Abstract
Background
The clinical presentation of Malassezia folliculitis (MF) can imitate acne vulgaris (AV), making it difficult to distinguish between the two conditions. Moreover, MF can coexist with AV in the same patient. The incidence of MF in patients clinically diagnosed with AV may be underestimated. This study aimed to determine the prevalence, associated factors, and clinical characterization of MF patients diagnosed with AV.
Materials and Methods
Three hundred twenty new acne patients were questioned regarding general information, including age, sex, itchy symptoms, and past treatment history with antibiotics and steroids within four weeks. Clinical presentations of AV (location and severity), dandruff, and seborrheic dermatitis were examined by a dermatologist. Cytologic studies to determine the abnormal proliferation of Malassezia yeasts were performed from pustules or, in the absence of pustules, comedo-like papules, and comedones. The smears were stained with methylene blue and evaluated under a light microscope by the researcher.
Results
The prevalence of MF in patients clinically diagnosed with AV was 28.8% (95% Confidence interval: CI = 23.8% - 33.7%), which can be classified as 24.7% were AV with MF and the remaining 4.1% were MF only. This study revealed that patients diagnosed with MF were 7.38 times more likely to have itchy symptoms than patients diagnosed with AV. MF patients had 8.89 times and 9.17 times higher risk of acneiform lesions on the scalp/ hairline and upper back than those who did not have MF, respectively.
Conclusion
This present study revealed a high prevalence of MF in patients clinically diagnosed with AV. Dermatologists should be aware of MF when encountering AV patients with acneiform lesions on the scalp/ hairline and upper back with pruritus. Diagnosis based on clinical presentations alone may lead to misdiagnosis. Methylene blue staining is easy to perform and beneficial to diagnose MF.
Keywords: Malassezia folliculitis, Pityrosporum folliculitis, acne vulgaris, methylene blue, yeast
Introduction
Acne vulgaris (AV) is characterized by a mixed eruption of comedones, papules, pustules, and nodules, mainly occurring on the face but may involve the neck, upper chest, and back. The pathology of AV is an inflammation of the pilosebaceous units triggered by excessive sebum production, the abnormal keratinization of follicular epithelium, the abnormal proliferation of Cutibacterium acnes, and host immune responses. However, even now, with a lot of pieces of knowledge about the pathogenesis of acne, the exact cause has yet to be discovered.1,2
Malassezia folliculitis (MF) results from an overgrowth of Malassezia yeasts, which are normal skin flora. The abnormal proliferation of yeast can cause inflammation of the hair follicles by two possible mechanisms. The first mechanism is caused by the lipase and phospholipase enzyme activity of Malassezia yeast, damaging skin barrier function and inducing irritation.3 The second mechanism is the ability of Malassezia yeast that can stimulate keratinocyte production of inflammatory cytokines via Toll-like receptor 2 and activate complement cascades by both the classical and alternative pathways.4,5
MF presented as small, monomorphic, itchy comedo-like papules and pustules, particularly on the hairline, face, and upper trunk. The clinical presentation of MF can imitate AV, making it difficult to distinguish between the two conditions. Moreover, MF can coexist with AV in the same patient. Patients with AV usually have increased sebum production, predisposing them to develop MF.6–9 Therefore, it is important to identify the existence of MF in patients clinically diagnosed with AV for further proper management.
This study aimed to determine the prevalence, associated factors, and clinical characterization of MF in patients clinically diagnosed with AV.
Patients and Methods
Patients
This prospective, observational, analytic study included all new patients clinically diagnosed with AV who attended the Dermatologic clinic of Mae Fah Luang hospital, Bangkok, during the rainy season in Thailand (from July to September 2022). All patients and a parent or legal guardian of patients under the age of 18 years had given their written informed consent. Three hundred twenty patients were questioned regarding general background information, including age, sex, itchy symptoms, and prior treatment history with antibiotics and steroids within four weeks. A dermatologist examined clinical presentations of AV (location and severity), dandruff, and seborrheic dermatitis.
