Abstract
Objective
The study investigates the diagnostic and prognostic significance of the prothrombin time/international normalized ratio (PT/INR) in patients with sepsis and septic shock.
Background
Sepsis may be complicated by disseminated intravascular coagulation (DIC). While the status of coagulopathy of septic patients is represented within the sepsis-3 definition by assessing the platelet count, less data regarding the prognostic impact of the PT/INR in patients admitted with sepsis and septic shock is available.
Methods
Consecutive patients with sepsis and septic shock from 2019 to 2021 were included. Blood samples were retrieved from day of disease onset (ie, day 0), as well as on day 1, 2, 4, 6 and 9 thereafter. Firstly, the diagnostic value of the PT/INR in comparison to the activated partial thromboplastin time (aPTT) was tested for septic shock compared to sepsis without shock. Secondly, the prognostic value of the PT/INR for 30-day all-cause mortality was tested. Statistical analyses included univariable t-tests, Spearman's correlations, C-statistics, Kaplan-Meier analyses and Cox proportional regression analyses.
Results
338 patients were included (56% sepsis without shock, 44% septic shock). The overall rate of all-cause mortality at 30 days was 52%. With an area under the curve (AUC) of 0.682 (p= .001) on day 0, the PT/INR revealed moderate discrimination of septic shock and sepsis without shock. Furthermore, PT/ INR was able to discriminate non-survivors and survivors at 30 days (AUC = 0.612; p = .001). Patients with a PT/INR >1.5 had higher rates of 30-day all-cause mortality than patients with lower values (mortality rate 73% vs 48%; log rank p = .001; HR = 2.129; 95% CI 1.494-3.033; p = .001), even after multivariable adjustment (HR = 1.793; 95% CI 1.343-2.392; p = .001). Increased risk of 30-day all-cause mortality was observed irrespective of concomitant thrombocytopenia.
Conclusion
The PT/INR revealed moderate diagnostic accuracy for septic shock but was associated with reliable prognostic accuracy with regard to 30-day all-cause mortality in patients admitted with sepsis and septic shock.
Keywords: sepsis, septic shock, PT/INR, coagulopathy, prognosis, mortality
Introduction
Sepsis remains one of the top ten leading causes of death and one of the main causes of intensive care unit admission (ICU) in the Western world.1 During the past decades, the incidence of sepsis has increased, which is related to an aging population with increased burden of comorbidities.2 Especially due to improved early-goal directed diagnostics and therapies, such as an early microbiological and biomarker assessment, as well as the early administration of antibiotic therapies, sepsis-related mortality was reduced from 47% from 1991–1995% to 29% from 2006–2009.1 With the publication of the “sepsis-3” criteria in 2016, the “Sequential Organ Failure Assessment” (SOFA) score was included for an early assessment of organ failure embedded in the diagnose making of sepsis.3
As a common sepsis-related complication, coagulopathies affect up to 80% of septic patients.4 Disseminated intravascular coagulation (DIC) as a result of uncontrolled activation of the clotting cascade, leading to clotting factor consumption and intravascular thrombosis, may result in both fatal thrombotic and haemorrhagic events.4 DIC was demonstrated to increase the risk of 28-day mortality among critically ill patients with corresponding mortality rates ranging from 20% to 50%.5 By now, the presence of DIC in the diagnosis-making of sepsis may be reflected only by the platelet count within the SOFA score.3 However, an improved identification and novel treatment options for sepsis-associated coagulopathies are of major interest. Recently, potential therapeutics modulating DIC, such as recombinant human soluble thrombomodulin6 and activated protein C failed to improve prognosis in septic shock.7
Routine laboratory parameters that may be helpful for the identification of DIC in sepsis include the platelet count, prothrombin time (PT), activated partial thromboplastin time (aPTT) and PT/international normalized ratio (PT/INR). Although some studies identified an association of the platelet count with the prognosis in critically ill patients,4,8–10 less data is available focusing on the prognostic impact of the PT/INR in septic patients. Moreover, studies investigating the prognostic value of the PT/INR with regard to sepsis-related outcomes commonly did not investigate the role of serial PT/INR measurements.3 Recently, increased PT/INR but not aPTT was observed in sepsis non-survivors as compared to survivors, suggesting the PT/INR – as standardized tool between laboratories and reagents – may reveal superior diagnostic and prognostic accuracy in patients with sepsis or septic shock.11
Therefore, the scope of the present study is to investigate the diagnostic role of the PT/INR regarding the identification of patients with septic shock, as well as to assess the prognostic role of the PT/INR on 30-day mortality in patients admitted with sepsis or septic shock.
Methods
Study Patients, Design and Data Collection
The present study prospectively included all consecutive patients presenting with sepsis or septic shock on admission to the internal ICU at the University Medical Center Mannheim, Germany, from June 2019 to January 2021. All relevant clinical data related to the index event were documented using the electronic hospital information system as well as the IntelliSpace Critical Care and anesthesia information system (ICCA, Philips, Philips GmbH Market DACH, Hamburg, Germany) implemented on the ICU, organizing patient data, including admission documents, vital signs, laboratory values, treatment data and consult notes.
The presence of sepsis without shock and septic shock, as well as important laboratory data, sepsis-related scores, hemodynamic measurements, ventilation parameters were assessed on disease onset (ie, day 0), as well as on days 2, 4, 6 and 9 thereafter. Further data being documented contained baseline characteristics, prior medical history, length of index hospital stay, data derived from imaging diagnostics, as well as pharmacological therapies. Documentation of source data was performed by intensivists and ICU nurses at the patients’ individual period of hospitalization during routine clinical care blinded to final data analyses.
The present study derived from an analysis of the “Mannheim Registry for Sepsis and Septic Shock” (MARSS-registry), representing a prospective single-center registry including consecutive patients presenting with sepsis or septic shock being acutely admitted to the ICU for internal medicine of the University Medical Center Mannheim (UMM), Germany (clinicaltrials.gov identifier: NCT05231720). The registry was carried out according to the principles of the declaration of Helsinki and was approved by the medical ethics committee II of the Medical Faculty Mannheim, University of Heidelberg, Germany.
Inclusion and Exclusion Criteria, Study Endpoints
For the present study, all consecutive patients with sepsis without shock and septic shock were included. Patients without measurement of the PT/INR on sepsis onset (ie, day 0) were excluded from the present study. No further exclusion criteria were applied.
Diagnosis of sepsis and septic shock were determined according to the “Third International Consensus Definition for Sepsis and Septic Shock” (ie, sepsis-3).3 Accordingly, sepsis was defined as life-threatening organ dysfunction, caused by a dysregulated host response to infection. Organ dysfunction is defined as an increase of ≥ 2 in the SOFA score. Septic shock was defined as persistent hypotension, despite adequate volume resuscitation, requiring vasopressors to maintain a mean arterial pressure (MAP) ≥ 65 mm Hg and a serum lactate ≥ 2 mmol/l.3 According to the sepsis-3 guidelines, patients were classified as “sepsis without shock” or “septic shock” at each day during the evaluated days during ICU hospitalization.
All-cause mortality at 30 days was documented using our electronic hospital information system and by directly contacting state resident registration offices (‘bureau of mortality statistics’). Identification of patients was verified by place of name, surname, day of birth, and registered living address. Information on 30-day all-cause mortality was available for all patients.
