Abstract
Purpose
Infants with macrosomia are more likely to be born to mothers with gestational diabetes mellitus (GDM). This study aimed to investigate the associations between maternal blood glucose levels and fetal weight, placental weight, and risk of macrosomia in mothers with GDM.
Patients and Methods
This retrospective study included 3211 singletons of mothers with GDM at the Shanghai First Maternity and Infant Hospital between January 2017 and December 2019. All women underwent an oral glucose tolerance test (OGTT) during the 24–28 weeks gestation period. Data on fetal and placental parameters were collected at delivery. Multiple linear regression models were used to evaluate the associations of maternal blood glucose levels with fetal weight and placental weight, while multiple logistic regression model was used to estimate the association between maternal blood glucose levels and the risk of macrosomia.
Results
The prevalence of GDM in our study was 7%. Fasting plasma glucose (FPG) was positively correlated with fetal weight (r2=0.0329, P<0.001), and macrosomia risk (odds ratio [OR], 2.42; 95% confidence interval [CI], 1.93–3.04; P<0.001). After adjusting for gestational age, the result remained significant (OR, 2.67; 95% CI, 2.11–3.38; P<0.001). In contrast, there was no significant relationship between 1-h plasma glucose (1hPG) or 2-h plasma glucose (2hPG) and fetal weight (P=0.18, P=0.46). Additionally, 1hPG or 2hPG was not strongly associated with macrosomia risk (OR, 0.95; 95% CI, 0.85–1.05; P=0.32 vs OR, 0.94; 95% CI, 0.85–1.05; P=0.28). Maternal blood glucose levels did not affect placental weight. The associations were similar in women carrying male and female fetuses.
Conclusion
Maternal fasting plasma glucose levels were strongly associated with increased birth weight and macrosomia risk. Our findings suggest that fasting plasma glucose may predict birth weight.
Keywords: gestational diabetes mellitus, blood glucose levels, weight, macrosomia
Introduction
Gestational diabetes mellitus (GDM) has been traditionally defined as a result of varying degrees of glucose intolerance during pregnancy.1 The incidence rate of GDM in the Western Pacific Region has increased in recent years, affecting 10.3% (range, 4.5–20.3%) of pregnancies.2 In addition, GDM increases the likelihood of long-term maternal and fetal complications, such as obesity, glucose metabolism disorders, and cardiovascular disease.2 Moreover, infants born to diabetic mothers have an increased risk of developing short-term complications.3
The most common short-term complication associated with GDM is macrosomia, which occurs in 15–45% of infants born to affected mothers. The incidence rate of macrosomia in mothers with GDM is three-fold higher than those without GDM.4 According to the Pedersen hypothesis, maternal serum glucose values are elevated in women with GDM.5 The placenta allows a significant quantity of blood glucose to enter fetal circulation; however, maternal or exogenous insulin is excluded.6 Consequently, macrosomia is caused by increased fetal fat stores due to hyperglycemia and fetal insulin secretion.7 As a result of macrosomia, neonates are more likely to be admitted to neonatal units and develop shoulder dystocia, clavicle fractures, and brachial plexus trauma. Cesarean birth, postpartum hemorrhage, and vaginal laceration are some of the maternal complications associated with macrosomia.8–12 During pregnancy, the intrauterine growth of the fetus is closely monitored as it is an important parameter for assessing neonatal health.13 It is crucial to pay attention to the correlation between fetal weight and maternal blood glucose levels in pregnancies with GDM. Many studies have reported on the effects of maternal blood glucose levels on fetal weight, placental weight, and risk of macrosomia.14,15 However, conflicting results are available in the literature. To our knowledge, data is limited on the effects of fasting plasma glucose (FPG), 1 h plasma glucose (1hPG), and 2 h plasma glucose (2hPG) on these parameters in GDM.
In this study, we examined the association between maternal blood glucose levels and fetal weight, placental weight, and risk of macrosomia in pregnant women with GDM.
