Abstract
The Precision Interventions for Severe and/or Exacerbation-Prone Asthma clinical trials network is actively assessing novel treatments for severe asthma during the coronavirus disease (COVID-19) pandemic and has needed to adapt to various clinical dilemmas posed by the COVID-19 pandemic. Pharmacologic interactions between established asthma therapies and novel drug interventions for COVID-19 infection, including antivirals, biologics, and vaccines, have emerged as a critical and unanticipated issue in the clinical care of asthma. In particular, impaired metabolism of some long-acting beta-2 agonists by the cytochrome P4503A4 enzyme in the setting of antiviral treatment using ritonavir-boosted nirmatrelvir (NVM/r, brand name Paxlovid) may increase risk for adverse cardiovascular events. Although available data have documented the potential for such interactions, these issues are largely unappreciated by clinicians who treat asthma, or those dispensing COVID-19 interventions in patients who happen to have asthma. Because these drug-drug interactions have not previously been relevant to patient care, clinicians have had no guidance on management strategies to reduce potentially serious interactions between treatments for asthma and COVID-19. The Precision Interventions for Severe and/or Exacerbation-Prone Asthma network considered the available literature and product information, and herein share our considerations and plans for treating asthma within the context of these novel COVID-19–related therapies.
Key words: Asthma, ritonavir, COVID-19, salmeterol, cytochrome P450, interaction, CYP3A4, long-acting beta-adrenergic agonist, corticosteroids
The Precision Interventions for Severe and/or Exacerbation-Prone Asthma (PrecISE Asthma) network, sponsored by the National Heart, Lung, and Blood Institute, is actively conducting phase II/proof-of-concept clinical trials of precision interventions in patients with severe and exacerbation-prone asthma.1 In this protocol, participants in the study cohort use, as part of their baseline controller therapy for asthma, combination inhalers that contain both an inhaled corticosteroid (ICS) and a long-acting selective beta (2)-adrenoceptor agonist (LABA). This controller therapy is provided without charge to participants in the form of fluticasone, 500 μg per inhalation, and salmeterol, 50 μg per inhalation (Advair, donated by GlaxoSmithKline), to be used twice daily. Interventions proposed within the trial include medications with potential for immunomodulation or immunosuppression, such as imatinib, an inhibitor of c-kit, and clazakizumab, an mAb directed against IL-6. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease 2019 [COVID-19]) pandemic of early 2020 coincided with the beginning of active recruitment for PrecISE Asthma, which was subsequently halted. The investigators of PrecISE Asthma were tasked with identifying institutionally and regionally appropriate policies for testing, masking, and conducting in-person procedures, with a focus on participant and staff safety. The PrecISE Asthma Safety Committee was responsible for identifying potential interactions between the asthma interventions in PrecISE Asthma and varied exposures, including vaccination for COVID-19, which was prioritized for this high-risk population. As the COVID-19 pandemic continues and therapies for this infection have evolved, a new clinical situation has emerged. We and the asthma community at large are encountering patients who contract SARS-CoV-2 infection and are being considered for therapy with ritonavir-boosted nirmatrelvir (NVM/r, brand name Paxlovid) treatment. However, the NVM/r prescribing information warns of drug-drug interactions with any agent metabolized by cytochrome enzyme P4503A4 (CYP3A4).2 Popular COVID-19 advisory websites such as the Ontario Science Table 3 (https://covid19-sciencetable.ca) and the University of Liverpool COVID-19 Drug Interactions website4 (https://www.covid19-druginteractions.org) specifically warn to avoid the use of salmeterol when prescribing NVM/r for COVID-19 infection.
Table I.
Risk of CYP3A4 inhibitors with asthma medication
| Representative CYP3A4 inhibitors | Drug class of asthma medication |
Asthma medications |
||
|---|---|---|---|---|
| Likely to be affected by CYP3A4 inhibition | Possibly affected by CYP3A4 inhibition∗ | Unlikely to be affected by CYP3A4 inhibition | ||
| Ritonavir, and other protease inhibitors | LABA | Salmeterol Vilanterol | Olodaterol Formoterol |
|
| Ketoconazole, and other imidazole antifungals | Short-acting beta2-adrenergic agonist | Albuterol | ||
| Clarithromycin, and other macrolide antibiotics | Corticosteroid | Fluticasone | Methylprednisolone Budesonide Mometasone Triamcinolone |
Beclomethasone Prednisone |
| Cimetidine, Ranitidine | Long-acting muscarinic antagonist | Tiotropium Umeclidinium Aclidinium |
||
| Diltiazem | Macrolide antibiotic | Clarithromycin | Azithromycin | |
| Cyclosporine | Other | Montelukast Theophylline† |
||
| Fluoxetine Grapefruit Juice |
Asthma biologics | Omalizumab Mepolizumab Reslizumab Benralizumab Dupilumab Tezepelumab |
||
Monitoring for side effects during use of strong CYP3A4 inhibitor is suggested.
Theophylline serum levels may decrease due to ritonavir induction of CYP1A2.
The PrecISE Asthma Safety Committee has carefully reviewed this interaction and has considered available literature regarding other potential drug-drug interactions for the treatment of COVID-19 in patients with asthma and has created recommendations for managing these interactions for our research study participants. Herein, we share our findings and offer management recommendations for implementation by providers who treat patients with asthma.
Overview of cytochrome p450 metabolism and paxlovid
The cytochrome p450 family of super enzymes functions as monooxygenases, oxidizing a large number of compounds and xenobiotics including fatty acids, steroid hormones, naturally occurring chemical compounds, and pharmaceuticals.5 , 6 These enzymes are responsible for a large amount of drug metabolism and clearance, along with some prodrug activation. A number of drugs and other compounds, such as chemicals found in grapefruit juice (furanocoumarins), can alter the activity of cytochrome P450 enzymes. Increasing or inhibiting enzyme activity can markedly affect the pharmacokinetics and risk profiles of medications metabolized by those enzymes.
