Abstract
Coronavirus disease 2019 (COVID-19) booster vaccination has been implemented globally in the midst of surges in infection due to the Delta and Omicron variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The objective of the present study was to present a framework to estimate the proportion of the population that is immune to symptomatic SARS-CoV-2 infection with the Omicron variant (immune proportion) in Japan, considering the waning of immunity resulting from vaccination and naturally acquired infection. We quantified the decay rate of immunity against symptomatic infection with Omicron conferred by the second and third doses of COVID-19 vaccine. We estimated the current and future vaccination coverage for the second and third vaccine doses from February 17, 2021 to August 1, 2022 and used data on the confirmed COVID-19 incidence from February 17, 2021 to April 10, 2022. From this information, we estimated the age-specific immune proportion over the period from February 17, 2021 to August 1, 2022. Vaccine-induced immunity, conferred by the second vaccine dose in particular, was estimated to rapidly wane. There were substantial variations in the estimated immune proportion by age group because each age cohort experienced different vaccination rollout timing and speed as well as a different infection risk. Such variations collectively contributed to heterogeneous immune landscape trajectories over time and age. The resulting prediction of the proportion of the population that is immune to symptomatic SARS-CoV-2 infection could aid decision-making on when and for whom another round of booster vaccination should be considered. This manuscript was submitted as part of a theme issue on “Modelling COVID-19 and Preparedness for Future Pandemics”.
Keywords: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Vaccine effectiveness, Waning immunity, Mathematical model, Statistical model
1. Introduction
At present (October 2022), more than 2 years have passed since the World Health Organization (WHO) declared the global pandemic of coronavirus disease 2019 (COVID-19) (Cucinotta and Vanelli, 2020), and yet COVID-19 is still affecting our lives. Many countries initially implemented public health and social measures, such as lockdown and social distancing, because only non-specific countermeasures were available, and subsequently shifted their intervention strategies to incorporate vaccination from December 2020 (Haas et al., 2021, Hall et al., 2021, Mathieu et al., 2021, Thompson et al., 2021). The worldwide vaccination rollouts brought hope that the local epidemics could be suppressed more efficiently and regional or temporal herd immunity could be reached (Grauer et al., 2020). However, reports of breakthrough infections soon appeared (Bergwerk et al., 2021, Brown et al., 2021, CDC COVID-19 Vaccine Breakthrough Case Investigations Team, 2021, Juthani et al., 2021), and evidence suggests that the available vaccines have substantially lower effectiveness against the newly emerged variants, including the Delta variant (B.1.617) and later the Omicron variant (B.1.1.529), which quickly became the dominant variants worldwide in 2021 and early 2022, respectively (Andrews et al., 2022, Kahn et al., 2022, Loconsole et al., 2022, Lopez Bernal et al., 2021, Madhi et al., 2022, Mohapatra et al., 2022, Seppälä et al., 2021, Tseng et al., 2022). It was also found that vaccine-induced immunity wanes rapidly after the second and even third doses (Andrews et al., 2022, Ferdinands et al., 2022, Tseng et al., 2022). Out of concern for the evident waning immunity, some countries have already started offering a fourth dose of COVID-19 vaccine to increase the level of protection (Bar-On et al., 2022, Centers for Disease Control and Prevention (CDC), 2022, Health Security Agency, 2022).
Japan launched its COVID-19 vaccination campaign on February 17, 2021, mainly using the messenger (m)RNA vaccines BNT162b2 and mRNA-1273 (Sasanami et al., 2022a). Healthcare workers were prioritized as the first group to receive the vaccines. The eligible population was then expanded on April 12, 2021 to include people aged ≥ 65 years, and later was further expanded to include younger members of the population in a descending manner. Japan achieved high vaccination coverage; overall, more than 75% of the population received the second dose of COVID-19 vaccine, and approximately 95% of the people aged ≥ 65 years became fully vaccinated (Fig. 1 ) (Digital Digital Agency, 2022, Prime Minister’s Office of Japan, 2022). A third dose of mRNA COVID-19 vaccine became available on December 1, 2021 to boost the immunity among healthcare workers and people who had received the second dose more than 6 or 7 months before. As of May 2022, Japan is considering another round of boosters (i.e., a fourth COVID-19 vaccine dose) using mRNA vaccines for people aged ≥ 60 years or those who have underlying health conditions and received their third dose more than 5 months previously (Ministry of Health Labour and Welfare, 2022a).
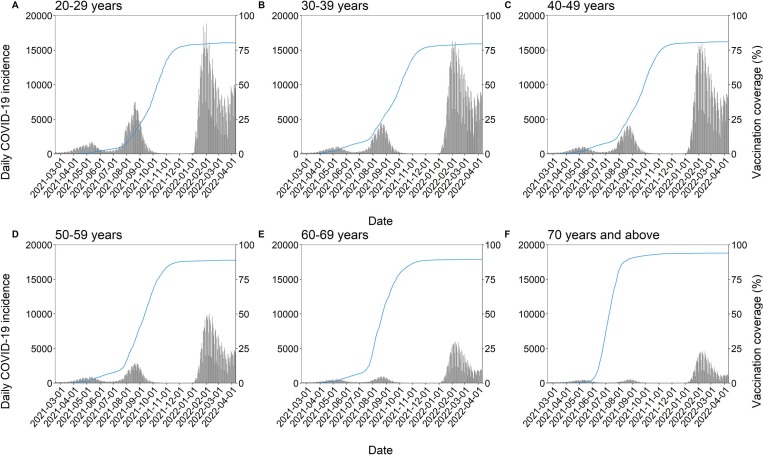
Fig. 1.
The age-specific number of confirmed COVID-19 cases and second vaccine dose coverage in Japan from February 17, 2021 to April 10, 2022. (A–F) The black bars show the confirmed COVID-19 incidence among people aged 20–29 (A), 30–39 (B), 40–49 (C), 50–59 (D), 60–69 (E), and ≥ 70 (F) years. The blue line represents the estimated second vaccine dose coverage; it should be noted that the age grouping of COVID-19 vaccination is slightly different from that of COVID-19 incidence, i.e., aged 15–24 (A), 25–34 (B), 35–44 (C), 45–54 (D), 55–64 (E), and ≥ 65 (F) years. The methods applied for estimating the second vaccine dose coverage are described elsewhere (Sasanami et al., 2022b), and we estimated the coverage until March 13, 2022, when the daily proportion of the population that was newly vaccinated in each age group became lower than 0.01% of the age-specific population and the coverage was deemed to have plateaued.
Japan has experienced a substantially lower number of COVID-19 cases and deaths compared with many Western countries (Ritchie et al., 2020). By the end of 2021, there was<5% of the cumulative risk of confirmed COVID-19 cases, and approximately 18,000 deaths in total had been reported as of May 16, 2022 (Ministry of Health Labour and Welfare, 2021a). However, after the Omicron variant became dominant in Japan, the country experienced its worst hit to date (sixth wave of the pandemic), with a maximum daily incidence reaching approximately 100,000 confirmed cases in early February 2022 (Fig. 1) (Ministry of Health Labour and Welfare, 2022b). This surge should have conferred naturally acquired immunity among infected individuals. The impacts of such natural infection-induced immunity in combination with the intrinsic waning effects of the second and third vaccine doses have complicated monitoring the immune landscape, and to our knowledge there is no study thus far that regularly estimates the population-level immunity against symptomatic infection considering these effects.