Clinical Grading of the Lesions
The severity grading of acne was assessed by a dermatologist based on the Leeds revised acne grading system.10 Mild acne symptoms are mostly presented with comedones, inflammatory papules, and pustules with less than 10 lesions. Moderate acne symptoms include inflammatory papules with more than 10 lesions and/or inflammatory nodules with less than 5 lesions. Severe acne symptoms include numerous inflammatory papules, pustules, and/or nodulocystic lesions or sinus tracts.
Microscopic Examination
All patients diagnosed with AV underwent cytologic studies for the presence of Malassezia yeasts. Samples were obtained from pustules or, in the absence of pustules, comedo-like papules and comedones from different parts of the face (forehead, right cheek, left cheek, chin) by comedone extractors. Three different sampling sites were collected for each patient to ensure adequate randomized specimens for microscopic examination. Methylene blue was applied to a smear for 30 seconds, rapid decolorization with water, and drop immersion oil before being covered with a coverslip. The smears were evaluated under a light microscope by the researcher. The total number of spores was counted wherever the smears showed the highest number. The result of methylene blue staining was interpreted as follows: if more than 30 spores were counted, the smear was marked as MF. If there were only numerous bacillus bacteria, it was interpreted as AV. The smear was categorized as AV with MF if both criteria were met.
Statistical Analysis
Demographic data and the prevalence of MF in clinically diagnosed acne vulgaris patients were analyzed by using descriptive statistics such as frequency and percentage. In the case of numeric data, the mean and standard deviations of both groups were reported. Chi-square or Fisher’s exact test (categorical data) and independent t-test (numeric data) were used to compare the MF positive and MF negative groups. Simple and multiple logistic regression models were used to compare the positive and negative MF groups in univariable and multivariable analyses. Factors variables with p value less than 0.05 from the univariable analysis were entered into a backward stepwise, multiple logistic regression model. Crude and adjusted odds ratio (OR) with a 95% confidence interval (CI) had been reported with significant factors with p value less than 0.05 in the final analysis. All data were categorized and analyzed using IBM SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, USA).
Results
Patients
This study enrolled 320 new patients clinically diagnosed with AV and classified as 238 females (74.4%) and 82 males (25.6%). The average mean (standard deviation, SD) age at diagnosis was 25.6 (±7.6) years. All patients aged 11 to 50 years were divided into two groups, 94 patients (29.4%) aged less than 20 and 226 patients (70.6%) aged 20 to 50. All patients lived in Bangkok, the central part of Thailand. This study was performed in the rainy season, during which the average temperatures range from 28.6 to 29 °C, and the humidity ranges from 78 to 81%.
Clinical Presentations
One hundred eighty-four patients (57.5%) presented with moderate severity of AV, 113 patients (35.3%) and 23 patients (7.2%) presented with mild and severe forms of AV, respectively. Two hundred forty-eight patients (77.5%) had acneiform lesions in more than one area of the face and/or the body. The common locations of acneiform lesions were the cheeks (79.4%), chin (60.6%), and forehead (55.9%). Forty-six patients (14.4%) reported itchy symptoms. Dandruff was detected in 44 patients (13.8%), while seborrheic dermatitis was found in 14 patients (4.4%) at the same time as clinically diagnosed with AV.
Associated Factors
One hundred sixty-three patients (50.9%) were using clindamycin, 114 patients (35.6%) and 14 patients (4.4%) were using benzoyl peroxide and topical retinoids, respectively. Many of these patients were using more than one topical treatment concurrently. Twenty patients (6.3%) reported steroid use (topical or oral), while 15 patients (4.7%) had previously received oral antibiotic therapy within four weeks.
