Abstract
Background:
We evaluated the performance of single-nucleotide polymorphism (SNP) genotyping arrays OncoScan (Thermo Fisher Scientific, San Diego, CA) and Infinium CytoSNP-850K (CytoSNP; Illumina, Waltham, MA) for assessing homologous recombination deficiency (HRD) genomic instability.
Methods:
DNA (pretreatment samples) across 20 tumor types was evaluated with OncoScan, CytoSNP, and the clinically validated HRD test. Copy number variation (CNV) and loss of heterozygosity (LOH) analyses were performed with ASCATv2.5.1. Aggregate HRD genomic metrics included LOH, telomeric-allelic imbalance number (TAI), and large-scale state transition (LST). Associations between BRCA mutation (BRCAm) status and the clinically validated HRD test metric (dichotomized at a clinical cut-off) were evaluated using area under the receiver operating characteristic (AUROC); Spearman ρ was calculated for continuous metrics. CNV segmentation and HRD genomic metrics were calculated (n = 120, n = 106, and n = 126 for OncoScan, CytoSNP and clinically validated HRD test, respectively).
Results:
When assessed by SNP arrays, the genomic metric demonstrated good association with BRCAm (AUROC of HRD: OncoScan, 0.87; CytoSNP, 0.75) and the clinically validated test (cut-off, 42; AUROC of HRD: OncoScan, 0.92; CytoSNP, 0.91). The genomic metrics demonstrated good correlation with the clinically validated aggregate HRD test metric (ρ: OncoScan, 0.82; CytoSNP, 0.81) and for each component (ρ: OncoScan, 0.68 [LOH], 0.76 [TAI], and 0.78 [LST]; CytoSNP, 0.59 [LOH], 0.77 [TAI], and 0.82 [LST]). HRD assessed by SNP genotyping arrays and the clinically validated test showed good correlation.
Conclusion:
OncoScan and CytoSNP may potentially identify most HRD-positive tumors with appropriate clinically relevant cut-offs.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-022-10197-z.
Keywords: BRCA, Loss of heterozygosity, Homologous recombination deficiency, Large-scale state transition, Telomeric-allelic imbalance number
Background
Ovarian cancer remains a significant cause of mortality; options are needed in both first-line treatment and subsequent treatment for advanced disease [1]. Homologous recombination deficiency (HRD) is linked to germline or somatic mutations in BRCA1 and/or BRCA2 (BRCAm) as well as other genomic changes (i.e., epigenetic), and approximately half of all advanced high-grade serous ovarian cancers (HGSOCs) are HRD positive [1, 2]. Accordingly, treatment strategies aim to target this pathway, specifically the enzyme poly(adenosine diphosphate-ribose) polymerase (PARP). PARP inhibitors (PARPis) were developed with the knowledge of their direct antitumor activity and their effects on HGSOC cells harboring mutations in genes implicated in the homologous recombination repair pathway, including BRCAm [3]. Specifically, PARPis prevent efficient single-strand DNA repair activity, leading to genomic instability and cellular death in BRCAm or HRD-affected cells [1]. Thus, various biomarkers have been investigated to identify patients likely to respond to PARPis [4].
The combination of the PARPi olaparib and the vascular endothelial growth factor inhibitor bevacizumab is approved by the US Food and Drug Administration (FDA) as maintenance therapy for HRD-positive advanced ovarian cancer [5]. The FDA contemporaneously approved a companion diagnostic for HRD assessment based on a next-generation sequencing (NGS) assay that determines HRD status using assessments of BRCAm and genomic instability, which includes loss of heterozygosity (LOH), telomeric-allelic imbalance number (TAI), and large-scale state transition (LST) [6, 7]. Using this assay, tumors are considered HRD positive if they have a deleterious or suspected deleterious BRCAm or genomic instability defined as an HRD score of ≥ 42 or both [6]. HRD status or BRCAm status as determined by the test is prognostic of outcome [7]. Patients with ovarian cancer with either a germline or somatic deleterious or suspected deleterious BRCAm have better outcomes (i.e., improved response rates, progression-free survival, or overall survival) than patients with non–BRCAm tumors [8–12]. Additionally, patients with a high HRD score as determined by the test had a more favorable prognosis than patients with low HRD scores [9, 12–14]. The presence of a BRCAm or a high HRD score, or both, was also predictive of a better response to PARPis as monotherapy or in combination with bevacizumab [8, 11–13, 15].