Statistical Methods
Quantitative data are presented as mean ± standard error of mean (SEM), median and interquartile range (IQR), and ranges depending on the distribution of the data. They were compared using the Student's t test for normally distributed data or the Mann-Whitney U test for nonparametric data. Deviations from a Gaussian distribution were tested by the Kolmogorov-Smirnov test. Qualitative data are presented as absolute and relative frequencies and were compared using the Chi-square test or the Fisher's exact test, as appropriate. Box plots for the PT/INR and aPTT were created for the comparisons of patients with sepsis and septic shock during the first week of sepsis on day 0, 2, 4, 6 and 9 thereafter. Spearman's rank correlation for nonparametric data was used to test the association of the PT/INR with medical and laboratory parameters on day 0.
Diagnostic Performance of the PT/INR and aPTT
C-statistics were applied with calculation of receiver operating characteristic (ROC) and the corresponding area under the curves (AUC) within the entire cohort to assess the discriminative performance of the aPTT and PT/INR for the diagnosis of septic shock and sepsis without shock at days 0, 2, 4, 6 and 9. AUCs for diagnostic performance were compared by the method of Hanley et al11 Additionally, using the positive predictive value (PPV) and true positive rate (TPR), precision recall (PR) curves and their corresponding AUCs were calculated to provide further insights into the diagnostic performance of the INR.
Prognostic Performance of the PT/INR and aPTT
C-statistics were applied with calculation of ROC and the corresponding AUC within the entire cohort for 30-day all-cause mortality at days 0, 2, 4, 6 and 9. AUCs for prognostic performance were compared by the method of Hanley et al12 Furthermore, using the positive predictive value (PPV) and true positive rate (TPR), PR-Curves and their corresponding AUCs were calculated to provide further insights into the prognostic performance of the INR.
Kaplan-Meier analyses according to the PT/INR were performed within the entire study cohort, as well as separated by patients with sepsis without shock and septic shock and by the presence and degree of thrombocytopenia on admission. Univariable hazard ratios (HR) were given together with 95% confidence intervals. Thereafter, multivariable Cox regression models were developed using the “forward selection” option.
Results of all statistical tests were considered significant for p ≤ .05. SPSS (Version 25, IBM, Armonk, New York) and GraphPad Prism (Version 9, GraphPad Software, San Diego, California) were used for statistics.
Results
Study Population
From a total of 361 patients with sepsis or septic shock, 23 patients without PT/INR measurement on day 0 were excluded. 338 patients were finally included, 56% were admitted with sepsis without shock and 44% with septic shock. As outlined in Table 1 (left panel), patients were median-aged at 70 years and most patients were males (64%). As compared to patients with sepsis without shock, the baseline heart rate (103 bpm vs 96 bpm; p = .034) was higher in patients with septic shock and patients with septic shock were admitted with lower systolic blood pressure (105 mm Hg vs 113 mm Hg; p = .001). In contrast, cardiovascular risk factors, as well as rates of coronary artery disease, atrial fibrillation, chronic kidney disease and congestive heart failure did not significantly differ between both groups. However, patients with septic shock revealed higher rates of concomitant malignancies (40% vs 28%; p = .027). Moreover, a left ventricular ejection fraction (LVEF) < 35% (20% vs 12%; p = .002) and the rate of cardiopulmonary resuscitation (CPR) (20% vs 6%; p = .001) was higher in the septic shock group.
Table 1.
Baseline Characteristics.
| All patients (n = 338) |
Sepsis (n = 190) |
Septic shock (n = 148) |
p value | ||||
|---|---|---|---|---|---|---|---|
| Age, median; (IQR) | 70 | (60-79) | 70 | (60-79) | 70 | (59-79) | .348 |
| Male sex, n (%) | 217 | (64.2) | 124 | (65.3) | 93 | (62.8) | .644 |
| Body mass index (kg/m2), median; (IQR) | 26.23 | (23.39-29.41) | 26.23 | (22.93-29.39) | 26.23 | (23.67-30.86) | .904 |
| Entry criteria, median; (IQR) | |||||||
| Body temperature (°C) | 36.7 | (36-37.5) | 36.8 | (36-37.5) | 36.5 | (35.8-37.5) | .087 |
| Heart rate (bpm) | 99 | (86-115) | 96 | (85-111) | 103 | (89-120) | .034 |
| Systolic blood pressure (mm Hg) | 110 | (94-130) | 113 | (99-132) | 105 | (88-125) | .001 |
| Respiratory rate (breaths/minute) | 21 | (18-26) | 21 | (18-26) | 20 | (17-27) | .882 |
| Cardiovascular risk factors, n (%) | |||||||
| Arterial hypertension | 218 | (64.7) | 122 | (64.2) | 96 | (65.3) | .853 |
| Diabetes mellitus | 116 | (34.4) | 64 | (33.7) | 52 | (35.4) | .746 |
| Hyperlipidemia | 96 | (28.6) | 47 | (24.7) | 49 | (33.6) | .076 |
| Smoking | 97 | (29.0) | 54 | (28.9) | 43 | (29.3) | .940 |
| Prior medical history, n (%) | |||||||
| Coronary artery disease | 116 | (34.4) | 61 | (32.1) | 55 | (37.4) | .309 |
| Congestive heart failure | 68 | (20.2) | 34 | (17.9) | 34 | (23.1) | .235 |
| Atrial fibrillation | 95 | (28.1) | 54 | (28.4) | 41 | (27.7) | .884 |
| Chronic kidney disease | 67 | (19.8) | 43 | (22.6) | 24 | (16.2) | .142 |
| COPD | 63 | (18.7) | 38 | (20.0) | 25 | (17.0) | .485 |
| Liver cirrhosis | 31 | (9.2) | 13 | (6.8) | 18 | (12.2) | .093 |
| Malignancy | 113 | (33.4) | 54 | (28.4) | 59 | (39.9) | .027 |
| Immunosuppression | 47 | (14.5) | 28 | (15.5) | 19 | (13.2) | .562 |
| LVEF at admission, n (%) | |||||||
| ≥ 55% | 126 | (40.4) | 75 | (42.4) | 51 | (37.8) | .002 |
| 54-45 | 86 | (27.6) | 60 | (33.9) | 26 | (19.3) | |
| 44-35% | 52 | (16.7) | 21 | (11.9) | 31 | (23.0) | |
| <35% | 48 | (15.4) | 21 | (11.9) | 27 | (20.0) | |
| Not documented | 26 | — | 13 | — | 13 | — | — |
| Cardiopulmonary resuscitation, n (%) | 41 | (12.1) | 11 | (5.8) | 30 | (20.3) | .001 |
| In-hospital | 12 | (3.6) | 3 | (1.6) | 9 | (6.1 | .001 |
| Out-of-hospital | 29 | (8.6) | 8 | (4.2) | 21 | (14.2) | |
Abbreviations: COPD, chronic obstructive pulmonary disease; IQR, interquartile range; LVEF, left ventricular ejection fraction.
Level of significance p < .05.
As demonstrated in Table 2, the acute physiology and chronic health evaluation (APACHE) II score (median 26 vs 22; p = .001), acute physiology score (median 18 vs 14; p = .001) and the SOFA score (median 13 vs 10; p = .001) were higher among patients with septic shock. A pulmonary infectious focus was most common in both groups (58% vs 60%; p = .390). Furthermore, serum lactate (3.5 mmol/L vs 1.3 mmol/L; p = .001), D-dimer levels (10.5 µg/L vs 3.8 µg/L; p = .001), as well as the PT/INR (1.3 vs 1.2; p = .001) were higher in patients with septic shock on day 0.