A Microscopic Examination of Methylene Blue Staining
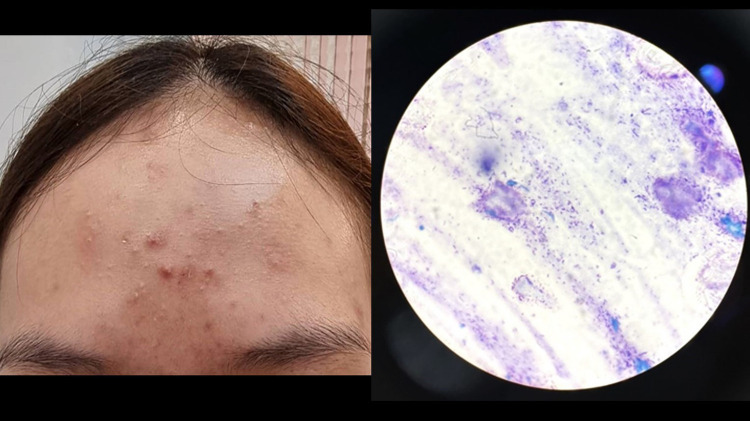
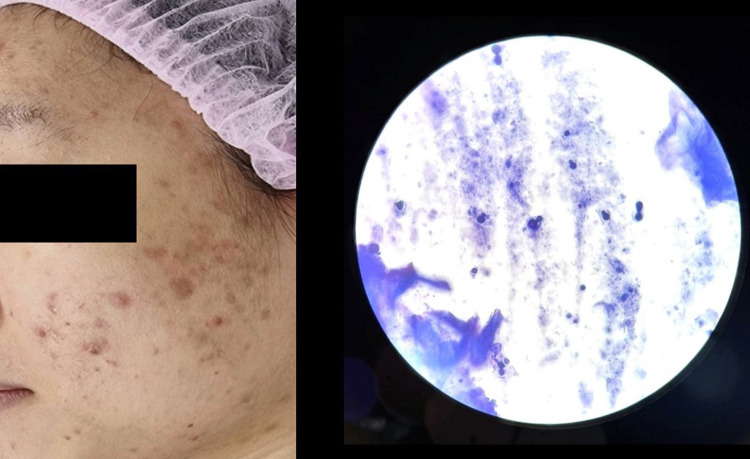
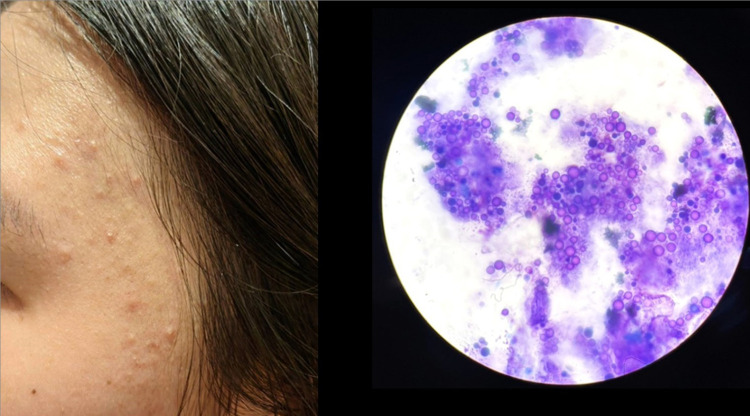
Methylene blue staining revealed that the prevalence of MF was 28.8% in patients clinically diagnosed with AV (95% Confidence interval: CI = 23.8% - 33.7%). There were 228 patients (71.2%) that had positive results for bacillus bacteria only (Figure 1), 79 patients (24.7%) had positive for both bacillus bacteria and spores (Figure 2), and 13 patients (4.1%) had positive results for spores (Figure 3).
Figure 1.
Acne vulgaris.
Note: Methylene blue staining revealed numerous bacillus bacteria (original magnification ×1000).
Figure 2.
Acne vulgaris with Malassezia folliculitis.
Note: Methylene blue staining demonstrated numerous bacillus bacteria with multiple blastospores (original magnification ×1000).
Figure 3.
Malassezia folliculitis.
