Abstract
The utility of pesticides in the agricultural field is unquestionable, but at the same time pesticide use presents serious hazards to the environment and the human health. For that reason, detection of pesticides and their biotransformation products in food is of utmost importance. According to previous studies, esterase‐based biosensors have been proposed as a viable and efficient solution for the detection of organophosphate pesticides. In this project, a double mutant of the thermostable esterase‐2 (EST2) from Alicyclobacillus acidocaldarius was studied as a potential biosensor, for its ability to detect residual amounts of pesticides. Initial characterisation of the enzyme was performed, that included determination of optimal pH, thermophilicity, as well as kinetic analysis. Subsequently, the enzyme was studied by enzymatic activity assays with and without the presence of various organophosphate compounds. The effect of the organophosphates on the enzymatic activity was measured and complete inhibition of the enzyme was observed after incubation with paraoxon. These experiments were followed by an additional method involving labelling of the enzyme with a fluorescent probe. In this case, the effect of different pesticides on the EST2 enzyme was monitored by measuring the fluorescence quenching upon addition to the enzyme. Fourteen compounds were screened with this method and significant fluorescence quenching was observed in the presence of paraoxon and methyl‐paraoxon when used in equimolar amounts with the enzyme in the range of nanomolar. This biosensor has been also used to test the presence of pesticides in real food samples, like fruits and juices. This research represents a starting point to develop effective fluorescence‐based biosensors aiming at the screening of mutants with different pesticide selectivity profiles. The use of this enzyme‐based biosensor can have applications in the field of food traceability as well as environmental monitoring, to control the presence of toxic chemicals, in particular organophosphate pesticides.
Keywords: EST2, biosensors, organophosphates, thermostable enzyme, fluorescence
1. Introduction
The use of pesticides in agricultural activity has become inevitable since the last century, but with that, the hazards to the entire ecosystem, including humans have increased. On top of the strict regulations imposed by European law, monitoring pesticide fate is always necessary. Especially in food, detection of pesticides and their biotransformation products is of utmost importance.
Organophosphate compounds (OPs) belong to a family of chemicals with widespread use as pesticides but at the same time present a hazard to human health by their accumulation in the environment and in food (Georgiadis et al., 2018). OPs act by irreversibly inhibiting acetylcholinesterase (AChE), thus interrupting the function of the pest nervous system. However, this mode of action is so ubiquitous that contact with OPs can affect many non‐target organisms including humans. Inhibition of AChE leads to accumulation of acetylcholine (Ach) at the synapses causing neurotoxicity followed by loss of metabolic balance which can ultimately cause death in absence of any effective prevention or treatment (Ranjan et al., 2018).
Traditional methods for the detection and quantification of OPs include gas chromatography (GC) and liquid chromatography coupled to mass spectrometry (LC–MS) (Park et al., 2021). Such methods can be highly sensitive and selective but on the other hand they are time consuming and require high levels of technical expertise to be applied. In recent years, attempts have been made for the development of fast, easy and cheap methodologies for the detection of pesticides, that are based on fluorescent biosensors (Carullo et al., 2015; Pundir et al., 2019). Towards that aim, cholinesterase (Pohanka et al., 2009) and carboxylesterase (Manco et al., 2009) biosensors have emerged during the last decades.
One such carboxylesterase, the thermostable esterase EST2 from the bacterium Alicyclobacillus acidocaldarius has recently been studied as a potential biosensor for the detection of OP pesticides (Febbraio et al., 2011). This enzyme is known to bind the OP paraoxon with high affinity (Febbraio et al., 2008) resulting in the irreversible inhibition of the enzymatic activity. This characteristic, combined with the tolerance of EST2 to high temperatures and the overall stability of the enzyme, make it a good candidate for biosensor studies. Previous studies have also investigated various mutants of EST2 for their properties (Pezzullo et al., 2013) and biosensor capabilities (Porzio et al., 2018).