Here, we tackle the issue of reconstructing the fraction of the population that is immune to symptomatic SARS-CoV-2 infection over time and age. The objective of the present study was to present a framework that enables us to monitor the immune landscape, specifically the proportion of people who are immune to symptomatic SARS-CoV-2 infection (immune proportion), and incorporates the abovementioned complexities. We estimated the age-specific immune proportion, which is deemed more informative as opposed to the overall immune proportion for planning public health interventions or effective booster campaigns for additional vaccination rounds in the future, given the evidence of heterogeneities not only in contact patterns but also in risk in different age groups (Antonelli et al., 2022, Jordan et al., 2020, Lovell-Read et al., 2022). This study also accounts for the buildup and waning of vaccine-induced immunity after boosting, which should improve the likelihood of selecting an optimal initiation time, and the initiation timing of a vaccination rollout is key to its success (Gavish et al., 2022).
2. Materials and methods
2.1. Epidemiological data
2.1.1. Vaccination registry data
We obtained Vaccination Record System (VRS) data from the Ministry of Health, Labour and Welfare, which recorded the daily number of vaccinees and their ages as aggregated into 5-year cohorts, for the period from February 17, 2021 to April 10, 2022. We also obtained Vaccination System (V-SYS) data from the website of the Prime Minister’s Office of Japan, which recorded the daily total number of distributed COVID-19 vaccine doses (in contrast with the number of doses actually administered) (Prime Minister’s Office of Japan, 2022).
2.1.2. Confirmed COVID-19 incidence data
We obtained two different pieces of data from the Ministry of Health, Labour and Welfare (Ministry of Health Labour and Welfare, 2022c): (1) the daily number of newly confirmed COVID-19 cases (with no information on age), and (2) the weekly number of confirmed COVID-19 cases by sex and age. We retrieved subsets of data for the period of interest, which spanned from February 17, 2021 to April 10, 2022. We modified dataset (1) to categorize the data into six age groups (): 20–29, 30–39, 40–49, 50–59, 60–69, and ≥ 70 years by assuming that the age distribution of COVID-19 cases reported in dataset (2) is constant throughout the week. Furthermore, we assumed that the actual COVID-19 incidence (i.e., including unconfirmed cases) was four times higher than the number reported in dataset (1) (Sanada et al., 2022). Henceforth, “age group” refers to the abovementioned six age categories.
2.1.3. Vaccine effectiveness data
We used published data on the vaccine effectiveness of BNT162b2 against symptomatic infection with Delta and Omicron variants, estimated in a test-negative case–control study conducted in England (Andrews et al., 2022). The study provides estimates for the second and third vaccine dose over time since vaccination, showing the evidence of waning protection levels.
2.1.4. Statistics for the Japanese population
We obtained statistics for the Japanese population, including age-specific and prefecture-specific populations, from the Portal Site of the Official Statistics of Japan (Ministry of Internal Affairs and Communications, 2022).
2.2. Modelling the protected fraction of the population
2.2.1. Quantifying waning vaccine effectivenes
To describe the waning immunity against infectious diseases including COVID-19, various decay functions have been employed in published modelling studies (Feng et al., 2022, Hogan et al., 2021, Khoury et al., 2021, Nishiura et al., 2006), but there is no general consensus or evidence that suggests the most plausible model to be used. We therefore employed the simplest, exponential function to model the decaying vaccine effectiveness against symptomatic infection with the Omicron variant induced by the second and third vaccine doses as follows:
| (1) |
where represents the vaccine effectiveness at days after the second or third vaccine dose; represents the maximum vaccine effectiveness against symptomatic infection with Omicron, and regulates the speed of decay for immunity among vaccinees. Note that the spike in effectiveness shortly after receiving a dose of vaccine is omitted, and thus, is a decreasing function.
We estimated the parameters and by fitting the exponential function to the data from a published study that reports vaccine effectiveness estimates against symptomatic infection with the Omicron variant, via the maximum likelihood method assuming Gaussian distributed errors, as a function of time since immunization (Andrews et al., 2022). From the published study, we specifically retrieved estimates for the effectiveness of BNT162b2, which the vast majority of the Japanese population received (Prime Minister’s Office of Japan, 2022). We obtained 95% confidence intervals (CIs) via the parametric bootstrapping method. Furthermore, we assumed that infection-induced immunity wanes in a manner identical to that of the effectiveness of the third vaccine dose, although it was assumed to confer perfect protection immediately after infection ().
2.2.2. Estimating age-specific vaccination coverage for the second and third doses
We estimated age-specific second and third vaccine dose coverage, by conducting all the computations shown below for each of the aforementioned age cohorts (i.e., the age-specific vaccination coverage was used for the following calculations). Because more than 98% of people in Japan who were vaccinated with the first dose received the second dose (Prime Minister’s Office of Japan, 2022), here, we considered the effectiveness conferred from the second and third doses only.
2.2.2.1. Estimating second vaccine dose coverage
We calculated the uptake of the second vaccine dose, mainly using VRS data, while accounting for the reporting delay in the data. We also employed V-SYS data to complement the missing parts of the VRS data. A detailed explanation of this approach is provided elsewhere (Sasanami et al., 2022b).
2.2.2.2. Estimating third vaccine dose coverage
To address the right truncation of the observed vaccination coverage, we fitted a logistic function to the third vaccine dose coverage data to estimate time-dependent coverage until April 10, 2022 and to predict it through August 1, 2022:
| (2) |
where represents the vaccination coverage at calendar time , which is the elapsed time since the start of the booster vaccination campaign (i.e., December 1, 2021); represents the speed of increase in the vaccination coverage; is the eventual booster vaccination coverage; and represents the duration required for the coverage to reach 50% of its maximum (. For the age groups 20–29 years, 30–39 years, and 40–49 years, we fixed to be identical to the vaccination coverage for the second dose in each age group reported by the Prime Minister’s Office of Japan as of April 11, 2022 (Prime Minister’s Office of Japan, 2022), i.e., 80.0%, 79.9%, and 83.0%, respectively. We fixed these values because there were still too few people vaccinated to estimate all three parameters (i.e., , , and ) for these age cohorts. By contrast, for the older age groups of 50–59 years, 60–69 years, and ≥ 70 years, all the parameters were estimated from empirical data. The abovementioned maximum likelihood estimation was performed on these vaccine rollout data.
2.2.3. Estimating the immune proportion
We estimated the age-specific immune proportion, accounting for waning vaccine effectiveness following the second and third vaccine doses and infection-induced immunity.
First, we expressed the population who obtained immunity from vaccination or infection as follows:
| (3) |
where indicates either the second or third vaccine dose or natural infection; represents the number of people who newly received a vaccine dose or were infected at calendar time (as described in 2.2.2.1–2. and 2.1.2); is the number of immune populations at time who were vaccinated or infected days ago; and is the immune decay function estimated in subsection 2.1. Integration over the characteristic line gives the solution of the McKendrick equation (3), i.e.,
| (4) |
for t > s. Therefore, the total immune population at time , is:
We then computed the immune proportion by summing the number of people who were immune owing to vaccination and infection:
| (6) |
where the summation in the right-hand side of the equation computes the number of persons who are immune owing to the second or third vaccine dose or infection, and we converted this value into a fraction by dividing it by the age-specific population size, denoted as . It should be noted that here we made the assumption that people who had lost immunity from their second vaccine dose regained immunity from booster vaccination or natural infection. In other words, under this assumption, the people who remained immune to symptomatic infection after their second vaccine dose had not yet received a third vaccine dose or been infected. This assumption enabled the simple summation in equation (6) and was deemed reasonable given the rapidity of the waning protection against the Omicron variant provided by the second vaccine dose and Japan’s vaccination scheme in which people generally became eligible for booster vaccination 6 or 7 months after receiving their second vaccine dose. The CIs for the estimated immune proportion were computed using the two sets of 1,000-parameter samples gained from the estimation of waning immunity from the second and third vaccine doses.