Note: Methylene blue staining showed numerous spores and blastospores (original magnification ×1000).
Of those patients diagnosed with MF, 60 patients (65.2%) were women, and 32 patients (34.8%) were men. The average mean (SD) age at diagnosis was 23.0 (±7.7). The common locations of MF were the forehead, cheeks, upper back, and scalp/ hairline. Comparison of associated factors between the MF positive group and MF negative group in patients clinically diagnosed with AV are presented in Table 1.
Table 1.
Comparison of Outcome Variables Between the Malassezia Folliculitis Positive Group and Malassezia Folliculitis Negative Group in Patients Clinically Diagnosed with Acne Vulgaris (n =320)
| Variables | MF Positive (n=92) | MF Negative (n=228) | p-value* |
|---|---|---|---|
| Age, mean (SD), years | 23.0(7.7) | 26.6(7.5) | 0.001 |
| Age < 20 years, n (%) | 42(45.6) | 52(22.8) | <0.001 |
| Male sex, n (%) | 32(34.8) | 50(21.9) | 0.017 |
| Itchy symptom, n (%) | 33(35.9) | 13(5.7) | <0.001 |
| Dandruff, n (%) | 29(31.5) | 15(6.6) | <0.001 |
| Underlying seborrheic dermatitis, n (%) | 7(7.6) | 10(4.4) | 0.245 |
| Disease severity of acne, n (%) | |||
| Grade 1, mild | 39(42.4) | 74(32.5) | 0.209 |
| Grade 2, moderate | 46(50.0) | 138(60.5) | |
| Grade 3, severe | 7(7.6) | 16(7.0) | |
| Location of acne, n (%) | |||
| Forehead | 77(83.7) | 102(44.7) | <0.001 |
| Scalp and hairline | 55(59.8) | 19(8.3) | <0.001 |
| Cheek | 71(71.2) | 183(80.3) | 0.536 |
| Chin | 50(54.3) | 144(63.2) | 0.144 |
| Neck | 15(16.3) | 13(5.7) | 0.002 |
| Upper chest | 23(25.0) | 6(2.6) | <0.001 |
| Upper back | 57(62.0) | 17(7.5) | <0.001 |
| Prior medication used, n (%) | |||
| Oral or topical steroid use | 10(10.8) | 10(4.4) | 0.002 |
| Oral antibiotics | 4(4.4) | 11(4.8) | 0.855 |
| Topical clindamycin | 36(39.1) | 127(55.7) | 0.007 |
| Topical benzoyl peroxide | 23(25.0) | 91(39.9) | 0.012 |
| Topical retinoid | 2(2.2) | 12(5.3) | 0.221 |
Notes: *Chi-square or Fisher’s exact test (categorical data) and independent t-test (numeric data) were used to compare the MF positive and MF negative groups.
Abbreviation: MF, Malassezia folliculitis.
Associated factors with p value less than 0.05 from the univariable analysis were entered into a backward stepwise, multiple logistic regression model. Significant factors with p value less than 0.05 in the final analysis were reported with crude and adjusted odds ratio (OR) with a 95% confidence interval (CI) (Table 2).
Table 2.