In this project, we studied the double mutant of EST2, named here ‘2m‐EST2’. This version of the enzyme has been modified to carry a cystein at position 35, where the fluorophore IAEDANS can be conjugated, and an additional mutation that was studied for potential diverse selectivity towards OPs. The enzyme was characterised and optimal conditions were determined. A set of available pesticides including paraoxon and other OPs were tested for fluorescence quenching of the double mutant. Indeed, the presence of paraoxon was detectable by fluorescence with a very low limit od detection (LOD) value, making this a good starting point for rapid detection of OPs.
2. Description of work programme
2.1. Aims
The aim of the project was to develop and study an enzyme‐based biosensor in the field of food traceability and monitoring, to control the presence of toxic chemicals, particularly organophosphate pesticides, in foods. As part of this working programme, the fellow was planned to develop a fluorescence biosensor for fast and sensitive monitoring of pesticides in water and food samples.
To this aim, several activities were planned, including the bioreceptor preparation, the characterisation of the enzyme, followed by labelling of the enzyme with a fluorescent probe, the testing and evaluation of all the parameters for efficient detection of different pesticides, and finally, testing of the optimised biosensor in real samples, such as water and/or food. In addition, the work plan allowed the fellow to apply and improve expertise in the fields of biochemistry, molecular biology, microbiology and risk assessment.
2.2. Activities/Methods
2.2.1. Bioreceptor preparation: overexpression and purification
The enzyme studied in this project was the thermostable double mutant of the carboxylesterase EST2 from A. acidocaldarius, called ‘2m‐EST2’. Preparations of the enzyme were made starting from an existing plasmid vector pT7‐7, containing the mutated esterase gene, that was used to chemically transform E. coli BL21 (DE3) cells. Upon induction of protein production, the enzyme was purified from the bacterial culture by using different steps: lysis by sonication, thermoprecipitation, anion exchange and gel filtration chromatography. The purity and activity of the enzyme were monitored through every step of the preparation process by enzymatic activity assay and analysis on SDS–PAGE, showing a > 95% final purity.
2.2.2. Enzymatic activity assays
Enzymatic activity assays were implemented to characterise the enzyme. The assays were performed by using as substrate the carboxylester p‐nitrophenyl caprylate (pNP‐C8). The enzyme hydrolyses this compound, and the activity was detected by following the release of the product (p‐nitrophenol) at 405 nm at the spectrophotometer (Cary 100 spectrophotometer (Varian, Australia)).
Standard assays were performed at 25°C, and complete characterisation was performed to determine various important parameters of the enzyme, including: the optimal pH and the optimal temperature for the esterase activity; the specific activity value and the kinetic parameters in the optimal defined conditions.
The standard assay has been used to evaluate the inhibition effect of different pesticides on the esterase activity of the enzyme.
2.2.3. Labelling of the enzyme with a fluorescence probe and fluorescence spectroscopy analyses
Fluorescence is a very useful and powerful tool for the analysis of biological systems. In this project, it was used to detect changes of fluorescence signal caused by the presence of different pesticides in the sample. For this purpose, the 2m‐EST2 enzyme was conjugated with the fluorescent probe 5‐({2‐[(iodoacetyl)amino]ethyl}amino)naphthalene‐1‐sulfonic acid (IAEDANS) that specifically binds to the cysteine residues, Cys35 (Figure 1).
Figure 1.
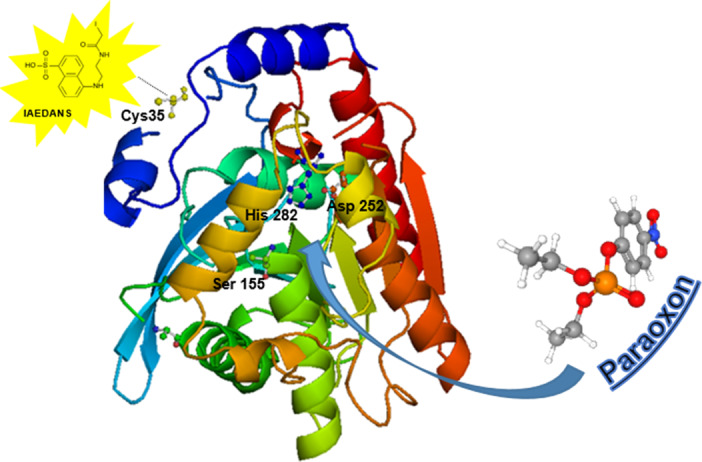
Crystal structure (pdb:1EVQ) of the thermophilic carboxylesterase EST2 from Alicyclobacillus acidocaldarius, with indication of the modified Cys35 residue where the fluorescence probe IAEDANS has been conjugated and the position where paraoxon interacts in the active site
After conjugation, the modified enzyme was purified by size exclusion chromatography to remove the excess of the unbound probe. The fluorescence of the labelled enzyme was measured by fluorescence spectroscopy using a spectrofluorimeter (Jasco‐FP‐8200). Measurements were recorded at emission wavelength of 472 nm, after excitation at 340 nm.
Subsequently, different concentrations of pesticides were added to the conjugated enzyme and new measurements were performed in order to determine the potential fluorescence quenching. An exhaustive list of all the available compounds that were used for these experiments can be found in Table A.1 (Appendix A).
Table A.1.
Complete list of the chemical compounds used for the fluorescence experiments of this project
| Name | MW (a) | Chemical structure | Preferable solvent (b) | |
|---|---|---|---|---|
| 1 | Glyphosate | 169.07 |