Table 2.
Sepsis-Related Data, Follow-up Data and Endpoints.
| All patients (n = 338) |
Sepsis without shock (n = 190) |
Septic shock (n = 148) |
p value | ||||
|---|---|---|---|---|---|---|---|
| Sepsis scores, median; (IQR) | |||||||
| DIC | 1 | (0-2) | 1 | (0-2) | 2 | (1-3) | .001 |
| Acute physiology score | 16 | (12-21) | 14 | (9-19) | 18 | (14-23) | .001 |
| APACHE II | 23 | (18-29) | 22 | (15-27) | 26 | (20-31) | .001 |
| SOFA | 11 | (8-13) | 10 | (7-12) | 13 | (10-15) | .001 |
| ISARIC-4C-Mortality score | 14 | (12-16) | 14 | (12-16) | 14 | (12-16) | .797 |
| Infection focus, n (%) | |||||||
| Pulmonary | 200 | (59.2) | 114 | (60.0) | 86 | (58.1) | .390 |
| Urogenital | 37 | (10.9) | 26 | (13.7) | 11 | (7.4) | |
| Intra-abdominal | 31 | (9.2) | 14 | (7.4) | 17 | (11.5) | |
| Wound | 2 | (.6) | 1 | (0.5) | 1 | (0.7) | |
| Catheter | 2 | (0.6) | 1 | (0.5) | 1 | (0.7) | |
| Unknown | 66 | (19.5) | 34 | (17.9) | 32 | (21.6) | |
| SARS-CoV-2 infection, n (%) | 36 | (10.7) | 28 | (14.7) | 8 | (5.4) | .006 |
| Multiple organ support during ICU | |||||||
| Vasopressor support norepinephrine, n (%) | 296 | (87.6) | 149 | (78.4) | 147 | (99.3) | .001 |
| Dosis norepinephrine (µg/ml; median (IQR)) | 46.6 | (5.3-153.2) | 21.8 | (1.1-100.1) | 87.6 | (22.4-268.3) | .001 |
| Dialysis during hospitalization, n (%) | 147 | (43.5) | 61 | (32.1) | 86 | (58.1) | .001 |
| Extracorporal membrane oxygenation, n (%) | 24 | (7.1) | 14 | (7.4) | 10 | (6.8) | .828 |
| Respiratory status | |||||||
| Mechanical ventilation, n (%) | 186 | (55.0) | 97 | (51.1) | 89 | (60.1) | .096 |
| Invasive mechanical ventilation, n(%) | 144 | (42.6) | 63 | (33.2) | 81 | (54.7) | .001 |
| Duration of mechanical ventilation (median; (IQR)) | 5 | (1-16) | 7 | (1-16) | 4 | (1-14) | .265 |
| PaO2/FiO2 ratio (median; (IQR)) | 197 | (137-291) | 196 | (141-293) | 202 | (134-291) | .549 |
| PaO2 (median; (IQR)) | 89 | (74-120) | 85 | (70-115) | 94 | (78-127) | .014 |
| Liver function | |||||||
| Acute liver failure, n (%) | 30 | (8.9) | 11 | (5.8) | 19 | (12.8) | .024 |
| Renal function, median; (IQR) | |||||||
| Serum creatinine (mg/dl) | 1.8 | (1.1-3) | 1.6 | (1-2.9) | 1.9 | (1.4-3.1) | .543 |
| GFR (ml/min) | 32.5 | (19.1-57.9) | 38.8 | (19.6-66.3) | 29.9 | (18.2-47.2) | .004 |
| Urine output (ml) | 800 | (238-1600) | 990 | (410-1733) | 555 | (100-1350) | .046 |
| Dialysis (days) | 0 | (0-4) | 0 | (0-3) | 2 | (0-5) | .039 |
| Baseline laboratory values, median; (IQR) | |||||||
| pH | 7.37 | (7.29-7.42) | 7.39 | (7.31-7.44) | 7.34 | (7.24-7.4) | .378 |
| Lactate (mmol/l) | 2 | (1.2-4) | 1.3 | (1-2) | 3.5 | (2.2-7.5) | .001 |
| Serum sodium (mmol/l) | 139 | (135-144) | 139 | (135-144) | 139 | (135-145) | .406 |
| Serum potassium (mmol/l) | 4.2 | (3.8-4.7) | 4.2 | (3.7-4.6) | 4.2 | (3.9-4.7) | .051 |
| Hemoglobin (g/dl) | 10 | (8.5-12.1) | 10 | (8.7-12.3) | 10 | (8.1-11.9) | .349 |
| WBC (106/ml) | 12.7 | (8.3-18.2) | 12.5 | (8.4-17.8) | 12.8 | (8-19.4) | .683 |
| Platelets (106/ml) | 176 | (107-264) | 190 | (125-262) | 156 | (88-267) | .231 |
| PT/INR | 1.2 | (1.1-1.4) | 1.2 | (1.1-1.3) | 1.3 | (1.1-1.6) | .001 |
| Fibrinogen (g/l) | 3.8 | (2.5-5.6) | 4.7 | (3.1-6.3) | 3.2 | (2.2-5) | .015 |
| D-dimer (mg/l) | 4.3 | (1.6-15.3) | 3.8 | (1.3-10.2) | 10.5 | (4.2-31.3) | .001 |
| AST (U/l) | 56 | (29-127) | 43 | (25-80.5) | 78 | (42.3-199.3) | .011 |
| ALT (U/l) | 31 | (17-72) | 27 | (16-56.8) | 38 | (19-93.5) | .061 |
| AST/ALT ratio | 1.8 | (1.1-2.5) | 1.5 | (0.9-2.2) | 2.1 | (1.4-3.1) | .001 |
| Bilirubin (mg/dl) | 0.9 | (0.5-1.7) | 0.8 | (0.4-1.4) | 1 | (0.6-2) | .087 |
| Troponin I (µg/l) | 0.2 | (0-1) | 0.1 | (0-0.6) | 0.5 | (0.1-1.9) | .301 |
| NT-pro BNP (pg/ml) | 2801 | (1004-7945) | 2268 | (789-7001) | 4500 | (1056- 11,754) | .158 |
| Procalcitonin (ng/ml) | 2.8 | (0.7-20.8) | 1.6 | (0.6-12.7) | 5.3 | (1-33.8) | .249 |
| CRP (mg/l) | 146 | (83-225) | 151 | (99-227) | 134 | (79-220) | .095 |
| Primary endpoint | |||||||
| All-cause mortality at 30 days, n (%) | 176 | (52.1) | 81 | (42.6) | 95 | (64.2) | .001 |
| Follow up data, n (%) | |||||||
| ICU time (days; median; (IQR)) | 8 | (3-18) | 10 | (4-20) | 6 | (3-17) | .148 |
| Death ICU, n (%) | 165 | (48.8) | 69 | (36.3) | 96 | (64.9) | .001 |
Abbreviations: ALT, alanine aminotransferase; APACHE II, acute physiology and chronic health evaluation II; AST, aspartate aminotransferase; NT-pro BNP, N-terminal prohormone of brain natriuretic peptide; CRP, C-reactive Protein; DIC, disseminated intravascular coagulation; GFR, glomerular filtration rate; ICU, intensive care unit; PT/INR, prothrombin time/ international normalized ratio; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; SOFA, sepsis-related organ failure assessment score; WBC, white blood cells.
