%, respectively) and month 0,2 cohort (53.1% vs 38.7%, respectively). No SAEs or MAEs in either cohort were considered vaccine related. One participant (male; 71 years old) died during the study due to myocardial infarction occurring 211 days after vaccination with 240 μg + CpG/Al(OH)3; the death was not considered to be vaccine related.
Table 1.
Participants Reporting AEs Within 12 Months after Vaccination (Primary Cohort) and Within 6 Months After Vaccination (Month 0,2 Cohort)
| Primary Cohorta | Month 0,2 Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RSVpreF 60 μg | RSVpreF 120 μg | RSVpreF 240 μg | Placebo | RSVpreF 240 μg | Placebo | |||||
| Adverse Events | Al(OH)3 (N = 32) | CpG/Al(OH)3 (N = 32) | Al(OH)3 (N = 32) | CpG/Al(OH)3 (N = 31) | Al(OH)3 (N = 31) | CpG/Al(OH)3 (N = 30) | Unadjuvanted (N = 32) | (N = 30) | CpG/Al(OH)3 (N = 32) | (N = 31) |
| Any AE | 19 (59.4) | 22 (68.8) | 19 (59.4) | 19 (61.3) | 21 (67.7) | 20 (66.7) | 20 (62.5) | 18 (60.0) | 21 (65.6) | 20 (64.5) |
| Vaccine-related AE | 1 (3.1) | 0 | 0 | 1 (3.2) | 0 | 0 | 0 | 0 | 1 (3.1) | 1 (3.2) |
| Severe AE | 5 (15.6) | 6 (18.8) | 3 (9.4) | 3 (9.7) | 8 (25.8) | 4 (13.3) | 3 (9.4) | 1 (3.3) | 5 (15.6) | 5 (16.1) |
| Life-threatening AE | 0 | 0 | 0 | 0 | 0 | 1 (3.3) | 0 | 0 | 1 (3.1) | 1 (3.2) |
| Immediate AEa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Medically attended AEb | 15 (46.9) | 19 (59.4) | 15 (46.9) | 16 (51.6) | 17 (54.8) | 18 (60.0) | 16 (50.0) | 15 (50.0) | 17 (53.1) | 12 (38.7) |
| Serious AE | 2 (6.3) | 4 (12.5) | 4 (12.5) | 4 (12.9) | 5 (16.1) | 4 (13.3) | 4 (12.5) | 3 (10.0) | 6 (18.8) | 5 (16.1) |
| AE leading to withdrawal | 0 | 0 | 0 | 0 | 0 | 1 (3.3) | 0 | 0 | 1 (3.1) | 3 (9.7) |
Data presented as n (%) for each category; n = number of participants with the specified characteristic. N = number of participants in the specified vaccine group. This value is the denominator for the percentage calculations.
Abbreviations: AE, adverse event; RSVpreF, stabilized respiratory syncytial virus prefusion F subunit vaccine.
Immediate AE refers to an AE reported in the 30-minute postvaccination observation period.
Medically attended AEs are nonserious AEs that result in evaluation at a medical facility (excludes serious AEs).