Furthermore, we conducted sensitivity analyses in which three different scenarios were assumed. Because, to our knowledge, there is no established evidence regarding the waning rate of immunity from natural infection, the first two sensitivity analyses examined the impacts of different decay rates for infection-induced immunity. We assumed that the waning speed was: i) identical to that from the second vaccine dose; or ii) dependent on the dominant SARS-CoV-2 variant in circulation. For assumption ii), we applied the decay rate for the second and third vaccine doses to infection that occurred during the Delta- (until 31 December 2021) and Omicron- (1 January 2022 onward) dominant periods, respectively (Ministry of Health Labour and Welfare, 2021b). The third sensitivity analysis explored the scenario in which neither emergence of the Omicron variant nor a booster vaccination campaign had occurred. For this analysis, we first estimated the time-dependent vaccine effectiveness from the second vaccine dose against symptomatic infection with Delta, based on data from a published study (Andrews et al., 2022), and then computed the immune proportion using the same methods described above. The results of the sensitivity analyses are documented in the Supplementary material (https://github.com/nishiurah/immunelandscape).
2.3. Validation
To assess the validity of our estimates, we conducted a real-time analysis, investigating if there is an association between the epidemic dynamics and the immune proportion using prefecture-level data.
2.3.1. COVID-19 incidence trend in each prefecture
We collected data on the incidence of SARS-CoV-2 infection in each prefecture from the Japan Broadcasting Corporation (Nippon Hoso Kyokai; NHK) website (NHK, 2022). As a snapshot evaluation for the purposes of this study, we used data from February 7 to April 10, 2022, when the sixth wave of COVID-19 hit Japan, and calculated the prefecture-specific risk of infection per 100,000 people and then fitted it to a simple exponential growth model. If the estimated growth rate was positive, the prefecture was considered to have an increasing risk of infection, whereas if negative, it was deemed that the prefecture had a decreasing risk. The prefectures for which the estimated coefficient was not statistically significantly different from 0 ( were removed from the analysis.
2.3.2. Estimating the prefecture-specific immune proportion
We applied the aforementioned method of computing the immune landscape to estimate the prefecture-specific immune proportion from symptomatic illness. Because of the limited data availability, this analysis specifically considered the immunity gained from the third vaccine dose alone. We did not account for the second dose because of its small impact on preventing symptomatic infection from Omicron around the time period of interest and also because of the waning effect of infection-acquired immunity, which was assumed to be negligible given the short time period of interest.
2.3.3. Comparison of the mean immune proportions according to the risk trend
Subsequently, we conducted the Wilcoxon rank sum test to compare the mean prefecture-specific immune proportion on April 10, 2022 according to the upward or downward risk trend.
All analyses were conducted in R (version 4.2.0), and the data and codes are provided in the Supplementary files (https://github.com/nishiurah/immunelandscape). Note that vaccination data used here are provided in a publicly available form (downloaded from the website of the Prime Minister’s Office of Japan [Prime Minister’s Office of Japan, 2022]).
3. Results
Fig. 1 shows the second vaccine dose coverage and the total number of confirmed SARS-CoV-2 infections during the period from February 17, 2021 to April 10, 2022. The rate at which vaccination was conducted exceeded 1 million doses per day during the second dose program; this was set as the goal for the third vaccine dose campaign as well, and the goal was achieved in early March 2022.
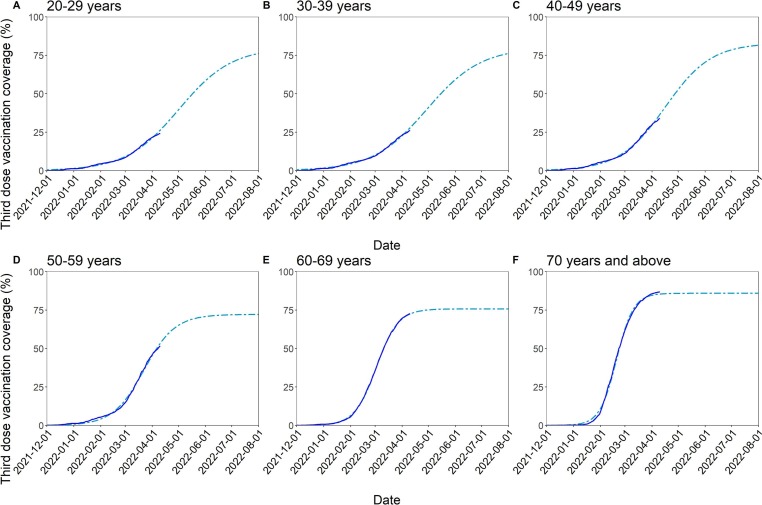
The most up-to-date coverage and the predicted booster vaccination coverage by age group are shown in Fig. 2 . The coverage started plateauing in the older age groups (specifically, the 60–69 and ≥ 70-year-old groups) at the time when our analysis was conducted. By contrast, it was estimated that it will take approximately another 4 months for the younger age groups to reach the maximum possible coverage if the vaccination speed remains the same.
Fig. 2.
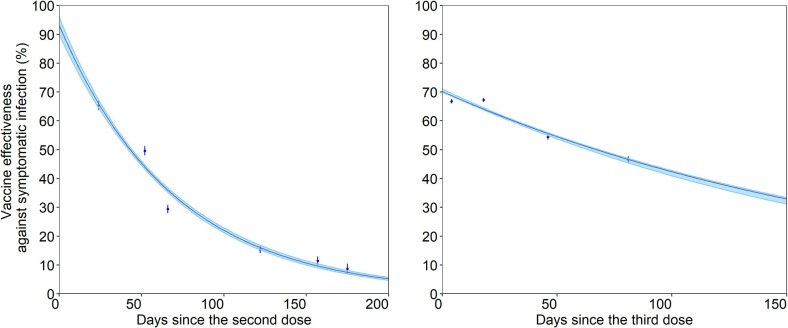
Estimated waning of vaccine-induced protection against symptomatic infection with the Omicron variant after the second (left) and third (right) vaccine doses. The blue lines show the estimated protected fraction, with the shaded area representing their 95% confidence intervals (CIs) derived by parametric bootstrapping. The points represent the empirically reported vaccine effectiveness against symptomatic infection, with error bars displaying their 95% CIs, according to a published study (Andrews et al., 2022).
The estimated waning vaccine effectiveness against symptomatic infection with the Omicron variant over time is shown in Fig. 3 . The parameters in the immune decay function (i.e., the maximum vaccine effectiveness) and (i.e., the waning rate of vaccine effectiveness) were estimated to be 0.93 (95% CI: 0.90–0.96) and 0.014 (95% CI: 0.014–0.015) for the second vaccine dose and 0.70 (95% CI: 0.70–0.71) and 0.0050 (95% CI: 0.0050–0.0055) for the third vaccine dose, respectively. The half-life of vaccine effectiveness was 50 days and 139 days for the second and third vaccine doses, respectively.
Fig. 3.