Level of significance p < .05. Bold type indicates statistical significance.
Association of the Baseline PT/INR with Clinical and Laboratory Data
Table 3 illustrates the correlation of the PT/INR on day 0 with clinical and laboratory data. The PT/INR significantly correlated with serum bilirubin (r = 0.397; p = .001), white blood cell count (WBC) (r = 0.111; p = .042), platelet count (r = −0.158; p = .004), procalcitonin (r = 0.206; p = .001) and serum creatinine (r = 0.217; p = .001). In line, PT/INR correlated with the SOFA score (r = 0.268; p = .001), acute physiology score (r = 0.195; p = .001), APACHE II score (r = 0.180; p = .001), as well as clinical parameters such as MAP (r = −0.203; p = .001) and mechanical ventilation days (r = −0.170; p = .002).
Table 3.
Univariate Correlations of the PT/INR with Laboratory and Clinical Parameters in all Patients (n = 338) at day 0.
| r | p value | |
|---|---|---|
| Age | 0.003 | .954 |
| BMI | −0.012 | .835 |
| Creatinine (mg/dl) | 0.217 | .001 |
| Bilirubin (mg/dl) | 0.397 | .001 |
| Hb (g/dl) | −0.182 | .001 |
| WBC (106/ml) | 0.111 | .042 |
| Platelet count (106/ml) | −0.158 | .004 |
| aPPT | 0.529 | .001 |
| Fibrinogen | −0.533 | .001 |
| CRP (mg/dl) | −0.069 | .209 |
| Procalcitonin (ng/ml) | 0.206 | .001 |
| PaO2/FiO2 ratio | 0.134 | .017 |
| MAP (mm Hg) | −0.203 | .001 |
| Intensive care days | −0.216 | .001 |
| Mechanical ventilation days | −0.170 | .002 |
| Renal replacement days | 0.068 | .213 |
| Catecholamine use | 0.095 | .081 |
| SOFA score | 0.268 | .001 |
| Acute Physiology score | 0.195 | .001 |
| APACHE II score | 0.180 | .001 |
Abbreviations: APACHE II, acute physiology and chronic health evaluation II; BMI, body mass index; CRP, C-reactive protein; PT/INR, prothrombin time/ international normalized ratio; aPPT, activated partial thromboplastin time; SOFA, sepsis-related organ failure assessment score; Hb, haemoglobin; WBC, white blood cells;
Level of significance p < .05. Bold type indicates statistical significance.
Diagnostic Performance of the PT/INR
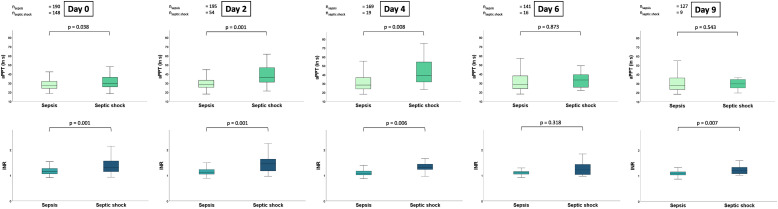
Box plots presenting the distribution of the aPTT and PT/INR in the presence of sepsis without shock or septic shock at day 0, 2, 4, 6 and day 9 are presented in Figure 1. Except for day 6 (33.4 vs 28.5 s; p = .873) and 9 (29.6 vs 27.8 s; p = .543), the aPTT was higher in patients with septic shock than in patients with sepsis without shock on day 0 (29.8 vs 27.6 s; p = .038), day 2 (36 vs 28.7 s; p = .001) and day 4 (39.1 vs 28.3 s; p = .008), respectively. Likewise, the PT/INR was increased in patients with septic shock during the first week on day 0 (1.3 vs 1.2; p = .001), day 2 (1.1 vs 1.5; p = .001), day 4 (1.31 vs 1.1; p = .006) and day 9 (1.1 vs 1.2; p = .007).
Figure 1.
Box plots demonstrating the distribution of the aPTT and PT/INR among patients with sepsis and septic shock during the first week of sepsis onset. Data is presented as median with interquartile ranges (boxes) and 5-95% percentiles (whiskers).
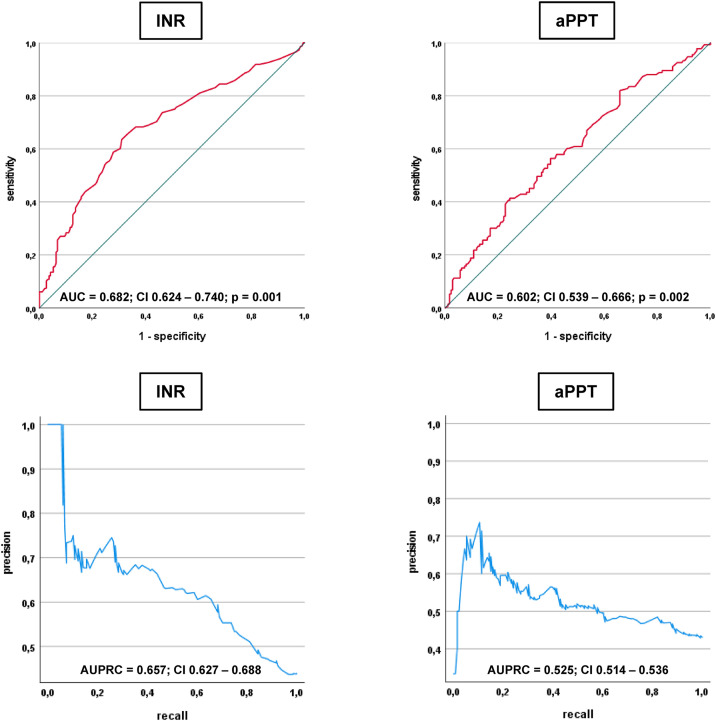
C-statistics revealed comparable diagnostic AUCs for both the PT/INR (AUC = 0.682) and aPTT (AUC = 0.602) to discriminate between patients with septic shock and sepsis on sepsis day 0 (Figure 2). In line, the PT/INR displayed a better AUPRC on sepsis day 0 than the aPPT (0.657 vs 0.525).
Figure 2.
Receiver operating characteristic (ROC) and precision recall analyses investigating the diagnostic performance of the PT/INR and the aPTT for the discrimination of septic shock as compared to sepsis without shock on day 0.
Focusing on the diagnostic value of the PT/INR compared to the aPTT during course of ICU hospitalization, a comparable diagnostic value of the aPTT and PT/INR was seen on all evaluated treatment days (range of AUCs for INR 0.630 to 0.798; range of AUCs for aPTT 0.502 to 0.761; p for AUC differences >.05) (Supplemental Table 1).
