Abstract
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease that frequently develops in patients with psoriasis (PsO) but can also occur spontaneously. As a result, PsA diagnosis and treatment is commonly delayed, or even missed outright due to the manifold of clinical presentations that patients often experience. This inevitably results in progressive articular damage to axial and peripheral joints and entheses. As such, patients with PsA frequently experience reduced expectancy and quality of life due to disability. More recently, research has aimed to improve PsA diagnosis and prognosis by identifying novel disease biomarkers.
Methods
Here, we conducted a systematic review of the published literature on candidate biomarkers for PsA diagnosis and prognosis in MEDLINE(Pubmed), EMBase and the Cochrane library with the goal to identify clinically applicable PsA biomarkers. Meta-analyses were performed when a diagnostic bone and cartilage turnover biomarker was reported in 2 or moredifferent cohorts of PsA and control.
Results
We identified 1444 publications and 124 studies met eligibility criteria. We highlighted bone and cartilage turnover biomarkers, genetic markers, and autoantibodies used for diagnostic purposes of PsA, as well as acute phase reactant markers and bone and cartilage turnover biomarkers for activity or prognostic severity purposes. Serum cartilage oligometrix metalloproteinase levels were significantly increased in the PsA sera compared to Healthy Control (HC) with a standardized mean difference (SMD) of 2.305 (95%CI 0.795-3.816, p=0.003) and compared to osteoarthritis (OA) with a SMD of 0.783 (95%CI 0.015-1.551, p=0.046). The pooled serum MMP-3 levels were significantly higher in PsA patients than in PsO patients with a SMD of 0.419 (95%CI 0.119-0.719; p=0.006), but no significant difference was highlighted when PsA were compared to HC. While we did not identify any new genetic biomarkers that would be useful in the diagnosis of PsA, recent data with autoantibodies appear to be promising in diagnosis, but no replication studies have been published.
Conclusion
In summary, no specific diagnostic biomarkers for PsA were identified and further studies are needed to assess the performance of potential biomarkers that can distinguish PsA from OA and other chronic inflammatory diseases.
Keywords: psoriatic arthritis, psoriatic biomarker, psoriasis, arthritis, meta-analysis
1 Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease that develops in up to 30% of patients with psoriasis (PsO), and can affect up to 0.7% of the general population (1, 2). PsA is characterized as affecting axial and peripheral joints and entheses, which can present clinically with diverse symptoms, often resulting in delayed diagnosis and treatment. PsA can lead to progressive articular damage, thus can be a source of impaired function, permanent disability, quality of life, and an increase in mortality (3, 4).
Through the Biomarkers Project, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) places critical emphasis on the research of biomarkers in its development strategy (5). A biomarker is defined as a characteristic that is objectively measured and evaluated as an indicator of pharmacologic responses, or normal or pathogenic biological processes, for a therapeutic intervention (6). Identification of specific biomarkers would improve early diagnosis and management of PsA in patients with joint pain and/or skin psoriasis. Although PsA can develop in up to 30% of PsO patients, the prevalence of undiagnosed PsA in patients with psoriasis is still estimated to be 10-15% (4). Although classification criteria are sometimes used by default, there are currently no diagnostic criteria or specific biomarkers available for PsA (4). Therefore, we sought to identify biomarkers for determining diagnosis and prognosis of PsA by conducting a systematic review and pairwise meta-analysis.
2 Methods
To conduct this research, we followed the guidelines and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting on studies evaluating healthcare interventions (7). PRISMA Checklist is provided in supplementary file 1. Ethics approval was not required under local legislation for this study.
2.1 Study selection
A systematic search of English-language literature was conducted in MEDLINE (via PubMed), EMBase, and the Cochrane Library dating from inception to March 1, 2022. We designed the search algorithm according to the Patient-Intervention-Comparison-Outcome-Time (PICOT) format. Search terms corresponded to MeSH or Emtree terms for “psoriatic arthritis” and “biomarkers” or “pharmacological biomarkers”. A manual search was also performed.
After searching with the pre-determined PICOT algorithm, study eligibility was ascertained after reading the title, keywords, and abstract. Once the articles of interest were identified, the full text was read to evaluate it according to the exclusion criteria, and to subsequently extract the necessary data. Inclusion criteria required the following: study must be an observational or interventional clinical trial published in English before March 2022; include an assessment of biomarker(s) in serum (including genetic biomarkers), synovial fluid, urine, or feces as diagnostic or prognostic factors; cohort must include patients with PsA according to a rheumatologist diagnosis, Moll and Wright criteria, or Classification Criteria for Psoriatic Arthritis (CASPAR).
Exclusion criteria were applied in a sequential order, and included an editorial or congress abstract; duplicates (between electronic databases or journals); non-English language full text; non-human, non-PsA, or pediatric (≤18 years old) populations; off-topic; not relevant for diagnosis and prognosis in PsA. We focused on prognostic factors of disease severity, regardless of treatment, and did not include prognostic factors of response to treatment, out of the scope.
Study results were highlighted in the main text if select biomarkers were mentioned in at least 2 publications. In addition, all included articles are presented in tabular form ( Tables 1 , 2 ).
Table 1.
Included Diagnostic Biomarkers Studies.
| References | Publication Year | Study Design | PsA (n) | Classification Criteria | Biomarkers | Methodology | Outcome |
|---|---|---|---|---|---|---|---|
| Bone and cartilage turnover biomarkers | |||||||
| Mânsson (8) | 2001 | Cross-sectional | 18 | Moll & Wright | COMP; BSP; Aggrecan | Comparison of COMP, BSP and Aggrecan level by ELISA method in the synovial fluid from 18 PsA and 43 RA. | Increased levels of COMP in PsA synovial fluid compared to RA population. |
| Farouk (9) | 2010 | Cross-sectional | 30 | CASPAR | COMP | Comparison of COMP level using ELISA with US on 30 PsA and 30 PsO | Moderate diagnosis accuracy of COMP to distinguish PsA of PsO with an AUC of 0.56. |
| Chandran (10) | 2010 | Cross-sectional | 26 | CASPAR | hsCRP; IL-12; p40Il12; IL-17; RANK-L; OPG; TNFSF14; MMP3; C2C; C1-2C; CPII; COMP | Comparison between 26 PsA, 26 PsO and 26 HC of several biomarker levels using ELISA methods. | Using of biomarkers panel consisting of hsCRP, OPG, TNFSF14 and CPII:C2C ratio, PsA can be distinguished from PsO with high accuracy (AUC: 0.90). |
| Cretu (11) | 2014 | Cross-sectional | 10 | CASPAR | Protein expression of synovial fluid | Liquid phase chromatography with mass-spectrometry of 10 PsA and 10 OA. | CRP, MMP-3, S100A9, EPO, M2BP, DEFA1, H4, H2AFX, ORM1, CD5L, PFN1 and C4BP were overexpressed in PsA synovial tissues. |
| Dolcino (12) | 2015 | Cross-sectional | 60 | CASPAR | Osteopontine; osteoactivin; fibronectin1; MMP3; CathepsinZ; IL-17 | Serum analysis of 60 PsA, 60 HC, 60 RA and 60 SpA after evaluation of genic expression in PBMC from 10 PsA and 10 HC. | Osteoactivin was significantly higher in PsA sera than in RA, SpA and HC sera, with 100% accuracy between PsA and HC. |
| Jadon (13) | 2017 | Cross-sectional | 200 | CASPAR | OPG; MMP3; Dkk-1; MCSF | ELISA determination of Dkk-1, MMP3; M-CSF and OPG levels in 200 PsA, 200 PsO, 157 SpA and 50 HC. | Biomarkers panel of Dkk-1; MCS, MMP3 and OPG was able to distinguish PsA and HC (AUC: 0.84). |
| Cretu (14) | 2017 | Cross-sectional | 100 | CASPAR | M2BP; CD5L; MPO; ITGB; CRP; MMP3 | Level measurement by ELISA of 100 PsA, 100 PsO and 100 HC. | ITGB5, CRP and M2BP levels were increased in PsA patients (Panel of these 3 biomarkers: AUC of 0.85) |
| Chandran (15) | 2019 | Cross-sectional | 73 | CASPAR | COMP; hyaluronan; resistin; adiponectin; adipsin; HGF; insulin; leptin; CRP; IL-8; IL-6; IL-1β; TNFα; MCP1; NGF | Serum analysis of 77 PsA, 201 OA and 76 HC by ELISA in a first discovery phase and then comparison of 4 biomarkers in a second validation phase of 73 PsA and 75 OA. | COMP, Resistin, MCP-1 and NGF used as a panel were able to distinguish PsA and OA populations with high accuracy (AUC: 0.99). |
| Diani (16) | 2019 | Cross-sectional | 50 | CASPAR | MMPs; TIMPs; OPG; RANK-L; CTX; Dkk-1; SOST; CTX-I; CTX-II; PINP; Chi3L1 | Serum analysis of osteoimmunologic biomarkers in 50 PsA, 50 PsO and 20 HC. | MMP2, MMP12, MMP13, TIMP2 and TIMP4 was able to distinguish PsA undergoing treatment from PsO. CHI3L1 and MMP10 was able to distinguish PsA not undergoing systemic treatment from PsO. |
| Waszczykowski (17) | 2020 | Cross-sectional | 24 | CASPAR | IL-18; IL-20; MMP-1; MMP-3; COMP; YKL-40; Aggrecan | ELISA analysis from 24 active PsA sera and 26 HC. | COMP, IL-18, MMP-3 and MMP-1 was able to distinguish PsA from HC. |
| Waszczykowski (18) | 2021 | Cross-sectional | 22 | CASPAR | IL-6; IL-18; IL-20; MMP-1; MMP-3; COMP; YKL-40; Aggrecan | ELISA analysis from 22 PsA sera, 22 OA and 23 HC. | Age-associated serum COMP and Aggrecan levels discriminate PsA from OA (AUC: 0.91) |
| Genetic biomarkers | |||||||
| Elkayam (19) | 2004 | Cross-sectional | 50 | Moll & Wright | HLA | HLA class I and II typing on 50 PsA from Israeli Jewish population. | PsA was associated with HLA-A3; HLA-B13; HLA-B38; HLA-DRB0101; HLA-DRB0301; and HLA-DRB0401 on Israeli Jewish population. HLA-B27 was not associated with PsA in this cohort. |
| Alenius (20) | 2004 | Cross-sectional | 120 | Physician diagnosis | CTLA4; TNF; loci 8q24; 16q21 gene polymorphisms | Genotyping of TNF locus 1q21 (PSORS4) and 3q21 (PSORS5), 8q24 loci, 16q21 loci and CTLA4 locus on 120 PsA patients and 90 HC. | No association reported in this paper. |
| Ravindran (21) | 2004 | Cross-sectional | 140 | Moll & Wright | IL-1a; IL-1b; R-IL-1 gene polymorphisms | Genotyping of IL-1a-889; Il-1b +3953 and IL-1R+970 on 140 PsA. | Major risk allele C of IL-1a-889 loci was associated with PsA. |
| Batliwalla (22) | 2005 | Cross-sectional | 19 | Moll & Wright | Microarray’s analysis | Gene expression profiling on peripheral blood cells in 19 PsA patients and 19 age- and sex-matched HC. | Decreased of Nucleoporine 62KDa and MAP3K3 expression was associated with PsA compared to control. |
| Stoeckman (23) | 2006 | Cross-sectional | 16 | Moll & Wright | Microarray’s analysis | Whole blood gene expression profiling on 16 PsA and 15 sex- and age-matched HC. | Zinc-finger protein 395, dead box polypeptide 28, pecanex-like 3, and PI3KC2B gene expressions were upregulated in PsA compared to HC. |
| Butt (24) | 2007 | Cross-sectional | 258 | Physician diagnosis | VEGF; FGF1; FGF2; EGF gene polymorphisms | Genotyping with MALDI-TOF spectrophotometry on 258 PsA and 154 HC. | rs3025039*T in VEGF+936 loci was protector of PsA. |
| Bowes (25) | 2011 | Cross-sectional | 1057 | CASPAR | IL-13 gene polymorphism (Alleles rs20541 and rs1800925) | Genotyping of rs20541 and rs1800925 on 1057 PsA, 778 PsO and 5575 HC. | Double alleles rs1800925*C/C and rs20541*G/G were significantly associated with PsA. |
| Eder (26) | 2011 | Cross-sectional | 555 | Moll & Wright | IL-13 gene polymorphism (Alleles rs20541; rs843; rs1800925) | Genotyping of rs20541, rs843 and rs1800925 single nucleotide polymorphisms on 555 PsA, 342 PsO and 217 HC. | rs20541 and rs843 polymorphism increased the risk of PsA in PsO patients. |
| Eder (27) | 2012 | Cross-sectional | 178 and their family | CASPAR | HLA-B and HLA-C | Family-based association study by HLA-genotyping on 178 PsA, 30 PsO and 561 first degree relatives. | HLA-B27, B-38, B-39 and HLA-C12 were associated to PsA compared to PsO. |
| Winchester (28) | 2012 | Cross-sectional | 359 | CASPAR | HLA-B and HLA-C | Comparison of the HLA-B and HLA-C alleles and haplotypes by HLA-genotyping on 359 PsA, 214 PsO and 1119 HC, divided in two cohort: discovery then validation. | HLA-B27 was associated to PsA. |
| Chandran (29) | 2013 | Cross-sectional | 678 | CASPAR | HLA alleles | HLA-genotyping on 678 PsA and 688 HC. | In comparison of HC, HLA-C*12/B*38 association, HLA-C*06/B*57 association and HLA-B*27 were associated to PsA. |
| Chandran (30) | 2014 | Cross-sectional | 678 | CASPAR | KIR2D and KIR3D gene polymorphism | KIR2D and KIR3D genotyping on 678 PsA and 688 HC. | The allele KIR2DS was significantly associated with PsA. |
| Zhang (31) | 2017 | Cross-sectional | 465 | CASPAR | 36 loci, including IL-12B; RUNX3; LCE gene polymorphisms | DNA Genotyping on 465 PsA and 421 HC using MALDI-TOF spectrophotometry. | Polymorphisms in IL-12B, RUNX3 and LCE genes were associated with an increased risk of PsA. |
| Ciancio (32) | 2017 | Cross-sectional | 39 | CASPAR | miR-21-5p | Micro-array expression analysis of 723 miRNA on 39 PsA, 26 RA and 16 HC then PCR analysis of miR-21-5p. | miR-21-5p was overexpressed in RA and PsA population. |
| Cascella (33) | 2017 | Cross-sectional | 500 | CASPAR | KIF3A and IL-4 gene polymorphism | RT-PCR of rs2227282 located in the IL-4 gene and rs2285700, rs10062446 and rs2897442, located in the KIF3A gene from 500 PsA, 426 PsO and 600 HC blood samples. | Except rs2285700 on KIF3A gene, the presence of SNPs increased susceptibility to PsA but not PsO. |
| Abji (34) | 2018 | Cross-sectional | 14 | CASPAR | Genetic expression of Th17 pathway | RT-PCR of 84 genes from synovial fluid samples from 14 PsA, and 9 OA. | MMP3, CCL1, IL-17C, IL-3, CXCL5, IL-6 and CX3CL1 genes were expressed more in samples from PsA compared to OA. |
| Chen (35) | 2019 | Cross-sectional | 111 | CASPAR | HLA class I and HLA DRB1 | HLA-Genotyping on 111 PsA and 207 HC from a Chinese Han population. | HLA-A*01/A*01 and HLA-C*06/C*02 were risk alleles for PsA. |
| Smith (36) | 2020 | Cross-sectional | 140 | CASPAR | HLA-c*06:02; B*44:02; B*27:05; B08:01; TNFRSF9; LCE3C/B; IL-23R; TNFAIP3; CSF2-P4HA2 genes | 11 genes reported to be associated with PsA or PsO were genotyped in 140 PsA, 403 PsO and 181 PsO patients with joint pain. | Low accuracy of this genetic association to PsA diagnosis. |