CYP3A4 is the most highly expressed isoform of the cytochrome p450 system in humans and is responsible for metabolism of a large proportion of common medications.7 The enzyme is highly expressed in the small intestine and in hepatocytes,5 , 6 , 8 but the lung also has significant expression.9 CYP3A4 has a broad capacity for oxidative metabolism and can accommodate various structurally diverse substrates.10 Polymorphisms in CYP3A4 may contribute to interperson variability in enzyme activity.7 , 11
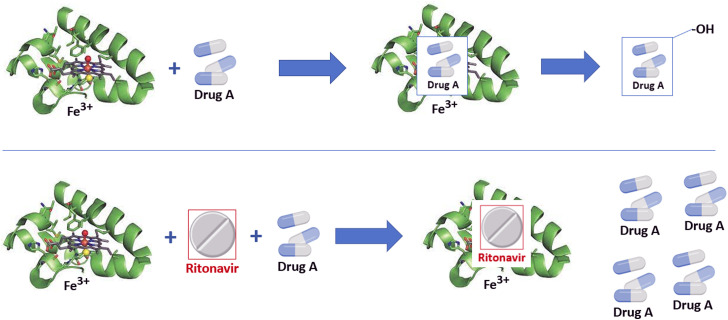
Ritonavir, a protease inhibitor originally developed as part of the highly active antiretroviral therapy for HIV treatment,12 , 13 is perhaps the most potent known inhibitor of CYP3A4 developed to date. This molecule has been been shown to irreversibly bind to the heme portion of the enzyme, preventing reduction functions and inhibiting binding of other molecules to the active site (Fig 1 ).10 Because of this, ritonavir acts as a pharmacologic boost to other drugs metabolized by CYP3A4, such as in the case of highly active antiretroviral therapy, where ritonavir enhances the pharmacokinetics of antiviral agents and thereby improves clinical efficacy.14 In a similar way, ritonavir added to nirmatrelvir, a protease inhibitor with activity against SARS-CoV-2 (NVM/r, Paxlovid), improves the clinical activity of the latter 5-fold through achieving higher sustained serum concentrations, and forms the basis of combination therapy in the treatment of early COVID-19 infection.15
Fig 1.
Example of ritonavir as cytochrome P4503A4 inhibitor. Drug A is substrate for cytochrome P4503A4. On binding to the heme element, a series of chemical reactions causes hydroxylation, thereby deactivating drug A or targeting it for further metabolism (upper panel). In the presence of ritonavir, or other CYP3A4 inhibitors, the drug substrate is unable to bind to the active site and drug A levels will accumulate (lower panel).
Inhibition of CYP3A4 by ritonavir occurs within 48 hours of dosing. Because the molecule irreversibly binds to the enzyme, de novo enzyme synthesis is required to restore CYP3A4 function. Marzolini et al16 recommend, on the basis of modeling data, that medications held during ritonavir therapy could be restarted 3 to 5 days after the last dose of ritonavir. These recommendations acknowledged that approximately 80% of enzyme function would return within 3 days, but that because of interpatient variability, a 5-day window would reduce the likelihood of interaction.
Also, ritonavir is known to induce expression of non-CYP3A4 enzymes, thereby reducing levels of medications metabolized through those pathways. Maximal induction occurs after approximately 1 week of therapy, but the extent of impact is dependent on the pharmacologic parameters and dosing of those affected medications, and less well quantified.17 Although the clinical significance of this induction is not well understood, it is thought to be of minimal impact given the short duration of NVM/r therapy for COVID-19.
Several other drugs are known to inhibit CYP3A4 and thus have the same considerations regarding drug dosing as does ritonavir. Indeed, antifungal agents such as ketoconazole, an imidazole drug, has been used as a standard agent to test the involvement of CYP3A4 in drug metabolism in preclinical pharmacokinetic investigations, and on binding to CYP3A4 has a structure similar to that of ritonavir-bound CYP3A4.10 Other common clinical agents with known activity against CYP3A4 that may be encountered in the treatment of patients with asthma include macrolide antibiotics such as erythromycin, clarithromycin, and azithromycin.18, 19, 20 The inhibitory effect of azithromycin on CYP3A4 may be less than that of other macrolides,21 and may be context specific, depending on the substrate. In summary, clinicians may encounter CYP3A4 inhibition with drugs other than ritonavir when treating patients with asthma.
Asthma medications and CYP3A4 inhibition
The potential risks of CYP3A4 inhibition on a CYP3A4-metabolized comedication relates to that drug’s mechanism of action and the range of its therapeutic window. The side effects of asthma medications will increase proportionally in the setting of higher sustained drug levels. For asthma medications, considerations regarding side effects will also rely on the duration of ritonavir therapy. In chronic antiretroviral treatment with ritonavir (eg, for the treatment of HIV infection), long-term asthma controller therapy should be carefully selected to minimize interactions and side effects. Table I summarizes the risk of CYP3A4 inhibitors on commonly used asthma medications.
Long-acting beta-agonists
Salmeterol, vilanterol, olodaterol, and formoterol are inhaled LABAs commonly prescribed in combination inhalers to treat patients with asthma and chronic obstructive pulmonary disease. Although previous Global INitiative for Asthma guidelines considered the use of different ICS-LABA combinations as equivalent, the most recent Global INitiative for Asthma guidelines recommend the use of ICS-formoterol combinations as “initial track” therapy for asthma and other LABAs combined with ICS as “alternative track” therapy.22 Yet, many patients with asthma receive ICS-LABA combinations that contain salmeterol or vilanterol as the LABA agent.
Systemic absorption of LABAs occurs from oropharyngeal membranes, the airway mucosa, and the gastrointestinal tract. Major potential side effects of systemic LABA exposure include cardiovascular toxicity such as tachycardia, palpitations, and prolonged QT interval.
Ritonavir will not affect the plasma concentrations of either formoterol or olodaterol because these drugs are not metabolized by CYP3A4 (non–CYP3A4-metabolized LABAs). Similarly, albuterol, a short-acting beta agonist, is not metabolized by CYP3A4 and has no known interaction with ritonavir.
In contrast, both salmeterol and vilanterol are metabolized by CYP3A4; thus, concentrations may be increased by CYP3A4 inhibition.23 , 24 Kempsford et al24 published the pharmacodynamic study of fluticasone furoate and vilanterol trifenatate with ketoconazole. In healthy participants, vilanterol plasma concentrations were increased by 65% with ketoconazole coadministration, with no change in heart rate but slight increase in mean QT interval. No participants had QT prolongation to intervals of concern. Salmeterol prescribing information describes a placebo-controlled, crossover study of 20 healthy subjects receiving salmeterol with or without ketoconazole.25 In this study, plasma exposure to salmeterol was increased 16-fold with ketoconazole administration. Three subjects had to discontinue coadministration because of adverse effects of QT prolongation or sinus tachycardia. Although mean heart rate and QT interval were not different between the placebo and ketoconazole groups, some individuals had outlier events. These data suggest that there will be individuals susceptible to potentially harmful cardiovascular effects of increased systemic salmeterol concentrations seen following CYP3A4 inhibition.
Risk management decisions
The PrecISE Asthma Steering Committee recognizes that the risk for adverse cardiac side effects from salmeterol or vilanterol during therapy with a CYP3A4 inhibitor such as ritonavir may vary with individual susceptibility factors. Clinicians are not able to predict which patients will develop an adverse therapeutic response, however, and there is an approximately 15% rate of significant adverse cardiovascular outcomes. Therefore, the PrecISE Asthma Safety Committee decided that the risk of continuing salmeterol or vilanterol therapy during treatment with ritonavir is unacceptable. Therefore, participants receiving ritonavir therapy as part of NVM/r for COVID-19 in the PrecISE Asthma protocol will discontinue the use of salmeterol- and vilanterol-containing inhalers as soon as possible after initiation of ritonavir. For patients with severe asthma who require the use of ICS/LABA for asthma control, we recommend alternate therapy using either budesonide-formoterol or mometasone-formoterol. For patients with less severe disease, ICSs with or without long-acting muscarinic antagonists could be used as alternatives to ICS/LABA therapy in the setting of NVM/r dosing, as discussed below. On the basis of aforementioned pharmacokinetics, patients should use this alternative therapy for at least 5 days after completing NVM/r dosing. Because NVM/r can be prescribed by physicians and pharmacists and is likely to be prescribed by a practitioner outside our research network, we provide anticipatory education to all participants about this interaction. We also acknowledge that payers impose prescribing limitations that may variably affect access to alternative inhalers; patient advocacy (eg, the use of “prior authorization” mechanisms) may be necessary to ensure that patients receive appropriate alternative medication in a timely manner.