Several tests are in development to assess HRD status; however, there is currently no standardized way to define, measure, or report HRD [16]. Further, many of these assays are complex, employing different approaches, data analysis algorithms, and cut-offs to assess HRD. This lack of harmonization across HRD assays highlights the importance of comparisons of available testing strategies; it could also introduce risk to patients if a clinical HRD test is reported or interpreted incorrectly relative to how HRD status has been clinically validated. Formal comparisons of available HRD assays are lacking, with the current study being one of the first few to investigate the utility of genomic instability score based on commercially available single-nucleotide polymorphism (SNP) arrays to assess HRD status in the support of PARPi use [17].
Genotyping microarrays that evaluate SNPs are among those being developed for HRD determination, which may provide an alternative to the NGS-based assay. SNP-based assays are designed to measure tumor-related copy number changes [9, 11, 18]. Herein, we evaluated the performance of two SNP-based assay platforms: OncoScan (Thermo Fisher Scientific, San Diego, CA) and Infinium CytoSNP-850K (Illumina, Waltham, MA), for assessing HRD genomic instability.
Methods
Clinical tumor samples
HRD was assessed from pretreatment archival tumor samples (formalin-fixed paraffin-embedded tissue) collected from clinical studies (KEYNOTE-001 [ClinicalTrials.gov Identifier: NCT01295827], KEYNOTE-012 [ClinicalTrials.gov Identifier: NCT01848834], KEYNOTE-028 [ClinicalTrials.gov Identifier: NCT02054806], KEYNOTE-055 [ClinicalTrials.gov Identifier: NCT02255097], KEYNOTE-061 [ClinicalTrials.gov Identifier: NCT02370498], KEYNOTE-086 [ClinicalTrials.gov Identifier: NCT02447003], KEYNOTE-100 [ClinicalTrials.gov Identifier: NCT02674061], and KEYNOTE-199 [ClinicalTrials.gov Identifier: NCT02787005]) funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, across various cancer types [19]. All patients provided written informed consent before enrollment in the clinical trials. All study protocols were consistent with the global standards of the International Conference on Harmonization Good Clinical Practices, the Council for International Organizations of Medical Sciences Public Policy Statement, Clinical Trial Ethics Sciences International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS, 2002), the Pharmaceutical Research and Manufacturers of America (PhRMA, 2009) Principles on Conduct of Clinical Trials, applicable local regulatory requirements, and the ethical principles that have their origin in the Declaration of Helsinki. All patient samples were analyzed anonymously and deidentified prior to use. The current analysis did not require IRB approval. All reference assay data were generated by Myriad Genetics at their Salt Lake City, UT, facility.
DNA extraction
Specimens passing pathology review were queued for DNA extraction by lysing cells from formalin-fixed paraffin embedded tissue by digestion with a proteinase K buffer followed by automated purification using the 96-well KingFisher Flex Magnetic Particle Processor (Thermo Fisher Scientific, San Diego, CA) [20].
Analyses
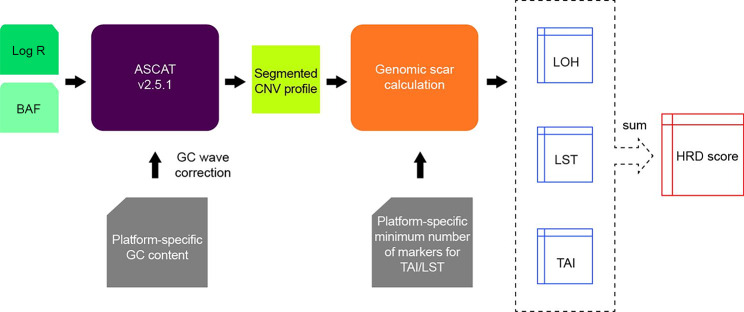
GC content is known to affect hybridization yield for Illumina sequencing, resulting in artifactual variations in the inferred copy number across the genome [21]. In our work, platform-specific (OncoScan or Infinium CytoSNP-850K) GC content files were first generated and input to ASCAT (version 2.5.1) for GC correction [22]. ASCAT was then used to evaluate copy number variation (CNV) and LOH (Fig. 1) using log R ratio and B-allele frequency of 205,647 autosomal markers for OncoScan and 789,872 autosomal markers for Infinium CytoSNP-850K, with GC wave correction [23]. Genomic metrics were further generated with default parameters using previously reported algorithms [24]. The aggregate HRD metric was the sum of the three components (LOH, TAI, and LST). LOH was defined as the number of regions longer than 15 megabases (Mb) but shorter than the whole chromosome, with a loss of one normal copy of a gene or a group of genes [25]. TAI was defined as the number of regions with allelic imbalance that extended to one of the subtelomeres but did not cross the centromere [26]. LST was defined as the number of chromosomal break points between adjacent regions of at least 10 Mb after filtering out regions shorter than 3 Mb [27]. Individual LOH, TAI, and LST scores were not available.