|
Water |
| 2 | Phosmet | 317.32 |

|
Organic solvents |
| 3 | Diazinon | 304.35 |

|
Organic solvents |
| 4 | Fensulfothion | 308.36 |

|
Water |
| 5 | Pirimiphos‐methyl | 305.33 |

|
Organic solvents |
| 6 | Dimethoate | 229.26 |

|
Water |
| 7 | Tolclofos‐methyl | 301.13 |

|
Organic solvents |
| 8 | Chlorpyriphos | 350.59 |

|
Organic solvents |
| 9 | Coumaphos | 362.77 |

|
Organic solvents |
| 10 | Parathion | 291.26 |

|
Organic solvents |
| 11 | Parathion‐methyl | 263.21 |

|
Organic solvents |
| 12 | Paraoxon‐methyl | 247.14 |

|
Organic solvents |
| 13 | Paraoxon‐ethyl | 275.2 |

|
Water |
| 14 | Famoxadone | 374.39 |

|
Organic solvents |
| 15 | Malathion | 330.36 |

|
Organic solvents |
| 16 | 4‐nitrophenol | 139.11 |

|
Water |
Molecular weight (g/mol).
For the compounds insoluble in water, acetonitrile was used as the solvent.
The inhibition detection method was further improved by performing a quicker analysis of pesticides screening on 96‐well plates, at a Microplate Reader (Victor Nivo, Pelkinelmer), at 25°C, by using two amounts of bioreceptor (80 and 160 pmoles). The specificity of the bioreceptor 2m‐EST2 towards oxo‐ and thio‐organophosphorus pesticides has been evaluated. Moreover, the enzyme, labelled and not, has been immobilised on PVDF membrane, and its stability has been evaluated over time.
2.2.4. Oxidation of thio‐organophosphorous pesticides
Some phosphorothionate compounds (including tolclofos, chlorpyrifos and parathion) were oxidised in the presence of N‐bromosuccinimide (NBS). The products of the oxidation reaction were subsequently tested both with fluorescence and with enzymatic activity assay, as oxidised compounds are expected to inhibit more efficiently the enzyme EST2.
3. Conclusions
The double mutant 2m‐EST2 of the carboxylesterase from A. acidocaldarius has been easily expressed and purified with high yields, by recovering an amount of 50 mg of pure enzyme starting from 4 L of bacterial culture. This amount was sufficient for all the experiments of this project, as the enzyme was quite stable over time, being fully active after more than 5 months. However, a second purification was performed for reproducibility purposes and for further confirmation of the results.
3.1. Characterisation of the enzyme
The characterisation of the 2m‐EST2 enzyme was performed to determine the optimal conditions for the carboxylesterase activity. As expected, since this is a thermophilic enzyme, the optimal temperature was determined to be 70°C. Additionally, the enzyme showed the best activity at optimal pH value of 7.0 in phosphate buffer.
Since the biosensor is intended for use at ambient temperature, the specific activity and the kinetic parameters were evaluated at both 70°C (optimal temperature) and 25°C (temperature of intended use). The observed kcat value at 70°C was 3 times higher, but the affinity towards the substrate pNP‐C8 was almost the same, around 15 μM, indicating that the active site maintains a good conformation at room temperature, compatible with its use as a biosensor.