Materials and Methods
Study Design and Participants
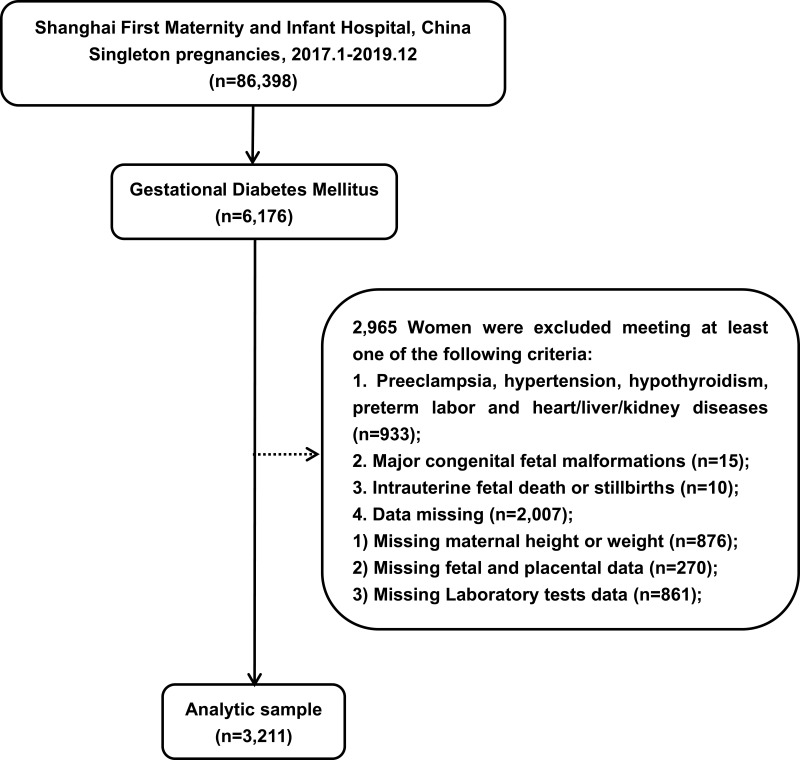
Between January 2017 and December 2019, 86,398 singleton pregnant women were hospitalized in the Shanghai First Maternity and Infant Hospital, School of Medicine of Tongji University, and 6176 women were diagnosed with GDM. The exclusion criteria were as follows (n=2965):
Preeclampsia, hypertension, hypothyroidism, preterm labor and heart/liver/kidney disease (n=933);
Major congenital fetal malformations (n=15);
Intrauterine fetal death or stillbirths (n=10);
Data missing (n=2007).
Finally, a total of 3211 women with GDM were included in this study. Through diet and behavior modification, all women achieved glycemic control. None of the patients were treated with insulin or metformin. This study was approved by the Scientific and Ethical Committee of the Shanghai First Maternity and Infant Hospital affiliated to Tongji University. All individuals provided verbal and written consent.
Data Collection
At each patient’s first visit during pregnancy (12–16 weeks gestation), an interview was held to obtain information on maternal age, height, pregnancy weight, gravidity, parity, and assisted reproductive technology (ART) utilization. Maternal blood glucose levels (FPG, 1hPG, and 2hPG), glycated hemoglobin (HbAlc), total cholesterol (TC), triglyceride (TG), apolipoprotein A1 (ApoA1), apolipoprotein B (ApoB), and ApoA1/ApoB (A1/B) were measured at 24–28 weeks gestation. Data on childbirth was acquired from our institution’s labor and delivery databases. These comprised gestational age (GA), infant birth weight, sex, length, placental weight, length and width of placenta, cord length, and mode of delivery.
Relative Definitions
The International Association of Diabetes and Pregnancy Study Groups (IADPSG) has established diagnostic standards for GDM.16 This criterion is met if any single threshold value of a 75 g or 2 h oral glucose tolerance test (OGTT) is reached or exceeded between 24–28 weeks gestation (fasting value, 5.1 mmol/L; 1 h value, 10.0 mmol/L; or 2 h value, 8.5 mmol/L).
During the first trimester of pregnancy, ultrasonography was used to measure the fetal length from the crown to rump to calculate GA, which corresponds to the last menstrual period of women. In this study, birth weight >4000 g was defined as macrosomia.17
Nurses weighed infants immediately after delivery using an electronic weight scale. A ruler was used to measure the length, width of placenta, and cord length. Using a computerized scale, placentas with attached membranes and umbilical cord were weighed.
We calculated body mass index (BMI) (kg/m2) by dividing the weight (kg) by the height 2 (m2).
The Ponderal Index (PI) was calculated as fetal weight / (fetal length)3 x 100.