Corticosteroids
National and international guidelines recommend the benefit of ICS therapy for prevention and control of asthma at all severity levels.22 , 26 Ideally, long-term use of locally acting, minimally absorbed ICSs with extensive first-pass metabolism and clearance by CYP3A4 is preferable to intermittent systemic steroid dosing both for the morbidity related to exacerbations and for the long-term risk of cumulative steroid systemic side effects. Indeed, maintenance and reliever therapy with ICS-formoterol preparation was shown to be safe, even at higher daily doses.27 With such evidence, the current 2022 Global INitiative for Asthma guidelines recommend the use of ICSs, in combination with formoterol when possible, as a primary controller medication to be initiated as soon as possible after asthma diagnosis.
It has been well established that ICSs can be absorbed into the bloodstream; as with LABA, this occurs via the oropharyngeal membranes and by transfer from the airway mucosa, peripheral airways, and the gastrointestinal tract.28 Generally, the amount of ICS in plasma leads to minimal side effects, but even with ordinary use, adrenal suppression can occur with side effects that include thinning skin, cataracts, and weight gain.29, 30, 31 These side effects may be dramatically increased by inhibition of drug metabolism, such as in the case of CYP3A4 inhibition by ritonavir. In 2008, Foisy et al32 published a literature review of adrenal suppression or Cushing’s syndrome from fluticasone therapy in the setting of chronic ritonavir use for highly active antiretroviral therapy. In this review, 25 published cases detailed characteristics of this interaction. Both intranasal and inhaled fluticasone preparations were implicated, and 10 cases were found in the pediatric age group. Onset of symptoms for patients taking inhaled fluticasone was as early as within the first month, but usually required 2 to 5 months after comedication. Most patients had biochemical evidence of adrenal suppression. Since that time, case reports continue to be published,33 reinforcing that this interaction may still be underappreciated by clinicians.34
Package inserts for fluticasone furoate– and fluticasone proprionate–containing inhalers warn of the potential for increased systemic exposure, generally expressed as the area under the curve of drug levels over time, during coadministration with ketoconazole, which strongly inhibits CYP3A4.25 , 35 In the aforementioned study by Kempsford et al,24 the authors found a 36% increase in steroid exposure with coadministration of ketoconazole, and an associated 27% reduction in mean serum cortisol, concluding the possibility for an increased risk of adverse reactions. Prescribing information for fluticasone propionate states that coadministration with ketoconazole increases plasma fluticasone propionate exposure and reduces plasma cortisol exposure; however, this had no effect on urinary excretion of cortisol.25 Similarly, fluticasone propionate nasal spray coadministered with ritonavir showed a marked increase in serum levels that approached 1 to 2 orders of magnitude and an 86% reduction in serum cortisol concentrations, suggesting clinically significant adrenal- suppressive effects.36 Seymour et al37 reviewed case reports of Cushing’s syndrome related to intranasal steroids in patients taking ritonavir. Most reported cases were seen with fluticasone intranasal preparations and were reported months after the onset of comedication.37
One study showed that exposure to orally administered budesonide was increased 6-fold with ketoconazole comedication.38 The prescribing information for budesonide-containing inhalers references these data to warn of possible interactions. Prescribing information for mometasone documents increased plasma concentrations in some patients during ketoconazole coadministration.39 A pharmacokinetic study of healthy volunteers suggested that beclomethasone blood levels were not substantially affected by coadministration of ritonavir, that is, without any clinically significant effects.40
The triamcinolone prescribing information cites case reports of clinically significant interactions with ritonavir; case studies available report adverse reactions with intraarticular and other injected forms of triamcinolone.41 Unlike the many ICS preparations, prednisone is not metabolized through CYP3A4 and does not influence the function of that enzyme.42 However, plasma concentrations of methylprednisolone may be increased by coadministration of a CYP3A4 inhibitor.43
Risk management decisions
The PrecISE Asthma Safety Committee carefully considered these data. The evidence supports that most inhaled and intranasal steroids are metabolized through CYP3A4, and coadministration of a potent CYP3A4 inhibitor causes increased serum levels of steroid and/or reduced serum cortisol levels, which may be clinically significant. Furthermore, the increased steroid exposure would increase the risk for adrenal insufficiency with longer duration of exposure. Adverse interactions of fluticasone preparations with strong CYP3A4 inhibitors are most commonly recognized; however, whether this is pharmacologic or reflects the wide use of these medications is unclear. Because of the relative importance of ICSs for the maintenance of asthma, in particular severe asthma, we determined that benefits of coadministration of ICSs with a 5-day course of NVM/r outweigh the risks of discontinuation of this controller medication. These benefits may be particularly practical given that recommendations for treatment of pulmonary COVID-19 infection include systemic corticosteroid administration for its anti-inflammatory benefits.44 , 45 However, for more long-term use of CYP3A4 inhibitors, careful monitoring for signs or symptoms of systemic steroid exposure is prudent, particularly for individuals using nasal and inhaled steroids. For these patients, fluticasone and combination inhalers containing fluticasone should probably be avoided.
Long-acting muscarinic antagonists
Only a small fraction of inhaled tiotropium absorbed systemically will be metabolized through CYP3A4; therefore, inhibitors such as ritonavir are unlikely to affect exposure.46 Umeclidinium is metabolized through cytochrome p450 2D6, and is therefore not affected by 3A4 inhibition.47 In vitro studies of aclidinium show that there is no interaction with cytochrome p450 enzymes.48
Azithromycin
As noted previously, azithromycin is also an inhibitor of CYP3A4 and may be used in some patients with asthma as an adjunct therapy.22 , 49 , 50 Although azithromycin itself can prolong the QT interval, particularly in patients with higher risks such as advanced age,51, 52, 53 in the cited asthma trials, there was no noted interaction between azithromycin and concomitant ICS-LABA therapy. Azithromycin itself is mainly excreted in the urine and feces unchanged.54 In addition, there are no known interactions between azithromycin and ritonavir.
