Abstract
Objectives
To assess improvement in the completeness of reporting coronavirus (COVID-19) prediction models after the peer review process.
Study Design and Setting
Studies included in a living systematic review of COVID-19 prediction models, with both preprint and peer-reviewed published versions available, were assessed. The primary outcome was the change in percentage adherence to the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) reporting guidelines between pre-print and published manuscripts.
Results
Nineteen studies were identified including seven (37%) model development studies, two external validations of existing models (11%), and 10 (53%) papers reporting on both development and external validation of the same model. Median percentage adherence among preprint versions was 33% (min-max: 10 to 68%). The percentage adherence of TRIPOD components increased from preprint to publication in 11/19 studies (58%), with adherence unchanged in the remaining eight studies. The median change in adherence was just 3 percentage points (pp, min-max: 0-14 pp) across all studies. No association was observed between the change in percentage adherence and preprint score, journal impact factor, or time between journal submission and acceptance.
Conclusions
The preprint reporting quality of COVID-19 prediction modeling studies is poor and did not improve much after peer review, suggesting peer review had a trivial effect on the completeness of reporting during the pandemic.
Keywords: Peer review, Reporting guidelines, Prediction modeling, COVID-19, TRIPOD, Adherence, Prognosis and diagnosis
What is new?
Key findings
-
•
In this study, we compared adherence to the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) statement for studies developing or validating coronavirus (COVID-19) prediction models with a preprint version available, before and after the peer review process.
-
•
The findings of this report demonstrate a poor quality of reporting of COVID-19 prediction modeling studies among preprint versions, which did not improve much following peer review.
-
•
Most TRIPOD items saw no change in the frequency of their reporting, with only the coverage of discussion items being substantially improved in the published version.
What this adds to what was known?
-
•
While it has previously been reported that the adherence to reporting guidelines for prediction modeling studies was poor, our findings also suggest that the peer review process had little impact on this adherence during the pandemic.
What is the implication and what should change now?
-
•
The implication of these findings is that a greater focus is needed on the importance of adhering to reporting guidelines by authors as well as checking during the editorial process.
1. Introduction
The coronavirus (COVID-19) pandemic presents a serious and imminent challenge to global health. Since the outbreak of COVID-19 in December 2019, in excess of 529 million cases have been confirmed in over 200 countries with over 6 million deaths [1]. Given the burden this pandemic has placed on health care systems around the world, efficient risk stratification of patients is crucial.
Diagnostic and prognostic prediction models combine multiple variables in order to estimate the risk that a specific event (e.g., disease or condition) is present (diagnostic) or will occur in the future (prognostic) in an individual [2]. In 2015, in an effort to improve the completeness of reporting of studies developing or validating prediction models, the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) statement was developed [2,3] and subsequently endorsed by several journals and included in the library of the enhancing the quality and transparency of health research (EQUATOR) network, an international initiative that seeks to promote transparent and accurate publishing via the use of reporting guidelines.
However, previous studies have demonstrated incomplete reporting of studies developing or validating prediction models [4,5]. One study assessing 170 prediction model studies, which was published just prior to the introduction of the TRIPOD statement in articles across a wide range of clinical domains, found the median percentage of TRIPOD items reported to be just 44% [4]. More recently, a living systematic review of all COVID-19-related prediction models critically evaluated over 230 models, and concluded that almost all of the models were poorly reported and were subject to a high risk of bias [5].
Poor reporting is considered to be a contributing factor to the high proportion of avoidable clinical research waste [6,7], an issue that was estimated to affect up to 85% of all research prior to the COVID-19 pandemic [8]. It has been suggested that research waste increased during the pandemic for a number of reasons, including the rise, in the widespread use of preprint servers to communicate COVID-19-related research findings, prior to journal peer review [9]. As COVID-19 swept the globe, the use of preprint servers to disseminate research findings skyrocketed in an effort to militate against delays in the dissemination of research that traditional publications faced. However, it was suggested that preprints prior to peer review led to irresponsible dissemination of flawed research and to such poor reporting that a critical appraisal of the methodology and trustworthiness of results became difficult [9]. The vast number of preprints made public during the pandemic provides a unique opportunity to investigate whether these concerns are justified. It is of interest to compare the completeness of reporting of COVID-19 prediction models between preprint and peer-reviewed publications to identify areas related to the development, validation, and subsequent reporting of prediction models which were improved upon following the peer review process and crucially, areas which were not improved.
We, therefore, investigated whether peer-reviewed articles are different in their completeness of reporting of COVID-19 prediction models as compared to non-peer reviewed versions of the same article. In particular, we aim to explore the adherence to the TRIPOD Statement for studies developing diagnostic or prognostic COVID-19 prediction models when released as a preprint and compare this to the published article following peer review.