3.2. Inhibition of enzymatic activity
The effect of various organophosphate compounds on the enzymatic activity of 2m‐EST2 was measured via enzymatic activity assays in the presence of the organophosphate pesticides paraoxon, parathion, tolclofos, chlorpyrifos and glyphosate. Paraoxon demonstrated complete inhibition of the enzyme, while no inhibition was observed for the remaining four pesticides (Figure 2).
Figure 2.
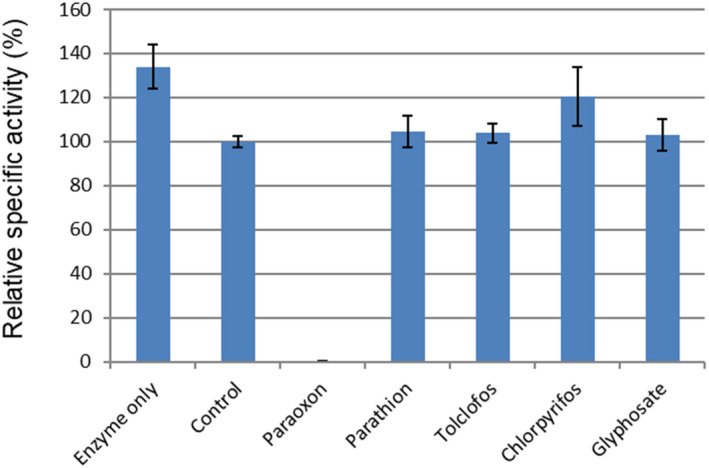
Comparative specific activity (%) relative to control assay containing 4% of corresponding solvent. Equimolar amounts of enzyme and organophosphate were incubated for 5 min at RT. After incubation, 3 pmol of enzyme were transferred into the assay mixture and activity was measured at 405 nm
3.3. Effect of the pesticides on fluorescence quenching
The enzyme was labelled with the fluorescent probe IAEDANS by means of a covalent bond with the residue Cys35. Upon this modification, the labelled enzyme was used to screen 16 different pesticides (presented in Appendix A) for their effect in fluorescence quenching, on a microplate reader. Paraoxon and methyl‐paraoxon displayed fluorescence quenching of 18 ± 1% and 14 ± 2% respectively, when used in equimolar amounts with the enzyme in the range of nanomolar. However, oxidation of thio‐OPs by NBS did not further improve the inhibition effect of these pesticides.
Therefore, a detailed fluorescence analysis was performed using paraoxon. A decrease in fluorescence intensity of the labelled enzyme was observed after paraoxon addition, demonstrating that the inhibition of 2m‐EST2 by paraoxon quenches its fluorescence, with addition of pesticide aliquots in the range from 20 to 200 pmol. A linear response was observed considering the ratio (I0/I) between the fluorescent intensity in the absence (I0) and presence (I) of increasing amounts of paraoxon. The estimated limit of detection (LOD) was relatively low, corresponding to 15 pmol of paraoxon.
3.4. Detection of pesticides in real food samples
The applicability of the developed biosensor was tested with real food samples (fruits and juice) as a model of food containing pesticides (Figure 3).
Figure 3.
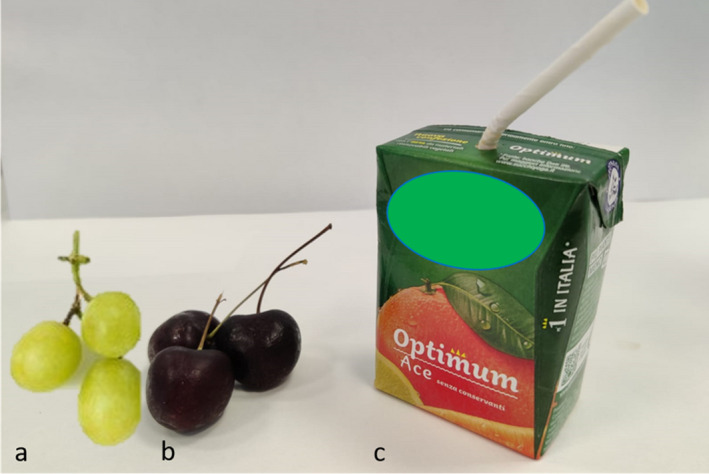
Real food samples used for testing model matrix by which pesticide can be extracted: (a) grapes; (b) cherries; (c) commercial juice. Paraoxon has been added and after extraction, the presence of pesticide has been detected by using the fluorescence‐labelled bioreceptor 2 m‐EST2
By using paraoxon as a pesticide, the compound was extracted in organic solvent from grapes, cherries and fruit juice, and the samples were analysed by using the thermostable bioreceptor 2m‐EST2‐IEADANS. The bioreceptor was able to detect the presence of pesticide in a range under 100 pmoles present in the foods. Detection was further confirmed by HPLC analysis.
3.5. Final conclusions
