Abstract
Polish raw‐milk cheeses produced in short supply chains may pose a threat to consumer safety due to pathogen presence. Listeria monocytogenes is a bacterium of great importance for the food safety of refrigerated RTE foods due to its ability to grow at refrigeration temperatures. During the EU‐FORA fellowship, a stochastic risk assessment was designed and executed to estimate the risk for consumers from L. monocytogenes in these products. The aim was to develop a probabilistic QMRA model that would incorporate the variability and uncertainty of the model's inputs such as prevalence, initial concentration levels, product intrinsic factors, domestic storage temperature and consumer behaviour. The project involved data collection and analysis, growth model selection, mathematical modelling and Monte Carlo analysis in R programming language. Microbiological and physicochemical testing were carried out throughout the year on two types of cheeses in combination with a domestic refrigerator temperature survey and accompanying consumption questionnaire. Collected data were fitted to probability distributions using R. The appropriate growth model for the pathogen was selected based on an inoculation study performed on one of the raw‐milk cheeses and the chosen mathematical model was written into the R script developed for the QMRA. The dose–response model used the ingested dose calculated from the modelled concentration of L. monocytogenes at the time of consumption and the single serving size from the questionnaire to estimate the probability of illness. The final risk was expressed as probability of listeriosis for Polish consumers per serving of raw‐milk cheese.
Keywords: Listeria monocytogenes, QMRA, raw‐milk cheese, risk assessment
1. Introduction
Polish cheese consumption is dominated by Polish white‐fresh cheeses, which account for 54% of total sales volume in the country (WORLD AND POLAND PER CAPITA CHEESE CONSUMPTION, n.d. ). The most popular among these are ‘twarog’, an acid‐set cheese, and ‘ser podpuszczkowy’, a rennet cheese, made from naturally acidified milk. These types of cheeses from unpasteurized cow's milk are commonly produced by small scale farms and sold at a local level by producers directly to customers (Lucey, n.d.). Consumer Interest in small‐scale locally produced cheeses, such as the aforementioned, has for the past years been on the rise in Poland (Soroka and Wojciechowska‐Solis, 2019 ).The biological risk associated with the consumption of such products has not been thoroughly assessed in Poland. The small scale of production by farmers and the use of unpasteurised milk raises concerns about the safety of these products. Products produced locally, often using traditional methods,14 are rarely controlled in terms of safety, and the farmers/producers themselves often lack extensive knowledge related to good hygiene and production practices.
Food of animal origin can be a very good substrate for the growth of microorganisms. Specifically, for fresh cheeses, the high nutrient content, high moisture, low‐temperature heat treatment and lack of additives, conduce to the growth of various microorganisms, including possibly dangerous pathogens, such as Listeria monocytogenes, that could pose a threat to consumers of these products. The small dose required for illness and the severity of listeriosis in humans warrant high vigilance for all RTE foods including cheeses (The European Union One Health 2019 Zoonoses Report, 2021; The European Union One Health 2020 Zoonoses Report, 2021).
Commission Regulation (EC) No 2073/2005 on microbiological criteria, distinguishes between RTE products able to support L. monocytogenes growth and those that inhibit growth. The physicochemical characteristics of these two products, pH and water activity (aw), in this case place them in category 1.2, for which two criteria apply. These are absence in 25 g at the end of production and < 100 CFU/g during its shelf life. These safety criteria are required to achieve the appropriate level of protection (ALOP) for EU consumers.
The Rapid Alert System for Food and Feed (RASFF) is a European system for reporting food safety issues within the Union, and is a key feature of EU food safety. The RASFF report for 2020 included 3 alerts regarding raw‐milk cheeses out of a total of 25. The Polish chief sanitary inspectorate (Główny Inspektorat Sanitarny), has enforced 6 recalls of cheeses due to detection of Listeria from 2019 to 2021 (Główny Inspektorat Sanitarny, 2021 ). These included cheeses made from raw‐milk, and traditional products. Pyz‐Łukasik et al studied the occurrence of L. monocytogenes in artisanal Polish cheeses and found the prevalence to be much higher than the European average reported by EFSA. Their findings suggest a prevalence of 6.2% for artisanal cheeses, thus warranting the assessment of listeriosis risk (Pyz‐Łukasik et al., 2021).
To ascertain the listeriosis risk associated with the consumption of such products, a quantitative microbiological risk assessment (QMRA) was conducted. QMRA is a mathematical modelling method used to estimate the risk from a hazard‐food combination for a specific population. The methodology involves the steps of hazard identification, hazard characterisation, exposure assessment and risk characterisation. The process aims to quantitatively describe the fate of the studied pathogen in the food from production to consumption and associate the consumers’ subsequent exposure with the risk of an adverse health outcome. Considering the variability and uncertainty associated with all the model inputs (food intrinsic factors, contamination levels, consumption patterns, etc.), the stochastic approach was selected to better portray real‐life scenarios. In the stochastic approach, the point estimate values of QMRA inputs are substituted by probability distributions describing variability. Subsequently the final outcome of the risk assessment (RA) is a probability distribution of risk.