2. Methods
This study is based on articles identified and included in a living systematic review of all COVID-19-related prediction models by Wynants et al. (third update, published January 2021) [5]. Studies were included in the living systematic review if they developed or validated a multivariable model or scoring system, based on individual participant-level data, to predict any COVID-19-related outcome. These models included three types of prediction models as follows diagnostic models to predict the presence or severity of COVID-19 in patients with suspected infection; prognostic models to predict the course of infection in patients with COVID-19; and prediction models to identify people in the general population at risk of COVID-19 infection or at risk of being admitted to hospital with the disease [5]. The latest update of the living systematic review included all publications identified from database searches repeatedly conducted up to 1st July 2020. Further details of the databases, search terms, and inclusion criteria used for the living review have been published previously [5]. Aspects of both the PRISMA (preferred reporting items for systematic reviews and meta-analyses) [10] and TRIPOD [2,3] guidelines were considered when reporting our study.
2.1. Inclusion criteria
For this study, we included all published articles (post peer review) from the latest version of the living systematic review [5], with an earlier preprint version of the same article available on either the arXiv, medRxiv, or bioRxiv server. Additionally, we included all preprint articles which were included in the living systematic review and were subsequently published in a peer-reviewed journal (search dates for published versions: July-September 2021). If more than one preprint version of a report was available on the respective server, the most recent version was reviewed. An exception was made for preprint versions posted after the date of submission to the journal to ensure the preprint version being included was drafted prior to the peer review. We included preprints published a maximum of 2 days following the date of the first submission to the journal (to allow a window for preprints to become available following submission to the server). We excluded reports with: missing information on the preprint upload date, manuscript first submission date, prediction models based upon only imaging/audio recording data, and substantial changes to the aims/objectives of the study between preprint and published reports.
2.2. Data extraction and calculating adherence to TRIPOD
The data extraction form (www.tripod-statement.org/wp-content/uploads/2020/01/TRIPOD-Adherence-assessment-form_V-2018_12.xlsx) consists of 22 main components which appear on the TRIPOD checklist [2,3]. They relate to items that should be reported in the title and abstract, introduction, methods, result, and discussion of a prediction modeling study, as well as additional information on the role of funding and supplementary material. Several of the 22 components are further split into items, that all need to be adhered to in order to achieve adherence to that specific component. For studies reporting on both model development and validation, there are a maximum of 36 relevant items of adherence. For studies focusing on model development only and for studies solely focused on external validation only, there are a maximum of 30 relevant items of adherence [2,3].
Data were extracted by one reviewer (MTH), from both the preprint and published version of the article. A second reviewer (LA) independently extracted data of both versions of nine articles (five randomly selected articles, plus all studies with increases in adherence from preprint to published version of >10% (three), and the one study which appeared to have a poorer adherence in the published version than the preprint). Minor discrepancies in data extraction of three of the preprint versions were observed; these were discussed and resolved between the two reviewers without the need for reference to a third opinion. Given the consistency in extraction between the two reviewers, it was concluded that single extraction on the remaining 10 articles was sufficient.
Based on the data extraction for the 21 main components of the TRIPOD checklist (excluding Q21 relating to supplementary information which is not included in the TRIPOD scoring calculation), both versions of each article were given an adherence score as detailed by Heus et al. [11]. A score of 1 was assigned if an item was adhered to in the article and a score of 0 was assigned for nonadherence. An article's overall TRIPOD adherence score was calculated by dividing the sum of the adhered TRIPOD items by the total number of applicable TRIPOD items for that article. Percentage adherence (%adherence) was then calculated. The above process was carried out for the preprint and the final published versions of each article.
2.3. Outcome measures and statistical analysis
The primary outcome of this study was the change in percentage adherence score between the preprint version and the published version of each article.
We summarized and described the percentage adherence from preprint and published versions of the same article and, in particular, the change in adherence (in percentage points, pp) between these two versions for each study. We further summarized the percentage adherence across all studies, by type (preprint and published manuscript). The proportions of preprints and published articles reporting individual components of the TRIPOD checklist were described, and items where reporting changed substantially between the two versions were identified. We assessed whether there was an association between the published version adherence score and the following factors of interest, after adjustment for preprint adherence score: (i) impact factor of the journal which published the article, (ii) time between the first submission to the journal and acceptance date, (iii) number of preprint updates made prior to journal submission, (iv) number of days between the start of the pandemic (11th March 2020 as per WHO declaration) and manuscript acceptance, or (v) the presence of a statement relating to the use of the TRIPOD reporting guidelines in the journal's instructions to authors. To quantify these associations, we fit a series of linear regression models with the adherence score of the published version (rescaled to be a decimal between 0 and 1) as the dependent variable and each of the above factors of interest (one at a time) as the independent variable. Models were adjusted for the baseline adherence score from the preprint version (rescaled to be a decimal between 0 and 1) in the regression model.
For reports that were published in a journal with an open peer review process, we also explored the following: (i) the background of the reviewers, (ii) the number of reviewers/peer review rounds, (iii) the explicit mention of TRIPOD guidelines/checklist within the peer review document, and (iv) whether key reporting items/elements on the TRIPOD checklist were raised by reviewers (even if TRIPOD was not explicitly mentioned).