The labelled 2m‐EST2 enzyme maintains activity that is inhibited at different extents by some of the organophosphate pesticides tested. More specifically, paraoxon results in complete inhibition of the enzymatic activity when used at equimolar ratio. Fluorescence quenching was also observed and increased linearly with the amounts of paraoxon added, with a LOD of 15 pmol. Compared to the enzymatic activity assays, a method utilising fluorescence, such as the one presented here, provides very rapid detection making this research a starting point to develop effective fluorescence enzyme‐based biosensors. This kind of bioreceptor showed high sensitivity and can have applications in the field of food traceability as well as environmental monitoring, to control the presence of toxic chemicals, in particular organophosphate pesticides.
4. Other activities
For the duration of the EU‐FORA programme, the fellow participated in weekly laboratory meetings in the presence of supervisors and other colleagues from the host lab, by presenting as speaker her own experimental results. The purpose of those meetings was to present weekly experimental progress, discuss the projects of the group and receive feedback or guidance.
Additionally, the fellow had the opportunity to attend the 23rd Bologna Winter School. ‘Structural Bioinformatics in the era of AlphaFold2’, an online event organised by the University of Bologna that included lectures spanning between 9 and 25 February 2022.
Thanks to the EU‐FORA programme, the fellow was also able to present the results of her research at ‘The Biochemistry Global Summit’ in Lisbon, Portugal, between 9 and 14 July 2022. This was an international congress combining the 25th IUBMB Congress, the 46th FEBS Congress and the 15th PABMB Congress. The submitted abstract was presented as a poster with title ‘A thermostable esterase as a biosensor for rapid and sensitive detection of pesticides’.
5. Disclaimer
Detailed results obtained from the method development, sample analysis and risk assessment are not included in this report to avoid certain copyright claims, as these results are intended for subsequent publication in peer‐reviewed articles.
Abbreviations
- 2m‐EST2
double mutant of EST2
- EST2
esterase‐2
- IAEDANS
5‐({2‐[(iodoacetyl)amino]ethyl}amino)naphthalene‐1‐sulfonic acid
- LC–MS
liquid chromatography coupled to mass spectrometry
- LOD
limit of detection
- NBS
N‐Bromosuccinimide
- OP
organophosphate
- pNP‐C8
p‐nitrophenyl caprylate
Appendix A – List of pesticides used in this study
A detailed list of all the pesticides used in this study is presented below (Table A.1). All 16 compounds were used in fluorescence quenching experiments, while a subset of the compounds was also used for inhibition of enzymatic activity as described in Section 3.2.
Suggested citation: Garefalaki V, Manco G and Porzio E, 2022. Use of biosensors for rapid and sensitive detection of pesticides in food samples for food safety chemical risk assessment. EFSA 2022;20(S2):e200922, 10 pp. 10.2903/j.efsa.2022.e200922