Immunogenicity
RSV Antibody Responses
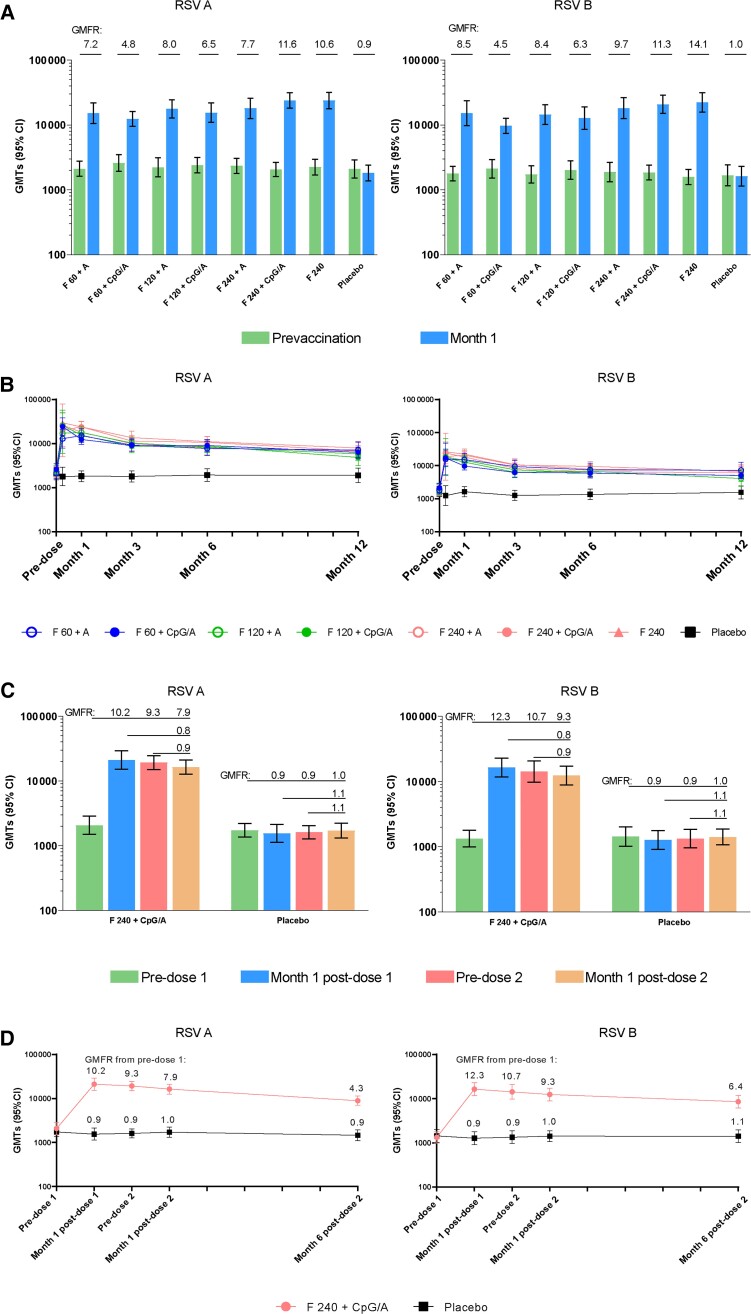
In the primary cohort, with all RSVpreF dose levels and formulations, RSV A and RSV B neutralizing geometric mean titers (GMTs) increased at 1 month after vaccination (geometric mean fold rises [GMFRs] of 4.8–11.6 and 4.5–14.1, respectively) (Figure 4). There was a modest trend of higher GMTs with increasing dose levels of the RSVpreF + CpG/Al(OH)3 formulations indicating a dose-response; however, there were no notable differences in neutralizing responses among the formulations, including those containing CpG/Al(OH)3. GMTs generally declined after 1 month; however, at 12 months after vaccination, they remained 2.1 to 3.5 times (RSV A) and 2.2 to 4.3 times (RSV B) the baseline GMTs.
Figure 4.
RSV A and RSV B neutralizing GMTs at month 1 (A and C) and through month 12 (B and D) in the primary cohort (A and B) and month 0,2 cohort (C and D). Error bars are 95% confidence intervals. Abbreviations: A, Al(OH)3; CI, confidence interval; CpG/A, CpG/Al(OH)3; F 60, 120, and 240, stabilized respiratory syncytial virus prefusion F subunit vaccine (dose levels 60, 120, and 240 µg, respectively); GMFR, geometric mean fold rise; GMT, geometric mean titer; RSV, respiratory syncytial virus.
As in the primary cohort, in the month 0,2 cohort, RSV A and RSV B neutralizing GMTs increased 1 month after the first dose in RSVpreF recipients (GMFRs, 10.2 and 12.3, respectively; Figure 4). No increase in GMTs (booster effect) was observed 1 month after the second dose (GMFRs, 0.9 for RSV A and B). After vaccination 1, GMTs generally declined after 1 month; however, through 6 months after vaccination 2, they remained 4.3 times (RSV A) and 6.4 times (RSV B) the baseline GMTs.