Recent trials of azithromycin and hydroxychloroquine in patients with COVID-19 have demonstrated QTc prolongation but not a significant risk of arrhythmogenesis55 , 56; however, these trials did not include patients with asthma receiving ICS-LABA therapy, and the interactions of these agents with QTc interval in the setting of COVID-19 infection has not been assessed. Given the lack of information on these interactions with this macrolide, clinical decision making regarding azithromycin dosing changes should account for the longer duration of action of this medication.
Montelukast
Montelukast is extensively metabolized by CYP3A4 and CYP2C8. However, in a trial of 11 healthy subjects, the effects of the gemfibrozil-itraconazole combination on the pharmacokinetics of montelukast did not differ from those of gemfibrozil alone. In humans, CYP2C8 is the dominant enzyme in the biotransformation of montelukast, accounting for about 80% of its metabolism.57 There are no published reports of changes in montelukast metabolism in the presence of CYP3A4 inhibitors and no known interactions between montelukast (or zafirlukast) and ritonavir.
Theophylline
Although theophylline is a drug used in the past for the treatment of asthma, its use has been deprecated in recent guidelines but may still be used by some clinicians.22 , 26 It is metabolized by the CYP1A family of cytochrome P450 isoforms; in contrast, CYP3A4 is not active in the in vitro metabolism of theophylline.58 Thus, there should be no change in theophylline serum concentrations in NVM/r therapy. However, ritonavir may decrease the serum concentration of theophylline derivatives, and ritonavir prescribing information indicates that cotreatment with theophylline may decrease theophylline exposure and efficacy such that therapeutic monitoring should be considered.59
Biologic therapies and COVID-19 risk and treatment
Biologic therapies using mAbs directed against cytokines important to type-2 inflammation have, over the past decade, been demonstrated to be useful in patients with severe asthma who are not controlled with ICS-LABA therapy and who have evidence of type-2 inflammation. No studies of interactions between CYP3A4 and IL-5–blocking mAbs such as mepolizumab, reslizumab, and benralizumab have been published. Similarly, there are no reports to date of interactions of the thymic stromal lymphopoietin–blocking mAb tezepelumab and CYP3A4, nor of the IgE-blocking mAb omalizumab. One study examined the pharmacokinetics of 5 different CYP450 substrates given before and 28 days after initiation of dupilumab (which blocks IL-4 and IL-13 through the IL-4 receptor alpha subunit), 300 mg every 2 weeks, in 14 patients with moderate to severe atopic dermatitis.60 Dupilumab therapy had no effect on the pharmacokinetics of the tested CYP450 substrates, and thus appears to be safe in this setting. Given the paucity of data to date, the decision to maintain or withhold biologic therapy in a patient with severe asthma who is being treated with NVM/r for COVID-19 infection should be done on a case-by-case basis, with consideration for the long half-life of biologic agents, and with prompt reporting of adverse events.
Considering the issue of COVID-19 and asthma biologic therapies more broadly, data generated to date generally support the relative safety of these therapies regarding both the risk of contracting COVID-19 infection and the risk of subsequent morbidity/mortality. In a national database in Israel, the use of biologics for asthma was associated neither with adverse outcomes nor with severe COVID-19.61 In a prospective study from Greece, 4.4% of patients with severe asthma on biologic therapy (omalizumab, mepolizumab, benralizumab) were diagnosed with COVID-19, and of these, 36% were hospitalized.62 However, there was no comparison group of patients with severe asthma not on biologic therapies. In a large asthma cohort in Spain with COVID-19 infection, biologic therapy for severe asthma was associated with better outcomes than severe asthma without biologics.63 , 64 Cohort studies report the overall safety of use of omalizumab during the pandemic, but in a nonasthmatic population.65 Additional investigation is clearly warranted with appropriate comparisons to patients with severe asthma who are not receiving biologic therapies.
One intriguing new idea is whether select biologic therapies for asthma may, themselves, have an anti–COVID-19 effect. Emerging evidence suggests that IL-13 is responsible in part for more severe COVID-19. One suggested mechanism relates to the induction of transmembrane protease, serine 2, necessary for SARS-CoV-2 receptor function, from IL-13 exposure in airway epithelial cell cultures.66 This is complementary to the induction of the spike protein target for viral entry, angiotensin-converting enzyme 2, by interferons released during viral infections.67 Donlan et al68 retrospectively examined an international COVID-19 cohort of approximately 350,000 cases, finding 81 patients who had received dupilumab before and independently of a COVID-19 diagnosis. They then matched these to patients not receiving the drug, and to patients with type-2 inflammatory diseases including asthma, atopic dermatitis, and rhinosinusitis. They found that dupilumab use was associated with lower circulating C-reactive protein, a marker of poor outcomes in COVID-19 infection,69 , 70 a lower risk for COVID-19–related death, and fewer respiratory complications such as mechanical ventilation. They confirmed this finding in a second validation cohort, and then used a mouse model of IL-13 neutralization and infection that suggested that the mechanism for the protective effect functions through deposition of hyaluronan in the lung, because these effects were abrogated by an IL-13 inhibitor. This early report supports the potential protective effects of biologic inhibitors against IL-13, such as dupilumab, for patients with asthma who contract COVID-19. These data run counter to data in primary human airway epithelial cells suggesting that IL-13 exposure decreases the expression of angiotensin-converting enzyme 2, a receptor known to be used by SARS-CoV-2 for cellular entry.66 , 71 Clearly, further studies are required before clinicians can understand the implication of IL-13–mediated inflammation in SARS-CoV-2 illness, and to consider the use of this or similar biologics as an active therapy against COVID-19.
Vaccination
One important question is whether any of the asthma biologic therapies influence the response to any of the currently authorized vaccines directed against COVID-19. In a series of 34 patients on asthma biologics—omalizumab, mepolizumab, reslizumab, and benralizumab—there was no impact of biologic therapy on 2 doses of the BNT162b2 mRNA vaccine response or durability.72 These findings support recommendations regarding other nonlive vaccines. For example, dupilumab administration does not impact Tdap or meningococcal polysaccharide vaccine antibody responses.73 Live-attenuated vaccines are avoided with dupilumab because of lack of data supporting safety.74 There are no data supporting the influence of mepolizumab, benralizumab, omalizumab, or tezepelumab on vaccine responses or safety.
Alternatives to the use of ritonavir-containing ANTI–COVID-19 therapies in asthma
The use of NVM/r in the treatment of COVID-19 infection has been a welcome addition to the therapeutic options available in the COVID-19 pandemic. For patients with asthma who are using salmeterol- or vilanterol-containing combination inhalers, alternative considerations include the use of molnupiravir instead of NVM/r in the first 5 days of a COVID-19 infection, or the mAb bebtelovimab, given as single injection within 7 days of disease onset. The susceptibility of future strains of SARS-CoV-2 will ultimately determine which medications are likely to be therapeutically active.