Reported and predicted booster vaccination coverage stratified by age group for December 1, 2021 to August 1, 2022. (A–F) The dark blue lines show the reported booster vaccination coverage until April 10, 2022, and the light blue dash-dotted line represents the projected booster vaccination coverage among people aged from 20 to 29 (A), 30–39 (B), 40–49 (C), 50–59 (D), 60–69 (E), and ≥ 70 (F) years.
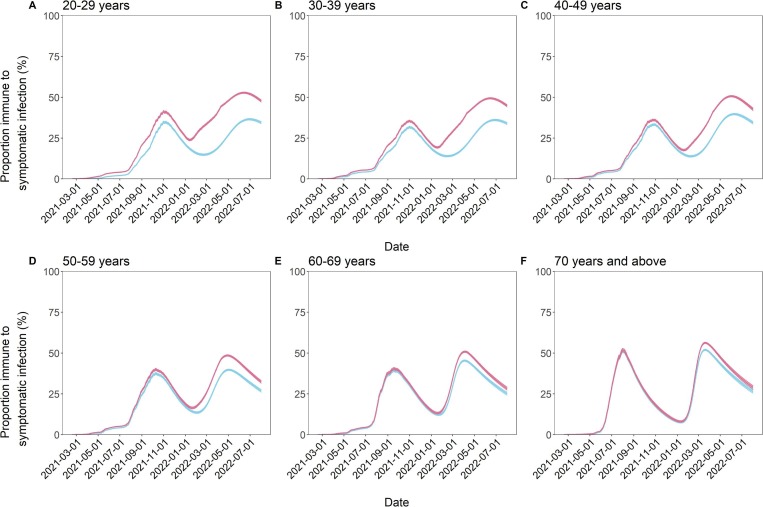
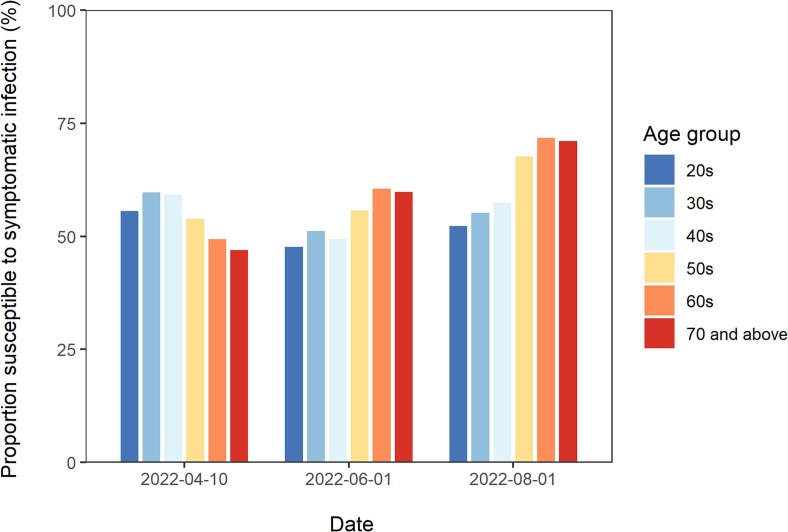
The estimated immune landscape notably varied by age group over the course of time (Fig. 4 and Table 1 ). The surge in COVID-19 cases substantially contributed to conferring immunity to the younger subset of the population. Such effects were most evident among those aged 20–29 years; by April 10, 2022, 21.0% (95% CI: 20.6–21.4) of this population was estimated to have acquired immunity solely from vaccination, but the addition of those who gained immunity from natural infection brought this value up to 44.4% (95% CI: 44.0–44.8). Incorporating infection-induced immunity, the immune proportion in this age group was predicted to be 52.3% (95% CI: 51.9–52.7) and 47.7% (95% CI: 47.1–48.4) on June 1 and August 1, 2022, respectively. The 30–39 and 40–49-year-old cohorts followed similar trends. By contrast, the immune proportions of the three older age groups were less impacted by infection. Among people aged ≥ 70 years, 48.6% (95% CI: 48.1–49.1) were estimated to have acquired immunity solely from vaccination, whereas the overall immune proportion (accounting for the effects of both vaccination and infection) in this group was 53.0% (95% CI: 52.5–53.5). As of April 10, 2022, a reduction in the immune proportion was already apparent, and the immune proportion was estimated to decline to 28.9% (95% CI: 27.9–30.0) by the summer of 2022.
Fig. 4.
Age-specific proportion of the population immune to symptomatic SARS-CoV-2 infection from February 17, 2021 to August 1, 2022. (A–F) The lines show the time-dependent proportion of people immune to symptomatic SARS-CoV-2 infection among those aged from 20 to 29 (A), 30–39 (B), 40–49 (C), 50–59 (D), 60–69 (E), and ≥ 70 (F) years, and the shaded areas are their 95% confidence intervals (CIs), computed via the bootstrapping method. The red lines reflect the scenarios in which the immunity from natural infection as well as the immunity from the second and third vaccine doses are considered, whereas the light blue lines reflect the scenarios in which only the immunity from the second and third vaccine doses is taken into account. The projection was performed on April 10, 2022, and the estimates afterward are from a model-based projection.
Table 1.
Age-specific proportion of the population susceptible to symptomatic SARS-CoV-2 infection on April 10, June 1, and August 1, 2022. The values in V and V + I represent the proportion of the population that is susceptible to symptomatic infection with the Omicron variant, considering immunity solely from vaccination (V) and immunity from both vaccination and natural infection (V + I), respectively. The projection was performed on April 10, 2022, and the estimates afterward are from a model-based projection. Numbers in parentheses show the 95% confidence intervals (CIs) as computed by the parametric bootstrap method.
| 2022/4/10 |
2022/6/1 |
2022/8/1 |
||||
|---|---|---|---|---|---|---|
| Age group | V (95% CI) | V + I (95% CI) | V (95% CI) | V + I (95% CI) | V (95% CI) | V + I (95% CI) |
| 20–29 | 79.0 (78.6–79.4) | 55.6 (55.2–56.0) | 65.7 (65.3–66.1) | 47.7 (47.3–48.1) | 65.5 (64.9–66.2) | 52.3 (51.6–52.9) |
| 30–39 | 78.9 (78.5–79.2) | 59.7 (59.3–60.1) | 65.9 (65.5–66.3) | 51.2 (50.8–51.6) | 66.0 (65.4–66.7) | 55.2 (54.5–55.9) |
| 40–49 | 73.8 (73.4–74.1) | 59.2 (58.8–59.6) | 60.5 (60.1–61.0) | 49.3 (48.9–49.8) | 65.6 (64.9–66.4) | 57.4 (56.6–58.2) |
| 50–59 | 63.7 (63.2–64.1) | 53.9 (53.5–54.3) | 63.2 (62.7–63.8) | 55.7 (55.2–56.3) | 73.2 (72.4–74.0) | 57.7 (57.3–58.9) |
| 60–69 | 54.9 (54.5–55.4) | 49.4 (48.9–49.9) | 64.8 (64.2–65.5) | 60.5 (59.9–61.3) | 74.9 (74.0–75.8) | 68.2 (66.8–68.5) |
| ≥70 | 51.4 (50.9–51.9) | 47.0 (46.5–47.5) | 63.3 (62.4–64.1) | 59.8 (59.0–60.7) | 73.6 (72.5–74.6) | 71.1 (70.0–72.1) |
A snapshot of the predicted immune landscape on April 10 (present time), June 1, and August 1, 2022 is presented in Fig. 5 . As indicated in Fig. 4, the susceptible proportion in the three younger age groups was estimated to decrease over the 4 months following April 10. However, it was estimated that there will be a considerable increase in the susceptible population among the older age groups. The specific values are listed in Table 1.