Prefusion F-binding IgG responses and kinetics for RSV A and RSV B generally paralleled neutralizing responses (Supplementary Figure 2). RSVpreF-binding IgG geometric mean concentrations increased robustly 1 month after an initial RSVpreF vaccination in both cohorts, with gradual reductions thereafter and with no boosting effect of a second dose in the month 0,2 cohort.
T- and B-Cell Responses
Interferon-γ (IFN-γ)+ T-cell response to RSVpreF measured by ELISpot peaked at 1 week after vaccination for all formulations evaluated (Supplementary Figure 3A). At 1 month after vaccination, T-cell responses declined but remained above baseline levels. No difference in T-cell response between dose levels or with and without CpG/Al(OH)3 was observed at 1 month after vaccination (Supplementary Figure 3A). Intracellular cytokine staining analysis confirmed the T-cell response was dominated by CD4 T cells (Supplementary Figure 3B–3D), with no measurable antigen-specific CD8+ T-cell response (data not shown). The CD4+ T-cell response consisted of RSV F-reactive IFN-γ+, interleukin-2 (IL-2)+, and IFN-γ+/IL-2+cells (Supplementary Figure 3B–3D). RSVpreF increased the frequency of RSV F-specific IgG+ memory B cells at 1 month after vaccination above baseline, suggesting a strong induction of memory B-cell response (Supplementary Figure 4). Similar to the T-cell response, the B-cell response did not differ across dose levels or formulations.
Response to SIIV
Immune responses to SIIV 1 month after vaccination, as measured by HAI and neutralizing titers, were generally similar or trended slightly lower among primary cohort participants who received SIIV concomitantly with RSVpreF compared with those who received SIIV with placebo (Supplementary Figure 5). Seroprotection and seroconversion rates to SIIV were similar in RSVpreF and placebo groups.
Nonvaccine Antigen-Binding IgG responses
Primary cohort participants were enrolled at the beginning of the RSV season, and month 6 postvaccination visits corresponded to the end of RSV season. Seroconversion by nonvaccine antigen-binding Ig, defined as ≥4-fold rise in Ig that bound any of 3 RSV nonvaccine antigens (Ga/Gb, matrix, nucleoprotein) at month 6 after vaccination, was observed in 9 of 212 participants (4.2%) in the RSVpreF groups and 10% in the placebo group. No participants in the month 0,2 cohort seroconverted to any RSV nonvaccine antigen.
DISCUSSION
In this older adult study, all RSVpreF formulations given as a single dose with concomitant SIIV or in a 2-dose series administered 2 months apart were generally well tolerated, with an acceptable safety profile. Although more RSVpreF recipients than placebo recipients reported local reactions, most were mild or moderate, and there were no vaccine-related SAEs or MAEs.
All RSVpreF vaccine candidates elicited robust and persistent serum neutralizing responses when administered alone or with SIIV. Serum RSV neutralizing titers generally peaked in the first month after the first dose and remained elevated above baseline through 6–12 months after vaccination. In the primary cohort, when RSVpreF was coadministered with SIIV, immune responses to SIIV were similar or trended lower compared with SIIV immunization alone. The sustained antibody responses observed in this study are consistent with data from a phase 1/2 study in older adults, which also supports durability of response for ≥12 months after vaccination [11]. Furthermore, all RSVpreF vaccine candidates induced a strong CD4+ T-cell response and memory B-cell response, in line with the robust immunogenicity of RSVpreF.