Prognostic Performance of the PT/INR
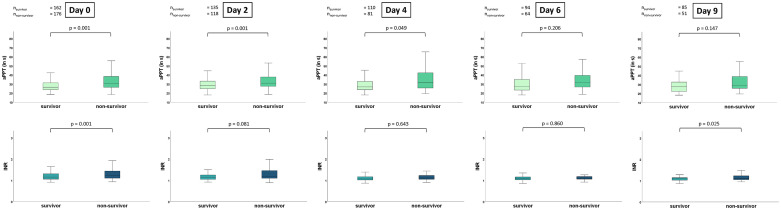
Overall risk of 30-day all-cause mortality was 52%. The PT/INR was significantly higher in non-survivors as compared to survivors on day 0 (median 1.2 (IQR 1.1-1.4) versus median 1.2 (IQR 1.1-1.3); p = .001) and day 9 (median 1.1 (1.1-1.2) versus 1.1 (1-1.2); p = .025). The PT/INR did not differ significantly between non-survivor and survivors on day 2 (median 1.2 (1.1-1.5) versus 1.1 (1.1-1.3); p = .081), day 4 (1.1 (1.1-1.2) versus 1.1 (1-1.2); p = .643) and day 6 (1.1 (1.1-1.2) versus 1.1 (1-1.2); p = .860). The aPPT was higher in non-survivors compared to survivors on day 0 (30.6 s (26.4-38.4) versus 26.5 s (23.8-31.5); p = .001), day 2 (31s (27.5-38) versus 28.5 s (24.8-33.4); p = .001) and day 4 (32s (25.5-42.5) versus 26.8 s (23.6-33.2); p = .049). However, the aPPT displayed no significant difference on day 6 (31.4 s (26.6-39.7) versus 27s (23.3-35.3); p = .206) and day 9 (29.2 s (25.5-38.7) versus 27.4 s (22.2-32.6); p = .147) of follow-up (Figure 3).
Figure 3.
Box plots demonstrating distribution of the aPTT and PT/INR during the first week of sepsis onset comparing 30-day survivors and non-survivors. Data is presented as median with interquartile ranges (boxes) and 5-95% percentiles (whiskers).
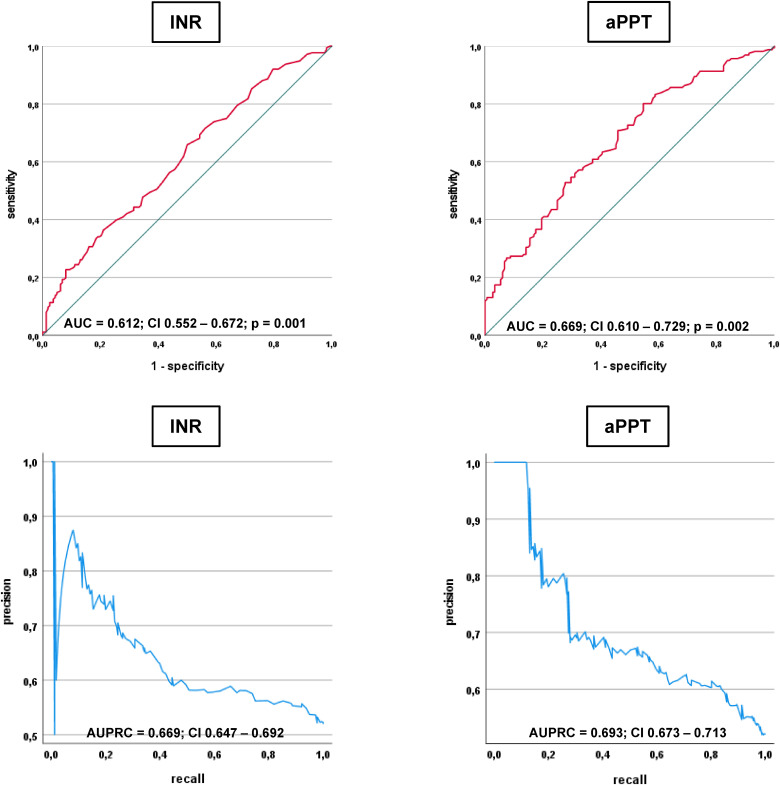
The prognostic AUC of the PT/INR on sepsis day 0 were comparable to the prognostic AUCs of the aPPT (0.612 vs 0.669) (Figure 4). The AUPRCs of both parameters were even closer (0.669 vs 0.693).
Figure 4.
Receiver operating characteristic (ROC) and precision recall analyses investigating the prognostic performance of the PT/INR and the aPTT with regard to 30-day all-cause mortality.
During course of ICU hospitalization, the prognostic AUCs of the PT/INR were statistically significant during the first 4 days of ICU treatment to predict all-cause mortality at 30 days (range of AUC 0.581 to 0.612). Of note, prognostic AUCs for aPTT were comparable to the PT/INR on the evaluated treatment days (Supplemental Table 2).
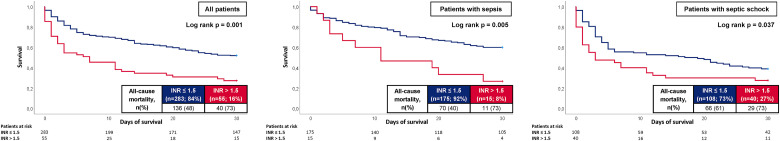
At 30 days, the primary endpoint of all-cause mortality occurred in 73% of the patients with PT/INR > 1.5% and 48% with PT/INR ≤ 1.5 on day 0. Accordingly, risk of all-cause mortality was higher in patients with PT/INR > 1.5 (log rank p = .001; HR = 2.129; 95% CI 1.494-3.033; p = .001) (Figure 5, left panel). Impaired all-cause mortality in patients with PT/INR > 1.5 was observed in both patients presenting with sepsis without shock (73% vs 40%, log rank p = .005; HR = 2.408; 95% CI 1.272-4.556; p = .007) and septic shock (73% vs 61%, log rank p = .037; HR = 1.561; 95% CI 1.008-2.417; p = .046) (Figure 5, middle and right panel).
Figure 5.
Kaplan-Meier curves for the PT/INR according to all-cause mortality at 30 days within the entire study cohort (left panel), in patients with sepsis (middle panel) and septic shock (right panel).
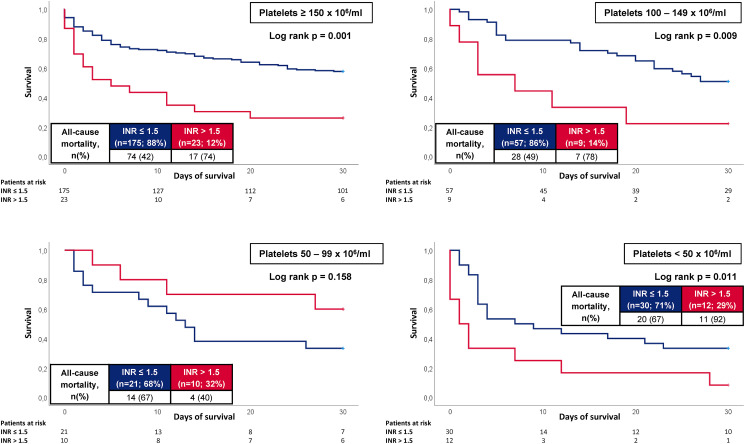
When stratified by concomitant platelet count on admission, PT/INR > 1.5 was especially associated with an increased risk of 30-day all-cause mortality in patients with platelet count ≥ 150 × 106/mL (74% vs 42%, log rank p = .001; HR = 2.528; 95% CI 1.487-4.297; p = .001), 100–149 × 106/mL (78% vs 49%, log rank p = .009; HR = 2.852; 95% CI 1.237-6.577; p = .014) and < 50 × 106/mL (92% vs 67%, log rank p = .011; HR = 2.431; 95% CI 1.157-5.110; p = .019), whereas the PT/INR had no prognostic impact in patients with a platelet count of 50–99 × 106/mL (log rank p = .158) (Figure 6).