3. Results
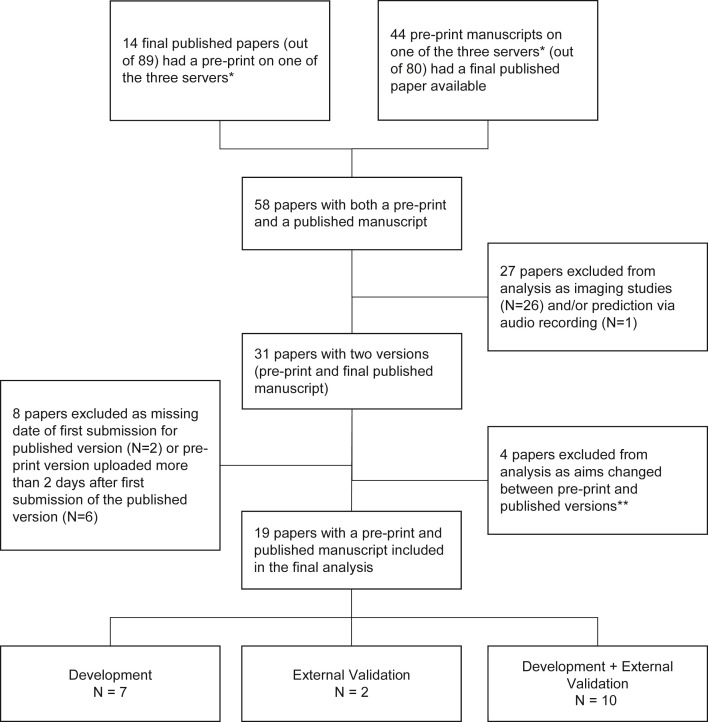
Of the 169 studies in the Wynants et al. review [5], 58 (34%) were identified as having both published and preprint versions. Of these, 26 papers related to diagnosis or prognosis through imaging, one concerned prediction via audio recording, and eight had either missing information on their initial submission date or uploaded the preprint version more than 2 days (min-max: 11 to 63 days) after submission of the published version and thus were excluded from our analyses. A further four studies made substantial changes in aims between preprint and publication, three of which contained an external validation in the published version which was previously not present in the preprint. These three authors were contacted in order to ascertain whether these amendments were preplanned; two of these studies replied and confirmed that the addition of the external validation was preplanned and added due to external datasets becoming available at a later date. All four of these studies were excluded from this study. This left 19 papers for our analyses as follows: 7 (37%) model development studies (possibly with internal validation), two external validation of an existing model (11%), and 10 (53%) papers reporting on both model development and external validation (see Fig. 1 ).
Fig. 1.
Flowchart of article screening and inclusion/exclusion criteria. ∗Preprint servers included were the arXiv, medRxiv, and bioRxiv servers. ∗∗Three of the excluded papers whose aims changed between preprint and published versions were due to the preprint version concentrating solely on model development, whereas the published version included an external validation in addition.
The majority of studies (17, 89%) uploaded only 1 preprint prior to publication, with one paper (5%) uploading two and one uploading three versions of their preprint (Table 1 ). The median number of days between first submission to the publishing journal and acceptance was 80 (lower quartile (LQ)–upper quartile (UQ): 40 to 187) and ranged from 22 to 259 days. The journal impact factor of published articles ranged from 2.7 to 39.9 with an average of 4.2 (LQ-UQ: 3.6 to 6.8), with most studies being published in journals with no reference to TRIPOD or EQUATOR on their websites (11 studies, 58%).
Table 1.
Descriptive statistics of the reports included within the analyses
| Variable | Median (lower quartile-upper quartile)/[minimum to maximum] |
|---|---|
| Preprint version percentage adherence | 33 (30–50)/[10 to 68] |
| Published version percentage adherence | 42 (31–57)/[10 to 71] |
| Change in percentage adherence | 3 (0–7)/[0 to 14] |
| Journal impact factor | 4 (4–7)/[3 to 40] |
| Number of days between first submission to the journal and acceptance date | 80 (40–187)/[22 to 259] |
| Number of preprint versions prior to journal submission | 1 (1–1)/[1 to 3] |
| Days between manuscript first submission and start of pandemica |
67 (35–141)/[12 to 202] |
| Half way down |
n (%) |
| Presence of statement relating to TRIPOD on journal website | |
| Yes | 6 (32) |
| Noa | 13 (68) |
| Preprint server | |
| ArXiv | 2 (11) |
| medRxiv | 16 (84) |
| bioRxiv | 1 (5) |
Abbreviations: TRIPOD, transparent reporting of a multivariable prediction model for individual prognosis or diagnosis; STARD, standards for reporting diagnostic accuracy studies.
Includes 2 papers published in journals mentioning alternative reporting guidelines (EQUATOR and STARD).