An adjuvanted influenza vaccine (FLUAD) is available for people ≥65 years old, and postmarketing studies have generally shown greater protection with the adjuvanted vaccine [15–17]. It was anticipated that inclusion of CpG bound to Al(OH)3 as an adjuvant for RSVpreF might increase immune responses in the elderly by overcoming immunosenescence. CpG is a toll-like receptor 9 agonist that can support elicitation of antigen-specific immunity. CpG has been well tolerated in clinical infectious disease vaccine trials and a CpG adjuvant is included in a recently licensed hepatitis B vaccine [18, 19]. However, no immune-boosting effect of CpG was observed in the current study compared with the RSVpreF formulations with or without Al(OH)3. There was also no increased immune response to a second dose of RSVpreF vaccine administered 2 months after the first. As a result, the study was terminated early, once all participants in the month 0,2 cohort completed at least 6 months of safety and immunogenicity follow-up after the second vaccination. The reason for lack of effect of CpG/Al(OH)3 is unknown. The same doses with and without Al(OH)3 were tested in another study and showed no significant differences between doses, or between formulations with and without Al(OH)3 [10, 11]. Based on those results and the observed lack of enhanced response of CpG/Al(OH)3-containing formulations in this study, the final formulation to be used in older adults was selected: 120 µg dose without Al(OH)3.
Given the high burden of RSV disease in older adults, there is substantial clinical need for an effective vaccine. Prior vaccine candidates have failed to protect against RSV-mediated lower respiratory tract disease in elderly individuals [7, 8]. For example, a nonprefusion stabilized RSV F subunit vaccine candidate did not meet its primary efficacy end point in older adults in a phase 3 trial [8]. An adenovirus vectored, prefusion F-based vaccine increased neutralizing titers up to 6-fold and provided approximately 51.9% protection against RSV infection and disease in healthy adults aged 18–50 years in a recent proof-of-concept human challenge study [20]. When combined with a prefusion F subunit, the adenovirus vectored prefusion F-based vaccine provided 80% protection against confirmed RSV-associated lower respiratory tract disease in a phase 2 study in adults ≥65 years old [21]. Although there is no established immune correlate of protection, RSV neutralizing titers in that study correlated with protection from infection. In the proof-of-concept study, neutralizing responses at 1 month were robust, with GMFRs of 4.8–11.6 for RSV A and 4.5–14.1 for RSV B. These are slightly lower than those observed in 65- to 85-year-old adults in the recent phase 1/2 trial of RSVpreF: 7.2–13.2 for RSV A and 6.9–14.9 for RSV B [11]. The neutralizing responses in the study reported here were durable, with neutralizing titers remaining 2.1–4.3 times the baseline titers at 12 months after a single dose. Therefore, despite the lack of effect of adjuvant in this study and discontinuation of development of a CpG/Al(OH)3-containing formulation, the high and durable neutralizing responses to unadjuvanted RSVpreF documented in this study make it a promising candidate to protect older adults from RSV disease.
Limitations include lack of power for statistical comparisons across groups. In addition, the study was conducted in Australian individuals 65–85 years old, with less racial and ethnic diversity compared with some other populations. Study strengths include the evaluation of multiple vaccine formulations and dose levels and high study completion rates despite the coronavirus disease 2019 (COVID-19) pandemic. The study provides evidence of a strong immune response to the RSVpreF candidates.
In conclusion, CpG/Al(OH)3 adjuvanted formulations did not further enhance the robust neutralizing antibody response and cellular immune response to RSVpreF in adults 65–85 years old. Our results, combined with results from previous studies showing that RSVpreF without adjuvant elicited a robust immune response, support the further clinical development of RSVpreF. The 120 µg dose level without Al(OH)3 was selected for evaluation in a human infectious RSV challenge study (NCT04785612) and a pivotal phase 3 efficacy trial (NCT05035212).
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
James Baber, Vaccine Clinical Research, Pfizer Inc, Sydney, Australia.
Mark Arya, Australian Clinical Research Network, Maroubra, Australia.
Yuben Moodley, School of Medicine, University of Western Australia, Perth, WA, Australia.
Anna Jaques, Vaccine Clinical Research, Pfizer Inc, Sydney, Australia.
Qin Jiang, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Kena A Swanson, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
David Cooper, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Mohan S Maddur, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Jakob Loschko, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Alejandra Gurtman, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Kathrin U Jansen, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
William C Gruber, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Philip R Dormitzer, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Beate Schmoele-Thoma, Vaccine Research and Development, Pfizer Pharma GmbH, Berlin, Germany.