Figure 6.
Kaplan-Meier curves for the PT/INR according to all-cause mortality at 30 days according to concomitant platelet count on admission.
Multivariable Cox Regression Analysis
After multivariable adjustment, a PT/INR > 1.5 was still associated with increased risk of all-cause mortality at 30 days (HR = 1.793; 95% CI 1.343-2.392; p = .001) (Table 4). Furthermore, especially increasing age (HR = 1.017; p = .011), CPR (HR = 2.080; p = .001), and platelet count (HR = .998; p = .003) were associated with increased all-cause mortality at 30 days. Even when stratified for the presence of sepsis without shock and septic shock, PT/INR > 1.5 was especially associated with increased risk of short-term death in patients with septic shock (HR = 1.458; 95% CI 1.034-2.056; p = .031), whereas PT/INR > 1.5 did not reach statistical significance in patients with sepsis without shock (HR = 2.542; 95% CI .866-7.463; p = .090) (Table 5).
Table 4.
Uni- and multivariable Cox regression analyses within the entire study cohort with regard to 30-day all-cause mortality.
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | 1.013 | 1.002-1.024 | .020 | 1.017 | 1.004-1.030 | .011 |
| Sex | 1.066 | 0.791-1.435 | .675 | 1.120 | 0.796-1.575 | .515 |
| LVEF | 1.236 | 1.083-1.411 | .002 | 1.113 | 0.959-1.293 | .158 |
| Liver cirrhosis | 1.512 | 0.985-2.321 | .058 | 1.233 | 0.704-2.159 | .464 |
| Malignancy | 1.206 | 0.894-1.627 | .221 | 1.176 | 0.821-1.684 | .376 |
| CPR | 1.665 | 1.283-2.160 | .001 | 2.080 | 1.332-3.247 | .001 |
| Respiratory rate >22/min | 0.928 | 0.696-1.236 | .609 | 0.893 | 0.637-1.254 | .515 |
| Systolic BP <100mm Hg | 1.111 | 0.822-1.500 | .493 | 0.818 | 0.578-1.159 | .259 |
| GCS <15 | 1.478 | 0.920-2.376 | .107 | 1.079 | 0.645-1.804 | .773 |
| CRP | 0.999 | 0.998-1.001 | .203 | 0.999 | 0.998-1.001 | .478 |
| Platelets | 0.998 | 0.997-0.999 | .002 | 0.998 | 0.996-0.999 | .003 |
| PT/INR >1.5 | 2.129 | 1.494-3.033 | .001 | 1.793 | 1.343-2.392 | .001 |
Abbreviations: LVEF, left ventricular ejection fraction; CPR, cardiopulmonary resuscitation; Systolic BP, systolic blood pressure; GCS, Glasgow Coma Scale; CRP, C-reactive protein; PT/INR, prothrombin time/ international normalized ratio.
Level of significance p < .05.
Table 5.
Multivariable Cox Regression Analysis with regard to 30-day all-cause mortality stratified by sepsis without shock and septic shock.
| Variables | Sepsis without shock | Septic shock | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | 1.020 | 1.000-1.042 | .054 | 1.012 | 0.994-1.030 | .180 |
| Sex | 1.016 | 0.595-1.735 | .952 | 1.211 | 0.760-1.927 | .420 |
| LVEF | 1.159 | 0.909-1.477 | .235 | 1.044 | 0.855-1.273 | .674 |
| Liver cirrhosis | 1.028 | 0.406-2.602 | .954 | 1.252 | 0.584-2.685 | .563 |
| Malignancy | 1.138 | 0.655-1.979 | .647 | 1.142 | 0.670-1.947 | .626 |
| CPR | 2.484 | 1.074-5.754 | .034 | 1.502 | 0.866-2.603 | .148 |
| Respiratory rate >22/min | 1.034 | 0.622-1.719 | .897 | 0.807 | 0.503-1.295 | .374 |
| Systolic BP <100mm Hg | 0.745 | 0.411-1.348 | .330 | 0.789 | 0.476-1.305 | .356 |
| GCS <15 | 1.171 | 0.543-2.526 | .688 | 1.072 | 0.515-2.235 | .852 |
| CRP | 0.999 | 0.997-1.002 | .500 | 1.000 | 0.998-1.002 | .838 |
| Platelets | 0.977 | 0.995-1.000 | .035 | 0.998 | 0.997-1.000 | .062 |
| PT/INR > 1.5 | 2.542 | 0.866-7.463 | .090 | 1.458 | 1.034-2.056 | .031 |
Abbreviations: LVEF, left ventricular ejection fraction; CPR, cardiopulmonary resuscitation; Systolic BP, systolic blood pressure; GCS, Glasgow Coma Scale; CRP, C-reactive protein; PT/INR, prothrombin time/ international normalized ratio.
Level of significance p < .05.
Discussion
The present study investigates the diagnostic and prognostic role of the PT/INR in patients with sepsis and septic shock. The data suggests an increased PT/INR in patients with septic shock as compared to patients with sepsis without shock. AUCs showed, that the PT/INR is an moderate dignostic tool for the diagnosis of septic shock. This was confirmed by the AUPRCs, which are more precise in study populations where the true positives (ie, septic shock) are less frequent than the true negatives (ie, sepsis without shock).13 In line, an increased risk of 30-day all-cause mortality was observed in patients with PT/INR > 1.5 on admission, which was observed in both patients with sepsis without shock and septic shock and which was still evident after multivariable adjustment. Finally, the prognostic impact of the PT/INR was irrespective of concomitant thrombocytopenia.
Coagulopathy reflects a common complication of sepsis or septic shock and ranges from hypercoagulability to DIC. In addition to the SOFA score that only includes the platelet count for the assessment of coagulopathies, the sepsis-induced coagulopathy (SIC) score also includes the PT/INR and the SOFA score for the prediction of 28-day all-cause mortality in sepsis and septic shock.14 Despite the high number of studies focusing on the platelet count for the diagnosis making of sepsis,4,7,15 limited data is available regarding the significance of the PT/INR for the prediction of septic shock. Within a retrospective study including 54 patients with sepsis related to acute obstructive pyelonephritis, an INR ≥ 1.20 was shown to be a predictor of septic shock within an univariable logistic regression analysis, which was no longer observed after multivariable adjustment.16 In line, the INR was shown to be an appropriate tool for the diagnosis of sepsis (AUC = 0.700) within a study by Czempik et al, including 217 critically ill patients with and without sepsis presenting with concomitant anaemia.11 However, in their study, patients were not stratified for the severity of sepsis. Zhang et al investigated the prognostic impact of the INR on the efficacy of diagnosis of sepsis in 108 patients with no-pulmonary sepsis, demonstrating that INR is a superior detection tool with an AUC of 0.918 as compared to neutrophil-lymphocyte count ratio (NLR), platelet count and quick SOFA (qSOFA) score.17 The present study confirms these findings, suggesting that the PT/INR represents an appropriate tool for the diagnosis of septic shock. In line with this, concomitant coagulopathy was shown to be a common complication in sepsis or septic shock (up to 84%) and to occur more frequently in patients with septic shook as compared to patients with sepsis.18 In line with this, the PT/INR was higher among patients with septic shock as compared to patients with sepsis during the first 10 days of ICU treatment within the present study and was shown to be an appropriate tool for the diagnosis of septic shock.