Fig. 5.
Predicted proportion of individuals susceptible to symptomatic SARS-CoV-2 infection on 10 April, 1 June 1, and August 1, 2022. The bars represent the proportion of individuals susceptible to symptomatic SARS-CoV-2 infection over time, according to the age groups 20–29, 30–39, 40–49, 50–59, 60–69, and ≥ 70 years, presented from left to right. The projection was performed on April 10, 2022, and the estimates afterward are from a model-based projection.
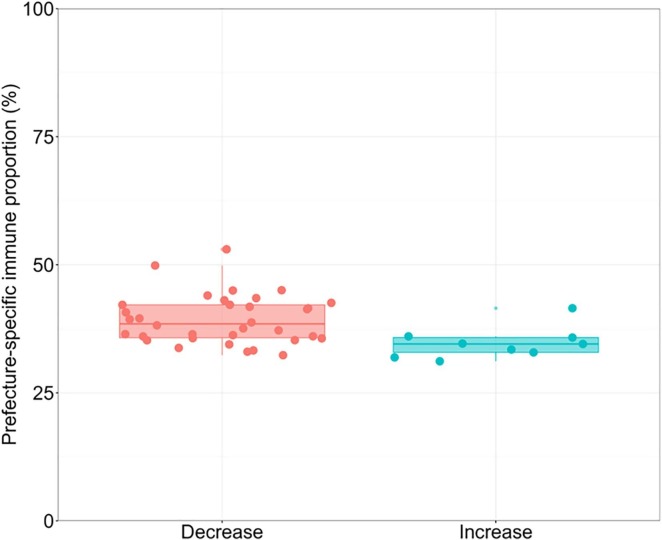
While abovementioned results dealt nationwide data as a single group, independent estimate of immune fraction was also obtained for each prefecture. Fig. 6 shows the prefecture-specific immune proportions grouped by epidemiological dynamics (i.e., by increasing or decreasing trend). The difference in the immune fraction against symptomatic infection between the two groups was 4.12% (95% CI: 1.39–7.64), indicating that the prefectures that had higher estimated immune proportions tended to be in the decreasing risk phase.
Fig. 6.
The prefecture-specific immune proportion according to the trend of infection with SARS-CoV-2. The box-plot shows the estimated immune proportion on April 10, 2022 among the prefectures that had decreasing and increasing risks of infection on the left and right, respectively, from February 7 to April 10, 2022. Each dot represents the estimated fraction immune in a single prefecture, and there are in total 47 prefectures in Japan. Grouping each prefecture as decreasing or increasing in its incidence was judged by the growth rate of incidence as on April 10, 2022 taking negative or positive values, respectively. The lines inside the box represent the median, and lower and upper box boundaries are the first and third quantiles, respectively. The whiskers indicate the interquartile range from the first and third quantiles.
4. Discussion
The present study presents the COVID-19 immune landscape in Japan, accounting for waning immunity from vaccination and natural infection. We quantified the decay rate of vaccine-induced protection against symptomatic infection, showing the dramatically rapid decrease in immunity against the Omicron variant among those who received only the second vaccine dose. As such, our results show that the estimated immune proportion has substantially declined over time since vaccination coverage plateaued. As expected, the immune proportion was boosted following the third vaccine rollout, although it was estimated that some older age groups have already begun experiencing a decrease in their booster dose-induced immunity. The recent surge in infection, caused by the Omicron variant in particular, contributed to a considerable increase in the immune proportion among young age groups compared with the older population. Our results predict that by August 2022, there will be a substantial increase in the susceptible proportion among the older age groups. Lastly, we showed that the estimated immune proportion was associated with the infection risk trend by comparing the growth trend by prefecture in Japan, as a simple validation of our overall analysis framework. Although previous studies have quantified waning immunity against infectious diseases (Feng et al., 2022, Hogan et al., 2021, Khoury et al., 2021, Nishiura et al., 2006), to our knowledge, this is the first study to report estimates of the immune proportion at the population-level.
We provide a framework that allows for estimation of the proportion of people that are immune to symptomatic SARS-CoV-2 infection in real time, accounting for prior immunity waning in the midst of expanding booster vaccination and a surge in infection. The half-life of the effectiveness of the second vaccine dose was estimated to be substantially shorter than that of the third dose and this dropped to < 10% in 6 months. This supports the decision made by many countries to encourage people to get a booster dose within no more than 6 months after receiving their second vaccine dose. The estimated rollout rate and goal (plateaued value) of booster vaccination coverage allowed us to estimate the current immune landscape in real time and to project the future trajectory. Such estimations could be particularly useful for countries in which the booster vaccine rollout has just begun. To deal with the great uncertainty regarding the effectiveness of infection-induced immunity, we examined different scenarios regarding the waning rate of infection-induced immunity. The results showed that different immunity decay rates could provide notably different immune landscapes over time. It was suggested that if the infection-induced immunity decays rapidly in the same manner as the second vaccine dose-induced immunity, the past surge in infection will not necessarily contribute to a substantial long-term gain in the immune proportion (see Supplementary material).
It was vital to account for the age group when estimating and projecting the trajectory of the immune landscape. Each age cohort experienced different vaccination coverage timings, rollout rates, and goals (plateaued values), as well as different risks of natural infection with SARS-CoV-2, and these factors collectively contributed to substantial variations in the age-specific immune proportion. The older age groups received a second vaccine dose earlier, and the vaccination coverage increased more quickly in these age groups compared with the younger age groups. They experienced many fewer cases of infection and thus had lower proportions of individuals who acquired immunity from infection, perhaps owing to risk awareness and more cautious behaviors among the individuals in these age groups, in response to the finding that older people are at greater risk of complications and death compared with younger individuals. These factors contributed to a more rapid increase in the immune proportion in the older age groups but also to a subsequent dramatic decrease. It was predicted that the immune fraction could decline to a low level of < 30% among people aged ≥ 70 years by the summer of 2022. Given that they were at higher risk of severe outcomes, the older generation were considered to be the priority in the next round of the booster vaccination campaign, and indeed, the government of Japan implemented fourth dose booster vaccinations for the older population and for those with underlying comorbidities. By contrast, although the younger population had lower vaccination coverage, a steep upsurge in COVID-19 incidence was observed, resulting in a higher immune fraction compared with the older population. Understanding the age-specific immune landscape could aid decision-making regarding the commencement timing and target age groups of future vaccination campaigns, while providing valuable insights into the COVID-19 transmission dynamics in a country.