| Caputo (37) | 2020 | Cross-sectional | 424 | CASPAR | SNPs of COL6A5 (rs12488457 A/C); COL8A1 (rs13081855 G/T); COL10A1 (rs3812111 A/T); miR146A (rs2910164 C/G) | Genotyping of blood sample from 424 PsA, 394 PsO and 600 HC from Italian population. | rs13081855*T, rs12488457*C and rs2910164*T were associated with PsA. |
| Lin SH (38) | 2020 | Cross-sectional | 40 | CASPAR | miR-941 and miR-1466-p | Expression of miR941 and miR1466-5p measured in 40 PsA, 40 PsO and 40 HC blood samples. | Higher miR-941 expression in PsA samples than PsO or HC. |
| Pasquali (39) | 2020 | Cross-sectional | 28 | CASPAR | Extra-vesicular micro-RNA | miRCURY™ exosome isolation kit was used to compare 14 PsA and 15 PsO blood samples in the discovery phase and then 24 PsA and 25 PsO in the validation phase. | Plasma extravesicular « let-7b-5p » and « miR-30e-5p » were significantly lower in PsA compared to PsO in validation phase. |
| Wade (40) | 2020 | Cross-sectional | 31 | CASPAR | Micro RNA signature | miRNA panel was assessed using miRNA Fireplex assay in sera of 31 PsA and 20 HC. | miR-221-3p, miR-130a-3p, miR-146a-3p, miR-26a-5p, miR-151a-5p and miR-21-5p were promising candidate biomarkers to distinguish PsA from HC. |
| Iwaszko (41) | 2021 | Cross-sectional | 126 | CASPAR | IL-33 gene polymorphisms (rs16924159; rs10975519; rs7044343) | PCR analysis of 126 PsA sera, 143 SpA, 466 RA and 229 HC. | These SNPs within the IL-33 gene were not useful for PsA diagnosis. |
| Cheleschi (42) | 2022 | Case-control | 50 | CASPAR | Selected mi-RNA, pro-inflammatory cytokines and adipokines | RT-PCR and ELISA analysis in blood samples from 50 PsA, 50 RA and 50 HC. | Increased expression of miR-140 and serum leptin in PsA compared to RA. |
| Autoantibodies | |||||||
| Calzavara-Pinton (43) | 1999 | Cross-sectional | 76 | Moll & Wright | Anti-CCP autoantibodies | Indirect immunofluorescence test on 76 PsA sera, 38 PsO, 159 RA, 119 non- inflammatory rheumatic diseases and 204 HC. | Anti-CCP autoantibodies were specific of RA but were present in a small number of PsA cases. |
| Chou (44) | 2010 | Cross-sectional | 13 | CASPAR | IgG anti-agalctosyl autoantibodies | ELISA analysis on 13 PsA, 30 SpA, 22 RA and 25 HC. | IgG anti-agalactosyl autoantibodies were present in higher quantities in patients with RA, SpA and PsA serum compared to HC. |
| Dalmády (45) | 2013 | Cross-sectional | 46 | CASPAR | Anti-MCV autoantibodies | Serum analysis by ELISA on 46 PsA, 42 PsO and 40 HC. | Anti-MCV autoantibodies were more represented in PsA patients than in those with PsO and HC. |
| Dolcino (46) | 2014 | Cross-sectional | 100 | CASPAR | Anti-PsA peptide (TNRRGRGSPGAL) autoantibodies | Serum analysis of 100 PsA, 200 RA, 30 PsO, 30 LES, 30 Sjogren syndrome, 30 SpA, 30 scleroderma and 50 HC. | Anti-NRAP autoantibodies were highly associated with PsA compared to PsO, RA CCP+ or CCP-, HC and the others rheumatic diseases included. |
| Hu (47) | 2018 | Cross-sectional | 12 | Physician diagnosis | AC anti-SIRT1 autoantibodies | ELISA analysis on 12 PsA, 94 RA, 185 SpA and 87 HC. | Anti-SIRT1 autoantibodies were expressed higher in patients with SpA and PsA compared to patients with RA and HC but it seems to be more specific of patients with SpA. |
| Frasca (48) | 2018 | Cross-sectional | 32 | CASPAR | Anti-LL37 carbamylated (carb) autoantibodies and Anti-LL37 citrullinated (cit) autoantibodies | Serum analysis using ELISA from 32 PsA, 24 PsO and 12 HC. | Anti-LL37 cit were associated to psoriatic disease, while anti-LL37carb were more specific to PsA. |
| Yuan (49) | 2019 | Cross-sectional | 22 | Physician diagnosis | Anti-ADAMSTS5 and anti-LL37 autoantibodies | ELISA analysis of 22 PsA and 32 PsO blood samples. | IgG anti-LL37 and anti-ADAMTS5 autoantibodies distinguished PsA from PsO. |
| Vinci (50) | 2020 | Cross-sectional | 69 | CASPAR | IgA Anti-oxPTMCII autoantibodies | ELISA analysis on 69 PsA, 60 RA, 242 SpA, 35 PsO, 48 UA, 19 FM, and 178 HC. | IgG anti-oxPTMCII were associated with RA while IgA anti-oxPTMCII were associated with SpA, PsA and SpA associated with inflammatory bowel disease. |
| Other biomarkers | |||||||
| Veale (51) | 1993 | Cross-sectional | 15 | Benett criteria | ELAM-1; ICAM-1; VCAM-1 | Immunohistochemistry analysis of synovial tissue from 15 PsA and 15 RA. | Increased ELAM-1 expression in RA synovial samples compared to PsA. |
| Szodoray (52) | 2007 | Cross-sectional | 43 | Moll & Wright | Panel of 23 different biomarkers: VEGF, EGF; IL-10; IL-13; IFNα; MIP1α (CCL3); MIP1β (CCL4); Eotaxin (CCL11); IL12p-40 | ELISA analysis on 43 PsA and 25 HC. | Overexpression of IFNα and IL-10 in PsA. Under expression of G-CSF, CCL4, CCL11, IL-13, EGF, VEGF and FGF in PsA. |
| Firuzi (53) | 2008 | Cross-sectional | 16 | Moll & Wright | Carbonyl (CO) and Sulfhydryl (SH) groups | Serum and Synovial analysis using spectrophotometry on 16 PsA, 18 RA and 15 OA. | Decreased SH-group in RA and PsA synovial samples compared to OA. |
| Hansson (54) | 2014 | Prospective | 65 | CASPAR | Calprotectin S100A8/S100A9 | Serum analysis of 65 PsA and 31 HC. | ROC analysis of calprotectin S100A8/A9 revealed an AUC of 0.87 to distinguish PsA from HC. |
| Bosè (55) | 2014 | Cross-sectional | 30 | CASPAR | IL-2 | Cytokine expression assessed on plasma circulating T-cells of 30 PsA, 21 PsO and 24 HC. | IL-2 expression was significantly associated with PsA. |
| Maejima (56) | 2014 | Cross-sectional | 12 | CASPAR | Moesin; K17; ANXA1; STIP-1. | Level assessment in 12 PsA, 31 PsO and 13 HC sera using dot blot analysis. | Significant increase in K17 and STIP-1 in PsA compared to PsO and HC. |
| Kim (57) | 2015 | Cross-sectional | 25 | CASPAR | Ratio PNN/Ly et PLQ/Ly | Compared between 25 PsA, 111 PsO, and 94 HC. | An increase in the PNN/Ly ratio and PLQ/Ly ratio was predictive of PsA. |
| Armas-González (58) | 2015 | Cross-sectional | 15 | CASPAR | B-cell protein expression profiling | Flow cytometry analysis on 15 PsA and 13 RA. | B-cells in RA synovial samples expressed more MHC class II molecules than those in PsA. |
| Amin (59) | 2016 | Prospective | 20 | Moll & Wright | RANK-L | Level measurement by ELISA of RANK-L from 20 PsA, 40 PsO and 20 HC. | Low accuracy of RANK-L to discriminate PsA from PsO (AUC: 0.66). |
| Gudmann (60) | 2016 | Cross-sectional | 101 | CASPAR | ProC2 and C-col10 | ELISA analysis on 110 SpA, 101 PsA and 118 HC. | Increased serum ProC2 concentration in PsA and SpA. |
| Muntyanu (61) | 2016 | Cross-sectional | 40 | CASPAR | CXCL10 | Serum analysis of 40 PsA, 14 OA, 11 RA and 8 gouts. | CXCL10 titers were higher in PsA synovial fluid than in gout and OA. No difference from RA. |
| Abji (62) | 2016 | Prospective | 620 | CASPAR | CXCL10 | Monitoring the variation of CXCL10 titres in sera from 620 PsO. | Mean level of CXCL10 was higher in sera from PsA converter compared to non-converter. |
| Reindl (63) | 2016 | Cross-sectional | 33 | CASPAR | 15 serum biomarkers issued in a discovery phase | ELISA analysis of serum from 33 PsA, 100 PsO and 25 HC. | Complement 3, Polymeric Immunoglobulin Receptor, Plasma Kallikrein and Zn-a2-glycoprotein were significantly higher in PsA sera compared to PsO and HC. |
| Alonso (64) | 2016 | Cross-sectional | 200 | Physician diagnosis | Urinary biomarker panel | Urine metabolome of 200 PsA, 200 RA, 200 PsO, 200 SLE, 200 Crohn’s disease, and 200 HC analysed using nuclear magnetic resonance. | Urine metabolome expression was different in PsA compared to RA. |
| Grossi (65) | 2017 | Cross-sectional | 18 | CASPAR | Calprotectin S100A8/S100A9 | Assessment of serum calprotectin mean concentration from 18 PsA, 49 RA, 21 SpA and 73 HC. | High accuracy to Calprotectin S100A8/A9 to distinguish PsA from HC. No difference between PsA and SpA. |
| Ausavarungnirun (66) | 2017 | Cross-sectional | 55 | CASPAR | ESR and hsCRP | Inflammatory markers determination in serum from 55 PsA and 55 PsO. | Increased VS and hsCRP levels in PsA compared to PsO. |
| Maejima (67) | 2017 | Cross-sectional | 11 | CASPAR | VCP | Serum analysis using Reverse-phase protein array from 11 PsA, 23 PsO and 11HC. | VCP was significantly increased in PsA compared to PsO and HC. |
| Farrag (68) | 2017 | Cross-sectional | 21 | CASPAR | Il-34 | ELISA analysis from 21 PsA, 24 PsO and 20 HC blood samples. | IL-34 concentration was able to distinguish PsA from PsO and HC (AUC: 0.90). |
| Sinkeviciute (69) (67) | 2020 | Prospective | 111 | CASPAR | PROM | ELISA analysis of 11 PsA and 55 HC. | PROM was associated with PsA but ROC analysis described low accuracy (AUC: 0.64). |
| Abji (70) | 2020 | Prospective | 29 | CASPAR | CXCL10 | Monitoring of serum CXCL10 levels in 644 PsO patients to compare PsA converters (n=29) and matched non-converters (n=52). | Decrease in serum CXCL10 titers in PsA converters before and after conversion to PsA. |
| Esawy (71) | 2020 | Cross-sectional | 76 | Moll & Wright | Plasma Gelsolin | Serum analysis by ELISA of 76 PsA, 40 PsO and 40 age and sex-matched HC. | Gelsolin was able to discriminate PsA from PsO (AUC: 0.91) and HC (AUC: 0.98). |
| Souto-carneiro (72) | 2020 | Cross-sectional | 73 | Physician diagnosis | Serum metabolome and lipidome (7 lipids groups and 24 different metabolites) | Proton nuclear magnetic resonance analysis of 73 PsA sera and 49 seronegative RA. | Construction of a predictive model consisting of the lipid ratio and the expression of metabolites made it possible to distinguish RA from PsA (AUC: 0.85). |
| Cuervo (73) | 2021 | Cross-sectional | 35 | CASPAR | Mast-cells CD117 and fibroblasts (hsp47) in synovial fluid | Immunohistochemical analysis of cell types from synovial samples of 35 PsA, 39 RA and 31 UA (19 evolving to RA and 12 evolving to PsA). | Higher mast cell and fibroblastic density were associated with PsA progression. |
| Leijten (74) | 2021 | Cross-sectional | 20 | CASPAR | 951 unique proteins | Serum proteomic analyses from 20 PsA samples, 20 PsO, 19 SpA and 20 HC. | No difference in proteomic expression between PsO and PsA but 68 expressed proteins differ compared to HC. |
| Kishikawa (75) | 2021 | Cross-sectional | 42 | CASPAR | Plasma metabolome | Plasma-metabolite profiles investigated in 42 blood samples from PsA, 50 PsO and 38 HC using dual approach by CE-TOFMS and LC-TOFMS. | In PsA compared to PsO: Increased levels of all saturated fatty acid and tyramine level. Decreased levels of mucic acid. |
| Fuentelsaz-Romero (76) | 2021 | Retrospective | 9 | CASPAR | Macrophage polarization | Analysis of GM-CSF expression and macrophage polarization in synovial tissue from 8 UA evolving to RA, 9 UA evolving to PsA, 16 persistent UA, 12 established RA and 10 persistent PsA. | CD163+ CD209+ macrophages were more abundant in synovial tissues from PsA and HC compared to RA and persistent UA. |
| Zhu J (77). | 2021 | Cross-sectional | 4 | CASPAR | Proteome profile of peripheral blood mononuclear cells | Blood samples analysis firstly using mass-spectrometry then using western-blot from 4 PsA, 4 PsO and 4 HC. | Higher SIRT2 expression in PBMC from PsA than PsO and HC. |
| Looby (78) | 2021 | Retrospective | 30 | CASPAR | Metabolomics | Monitoring of metabolite expression by mass spectrometry in 30 PsA, 20 PsO (10 converted to PsA and 10 non-converted to PsA), and 10 HC. | 1,11-undecanedicarboxylic acid expression differed between PsA patients and HC. |
| Leijten (79) | 2021 | Cross-sectional | 21 | CASPAR | CD8+ CCR10+ T-cells | Flow cytometry of PBMC from 21 patients with PsA, 21 with PsO, 16 with SpA and 20 HC. | CD8+CCR10+ T-cells were more represented in PsA sera compared to HC. |
| Ek (80) | 2021 | Cross-sectional | 1025 | NA | 21 inflammatory biomarkers | 21 biomarkers were assessed in 18 different inflammatory disease populations from the UK biobank. | No biomarker measured was associated with PsA diagnosis. |
| Wang N (81) | 2022 | Cross-sectional | 27 | ACR criteria | Fecal metabolites | Evaluation of metabolic profile of fecal samples from 27 PsA patients, 29 with RA and 36 HC were analysed using liquid chromatography and completed by mass spectrometry. | 5 fecal metabolites (α/β-turmerone, glycerol 1-hexadecanoate, dihydrosphingosine, pantothenic acid and glutamine) are potential PsA biomarkers. |
| Marzaioli (82) | 2022 | Cross-sectional | 37 | NA | Dendritic cells CD209/CD14+ and its cytokine expression | Flow cytometry in blood sample from 37 PsA, 62 RA, 6 OA and 11 HC and transcriptional analyses by qRT-PCR. | Higher concentration of CD209/CD14+ dendritic cells from patients with PsA and RA compared to HC. No difference of CD209+ transcriptional expression between PsA and RA. |
| Mc Ardle (83) | 2022 | Cross-sectional | 95 | CASPAR | Serum proteome | Comparison of protein expression from 95 PsA sera and 72 RA. | A panel of select proteins was able to distinguish PsA from RA (AUC: 0.79). |
PsA, psoriatic arthritis; PsO, psoriasis; HC, Healthy control; SLE, Systemic lupus erythematosus; UA, Undifferentiated Arthritis; FM, fibromyalgia; ELISA, Enzyme-linked immune absorbent assay; RT-PCR, reverse transcription and polymerase chain reaction; MAP3K3, MAP kinase 3; PI3KC2B, phosphoinositide-3-kinase, class 2, beta polypeptide; COMP, Cartilage Oligomeric MetalloProteinase; MMP3, Matrix MetalloProteinase-3; RA, Rheumatoid arthritis; BSP, Bone Sialo-protein; AUC, Area under the curve; IL, Interleukin; OPG, Osteoprotegerin; TNFSF, Tumor Necrosis Factor Super Family; C2C, Col2-3/4 long mono; CPII, Pro-collagen 2 peptide; C1-2C, Col2-3/4 short; Pro-C2 (or PIIBNP), N-terminal propeptide of the pro-collagen IIB slice variant; Dkk1, Dikkopf-1; MCSF, Macrophage colony stimulating factor; M2BP, Mac-2 binding protein; CD5L, CD5 Like protein; MPO, Myeloperoxydase; CRP, C-reactive protein; TNF, Tumor necrosis factor; NGF, Nerve Grow Factor; Chi2L3, Chitinase like 3 protein; PROM, Matrix metalloproteinase-cleaved Prolargin; HLA, Human leukocyte antigen; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; VEGF, Vascular endothelial growth factor; FGF, Fibroblast growth factor; EGF, Endothelial growth factor; ELAM1, Endothelial leukocyte adhesion molecule 1; ICAM-1, Intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1; K17, Keratin 17; ANXA1, Annexin A1; STIP-1, Stress-induced phosphoprotein-1; PNN, polynuclear; CXCL-10, CXC-motif Chemokine Ligand-10; ESR, Erythrocyte sedimentation rate; hsCRP, High sensitive C- reactive protein; VCP, Valosin-containing protein; oxPTMCII, Oxidized collagen type II; CE-TOFMS, Capillary electrophoresis time-of-flight mass spectrometry; LC-TOFMS, Liquid chromatography time-of-flight mass spectrometry.
Table 2.