Fig. 1.
Single-nucleotide polymorphism array data processing diagram. CNV, copy number variation; HRD, homologous recombination deficiency;
LOH, loss of heterozygosity; LST, large-scale state transition; TAI, telomeric-allelic imbalance number
The associations between genomic metrics that use BRCAm status (defined as deleterious or suspected deleterious mutation) and the clinically validated HRD status test (myChoice CDx) metric (dichotomized at clinical cut-off) were calculated using the area under the receiver operating characteristic (AUROC) curve [28]. AUROC is a descriptive analysis; an AUROC value of < 0.5 is indicative of a negative relationship with the presence of a BRCAm, whereas an AUROC value of > 0.5 is indicative of a positive relationship with the presence of a BRCAm when the confidence intervals (CIs) in either case do not overlap 0.5. Correlations between continuous metrics were assessed using Spearman and Pearson rank correlation coefficients. CIs of AUROC and correlation coefficients were estimated by the R package pROC (version 1.13.0) and psychometric (version 2.2), respectively. Due to the small sample size of biomarker positive samples (BRCAm or HRD positive) in our dataset, area under the precision-recall curve (AUPRC) was also calculated using R package (version 0.11.2). The proportion of biomarker positive samples was taken as baseline value for AUPRC rather than 0.5 for AUROC.
The analytical parameters used in this study can be implemented by individual laboratories using a publicly available algorithm to evaluate HRD locally [23, 24].
Downsampling
To investigate the relationship between the number of SNP markers and HRD assessment, downsampling of SNP markers was performed to prespecified proportions: 0.25%, 0.5%, 1%, 2.5%, 5%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, and 90%. Downsampling was conducted randomly 10 times for each proportion, and the median was calculated to represent the HRD score for the downsampling proportion.
Results
Clinical tumor samples
Pretreatment archival tumor samples from 126 patients across 20 different tumor types were included in the analysis (Supplementary Table S1, Supplementary Table S2). Of these, CNV segmentation and genomic metrics were successfully calculated for 120 OncoScan, 106 Infinium CytoSNP-850K, and 126 clinically validated test samples.
Association between genomic metrics per OncoScan or Infinium CytoSNP-850K and BRCAm status
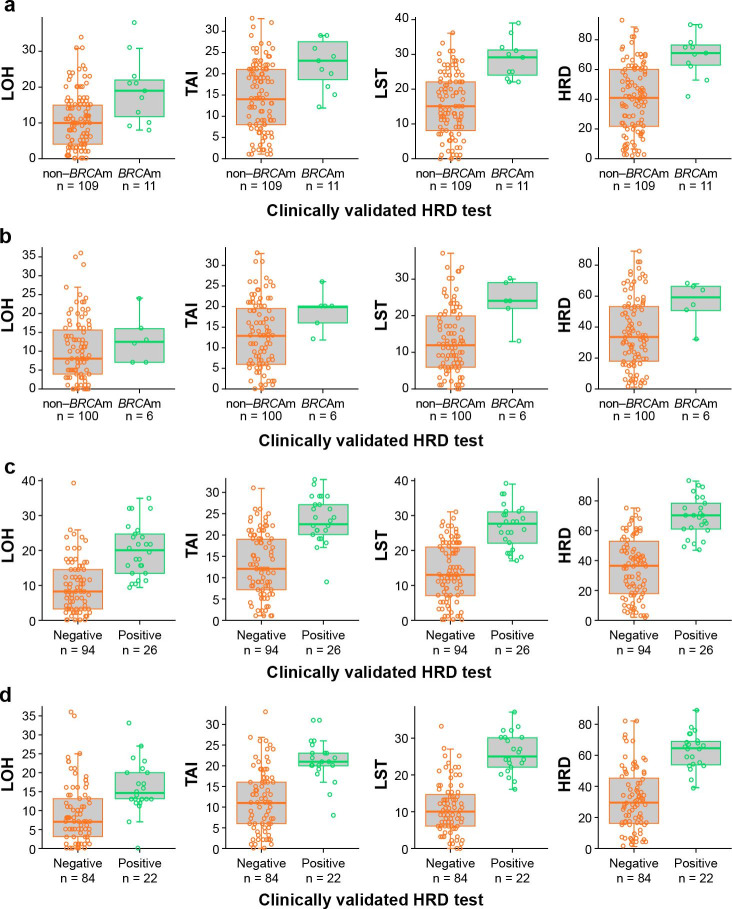
Of the 120 patients included in the OncoScan analysis, 109 had non–BRCAm tumors, and 11 had BRCAm tumors as determined by the clinically validated HRD test. Patient-level LOH, TAI, LST, and HRD scores without downsampling per OncoScan by BRCAm status are shown in Fig. 2A. The medians of the genomic metrics assessed using OncoScan were all numerically higher in patients with BRCAm versus non–BRCAm tumors.