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements This report is funded by EFSA as part of the EU‐FORA programme; grant GP/EFSA/AFSCO/2020/04 to E. Porzio (IBBC‐CNR).
Approved: 31 August 2022
References
- Carullo P, Cetrangolo GP, Mandrich L, Manco G and Febbraio F, 2015. Fluorescence spectroscopy approaches for the development of a real‐time organophosphate detection system using an enzymatic sensor. Sensors (Basel), 15, 3932–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio F, D'Andrea SE, Mandrich L, Merone L, Rossi M, Nucci R and Manco G, 2008. Irreversible inhibition of the thermophilic esterase EST2 from Alicyclobacillus acidocaldarius. Extremophiles, 12, 719–728. [DOI] [PubMed] [Google Scholar]
- Febbraio F, Merone L, Cetrangolo GP, Rossi M, Nucci R and Manco G, 2011. Thermostable esterase 2 from Alicyclobacillus acidocaldarius as biosensor for the detection of organophosphate pesticides. Analytical Chemistry, 83, 1530–1536. [DOI] [PubMed] [Google Scholar]
- Georgiadis G, Mavridis C, Belantis C, Zisis IE, Skamagkas I, Fragkiadoulaki I, Heretis I, Tzortzis V, Psathakis K, Tsatsakis A and Mamoulakis C, 2018. Nephrotoxicity issues of organophosphates. Toxicology, 406–407, 129–136. [DOI] [PubMed] [Google Scholar]
- Manco G, Nucci R and Febbraio F, 2009. Use of esterase activities for the detection of chemical neurotoxic agents. Protein Peptides Letters, 16, 1225–1234. [DOI] [PubMed] [Google Scholar]
- Park E, Lee J, Lee J, Lee J, Lee HS, Shin Y and Kim JH, 2021. Method for the simultaneous analysis of 300 pesticide residues in hair by LC‐MS/MS and GC‐MS/MS, and its application to biomonitoring of agricultural workers. Chemosphere, 277, 130215. [DOI] [PubMed] [Google Scholar]
- Pezzullo M, Del Vecchio P, Mandrich L, Nucci R, Rossi M and Manco G, 2013. Comprehensive analysis of surface charged residues involved in thermal stability in Alicyclobacillus acidocaldarius esterase 2. Protein Engineering, Design and Selection, 26, 47–58. [DOI] [PubMed] [Google Scholar]
- Pohanka M, Musilek K and Kuca K, 2009. Progress of biosensors based on cholinesterase inhibition. Current Medicinal Chemistry, 16, 1790–1798. [DOI] [PubMed] [Google Scholar]
- Porzio E, Bettazzi F, Mandrich L, Del Giudice I, Restaino OF, Laschi S, Febbraio F, De Luca V, Borzacchiello MG, Carusone TM, Worek F, Pisanti A, Porcaro P, Schiraldi C, De Rosa M, Palchetti I and Manco G, 2018. Innovative biocatalysts as tools to detect and inactivate nerve agents. Scientific Reports, 8, 13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pundir CS, Malik A and Preety , 2019. Bio‐sensing of organophosphorus pesticides: a review. Biosensors and Bioelectronics, 140, 111348. [DOI] [PubMed] [Google Scholar]
- Ranjan A, Chauhan A and Jindal T, 2018. In‐silico and in‐vitro evaluation of human acetylcholinesterase inhibition by organophosphates. Environmental Toxicology and Pharmacology, 57, 131–140. [DOI] [PubMed] [Google Scholar]