3.1. Changes in completeness of reporting following peer review
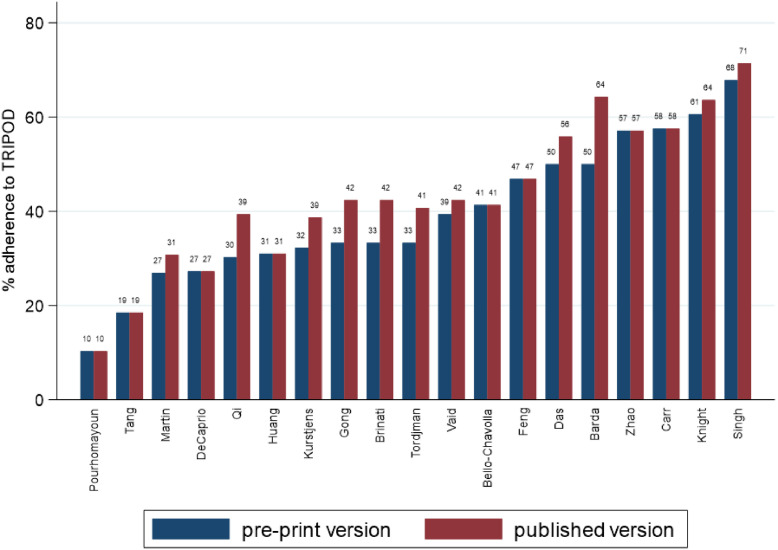
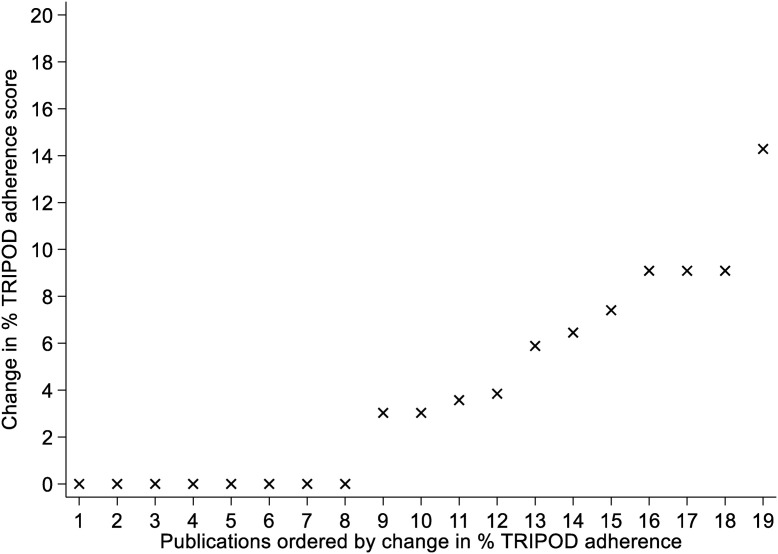
Adherence to TRIPOD was highest on average in the published manuscripts, with a median adherence of 42% (LQ-UQ: 31% to 57%), ranging from a low of 10% to a high of 71%. In contrast, median adherence across preprints was 33% (LQ-UQ: 30% to 50%), ranging from 10% to 68%. Overall adherence was low, with only 4 preprints and 6 published manuscripts adhering to more than 50% of the items in the TRIPOD checklist (see Fig. 2 ). Adherence increased from preprint to publication in 11 (58%) of the studies assessed, with a median change in adherence of 3 percentage points (pp) across all 19 studies (see Fig. 3 ; LQ-UQ: 0 to 7). While no study had a lower adherence overall in their published manuscript than in their preprint, 8 (42%) of the studies assessed showed no change in total percentage adherence following peer review.
Fig. 2.
TRIPOD adherence percent score for preprint and published versions of the 19 included studies.
Fig. 3.
Change in TRIPOD adherence score across publications.
Unsurprisingly, there was a strong positive correlation between preprint and published version adherence scores (Pearson's correlation coefficient = 0.96). After adjusting for the preprint adherence score, there was no evidence of meaningful or statistically significant associations between published article adherence score and journal impact factor, time between journal submission and acceptance, the number of preprints uploaded prior to journal submission, the number of days between the start of the pandemic and manuscript acceptance, or the presence of a statement relating to the use of the TRIPOD in the journal's instructions to authors (Table 2 ).
Table 2.
Regression coefficients from models assessing associations between published article adherence scores and a range of variables
| Model | Covariate | Coefficient (95% CI) |
|---|---|---|
| Model 1 | Preprint score | 1.01 (0.87 to 1.16) |
| Intercept | 0.03 (−0.03 to 0.10) | |
| Model 2 | Journal impact factor | 0.00 (−0.003 to 0.003) |
| Preprint score | 1.01 (0.83 to 1.19) | |
| Intercept | 0.04 (−0.03 to 0.11) | |
| Model 3 | Time between journal submission and acceptance (per 28-day period) | −0.005 (−0.01 to 0.004) |
| Preprint score | 1.01 (0.86 to 1.15) | |
| Intercept | 0.06 (−0.02 to 0.13) | |
| Model 4 | Number of preprints uploaded prior to journal submission | −0.03 (−0.07 to 0.02) |
| Preprint score | 1.03 (0.88 to 1.18) | |
| Intercept | 0.06 (−0.01 to 0.13) | |
| Model 5 | Days between manuscript first submission and start of pandemica (per 28-day period) | −0.008 (−0.02 to 0.003) |
| Preprint score | 1.06 (0.90 to 1.21) | |
| Intercept | 0.04 (−0.02 to 0.10) | |
| Model 6 | Statement on the use of the TRIPOD in the journal's instructions to authors | |
| No | (reference group) | |
| Yes | −0.002 (−0.05 to 0.05) | |
| Preprint score | 1.01 (0.86 to 1.17) | |
| Intercept | 0.03 (−0.03 to 0.10) |
Abbreviations: TRIPOD, transparent reporting of a multivariable prediction model for individual prognosis or diagnosis.