Statistical Analysis
We identified the mean ± SD, median (interquartile spacing), and minimum and maximum values for each continuous variable, and Wilcoxon rank sum test was used for intergroup comparisons. Categorical variables are expressed as number (percentages), and Chi-square test was used to analyze intergroup differences. Multiple linear regression models were used to evaluate the associations of maternal blood glucose levels with fetal weight and placental weight, while multiple logistic regression model was used to estimate the association between maternal blood glucose levels and the risk of macrosomia. The associations were examined after adjusting for gestational age, fetal sex, maternal age, and pregnancy BMI, or without any adjustment. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, North Carolina).
Results
Pregnant Women with GDM: Prevalence and Features
A total of 6176 of the 86,398 singleton pregnant women in our study were diagnosed with GDM based on the IADPFG standards. The prevalence of GDM in our study was 7%. A total of 3211 women with GDM were analyzed based on the inclusion criteria (Figure 1).
Figure 1.
Flow chart of women with GDM included in this study.
Table 1 summarizes the main characteristics of maternal, fetal, and placental data at delivery, and laboratory tests. The mean ± SD of the maternal age, elderly pregnancy rate, and pregnancy BMI was 32±4 years, 27.7%, and 26.8±3.2 kg/m2, respectively. Multigravida and multiparity rates were 55.9% and 0.9%, respectively. Of the women, the mean ± SD gestational age was 39.3±0.8 weeks and 52.38% of the infants were male. The mean weight of the fetus and placenta was 3366 g and 547 g, respectively, and 192 fetuses (6%) were diagnosed with macrosomia. Median (range) glucose values for FPG, 1hPG, and 2hPG were 4.7 (3–8), 9.8 (4.3–15.3), and 8.5 (3.7–14.2) mmol/L, respectively, on the OGTT results. Approximately half of all pregnancies were delivered by cesarean section.
Table 1.
Clinical Characteristics of Pregnant Women with GDM (N=3211)
| Variables | N (%) | Mean ± SD | Median (IQR) | Min, Max |
|---|---|---|---|---|
| Age(years) | 32±4 | 32 (29, 35) | 19, 51 | |
| Elderly pregnant, n (%, ≥35) | 889 (27.7) | |||
| Pregnancy BMI (kg/m2) | 26.8±3.2 | 26.5 (24.5, 28.8) | (15.6, 38.9) | |
| Multigravida, n (%) | 1796 (55.9) | |||
| Multiparous, n (%) | 28 (0.9) | |||
| Gestational age(weeks) | 39.2±0.8 | 39.3 (38.6, 39.9) | (37.0, 41.3) | |
| Male fetus (%) | 1682 (52.38) | |||
| Weight(g) | 3366±393 | 3350 (3100, 3615) | (1905, 5200) | |
| Macrosomia, n (%, >4000g) | 192 (6.0) | |||
| Length(cm) | 50±0.5 | 50 (50, 50) | (43, 60) | |
| PI(g/cm3) | 2.7±0.3 | 2.7 (2.5, 2.9) | (1.4, 4.2) | |
| ART, n (%) | 398 (12.4) | |||
| Placental weight(g) | 547±68.5 | 545 (500, 595) | (220, 1020) | |
| Placental length(cm) | 19±2.4 | 19 (18, 20) | (8, 60) | |
| Placental width(cm) | 17.63±2.04 | 18 (17, 18) | (8, 50) | |
| The cord length(cm) | 55±7 | 55 (50, 60) | (6, 110) | |
| F/P weight | 6.2±1 | 6.2 (5.6, 6.8) | (3.8, 13.5) | |
| Cesarean section, n (%) | 1488 (46.3) | |||
| FPG (mmol/L) | 4.7±0.6 | 4.6 (4.3, 5.1) | (3, 8) | |
| 1hPG (mmol/L) | 9.8±1.3 | 10.0 (9.0, 10.5) | (4.3, 15.3) | |
| 2hPG (mmol/L) | 8.5±1.4 | 8.6 (7.6, 9.2) | (3.7, 14.2) | |
| HbAlc (%) | 5.3±0.4 | 5.2 (5.0, 5.5) | (3.0, 11.9) | |
| TC (mmol/L) | 5.8±1.2 | 5.7 (4.9, 6.6) | (1.97, .78) | |
| TG (mmol/L) | 3.4±1.9 | 3.0 (2.2, 4.1) | (0.4, 27.4) | |
| ApoA1 (g/L) | 1.8±0.4 | 1.9 (1.6, 2.1) | (0.3, 2.8) | |
| ApoB (g/L) | 1.2±0.3 | 1.2 (0.9, 1.4) | (0.3, 2.3) | |
| A1/B | 1.7±0.5 | 1.6 (1.3, 1.9) | (0.4, 5.5) |
Abbreviations: BMI, body mass index; PI, Ponderal Index; ART, assisted reproductive technology; F/P, fetal/placental; FPG, fasting plasma glucose; 1hPG, 1-hour plasma glucose; 2hPG, 2-hour plasma glucose; HbAlc, glycated hemoglobin; TC, total cholesterol; TG, triglyceride; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; A1/B, ApoA1/ApoB.