Fig. 2.
Patient-level genomic metrics by BRCAm or HRD status with the clinically validated HRD test. (A) OncoScan and (B) Infinium CytoSNP-850K by BRCAm status, and (C) OncoScan and (D) Infinium CytoSNP-850K by HRD status. Orange indicates non–BRCAm tumors or HRD negative tumors by the clinically validated HRD test; green indicates deleterious BRCAm tumors or tumors deemed HRD positive by the clinically validated HRD test. BRCAm, mutation in BRCA1, BRCA2, or both; HRD, homologous recombination deficiency; LOH, loss of heterozygosity; LST, large-scale state transition; TAI, telomeric-allelic imbalance number
Of the 106 patients included in the Infinium CytoSNP-850K analysis, 100 had non–BRCAm tumors and six had BRCAm tumors as determined by the clinically validated HRD test. Patient-level LOH, TAI, LST, and HRD scores without downsampling per Infinium CytoSNP-850K by BRCAm status are shown in Fig. 2B. The medians of the genomic metrics assessed using Infinium CytoSNP-850K were all numerically higher in patients with BRCAm versus non–BRCAm tumors.
Good association was seen between genomic metrics as a continuous variable and with the presence of a BRCAm (determined by the clinically validated HRD test) when assessed using SNP genotyping arrays (Table 1, Supplementary Table S3). When assessed using OncoScan, the AUROC of HRD score versus the presence of a BRCAm was 0.87 (95% CI 0.77–0.97); the AUROC of the individual HRD component score versus the presence of a BRCAm was 0.76 (95% CI 0.62–0.89) for LOH, 0.78 (95% CI 0.65–0.90) for TAI, and 0.89 (95% CI 0.82–0.96) for LST. When assessed using Infinium CytoSNP-850K, the AUROC of HRD score versus the presence of a BRCAm was 0.75 (95% CI 0.61–0.89); the AUROC of the individual HRD components versus the presence of a BRCAm was 0.64 (95% CI 0.46–0.82) for LOH, 0.72 (95% CI 0.57–0.87) for TAI, and 0.83 (95% CI 0.70–0.95) for LST.
Table 1.
AUROC of genomic metrics as a continuous variable
| AUROC (95% CI) | OncoScan n = 120 |
Infinium CytoSNP-850K n = 106 |
|---|---|---|
| With deleterious BRCA m | ||
| LOH versus BRCAm | 0.76 (0.62–0.89) | 0.64 (0.46–0.82) |
| TAI versus BRCAm | 0.78 (0.65–0.90) | 0.72 (0.57–0.87) |
| LST versus BRCAm | 0.89 (0.82–0.96) | 0.83 (0.70–0.95) |
| HRDa versus BRCAm | 0.87 (0.77–0.97) | 0.75 (0.61–0.89) |
| With the clinically validated HRD test (cut-off, 42) | ||
| LOH versus clinically validated HRD test | 0.85 (0.77–0.92) | 0.78 (0.67–0.88) |
| TAI versus clinically validated HRD test | 0.86 (0.79–0.93) | 0.86 (0.78–0.93) |
| LST versus clinically validated HRD test | 0.89 (0.82–0.95) | 0.95 (0.90–0.99) |
| HRDa versus clinically validated HRD test | 0.92 (0.87–0.97) | 0.91 (0.85–0.96) |
aHRD is the sum of LOH, TAI, and LST
Abbreviations: AUROC, area under the receiver operating characteristic; BRCAm, mutation in BRCA1, BRCA2, or both; CI, confidence interval; HRD, homologous recombination deficiency; LOH, loss of heterozygosity; LST, large-scale state transition; TAI, telomeric-allelic imbalance number
Association between genomic metrics per OncoScan or Infinium CytoSNP-850K and HRD status as determined by the clinically validated test
Of the 120 patient samples included in the OncoScan analysis, 94 were HRD negative, and 26 were HRD positive by the clinically validated HRD test (cut-off 42). Patient-level LOH, TAI, LST, and HRD scores without downsampling per OncoScan by HRD status using the clinically validated HRD test are shown in Fig. 2C.
Of the 106 patient samples included in the Infinium CytoSNP-850K analysis, 84 were HRD negative, and 22 were HRD positive by the clinically validated HRD test. Patient-level LOH, TAI, LST, and HRD scores per Infinium CytoSNP-850K by HRD status using the clinically validated HRD test are shown in Fig. 2D.