Molnupiravir is a small-molecule ribonucleoside prodrug of N-hydroxycytidine, which has demonstrated antiviral and clinical activity in nonhospitalized patients infected with COVID-19. This drug functions through its incorporation into the viral genome and thereby increasing errors in the viral RNA sequences during replication, and therefore has some innate protection against development of resistance.75 , 76 Use of the drug, 800 mg twice daily for 5 days, within 5 days of the time of diagnosis of mild to moderate COVID-19 infection with at least 1 risk factor for disease progression was shown to reduce risk of hospitalization in unvaccinated adults.77 Similarly, treatment with molnupiravir versus placebo in outpatient and hospitalized patients with COVID infection led to decreased need for respiratory interventions and quicker normalization of biomarkers such as C-reactive protein.78 In neither of these studies were data for participants with asthma provided. Molnupiravir now has been approved for emergency use in the United States for the treatment of COVID-19. Although the preferred antiviral therapy for each circulating strain of COVID-19 will change with susceptibility factors, as of fall 2022, the Centers for Disease Control and Prevention recommends molnupiravir as second line to NVM/r therapy.79 Molnupiravir is not metabolized by nor affects the CYP3A4 enzyme and there are no known reported interactions with asthma medications.
Bebtelovimab is an mAb that binds to and neutralizes the receptor-binding domain of the spike protein of SARS-CoV-2.80 This treatment was approved by the Food and Drug Administration for emergency use of mild to moderate COVID-19 in adolescents and adults who are considered to be high risk for progression of disease or are unable to use alternative therapies. This therapy maintains full potency against and may be useful with the current wave of Omicron BA.4 and BA.5 variants.80 , 81 This biologic is not metabolized through cytochrome P450 and therefore unlikely to be affected by drugs metabolized through, or inhibiting, these pathways. Interactions with asthma medications, including asthma biologics, have not been reported to date in the use of bebtelovimab.
Several other anti–COVID-19 mAb and small-molecule therapies are in development as of the publishing of this article; clinicians should be aware of the potential for interactions with asthma medications.
Risk management decisions
The PrecISE Asthma Safety Committee carefully considered the use of these antiviral agents with regard to both safety, with and without salmeterol-containing combination asthma inhaler therapy, and overall efficacy. Direct comparative data for these therapies now are only beginning to emerge. One recent meta-analysis suggests some superiority of NVM/r over molnupiravir therapy for outpatients with COVID-19 infection.82 With this in mind, we determined that when patients with asthma are newly infected with COVID-19, NVM/r should be first-line therapy with the necessary concomitant change in their combination inhaler to one containing formoterol (rather than salmeterol or vilanterol). We believe this is a better choice than providing molnupiravir therapy and continuing their salmeterol- or vilanterol-containing combination inhaler. Such a decision would provide patients with better and evidence-based treatment for the infection while avoiding the concerns regarding inhibition of CYP3A4.
Although not regarded as a therapy for COVID-19 and thus not presenting an immediate problem in patients with asthma who may take CYP3A4 inhibitors, one other antibody therapy directed against COVID-19 deserves comment. Tixagevimab and cilgavimab, provided as a combination injection (Evusheld), is approved as preexposure prophylaxis for COVID-19. This agent has been demonstrated to be particularly useful in immunocompromised patients who are either at risk for severe outcomes following COVID-19 infection or who are at risk of an inadequate response to vaccination.83, 84, 85 In the trials by Levin et al83 and Montgomery et al,84 participants with a diagnosis of asthma were included but response data were not provided separately for this asthma subgroup. Interactions with asthma medications and biologics have not been reported to date in the use of this combination therapy, and neither agent in this combination is apparently metabolized by cytochrome P450.
Future directions
To reduce the knowledge gaps mentioned throughout this rostrum, higher quality observational and interventional data on drug-drug interactions will be needed. There is a particular need for studies that examine drug interactions in “real-world” settings and among patients who have chronic lung diseases such as asthma. Pharmacogenetic analysis of CYP3A4, and other highly prevalent metabolic factors, might be a useful tool for risk stratification of individuals who are at higher risk of adverse drug reactions and could be studied for clinical application. Finally, implementation studies will need to address how best to bring alternative therapies and management to patients affected by these drug-drug interactions, particularly in the setting of clinical trials such as PrecISE Asthma and in managed care.
Conclusions
The PrecISE Asthma network identified potentially dangerous medication interactions that could affect patients with severe asthma who receive treatment for COVID-19 infection, and for which clear drug management recommendations were not available. The PrecISE Asthma Safety Committee, on behalf of the network, took into consideration the available literature, product information, and recommendations from expert contacts. Although not intended to be broad recommendations or evidence-based guidelines, we offer this information to clinicians and researchers who may grapple with the clinical questions raised.
For patients with asthma and COVID-19 infection, the most clinically relevant and potentially dangerous drug-drug interactions exist between the LABAs salmeterol and vilanterol and the CYP3A4 inhibitor ritonavir, in NVM/r (Paxlovid). The pharmacodynamics of CYP3A4 inhibition by ritonavir support a recommendation that these inhaled LABA agents should be switched to an alternative, such as formoterol, for the duration of NVM/r (Paxlovid) therapy and at least 5 days after completion of NVM/r therapy to reduce the risk of cardiovascular adverse events. ICS metabolism, particularly for fluticasone, can be similarly impaired; however, the short-term side effects with a 5-day course of NVM/r are less likely to be clinically significant. Fortunately, there are no other recognized significant adverse interactions between asthma medications and treatments for COVID-19. However, clinicians should remain vigilant and aware of potential drug-drug interactions as new anti–COVID-19 therapies become available. Websites that review these interactions3 , 4 and offer other advice on therapies and vaccination are especially useful in this regard.86
Footnotes
The PrecISE Asthma study is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health (grant nos. U24 HL138998, 1UG1HL139054, 1UG1HL139098, 1UG1HL139106, 1UG1HL139117, 1UG1HL139118, 1UG1HL139119, 1UG1HL139123, 1UG1HL139124, 1UG1HL139125, 1UG1HL139126, and 1UG1HL146002). Support for site institutional infrastructure came from National Institute of Health Clinical & Translational Science Award (grant nos. UL1TR002451 [Harvard], UL1TR000427 [University of Wisconsin], UL1TR002366 [University of Kansas], UL1TR002389 [University of Chicago], UL1TR002489 [University of North Carolina], UL1TR002548 [Cleveland Clinic], and UL1TR001442 [University of California, San Diego]). The study also gratefully acknowledges receiving contributed product from Vitaeris, owned and operated by the CSL Group (clazakizumab), Vitaflo (MCT), Sun Pharma (imatinib), OM Pharma, a Vifor Pharma Group Company (OM-85, BronchoVaxom), Incyte (itacitinib), Laurel Venture (cavosonstat), and GlaxoSmithKline (Advair Diskus and Ventolin).