Relationship Between OGTT Results and Fetal, Placental Weight Among Women with GDM
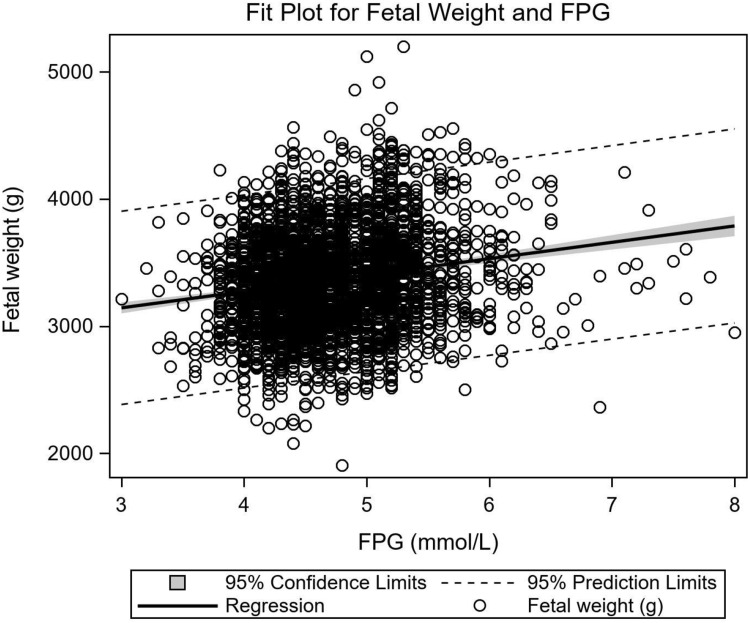
The relationship between OGTT-FPG and fetal weight was strongly positive (linear regression: y=2757.38+128.83x, r2=0.033, P<0.001) (Figure 2), and remained unchanged after adjusting for gestational age, fetal sex, maternal age, and pregnancy BMI at 12–16 weeks gestation. No significant relationships were observed between 1hPG or 2hPG and fetal weight (P=0.18, P=0.46). Placental weight has no significant relationship with OGTT results, including FPG (P=0.2), 1hPG (P=0.48), and 2hPG (P=0.22).
Figure 2.
Relationship between fetal weight and OGTT-FPG results. Fetal weight in relation to OGTT-FPG values in pregnancies with GDM (linear regression: y=2757.38+128.83x, r2=0.033, P<0.001).
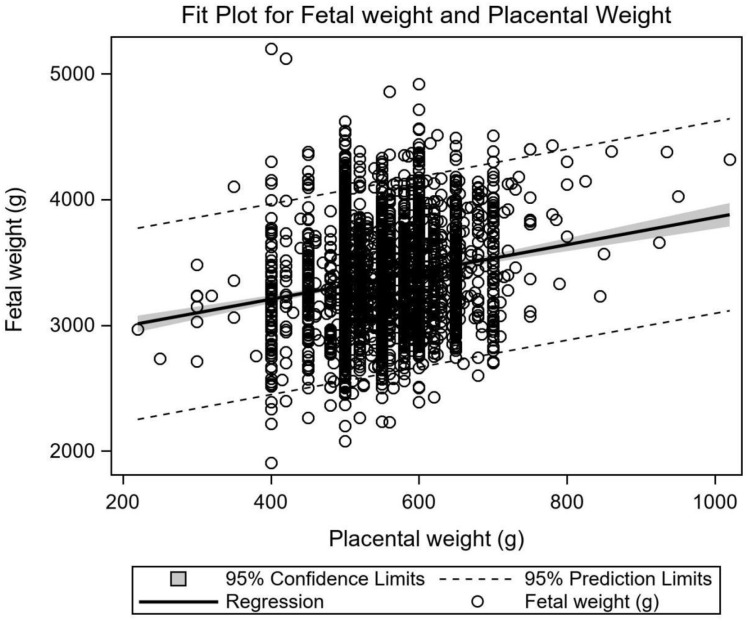
Figure 3 illustrates the significant relationship between fetal and placental weight (linear regression: y=2772.67+1.08x, r2=0.035, P<0.001).