Included Prognostic Biomarkers Studies.
| References | Publication year | Study design | PsA (n) | Classification criteria | Biomarkers | Methodology | Outcome |
|---|---|---|---|---|---|---|---|
| Acute Phase Reactant Biomarkers | |||||||
| Helliwell (84) | 1991 | Cross-sectional | 36 | Moll & Wright | Cytidine deaminase; ESR; CRP; histidine | Concentration assessment in 36 PsA blood samples. | Better correlation with disease activity was ESR. |
| Mchugh (85) | 2003 | Prospective | 87 | Moll & Wright | ESR | 5-year follow-up of a cohort of 87 PsA patients. | Correlation between high initial ESR level and pejorative disease progression. |
| Bandinelli (86) | 2015 | Cross-sectional | 112 | CASPAR | ESR; CRP; Anti-CCP; HLA | US examination, HLA typing and serum analysis of 112 patients with PsA. | US abnormalities were associated with higher mean serum CRP or ESR levels and expression of HLA-B27, B-35, B38, Cw*6 and DR4. |
| Bone and cartilage biomarkers | |||||||
| Kane (87) | 2003 | Retrospective | 22 | Moll & Wright | MRP8; MRP 14 | ELISA analysis of synovial and serum sample from 22 patients with PsA, 11 with RA 15 with SpA. | Serum MRP8 and MRP14 concentrations were markers of disease activity in PsA, RA and SpA. |
| Skoumal (88) | 2008 | Cross-sectional | 64 | Moll & Wright | COMP | ELISA analysis of serum from 64 patients with PsA and 39 with PsO. | COMP concentration was significantly correlated with CRP, ESR, TCJ and SCJ. |
| Dalbeth (89) | 2010 | Cross-sectional | 38 | CASPAR | Dkk-1; M-CSF; RANK-L; OPG | Serum analysis from 38 PsA, 10 PsO and 12 HC. | RANK-L and M-CSF are correlated with articular radiographic damage in PsA. |
| Van kuijk (90) | 2010 | Prospective | 24 | CASPAR | CPII; PINP; MIA; MMP-3; C2C; COMP; osteocalcin; NTX-1; CTX-1 | Serum analysis using ELISA for 12 weeks follow-up of 24 PsA in an adalimumab study. | Decreased MMP-3 concentration was associated with DAS28 improvement. |
| Ramonda (91) | 2013 | Prospective | 43 | CASPAR | MMP3; VEGF; PTX3; hsCRP | ELISA analysis on 43 HC and 43 PsA at inclusion and 24 weeks after anti-TNF introduction. | Decrease in biomarker levels during follow-up. |
| Waszczykowski (18) | 2021 | Cross-sectional | 24 | CASPAR | IL-18; IL-20; MMP-1; MMP-3; COMP; YKL-40; Aggrecan | ELISA analysis from 24 patients with active PsA sera and 26 HC. | COMP level was significantly correlated with TJC and SJC. |
| Chung (92) | 2021 | Cross-sectional | 69 | CASPAR | Dkk-1 | ELISA analysis from 69 PsA sera, 39 RA and 21 HC. Radiographic hands erosions and sacroiliitis were assessed. | Bone erosions, sacroiliitis and SCJ were correlated with elevated serum levels of Dkk-1. |
| Genetic biomarkers | |||||||
| Alenius (93) | 2002 | Cross-sectional | 88 | Moll & Wright | HLA-A; HLA-B; HLA-Cw*; HLA-DRB1*; HLA-DQB1* | HLA Genotyping of 88 PsA and 1085 HC. | HLA-B37 and HLA-B62 were markers of erosions and deformations in PsA. |
| Iwaszko (41) | 2021 | Cross-sectional | 126 | CASPAR | IL-33 gene polymorphisms (rs16924159; rs10975519; rs7044343) | PCR analysis in 126 PsA, 143 AS, 466 RA and 229 HC. | No correlation between SNPs within IL-33 gene and CRP or BASDAI in PsA. |
| Autoantibodies | |||||||
| Korendowych (94) | 2005 | Cross-sectional | 126 | Physician diagnosis | Anti-CCP | Immunofluorescence analysis from 126 PsA sera. | All PsA CCP+ had radiographic damages and significantly higher SJC. |
| Perez-alamino (95) | 2014 | Prospective | 81 | CASPAR | Anti-CCP | ELISA assessment from 81 PsA sera. | PsA CCP+ had more erosive damage and higher polyarticular forms. |
| Ibrahim (96) | 2018 | Cross-sectional | 45 | CASPAR | Anti-CarP autoantibodies | ELISA analysis of sera from 45 patients with PsA and 45 HC. | Anti-CarP autoantibodies were associated to both clinical and US activity in PsA. |
| Frasca (48) | 2018 | Cross-sectional | 32 | CASPAR | Anti-LL37 carbamylated autoantibodies and Anti-LL37 citrullinated autoantibodies | ELISA analysis from 32 PsA sera, 24 PsO, and 12 HC. | Correlation with DAS44 in PsA. |
| Other biomarkers | |||||||
| Elkayam (97) | 2000 | Cross-sectional | 34 | Moll & Wright | Hyaluronic acid | Radiometric assay of sera 34 patients with PsA, 34 with RA and 49 with HC. | Hyaluronic acid level was correlated to PASI Score (r: 0.87). |
| Elkayam (98) | 2000 | Cross-sectional | 34 | Moll & Wright | IL-10; IL-6; IL-1ra; IL-2R | ELISA analysis of serum samples from 34 patients with PsA and 10 HC. | Increase in IL-1Ra titers associated with joint activity in PsA. |
| Foell (87) | 2003 | Prospective | 22 | Moll & Wright | Calprotectin S100A12 | Serum analysis before and after MTX introduction of 22 PsA patients, 9 RA patients, 11 SpA patients and 30 HC. | Calprotectin S100A12 concentration was modestly correlated with ESR and Richie index. |
| Rooney (99) | 2004 | Prospective | 5 | Moll & Wright | IL-18 | Monitoring expression in synovial tissues from 5 patients with PsA, 11 with RA, 9 with UA and 2 with reactive arthritis before MTX or Salazopyrine introduction, repeated after 12 months delay. | IL-18 expression correlated with CRP circulating levels in all groups before treatment. Decrease expression of IL-18 after treatment correlated with a decrease in CRP levels. |
| Fink (100) | 2007 | Cross-sectional | 28 | Moll & Wright | VEGF | ELISA analysis of sera from 28 patients with PsA and 9 HC. | Higher VEGF titres in the active PsA subgroup. |
| Madland (101) | 2007 | Cross-sectional | 119 | Physician diagnosis | ESR; hsCRP; Calprotectin S100A8/A9; Calprotectin S100A12 | Serum analysis from 119 PsA patient blood samples. | Calprotectin S100A8/A9 was inferior to ESR and CRP to assess disease activity but was a better marker of radiographic damage. |
| Szodoray (52) | 2007 | Cross-sectional | 43 | Moll & Wright | Panel of 23 different biomarkers | ELISA analysis from 43 patients with PsA sera and 25 HC. | EGF, IFNα, VEGF, CCL3 and IL-12 p-40 levels correlated with disease activity. |
| Alenius (102) | 2009 | Cross-sectional | 134 | Moll & Wright | IL-6; IL-2R | Serum analysis from 134 patients with PsA and 85 PsO patients using ELISA. | IL-6 is associated to ESR, CRP and TCJ. |
| Pongratz (103) | 2010 | Cross-sectional | 52 | CASPAR | BAFF and testosterone | Serum analysis from 53 PsA patient blood samples. | In male patients with PsA, the BAFF/testosterone ratio was significantly correlated with DAS28. |
| Celis (104) | 2011 | Cross-sectional | 46 | CASPAR | miRNA expression and panel of 21 cytokines. | ELISA analysis associated to quantitative RT-PCR of synovial tissues from 46 PsA. | Association between synovial IL-6 and CCL20 levels and circulating CRP levels. |
| Przepiera-będzak (105) | 2013 | Cross-sectional | 80 | CASPAR | VEGF; EGF; FGFβ; FGFα | Serum analysis on 80 PsA patient samples, 18 with SAPHO and 20 HC. | VEGF and CRP are correlated. VEGF is not associated with several disease activity score. |
| Jensen (106) | 2011 | Prospective | 42 | Moll & Wright | YKL-40 (Chi3l1); hsCRP | Serum analysis on 42 patients with PsA and 6 with PsO before and after adalimumab introduction. | YKL40 is decreased in responder PsA patients. |
| Eissa (107) | 2013 | Cross-sectional | 50 | CASPAR | Kallikreins | Serum analysis on 50 patients with PsA, 50 PsO patients and 26 HC. | Kallikrein 8 and Kallikrein 6 are associated with PASI in PsA patients. |
| Kayikci (108) | 2013 | Cross-sectional | 48 | CASPAR | Il-17; Il-22; Il-23 | ELISA analysis on 48 patients with PsA, 20 with PsO and 19 HC. | Il-17 is weakly correlated with TJC. |
| Hansson (54) | 2014 | Prospective | 65 | CASPAR | Calprotectin S100A8/S100A9 | ELISA analysis in sera from 65 patents with PsA and 31 HC. | Calprotectin was significantly increased in polyarticular form of PsA. |
| Ahmed (109) | 2015 | Cross-sectional | 26 | CASPAR | YKL-40 (Chi3l1) | ELISA analysis of blood samples from 26 pateints with PsA, 22 with PsO and 30 HC. | YKL40 was significantly correlated with CPDAI in PsA patients. |
| Husakova (110) | 2015 | Cross-sectional | 40 | Physician diagnosis | Prolactin | Immunoradiometric assay on sera of 70 patients with PsO, 40 with PsA and 27 HC. | No correlation was found with disease activity. |
| Matt (111) | 2015 | Cross-sectional | 23 | CASPAR | Fc receptor of Monocyte-cells | Flow cytometru on sera of 23 patients with PsA and 32 HC. | High titer of CD64+ cells was significantly correlated with DAS28. |
| Peled (112) | 2015 | Cross-sectional | 20 | CASPAR | PD1 | Flow cytometry on 20 patients with PsA and 15 HC. | A low titre of T-cell presenting PD1 is associated with a low DAS28 score. |
| Dikbas (113) | 2016 | Cross-sectional | 28 | CASPAR | Adipocytokine: Visfatin; Resistin; Adiponectin | ELISA analysis on 28 PsA patients and 39 HC. | No correlation found. |
| Munk (114) | 2016 | Cross-sectional | 101 | CASPAR | PIIANP; C2M | ELISA analysis of 110 patients with SpA, 101 with PsA and 96 HC. | No correlation found. |
| Alonso (64) | 2016 | Cross-sectional | 200 | Physician diagnosis | Urinary biomarker panel | Nuclear magnetic resonance analysis of urine samples from 200 patients with PsA, 200 with RA, 200 with PsO, 200 with SLE, 200 with Crohn’s disease, and 200 HC. | Increased urine levels of citrate in active PsA. |
| Inciarte-mundo (115) | 2016 | Cross-sectional | 50 | CASPAR | Calprotectin and TNF trough serum levels | Serum analysis from 50 patients with PsA and 42 with RA. | High Calprotectin titers were associated with US activity in both PsA and RA patients. |
| Przepiera (116) | 2016 | Cross-sectional | 76 | CASPAR | IL-18; Fetuin A; ICAM-1; ET-1 | ELISA analysis of sera from 76 patients with PsA sera, 81 with SpA and 34 with SAPHO. | ET-1 levels were correlated to DAS28 in PsA. IL-18 levels were associated to BASDAI. |
| Kiliç (117) | 2017 | Cross-sectional | 116 | CASPAR | MPV | Serum analysis from 116 PsA patients, 41 PsO patients and 90 HC. | Skin disease severity was associated to high MPV concentration in patients with PsA. |
| Farrag (68) | 2017 | Cross-sectional | 21 | CASPAR | IL-34 | ELISA assessment in sera from 21 patients with PsA, 24 with PsO and 20 HC. | Association between IL-34 and disease articular and PASI, CPDAI, and BASDAI. |
| Inciarte-mundo (118) | 2018 | Prospective | 51 | CASPAR | Calprotectin; TNF trough serum levels | Serum analysis at baseline and relapse of 47 PsA patients and 56 with RA treated with anti-TNF. | High Calprotectin concentration at baseline was associated with relapse in both PsA and RA patients. |
| Wade (119) | 2018 | Cross-sectional | N.A. | N.A. | Polyfunctional T-cells | Flow cytometry analysis on patients with PsA. | Th1 and Th17 pathways were associated with DAPSA. |
| Coras (120) | 2019 | Cross-sectional | 41 | CASPAR | Eicosanoids | Assessment using liquid phase chromatography and mass spectrometry on 41 patients with PsA. | 9 pro-inflammatory eicosanoids were associated to DAS28 while 18 anti-inflammatory eicosanoids were inversely correlated with it. |
| Colak (121) | 2019 | Cross-sectional | 50 | CASPAR | VASPIN; NGAL; Apolipoprotein | Serum analysis on 50 patients with PsA and 36 HC. | No significant correlation found with disease activity. |
| Sakellariou (122) | 2019 | Cross-sectional | 78 | CASPAR | Calprotectin | ELISA analysis from 78 patients with PsA sera and 78 with RA. | Modest correlation with US activity score. |
| Coras (123) | 2019 | Cross-sectional | 38 | CASPAR | Cholin metabolites | Liquid phase chromatography and mass spectrometry analysis on 38 PsA. | Trimethylamine-N-oxide was correlated to DAS28, CPDAI and BSA. |
| Ozisler (124) | 2020 | Retrospective | 47 | CASPAR | RDW | Blood samples analysed from 47 patients with PsA and 56 HC. | RDW was modestly correlated to DAS28, CRP and ESR in PsA patients. |
| Arias de la rosa (125) | 2020 | Cross-sectional | 80 | CASPAR | Complement C3 | Serum analysis on 80 patients with PsA, 200 with RA, 150 with SpA and 100 HC. | Higher C3 concentration in PsA population with DAS28> 5.1. |
| Esawy (71) | 2020 | Cross-sectional | 76 | CASPAR | Plasma Gelsolin | ELISA analysis of sera from 76 patients with PsA, 40 with PsO and 40 age- and sex-matched HC. | Negative correlation between Gelsolin titers and DAPSA or CPDAI. |
| Jarlborg (126) | 2020 | Cross-sectional | 237 | CASPAR | Calprotectin S100A8/S100A9 | Serum analysis on 969 RA patient blood samples, 451 patients with SpA, 237 PsA patients and 72 HC. | No correlation founded with disease activity in PsA patients. |
| Medvedeva (127) | 2020 | Prospective | 80 | N.A. | 155 different proteins | Immunoassay on sera from 80 pateints with PsA, 175 with SpA and 93 with PsO during different apremilast’s phase 3 studies. | No correlation founded with disease activity in PsA patients. |
| Wcisło-dziadecka (128) | 2020 | Prospective | 6 | CASPAR | TNF- α; TNFR1; TNFR2 | Mass spectrometry analysis from blood samples in 6 patients with PsA treated with adalimumab. | Serum TNF higher titers was associated with lower DAS28. |
| Boyd (129) | 2020 | Cross-sectional | 45 | CASPAR | Panel of 12 biomarkers | Immunoassays analysis in blood samples from 45 patients with PsA. | Moderate correlation between YKL-40, IL-6, ICAM-1, and composite score (SAA CDAI, SDAI, DAS28, HAQ and MDA). |
| Leijten (74) | 2021 | Prospective | 20 | CASPAR | 951 unique proteins | Serum proteomic analyses from 20 patients with PsA, 20 with PsO, 19 with AS and 20 HC. | ICAM-1 and CCL-18 levels were correlated with SCJ. PI3 correlated with PASI score. |
| Coras (130) | 2021 | Cross-sectional | 19 | CASPAR | Oxylipins profile | Reverse-phase chromatography analyses in sera from 19 patients with PsA and 20 with PsO. | Oxylipins profile correlated with skin activity in PsO and PsA patients but not with articular activity in PsA patients. |
PsA, psoriatic arthritis; PsO, psoriasis; HC, Healthy control; OA, Osteoarthritis; RA, Rheumatoid arthritis; LES, lupus erythematosus systemic; UA, Undifferentiated Arthritis; SpA, Spondyloarthritis; SAPHO, Synovitis Acne Pustulosis Hyperostosis Osteitis; CASPAR, Classification criteria for Psoriatic Arthritis; PASI, Psoriatic Area and Severity Index; BSA, Body Surface Area; DAS, Disease Activity Score; CPDAI, composite psoriatic disease activity index; DAPSA, Disease Activity in PSoriatic Arthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; ELISA, Enzyme-linked immune absorbent assay; MTX, Methotrexate; MRP, Myeloid Related Protein; IL, Interleukin; TNFSF, Tumor Necrosis Factor Super Family; CRP, C-reactive protein; TNF, Tumor necrosis factor; HLA, Human leukocyte antigen; VEGF, Vascular endothelial growth factor; Anti-CCP, Anti-cyclic citrullinated protein; anti-LL37, anti-cathelecidin; Anti-ADAMTS5, A disintegrin and metalloproteinase with thrombospondin motifs; ICAM-1, Intercellular adhesion molecule 1; ESR, Erythrocyte sedimentation rate; hsCRP, High sensitive c reactive protein; IFN, Interferon; COMP, Cartilage Oligomeric MetalloProteinase; TCJ, Tender Joint Count; SCJ, Swollen Joint Count; Blys, B lymphocyte Stimulator; BAFF, B cells activating factor belonging to TNF Family; M-CSF, Macrophage colony stimulating factor; MIA, melanoma inhibitory activity; NTX, N-terminal cross-linked telopeptide of type I collagen; ITCP, C-telopeptide of Type I Collagen; RT-PCR, reverse transcription and polymerase chain reaction; CCL-x, chemokine ligand-x; Chi3L1, Chitinase 3 like protein; PTX3, Pentraxin 3; PIIANP, procollagen IIA N-Terminal peptide; C2M, Matrix metalloproteinase-generated type II collagen fragment; MPV, Mean platelet volume; PD1, Programmed Death-1; ET-1, Endothelin 1; VASPIN, visceral adipose tissue-derived serine protease inhibitor; NGAL, Neutrophil-gelatinase associated lipocalin; Anti-CarP, Anti-Carbamethylated protein; RDW, Red blood cell distribution width; MMP3, Matrix MetalloProteinase-3; MDA, Minimal activity disease.
2.2 Data extraction
All data was extracted into a standardized spreadsheet. For each article, we collected the data according to a pre-specified strategy. Collected information included the year of publication, name of the first author, geographical area, study design, population age and sex, disease duration, how the PsA population was determined (classification criteria used or therapist diagnosis), biomarkers investigated, primary study methodology, proportion of patients using corticosteroid and/or non-steroidal anti-inflammatory drugs (NSAIDs), proportions of patients using conventional disease modifying anti-rheumatic drugs (DMARDs; i.e., methotrexate, salazopyrine or leflunomide), and biological DMARDs. Study objectives (diagnosis or prognosis), primary outcomes, and control groups (i.e. cutaneous psoriasis, rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, undifferentiated arthritis, osteoarthritis or healthy control (HC)) were also recorded. For all extracted data, a central value (mean or median) and variability (standard deviation or interquartile range) was collected. Study quality and risk of bias was assessed using the Newcastle Ottawa Scale for assessing the quality of non-randomized studies in meta-analysis (131).