Univariable and Multivariable Analysis to Compare Between Malassezia Folliculitis Positive Group and Malassezia Folliculitis Negative Group
| Variables | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| Crude Odds Ratio (OR) | 95% CI of Crude OR | p-value* | Adjusted Odds Ratio (OR) | 95% CI of Adjusted OR | p-value** | |
| Age < 20 years | 2.84 | 1.70–4.75 | <0.001 | |||
| Male sex | 1.90 | 1.11–3.23 | 0.018 | |||
| Itchy symptom | 9.25 | 4.58–18.69 | <0.001 | 7.38 | 2.87–18.99 | <0.001 |
| Dandruff | 6.54 | 3.30–12.95 | <0.001 | 3.07 | 1.07–8.83 | 0.038 |
| Forehead | 6.34 | 3.44–11.69 | <0.001 | 2.69 | 1.15–6.28 | 0.022 |
| Scalp and hairline | 16.35 | 8.73–30.64 | <0.001 | 8.89 | 3.90–20.28 | <0.001 |
| Neck | 3.22 | 1.47–7.08 | 0.004 | |||
| Upper chest | 12.33 | 4.83–31.52 | <0.001 | 4.52 | 1.17–17.52 | 0.029 |
| Upper back | 20.21 | 10.56–38.68 | <0.001 | 9.17 | 3.097–21.21 | <0.001 |
| Oral or topical steroid use | 2.66 | 1.07–6.62 | 0.036 | |||
| Topical clindamycin | 0.51 | 0.31–0.84 | 0.008 | |||
| Topical benzoyl peroxide | 0.50 | 0.29–0.86 | 0.013 | |||
Notes: *Simple and multiple logistic regression models were used to compare the positive and negative MF groups in univariable and multivariable analyses. **Factors variables with p value less than 0.05 from the univariable analysis were entered into a backward stepwise, multiple logistic regression model. Crude and adjusted odds ratio (OR) with 95% confidence interval (CI) had been reported with significant factors with p value less than 0.05 in the final analysis.
Abbreviations: OR, odds ratio; CI, confidence interval.
Discussion
MF is often misdiagnosed as AV and can coexist with AV in the same patient.11–13 This study reveals that the prevalence of MF in Thai patients clinically diagnosed with AV was 28.8%, which was higher than a previous study in Turkey and Korea, where the prevalence of MF among patients with AV was reported to be 25.3% and 25%, respectively.7,14 The prevalence of MF in AV patients is significantly higher than the prevalence of MF in the population worldwide, which has been reported to be only 1–16.5%.15 In addition, The coexistence of AV and MF was detected in 79 patients (24.7%) of all patients clinically diagnosed with AV.
Comparing the MF positive and MF negative groups demonstrated that the age younger than 20, itchy symptoms, dandruff, and acneiform lesions on the forehead, scalp/ hairline, upper chest, and upper back, had a significant association with MF (p<0.001). While gender, underlying seborrheic dermatitis, the severity of acne, and prior steroid and antibiotic use did not show a significant association with MF (p>0.001). This information confirms existing knowledge about MF that it is more common in young adults who present as tiny, uniform, itchy papules, and pustules, particularly on the forehead, hairline, upper chest, and back.6,15 The results did not show a significant association between MF and seborrheic dermatitis but revealed a significant association with dandruff (p <0.001). Dandruff is considered a variant of seborrheic dermatitis.16,17 Steroid and antibiotics use (both topical and oral) within one month did not have a significant association with MF (p>0.001), which may be caused by the duration of usage not being long enough to make the substantial relationship as previous studies.9,18 This study also demonstrated that the cheek was another common location for MF. Numerous bacillus bacteria were usually found in the same smears from acneiform lesions on the cheeks of MF patients, indicating that the cheek location is often the coexistence of AV and MF.
After being tested with multiple logistic regression models, only three statistically significant factors were associated with MF. They were those who had MF had 7.38 times more likely to have itchy symptoms than those who did not have MF. The MF patients had 8.89 times and 9.17 times higher risk of acneiform lesions on the scalp/ hairline and upper back than those who did not have MF, respectively.