There are some limitations in the present study. First, we did not consider the immune protection against severe illness and death, or the potentially different levels of immune response and decay rates of immunity according to age group because of a serious shortage of accumulated evidence regarding the effectiveness of this induced immunity and its duration against the Omicron variant, especially the variants-of-concern that emerged from the Omicron group (e.g., BA.4 and BA.5). Statistical estimation in this regard would be helpful, particularly when the main aim of booster vaccination is to prevent these outcomes (i.e., severe disease or death). Second, we imposed an assumption that the actual COVID-19 incidence is four times higher than the number of reported cases. Although this estimate rests on a published statistical estimate, the figure could be biased because the ascertainment rate can be expected to vary over time owing to the epidemic situation. For example, the ascertainment rate could depend on the testing and contact tracing capacity and the healthcare-seeking behaviors among the public (Sen et al., 2021); thus, the bias could have been elevated by a surge of cases with the Omicron variant. Third, although we estimated the future vaccination coverage and used these estimates to predict the immune proportions over the next 4 months, we did not make such predictions for infection-induced immunity, and thus, the fraction of the population that has immunity from naturally acquired infections should be deemed the minimum bound. The time-dependent proportion of individuals with infection-induced immunity reflects the number of individuals who were infected with SARS-CoV-2 at the time the analysis was conducted; if a substantial number of infections occur in the future, it could provide a very different immune landscape. Lastly, the lack of evidence limited our study as we did not consider those who gained immunity from both previous infections and following vaccinations, which recently appears to confer a greater neutralizing response than those who were immunized by a natural infection alone or vaccination alone.
Despite the limitations described above, the present study provides a simple and easily tractable framework that enables an estimation of the proportion of the population that was immune to symptomatic SARS-CoV-2 infection during the time that the booster vaccination coverage was rapidly increasing and the country was experiencing a surge of infection with the Omicron variant. In the future, the estimated decay rate of immunity and the projected age-specific immune proportions will aid our understanding of the possible risk groups and heterogeneous transmission dynamics of COVID-19 as well as our decision-making regarding when a rollout of booster vaccination should commence and the target population.
Funding sources
H.N. received funding from Health and Labour Sciences Research Grants (20CA2024, 20HA2007, 21HB1002, and 21HA2016); the Japan Agency for Medical Research and Development (JP20fk0108140, JP20fk0108535, and JP21fk0108612); the Japan Society for the Promotion of Science (JSPS) KAKENHI (21H03198 and 22K19670); the Environment Research and Technology Development Fund (JPMEERF20S11804) of the Environmental Restoration and Conservation Agency of Japan; Kao Health Science Research; Daikin GAP fund program of Kyoto University; and the Japan Science and Technology Agency SICORP program (JPMJSC20U3 and JPMJSC2105) and RISTEX program for Science of Science, Technology and Innovation Policy (JPMJRS22B4). T.K. received funding from the JSPS KAKENHI (21K10495), and K.H. received funding from the JSPS KAKENHI (20K18953) and The Health Care Science Institute (IKEN).
Declaration of competing interest
All authors declare no conflicts of interest with regards to this paper.
CRediT authorship contribution statement
Misaki Sasanami: . Marie Fujimoto: Validation. Taishi Kayano: . Katsuma Hayashi: . Hiroshi Nishiura: .
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We thank the local governments, public health centers, and institutes for surveillance, laboratory testing, epidemiological investigation, and data collection. We thank Katie Oakley, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. The funders had no role in the study design, data collection and analysis, the decision to publish, or preparation of this manuscript.
Ethical approval statement
The present study used publicly available data from the Ministry of Health, Labour and Welfare, and all data were de-identified prior to analysis. This study was approved by the Medical Ethics Board of the Graduate School of Medicine, Kyoto University (R2676).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtbi.2022.111384.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- N. Andrews J. Stowe F. Kirsebom S. Toffa T. Rickeard E. Gallagher C. Gower M. Kall N. Groves A.-M. O’Connell D. Simons P.B. Blomquist A. Zaidi S. Nash I.B.A. Aziz N., Thelwall, S., Dabrera, G., Myers, R., Amirthalingam, G., Gharbia, S., Barrett, J.C., Elson, R., Ladhani, S.N., Ferguson, N., Zambon, M., Campbell, C.N.J., Brown, K., Hopkins, S., Chand, M., Ramsay, M., Lopez Bernal, J., Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant New England Journal of Medicine 1–15 2022 10.1056/nejmoa2119451. [DOI] [PMC free article] [PubMed]
- Antonelli M., Penfold R.S., Merino J., Sudre C.H., Molteni E., Berry S., Canas L.S., Graham M.S., Klaser K., Modat M., Murray B., Kerfoot E., Chen L., Deng J., Österdahl M.F., Cheetham N.J., Drew D.A., Nguyen L.H., Pujol J.C., Hu C., Selvachandran S., Polidori L., May A., Wolf J., Chan A.T., Hammers A., Duncan E.L., Spector T.D., Ourselin S., Steves C.J. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Amir O., Freedman L., Alroy-Preis S., Ash N., Huppert A., Milo R. Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. N. Engl. J. Med. 2022;1–9 doi: 10.1056/nejmoa2201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., Mandelboim M., Levin E.G., Rubin C., Indenbaum V., Tal I., Zavitan M., Zuckerman N., Bar-Chaim A., Kreiss Y., Regev-Yochay G. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021;385:1474–1484. doi: 10.1056/nejmoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.M., Vostok J., Johnson H., Burns M., Gharpure R., Sami S., Sabo R.T., Hall N., Foreman A., Schubert P.L., Gallagher G.R., Fink T., Madoff L.C., Gabriel S.B., MacInnis B., Park D.J., Siddle K.J., Harik V., Arvidson D., Brock-Fisher T., Dunn M., Kearns A., Laney A.S. Outbreak of SARS-CoV-2 infections, including COVID-19 Vaccine breakthrough infections, associated with large public gatherings — Barnstable County, Massachusetts, July 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70:1059–1062. doi: 10.15585/mmwr.mm7031e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC COVID-19 Vaccine Breakthrough Case Investigations Team COVID-19 Vaccine Breakthrough Infections Reported to CDC —. MMWR Morb. Mortal. Wkly Rep. 2021;70:792–793. doi: 10.15585/mmwr.mm7021e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2022. CDC Recommends Additional Boosters for Certain Individuals [WWW Document]. URL https://www.cdc.gov/media/releases/2022/s0328-covid-19-boosters.html#:∼:text=“Today%2C CDC expanded eligibility for,to increase their protection further.
- Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomedica. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digital Agency, 2022. COVID-19 vaccination data [WWW Document]. URL https://info.vrs.digital.go.jp/dashboard/#overview.