2.3 Statistical analysis
Meta-analyses were performed when a diagnostic bone and cartilage turnover biomarker was reported in 2 or more different cohorts of PsA and control. Levels of biomarkers in PsA and control populations, means differences (MD) and standard deviation (SD) were extracted. If necessary, we converted median and interquartile in MD and SD using previously published methods (132). To perform sensitivity analyses, we applied a random effects models using the “one-study-removed” method as soon as there were more than two publications. Difference effect sizes were ascertained with the standardized mean difference (SMD) and its 95% confidence intervals (CI). A positive SMD confirmed a higher biomarker level in PsA than the control population. Magnitude of SMD was characterized as small (< 0.40), moderate (0.41 to 0.69) or large (> 0.70) (133).
3 Results
We identified 1495 records extracted from the PubMed/MEDLINE (n=514), EMBASE (n=919), and Cochrane Library (n=62) databases. After a manual search, 4 additional publications were included.
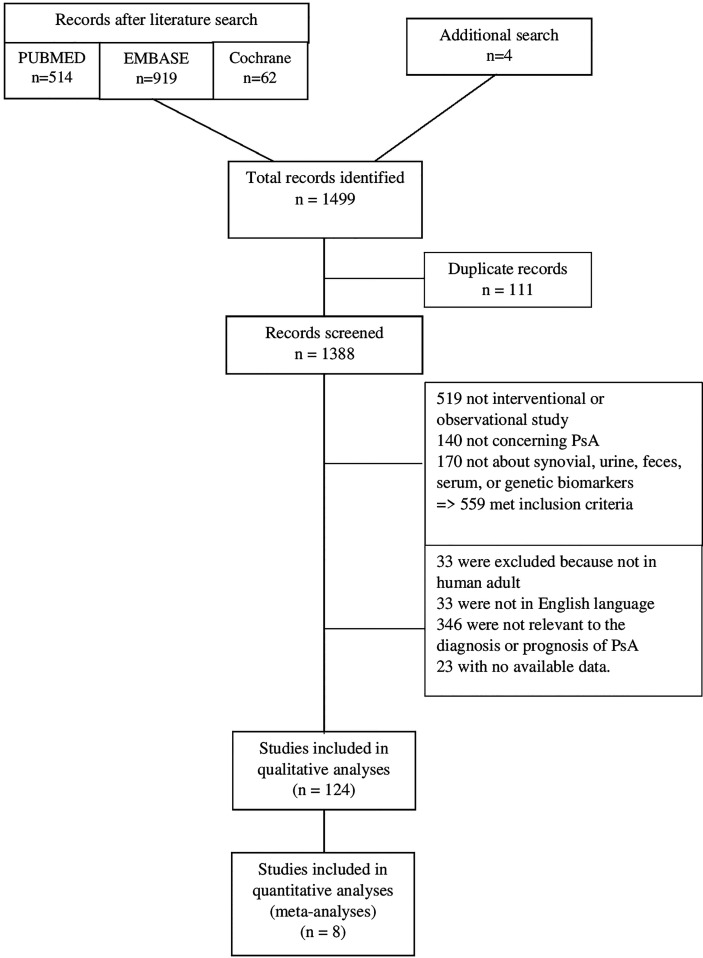
After removal of 111 duplicates, 1388 articles were screened and 559 met inclusion criteria. Subsequently, 346 publications were excluded because they did not report specific diagnostic or prognostic data for the biomarkers. Ultimately, a total of 124 studies, published from 1993 to 2022, met the eligibility criteria and were included in the qualitative analysis ( Figure 1 ).
Figure 1.
Flowchart of the study selection.
Sixty-eight studies evaluated biomarkers for diagnostic purposes, 48 evaluated biomarkers for activity or prognostic severity purposes, and 8 publications studied both diagnostic and prognostic biomarkers ( Tables 1 , 2 ). An assessment of bias risk was performed for each study and is available in Tables 3 , 4 .
Table 3.
Assessment of bias risk using NOS scale for case-control studies.
| Selection | Comparability | Exposure | ||||||
|---|---|---|---|---|---|---|---|---|
| References | Is the case definition adequate? | Representatives of the case | Controls selection | Controls definition | Comparability of cases and controls based on the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate |
| Abji, 2017 (34) | A* | A* | B | A* | A* | D | A* | C |
| Ahmed, 2014 (109) | A* | B | A* | A* | AB** | D | A* | C |
| Alenius, 2002 (93) | A* | B | B | A* | D | A* | C | |
| Alenius, 2004 (20) | A* | B | A* | B | B* | D | A* | C |
| Alenius, 2009 (102) | A* | B | B | A* | D | A* | C | |
| Alonso, 2016 (64) | C | B | B | B | B* | D | A* | C |
| Amin, 2016 (59) | A* | A* | C | A* | A* | D | A* | C |
| Arias de la rosa, 2020 (125) | A* | B | B | A* | A* | D | A* | C |
| Armas-Gonzales, 2015 (58) | A* | B | B | A* | D | A* | C | |
| Ausavarungnirun, 2017 (66) | A* | A* | B | A* | AB** | D | A* | C |
| Bandinelli, 2015 (86) | A* | B | B | D | A* | C | ||
| Batliwalla, 2006 (22) | A* | B | A* | B | A* | D | A* | C |
| Bosè, 2014 (55) | A* | B | B | B | D | A* | C | |
| Bowes, 2011 (25) | A* | A* | B | B* | D | A* | C | |
| Boyd, 2020 (129) | A* | A* | A* | B | D | A* | C | |
| Butt, 2007 (24) | A* | A* | A* | A* | B* | D | A* | C |
| Calzavara, 1999 (43) | A* | B | C | B | D | A* | C | |
| Caputo, 2020 (37) | A* | A* | B | A* | A* | D | A* | C |
| Cascella, 2017 (33) | A* | A* | B | A* | D | A* | C | |
| Celis, 2011 (104) | A* | B | D | C | ||||
| Chandran, 2010 (10) | A* | A* | B | A* | AB** | D | A* | C |
| Chandran, 2014 (30) | A* | B | B | A* | A | D | A* | C |
| Chandran, 2019 (15) | A* | B | B | A* | A* | D | A* | C |
| CheleschI, 2021 (42) | A* | A* | B | A* | A* | C | A* | C |
| Chen, 2019 (35) | A* | B | C | A* | B* | D | A* | C |
| Chou, 2010 (44) | A* | A* | B | A* | A | D | A* | C |
| Chung, 2021 (92) | A* | A* | C | A* | A* | D | A* | C |
| Ciancio, 2017 (32) | A* | B | C | A* | D | A* | C | |
| Colak, 2018 (121) | A* | A* | D | |||||
| Coras, 2019 (120) | A* | B | D | |||||
| Coras, 2019 (123) | A* | B | D | |||||
| Coras, 2021 (130) | A* | A* | A* | B | A* | D | A* | C |
| Cretu, 2014 (11) | A* | B | B | A* | AB** | D | A* | C |
| Cretu, 2018 (24) | A* | B | A* | A* | B* | D | A* | C |
| Cuervo, 2021 (73) | A* | A* | B | A* | B* | D | A* | C |
| Dalbeth, 2010 (89) | A* | B | B | A* | D | A* | C | |
| Dalmády 2013 (45) | A* | A* | A* | A* | D | A* | C | |
| Diani, 2019 (16) | A* | A* | C | B | A* | D | A* | C |
| Dikbas, 2014 (113) | A* | B | C | B | D | A* | C | |
| Dolcino, 2014 (46) | A* | B | C | A* | B* | D | A* | C |
| Dolcino, 2015 (12) | A* | A* | C | A* | AB** | D | A* | C |
| Eder, 2011 (26) | A* | A* | A* | A* | D | A* | C | |
| Eder, 2012 (27) | A* | B | B | B | D | A* | C | |
| Eissa, 2013 (107) | B | B | B | B | AB** | D | A* | C |
| Ek, 2021 (80) | B | A* | B | A* | A* | D | A* | C |
| Elkayam, 2000 (98) | A* | B | B | D | A* | C | ||
| Elkayam, 2000 (97) | A* | B | C | B | D | A* | C | |
| Elkayam, 2004 (19) | A* | A* | C | B | D | A* | C | |
| Esawy, 2020 (71) | A* | A* | B | A* | D | A* | C | |
| Farouk, 2010 (9) | A* | A* | A* | B | AB** | D | A* | C |
| Farrag, 2017 (68) | A* | A* | C | A* | AB** | D | A* | C |
| Fink, 2007 (100) | A* | B | C | A* | D | A* | C | |
| Firuzi, 2008 (53) | A* | B | C | A* | D | A* | C | |
| Foell, 2003 (87) | B | B | C | B | D | A* | C | |
| Frasca, 2018 (48) | A* | B | B | A* | A* | D | A* | C |
| Grossi, 2017 (65) | A* | A* | C | A* | D | A* | C | |
| Gudmann, 2016 (60) | A* | A* | A* | B | B* | D | A* | C |
| Hansson, 2014 (54) | A* | B | C | B | AB** | D | A* | C |
| Helliwell, 1991 (84) | A* | B | D | |||||
| Hu, 2018 (47) | A* | B | C | A* | A* | D | A* | C |
| Husakova, 2015 (101) | A* | B | C | B | A* | D | A* | C |
| Ibrahim, 2017 (96) | A* | B | C | B | A* | D | A* | C |
| Inciarte-Mundo, 2016 (115) | A* | B | B | A | B* | D | A* | C |
| Jadon, 2017 (13) | A* | A* | A* | A* | AB** | D | A* | C |
| Jarlborg, 2020 (126) | B | A* | B | A* | A* | D | A* | C |
| Jensen, 2012v (106) | A* | B | B | A* | A* | D | A* | C |
| Kane, 2003 (87) | A* | B | B | A* | D | A* | C | |
| Kayikci, 2013 (97) | A* | A* | C | B | D | A* | C | |
| Kim, 2015 (57) | A* | A* | B | A* | AB** | D | A* | C |
| Kishikawa, 2021 (75) | A* | A* | B | B | A* | C | A* | C |
| Korendowych, 2005 (94) | A* | B | D | |||||
| Leijten E, 2021 (74) | A* | A* | B | B | A* | C | A* | C |
| Leijten EF, 2021 (79) | A* | A* | B | A* | A* | C | A* | C |
| Lin, 2020 (38) | A* | B | C | A* | D | A* | C | |
| Looby, 2021 (78) | A* | A* | B | A* | A* | D | A* | C |
| Madland, 2007 (101) | A* | B | D | |||||
| Maejima, 2014 (56) | A* | B | C | A* | A* | A* | C | |
| Maejima, 2017 (67) | B | A* | C | B | D | A* | C | |
| Maejima, 2017 (118) | A* | B | B | B | D | A* | C | |
| Mansson, 2001 (8) | A* | A* | A* | A* | D | A* | C | |
| Marzaioli, 2022 (82) | A* | A* | B | A* | A* | D | A* | C |
| Matt, 2015 (111) | A* | B | C | B | A* | D | A* | C |
| Mc Ardle, 2021 (83) | A* | A* | B | A* | A* | D | A* | C |
| McHugh, 2003 (85) | A* | B | A* | |||||
| Medvedeva, 2020 (127) | A* | B | B | A | A* | A* | C | |
| Munk, 2015 (114) | A* | B | C | B | A* | D | A* | C |
| Muntyanu, 2016 (61) | A* | A* | B | A* | D | A* | C | |
| Ozisler, 2019 (124) | A* | B | C | B | A* | D | A* | C |
| Pasquali, 2020 (39) | A* | A* | B | A* | A* | D | A* | C |
| Peled, 2015 (112) | A* | B | B | A* | B* | D | A* | C |
| Perez-Alamino, 2014 (95) | A* | B | D | |||||
| Pongratz, 2010 (103) | A* | A* | D | |||||
| Przepiera-Będzak, 2013 (105) | A* | B | C | B | A* | D | A* | C |
| Przepiera-Będzak, 2016 (116) | A* | B | C | B | A* | D | A* | C |
| Ramonda, 2013 (91) | A* | A* | C | B | A* | D | A* | C |
| Ravindran, 2004 (21) | A* | A* | A* | B | D | A* | C | |
| Reindl, 2016 (63) | A* | B | C | B | D | A* | C | |
| Rooney, 2004 (99) | A* | B | C | B | D | A* | C | |
| Sakellariou, 2019 (122) | A* | B | B | B | A* | D | A* | C |
| Sinkeviciute, 2020 (69) | A* | B | C | B | D | A* | C | |
| Skoumal, 2008 (88) | A* | B | B | A* | D | A* | C | |
| Smith, 2020 (36) | A* | B | A* | A* | D | A* | C | |
| Souto-Carneiro, 2020 (72) | B | B | B | B | B* | D | A* | C |
| Stoeckmann, 2006 | A* | B | C | A* | A* | D | A* | C |
| Szodoray, 2007 (52) | A* | B | C | A* | AB** | D | A* | C |
| van Kuijk, 2010 (90) | A* | A* | D | C | ||||
| Veale, 1993 (51) | A* | B | C | A* | AB** | D | A* | C |
| Vinci, 2020 (50) | A* | B | B | B | D | A* | C | |
| Wade, 2018 (119) | A* | B | B | B | D | A* | C | |
| Wang N., 2022 (81) | A* | A* | B | A* | A* | D | A* | C |
| Waszczykowski, 2020 (17) | A* | A* | C | A* | A* | D | A* | C |
| Waszczykowski, 2021 (18) | A* | B | C | A* | A* | D | A* | C |
| Wcisło-Dziadecka, 2020 (128) | B | B | C | A* | D | A* | C | |
| Winchester, 2012 (28) | A* | A* | B | A* | B* | D | A* | C |
| Yuan, 2019 (49) | A* | B | B | B | AB** | D | A* | C |
| Zhang, 2016 (31) | A* | B | A* | A* | B* | D | A* | C |
| Zhu, 2021 (77) | A* | A* | B | B | A* | D | A* | C |
Selection. 1) Is the case definition adequate? A: yes, with independent validation*; B: yes, eg record linkage or based on self-reports; C: no description.
2) Representativeness of the cases A) consecutive or obviously representative series of cases*; B) potential for selection biases or not stated.
3) Selection of Controls A: community controls*; B: hospital controls; C: no description.
4) Definition of Controls A: no history of disease (endpoint)*; B: no description of source.
Comparability. Comparability of cases and controls on the basis of the design or analysis A: study controls for …*; B: study controls for any additional factor*.
Exposure. 1) Ascertainment of exposure A: secure record (eg surgical records)*; B: structured interview where blind to case/control status; C: interview not blinded to case/control status; D: written self-report or medical record only; E: no description.
2) Same method of ascertainment for cases and controls A: yes*; B: no.
3) Non-Response rate A: Same rate for both groups*; B: non respondents described; C: rate different and no designation.
Table 4.
Assessment of bias risk using NOS scale for cohort study.
| References | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of the study | Comparability of cohorts based on the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts |
|---|---|---|---|---|---|---|---|---|
| Abji F, 2020 (70) | B* | A* | A* | A* | A* | B* | ||
| Abji F, 2020 (BMJ) (70) | B* | A* | A* | A* | B* | A* | A* | D |
| Chandran, 2013 (29) | B* | B* | A* | A* | A* | A* | ||
| Fuentelsaz-Romero, 2021 (76) | B* | A* | A* | A* | A* | A* | C | D |
| Inciarte-Mundo, 2018 (118) | B* | A* | B* | A* | B* | A* | A* | B* |
| Iwaszko, 2021 (41) | A* | A* | A* | A* | A* | A* | A* | A* |
| Wade, 2020 (40) | A* | A* | B* | A* | A* | B* | A* | D |
1) Representativeness of the exposed cohort A: truly representative of the average in the community*; B: somewhat representative of the average in the community*; C: selected group of users eg nurses, volunteers; D: no description of the derivation of the cohort.
2) Selection of the non-exposed cohort A: drawn from the same community as the exposed cohort* B: drawn from a different source; C: no description of the derivation of the non-exposed cohort.
3) Ascertainment of exposure A: secure record (eg surgical records)*; B: structured interview*; C: written self report; D: no description.
4) Demonstration that outcome of interest was not present at start of study A: yes* B: no.
5) Comparability of cohorts on the basis of the design or analysis A: study controls for … *; B: study controls for any additional factor*.
6) Assessment of outcome A: independent blind assessment*; B: record linkage*; C: self report; D: no description.
7) Was follow-up long enough for outcomes to occur A: yes (select an adequate follow up period for outcome of interest)*; B: no.
8) Adequacy of follow up of cohorts A: complete follow up - all subjects accounted for*; B: subjects lost to follow up unlikely to introduce bias*; C: follow up rate < ::% (select an adequate %) and no description of those lost; D: no statement.
3.1 Diagnostic biomarkers
3.1.1 Bone and cartilage turnover biomarkers
Sixteen articles evaluated biomarkers associated with bone and cartilage turnover for their potential as PsA diagnostic biomarkers. All the studies assessing diagnostic biomarkers in our systematic review, including those assessing biomarkers panels, are listed in chronological order of publication in Table 1 . For meta-analyses, we considered only clearly identified individual biomarkers, and not panels of biomarkers. The two mainly assessed biomarkers were Cartilage Oligometrix MetalloProteinase (COMP) and Matrix MetalloProteinase-3 (MMP3).