Figure 3.
Relationship between fetal and placental weight. Fetal weight in relation to placental weight in pregnancies with GDM (linear regression: y=2772.67+1.08x, r2=0.035, P<0.001).
Relationship Between OGTT Results and Risk of Macrosomia
The relationship between OGTT results and macrosomia risk is presented in Table 2. For each unit increase in FPG, the risk of macrosomia increased 1.42-fold (OR, 2.42; 95% CI, 1.93–3.04; P<0.001). After adjusting for gestational age, the macrosomia risk increased 1.67-fold (OR, 2.67; 95% CI, 2.11–3.38; P<0.001). After adjusting for fetal sex, maternal age and pregnancy BMI, the macrosomia risk increased 1.06-fold (OR, 2.06; 95% CI, 1.59–2.66; P<0.001).
Table 2.
Relationship Between OGTT Results and the Risk of Macrosomia
| Variables | Algorithm A | Algorithm B* | Algorithm C# | |||
|---|---|---|---|---|---|---|
| P value | OR and 95% CI | P value | OR and 95% CI | P value | OR and 95% CI | |
| FPG | <0.0001 | 2.42(1.93–3.04) | <0.0001 | 2.67(2.11–3.38) | <0.0001 | 2.06(1.59–2.66) |
| 1hPG | 0.3152 | 0.95(0.85–1.05) | 0.6544 | 0.98(0.87–1.09) | 0.6366 | 0.97(0.87–1.09) |
| 2hPG | 0.2840 | 0.94(0.85–1.05) | 0.4776 | 0.96(0.86–1.07) | 0.4466 | 1.04(0.94–1.16) |
Notes: *After adjusting for gestational age. #After adjusting for fetal sex, maternal age and pregnancy BMI.
Abbreviations: FPG, fasting plasma glucose; 1hPG, 1-hour plasma glucose; 2hPG, 2-hour plasma glucose; OR, odds ratio; 95% CI, 95% confidence interval.
However, neither the 1hPG nor 2hPG was strongly associated with macrosomia risk (OR, 0.95; 95% CI, 0.85–1.05; P=0.32 vs OR, 0.94; 95% CI, 0.85–1.05; P=0.28).
Comparison of Male and Female Fetuses
Table 3 compares 3211 pregnant women who carried a male or female fetus (n=1682 and n=1529, respectively). Male fetus weight, length, PI, and fetal/placental weight (F/P) at delivery were significantly higher than that in the female fetus (P<0.01), as expected.
Table 3.
Comparison of Data Between Male and Female Fetus
| Variables | Male Fetus (N=1682) | Female Fetus (N=1529) | P value |
|---|---|---|---|
| Fetal Weight | 3400 (3130, 3660) | 3295 (3060, 3560) | <0.01 |
| Fetal Length | 50 (50, 50) | 50 (50, 50) | <0.01 |
| PI | 2.7 (2.5, 2.9) | 2.6 (2.5, 2.9) | <0.01 |
| Placental weight | 540 (500, 590) | 545 (500, 595) | 0.94 |
| F/P weight | 6.2 (5.7, 6.9) | 6.1 (5.5, 6.7) | <0.01 |
| Cesarean section, n (%) | 802 (47.7) | 686 (44.9) | 0.11 |
| FPG (mmol/L) | 4.7 (4.3, 5.1) | 4.6 (4.3, 5.1) | 0.73 |
| 1hPG (mmol/L) | 10.0 (9.0, 10.6) | 10.0 (9.1, 10.5) | 0.58 |
| 2hPG (mmol/L) | 8.6 (7.7, 9.2) | 8.6 (7.6, 9.2) | 0.84 |
| HbAlc (%) | 5.2 (5.0, 5.5) | 5.2 (5.0, 5.5) | 0.03 |
Abbreviations: PI, Ponderal Index; F/P, fetal/placental; FPG, fasting plasma glucose; 1hPG, 1-hour plasma glucose; 2hPG, 2-hour plasma glucose; HbAlc, glycated hemoglobin.