The pustular lesions of MF can be confused with AV. MF can be diagnosed by clinical presentation compatible with laboratory tests such as direct microscopy, histopathology, and fungal culture. A direct microscopic examination for the abnormal proliferation of Malassezia yeasts is positive more often than histology.19,20 The culture of Malassezia yeasts typically requires special media and time consuming, so it is not routinely performed. Another from that, Malassezia yeasts are normal skin flora, and culture results may frequently be false positive.21,22 This study used methylene blue stains to identify abnormal proliferation of Malassezia yeasts and bacillus bacteria, enabling a rapid diagnosis for MF and AV. This method is inexpensive, easily performed at the outpatient clinic, and can reveal the causes of folliculitis. Based on cytologic diagnostic criteria for MF, sampling from 3 pustules, if more than 30 spores are found, it is considered positive for MF.13,23 Methylene blue staining can show many spores, blastospores, footprint-shaped spores (budding yeasts), keratinocytes, and WBCs, as reported in the histopathology of MF.20
There were three limitations of this study: First, we did not collect data about genetics, hormonal abnormalities, and history of taking the hormonal pill; Second, we did not perform bacterial and fungal cultures; Third, we did not report the effect of antifungal treatment in MF positive group, as it was not the primary objective of this study, however, all patients showed clinical improvement within two weeks after starting treatment with oral fluconazole 100–200 mg daily, and/or topical 2% ketoconazole cream applied twice daily.
Conclusion
This present study revealed the prevalence of MF in patients clinically diagnosed with AV was 28.8%. MF can imitate and coexist with AV. Dermatologists should be aware of MF when encountering AV patients with acneiform lesions on the scalp/ hairline and upper back with pruritus. Diagnosis based on clinical presentations alone may lead to misdiagnosis. Methylene blue staining is easy to perform and beneficial to diagnose MF.
Acknowledgments
All authors would like to thank the participants for their participation and the School of Antiaging and Regenerative Medicine, Mae Fah Luang University, for their research facilities.
Funding Statement
This study did not receive any funding.
Data Sharing Statement
Unavailable data, but the reader can personally request to access the data via the personal email of the authors.
Statement of Ethics
The present study was conducted in accordance with the World Medical Association Declaration of Helsinki. All subjects and a parent or legal guardian of subjects under the age of 18 years had given their written informed consent. The study protocol was reviewed and approved by the Ethical Research Committee of Mae Fah Luang University, approval number COA 104/2022.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Akaza N, Takasaki K, Nishiyama E., et al. The Microbiome in Comedonal Contents of Inflammatory Acne Vulgaris is Composed of an Overgrowth of Cutibacterium Spp. and Other Cutaneous Microorganisms. Clin Cosmet Investig Dermatol. 2022;15:2003–2012. doi: 10.2147/CCID.S379609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chilicka K, Rusztowicz M, Rogowska AM, et al. Efficacy of hydrogen purification and cosmetic acids in the treatment of acne vulgaris: a preliminary report. J Clin Med. 2022;11(21):6269. doi: 10.3390/jcm11216269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faergemann J, Bergbrant IM, Dohsé M, Scott A, Westgate G. Seborrhoeic dermatitis and Pityrosporum (Malassezia) folliculitis: characterization of inflammatory cells and mediators in the skin by immunohistochemistry. Br J Dermatol. 2001;144(3):549–556. doi: 10.1046/j.1365-2133.2001.04082 [DOI] [PubMed] [Google Scholar]