It is well known that GC content can skew analyses, and thus, we performed an analysis with GC correction. The genomic metric as a continuous variable with the genotyping assays with GC correction demonstrated good association with the clinically validated test at a cut-off of 42 (Table 1, Supplementary Table S3). When assessed using OncoScan, the AUROC of HRD score versus the clinically validated test (cut-off 42) was 0.92 (95% CI 0.87–0.97); the AUROC of individual HRD component score versus the clinically validated test was 0.85 (95% CI 0.77–0.92) for LOH, 0.86 (95% CI 0.79–0.93) for TAI, and 0.89 (95% CI 0.82–0.95) for LST. When assessed using Infinium CytoSNP-850K, the AUROC of HRD score versus the clinically validated test (cut-off 42) was 0.91 (95% CI 0.85–0.96); the AUROC of individual HRD component score versus the clinically validated test was 0.78 (95% CI 0.67–0.88) for LOH, 0.86 (95% CI 0.78–0.93) for TAI, and 0.95 (95% CI 0.90–0.99) for LST.
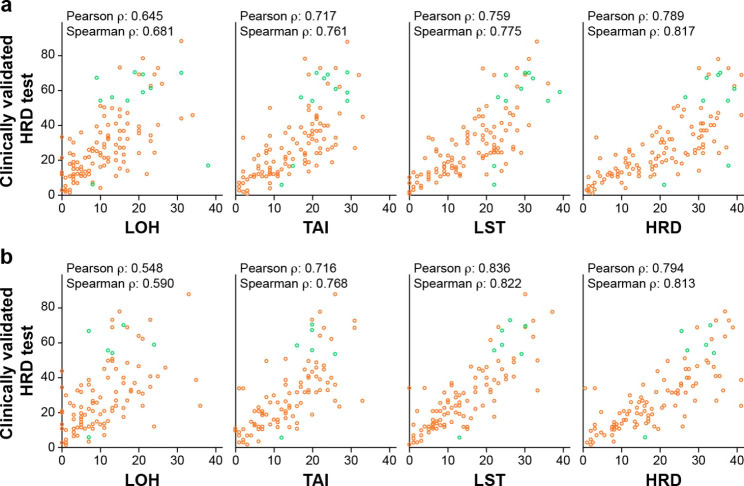
Correlation between genomic metrics as a continuous variable per OncoScan or Infinium CytoSNP-850K and the clinically validated HRD test
The genomic metric as a continuous variable with GC correction and without downsampling showed good correlation between the SNP genotyping assays and the clinically validated HRD test metric (Spearman ρ: OncoScan, 0.82 [95% CI 0.75–0.87] and Pearson ρ: OncoScan, 0.79, Fig. 3A; Infinium CytoSNP-850K, 0.81 [95% CI 0.74–0.87] and 0.79, Fig. 3B). Without GC correction, the correlation was poorer, particularly for HRD score derived on the Infinium CytoSNP-850K platform (Spearman ρ: OncoScan, 0.80 [95% CI 0.71–0.87]; Infinium CytoSNP-850K, 0.58 [95% CI 0.42–0.70]) (Supplementary Fig. S1). Among individual scores, LST had the highest correlation with HRD status for both OncoScan and Infinium CytoSNP-850K, consistent with the previously reported selection of LST as HRD score [29].
Fig. 3.