We compared the two groups based on mean glucose levels at each OGTT time point, to identify a possible independent relationship between fetal sex and maternal glucose metabolism. There were no significant intergroup differences in FPG, 1hPG, or 2hPG (P=0.73, P=0.58, P=0.84).
Discussion
Metabolic alterations in pregnant women with GDM are heterogeneous; thus, they may have different fetal outcomes. Aberrant glucose metabolism during pregnancy, particularly impaired fasting glucose, may operate as an independent risk factor for an abnormal birth weight of newborns.18 In our study, macrosomia risk and birth weight were correlated with maternal fasting plasma glucose values.
According to previous reports, macrosomia is the most frequent complication of GDM and is linearly correlated with maternal plasma glucose levels.19 Our study observed that FPG was related to a higher risk of macrosomia in neonates. Notably, our data demonstrated a significantly positive relationship between FPG and fetal weight, which is consistent to the Pedersen hypothesis. However, 1hPG and 2hPG were not associated with fetal weight. Previously, a substantial negative connection was observed between 2hPG and fetal weight (r2=0.063, P<0.001).15 Impaired FPG and glucose tolerance (IGT) with higher 1 h or 2 h plasma glucose levels on an OGTT are caused by different mechanisms in the general population, although isolated FPG is a typical sign of insulin resistance and is frequently associated with the inability of insulin to reduce hepatic glucose output.20 Individuals with isolated IGT display more severe impairments in beta cell activity with a relative deficit in glucose-induced insulin secretion.21 Given the differing physiological bases of FPG and IGT, pregnancy outcomes and fetal complications, particularly fetal weight, are expected to vary. Fetal weight may be differently and independently affected by fasting, 1 h and 2 h glucose values.
Studies on the association between GDM and placental weight have produced mixed findings.14,22,23 OGTT-2h glucose values and placental weight showed a strong correlation in two studies.15,24 Hyperglycemia causes an increase in the concentration of insulin in the fetal-placental circulation in pregnancies with GDM.25 The placenta synthesizes insulin and insulin-like growth factors to regulate placental growth.26 However, in our study, there was no discernible relationship between the OGTT results and placental weight. Our study participants were diagnosed after 24 weeks gestation, and their plasma glucose levels were controlled through strict diet and exercise, as recommended by a doctor. In addition, a previous study showed that placental weight was not related to HbA1c levels.14 This suggests that treatment could impact the relationship between maternal plasma glucose levels and placental weight, which may explain why our data did not indicate any association between the two groups.
Moreover, our data indicated that fetal weight was significantly correlated with placental weight, which was consistent with previous studies.27,28 Additionally, we divided our study population into those carrying male and female fetuses. OGTT results showed no significant intergroup differences. Consequently, fetal sex may not affect maternal glucose metabolism during pregnancy with GDM, which agrees with a previous finding.29 In contrast, some studies have previously suggested an association between fetal sex and GDM.30–34 The differences between these results may be due to the use of diverse diagnostic criteria for GDM and various ethnicities of the participants.
This study had limitations. First, nutritional status and gestational weight gain may have a significant impact on fetal growth and weight. These variables were not assessed in this study. However, we believe that such a relationship is credible. When adjusting for gestational age, fetal sex, maternal age, and pregnancy BMI, the relationship described above remained unchanged. Second, our study included pregnant women with GDM who had achieved glycemic control through diet and behavioral modifications. We did not include women with normal glucose metabolism as a control group. Therefore, we only focused on the relationship between maternal glucose metabolism and fetal weight, placental weight, and the association between maternal glucose metabolism and fetal sex in pregnancy with GDM. Third, we did not consider other adverse pregnancy outcomes, such as shoulder dystocia, delivery injuries, or the requirement for neonatal intensive care.
Conclusion
In conclusion, our results demonstrate significant associations between maternal FPG levels and higher birth weight, risk of macrosomia in pregnancies with GDM. Our findings indicate that FPG may be a stronger independent predictor of birth weight than 1hPG and 2hPG. Additionally, FPG was not highly associated with placental weight. Additional research is required to assess the association between maternal plasma glucose levels and other pregnancy outcomes.
Acknowledgments
We thank all the study participants for the provision of the data used.