Notes
Acknowledgments . Editorial/medical writing support was provided by Sheena Hunt, PhD, of ICON (Blue Bell, PA), and was funded by Pfizer Inc.
Financial support . This work was supported by Pfizer Inc. Funding to pay the Open Access publication charges for this article was provided by Pfizer Inc.
Data sharing . Upon request and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
- 1. Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 2013; 132:e341–8. [DOI] [PubMed] [Google Scholar]
- 2. Sundaram ME, Meece JK, Sifakis F, Gasser RA Jr, Belongia EA. Medically attended respiratory syncytial virus infections in adults aged ≥ 50 years: clinical characteristics and outcomes. Clin Infect Dis 2014; 58:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wyffels V, Kariburyo F, Gavart S, Fleischhackl R, Yuce H. A real-world analysis of patient characteristics and predictors of hospitalization among US Medicare beneficiaries with respiratory syncytial virus infection. Adv Ther 2020; 37:1203–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention . Respiratory syncytial virus infection (RSV). Trends and surveillance. https://www.cdc.gov/rsv/research/us-surveillance.html. Accessed 02 November 2020.
- 6. Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis 2004; 189:233–8. [DOI] [PubMed] [Google Scholar]
- 7. Falloon J, Yu J, Esser MT, et al. An adjuvanted, postfusion F protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. J Infect Dis 2017; 216:1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Novovax . Novavax announces topline RSV F vaccine data from two clinical trials in older adults. https://ir.novavax.com/2016-09-25-Novavax-Announces-Topline-RSV-F-Vaccine-Data-from-Two-Clinical-Trials-in-Older-Adults. Accessed 7 September 2021. [Google Scholar]
- 9. Domachowske JB, Anderson EJ, Goldstein M. The future of respiratory syncytial virus disease prevention and treatment. Infect Dis Ther 2021; 10:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walsh EE, Falsey AR, Scott DA, et al. A randomized phase 1/2 study of a respiratory syncytial virus prefusion F vaccine. J Infect Dis 2022; 225:1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falsey AR, Walsh EE, Scott DA, et al. Phase 1/2 randomized study of the immunogenicity, safety and tolerability of an RSV prefusion F vaccine in adults with concomitant inactivated influenza vaccine. J Infect Dis 2022; 225:2056–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB. Immunosenescence and human vaccine immune responses. Immun Ageing 2019; 16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boraschi D, Italiani P. Immunosenescence and vaccine failure in the elderly: strategies for improving response. Immunol Lett 2014; 162:346–53. [DOI] [PubMed] [Google Scholar]
- 14. US Food and Drug Administration . Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. https://www.fda.gov/media/73679/download. Accessed 1 April 2020.
- 15. Domnich A, Arata L, Amicizia D, Puig-Barbera J, Gasparini R, Panatto D. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: a systematic review and meta-analysis. Vaccine 2017; 35:513–20. [DOI] [PubMed] [Google Scholar]
- 16. Frey SE, Reyes MR, Reynales H, et al. Comparison of the safety and immunogenicity of an MF59(R)-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine 2014; 32:5027–34. [DOI] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention . Adjuvanted flu vaccine. https://www.cdc.gov/flu/prevent/adjuvant.htm. Accessed 7 September 2021.
- 18. Scheiermann J, Klinman DM. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 2014; 32:6377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schillie S, Harris A, Link-Gelles R, Romero J, Ward J, Nelson N. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep 2018; 67:455–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sadoff J, De Paepe E, DeVincenzo J, et al. Prevention of respiratory syncytial virus infection in healthy adults by a single immunization of Ad26. RSV.preF in a human challenge study. J Infect Dis 2022; 226:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson & Johnson . Janssen announces phase 2b data demonstrating its investigational RSV adult vaccine provided 80% protection against lower respiratory infections in older adults. https://www.jnj.com/janssen-announces-phase-2b-data-demonstrating-its-investigational-rsv-adult-vaccine-provided-80-protection-against-lower-respiratory-infections-in-older-adults. Accessed 11 November 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.