Correlation with GC correction between genomic metrics and the clinically validated HRD test. (A) OncoScan. (B) Infinium CytoSNP-850K. Orange circles indicate non–BRCAm tumors; green circles indicate deleterious BRCAm tumors. BRCAm, mutation in BRCA1, BRCA2, or both; HRD, homologous recombination deficiency; LOH, loss of heterozygosity; LST, large-scale state transition; TAI, telomeric-allelic imbalance number
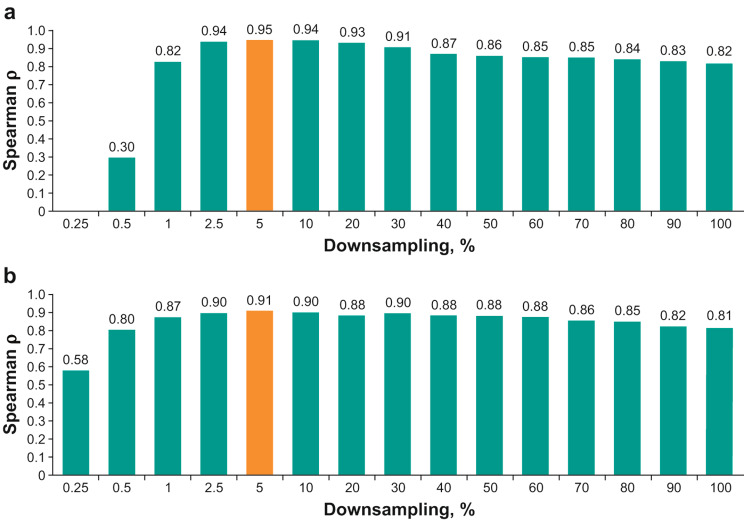
Another metric that can affect the accuracy of determining contiguous regions of genomic LOH is the density of SNPs; the use of too few SNPs may underestimate LOH by not using information from enough loci, whereas using too many SNPs may overestimate the LOH by fitting noisy neighboring loci. We evaluated the effect of downsampling on the accuracy of calculating the genomic metrics; HRD sum score concordance between the SNP array platforms and the clinically validated HRD test was thus further optimized by SNP downsampling (Fig. 4). The optimal (minimum) SNP downsampling factor (maximum correlation with the clinically validated HRD test while maintaining nearly full ASCAT evaluability) was 5% for both OncoScan (median Spearman ρ 0.95) and Infinium CytoSNP-850K (median Spearman ρ 0.91).
Fig. 4.
Correlation between HRD sum score by SNP genotyping assays and the clinically validated HRD test following SNP downsampling. (A) OncoScan. (B) Infinium CytoSNP-850K. HRD, homologous recombination deficiency; SNP, single-nucleotide polymorphism
Discussion
Given that HRD assessment has demonstrated both predictive and prognostic values for patients with advanced solid tumors receiving PARPis, including patients with ovarian cancer, several HRD testing strategies are being developed to identify patients likely to benefit from this treatment [2, 13, 30]. Despite the availability of several clinically validated companion HRD assays [2, 31], a gold standard HRD test has not been uniformly accepted, and currently available HRD tests face several limitations regarding more dynamic and more uniform testing harmonization. SNP-based strategies were the basis of most currently used HRD genomic assays that evaluated copy number, many of which use proprietary scoring methods and predefined cut-offs that have not been fully validated or adopted for use in clinical studies [2]. In the current study, common genomic metrics including HRD score as a continuous variable assessed by SNP genotyping assays (OncoScan; Infinium CytoSNP-850K) showed good correlation with the clinically validated HRD test metric. Notably, GC correction did not show much effect on OncoScan results. Composite scoring based on LOH, TAI, and LST and use of different cut-offs to evaluate HRD status have been assessed in several clinical studies [9, 11, 12, 15, 30, 32]. Such studies investigated the predictive value of various HRD testing strategies and the value of demonstrating clinically significant association between HRD status and survival benefits, particularly in patients with BRCAm tumors.
Differing HRD measures and cut-offs also pose an important challenge and require harmonization for guiding treatment decisions. For instance, one of the commonly used HRD tests is thought to use an unweighted sum of TAI, LST, and LOH and a dichotomous threshold using a cut-off of 42 based on a training cohort to determine high or low genomic instability score. Yet another common test uses NGS to determine high or low LOH with a predefined cut-off of ≥ 16%. The clinical utility for which various HRD tests are currently stratified further complicates harmonization. For some, but not all, tests, stratification is based on the test’s respective performance in predicting clinical benefit from PARPis rather than their absolute ability to detect HRD. Recent studies have suggested that combining scores from different tests may enhance HRD testing competence [2].
A key limitation shared by gene panel–based HRD tests and SNP-based arrays is that they provide a snapshot of past mutational processes and may not provide an accurate representation of the current activity of DNA repair mechanisms [2, 33]. None of the currently available DNA sequencing tests have the capability to assess the presence of known mechanisms of clinical resistance to PARPis [2]. Regarding limitations of the current analysis, the study was biased by selection of samples with known success toward DNA yield. In addition, we used samples that were available and which met our need and only reported analytical concordance without any clinical response data to PARPis.