Start of pandemic was defined as 11/03/2020, corresponding to the date the WHO declared COVID-19 as a pandemic.
The median adherence of published articles was similar across the 6 journals that mentioned TRIPOD on their website, and the 13 journals that did not mention TRIPOD on their website (44% vs. 42%, respectively). Median adherence was 49% in the 2 papers published in journals mentioning alternative reporting guidelines (EQUATOR [12] and Standards for Reporting Diagnostic accuracy studies [13]) and 40% for 11 papers in journals with no reference to any reporting guidelines.
3.2. Reporting of individual TRIPOD items
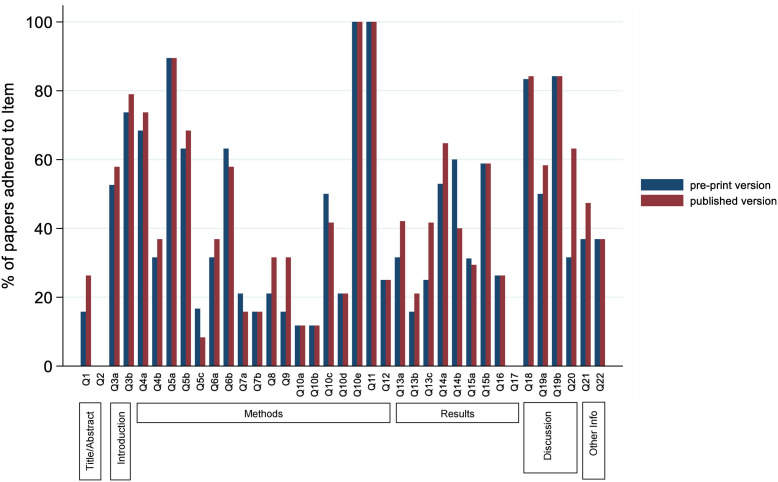
At preprint, only 5/36 TRIPOD-adherence items were reported in more than 75% of the included studies (6/36 items for published manuscripts), with nine items reported in less than 20% (fewer than three) of either published or preprint manuscripts (see Supplementary Table S2). Five items were reported less frequently in publications than in preprints (Fig. 4 , Supplementary Table S3), including those surrounding details on how predictors were measured and reporting of the full-model equation. Additional details are given in Appendix 1 of supplementary material.
Fig. 4.
Proportion of papers which adhered to the individual components of adherence in the preprint and published versions.
3.3. Assessment of open peer review
Open peer review was available for only 5/19 manuscripts [[14], [15], [16], [17], [18]]. Peer reviewers asked for a median of 4 TRIPOD items (min-max: 2 to 8), but only 1 reviewer mentioned TRIPOD explicitly. Additional results are given in Appendix 2 of supplementary material.
4. Discussion
In this study, we have compared adherence to the TRIPOD statement for studies developing or validating COVID-19 prediction models with a preprint version available, before and after the peer review process. Peer-reviewed articles showed low adherence and a modest improvement in adherence to TRIPOD-reporting items compared to preprint versions of the same article.
Most TRIPOD items saw no change in the frequency of their reporting, with only the coverage of discussion items (including implications of research and the availability of supplementary resources) being substantially improved in the published version. This highlights the need for a greater focus on the importance of adherence to reporting guidelines by authors and checking during the editorial process.
4.1. Comparison to other studies
Previous studies have shown that reporting of prediction model research has generally been poor across medical disciplines [6,19,20]. This contributes to a vast amount of avoidable research waste [7,8], as the lack of transparency may obscure study biases and hinder reproducibility of the reported results. The results of the current analysis, where we observed a median adherence of 42% (LQ-UQ: 31 to 57%) among published articles, were in line with findings from an earlier study by Heus et al. [4], which reported a median adherence of 44% (LQ-UQ: 35 to 52) in 170 models across 37 clinical domains, in articles published prior to the publishing of the TRIPOD statement. Since the introduction of the TRIPOD statement [2,3], continued poor reporting has been observed [[21], [22], [23]], with the present analysis suggesting that current reporting quality in the field of COVID-19 prediction is no different to the situation prior to publication of the TRIPOD statement. This is likely due to the less rigorous peer review and editorial process for COVID-19 articles during the pandemic. When considering the reporting of individual TRIPOD items, our findings are consistent with similar reviews in different clinical areas. For example, Jiang et al. 2020, when discussing reporting completeness of published models for melanoma prediction, also concluded that titles and abstracts were among the worst-reported items from the TRIPOD checklist, and that the discussion items (such as giving an overall interpretation of the results, item 19b) were most commonly reported [24].