Disclosure of potential conflict of interest: T. F. Carr has served in an advisory role or as a consultant for AstraZeneca, Genentech, GlaxoSmithKline (GSK), Novartis, and Regeneron and as a writer and editor for Wolters Kluwer UpToDate. M. Kraft has received research funding paid to her institution from the National Institutes of Health, ALA, Sanofi, AstraZeneca, Synairgen, and Janssen; served on an advisory board for AstraZeneca, Sanofi, Chiesi, and Synairgen; has received fees for speaking for Chiesi, AstraZeneca, and Sanofi; is cofounder and CMO, RaeSedo, Inc, a company evaluating peptidomimetics for treatment of inflammatory lung disease; and payments from Elsevier for UptoDate, where she is a section editor for asthma. W. Phipatanakul has served in advisory capacity for Genentech, Novartis, Sanofi, Regenron, Teva, Astra Zeneca, and GSK. S. J. Szefler reports consultant fees paid to the university from AstraZeneca, GSK, Moderna, OM Pharma, Propeller Health, Regeneron Pharmaceuticals, Inc, and Sanofi. A. A. Zeki reports serving as CSO for InStatin, Inc; and on Sanofi/Regeneron consulting and advisory boards. D. P. Beden has served as a consultant for Teva and GSK and receives grant support from the National Heart, Lung, and Blood Institute, National Institute of Environmental Health Sciences, National Institute of Allergy and Infectious Diseases, Department of Defense, and Environmental Protection Agency. S. R. White has served as a consultant and speaker for AstraZeneca, Sanofi, and Regeneron. M. L. Fajt has no relevant conflicts of interest.
References
- 1.Georas S.N., Wright R.J., Ivanova A., Israel E., LaVange L.M., Akuthota P., et al. The Precision Interventions for Severe and/or Exacerbation-Prone (PrecISE) Asthma Network: an overview of network organization, procedures, and interventions. J Allergy Clin Immunol. 2022;149:488–516.e9. doi: 10.1016/j.jaci.2021.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paxlovid [package insert] Pfizer, Inc; New York, NY: 2022. [Google Scholar]
- 3.Science Table COVID-19 Advisory for Ontario. https://covid19-sciencetable.ca Available at:
- 4.COVID-19 drug interactions: University of Liverpool. https://www.covid19-druginteractions.org Available at:
- 5.Li A.P., Kaminski D.L., Rasmussen A. Substrates of human hepatic cytochrome P450 3A4. Toxicology. 1995;104:1–8. doi: 10.1016/0300-483x(95)03155-9. [DOI] [PubMed] [Google Scholar]
- 6.Guengerich F.P. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Zanger U.M., Klein K. Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): advances on polymorphisms, mechanisms, and clinical relevance. Front Genet. 2013;4:24. doi: 10.3389/fgene.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leeder J.S., Gaedigk A., Lu X., Cook V.A. Epitope mapping studies with human anti-cytochrome P450 3A antibodies. Mol Pharmacol. 1996;49:234–243. [PubMed] [Google Scholar]
- 9.Anttila S., Hukkanen J., Hakkola J., Stjernvall T., Beaune P., Edwards R.J., et al. Expression and localization of CYP3A4 and CYP3A5 in human lung. Am J Respir Cell Mol Biol. 1997;16:242–249. doi: 10.1165/ajrcmb.16.3.9070608. [DOI] [PubMed] [Google Scholar]
- 10.Sevrioukova I.F., Poulos T.L. Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir. Proc Natl Acad Sci U S A. 2010;107:18422–18427. doi: 10.1073/pnas.1010693107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishna D.R., Shekar M.S. Cytochrome P450 3A: genetic polymorphisms and inter-ethnic differences. Methods Find Exp Clin Pharmacol. 2005;27:559–567. doi: 10.1358/mf.2005.27.8.928310. [DOI] [PubMed] [Google Scholar]
- 12.Danner S.A., Carr A., Leonard J.M., Lehman L.M., Gudiol F., Gonzales J., et al. A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. European-Australian Collaborative Ritonavir Study Group. N Engl J Med. 1995;333:1528–1533. doi: 10.1056/NEJM199512073332303. [DOI] [PubMed] [Google Scholar]
- 13.Markowitz M., Saag M., Powderly W.G., Hurley A.M., Hsu A., Valdes J.M., et al. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med. 1995;333:1534–1539. doi: 10.1056/NEJM199512073332204. [DOI] [PubMed] [Google Scholar]
- 14.Xu L., Desai M.C. Pharmacokinetic enhancers for HIV drugs. Curr Opin Investig Drugs. 2009;10:775–786. [PubMed] [Google Scholar]
- 15.Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marzolini C., Kuritzkes D.R., Marra F., Boyle A., Gibbons S., Flexner C., et al. Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications. Clin Pharmacol Ther. 2022;112:1191–1200. doi: 10.1002/cpt.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramsden D., Fung C., Hariparsad N., Kenny J.R., Mohutsky M., Parrott N.J., et al. Perspectives from the Innovation and Quality Consortium Induction Working Group on factors impacting clinical drug-drug interactions resulting from induction: focus on cytochrome 3A substrates. Drug Metab Dispos. 2019;47:1206–1221. doi: 10.1124/dmd.119.087270. [DOI] [PubMed] [Google Scholar]
- 18.Rowland Yeo K., Walsky R.L., Jamei M., Rostami-Hodjegan A., Tucker G.T. Prediction of time-dependent CYP3A4 drug-drug interactions by physiologically based pharmacokinetic modelling: impact of inactivation parameters and enzyme turnover. Eur J Pharm Sci. 2011;43:160–173. doi: 10.1016/j.ejps.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Westphal J.F. Macrolide-induced clinically relevant drug interactions with cytochrome P-450A (CYP) 3A4: an update focused on clarithromycin, azithromycin and dirithromycin. Br J Clin Pharmacol. 2000;50:285–295. doi: 10.1046/j.1365-2125.2000.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou S.F. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 2008;9:310–322. doi: 10.2174/138920008784220664. [DOI] [PubMed] [Google Scholar]
- 21.Polasek T.M., Miners J.O. Quantitative prediction of macrolide drug-drug interaction potential from in vitro studies using testosterone as the human cytochrome P4503A substrate. Eur J Clin Pharmacol. 2006;62:203–208. doi: 10.1007/s00228-005-0091-x. [DOI] [PubMed] [Google Scholar]
- 22.Global INitiative for Asthma. Global strategy for asthma management and prevention. Available at: www.ginasthma.org. Accessed October 14, 2022.