Funding Statement
This work was supported by the Shanghai Natural Science Foundation (No.19ZR1440300) and the Shanghai Science and Technology Commission Innovation Plan (No.201409004500).
Ethics Approval and Consent to Participate
This study was approved by the Scientific and Ethical Committee of the Shanghai First Maternity and Infant Hospital affiliated to Tongji University. All participants were informed about the purpose of the study, in accordance with the Declaration of Helsinki. All individuals provided verbal and written consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Poomalar GK. Changing trends in management of gestational diabetes mellitus. World J Diabetes. 2015;6(2):284–295. doi: 10.4239/wjd.v6.i2.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):47. doi: 10.1038/s41572-019-0098-8 [DOI] [PubMed] [Google Scholar]
- 3.Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. 2018;29(11):743–754. doi: 10.1016/j.tem.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 4.Fadl HE, Ostlund IK, Magnuson AF, Hanson US. Maternal and neonatal outcomes and time trends of gestational diabetes mellitus in Sweden from 1991 to 2003. Diabet Med. 2010;27(4):436–441. doi: 10.1111/j.1464-5491.2010.02978.x [DOI] [PubMed] [Google Scholar]
- 5.Pedersen J. Diabetes and pregnancy; blood sugar of newborn infants during fasting and glucose administration. Ugeskr Laeger. 1952;114(21):685. [PubMed] [Google Scholar]
- 6.Scholl TO, Sowers M, Chen X, Lenders C. Maternal glucose concentration influences fetal growth, gestation, and pregnancy complications. Am J Epidemiol. 2001;154(6):514–520. doi: 10.1093/aje/154.6.514 [DOI] [PubMed] [Google Scholar]
- 7.Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011;204(6):479–487. doi: 10.1016/j.ajog.2010.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Sullivan EP, Avalos G, O’Reilly M, et al. Atlantic Diabetes in Pregnancy (DIP): the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia. 2011;54(7):1670–1675. doi: 10.1007/s00125-011-2150-4 [DOI] [PubMed] [Google Scholar]
- 9.Sudasinghe BH, Wijeyaratne CN, Ginige PS. Long and short-term outcomes of Gestational Diabetes Mellitus (GDM) among South Asian women - a community-based study. Diabetes Res Clin Pract. 2018;145:93–101. doi: 10.1016/j.diabres.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 10.Murray SR, Reynolds RM. Short- and long-term outcomes of gestational diabetes and its treatment on fetal development. Prenat Diagn. 2020;40(9):1085–1091. doi: 10.1002/pd.5768 [DOI] [PubMed] [Google Scholar]
- 11.Mitanchez D, Yzydorczyk C, Siddeek B, Boubred F, Benahmed M, Simeoni U. The offspring of the diabetic mother--short- and long-term implications. Best Pract Res Clin Obstet Gynaecol. 2015;29(2):256–269. [DOI] [PubMed] [Google Scholar]
- 12.Al-Khalifah R, Al-Subaihin A, Al-Kharfi T, Al-Alaiyan S, Alfaleh KM. Neonatal short-term outcomes of gestational diabetes mellitus in Saudi mothers: a retrospective cohort study. J Clin Neonatol. 2012;1(1):29–33. doi: 10.4103/2249-4847.92241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGuire SF. Understanding the implications of birth weight. Nurs Womens Health. 2017;21(1):45–49. doi: 10.1016/j.nwh.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 14.Clarson C, Tevaarwerk GJ, Harding PG, Chance GW, Haust MD. Placental weight in diabetic pregnancies. Placenta. 1989;10(3):275–281. doi: 10.1016/0143-4004(89)90028-3 [DOI] [PubMed] [Google Scholar]
- 15.Taricco E, Radaelli T, Nobile de Santis MS, Cetin I. Foetal and placental weights in relation to maternal characteristics in gestational diabetes. Placenta. 2003;24(4):343–347. doi: 10.1053/plac.2002.0913 [DOI] [PubMed] [Google Scholar]
- 16.Zhu WW, Yang HX. Diagnosis of gestational diabetes mellitus in China. Diabetes Care. 2013;36(6):e76. doi: 10.2337/dc12-2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu L, Zhang R, Zhang S, et al. 中国不同胎龄新生儿出生体重曲线研制 [Chinese neonatal birth weight curve for different gestational age]. Zhonghua Er Ke Za Zhi. 2015;53(2):97–103. Chinese. [PubMed] [Google Scholar]