Only 2 studies included in the present analysis were focused specifically on an external validation of an existing model; one of which had the best adherence and the other being among the reports with the poorest adherence. These findings are in agreement with previous research suggesting that external validation of multivariable prediction models is reported as equally poorly as model development studies [25].
Regarding the change in completeness of reporting between preprint and published manuscripts of the same article, our analyses showed an improvement in reporting between preprint and published versions in 11/19 (58%) studies with a median improvement across all included studies of just 3 pp. While this small magnitude of improvement in completeness of reporting is in line with findings by Carneiro et al., where a difference in percentage reporting score of 4.7pp was observed between preprints and published articles in 2016 [26], it is in contrast to findings from other prepandemic studies which concluded substantially improved quality of reporting in the published manuscript [27,28].
Furthermore, while the main analysis focused on comparing overall adherence between preprint and published reports, several individual TRIPOD items were reported less frequently in the published version than the preprint, with other items being reported more frequently.
Of the studies included in this review, 12 out of 19 were assessed for risk of bias using the Prediction model Risk Of Bias ASsessment Tool at both preprint and publication, as a part of the COVID-19 living systematic review by Wynants et al. [5]. It was established that all 12 studies were at high risk of bias at preprint, while 11 out of 12 were at high risk of bias at publication. Only Carr et al. (2021) [15] improved in overall risk of bias category between preprint and publication, due to the inclusion of an external validation and the resolution of some unclear reporting in the published report. While this study was among the better-reported studies included in our analysis, the percentage adherence in fact did not change between preprint and publication. Given the strong overlap between the PROBAST risk of bias assessment and the TRIPOD checklist, with many of the items in the TRIPOD checklist being a requirement to conclude low risk of bias, it is unsurprising that our assessment of TRIPOD adherence was highly consistent with the risk of bias assessment.
Given the limited number of open peer reviews available for our included studies, we were unable to draw any meaningful conclusions about the impact of the background of the reviewers, or whether reviewers identified key reporting issues, on changes to TRIPOD adherence. Previously, reviewers from statistical backgrounds have been seen to improve reporting in biomedical articles, over-and-above using solely field experts [29], and targeted reviews to identify missing items from reporting guidelines were found to improve manuscript quality [30]. However, the available open reviews suggest that statistical review of prediction models seems to be rare in the field of COVID-19 prediction, and just one of the peer reviewers’ comments included mention of the TRIPOD checklist.
4.2. Strengths and limitations of this study
The current study has several strengths. Firstly, we obtained an objective assessment of reporting quality, using the TRIPOD adherence checklist (www.tripod-statement.org/wp-content/uploads/2020/01/TRIPOD-Adherence-assessment-form_V-2018_12.xlsx) to assess the adherence for all included articles. Furthermore, extraction of adherence information was performed by medical statisticians with the familiarity of the TRIPOD checklist and extensive experience in the field of prediction modeling research, to minimize the risk of incorrect assessment. Finally, this study included all studies from the latest version of a living systematic review of COVID-19 prediction models with an earlier preprint version of the same article available, and thus gives a complete view of state of the current literature.
There are also a number of limitations to the current study in particular, given the TRIPOD adherence checklist can be quite unforgiving in certain aspects. When calculating adherence, each of the 22 TRIPOD components are given the same weighting and importance, for example a clearly reported title is given equal importance to clearly reported statistical methodology. Additionally, despite some of 22 items requiring only a single statement to achieve adherence, others required reporting of up to 12 separate subitems to successfully adhere. While poor reporting for some TRIPOD components may hide possible sources of biasor make the research question unclear on first reading, others could lead to research being impossible to replicate. For example, a study with inadequate reporting of the statistical methods and results sections would likely prevent the study from being replicated or the developed model from being externally validated, whereas poor reporting of the title and/or abstract would likely not have such severe consequences.
Secondly, this study reports on the differences in reporting quality between preprints and published peer-reviewed articles for a relatively small set, limited to COVID-19 prediction models published at the beginning of the pandemic, where time pressures may have played more of a role on reporting than other studies before the pandemic, reducing our ability to generalize our conclusions. The (lack of) detectable improvement is not necessarily attributable to the peer review process. Authors or editors may initiate changes without probes from peer reviewers, and authors or editors may ignore or overrule peer review requests (for example, to adhere to word limits). Furthermore, recent studies have discussed the increasing importance of preprints in the dissemination of research during the COVID-19 pandemic, in particular addressing concerns around the lower quality of preprints when compared to peer-reviewed manuscripts and highlighting the importance of social media platforms and comments sections of preprint servers in the informal peer review of research [31,32]. With open peer review available for only 4 of the 19 studies, we were limited in our ability to ascertain whether changes in reporting completeness were a result of formal peer review or a result of journal requirements, editorial processes, or authors’ reformatting or editing of their report for submission. Nonetheless, our assessment of the available open peer-review reports indicates that peer reviewers generally requested a small number of TRIPOD items in studies with considerable shortcomings in reporting, indicating that peer reviewers might not detect all omissions.