- 23.Cazzola M., Testi R., Matera M.G. Clinical pharmacokinetics of salmeterol. Clin Pharmacokinet. 2002;41:19–30. doi: 10.2165/00003088-200241010-00003. [DOI] [PubMed] [Google Scholar]
- 24.Kempsford R., Allen A., Bal J., Rubin D., Tombs L. The effect of ketoconazole on the pharmacokinetics and pharmacodynamics of inhaled fluticasone furoate and vilanterol trifenatate in healthy subjects. Br J Clin Pharmacol. 2013;75:1478–1487. doi: 10.1111/bcp.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Advair [package insert] GlaxoSmithKline; Research Triangle Park, NC: 2008. [Google Scholar]
- 26.Expert Panel Working Group of the National Heart, Lung, and Blood Institute, coordinated National Asthma Education and Prevention Program Coordinating Committee. Cloutier M.M., Baptist A.P., et al. 2020 Focused Updates to the Asthma Management Guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146:1217–1270. doi: 10.1016/j.jaci.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddel H.K., Bateman E.D., Schatz M., Krishnan J.A., Cloutier M.M. A practical guide to implementing SMART in asthma management. J Allergy Clin Immunol Pract. 2022;10:S31–S38. doi: 10.1016/j.jaip.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Selroos O., Halme M. Effect of a volumatic spacer and mouth rinsing on systemic absorption of inhaled corticosteroids from a metered dose inhaler and dry powder inhaler. Thorax. 1991;46:891–894. doi: 10.1136/thx.46.12.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broersen L.H., Pereira A.M., Jorgensen J.O., Dekkers O.M. Adrenal insufficiency in corticosteroids use: systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:2171–2180. doi: 10.1210/jc.2015-1218. [DOI] [PubMed] [Google Scholar]
- 30.Allen D.B. Effects of inhaled steroids on growth, bone metabolism, and adrenal function. Adv Pediatr. 2006;53:101–110. doi: 10.1016/j.yapd.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Dluhy R.G. Clinical relevance of inhaled corticosteroids and HPA axis suppression. J Allergy Clin Immunol. 1998;101:S447–S450. doi: 10.1016/s0091-6749(98)70157-5. [DOI] [PubMed] [Google Scholar]
- 32.Foisy M.M., Yakiwchuk E.M., Chiu I., Singh A.E. Adrenal suppression and Cushing’s syndrome secondary to an interaction between ritonavir and fluticasone: a review of the literature. HIV Med. 2008;9:389–396. doi: 10.1111/j.1468-1293.2008.00579.x. [DOI] [PubMed] [Google Scholar]
- 33.Tiruneh F., Awan A., Didana A., Doshi S. Preventing Cushing: iatrogenic Cushing syndrome due to ritonavir-fluticasone interaction. Cureus. 2017;9:e1484. doi: 10.7759/cureus.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brennan V., Martin-Grace J., Greene G., Heverin K., Mulvey C., McCartan T., et al. The contribution of oral and inhaled glucocorticoids to adrenal insufficiency in asthma. J Allergy Clin Immunol Pract. 2022;10:2614–2623. doi: 10.1016/j.jaip.2022.05.031. [DOI] [PubMed] [Google Scholar]
- 35.Breo [package insert] GlaxoSmithKline; Research Triangle Park, NC: 2019. [Google Scholar]
- 36.Flonase [package insert] GlaxoSmithKline; Research Triangle Park, NC: 2003. [Google Scholar]
- 37.Seymour N., Robinson M., Richardson D., Mohammed H., Williams D., McGilligan J.A. Prescribing intranasal steroids in HIV-positive patients: systematic review of the literature. J Laryngol Otol. 2021;135:755–758. doi: 10.1017/S0022215121001791. [DOI] [PubMed] [Google Scholar]
- 38.Seidegard J. Reduction of the inhibitory effect of ketoconazole on budesonide pharmacokinetics by separation of their time of administration. Clin Pharmacol Ther. 2000;68:13–17. doi: 10.1067/mcp.2000.106895. [DOI] [PubMed] [Google Scholar]
- 39.Asmanex [package insert] Organon, Inc; Jersey City, NJ: 2021. [Google Scholar]
- 40.Boyd S.D., Hadigan C., McManus M., Chairez C., Nieman L.K., Pau A.K., et al. Influence of low-dose ritonavir with and without darunavir on the pharmacokinetics and pharmacodynamics of inhaled beclomethasone. J Acquir Immune Defic Syndr. 2013;63:355–361. doi: 10.1097/QAI.0b013e31829260d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadarangani S., Berg M.L., Mauck W., Rizza S. Iatrogenic Cushing syndrome secondary to ritonavir-epidural triamcinolone interaction: an illustrative case and review. Interdiscip Perspect Infect Dis. 2014;2014 doi: 10.1155/2014/849432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcantonio E.E., Ballard J., Gibson C.R., Kassahun K., Palamanda J., Tang C., et al. Prednisone has no effect on the pharmacokinetics of CYP3A4 metabolized drugs— midazolam and odanacatib. J Clin Pharmacol. 2014;54:1280–1289. doi: 10.1002/jcph.338. [DOI] [PubMed] [Google Scholar]
- 43.Varis T., Kaukonen K.M., Kivisto K.T., Neuvonen P.J. Plasma concentrations and effects of oral methylprednisolone are considerably increased by itraconazole. Clin Pharmacol Ther. 1998;64:363–368. doi: 10.1016/S0009-9236(98)90066-2. [DOI] [PubMed] [Google Scholar]
- 44.Meyerowitz E.A., Sen P., Schoenfeld S.R., Neilan T.G., Frigault M.J., Stone J.H., et al. Immunomodulation as treatment for severe coronavirus disease 2019: a systematic review of current modalities and future directions. Clin Infect Dis. 2021;72:e1130–e1143. doi: 10.1093/cid/ciaa1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner C., Griesel M., Mikolajewska A., Mueller A., Nothacker M., Kley K., et al. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;8:CD014963. doi: 10.1002/14651858.CD014963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spiriva [package insert] Boehringer Ingelheim Pharmaceuticals I. Ridgefield, CT; 2021. [Google Scholar]
- 47.Incruse [package insert] GlaxoSmithKline; Research Triangle Park, NC: 2019. [Google Scholar]
- 48.Tudorza [package insert] Wilmington, DE: AstraZeneca, Inc; 2012. [Google Scholar]
- 49.Brusselle G.G., Vanderstichele C., Jordens P., Deman R., Slabbynck H., Ringoet V., et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322–329. doi: 10.1136/thoraxjnl-2012-202698. [DOI] [PubMed] [Google Scholar]
- 50.Gibson P.G., Yang I.A., Upham J.W., Reynolds P.N., Hodge S., James A.L., et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659–668. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- 51.Choi Y., Lim H.S., Chung D., Choi J.G., Yoon D. Risk evaluation of azithromycin-induced QT prolongation in real-world practice. BioMed Res Int. 2018;2018 doi: 10.1155/2018/1574806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mosholder A.D., Mathew J., Alexander J.J., Smith H., Nambiar S. Cardiovascular risks with azithromycin and other antibacterial drugs. N Engl J Med. 2013;368:1665–1668. doi: 10.1056/NEJMp1302726. [DOI] [PubMed] [Google Scholar]
- 53.Ray W.A., Murray K.T., Hall K., Arbogast P.G., Stein C.M. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peters D.H., Friedel H.A., McTavish D. Azithromycin. A review of its antimicrobial activity, pharmacokinetic properties and clinical efficacy. Drugs. 1992;44:750–799. doi: 10.2165/00003495-199244050-00007. [DOI] [PubMed] [Google Scholar]