Conclusion
To our knowledge, our study is the first to demonstrate that there is an optimal number of SNP markers used to calculate HRD metrics that maximizes associations with other HRD-related orthogonal markers, and that number is not the maximum on either platform, but rather a fraction. A possible explanation for these findings may be an abundance of SNP markers that would bring more noise for copy number segmentation, which is a fundamental part of HRD metrics calculation. Our study demonstrated that GC content correction had a significantly different effect on OncoScan and CytoSNP data; it also showed that the correlation between median GC content and log R ratio (a measure of total signal intensity) for each 1-Mb region of genome was much higher for CytoSNP than OncoScan data (Wilcoxon rank test, P < 0.01) [34]. This might be due to variable input DNA quantity for the CytoSNP assay (data not shown) compared with the OncoScan assay for which uniform DNA quantity was used, which was concordant with previous reports [35]. Taken together, tests based on commercially available SNP-based platforms may potentially be able to identify most HRD-positive tumors (as defined by clinically approved assays), as demonstrated by the high analytical concordance between OncoScan and CytoSNP-850K and the clinically validated test (> 0.90); however, appropriate clinically relevant cut-offs must be determined.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients and their families and caregivers as well as the primary investigators and site personnel for participating in each study. Medical writing and/or editorial assistance was provided by Dominic Singson, MD, and Lei Bai, PhD, of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Abbreviations
- AUROC
area under the receiver operating characteristic
- BRCAm
mutation in BRCA1, BRCA2, or both
- CNV
copy number variation
- DNA
deoxyribonucleic acid
- FDA
US Food and Drug Administration
- HGSOC
high-grade serous ovarian cancer
- HRD
homologous recombination deficiency
- LOH
loss of heterozygosity
- LST
large-scale state transition
- NGS
next-generation sequencing
- PARP
poly(adenosine diphosphate-ribose) polymerase
- PARPi
poly(adenosine diphosphate-ribose) polymerase inhibitor
- SNP
single-nucleotide polymorphism
- TAI
telomeric-allelic imbalance number
Authors’ contributions
RC, GA, PQ, and MJM contributed to the conception, design, or planning of the study. XQL, CC, and PQ contributed to the analysis of the data. RC, CC, AA, and PQ contributed to the acquisition of the data. RC, XQL, PQ, and MJM contributed to the interpretation of the results. RC, XQL, and MJM contributed to the drafting of the manuscript. RC, XQL, GA, CC, AA, PQ, and MJM contributed to the critical review or revision of the manuscript for important intellectual content. All authors approved the final manuscript.
Funding
Funding for this study was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and is part of an alliance between AstraZeneca and Merck & Co, Inc., Rahway, NJ, USA.
Data availability statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Declarations
Ethics approval and consent
All patients provided written informed consent before enrollment in the clinical trials. The study protocols and all amendments were approved by the institutional review board or ethics committee at each institution. The studies were conducted in accordance with the protocols, their amendments, the ethical principles originating from the Declaration of Helsinki and Good Clinical Practice guidelines.
Conflict of interest
RC is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and has stock ownership interests in Merck & Co., Inc., Rahway, NJ, USA.
XQL is an employee of MSD R&D (China) Co. Ltd., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
GA is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and has stock ownership interests in Merck & Co., Inc., Rahway, NJ, USA.
CC is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
AA is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
PQ is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and has stock ownership interests in Merck & Co., Inc., Rahway, NJ, USA.
MJM is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, has stock ownership interests in Merck & Co., Inc., Rahway, NJ, USA as well as Fulgent Genetics Inc and Q2 Holdings Inc.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bouberhan S, Philp L, Hill S, Al-Alem LF, Rueda B. Exploiting the prevalence of homologous recombination deficiencies in high-grade serous ovarian cancer. Cancers (Basel) 2020;12:1206. doi: 10.3390/cancers12051206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller RE, Leary A, Scott CL, et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann Oncol. 2020;31:1606–22. doi: 10.1016/j.annonc.2020.08.2102. [DOI] [PubMed] [Google Scholar]
- 3.Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 4.Thomas A, Murai J, Pommier Y. The evolving landscape of predictive biomarkers of response to PARP inhibitors. J Clin Invest. 2018;128:1727–30. doi: 10.1172/JCI120388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynparza (olaparib) tablets, for oral use. Prescribing information. AstraZeneca Pharmaceuticals LP; 2020.
- 6.Arora S, Balasubramaniam S, Zhang H, et al. FDA approval summary: olaparib monotherapy or in combination with bevacizumab for the maintenance treatment of patients with advanced ovarian cancer. Oncologist. 2021;26:e164–72. doi: 10.1002/onco.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myriad Genetic Laboratories Inc. myChoice CDx technical information. https://myriad-web.s3.amazonaws.com/myChoiceCDx/downloads/myChoiceCDxTech.pdf. Accessed 17 February 2022.