4.3. Implications for practice and areas for future research
All prediction modeling studies included in this review showed incompleteness in their reporting, with only some improvement evident after publication. This suggests the importance of reporting quality is currently largely overlooked during the publishing process for prediction models.
While it is primarily the author's responsibility to ensure that their prediction modeling study is reported transparently, including all important aspects of model development and/or validation, it is currently unclear whether this responsibility extends beyond the authors onto journal editors and peer reviewers. While many journals do include some form of reporting checklists as a submission requirement, these are often unspecific or even irrelevant to the research questions posed in prediction modeling. Inclusion of relevant reporting guidelines as a submission requirement for journals (and preprint servers) publishing prediction research could improve this state of affairs considerably. In addition to this inclusion within the submission guidelines for authors, journals should make it explicit whether the checking of completeness of reporting within a manuscript being reviewed is a task for the editors or peer reviewers. If the latter, they should engage individuals with prediction modeling expertize in the peer review process and/or remind invited peer reviewers of prediction modeling papers of the available reporting checklists, such as TRIPOD, to check the completeness of reporting within the manuscript being reviewed.
5. Conclusions
Preprint and published prediction model studies for COVID-19 are poorly reported, and there is little improvement in the completeness of reporting after peer review and subsequent publication. These findings suggest limited impact of peer review on the completeness of reporting during the COVID-19 pandemic in the field of prediction research. This highlights the need for journals to make adherence to the TRIPOD checklist a submission requirement for prediction modeling studies in this field and to engage more risk prediction methodology experts in the peer review process.
Acknowledgments
We would like to thank the peer reviewers and editors for their helpful feedback and comments which helped improve the article.
Footnotes
Ethics approval and consent to participate: Not Applicable.
Consent for publication: Not Applicable.
Availability of data and materials: The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interests: The authors confirm we have no competing interests.
Funding: MTH is supported by St. George's, University of London. LA, RDR, and GSC were supported by funding from the MRC Better Methods Better Research panel (grant reference: MR/V038168/1). GSC was also supported by Cancer Research UK (programme grant: C49297/A27294). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Author Contributions: Conceptualization–MTH, MvS, GSC, EWS, JBR, RDR, BVC, LW. Data Curation–MTH, LA, CW, LW. Formal Analysis–MTH, LA, LW. Investigation–MTH, LA, LW. Methodology–MTH, LA, MvS, GSC, EWS, JBR, RDR, BVC, LW. Project Administration–MTH. Writing–Original Draft–MTH, LA, LW. Writing–Review&Editing–All Authors.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jclinepi.2022.12.005.
Appendix A. Supplementary Data
References
- 1.COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus/?utm_campaign=homeAdvegas1 Available at.
- 2.Moons K.G., Altman D.G., Reitsma J.B., Ioannidis J.P., Macaskill P., Steyerberg E.W., et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–W73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 3.Collins G.S., Reitsma J.B., Altman D.G., Moons K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. BMC Med. 2015;13(1):1. doi: 10.1186/s12916-014-0241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heus P., Damen J.A., Pajouheshnia R., Scholten R.J., Reitsma J.B., Collins G.S., et al. Poor reporting of multivariable prediction model studies: towards a targeted implementation strategy of the TRIPOD statement. BMC Med. 2018;16(1):1–12. doi: 10.1186/s12916-018-1099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wynants L., Van Calster B., Collins G.S., Riley R.D., Heinze G., Schuit E., et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins G.S., Omar O., Shanyinde M., Yu L.M. A systematic review finds prediction models for chronic kidney disease were poorly reported and often developed using inappropriate methods. J Clin Epidemiol. 2013;66:268–277. doi: 10.1016/j.jclinepi.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Glasziou P., Altman D.G., Bossuyt P., Boutron I., Clarke M., Julious S., et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet. 2014;383:267–276. doi: 10.1016/S0140-6736(13)62228-X. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers I., Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet. 2009;374:86–89. doi: 10.1016/S0140-6736(09)60329-9. [DOI] [PubMed] [Google Scholar]
- 9.Glasziou P.P., Sanders S., Hoffmann T. Waste in covid-19 research. BMJ. 2020;369:m1847. doi: 10.1136/bmj.m1847. [DOI] [PubMed] [Google Scholar]
- 10.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Heus P., Damen J.A.A.G., Pajouheshnia R., Scholten R.J.P.M., Reitsma J.B., Collins G.S., et al. Uniformity in measuring adherence to reporting guidelines: the example of TRIPOD for assessing completeness of reporting of prediction model studies. BMJ Open. 2019;9(4):e025611. doi: 10.1136/bmjopen-2018-025611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enhancing the QUAlity and transparency of health research. https://www.equator-network.org/ Available at.