Compared to the PT/INR, the aPTT is typically prolonged in moderate to severe deficit of all coagulation factors except for factor VII and XIII and is not standardized between laboratories and reagents. In patients with sepsis or septic shock, inflammation and activation of monocytes leads to excessive thrombin generation which on the one hand leads to platelet activation and consumption of coagulation inhibitors which promotes a hypercoagulable state, but on the other hand, may result into consumption of platelets, clotting factors and fibrinogen causing haemorrhagic events.19 This results into an increased PT/INR, which may be an important predictor for the formation of microthrombi at an advanced stage of septic shock.20 Within the present study, the PT/INR was significantly higher among non-survivors on day 0 and 9. This may be related to the dynamic clinical improvement/impairment in patients with sepsis. Within the present study, 38% of patients with initial septic shock showed clinical improvement to sepsis without shock within the first 24 h of ICU admission, which in line may result in lower PT/INR values.
Commonly, studies investigating the prognostic role of the INR on all-cause mortality included critically ill patients and did not focus on patients with sepsis or septic shock. For instance, PT/INR was demonstrated to increase the risk of all-cause mortality in patients admitted with trauma21,22 or critical illness.11 In contrast, only a few studies investigated the role of the PT/INR in patients presenting with sepsis or septic shock. Ling et al demonstrated an increased risk of all-cause mortality in 106 patients with sepsis as a consequence of limb necrosing fasciitis in the presence of an INR > 1.5 using multivariable logistic regression analyses.23 In line with this study, Liu et al found that an increased INR was associated with an increased risk of 28-day all-cause mortality in 66 patients with sepsis or septic shock. However, sequential assessment of the INR during the course of sepsis or septic shock was beyond the scope of their study.24 The prognostic value of DIC (defined as increased INR and low platelet count) was investigated within a retrospective study by Lyons et al, including 6148 patients from 2010 to 2015. They demonstrated an incremental increase of in-hospital mortality depending on the stage of DIC, which may reflect more advanced stages of septic shock resulting into multi-organ failure.4 However, their findings were not stratified for thrombocytopenia and increased INR.25 Interestingly, within the present study, PT/INR was shown to be a predictor of 30-day all-cause mortality in both patients with normal platelet count and those with severe thrombocytopenia, which may underline the importance of the PT/INR for the prediction of sepsis-related prognosis irrespective of the degree of concomitant coagulopathy. Intensivists working on an internal ICU are increasingly dependent on valuable blood derived biomarkers,26–28 in order to improve early decision making and risk-stratification of those patients endangered for increased short-term mortality during daily clinical practice. Recently, established biomarkers reflecting different organ-system damage as a consequence of sepsis or septic shock have shown poor to moderate prognostic values in patients with sepsis or septic shock. For instance, C-reactive protein and procalcitonin were found to have moderate discrimination for bacteremia in 459 patients with suspected infection (AUC 0.68 and 0.65), whereas the predictive value for 28-day all-cause mortality was even inferior to systolic blood pressure and pulse oximetry.29 A recently published hypothesis-generating meta-analysis suggested that N-terminal pro-B-type natriuretic peptide (NT-proBNP) may be associated with prognosis in sepsis or septic shock (AUC = 0.787). However, patients’ characteristics significantly differed among the 35 included studies.30 In line with the moderate AUCs for biomarkers, the AUCs for established sepsis-scores were shown to be rather poor within various studies. For instance, the qSOFA score and the SOFA score were shown to have moderate prognostic accuracy (AUC 0.547 and 0.686) within a sub-study of the “Medical Information Mart for Intensive Care III database” (MIMIC III).31 In addition to the evaluation of novel biomarkers for a better discrimination of mortality in sepsis or septic shock, research on innovative experimental biomarkers needs to be accomplished by the revaluation of established markers, such as aPTT, platelet count and the PT/INR in the setting of patients suffering from sepsis and septic shock. The latter are available in most ICUs worldwide, are easy to measure and therefore more cost-effective. Hence, their diagnostic and prognostic value is strongly recommended to be revaluated in the current era of intensive care medicine including the application of the latest sepsis-3 definitions.3
Often characterized by concomitant coagulopathies, the prognostic impact of the PT/INR has gained more significance in patients with the coronavirus disease 2019 (COVID-19). The PT/INR was shown to be increased in COVID-19 non-survivors, as well as in patients with severe COVID-19 sepsis within a large meta-analysis including 7440 patients.32,33 However, within the present study including patients from 2019 to 2021, only 11% of the patients were admitted with COVID-19, therefore sub-analyses investigating the diagnostic and prognostic role of the PT/INR were not possible. Although PT/INR was significantly associated with mortality in COVID-19, further studies are necessary to investigate the diagnostic accuracy of the PT/INR to identify COVID-19 patients at increased risk of ICU treatment and death.
In conclusion, the present study demonstrated that the PT/INR reflects a moderate diagnostictool for the diagnosis of septic shock and sepsis. Furthermore, the PT/INR was shown to be an appropriate prognostic tool with regard to 30-day all-cause mortality. PT/INR > 1.5 was associated with an increased risk of 30-day all-cause mortality, which was observed both in patients with sepsis and septic shock. Finally, the prognostic impact of the PT/INR was demonstrated irrespective of concomitant thrombocytopenia on admission.
Study Limitations
This study has several limitations. Due to the single-centre and observational study design, results may be influenced by measured an unmeasured cofounding, although we adjusted for potential cofounders using multivariable Cox regression. The stages of concomitant liver disease (such as Child-Pugh and Model for End-stage Liver Disease (MELD) score) and were only available for minor part of the study population and therefore beyond the scope of the present study. With regard to the diagnostic value of the PT/INR, no control group with healthy individuals was considered. Finally, the association of the PT/INR with regard to long-term outcomes was beyond the scope of the present study.