- Feng, A., Obolski, U., Stone, L., He, D., 2022. Modelling COVID-19 Vaccine Breakthrough Infections in Highly Vaccinated Israel – the effects of waning immunity and third vaccination dose. medRxiv, https://doi.org/10.1101/2022.01.08.22268950. [DOI] [PMC free article] [PubMed]
- Ferdinands J.M., Rao S., Dixon B.E., Mitchell P.K., DeSilva M.B., Irving S.A., Lewis N., Natarajan K., Stenehjem E., Grannis S.J., Han J., McEvoy C., Ong T.C., Naleway A.L., Reese S.E., Embi P.J., Dascomb K., Klein N.P., Griggs E.P., Konatham D., Kharbanda A.B., Yang D.-H., Fadel W.F., Grisel N., Goddard K., Patel P., Liao I.-C., Birch R., Valvi N.R., Reynolds S., Arndorfer J., Zerbo O., Dickerson M., Murthy K., Williams J., Bozio C.H., Blanton L., Verani J.R., Schrag S.J., Dalton A.F., Wondimu M.H., Link-Gelles R., Azziz-Baumgartner E., Barron M.A., Gaglani M., Thompson M.G., Fireman B. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance — VISION Network, 10 States, Aug. MMWR Morb. Mortal. Wkly Rep. 2022;71:255–263. doi: 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavish N., Yaari R., Huppert A., Katriel G. Population-level implications of the Israeli booster campaign to curtail COVID-19 resurgence. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.abn9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grauer J., Löwen H., Liebchen B. Strategic spatiotemporal vaccine distribution increases the survival rate in an infectious disease like Covid-19. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-78447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., Brooks N., Smaja M., Mircus G., Pan K., Southern J., Swerdlow D.L., Jodar L., Levy Y., Alroy-Preis S. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, Victoria Jane, Foulkes, Sarah, Saei, Ayoub, Andrews, Nick, Oguti, Blanche, Charlett, Andre, Wellington, Edgar, Stowe, J., Gillson, Natalie, Atti, Ana, Islam, Jasmin, Karagiannis, Ioannis, Munro, Katie, Khawam, Jameel, Chand, Meera A., Brown, Colin S., Ramsay, Mary, Lopez-Bernal, Jamie, Hopkins, Susan, Andrews, N., Atti, A., Aziz, H., Brooks, T., Brown, C. S., Camero, D., Carr, C., Chand, M. A., Charlett, A., Crawford, H., Cole, M., Conneely, J., D’Arcangelo, S., Ellis, J., Evans, S., Foulkes, S., Gillson, N., Gopal, R., Hall, L., Hall, V. J., Harrington, P., Hopkins, S., Hewson, J., Hoschler, K., Ironmonger, D., Islam, J., Kall, M., Karagiannis, I., Kay, O., Khawam, J., King, E., Kirwan, P., Kyffin, R., Lackenby, A., Lattimore, M., Linley, E., Lopez-Bernal, J., Mabey, L., McGregor, R., Miah, S., Monk, E.J.M., Munro, K., Naheed, Z., Nissr, A., O’Connell, A.M., Oguti, B., Okafor, H., Organ, S., Osbourne, J., Otter, A., Patel, M., Platt, S., Pople, D., Potts, K., Ramsay, M., Robotham, J., Rokadiya, S., Rowe, C., Saei, A., Sebbage, G., Semper, A., Shrotri, M., Simmons, R., Soriano, A., Staves, P., Taylor, S., Taylor, A., Tengbe, A., Tonge, S., Vusirikala, A., Wallace, S., Wellington, E., Zambon, M., Corrigan, D., Sartaj, M., Cromey, L., Campbell, S., Braithwaite, K., Price, L., Haahr, L., Stewart, S., Lacey, E.D., Partridge, L., Stevens, G., Ellis, Y., Hodgson, H., Norman, C., Larru, B., Mcwilliam, S., Roynon, A., Northfield, J., Winchester, S., Cieciwa, P., Pai, A., Bakker, P., Loughrey, C., Watt, A., Adair, F., Hawkins, A., Grant, A., Temple-Purcell, R., Howard, J., Slawson, N., Subudhi, C., Davies, S., Bexley, A., Penn, R., Wong, N., Boyd, G., Rajgopal, A., Arenas-Pinto, A., Matthews, R., Whileman, A., Laugharne, R., Ledger, J., Barnes, T., Jones, C., Osuji, N., Chitalia, N., Bailey, T., Akhtar, S., Harrison, G., Horne, S., Walker, N., Agwuh, K., Maxwell, V., Graves, J., Williams, S., O’Kelly, A., Ridley, P., Cowley, A., Johnstone, H., Swift, P., Democratis, J., Meda, M., Brake, S., Gunn, J., Selassi, A., Hams, S., Irvine, V., Chandrasekaran, B., Forsyth, C., Radmore, J., Thomas, C., Brown, K., Roberts, S., Burns, P., Gajee, K., Lewis, T., Byrne, T.M., Sanderson, F., Knight, S., Macnaughton, E., Burton, B.J.L., Smith, H., Chaudhuri, R., Aeron-Thomas, J., Hollinshead, K., Shorten, R.J., Swan, A., Favager, C., Murira, J., Baillon, S., Hamer, S., Shah, A., Russell, J., Brennan, D., Dave, A., Chawla, A., Westwell, F., Adeboyeku, D., Papineni, P., Pegg, C., Williams, M., Ahmad, S., Horsley, A., Gabriel, C., Pagget, K., Maloney, G., Ashcroft, J., Del Rosario, I., Crosby-Nwaobi, R., Flanagan, D., Dhasmana, D., Fowler, S., Cameron, E., Prentice, L., Sinclair, C., Bateman, V., McLelland-Brooks, K., Ho, A., Murphy, M., Cochrane, A., Gibson, A., Black, K., Tempeton, K., Donaldson, S., Coke, L., Elumogo, N., Elliott, J., Padgett, D., Cross, A., Mirfenderesky, M., Joyce, S., Sinanovic, I., Howard, M., Cowling, P., Brazil, M., Hanna, E., Abdelrazik, A., Brand, S., Sheridan, E.A., Wadams, B., Lloyd, A., Mouland, J., Giles, J., Pottinger, G., Coles, H., Joseph, M., Lee, M., Orr, S., Chenoweth, H., Browne, D., Auckland, C., Lear, R., Mahungu, T., Rodger, A., Warren, S., Brooking, D., Pai, S., Druyeh, R., Smith, E., Stone, S., Meisner, S., Delgado, D., Underhill, E., Keen, L., Aga, M., Domingos, P., Gormley, S., Kerrison, C., Birch, S., DeSilva, T., Allsop, L., Ambalkar, S., Beekes, M., Jose, S., Tomlinson, J., Painter, S., Price, C., Pepperell, J., James, K., Trinick, T., Moore, L., Day, J., Boulos, A., Knox, I., Defever, E., McCracken, D., Gray, K., Houston, A., Planche, T., Pritchard Jones, R., Wycherley, D., Bennett, S., Marrs, J., Nimako, K., Stewart, B., Bain, S.C., Kalakonda, N., Khanduri, S., Ashby, A., Holden, M., Mahabir, N., Harwood, J., Payne, B., Court, K., White, N., Longfellow, R., Hughes, L.E., Green, M.E., Halkes, M., Mercer, P., Roebuck, A., Wilson-Davies, E., Gallego, L., Lazarus, R., Aldridge, N., Berry, L., Game, F., Reynolds, T., Holmes, C., Wiselka, M., Higham, A., Booth, M., Duff, C., Alderton, J., Hilton, D., Powell, J., Jackson, A., Plant, A.J., Ahmed, N., Chin, T., Qazzafi, M.Z., Moody, A.M., Tilley, R.E., Donaghy, T., O’Kane, M., Shipman, K., Sierra, R., Parmar, C., Mills, G., Harvey, D., Huang, Y.W.J., Birch, J., Robinson, L., Board, S., Broadley, A., Laven, C., Todd, N., Eyre, D.W., Jeffery, K., Dunachie, S., Duncan, C., Klenerman, P., Turtle, L., Baxendale, H., Heeney, J.L., 2021. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. The Lancet 397, 1725–1735. https://doi.org/10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed]
- UK Health Security Agency, 2022. Almost 80% of eligible over-75s receive spring booster [WWW Document].
- Hogan, A.B., Wu, S.L., Doohan, P., Watson, O.J., Winskill, P., Charles, G., Barnsley, G., Riley, E.M., Khoury, D.S., Ferguson, N.M., Ghani, A.C., 2021. Report 48 - The value of vaccine booster doses to mitigate the global impact of the Omicron SARS-CoV-2 variant.