3.1.1.1 Cartilage oligometrix metalloproteinase (COMP)
Increases of serum COMP levels by one unit resulted in an increased PsO odds ratio (OR) of 1.001 (95% CI=1.000-1.002, p=0.04), but not for PsA (OR=1.00; 95% CI=0.999-1.002, p=0.47) when comparing PsA to both PsO and HC (10). More recently, a cross-sectional study including patients with PsA, osteoarthritis (OA) and HC demonstrated in its primary discovery phase that COMP levels were significantly higher in sera of the PsA population than that of the OA population (OR=1.24; 95% CI=1.06-1.46, p=0.0062) (15). In the validation phase, serum COMP levels were not significantly different between PsA and OA populations (217.3 ng/mL vs 210.3 ng/mL, respectively p=0.344) (15). The diagnostic value of COMP was assessed in two cross-sectional studies. In the first study, serum COMP levels were significantly higher in patients with PsA (2645.3 ± 489.5 ng/mL) than in the HC population (835.9 ± 434.6 ng/mL) and clearly distinguished the 2 populations (Area Under the Curve [AUC]=0.96, 95% CI Not Available [NA]) (17). The second study compared the biomarker in PsA, OA, and HC and reported significantly higher levels in the sera of PsA patients than in the other two populations (18). COMP levels were also reported to be significantly higher in PsA synovial fluid compared to rheumatoid arthritis (RA), even in the presence of joint destruction (8).
The four studies comparing serum COMP levels between PsA and HC were included in a meta-analysis (10, 15, 17, 18). COMP was significantly increased in the serum of the PsA population, and the effect size measured by SMD was 2.305 (95% CI=0.80-3.81, p=0.003; Figure 2 ). When COMP levels were compared between PsA and OA, the 3-study meta-analysis reported a significant SMD of 0.78 (95% CI=0.02-1.55, p=0.046) (15, 18).
Figure 2.
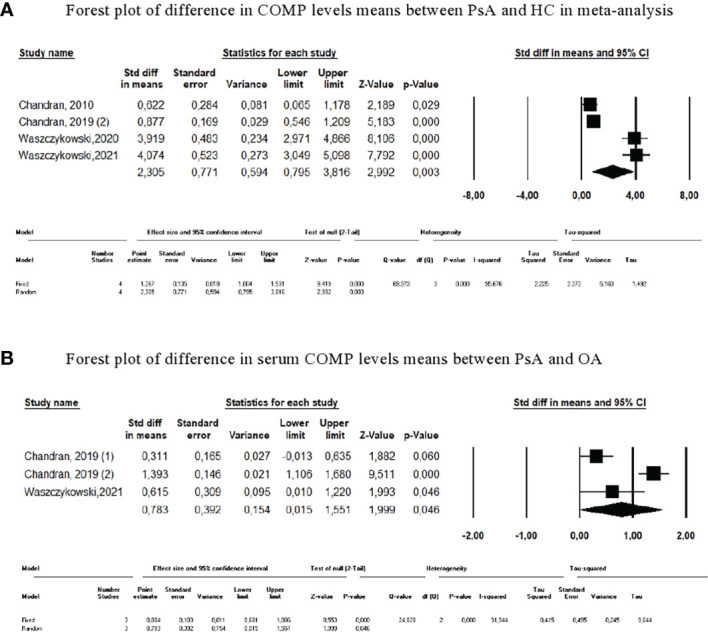
Meta-analysis of COMP levels. (A) Forest plot of difference in COMP levels means between PsA and HC in meta-analysis. (B) Forest plot of difference in serum COMP levels means between PsA and OA.
Due to the heterogeneity of the results, sensitivity analyses were also performed. The results remained unchanged after each study was excluded serially. The difference in COMP levels between PsA and PsO was tested in only one study (10).
3.1.1.2 Matrix metalloproteinase-3 (MMP3)
The results are more discordant when considering serum levels of MMP3, also known as stromelysin-1. Five cross-sectional studies revealed that MMP3 levels were significantly higher in patients with PsA than in HC (12–14, 17, 18) or in patients with PsO (13). The accuracy of MMP3 to distinguish PsA from PsO (AUC=0.70, 95% CI=0.65-0.75) and PsA from HC (AUC=0.66, 95% CI=0.59-0.74) was moderate. Other studies did not report any difference in MMP3 levels between PsA and HC (16) or PsO (14). The latter study described an increased concentration of MMP3 in PsA sera compared to PsO, with a significant OR of 1.59 (95% CI=1.21-2.11); however, this disappeared after multivariate regression (14). In a recent study, serum MMP3 levels from PsA compared to PsO and HC were not significantly different. Only one study compared PsA and OA, and did not demonstrate any difference in serum levels of MMP3 (18).
In total, 6 studies compared serum MMP3 levels between PsA and HC (10, 13, 14, 16–18) and 4 studies compared levels between PsA and PsO (10, 13, 14, 16). The pooled results displayed a higher, but not significant, level of MMP3 in sera of PsA patients compared to HC (SMD=0.450, 95% CI=-0.080-0.981, p=0.096; Figure 3 ). MMP3 was significantly higher in PsA compared to PsO (SMD=0.419, 95% CI=0.119-0.719; p=0.006). Results were confirmed with sensitivity analyses. No significant SMD was found when pooled MMP3 levels of PsO and HC were compared (SMD=-0.118 [95% CI=-0.693-0.457], p=0.689).
Figure 3.
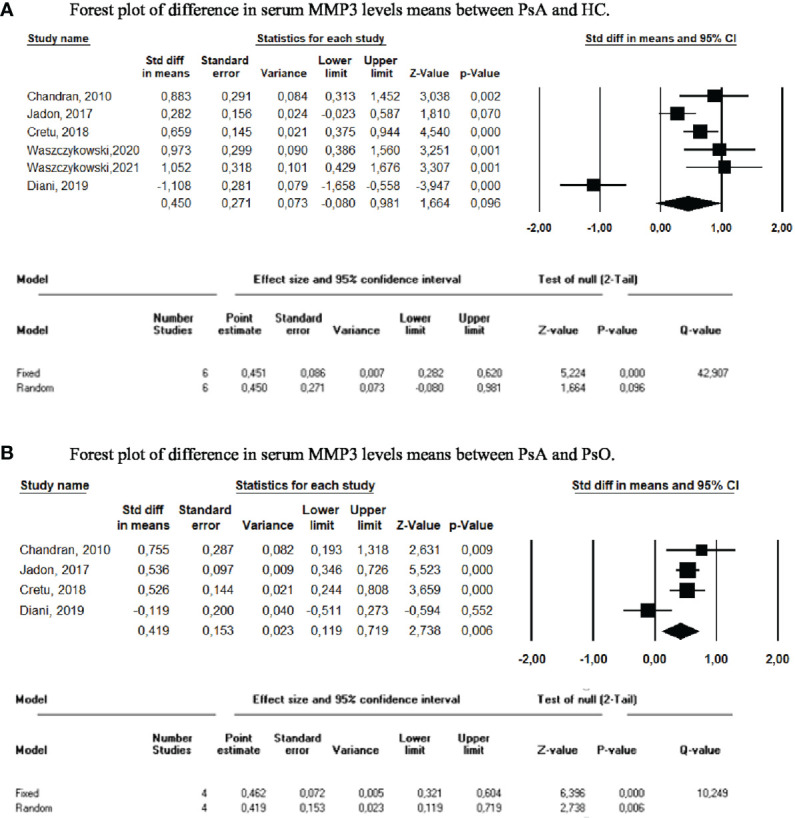
Meta-analysis of serum MMP3 levels. (A) Forest plot of difference in serum MMP3 levels means between PsA and HC. (B) Forest plot of difference in serum MMP3 levels means between PsA and PsO.
3.1.1.3 Receptor activator of nuclear kappa-B ligand (RANK-L)
RANK-L was also assessed as a diagnostic biomarker in three studies. Two studies reported significantly higher serum RANK-L levels in PsA patients compared with HC (59, 134), whereas the remaining study observed the opposite (16). The same three studies also compared RANK-L levels between PsA and PsO, with little or no difference detected between the two populations. The only study reporting significantly higher levels in PsA patients concluded that RANK-L was inaccurate for differentiating PsA from PsO (AUC=0.66 [95% CI NA]) (59). In the meta-analysis of these three publications, comparison of RANKL levels between PsA and HC and between PsA and PsO (10, 16, 59), the SMDs were -0.106 (95% CI=-3.75 -3.36, p=0.952) and 0.315 (95% CI=-0.391-1.021, p=0.382), respectively ( Figure 4 ). Results did not change upon performance of sensitivity analyses.
Figure 4.
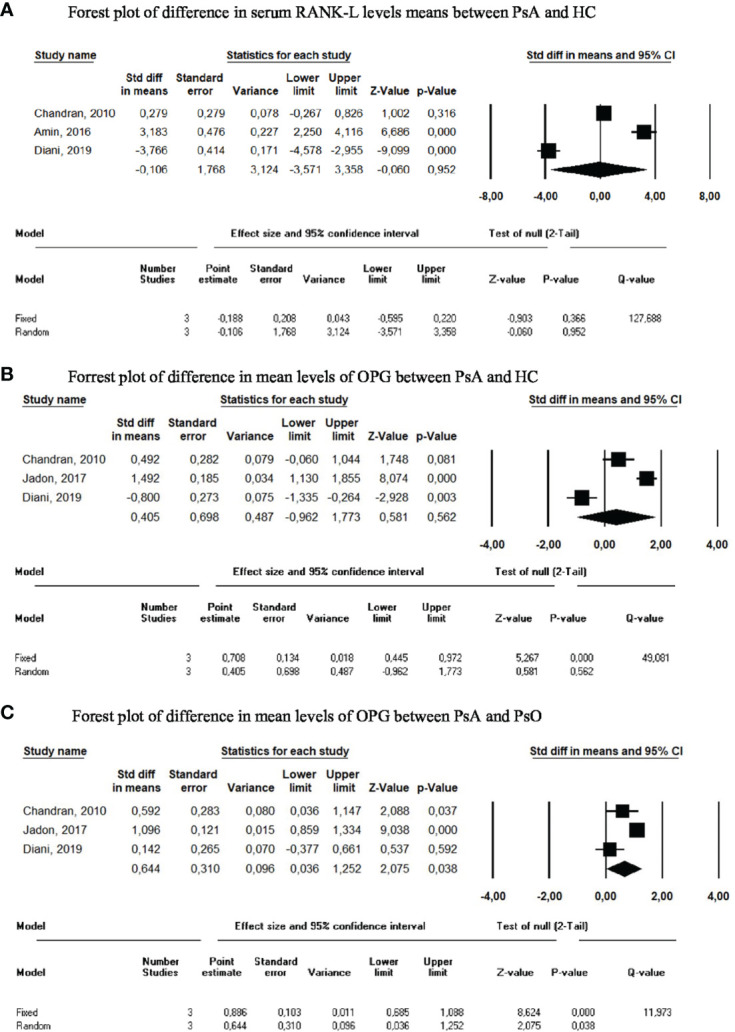
Meta-analysis of circulating biomarkers. (A) Forest plot of difference in serum RANK-L levels means between PsA and HC. (B) Forrest plot of difference in mean levels of OPG between PsA and HC. (C) Forrest plot of difference in mean levels of OPG between PsA and PsO.
3.1.1.4 Osteoprotegerin (OPG)
OPG serum levels were significantly increased in a small PsA cohort (n=26 per group) compared with PsO and HC (10). However, levels were not different in a larger PsA cohort (n=200 per group) compared to PsO and HC (13), nor in another smaller PsA cohort (n=50) compared to PsO (n=50) and HC (n=20) (16). In our meta-analysis, we calculated an SMD of 0.405 (95%CI -0.962-1.773, p=0.562) when PsA was compared to HC, and an SMD of 0.644 (95%CI 0.036-1.252, p=0.038) when PsA was compared to PsO ( Figure 4 ).
3.1.1.5 Dickkopf-1 (Dkk-1)
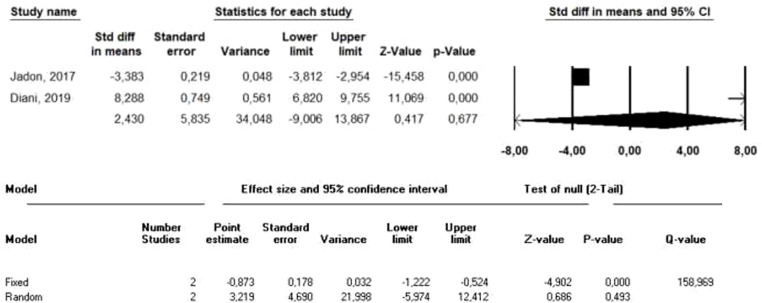
Dkk-1 serum levels were studied in two publications with opposing results (13, 16). After meta-analysis, SMD in Dkk-1 levels between PsA and HC was 3.22 (95% CI=-5.974-12.412, p=0.493; Figure 5 ). When patients with PsA were compared to patients with PsO, the SMD was 0.992 (95% CI=-0.89-2.87, p=0.301).
Figure 5.
Forest plot of difference in Dkk-1 levels means between PsA and HC in meta-analysis.
3.1.2 Genetic biomarkers
Human Leucocyte Antigen (HLA) was studied in 6 publications (19, 27–29, 35, 36). A family-based study reported an association between PsA and various HLA alleles: HLA-B*27, HLA-B*38, HLA-B*39, and HLA-C*12 (27). HLA-B*27 association with PsA was additionally described in a large cross-sectional study, while HLA-C*06 was found to be associated with skin damage and less prevalent musculoskeletal developmental phenotypes (28). Another study described an association between PsA and HLA-B*27, HLA haplotype C*12/B*38m and HLA-C*06/B*57 (29). Interestingly, HLA-B27 was not associated with PsA in the Jewish Israeli population (19), though HLA-A*01/A*01 and HLA-C*06/C*02 were risk alleles for PsA in the Chinese Han population (35).
Genetic polymorphisms were also explored in 9 different articles (20, 21, 24–26, 30, 31, 33, 41). Polymorphisms of the gene Il-13 were examined in two studies, which reported that rs1800925*C and rs20641*G were significantly associated with PsA in a PsO population (25, 26). However, one study reported the association between rs1800925 polymorphism and PsA only in the smoker population (26).
3.1.3 Autoantibodies
In our systematic literature review, autoantibodies were explored in 8 articles (43–50). They focused on different autoantibodies, preventing meta-analysis. Among these autoantibodies, those of interest are outlined below. It should be noted that none of these autoantibodies have been evaluated or validated in any additional PsA cohorts beyond those described here.
Anti-Cyclic citrullinated peptides (CCP) were present in some PsA patients (7.9%) (43). Anti-Mutated citrullinated vimentin (MCV) levels were significantly higher in PsA sera (24%) than PsO (8%) (45). The novel autoantibody named anti-PsA peptide was identified after screening of a random synthetic peptide library with pooled immunoglobulins derived from 30 patients with recent onset PsA. Anti-PsA shares a sequence homology with Nebullin Related Anchoring Protein (N-RAP) (46). A peptide corresponding to N-RAP sequence was synthetized and tested by ELISA. Anti-NRAP autoantibodies were recognized by 83% of PsA sera, versus 7% of rheumatoid arthritis (RA) anti-CCP positive, 4% of RA anti-CCP negative, 3.3% of PsO, and none of other rheumatic diseases included in this study (46).
Anti-A Disintegrin and MetalloproteinaSe with ThromboSpondin motifs 5 (ADAMSTS5) and anti-Cathelicidin LL37 (LL37) IgG autoantibodies were assessed for differentiating PsA from PsO. The ROC analysis reported an AUC of 0.84 (95% CI=NA, p<0.1) for anti-ADAMSTS5 autoantibodies and 0.87 (95% CI=NA, p<0.01) for anti-LL37 autoantibodies (49). Expression of Carbamethylated anti-LL37 was significantly higher in PsA sera (median=0.66, IQR=0.439) than PsO (median=0.43, IQR=0.47, p=0.02) and HC (median=0.158, IQR=0.099, p=0.0001) (48). Finally, higher IgA anti-oxidized collagen type II (oxPTMCII) autoantibodies were detected in PsA (84%, n=33/39) and axial spondyloarthritis (SpA, 47%, n=79/165) sera compared to HC (0%, n=0/28) (50).
3.1.4 Other biomarkers
Two additional biomarkers unrelated to the above categories were also assessed in at least 2 publications.
3.1.4.1 C-X-C motif chemokine ligand 10 (CXCL10)
CXCL10 rates were overexpressed in synovial fluid of PsA versus gout or SpA, but rates were similar to those of RA (61). A prospective follow-up of patients with PsO reported both higher baseline serum CXCL10 levels in patients who subsequently developed PsA as compared with those who did not, and a significant decrease in CXCL10 levels from the year before to the year after PsA onset (70).
3.1.4.2 Calprotectin S100A8/S100A9
A serum calprotectin S100A8/S100A9 with a cut-off of 475 ng/mL was able to discriminate PsA from HC with a 93.3% specificity and 75.0% sensitivity (54). Serum calprotectin levels were increased in PsA but also in other inflammatory arthritis (RA, axial SpA) compared to controls (i.e., OA, fibromyalgia, and undifferentiated arthralgia), with good accuracy for distinguishing inflammatory arthritis from controls (AUC=0.964, 95% CI=NA) (65). Calprotectin levels in each subpopulation were not available for a meta-analysis.