- 13.Bossuyt P.M., Reitsma J.B., Bruns D.E., Gatsonis C.A., Glasziou P.P., Irwig L., et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das A.K., Mishra S., Saraswathy Gopalan S. Predicting CoVID-19 community mortality risk using machine learning and development of an online prognostic tool. PeerJ. 2020;8:e10083. doi: 10.7717/peerj.10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carr E., Bendayan R., Bean D., Stammers M., Wang W., Zhang H., et al. Evaluation and improvement of the national early warning score (NEWS2) for COVID-19: a multi-hospital study. BMC Med. 2021;19(1):23. doi: 10.1186/s12916-020-01893-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeCaprio D., Gartner J., McCall C.J., Burgess T., Garcia K., Kothari S., et al. Building a COVID-19 vulnerability index. J Med Artif Intell. 2020;3:15. [Google Scholar]
- 17.Knight S.R., Ho A., Pius R., Buchan I., Carson G., Drake T.M., et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barda N., Riesel D., Akriv A., Levy J., Finkel U., Yona G., et al. Developing a COVID-19 mortality risk prediction model when individual-level data are not available. Nat Commun. 2020;11(1):4439. doi: 10.1038/s41467-020-18297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins G.S., Mallett S., Omar O., Yu L.M. Developing risk prediction models for type 2 diabetes: a systematic review of methodology and reporting. BMC Med. 2011;9:103. doi: 10.1186/1741-7015-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouwmeester W., Zuithoff N.P., Mallett S., Geerlings M.I., Vergouwe Y., Steyerberg E.W., et al. Reporting and methods in clinical prediction research: a systematic review. PLoS Med. 2012;9(5):1–12. doi: 10.1371/journal.pmed.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damen J.A., Hooft L., Schuit E., Debray T.P., Collins G.S., Tzoulaki I., et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. doi: 10.1136/bmj.i2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen Z., Guo Y., Xu B., Xiao K., Peng T., Peng M. Developing risk prediction models for postoperative pancreatic fistula: a systematic review of methodology and reporting quality. Indian J Surg. 2016;78(2):136–143. doi: 10.1007/s12262-015-1439-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zamanipoor Najafabadi A.H., Ramspek C.L., Dekker F.W., Heus P., Hooft L., Moons K.G.M., et al. TRIPOD statement: a preliminary pre-post analysis of reporting and methods of prediction models. BMJ Open. 2020;10(9):e041537. doi: 10.1136/bmjopen-2020-041537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang M.Y., Dragnev N.C., Wong S.L. Evaluating the quality of reporting of melanoma prediction models. Surgery. 2020;168:173–177. doi: 10.1016/j.surg.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Collins G.S., de Groot J.A., Dutton S., Omar O., Shanyinde M., Tajar A., et al. External validation of multivariable prediction models: a systematic review of methodological conduct and reporting. BMC Med Res Methodol. 2014;14:40. doi: 10.1186/1471-2288-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carneiro C.F.D., Queiroz V.G.S., Moulin T.C., Carvalho C.A.M., Haas C.B., Rayêe D., et al. Comparing quality of reporting between preprints and peer-reviewed articles in the biomedical literature. Res Integr Peer Rev. 2020;5(1):16. doi: 10.1186/s41073-020-00101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierie J.-P.E.N., Walvoort H.C., Overbeke A.J.P.M. Readers' evaluation of effect of peer review and editing on quality of articles in the Nederlands Tijdschrift voor Geneeskunde. Lancet. 1996;348:1480–1483. doi: 10.1016/S0140-6736(96)05016-7. [DOI] [PubMed] [Google Scholar]
- 28.Goodman S., Berlin J., Fletcher S., Fletcher R. Manuscript quality before and after peer review and editing at annals of internal medicine. Ann Intern Med. 1994;121:11–21. doi: 10.7326/0003-4819-121-1-199407010-00003. [DOI] [PubMed] [Google Scholar]
- 29.Cobo E., Selva-O'Callagham A., Ribera J.-M., Cardellach F., Dominguez R., Vilardell M. Statistical reviewers improve reporting in biomedical articles: a randomized trial. PLoS One. 2007;2:e332. doi: 10.1371/journal.pone.0000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cobo E., Cortés J., Ribera J.M., Cardellach F., Selva-O’Callaghan A., Kostov B., et al. Effect of using reporting guidelines during peer review on quality of final manuscripts submitted to a biomedical journal: masked randomised trial. BMJ. 2011;343:d6783. doi: 10.1136/bmj.d6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraser N., Brierley L., Dey G., Polka J.K., Pálfy M., Nanni F., et al. The evolving role of preprints in the dissemination of COVID-19 research and their impact on the science communication landscape. PLoS Biol. 2021;19(4):e3000959. doi: 10.1371/journal.pbio.3000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brierley L., Nanni F., Polka J.K., Dey G., Pálfy M., Fraser N., et al. Tracking changes between preprint posting and journal publication during a pandemic. PLoS Biol. 2022;20(2):e3001285. doi: 10.1371/journal.pbio.3001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.