- 55.Maraj I., Hummel J.P., Taoutel R., Chamoun R., Workman V., Li C., et al. Incidence and determinants of QT interval prolongation in COVID-19 patients treated with hydroxychloroquine and azithromycin. J Cardiovasc Electrophysiol. 2020;31:1904–1907. doi: 10.1111/jce.14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Connell T.F., Bradley C.J., Abbas A.E., Williamson B.D., Rusia A., Tawney A.M., et al. Hydroxychloroquine/azithromycin therapy and QT prolongation in hospitalized patients with COVID-19. JACC Clin Electrophysiol. 2021;7:16–25. doi: 10.1016/j.jacep.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karonen T., Neuvonen P.J., Backman J.T. CYP2C8 but not CYP3A4 is important in the pharmacokinetics of montelukast. Br J Clin Pharmacol. 2012;73:257–267. doi: 10.1111/j.1365-2125.2011.04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ha H.R., Chen J., Freiburghaus A.U., Follath F. Metabolism of theophylline by cDNA-expressed human cytochromes P-450. Br J Clin Pharmacol. 1995;39:321–326. doi: 10.1111/j.1365-2125.1995.tb04455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Novir (ritonavir) [package insert] Abbott Laboratories; North Chicago, IL: 2017. [Google Scholar]
- 60.Davis J.D., Bansal A., Hassman D., Akinlade B., Li M., Li Z., et al. Evaluation of potential disease-mediated drug-drug interaction in patients with moderate-to-severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther. 2018;104:1146–1154. doi: 10.1002/cpt.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adir Y., Humbert M., Saliba W. COVID-19 risk and outcomes in adult asthmatic patients treated with biologics or systemic corticosteroids: nationwide real-world evidence. J Allergy Clin Immunol. 2021;148:361–367.e13. doi: 10.1016/j.jaci.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papaioannou A.I., Fouka E., Tzanakis N., Antoniou K., Samitas K., Zervas E., et al. SARS-CoV-2 infection in severe asthma patients treated with biologics. J Allergy Clin Immunol Pract. 2022;10:2588–2595. doi: 10.1016/j.jaip.2022.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Izquierdo J.L., Soriano J.B. Biologics may have a beneficial effect in asthma patients with COVID-19. Eur Respir J. 2021;58 doi: 10.1183/13993003.01076-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Izquierdo J.L., Almonacid C., Gonzalez Y., Del Rio-Bermudez C., Ancochea J., Cardenas R., et al. The impact of COVID-19 on patients with asthma. Eur Respir J. 2021;57 doi: 10.1183/13993003.03142-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Passante M., Napolitano M., Dastoli S., Bennardo L., Fabbrocini G., Nistico S.P., et al. Safety of omalizumab treatment in patients with chronic spontaneous urticaria and COVID-19. Dermatol Ther. 2021;34 doi: 10.1111/dth.15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kimura H., Francisco D., Conway M., Martinez F.D., Vercelli D., Polverino F., et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146:80–88.e8. doi: 10.1016/j.jaci.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sajuthi S.P., DeFord P., Li Y., Jackson N.D., Montgomery M.T., Everman J.L., et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat Commun. 2020;11:5139. doi: 10.1038/s41467-020-18781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Donlan A.N., Sutherland T.E., Marie C., Preissner S., Bradley B.T., Carpenter R.M., et al. IL-13 is a driver of COVID-19 severity. JCI Insight. 2021;6 doi: 10.1172/jci.insight.150107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J., Wang L., Liu C., Wang Z., Lin Y., Dong X., et al. Exploration of prognostic factors for critical COVID-19 patients using a nomogram model. Sci Rep. 2021;11:8192. doi: 10.1038/s41598-021-87373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu R., Cui J., Hu L., Wang Y., Wang T., Ye D., et al. Development and validation of a simplified nomogram predicting individual critical illness of risk in COVID-19: a retrospective study. J Med Virol. 2021;93:1999–2009. doi: 10.1002/jmv.26551. [DOI] [PubMed] [Google Scholar]
- 71.Jackson D.J., Busse W.W., Bacharier L.B., Kattan M., O’Connor G.T., Wood R.A., et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146:203–206.e3. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Podrazil M., Taborska P., Stakheev D., Rataj M., Lastovicka J., Vlachova A., et al. Effectiveness and durability of mRNA vaccine-induced SARS-CoV-2-specific humoral and cellular immunity in severe asthma patients on biological therapy. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.892277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blauvelt A., Sofen H., Papp K., Gooderham M., Tyring S., Zhao Y., et al. Tildrakizumab efficacy and impact on quality of life up to 52 weeks in patients with moderate-to-severe psoriasis: a pooled analysis of two randomized controlled trials. J Eur Acad Dermatol Venereol. 2019;33:2305–2312. doi: 10.1111/jdv.15862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinez-Cabriales S.A., Kirchhof M.G., Constantinescu C.M., Murguia-Favela L., Ramien M.L. Recommendations for vaccination in children with atopic dermatitis treated with dupilumab: a consensus meeting, 2020. Am J Clin Dermatol. 2021;22:443–455. doi: 10.1007/s40257-021-00607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kabinger F., Stiller C., Schmitzova J., Dienemann C., Kokic G., Hillen H.S., et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021;28:740–746. doi: 10.1038/s41594-021-00651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson M.G., Puenpatom A., Moncada P.A., Burgess L., Duke E.R., Ohmagari N., et al. Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19: a randomized, placebo-controlled trial. Ann Intern Med. 2022;175:1126–1134. doi: 10.7326/M22-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Molnupiravir. Bethesda, Md: National Institutes of Health; 2022. https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/molnupiravir/ Available at:
- 80.Westendorf K., Zentelis S., Wang L., Foster D., Vaillancourt P., Wiggin M., et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022;39 doi: 10.1016/j.celrep.2022.110812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Q., Guo Y., Iketani S., Nair M.S., Li Z., Mohri H., et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608:603–608. doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wen W., Chen C., Tang J., Wang C., Zhou M., Cheng Y., et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19—a meta-analysis. Ann Med. 2022;54:516–523. doi: 10.1080/07853890.2022.2034936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levin M.J., Ustianowski A., De Wit S., Launay O., Avila M., Templeton A., et al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386:2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Montgomery H., Hobbs F.D.R., Padilla F., Arbetter D., Templeton A., Seegobin S., et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2022;10:985–996. doi: 10.1016/S2213-2600(22)00180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nguyen Y., Flahault A., Chavarot N., Melenotte C., Cheminant M., Deschamps P., et al. Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients. Clin Microbiol Infect. 2022;28:1654.e1–1654.e4. doi: 10.1016/j.cmi.2022.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.American Academy of Allergy, Asthma & Immunology. Resources for A/I clinicians during the COVID-19 pandemic, Available at: https://education.aaaai.org/resources-for-a-i-clinicians/covid-19. Accessed October 14, 2022.