3.2 Prognostic biomarkers
Prognostic biomarkers, including markers of disease activity, were less frequently assessed than diagnostic biomarkers ( Table 2 ). As such, the data collected were insufficient to perform meta-analyses.
3.2.1 Acute phase reactant biomarkers
One study concluded that erythrocyte sedimentation rate (ESR) was better correlated with Ritchie’s index, tender joint count (TJC) and swollen joint count (SJC) than C-Reactive Protein (CRP) in a 36 patient PsA cohort (84). These potential prognosis markers were explored in a 5-year follow-up prospective study. The 36 patients with PsA demonstrated a disease duration ranging from 1-40 years, and baseline ESR was associated with structural progression (85). Both ESR and CRP were also associated with UltraSonography (US) which are signs of active synovitis (86).
3.2.2 Bone and cartilage turnover biomarkers
Serum COMP levels were correlated with acute phase reactants and disease activity (TJC, r=0.60, p<0.001) and SJC, r=0.75, p<0.0001) (18, 88). A decrease in MMP3 serum levels in PsA patients receiving adalimumab was correlated with Disease Activity Score (DAS)-28 improvement (90).
3.2.3 Autoantibodies
In a cross-sectional study, positivity for anti-CCP antibodies was associated with more radiographic damage and polyarticular phenotypes (95). Anti-LL37 autoantibodies were also described to correlate with disease activity in PsA (48). A strong correlation between Anti-Carbamethylated Protein (CarP) antibody levels and both clinical and ultrasonographic activity was described (correlation between anti-CarP and DAS-28 (r=0.96), CRP (r=0.97), ESR (r=0.97) and US power Doppler+synovitis with a Pearson coefficient >0.97) (96).
3.2.4 Other biomarkers
3.2.4.1 Serum calprotectin S100A8/A9
Calprotectin S100A8/A9 plasma levels were not correlated with disease activity (122, 126). Although, one study reported calprotectin S100A8/A9 levels significantly increased in the polyarticular phenotype of PsA, and were correlated with SJC (54). Contradictory results were reported regarding correlation with US synovitis in greyscale and power Doppler analyses (115, 122). High levels of calprotectin were also associated with relapse at 1-year (118).
3.2.4.2 YKL40
Serum concentration of YKL40, also named Chitinase like-3 protein (Chi3L1) was significantly correlated with disease activity (r=0.848, p<0.001) (109). It was also sensitive to changes, with a significant decrease in serum of good responders to TNF inhibitor (106).
4 Discussion
Our systematic review of the literature shows that few biomarkers are currently available to guide clinical practice in the diagnosis and prognosis of PsA. This review has highlighted COMP and MMP3 as two potential serum biomarkers for the diagnosis of PsA. However, the discriminative qualities of these bone and cartilage remodeling markers have been revealed as insufficient for clinical use.
The meta-analysis showed that COMP was able to differentiate patients with PsA from those with OA or from healthy subjects. However, serum levels varied widely between studies. One study reported mean COMP rates were 10-fold lower in the PsA, OA, and HC populations compared with those observed in the other studies, while methodologically, only the commercial ELISA kits differed between studies (15). The success of COMP in distinguishing PsA from PsO was different across studies, and the absence of numerical data prevented performance of a meta-analysis and to deduce its efficacy in this application.
The pooled serum MMP-3 levels were significantly higher in PsA patients than in PsO patients. However, the meta-analysis showed that they did not appropriately distinguish patients with PsA from healthy subjects. Similarly, the only publication that studied MMP3 serum levels in PsA and OA reported no difference between the two (134). MMP3 levels were increased in OA when compared to HC, but do not appear to be a reliable biomarker, particularly as their assessment in a multiplex system reported results contrary to those found in this analysis (i.e., lower MMP3 serum levels in patients with PsA and PsO than in healthy subjects) (16). None of the remaining bone remodeling or cartilage markers demonstrated any ability to differentiate patients with PsA from controls. However, all the studies included in the analysis were cross-sectional studies, with patients whose diagnosis was pre-existing and whose disease duration was not considered.
We have not identified any new genetic biomarkers useful in the diagnosis of PsA since the last meta-analyses on the subject, and these analyses on genetic polymorphisms had not identified any useful biomarkers for the practical diagnosis of PsA (135, 136). Our systematic review did not identify all publications on HLA and PsA association, which may be due to the fact that our search algorithm was not specifically focused on genetics. The major loci of interest have historically been MHC region and HLA genes, of which certain alleles, primarily HLA-C*06 and HLA-B*27, are carried by about 20-35% of PsA patients (137). Recently, HLA-B27 has been identified as a marker of the axial PsA phenotype, and HLA-C*06 as a marker of the peripheral PsA phenotype (138). Furthermore, both systematic review for PsA biomarkers and a recent meta-analysis examining HLA association in PsA patients confirmed a significant increase in the risk of PsA in HLA-C*02 and C*12 populations (139, 140). Specific non-HLA PsA variants have been identified in GWAS studies, including in the IL12B, NOS2 and IFIH1 regions as reported in a systematic review published in 2015 (141). Several signaling pathways possibly implicated in the pathogenesis of PsA were presented in a recent systematic review published in 2020 (142).
Data concerning autoantibodies in PsA remains sparse in the literature. While some data appears promising, no replication studies have been published. Anti-CCP antibodies are associated with polyarticular phenotypes and structural lesions, and have been shown to be markers of severity rather than diagnosis. Indeed, a recent study reported a correlation between anti-CCP antibodies in PsA and pulmonary manifestations (143).
This systematic review of the literature has several limitations. Only patients with PsA were included while studies involving patients with SpA were excluded, which may have incidentally excluded patients with psoriatic forms of axial disease. The search algorithm also did not include imaging biomarkers, although combining imaging with other biomarkers might help to better define PsA (144). In addition, we choose to focus on diagnosis and prognosis biomarkers unrelated to treatment and did not include predictive biomarkers of treatment response. Finally, our search equation did not allow us to highlight the numerous studies concerning genetics biomarkers in psoriatic arthritis either and should be a focus of a future systemic review.
In summary, this review was broad, with more than 50 studies included since the prior systematic review on diagnostic and prognostic biomarkers in PsA (139). No specific diagnostic biomarkers for PsA were identified, despite the fact that this was the first meta-analyses to assess COMP and MMP3. The search for autoantibodies in PsA appears promising but requires additional confirmatory studies. Further studies are also needed to assess the performance of potential biomarkers that can distinguish PsA from OA and other chronic inflammatory diseases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
TW: conception and design of the study, acquisition of data, analysis of data, original draft preparation, data curation, reviewing and editing. NB: conception and design of the study, editing LB: analysis of data, stats PL: editing, supervision TP: conception and design of the study, analysis of data, drafting manuscript, supervision, reviewing and editing All authors reviewed and approved the final draft of the manuscript.
Acknowledgments
Mrs. Catherine Weill assisted with the Embase bibliographic research. JetPub Scientific Communications LLC provided editorial assistance to the authors during preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Scotti L, Franchi M, Marchesoni A, Corrao G. Prevalence and incidence of psoriatic arthritis: A systematic review and meta-analysis. Semin Arthritis Rheumatol (2018) 48(1):28–34. doi: 10.1016/j.semarthrit.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 2. Mease PJ, Gladman DD, Papp KA, Khraishi MM, Thaçi D, Behrens F, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol (2013) 69(5):729–35. doi: 10.1016/j.jaad.2013.07.023 [DOI] [PubMed] [Google Scholar]
- 3. Taylor WJ. Impact of psoriatic arthritis on the patient: Through the lens of the WHO international classification of functioning, health, and disability. Curr Rheumatol Rep (2012) 14(4):369–74. doi: 10.1007/s11926-012-0263-5 [DOI] [PubMed] [Google Scholar]
- 4. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med (2017) 376(10):957–70. doi: 10.1056/nejmra1505557. [DOI] [PubMed] [Google Scholar]
- 5. Duffin KC, FitzGerald O, Kavanaugh A, Mease PJ, Merola JF, Ogdie A, et al. GRAPPA 2017 project report. J Rheumatol Suppl (2018) 94:48–51. doi: 10.3899/jrheum.180139 [DOI] [PubMed] [Google Scholar]
- 6. Atkinson AJ, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther (2001) 69(3):89–95. doi: 10.1067/mcp.2001.113989 [DOI] [PubMed] [Google Scholar]
- 7. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev (2021) 10(1):1–11. doi: 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Månsson B, Gülfe A, Geborek P, Heinegård D, Saxne T. Release of cartilage and bone macromolecules into synovial fluid: differences between psoriatic arthritis and rheumatoid arthritis. Ann Rheum Dis (2001) 60(1):27–31. doi: 10.1136/ard.60.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farouk HM, Mostafa AAA, Youssef SS, Elbeblawy MMS, Assaf NY, Elokda ESE. Value of entheseal ultrasonography and serum cartilage oligomeric matrix protein in the preclinical diagnosis of psoriatic arthritis. Clin Med Insights Arthritis Musculoskelet Disord (2010) 3:7–14. doi: 10.4137/CMAMD.S4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chandran V, Cook RJ, Edwin J, Shen H, Pellett FJ, Shanmugarajah S, et al. Soluble biomarkers differentiate patients with psoriatic arthritis from those with psoriasis without arthritis. Rheumatology (2010) 49(7):1399–405. doi: 10.1093/rheumatology/keq105 [DOI] [PubMed] [Google Scholar]
- 11. Cretu D, Prassas I, Saraon P, Batruch I, Gandhi R, Diamandis EP, et al. Identification of psoriatic arthritis mediators in synovial fluid by quantitative mass spectrometry. Clin Proteomics (2014) 11(1):1–12. doi: 10.1186/1559-0275-11-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dolcino M, Ottria A, Barbieri A, Patuzzo G, Tinazzi E, Argentino G, et al. Gene expression profiling in peripheral blood cells and synovial membranes of patients with psoriatic arthritis. PloS One (2015) 10(6):e0128262. doi: 10.1371/journal.pone.0128262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jadon DR, Sengupta R, Nightingale A, Lu H, Dunphy J, Green A, et al. Serum bone-turnover biomarkers are associated with the occurrence of peripheral and axial arthritis in psoriatic disease: A prospective cross-sectional comparative study. Arthritis Res Ther (2017) 19(1):1–10. doi: 10.1186/s13075-017-1417-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cretu D, Gao L, Liang K, Soosaipillai A, Diamandis EP, Chandran V. Differentiating psoriatic arthritis from psoriasis without psoriatic arthritis using novel serum biomarkers. Arthritis Care Res (2018) 70(3):454–61. doi: 10.1002/acr.23298 [DOI] [PubMed] [Google Scholar]
- 15. Chandran V, Abji F, Perruccio AR, Gandhi R, Li S, Cook RJ, et al. Serum-based soluble markers differentiate psoriatic arthritis from osteoarthritis. Ann Rheum Dis (2019) 78(6):796–801. doi: 10.1136/annrheumdis-2018-214737 [DOI] [PubMed] [Google Scholar]
- 16. Diani M, Perego S, Sansoni V, Bertino L, Gomarasca M, Faraldi M, et al. Differences in osteoimmunological biomarkers predictive of psoriatic arthritis among a Large Italian cohort of psoriatic patients. Int J Mol Sci (2019) 20(22):5617. doi: 10.3390/ijms20225617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waszczykowski M, Bednarski I, Narbutt J, Waszczykowska E, Lesiak A, Fabiś J. Interleukin-18, interleukin-20, and matrix metalloproteinases (MMP-1, MMP-3) as markers of psoriatic arthritis disease severity and their correlations with biomarkers of inflammation and turnover of joint cartilage. Adv Dermatol Allergology/Postępy Dermatologii i Alergologii (2020) 37(6):1001–8. doi: 10.5114/ada.2020.94903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waszczykowski M, Fabiś-Strobin A, Bednarski I, Lesiak A, Narbutt J, Fabiś J. Serum biomarkers of inflammation and turnover of joint cartilage can help differentiate psoriatic arthritis (PsA) patients from osteoarthritis (OA) patients. Diagnostics (2021) 11(1):52. doi: 10.3390/diagnostics11010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elkayam O, Segal R, Caspi D. Human leukocyte antigen distribution in Israeli patients with psoriatic arthritis. Rheumatol Int (2004) 24(2):93–7. doi: 10.1007/s00296-003-0325-0 [DOI] [PubMed] [Google Scholar]
- 20. Alenius GM, Friberg C, Nilsson S, Wahlström J, Dahlqvist SR, Samuelsson L. Analysis of 6 genetic loci for disease susceptibility in psoriatic arthritis. J Rheumatol (2004) 31(11):2230–5. [PubMed] [Google Scholar]
- 21. Ravindran JS, Owen P, Lagan A, Lewis J, Korendowych E, Welsh K, et al. Interleukin 1α, interleukin 1β and interleukin 1 receptor gene polymorphisms in psoriatic arthritis. Rheumatology (2004) 43(1):22–6. doi: 10.1093/rheumatology/keg443 [DOI] [PubMed] [Google Scholar]
- 22. Batliwalla FM, Li W, Ritchlin CT, Xiao X, Brenner M, Laragione T, et al. Microarray analyses of peripheral blood cells identifies unique gene expression signature in psoriatic arthritis. Mol Med (2005) 11(1–12):21–9. doi: 10.2119/2006-00003.Gulko [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stoeckman AK, Baechler EC, Ortmann WA, Behrens TW, Michet CJ, Peterson EJ. A distinct inflammatory gene expression profile in patients with psoriatic arthritis. Genes Immun (2006) 7(7):583–91. doi: 10.1038/sj.gene.6364334 [DOI] [PubMed] [Google Scholar]
- 24. Butt C, Lim S, Greenwood C, Rahman P. VEGF, FGF1, FGF2 and EGF gene polymorphisms and psoriatic arthritis. BMC Musculoskelet Disord (2007) 8(1):1–7. doi: 10.1186/1471-2474-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bowes J, Eyre S, Flynn E, Ho P, Salah S, Warren RB, et al. Evidence to support IL-13 as a risk locus for psoriatic arthritis but not psoriasis vulgaris. Ann Rheum Dis (2011) 70(6):1016–9. doi: 10.1136/ard.2010.143123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eder L, Chandran V, Pellett F, Pollock R, Shanmugarajah S, Rosen CF, et al. IL13 gene polymorphism is a marker for psoriatic arthritis among psoriasis patients. Ann Rheum Dis (2011) 70(9):1594–8. doi: 10.1136/ard.2010.147421 [DOI] [PubMed] [Google Scholar]
- 27. Eder L, Chandran V, Pellett F, Shanmugarajah S, Rosen CF, Bull SB, et al. Differential human leucocyte allele association between psoriasis and psoriatic arthritis: a family-based association study. Ann Rheum Dis (2012) 71(8):1361–5. doi: 10.1136/annrheumdis-2012-201308 [DOI] [PubMed] [Google Scholar]
- 28. Winchester R, Minevich G, Steshenko V, Kirby B, Kane D, Greenberg DA, et al. HLA associations reveal genetic heterogeneity in psoriatic arthritis and in the psoriasis phenotype. Arthritis Rheum (2012) 64(4):1134–44. doi: 10.1002/art.33415 [DOI] [PubMed] [Google Scholar]
- 29. Chandran V, Bull SB, Pellett FJ, Ayearst R, Rahman P, Gladman DD. Human leukocyte antigen alleles and susceptibility to psoriatic arthritis. Hum Immunol (2013) 74(10):1333–8. doi: 10.1016/j.humimm.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 30. Chandran V, Bull SB, Pellett FJ, Ayearst R, Pollock RA, Gladman DD. Killer-cell immunoglobulin-like receptor gene polymorphisms and susceptibility to psoriatic arthritis. Rheumatology (2014) 53(2):233–9. doi: 10.1093/rheumatology/ket296 [DOI] [PubMed] [Google Scholar]