Supplemental Material
Supplemental material, sj-docx-1-cat-10.1177_10760296221137893for Diagnostic and Prognostic Significance of the Prothrombin Time/International Normalized Ratio in Sepsis and Septic Shock by Tobias Schupp, Kathrin Weidner, Jonas Rusnak, Schanas Jawhar, Jan Forner, Floriana Dulatahu, Lea Marie Brück, Ursula Hoffmann, Thomas Bertsch, Julian Müller, Christel Weiß, Ibrahim Akin and Michael Behnes in Clinical and Applied Thrombosis/Hemostasis
Supplemental material, sj-docx-2-cat-10.1177_10760296221137893for Diagnostic and Prognostic Significance of the Prothrombin Time/International Normalized Ratio in Sepsis and Septic Shock by Tobias Schupp, Kathrin Weidner, Jonas Rusnak, Schanas Jawhar, Jan Forner, Floriana Dulatahu, Lea Marie Brück, Ursula Hoffmann, Thomas Bertsch, Julian Müller, Christel Weiß, Ibrahim Akin and Michael Behnes in Clinical and Applied Thrombosis/Hemostasis
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article
ORCID iD: Tobias Schupp https://orcid.org/0000-0001-8171-7617
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: A comparative meta-analysis. Crit Care Med. 2014;42(3):625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De La Rica AS, Gilsanz F, Maseda E. Epidemiologic trends of sepsis in western countries. Ann Transl Med. 2016;4(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer M, Deutschman CS, Seymour CWet al. The third international consensus definitions for sepsis and septic shock (sepsis-3). Jama. 2016;315(8):801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greco E, Lupia E, Bosco O, Vizio B, Montrucchio G. Platelets and multi-organ failure in sepsis. Int J Mol Sci. 2017;18(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gando S, Saitoh D, Ogura Het al. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: Results of a multicenter, prospective survey. Crit Care Med. 2008;36(1):145-150. [DOI] [PubMed] [Google Scholar]
- 6.Vincent JL, Francois B, Zabolotskikh Iet al. Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: The SCARLET randomized clinical trial. Jama. 2019;321(20):1993-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055–2064. [DOI] [PubMed]
- 8.Claushuis TA, van Vught LA, Scicluna BPet al. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood. 2016;127(24):3062-3072. [DOI] [PubMed] [Google Scholar]
- 9.Semeraro F, Colucci M, Caironi Pet al. Platelet drop and fibrinolytic shutdown in patients with sepsis. Crit Care Med. 2018;46(3):e221-e2e8. [DOI] [PubMed] [Google Scholar]
- 10.Thachil J, Warkentin TE. How do we approach thrombocytopenia in critically ill patients? Br J Haematol. 2017;177(1):27-38. [DOI] [PubMed] [Google Scholar]
- 11.Czempik PF, Herzyk J, Wilczek D, Krzych ŁJ. Hematologic system dysregulation in critically ill septic patients with anemia-a retrospective cohort study. Int J Environ Res Public Health. 2022;19(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839-843. [DOI] [PubMed] [Google Scholar]
- 13.Ozenne B, Subtil F, Maucort-Boulch D. The precision--recall curve overcame the optimism of the receiver operating characteristic curve in rare diseases. J Clin Epidemiol. 2015;68(8):855-859. [DOI] [PubMed] [Google Scholar]
- 14.Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: A retrospective analysis of a nationwide survey. BMJ Open. 2017;7(9):e017046-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akca S, Haji-Michael P, de Mendonça A, Suter P, Levi M, Vincent JL. Time course of platelet counts in critically ill patients. Crit Care Med. 2002;30(4):753-756. [DOI] [PubMed] [Google Scholar]
- 16.Kamei J, Nishimatsu H, Nakagawa Tet al. Risk factors for septic shock in acute obstructive pyelonephritis requiring emergency drainage of the upper urinary tract. Int Urol Nephrol. 2014;46(3):493-497. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Du HM, Cheng MX, He FM, Niu BL. Role of international normalized ratio in nonpulmonary sepsis screening: An observational study. World J Clin Cases. 2021;9(25):7405-7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jhang WK, Park SJ. Evaluation of sepsis-induced coagulopathy in critically ill pediatric patients with septic shock. Thromb Haemost. 2021;121(4):457-463. [DOI] [PubMed] [Google Scholar]
- 19.Papageorgiou C, Jourdi G, Adjambri Eet al. Disseminated intravascular coagulation: An update on pathogenesis, diagnosis, and therapeutic strategies. Clin Appl Thromb Hemost. 2018;24(9_suppl):8s-28s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levi M. Diagnosis and treatment of disseminated intravascular coagulation. Int J Lab Hematol. 2014;36(3):228-236. [DOI] [PubMed] [Google Scholar]
- 21.Verma A, Kole T. International normalized ratio as a predictor of mortality in trauma patients in India. World J Emerg Med. 2014;5(3):192-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peltan ID, Vande Vusse LK, Maier RV, Watkins TR. An international normalized ratio-based definition of acute traumatic coagulopathy is associated with mortality, venous thromboembolism, and multiple organ failure after injury. Crit Care Med. 2015;43(7):1429-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling XW, Lin K, Jiang XQet al. et al. International normalised ratio as an independent predictor of mortality in limb necrotising fasciitis with sepsis. Ann R Coll Surg Engl. 2021;103(1):35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Bai C, Li Bet al. Mortality prediction using a novel combination of biomarkers in the first day of sepsis in intensive care units. Sci Rep. 2021;11(1):1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons PG, Micek ST, Hampton N, Kollef MH. Sepsis-associated coagulopathy severity predicts hospital mortality. Crit Care Med. 2018;46(5):736-742. [DOI] [PubMed] [Google Scholar]
- 26.Behnes M, Bertsch T, Lepiorz Det al. Diagnostic and prognostic utility of soluble CD 14 subtype (presepsin) for severe sepsis and septic shock during the first week of intensive care treatment. Crit Care. 2014;18(5):507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giannakopoulos K, Hoffmann U, Ansari Uet al. et al. The use of biomarkers in sepsis: A systematic review. Curr Pharm Biotechnol. 2017;18(6):499-507. [DOI] [PubMed] [Google Scholar]
- 28.Hamed S, Behnes M, Pauly Det al. Pentraxin-3 predicts short- and mid-term mortality in patients with sepsis and septic shock during intensive care treatment. Clin Lab. 2018;64(6):999-1011. [DOI] [PubMed] [Google Scholar]
- 29.Gornet M, Leroux P, Ramont Let al. Lack of admission biomarkers’ clinical utility in outcomes prediction in patients suspected with infection in the emergency department. Am J Emerg Med. 2021;47:109-114. [DOI] [PubMed] [Google Scholar]
- 30.Vallabhajosyula S, Wang Z, Murad MHet al. Natriuretic peptides to predict short-term mortality in patients with sepsis: A systematic review and meta-analysis. Mayo Clin Proc Innov Qual Outcomes. 2020;4(1):50-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Meng Z, Li Yet al. et al. Prognostic accuracy of the serum lactate level, the SOFA score and the qSOFA score for mortality among adults with sepsis. Scand J Trauma Resusc Emerg Med. 2019;27(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zinellu A, Paliogiannis P, Carru C, Mangoni AA. INR And COVID-19 severity and mortality: A systematic review with meta-analysis and meta-regression. Adv Med Sci. 2021;66(2):372-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tahir Huyut M, Huyut Z, İlkbahar F, Mertoğlu C. What is the impact and efficacy of routine immunological, biochemical and hematological biomarkers as predictors of COVID-19 mortality? Int Immunopharmacol. 2022;105:108542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cat-10.1177_10760296221137893for Diagnostic and Prognostic Significance of the Prothrombin Time/International Normalized Ratio in Sepsis and Septic Shock by Tobias Schupp, Kathrin Weidner, Jonas Rusnak, Schanas Jawhar, Jan Forner, Floriana Dulatahu, Lea Marie Brück, Ursula Hoffmann, Thomas Bertsch, Julian Müller, Christel Weiß, Ibrahim Akin and Michael Behnes in Clinical and Applied Thrombosis/Hemostasis
Supplemental material, sj-docx-2-cat-10.1177_10760296221137893for Diagnostic and Prognostic Significance of the Prothrombin Time/International Normalized Ratio in Sepsis and Septic Shock by Tobias Schupp, Kathrin Weidner, Jonas Rusnak, Schanas Jawhar, Jan Forner, Floriana Dulatahu, Lea Marie Brück, Ursula Hoffmann, Thomas Bertsch, Julian Müller, Christel Weiß, Ibrahim Akin and Michael Behnes in Clinical and Applied Thrombosis/Hemostasis