- 18.Goldstein RF, Abell SK, Ranasinha S, et al. Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018;16(1):153. doi: 10.1186/s12916-018-1128-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metzger BE, Lowe LP, et al; Group HSCR. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 20.Tripathy D, Carlsson M, Almgren P, et al. Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the botnia study. Diabetes. 2000;49(6):975–980. doi: 10.2337/diabetes.49.6.975 [DOI] [PubMed] [Google Scholar]
- 21.Hanefeld M, Koehler C, Fuecker K, et al. Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in impaired glucose tolerance for atherosclerosis and diabetes study. Diabetes Care. 2003;26(3):868–874. doi: 10.2337/diacare.26.3.868 [DOI] [PubMed] [Google Scholar]
- 22.Evers IM, Nikkels PG, Sikkema JM, Visser GH. Placental pathology in women with type 1 diabetes and in a control group with normal and large-for-gestational-age infants. Placenta. 2003;24(8–9):819–825. doi: 10.1016/S0143-4004(03)00128-0 [DOI] [PubMed] [Google Scholar]
- 23.Baptiste-Roberts K, Salafia CM, Nicholson WK, Duggan A, Wang NY, Brancati FL. Maternal risk factors for abnormal placental growth: the national collaborative perinatal project. BMC Pregnancy Childbirth. 2008;8:44. doi: 10.1186/1471-2393-8-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lao TT, Lee CP, Wong WM. Placental weight to birthweight ratio is increased in mild gestational glucose intolerance. Placenta. 1997;18(2–3):227–230. doi: 10.1016/S0143-4004(97)90097-7 [DOI] [PubMed] [Google Scholar]
- 25.Jiang S, Teague AM, Tryggestad JB, Lyons TJ, Chernausek SD. Fetal circulating human resistin increases in diabetes during pregnancy and impairs placental mitochondrial biogenesis. Mol Med. 2020;26(1):76. doi: 10.1186/s10020-020-00205-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiden U, Glitzner E, Hartmann M, Desoye G. Insulin and the IGF system in the human placenta of normal and diabetic pregnancies. J Anat. 2009;215(1):60–68. doi: 10.1111/j.1469-7580.2008.01035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nascente LMP, Grandi C, Aragon DC, Cardoso VC. Placental measurements and their association with birth weight in a Brazilian cohort. Rev Bras Epidemiol. 2020;23:e200004. doi: 10.1590/1980-549720200004 [DOI] [PubMed] [Google Scholar]
- 28.Grandi C, Veiga A, Mazzitelli N, Cavalli Rde C, Cardoso V. Placental growth measures in relation to birth weight in a Latin American population. Rev Bras Ginecol Obstet. 2016;38(8):373–380. doi: 10.1055/s-0036-1586721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cosson E, Diallo A, Docan M, et al. Fetal gender is not associated with either gestational diabetes mellitus or placental weight: a cohort study. Diabetes Metab. 2016;42(4):276–279. doi: 10.1016/j.diabet.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 30.Sheiner E, Levy A, Katz M, Hershkovitz R, Leron E, Mazor M. Gender does matter in perinatal medicine. Fetal Diagn Ther. 2004;19(4):366–369. doi: 10.1159/000077967 [DOI] [PubMed] [Google Scholar]
- 31.Retnakaran R, Shah BR. Fetal sex and the natural history of maternal risk of diabetes during and after pregnancy. J Clin Endocrinol Metab. 2015;100(7):2574–2580. doi: 10.1210/jc.2015-1763 [DOI] [PubMed] [Google Scholar]
- 32.Retnakaran R, Kramer CK, Ye C, et al. Fetal sex and maternal risk of gestational diabetes mellitus: the impact of having a boy. Diabetes Care. 2015;38(5):844–851. doi: 10.2337/dc14-2551 [DOI] [PubMed] [Google Scholar]
- 33.Ehrlich SF, Eskenazi B, Hedderson MM, Ferrara A. Sex ratio variations among the offspring of women with diabetes in pregnancy. Diabet Med. 2012;29(9):e273–e278. doi: 10.1111/j.1464-5491.2012.03663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend Med. 2007;4(1):19–30. doi: 10.1016/S1550-8579(07)80004-0 [DOI] [PubMed] [Google Scholar]