- 31. Zhang Z, Yuan J, Tian Z, Xu J, Lu Z. Investigation of 36 non-HLA (human leucocyte antigen) psoriasis susceptibility loci in a psoriatic arthritis cohort. Arch Dermatol Res (2017) 309(2):71–7. doi: 10.1007/s00403-016-1706-z [DOI] [PubMed] [Google Scholar]
- 32. Ciancio G, Ferracin M, Saccenti E, Bagnari V, Farina I, Furini F, et al. Characterisation of peripheral blood mononuclear cell microRNA in early onset psoriatic arthritis. Clin Exp Rheumatol (2017) 35:113–21. [PubMed] [Google Scholar]
- 33. Cascella R, Strafella C, Ragazzo M, Manzo L, Costanza G, Bowes J, et al. KIF3A and IL-4 are disease-specific biomarkers for psoriatic arthritis susceptibility. Oncotarget (2017) 8(56):95401. doi: 10.18632/oncotarget.20727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abji F, Pollock RA, Liang K, Chandran V, Gladman DD. Th17 gene expression in psoriatic arthritis synovial fluid and peripheral blood compared to osteoarthritis and cutaneous psoriasis. Clin Exp Rheumatol (2017) 36(3):486–9. [PubMed] [Google Scholar]
- 35. Chen J, Yang F, Zhang Y, Fan X, Xiao H, Qian W, et al. HLA-A*01:01 in MHC is associated with psoriatic arthritis in Chinese han population. Arch Dermatol Res (2019) 311(4):277–85. doi: 10.1007/s00403-019-01902-3 [DOI] [PubMed] [Google Scholar]
- 36. Smith MP, Ly K, Thibodeaux Q, Beck K, Yang E, Sanchez I, et al. Evaluation of a genetic risk score for diagnosis of psoriatic arthritis. J Psoriasis Psoriatic Art (2020) 5(2):61–7. doi: 10.1177/2475530320910814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caputo V, Strafella C, Termine A, Campione E, Bianchi L, Novelli G, et al. RNAseq-based prioritization revealed COL6A5, COL8A1, COL10A1 and MIR146A as common and differential susceptibility biomarkers for psoriasis and psoriatic arthritis: Confirmation from genotyping analysis of 1417 Italian subjects. Int J Mol Sci (2020) 21(8):2740. doi: 10.3390/ijms21082740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin SH, Ho JC, Li SC, Cheng YW, Yang YC, Chen JF, et al. Upregulation of miR-941 in circulating CD14+ monocytes enhances osteoclast activation via WNT16 inhibition in patients with psoriatic arthritis. Int J Mol Sci (2020) 21(12):4301. doi: 10.3390/ijms21124301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pasquali L, Svedbom A, Srivastava A, Rosén E, Lindqvist U, Ståhle M, et al. Circulating microRNAs in extracellular vesicles as potential biomarkers for psoriatic arthritis in patients with psoriasis. J Eur Acad Dermatol Venereology (2020) 34(6):1248–56. doi: 10.1111/jdv.16203 [DOI] [PubMed] [Google Scholar]
- 40. Wade SM, McGarry T, Wade SC, Fearon U, Veale DJ. Serum MicroRNA signature as a diagnostic and therapeutic marker in patients with psoriatic arthritis. J Rheumatol (2020) 47(12):1760–7. doi: 10.3899/jrheum.190602 [DOI] [PubMed] [Google Scholar]
- 41. Iwaszko M, Wielińska J, Świerkot J, Kolossa K, Sokolik R, Bugaj B, et al. IL-33 gene polymorphisms as potential biomarkers of disease susceptibility and response to TNF inhibitors in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis patients. Front Immunol (2021) 12:2168. doi: 10.3389/fimmu.2021.631603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheleschi S, Tenti S, Bedogni G, Fioravanti A. Circulating mir-140 and leptin improve the accuracy of the differential diagnosis between psoriatic arthritis and rheumatoid arthritis: a case-control study. Trans Res (2022) 239:18–34. doi: 10.1016/j.trsl.2021.08.001 [DOI] [PubMed] [Google Scholar]
- 43. Calzavara-Pinton PG, Franceschini F, Manera C, Zane C, Prati E. Incidence of antiperinuclear factor in patients with psoriatic arthritis. Adv Exp Med Biol (1999) 455:215–20. doi: 10.1007/978-1-4615-4857-7_31 [DOI] [PubMed] [Google Scholar]
- 44. Chou CL, Wu MJ, Yu CL, Lu MC, Hsieh SC, Wu TH, et al. Anti-agalactosyl IgG antibody in ankylosing spondylitis and psoriatic arthritis. Clin Rheumatol (2010) 29(8):875–81. doi: 10.1007/s10067-010-1413-7 [DOI] [PubMed] [Google Scholar]
- 45. Dalmády S, Kiss M, Képíró L, Kovács L, Sonkodi G, Kemény L, et al. Higher levels of autoantibodies targeting mutated citrullinated vimentin in patients with psoriatic arthritis than in patients with psoriasis vulgaris. Clin Dev Immunol (2013) 2013:474028. doi: 10.1155/2013/474028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dolcino M, Lunardi C, Ottria A, Tinazzi E, Patuzzo G, Puccetti A. Crossreactive autoantibodies directed against cutaneous and joint antigens are present in psoriatic arthritis. PloS One (2014) 9(12):e115424. doi: 10.1371/journal.pone.0115424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu Q, Sun Y, Li Y, Shi H, Teng J, Liu H, et al. Anti-SIRT1 autoantibody is elevated in ankylosing spondylitis: A potential disease biomarker. BMC Immunol (2018) 19(1):1–8. doi: 10.1186/s12865-018-0280-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Frasca L, Palazzo R, Chimenti MS, Alivernini S, Tolusso B, Bui L, et al. Anti-LL37 antibodies are present in psoriatic arthritis (PsA) patients: New biomarkers in PsA. Front Immunol (2018) 9(SEP):1936. doi: 10.3389/fimmu.2018.01936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yuan Y, Qiu J, Lin ZT, Li W, Haley C, Mui UN, et al. Identification of novel autoantibodies associated with psoriatic arthritis. Arthritis Rheumatol (2019) 71(6):941–51. doi: 10.1002/art.40830 [DOI] [PubMed] [Google Scholar]
- 50. Vinci C, Infantino M, Raturi S, Tindell A, Topping LM, Strollo R, et al. Immunoglobulin a antibodies to oxidized collagen type II as a potential biomarker for the stratification of spondyloarthritis from rheumatoid arthritis. Scandinavian J Rheumatol (2020) 49(4):281–91. doi: 10.1080/03009742.2020.1713395 [DOI] [PubMed] [Google Scholar]
- 51. Veale D, Yanni G, Rogers S, Barnes L, Bresnihan B, Fitzgerald O. Reduced synovial membrane macrophage numbers, elam-1 expression, and lining layer hyperplasia in psoriatic arthritis as compared with rheumatoid arthritis. Arthritis Rheum (1993) 36(7):893–900. doi: 10.1002/art.1780360705 [DOI] [PubMed] [Google Scholar]
- 52. Szodoray P, Alex P, Chappell-Woodward CM, Madland TM, Knowlton N, Dozmorov I, et al. Circulating cytokines in Norwegian patients with psoriatic arthritis determined by a multiplex cytokine array system. Rheumatology (2007) 46(3):417–25. doi: 10.1093/rheumatology/kel306 [DOI] [PubMed] [Google Scholar]
- 53. Firuzi O, Spadaro A, Spadaro C, Riccieri V, Petrucci R, Marrosu G, et al. Protein oxidation markers in the serum and synovial fluid of psoriatic arthritis patients. J Clin Lab Anal (2008) 22(3):210–5. doi: 10.1002/jcla.20243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hansson C, Eriksson C, Alenius GM. S-calprotectin (S100A8/S100A9): A potential marker of inflammation in patients with psoriatic arthritis. J Immunol Res (2014) 2014:696415. doi: 10.1155/2014/696415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bosè F, Capsoni F, Molteni S, Raeli L, Diani M, Altomare A, et al. Differential expression of interleukin-2 by anti-CD3-stimulated peripheral blood mononuclear cells in patients with psoriatic arthritis and patients with cutaneous psoriasis. Clin Exp Dermatol (2014) 39(3):385–90. doi: 10.1111/ced.12251 [DOI] [PubMed] [Google Scholar]
- 56. Maejima H, Nagashio R, Yanagita K, Hamada Y, Amoh Y, Sato Y, et al. Moesin and stress-induced phosphoprotein-1 are possible sero-diagnostic markers of psoriasis. PloS One (2014) 9(7):e101773. doi: 10.1371/journal.pone.0101773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim DS, Shin D, Lee MS, Kim HJ, Kim DY, Kim SM, et al. Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J Dermatol (2016) 43(3):305–10. doi: 10.1111/1346-8138.13061 [DOI] [PubMed] [Google Scholar]
- 58. Armas-González E, Díaz-Martín A, Domínguez-Luis MJ, Arce-Franco MT, Herrera-García A, Hernández-Hernández MV, et al. Differential antigen-presenting b cell phenotypes from synovial microenvironment of patients with rheumatoid and psoriatic arthritis. J Rheumatol (2015) 42(10):1825–34. doi: 10.3899/jrheum.141577 [DOI] [PubMed] [Google Scholar]
- 59. Amin TE, Elfar NN, Ghaly NR, Hekal MM, Hassan AM, Elsaadany HM. Serum level of receptor activator of nuclear factor kappa-b ligand in patients with psoriasis. Int J Dermatol (2016) 55(5):e227–33. doi: 10.1111/ijd.13159 [DOI] [PubMed] [Google Scholar]
- 60. Gudmann NS, Munk HL, Christensen AF, Ejstrup L, Sørensen GL, Loft AG, et al. Chondrocyte activity is increased in psoriatic arthritis and axial spondyloarthritis. Arthritis Res Ther (2016) 18(1):1–9. doi: 10.1186/s13075-016-1040-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Muntyanu A, Abji F, Liang K, Pollock RA, Chandran V, Gladman DD. Differential gene and protein expression of chemokines and cytokines in synovial fluid of patients with arthritis. Arthritis Res Ther (2016) 18(1):1–10. doi: 10.1186/s13075-016-1196-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Abji F, Pollock RA, Liang K, Chandran V, Gladman DD. Brief report: CXCL10 is a possible biomarker for the development of psoriatic arthritis among patients with psoriasis. Arthritis Rheumatol (2016) 68(12):2911–6. doi: 10.1002/art.39800 [DOI] [PubMed] [Google Scholar]
- 63. Reindl J, Pesek J, Krüger T, Wendler S, Nemitz S, Muckova P, et al. Proteomic biomarkers for psoriasis and psoriasis arthritis. J Proteomics. (2016) 140:55–61. doi: 10.1016/j.jprot.2016.03.040 [DOI] [PubMed] [Google Scholar]
- 64. Alonso A, Julià A, Vinaixa M, Domènech E, Fernández-Nebro A, Cañete JD, et al. Urine metabolome profiling of immune-mediated inflammatory diseases. BMC Med (2016) 14(1):133. doi: 10.1186/s12916-016-0681-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Grossi V, Infantino M, Manfredi M, Meacci F, Bellio E, Bellio V, et al. A proposed serum calprotectin IgG cut-off level for diagnosing inflammatory arthritis. Curr Rheumatol Rev (2017) 13(2):93–97. doi: 10.2174/1573397112666160629085231 [DOI] [PubMed] [Google Scholar]
- 66. Ausavarungnirun R, Intarasupht J, Nakakes A, Rojanametin K. Nail abnormalities, quality of life and serum inflammatory marker in psoriatic arthritis compare to psoriasis without arthritis. J Med Assoc Thai (2017) 100:1021. [Google Scholar]
- 67. Maejima H, Kobayashi M, Yanagita K, Hamada Y, Nagashio R, Sato Y, et al. Valosin-containing protein is a possible sero-diagnostic marker of psoriatic arthritis. Biomed Res (2017) 28(1):442–6. [Google Scholar]
- 68. Farrag DA, Asaad MK, Ghobrial CK. Evaluation of IL-34 in psoriasis and psoriatic arthritis patients: Correlation with disease activity and severity. Egyptian Rheumatologist. (2017) 39(1):25–31. doi: 10.1016/j.ejr.2016.05.008 [DOI] [Google Scholar]
- 69. Sinkeviciute D, Skovlund Groen S, Sun S, Manon-Jensen T, Aspberg A, Önnerfjord P, et al. A novel biomarker of MMP-cleaved prolargin is elevated in patients with psoriatic arthritis. Sci Rep (2020) 10(1):1–10. doi: 10.1038/s41598-020-70327-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Abji F, Lee KA, Pollock RA, Machhar R, Cook RJ, Chandran V, et al. Declining levels of serum chemokine (C-X-C motif) ligand 10 over time are associated with new onset of psoriatic arthritis in patients with psoriasis: a new biomarker? Br J Dermatol (2020) 183(5):920–7. doi: 10.1111/bjd.18940 [DOI] [PubMed] [Google Scholar]
- 71. Esawy MM, Makram WK, Albalat W, Shabana MA. Plasma gelsolin levels in patients with psoriatic arthritis: a possible novel marker. Clin Rheumatol (2020) 39(6):1881–8. doi: 10.1007/s10067-020-04959-y [DOI] [PubMed] [Google Scholar]
- 72. Souto-Carneiro M, Tóth L, Behnisch R, Urbach K, Klika KD, Carvalho RA, et al. Differences in the serum metabolome and lipidome identify potential biomarkers for seronegative rheumatoid arthritis versus psoriatic arthritis. Ann Rheum Dis (2020) 79(4):499–506. doi: 10.1136/annrheumdis-2019-216374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cuervo A, Celis R, Julià A, Usategui A, Faré R, Ramírez J, et al. Synovial immunohistological biomarkers of the classification of undifferentiated arthritis evolving to rheumatoid or psoriatic arthritis. Front Med (Lausanne). (2021) 8:417. doi: 10.3389/fmed.2021.656667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Leijten E, Tao W, Pouw J, van Kempen T, Olde Nordkamp M, Balak D, et al. Broad proteomic screen reveals shared serum proteomic signature in patients with psoriatic arthritis and psoriasis without arthritis. Rheumatology (2021) 60(2):751–61. doi: 10.1093/rheumatology/keaa405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kishikawa T, Arase N, Tsuji S, Maeda Y, Nii T, Hirata J, et al. Large-Scale plasma-metabolome analysis identifies potential biomarkers of psoriasis and its clinical subtypes. J Dermatol Sci (2021) 102(2):78–84. doi: 10.1016/j.jdermsci.2021.03.006 [DOI] [PubMed] [Google Scholar]
- 76. Fuentelsaz-Romero S, Cuervo A, Estrada-Capetillo L, Celis R, García-Campos R, Ramírez J, et al. GM-CSF expression and macrophage polarization in joints of undifferentiated arthritis patients evolving to rheumatoid arthritis or psoriatic arthritis. Front Immunol (2021) 11:3940. doi: 10.3389/fimmu.2020.613975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhu J, Han L, Liu R, Zhang Z, Huang Q, Fang X, et al. Identification of proteins associated with development of psoriatic arthritis in peripheral blood mononuclear cells: a quantitative iTRAQ-based proteomics study. J Transl Med (2021) 19(1):1–9. doi: 10.1186/s12967-021-03006-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Looby N, Roszkowska A, Reyes-Garcés N, Yu M, Bączek T, Kulasingam V, et al. Serum metabolic fingerprinting of psoriasis and psoriatic arthritis patients using solid-phase microextraction–liquid chromatography–high-resolution mass spectrometry. Metabolomics (2021) 17(7):1–12. doi: 10.1007/s11306-021-01805-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Leijten EF, van Kempen TS, Olde Nordkamp MA, Pouw JN, Kleinrensink NJ, Vincken NL, et al. Tissue-resident memory CD8+ T cells from skin differentiate psoriatic arthritis from psoriasis. Arthritis Rheumatol (2021) 73(7):1220–32. doi: 10.1002/art.41652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ek WE, Karlsson T, Höglund J, Rask-Andersen M, Johansson Å. Causal effects of inflammatory protein biomarkers on inflammatory diseases. Sci Adv (2021) 7(50):4359. doi: 10.1126/sciadv.abl4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang N, Yang L, Shang L, Liang Z, Wang Y, Feng M, et al. Altered fecal metabolomics and potential biomarkers of psoriatic arthritis differing from rheumatoid arthritis. Front Immunol (2022) 13. doi: 10.3389/fimmu.2022.812996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Marzaioli V, Canavan M, Floudas A, Flynn K, Mullan R, Veale DJ, et al. CD209/CD14+ dendritic cells characterization in rheumatoid and psoriatic arthritis patients: Activation, synovial infiltration, and therapeutic targeting. Front Immunol (2022) 12. doi: 10.3389/fimmu.2021.722349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mc Ardle A, Kwasnik A, Szentpetery A, Hernandez B, Parnell A, de Jager W, et al. Identification and evaluation of serum protein biomarkers that differentiate psoriatic arthritis from rheumatoid arthritis. Arthritis Rheumatol (2022) 74(1):81–91. doi: 10.1002/art.41899 [DOI] [PubMed] [Google Scholar]
- 84. Helliwell PS, Marchesoni A, Peters M, Platt R, Wright V. Cytidine deaminase activity, c reactive protein, histidine, and erythrocyte sedimentation rate as measures of disease activity in psoriatic arthritis. Ann Rheum Dis (1991) 50(6):362–5. doi: 10.1136/ard.50.6.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. McHugh NJ, Balachrishnan C, Jones SM. Progression of peripheral joint disease in psoriatic arthritis: a 5-yr prospective study. Rheumatology (2003) 42(6):778–83. doi: 10.1093/rheumatology/keg217 [DOI] [PubMed] [Google Scholar]
- 86. Bandinelli F, Denaro V, Prignano F, Collaku L, Ciancio G, Matucci-Cerinic M, et al. Ultrasonographic wrist and hand abnormalities in early psoriatic arthritis patients: correlation with clinical, dermatological, serological and genetic indices. Clin Exp Rheumatol (2015) 33(3):330–5. [PubMed] [Google Scholar]