- 4.Baroni A, Orlando M, Donnarumma G, et al. Toll-like receptor 2 (TLR2) mediates intracellular signaling in human keratinocytes in response to Malassezia furfur. Arch Dermatol Res. 2006;297(7):280–288. doi: 10.1007/s00403-005-0594-4 [DOI] [PubMed] [Google Scholar]
- 5.Ljubojević S, Skerlev M, Lipozencić J, Basta-Juzbasić A. The role of Malassezia furfur in dermatology. Clin Dermatol. 2002;20(2):179–182. doi: 10.1016/s0738-081x(01)00240-1 [DOI] [PubMed] [Google Scholar]
- 6.Paichitrojjana A. Malassezia folliculitis: a review article. J Med Assoc Thai. 2022;105(2):160–167. doi: 10.35755/jmedassocthai.2022.02.13268 [DOI] [Google Scholar]
- 7.Pürnak S, Durdu M, Tekindal MA, Güleç AT, Seçkin D. The Prevalence of Malassezia Folliculitis in Patients with Papulopustular/Comedonal Acne, and Their Response to Antifungal Treatment. Skinmed. 2018;16(2):99–104. [PubMed] [Google Scholar]
- 8.Ayers K, Sweeney SM, Wiss K. Pityrosporum folliculitis: diagnosis and management in 6 female adolescents with acne vulgaris. Arch Pediatr Adolesc Med. 2005;159(1):64–67. doi: 10.1001/archpedi.159.1.64 [DOI] [PubMed] [Google Scholar]
- 9.Youn NH, Cha SH, Park SD. Malassezia Yeasts in Acne Vulgaris. Korean J Dermatol. 2002;1;1453–1460. [Google Scholar]
- 10.O’brien SC, Lewis JB, Cunliffe WJ. The Leeds revised acne grading system. J Dermatol Treat. 2009;9(4):215–220. doi: 10.3109/09546639809160698 [DOI] [Google Scholar]
- 11.Durdu M, Güran M, Ilkit M. Epidemiological characteristics of Malassezia folliculitis and use of the May-Grünwald-Giemsa stain to diagnose the infection. Diagn Microbiol Infect Dis. 2013;76(4):450–457. doi: 10.1016/j.diagmicrobio.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 12.Prindaville B, Belazarian L, Levin NA, Wiss K. Pityrosporum folliculitis: a retrospective review of 110 cases. J Am Acad Dermatol. 2018;78(3):511–514. doi: 10.1016/j.jaad.2017.11.022 [DOI] [PubMed] [Google Scholar]
- 13.Tu WT, Chin SY, Chou CL, et al. Utility of Gram staining for diagnosis of Malassezia folliculitis. J Dermatol. 2018;45(2):228–231. doi: 10.1111/1346-8138.14120 [DOI] [PubMed] [Google Scholar]
- 14.Kang SH, Kim HU. The isolation of malassezia yeasts in the comedones of acne vulgaris. Korean J Med Mycol. 1999;4(1):33–39. [Google Scholar]
- 15.Henning MA, Jemec GB, Saunte DM. Malassezia folliculitis. Ugeskr Laeger. 2020;182(47):V08200572. [PubMed] [Google Scholar]
- 16.Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3(2). doi: 10.13188/2373-1044.1000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudramurthy SM, Honnavar P, Dogra S, Yegneswaran PP, Handa S, Chakrabarti A. Association of Malassezia species with dandruff. Indian J Med Res. 2014;139(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- 18.Yu HJ, Lee SK, Son SJ, Kim YS, Yang HY, Kim JH. Steroid acne vs. Pityrosporum folliculitis: the incidence of Pityrosporum ovale and the effect of antifungal drugs in steroid acne. Int J Dermatol. 1998;37(10):772–777. doi: 10.1046/j.1365-4362.1998.00229 [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Razek M, Fadaly G, Abdel-Raheim M. Pityrosporum (Malassezia) folliculitis in Saudi Arabia–diagnosis and therapeutic trials. Clin Exp Dermatol. 1995;20(5):406–409. doi: 10.1111/j.1365-2230.1995.tb01358 [DOI] [PubMed] [Google Scholar]
- 20.Durdu M, Ilkit M. First step in the differential diagnosis of folliculitis: cytology. Crit Rev Microbiol. 2013;39(1):9–25. doi: 10.3109/1040841X.2012.682051 [DOI] [PubMed] [Google Scholar]
- 21.Grice EA, Dawson TL. Host-microbe interactions: malassezia and human skin. Curr Opin Microbiol. 2017;40:81–87. doi: 10.1016/j.mib.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 22.Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev. 2012;25(1):106–141. doi: 10.1128/CMR.00021-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki C, Hase M, Shimoyama H, Sei Y. Treatment Outcomes for Malassezia Folliculitis in the Dermatology Department of a University Hospital in Japan. Med Mycol J. 2016;57(3):E63–6. doi: 10.3314/mmj.16-00003 [DOI] [PubMed] [Google Scholar]