- Jordan R.E., Adab P., Cheng K.K. Covid-19: Risk factors for severe disease and death. The BMJ. 2020;368:1–2. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- Juthani P.V., Gupta A., Borges K.A., Price C.C., Lee A.I., Won C.H., Chun H.J. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect. Dis. 2021;21:1485–1486. doi: 10.1016/S1473-3099(21)00558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn F., Bonander C., Moghaddassi M., Rasmussen M., Malmqvist U., Inghammar M., Björk J. Risk of severe COVID-19 from the Delta and Omicron variants in relation to vaccination status, sex, age and comorbidities - surveillance results from southern Sweden, July 2021 to January 2022. Euro Surveill. 2022;27:1–6. doi: 10.2807/1560-7917.ES.2022.27.9.2200121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Loconsole, D., Centrone, F., Sallustio, A., Accogli, M., Casulli, D., Sacco, D., Zagaria, R., Morcavallo, C., Chironna, M., 2022. Characteristics of the First 284 Patients Infected with the SARS-CoV-2 Omicron BA . 2 Subvariant at a Single Center in the Apulia Region of Italy , January – March 2022 4–11. [DOI] [PMC free article] [PubMed]
- Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., Myers R., Campbell C.N.J., Amirthalingam G., Edmunds M., Zambon M., Brown K.E., Hopkins S., Chand M., Ramsay M. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021;385:585–594. doi: 10.1056/nejmoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell-Read F.A., Shen S., Thompson R.N. Estimating local outbreak risks and the effects of non-pharmaceutical interventions in age-structured populations: SARS-CoV-2 as a case study. J. Theor. Biol. 2022;535 doi: 10.1016/j.jtbi.2021.110983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhi S.A., Kwatra G., Myers J.E., Jassat W., Dhar N., Mukendi C.K., Nana A.J., Blumberg L., Welch R., Ngorima-Mabhena N., Mutevedzi P.C. Population Immunity and Covid-19 Severity with Omicron Variant in South Africa. N. Engl. J. Med. 2022 doi: 10.1056/nejmoa2119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., Giattino C., Rodés-Guirao L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021;5:947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- Ministry of Health Labour and Welfare The situation analysis on COVID-19 and response from MHLW (2021/12/31) [WWW Document] 2021 https://www.mhlw.go.jp/stf/newpage_23137.html.
- Ministry of Health Labour and Welfare, 2021b. Advisory board for COVID-19 control, 65th [WWW Document]. URL https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00294.html.
- Ministry of Health Labour and Welfare, 2022a. Notice about fourth dose vaccine.
- Ministry of Health Labour and Welfare, 2022b. Open data [WWW Document]. URL https://www.mhlw.go.jp/stf/covid-19/open-data.html.
- Ministry of Health Labour and Welfare, 2022c. Visualizing the data: information on COVID-19 infections [WWW Document]. URL https://covid19.mhlw.go.jp/en/.
- Ministry of Internal Affairs and Communications, 2022. Portal Site of Official Statistics of Japan (e-Stat) [WWW Document]. URL https://www.e-stat.go.jp/en/about.
- Mohapatra R.K., Tiwari R., Sarangi A.K., Sharma S.K., Khandia R., Saikumar G., Dhama K. Twin combination of Omicron and Delta variants triggering a tsunami wave of ever high surges in COVID-19 cases: A challenging global threat with a special focus on the Indian subcontinent. J. Med. Virol. 2022;94:1761–1765. doi: 10.1002/jmv.27585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhk SARS-CoV-2 [WWW Document] 2022. https://www3.nhk.or.jp/news/special/coronavirus/ accessed 9.24.22.
- Nishiura H., Schwehm M., Eichner M. Still protected against smallpox? Estimation of the duration of vaccine-induced immunity against smallpox. Epidemiology. 2006;17:576–581. doi: 10.1097/01.ede.0000229196.41862.c2. [DOI] [PubMed] [Google Scholar]
- Prime Minister’s Office of Japan About COVID-19 vaccination [WWW Document] 2022 https://www.kantei.go.jp/jp/headline/kansensho/vaccine.html.
- Ritchie, H., Mathieu, E., Rodés-Guirao, L., Appel, C., Giattino, C., Ortiz-Ospina, E., Hasell, J., Macdonald, B., Beltekian, D., Roser, M., 2020. Coronavirus Pandemic (COVID-19) [WWW Document]. URL https://ourworldindata.org/coronavirus#coronavirus-country-profiles (accessed 4.1.22).
- Sanada T., Honda T., Yasui F., Yamaji K., Munakata T., Yamamoto N., Kurano M., Matsumoto Y., Kohno R., Toyama S., Kishi Y., Horibe T., Kaneko Y., Kakegawa M., Fukui K., Kawamura T., Daming W., Qian C., Xia F., He F., Yamasaki S., Nishida A., Harada T., Higa M., Tokunaga Y., Takagi A., Itokawa M., Kodama T., Kohara M. Serologic Survey of IgG Against SARS-CoV-2 Among Hospital Visitors Without a History of SARS-CoV-2 Infection in Tokyo, 2020–2021. J. Epidemiol. 2022;32:105–111. doi: 10.2188/jea.JE20210324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasanami M., Kayano T., Nishiura H. The number of COVID-19 clusters in healthcare and elderly care facilities averted by vaccination of healthcare workers in Japan, February – June 2021. Math. Biosci. Eng. 2022;19:2762–2773. doi: 10.3934/mbe.2022126. [DOI] [PubMed] [Google Scholar]
- Sasanami M., Kayano T., Nishiura H. Monitoring the COVID-19 immune landscape in Japan. Int. J. Infect. Dis. 2022;122:300–306. doi: 10.1016/j.ijid.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen P., Yamana T.K., Kandula S., Galanti M., Shaman J. Burden and characteristics of COVID-19 in the United States during 2020. Nature. 2021;598:338–341. doi: 10.1038/s41586-021-03914-4. [DOI] [PubMed] [Google Scholar]
- Seppälä E., Veneti L., Starrfelt J., Danielsen A.S., Bragstad K., Hungnes O., Taxt A.M., Watle S.V., Meijerink H. Vaccine effectiveness against infection with the delta (b.1.617.2) variant, norway, april to august 2021. Eurosurveillance. 2021;26:1–6. doi: 10.2807/1560-7917.ES.2021.26.35.2100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M.G., Burgess J.L., Naleway A.L., Tyner H.L., Yoon S.K., Meece J., Olsho L.E.W., Caban-Martinez A.J., Fowlkes A., Lutrick K., Kuntz J.L., Dunnigan K., Odean M.J., Hegmann K.T., Stefanski E., Edwards L.J., Schaefer-Solle N., Grant L., Ellingson K., Groom H.C., Zunie T., Thiese M.S., Ivacic L., Wesley M.G., Lamberte J.M., Sun X., Smith M.E., Phillips A.L., Groover K.D., Yoo Y.M., Gerald J., Brown R.T., Herring M.K., Joseph G., Beitel S., Morrill T.C., Mak J., Rivers P., Harris K.M., Hunt D.R., Arvay M.L., Kutty P., Fry A.M., Gaglani M. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers — Eight U.S. Locations, December 2020–March. MMWR Morb. Mortal. Wkly Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng H.F., Ackerson B.K., Luo Y., Sy L.S., Talarico C.A., Tian Y., Bruxvoort K.J., Tubert J.E., Florea A., Ku J.H., Lee G.S., Choi S.K., Takhar H.S., Aragones M., Qian L. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med. 2022;19 doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.