- 8.Hodgson DR, Dougherty BA, Lai Z, et al. Candidate biomarkers of PARP inhibitor sensitivity in ovarian cancer beyond the BRCA genes. Br J Cancer. 2018;119:1401–9. doi: 10.1038/s41416-018-0274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stronach EA, Paul J, Timms KM, et al. Biomarker assessment of HR deficiency, tumor brca1/2 mutations, and ccne1 copy number in ovarian cancer: associations with clinical outcome following platinum monotherapy. Mol Cancer Res. 2018;16:1103–11. doi: 10.1158/1541-7786.MCR-18-0034. [DOI] [PubMed] [Google Scholar]
- 10.Isakoff SJ, Mayer EL, He L, et al. TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015;33:1902–9. doi: 10.1200/JCO.2014.57.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 12.Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–28. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 13.González-Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 14.Sharma P, Barlow WE, Godwin AK, et al. Impact of homologous recombination deficiency biomarkers on outcomes in patients with triple-negative breast cancer treated with adjuvant doxorubicin and cyclophosphamide (SWOG S9313) Ann Oncol. 2018;29:654–60. doi: 10.1093/annonc/mdx821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–64. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 16.Friends of Cancer Research. HRD Harmonization Project. https://friendsofcancerresearch.org/hrd/. Accessed 19 September 2022.
- 17.Timms KM, Mills GB, Perry M, Gutin A, Lanchbury J, Brown R. Comparison of genomic instability test scores used for predicting PARP activity in ovarian cancer. J Clin Oncol. 2020;38:1586. doi: 10.1200/JCO.2020.38.15_suppl.1586. [DOI] [Google Scholar]
- 18.Telli ML, Jensen KC, Vinayak S, et al. Phase II study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer with assessment of a tumor-based measure of genomic instability: PrECOG 0105. J Clin Oncol. 2015;33:1895–901. doi: 10.1200/JCO.2014.57.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aurora-Garg D, Albright A, Qiu P, et al. Large-scale evaluation of concordance of genomic scores in whole exome sequencing and foundation medicine comprehensive genomic platform across cancer types. J Immunother Cancer. 2019;7:172. [Google Scholar]
- 20.Foundation Medicine Inc. FDA Summary of Safety and Effectiveness Data (SSED). Published 30 November 2017. Accessed 30 November 2022. https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170019B.pdf.
- 21.Leo A, Walker AM, Lebo MS, Hendrickson B, Scholl T, Akmaev VR. A GC-wave correction algorithm that improves the analytical performance of aCGH. J Mol Diagnos. 2012;14:550–9. doi: 10.1016/j.jmoldx.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Cheng J, Vanneste E, Konings P, Voet T, Vermeesch JR, Moreau Y. Single-cell copy number variation detection. Genome Biol. 2011;12:R80. doi: 10.1186/gb-2011-12-8-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Loo P, Nordgard SH, Lingjærde OC, et al. Allele-specific copy number analysis of tumors. Proc Natl Acad Sci U S A. 2010;107:16910–5. doi: 10.1073/pnas.1009843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marquard AM, Eklund AC, Joshi T, et al. Pan-cancer analysis of genomic scar signatures associated with homologous recombination deficiency suggests novel indications for existing cancer drugs. Biomark Res. 2015;3:9. doi: 10.1186/s40364-015-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abkevich V, Timms KM, Hennessy BT, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107:1776–82. doi: 10.1038/bjc.2012.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birkbak NJ, Wang ZC, Kim JY, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2012;2:366–75. doi: 10.1158/2159-8290.CD-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popova T, Manié E, Rieunier G, et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 2012;72:5454–62. doi: 10.1158/0008-5472.CAN-12-1470. [DOI] [PubMed] [Google Scholar]
- 28.Yin J, Vogel RL. Using the ROC curve to measure association and evaluate Prediction accuracy for a binary outcome. Biomet Biostat Internat J. 2017;5:95–103. [Google Scholar]
- 29.Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571:576–9. doi: 10.1038/s41586-019-1382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 31.Ngoi NYL, Tan DSP. The role of homologous recombination deficiency testing in ovarian cancer and its clinical implications: do we need it? ESMO Open. 2021;6:100144. doi: 10.1016/j.esmoop.2021.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–61. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noordermeer SM, van Attikum H. PARP inhibitor resistance: a tug-of-war in BRCA-mutated cells. Trends Cell Biol. 2019;29:820–34. doi: 10.1016/j.tcb.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Lin CF, Naj AC, Wang LS. Analyzing copy number variation using SNP array data: protocols for calling CNV and association tests. Curr Protoc Hum Genet. 2013;79:unit 1.27. doi: 10.1002/0471142905.hg0127s79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diskin SJ, Li M, Hou C, et al. Adjustment of genomic waves in signal intensities from whole-genome SNP genotyping platforms. Nucleic Acids Res. 2008;36:e126. doi: 10.1093/nar/gkn556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.