- 87. Foell D, Kane D, Bresnihan B, Vogl T, Nacken W, Sorg C, et al. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology (2003) 42(11):1383–9. doi: 10.1093/rheumatology/keg385 [DOI] [PubMed] [Google Scholar]
- 88. Skoumal M, Haberhauer G, Fink A, Steiner A, Klingler A, Varga F, et al. Increased serum levels of cartilage oligomeric matrix protein in patients with psoriasis vulgaris: a marker for unknown peripheral joint involvement? Clin Exp Rheumatol (2008) 26(6):1087–90. [PubMed] [Google Scholar]
- 89. Dalbeth N, Pool B, Smith T, Callon KE, Lobo M, Taylor WJ, et al. Circulating mediators of bone remodeling in psoriatic arthritis: Implications for disordered osteoclastogenesis and bone erosion. Arthritis Res Ther (2010) 12(4):1–9. doi: 10.1186/ar3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. van Kuijk AWR, DeGroot J, Koeman RC, Sakkee N, Baeten DL, Gerlag DM, et al. Soluble biomarkers of cartilage and bone metabolism in early proof of concept trials in psoriatic arthritis: effects of adalimumab versus placebo. PloS One (2010) 5(9):1–5. doi: 10.1371/journal.pone.0012556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ramonda R, Modesti V, Ortolan A, Scanu A, Bassi N, Oliviero F, et al. Serological markers in psoriatic arthritis: promising tools. Exp Bio Med (2013) 238(12):1431–6. doi: 10.1177/1535370213506435 [DOI] [PubMed] [Google Scholar]
- 92. Chung Y, Li ZC, Sun XL, Liu YY, Shao M, Gan YZ, et al. Elevated serum dickkopf-1 is a biomarker for bone erosion in patients with psoriatic arthritis. Chin Med J (2021) 134(21):2583–8. doi: 10.1097/CM9.0000000000001612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Alenius GM, Jidell E, Nordmark L, Rantapää Dahlqvist S. Disease manifestations and HLA antigens in psoriatic arthritis in northern Sweden. Clin Rheumatol (2002) 21(5):357–62. doi: 10.1007/s100670200097 [DOI] [PubMed] [Google Scholar]
- 94. Korendowych E, Owen P, Ravindran J, Carmichael C, McHugh N. The clinical and genetic associations of anti-cyclic citrullinated peptide antibodies in psoriatic arthritis. Rheumatology (2005) 44(8):1056–60. doi: 10.1093/rheumatology/keh686 [DOI] [PubMed] [Google Scholar]
- 95. Perez-Alamino R, Garcia-Valladares I, Cuchacovich R, Iglesias-Gamarra A, Espinoza LR. Are anti-CCP antibodies in psoriatic arthritis patients a biomarker of erosive disease? Rheumatol Int (2014) 34(9):1211–6. doi: 10.1007/s00296-014-2956-8 [DOI] [PubMed] [Google Scholar]
- 96. Ibrahim SE, Morshedy NA, Farouk N, Louka AL. Anti-carbamylated protein antibodies in psoriatic arthritis patients: Relation to disease activity, severity and ultrasonographic scores. Egyptian Rheumatologist. (2018) 40(1):17–21. doi: 10.1016/j.ejr.2017.08.002 [DOI] [Google Scholar]
- 97. Elkayam O, Yaron I, Shirazi I, Yaron M, Caspi D. Serum levels of hyaluronic acid in patients with psoriatic arthritis. Clin Rheumatol (2000) 19(6):455–7. doi: 10.1007/s100670070005 [DOI] [PubMed] [Google Scholar]
- 98. Elkayam O, Yaron I, Shirazi I, Yaron M, Caspi D. Serum levels of IL-10, IL-6, IL-1ra, and sIL-2R in patients with psoriatic arthritis. Rheumatol Int (2000) 19(3):101–5. doi: 10.1007/s002960050111 [DOI] [PubMed] [Google Scholar]
- 99. Rooney T, Murphy E, Benito M, Roux-Lombard P, FitzGerald O, Dayer JM, et al. Synovial tissue interleukin-18 expression and the response to treatment in patients with inflammatory arthritis. Ann Rheum Dis (2004) 63(11):1393–8. doi: 10.1136/ard.2003.016428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fink AM, Cauza E, Hassfeld W, Dunky A, Bayer PM, Jurecka W, et al. Vascular endothelial growth factor in patients with psoriatic arthritis. Clin Exp Rheumatol (2007) 25(2):305–8. [PubMed] [Google Scholar]
- 101. Madland TM, Larsen A, Brun JG. S100 proteins calprotectin and S100A12 are related to radiographic changes rather than disease activity in psoriatic arthritis with low disease activity. J Rheumatol (2007) 34(10):2089–92. [PubMed] [Google Scholar]
- 102. Alenius GM, Eriksson C, Rantapää Dahlqvist S. Interleukin-6 and soluble interleukin-2 receptor alpha-markers of inflammation in patients with psoriatic arthritis? Clin Exp Rheumatol (2009) 27(1):120–3. [PubMed] [Google Scholar]
- 103. Pongratz G, Straub RH, Schölmerich J, Fleck M, Härle P. Serum BAFF strongly correlates with PsA activity in male patients only–is there a role for sex hormones? Clin Exp Rheumatol (2010) 28(6):813–9. [PubMed] [Google Scholar]
- 104. Celis R, Planell N, Fernández-Sueiro JL, Sanmartí R, Ramírez J, González-Álvaro I, et al. Synovial cytokine expression in psoriatic arthritis and associations with lymphoid neogenesis and clinical features. Arthritis Res Ther (2012) 14(2):1–9. doi: 10.1186/ar3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Przepiera-Będzak H, Fischer K, Brzosko M, Reumatologii K, Wewnętrznych C. Serum levels of angiogenic cytokines in psoriatic arthritis and SAPHO syndrome. Polish Arc Int Med (2013) 123:Pol Arch Med Wewn. doi: 10.20452/pamw.1772 [DOI] [PubMed] [Google Scholar]
- 106. Jensen P, Wiell C, Milting K, Poggenborg RP, Østergaard M, Johansen JS, et al. Plasma YKL-40: a potential biomarker for psoriatic arthritis? J Eur Acad Dermatol Venereol (2013) 27(7):815–9. doi: 10.1111/j.1468-3083.2012.04570.x [DOI] [PubMed] [Google Scholar]
- 107. Eissa A, Cretu D, Soosaipillai A, Thavaneswaran A, Pellett F, Diamandis A, et al. Serum kallikrein-8 correlates with skin activity, but not psoriatic arthritis, in patients with psoriatic disease. Clin Chem Lab Med (2013) 51(2):317–25. doi: 10.1515/cclm-2012-0251/html [DOI] [PubMed] [Google Scholar]
- 108. Kayikçi Ö, Pamuk ÖN, Pamuk GE, Arican Ö, Dönmez S. T Helper 17 cytokine profile in psoriatic arthritis and their relations with clinical findings. Yeni Tıp Dergisi (2014) 31(3):163–7. [Google Scholar]
- 109. Ahmed SF, Attia EAS, Saad AA, Sharara M, Fawzy H, el Nahrery EMA. Serum YKL-40 in psoriasis with and without arthritis; correlation with disease activity and high-resolution power Doppler ultrasonographic joint findings. J Eur Acad Dermatol Venereology (2015) 29(4):682–8. doi: 10.1111/jdv.12653 [DOI] [PubMed] [Google Scholar]
- 110. Husakova M, Lippert J, Stolfa J, Sedova L, Arenberger P, Lacinova Z, et al. Elevated serum prolactin levels as a marker of inflammatory arthritis in psoriasis vulgaris. BioMed Pap Med Fac Univ Palacky Olomouc Czech Repub (2015) 159(4):562–8. doi: 10.5507/bp.2015.033 [DOI] [PubMed] [Google Scholar]
- 111. Matt P, Lindqvist U, Kleinau S. Up-regulation of CD64-expressing monocytes with impaired FcγR function reflects disease activity in polyarticular psoriatic arthritis. Scandinavian J Rheumatol (2015) 44(6):464–73. doi: 10.3109/03009742.2015.1020864 [DOI] [PubMed] [Google Scholar]
- 112. Peled M, Strazza M, Azoulay-Alfaguter I, Mor A. Analysis of programmed death-1 in patients with psoriatic arthritis. Inflammation (2015) 38(4):1573–9. doi: 10.1007/s10753-015-0132-2 [DOI] [PubMed] [Google Scholar]
- 113. Dikbas O, Tosun M, Bes C, Tonuk SB, Aksehirli OY, Soy M. Serum levels of visfatin, resistin and adiponectin in patients with psoriatic arthritis and associations with disease severity. Int J Rheum Dis (2016) 19(7):672–7. doi: 10.1111/1756-185X.12444 [DOI] [PubMed] [Google Scholar]
- 114. Munk HL, Gudmann NS, Christensen AF, Ejstrup L, Sorensen GL, Loft AG, et al. Cartilage collagen type II seromarker patterns in axial spondyloarthritis and psoriatic arthritis: associations with disease activity, smoking and HLA-B27. Rheumatol Int (2016) 36(4):541–9. doi: 10.1007/s00296-015-3397-8 [DOI] [PubMed] [Google Scholar]
- 115. Inciarte-Mundo J, Ramirez J, Hernández MV, Ruiz-Esquide V, Cuervo A, Cabrera-Villalba SR, et al. Calprotectin and TNF trough serum levels identify power Doppler ultrasound synovitis in rheumatoid arthritis and psoriatic arthritis patients in remission or with low disease activity. Arthritis Res Ther (2016) 18(1):1–10. doi: 10.1186/s13075-016-1032-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Przepiera-Będzak H, Fischer K, Brzosko M. Serum interleukin-18, fetuin-a, soluble intercellular adhesion molecule-1, and endothelin-1 in ankylosing spondylitis, psoriatic arthritis, and SAPHO syndrome. Int J Mol Sci (2016) 17(8):1255. doi: 10.3390/ijms17081255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kiliç S, Reşorlu H, Işik S, Oymak S, Akbal A, Hiz MM, et al. Association between mean platelet volume and disease severity in patients with psoriasis and psoriatic arthritis. Adv Dermatol Allergol (2017) 34(2):126–30. doi: 10.5114/ada.2017.67076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Inciarte-Mundo J, Ramirez J, Hernández MV, Ruiz-Esquide V, Cuervo A, Cabrera-Villalba SR, et al. Calprotectin strongly and independently predicts relapse in rheumatoid arthritis and polyarticular psoriatic arthritis patients treated with tumor necrosis factor inhibitors: A 1-year prospective cohort study. Arthritis Res Ther (2018) 20(1):1–11. doi: 10.1186/s13075-018-1764-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wade SM, Canavan M, McGarry T, Low C, Wade SC, Mullan RH, et al. Association of synovial tissue polyfunctional T-cells with DAPSA in psoriatic arthritis. Ann Rheum Dis (2019) 78(3):350–4. doi: 10.1136/annrheumdis-2018-214138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Coras R, Kavanaugh A, Boyd T, Huynh Q, Pedersen B, Armando AM, et al. Pro- and anti-inflammatory eicosanoids in psoriatic arthritis. Metabolomics (2019) 15(4):1–9. doi: 10.1007/s11306-019-1527-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Colak S, Omma A, Sandikci SC, Yucel C, Omma T, Turhan T. Vaspin, neutrophil gelatinase-associated lipocalin and apolipoprotein levels in patients with psoriatic arthritis. Bratisl Lek Listy (2019) 120(1):65–9. doi: 10.4149/BLL_2019_010 [DOI] [PubMed] [Google Scholar]
- 122. Sakellariou G, Lombardi G, Vitolo B, Gomarasca M, Faraldi M, Caporali R, et al. Serum calprotectin as a marker of ultrasound-detected synovitis in early psoriatic and rheumatoid arthritis: results from a cross-sectional retrospective study. Clin Exp Rheumatol (2019) 37(3):429–36. [PubMed] [Google Scholar]
- 123. Coras R, Kavanaugh A, Boyd T, Huynh D, Lagerborg KA, Xu YJ, et al. Choline metabolite, trimethylamine n-oxide (TMAO), is associated with inflammation in psoriatic arthritis. Clin Exp Rheumatol (2019) 37(3):481–4. [PMC free article] [PubMed] [Google Scholar]
- 124. Ozisler C, Sandikci SC. Evaluation of red blood cell distribution width in patients with psoriatic arthritis. Egyptian Rheumatologist. (2020) 42(4):309–12. doi: 10.1016/j.ejr.2019.06.001 [DOI] [Google Scholar]
- 125. Arias de la Rosa I, Font P, Escudero-Contreras A, López-Montilla MD, Pérez-Sánchez C, Ábalos-Aguilera MC, et al. Complement component 3 as biomarker of disease activity and cardiometabolic risk factor in rheumatoid arthritis and spondyloarthritis. Ther Adv Chronic Dis (2020) 11:2040622320965067. doi: 10.1177/2040622320965067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jarlborg M, Courvoisier DS, Lamacchia C, Martinez Prat L, Mahler M, Bentow C, et al. Serum calprotectin: A promising biomarker in rheumatoid arthritis and axial spondyloarthritis. Arthritis Res Ther (2020) 22(1):1–11. doi: 10.1186/s13075-020-02190-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Medvedeva I v., Stokes ME, Eisinger D, LaBrie ST, Ai J, Trotter MWB, et al. Large-Scale analyses of disease biomarkers and apremilast pharmacodynamic effects. Sci Rep (2020) 10(1):1–11. doi: 10.1038/s41598-020-57542-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wcisło-Dziadecka D, Grabarek B, Kruszniewska-Rajs C, Swinarew A, Jasik K, Rozwadowska B, et al. Analysis of molecular and clinical parameters of 4-year adalimumab therapy in psoriatic patients. Adv Dermatol Allergol (2020) 37(5):736–45. doi: 10.5114/ada.2020.100484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Boyd TA, Eastman PS, Huynh DH, Qureshi F, Sasso EH, Bolce R, et al. Correlation of serum protein biomarkers with disease activity in psoriatic arthritis. Expert Rev Clin Immunol (2020) 16(3):335–41. doi: 10.1080/1744666X.2020.1729129 [DOI] [PubMed] [Google Scholar]
- 130. Coras R, Kavanaugh A, Kluzniak A, Holt D, Weilgosz A, Aaron A, et al. Differences in oxylipin profile in psoriasis versus psoriatic arthritis. Arthritis Res Ther (2021) 23(1):1–13. doi: 10.1186/s13075-021-02575-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute; (2021). Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 132. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol (2005) 5(1):1–10. doi: 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cohen J. Statistical power analysis for the behavioral sciences. In: Statistical power analysis for the behavioral sciences, 2nd ed. New York: Routledge; (1988). doi: 10.4324/9780203771587/statistical-power-analysis-behavioral-sciences-jacob-cohen [DOI] [Google Scholar]
- 134. Chandran V, Cook R, Pollock RA, Pellet F, Hua S, Gladman D. Soluble biomarkers predict response to anti-tumour necrosis factor (TNF) therapy in psoriatic arthritis (PsA). J Rheumatol (2010) 37(6):1282–3. doi: 10.1093/rheumatology/keq105 [DOI] [Google Scholar]
- 135. Loures MAR, Alves HV, de Moraes AG, Santos T da S, Lara FF, Neves JSF, et al. Association of TNF, IL12, and IL23 gene polymorphisms and psoriatic arthritis: meta-analysis. Expert Rev Clin Immunol (2019) 15(3):303–13. doi: 10.1080/1744666X.2019.1564039 [DOI] [PubMed] [Google Scholar]
- 136. Villalpando-Vargas FV, Rivera-Valdés JJ, Alvarado-Navarro A, Huerta-Olvera SG, Macías-Barragán J, Martínez-López E, et al. Association between IL-17A, IL-17F and IL-17RA gene polymorphisms and susceptibility to psoriasis and psoriatic arthritis: a meta-analysis. Inflammation Res (2021) 70(10–12):1201–10. doi: 10.1007/s00011-021-01514-6 [DOI] [PubMed] [Google Scholar]
- 137. Queiro R, Morante I, Cabezas I, Acasuso B. HLA-B27 and psoriatic disease: a modern view of an old relationship. Rheumatology (2016) 55(2):221–9. doi: 10.1093/rheumatology/kev296 [DOI] [PubMed] [Google Scholar]
- 138. Massy E, Pedini P, Pollet E, Martin M, Roudier J, Picard C, et al. Association study between HLA-a, -b, -c, -DRB1 alleles and psoriatic arthritis in southern France. Hum Immunol (2022) 83(6):515–20. doi: 10.1016/j.humimm.2022.04.001 [DOI] [PubMed] [Google Scholar]
- 139. Generali E, Scirè CA, Favalli EG, Selmi C. Biomarkers in psoriatic arthritis: a systematic literature review. Exp Rev Clin Immunol (2016) 12(6):651–60. doi: 10.1586/1744666X.2016.1147954 [DOI] [PubMed] [Google Scholar]
- 140. Shao LN, Wang N, Zhou SH, Wang Z. Associations between human leukocyte antigen c locus polymorphism and psoriatic arthritis in populations of European and middle Eastern descent: a meta-analysis. Ann Saudi Med (2020) 40(4):338–46. doi: 10.5144/0256-4947.2020.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Stuart PE, Nair RP, Tsoi LC, Tejasvi T, Das S, Kang HM, et al. Genome-wide association analysis of psoriatic arthritis and cutaneous psoriasis reveals differences in their genetic architecture. Am J Hum Genet (2015) 97(6):816–36. doi: 10.1016/j.ajhg.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chen J, Yuan F, Fan X, Wang Y. Psoriatic arthritis: A systematic review of non-HLA genetic studies and important signaling pathways. Int J Rheum Dis (2020) 23(10):1288–96. doi: 10.1111/1756-185X.13879 [DOI] [PubMed] [Google Scholar]
- 143. Hagiwara S, Tsuboi H, Terasaki T, Terasaki M, Toko H, Shimizu M, et al. Association of anti-cyclic citrullinated peptide antibody with clinical features in patients with psoriatic arthritis. Mod Rheumatol (2020) 30(2):365–72. doi: 10.1080/14397595.2019.1586085 [DOI] [PubMed] [Google Scholar]
- 144. Elliott A, McGonagle D, Rooney M. Integrating imaging and biomarker assessment to better define psoriatic arthritis and predict response to biologic therapy. Rheumatology (2021) 60(Supplement_6):vi38–52. doi: 10.1093/rheumatology/keab504 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.