2. Description of work programme
2.1. Aims
The working programme aimed to familiarise the fellow with all aspects of an QMRA by providing hand‐on experience in performing a RA of a traditional RTE food of animal origin. The fellow carried out activities spanning from relevant data collection to mathematical modelling in R. By applying a stochastic approach, emphasis was placed on describing the variability and uncertainty of most of the model inputs. Throughout the fellowship's duration the fellow undertook learning to code in R, an open‐source programming language, popular in the RA community. Thus, a custom R script was written to fit probability distributions to data, estimate kinetic parameters, model bacterial growth for L. monocytogenes and lactic acid bacteria (LAB), perform Monte Carlo analysis and produce a final risk estimate for listeriosis arising from the consumption of raw‐milk cheeses produced in short supply chains in Poland.
2.2. Activities/Methods
2.2.1. Prevalence estimation – Cyclical microbiological testing
Microbiological analyses were performed on 46 raw‐milk cheese samples (26 twarog and 20 ser podpuszczkowy) from a farmer/producer based in the Silesian voivodeship. Testing for L, monocytogenes was carried out according to EN ISO 11290‐1:2017 and EN ISO 11290‐2:2017 aiming to detect the presence, as concentrations were expected to be low. In cases of detection, L. monocytogenes was biochemically verified (LISTERIA‐ID Microgen Bioproducts, Camberley Surrey, UK). L. monocytogenes was detected in 3.85% of acid‐set cheeses and 10% of rennet cheeses, for a total product prevalence of 6.52%. In their study of Polish artisanal cheeses, Pyz‐Lukasik et al. estimated a mean prevalence of 6.2% for Listeria monocytogenes.
An important parameter affecting the fate of Listeria in dairy products is their indigenous lactic acid microflora which is characteristic of these cheeses, as they are produced without the addition of a starter culture. LAB act by acidifying the food matrix via lactic acid production, competing for resources and producing antimicrobial peptides such as bacteriocins. Listeria growth suppression due to the action of LAB is referred to in literature as the ‘Jameson Effect’ (Mellefont et al., 2008; Sip et al., 2012de Niederhäusern et al., 2020). Το include this important parameter in the final QMRA model, mesophilic LAB were enumerated following ISO 15214:2002.
Additionally, cyclical microbiological ISO standard‐based testing was carried out on samples for presence of Salmonella spp., Campylobacter spp., Clostridium perfringens, coagulase‐positive Staphylococcus aureus, Escherichia coli O157:H7, E. coli, total number of microorganisms, coliforms, Enterobacteriaceae, moulds and yeasts.
2.2.2. Product intrinsic factors
The ability of L. monocytogenes to grow in food and the rate at which it can proliferate is dependent on temperature, water activity, pH and organic acids such as lactic acid (Mellefont et al., 2008; Sip et al., 2012de Niederhäusern et al., 2020). The product intrinsic factors influencing bacterial growth were measured for every sample and used to better describe the batch‐to‐batch variability in product characteristics. Water activity was measured with an Aqualab 4TE metre (METER Group, Pullman Washington, USA) with ± 0.003 accuracy, and pH was measured with an accuracy of ± 0.01 using a FiveEasy Benchtop pH‐metre (Mettler Toledo, Columbus Ohio, USA). Additionally, cheese samples (n = 6) were sent to the institute's chemical department in Warsaw for lactic acid quantification by HPLC.
2.2.3. Domestic refrigerator temperature survey
Domestic refrigerated storage is a crucial part of the cold chain and proper storing temperatures contribute to food safety. Refrigerators can vary considerably and temperature fluctuates constantly during the cooling cycle. This variability in storage temperatures is of paramount importance in a stochastic quantitative microbial risk assessment. Since artisanal and traditional farmer products are mainly sold directly to consumers, the majority of storage time is spent in households. Due to this temperature variation, contaminated products may end up in an environment that could allow for additional growth of the pathogen, thus increasing the final dose consumed. The fact that L. monocytogenes is one of the few food‐borne pathogens that can adapt and grow slowly under refrigeration temperatures emphasised the need for domestic storage temperature data (Taoukis et al., n.d.; Tasara and Stephan, 2006). As there was no data regarding storage temperatures in Poland in the literature, a refrigerator temperature survey was conducted in order to incorporate this source of variability into the exposure assessment. The survey included 78 inhabitants of Lodz, Poland, and measured the temperature of the middle shelf with a data logger (LogTag model TRIX‐8) at 5‐min intervals for 24 h. The results of this survey are under consideration for publication for use in future risk assessments.
2.2.4. Consumer questionnaire
Alongside the temperature survey, a consumption questionnaire was provided to participants. Questions were related to personal characteristics of the consumers, cheese storage habits, consumption patterns and refrigerator use. An English version of the questionnaire is provided in the Appendix A. The received questionnaires (n = 56) were used to estimate the variability in portion size (single serving consumed in one sitting), and time of consumption for consumers of these traditional products. The information collected was incorporated into the final model.
2.2.5. Inoculation study – model selection
To select an appropriate predictive microbiology model for L. monocytogenes in the fresh raw‐milk cheeses, a 10‐day inoculation study was performed on ser podpuszczkowy. A L. monocytogenes inoculum strain mix was prepared from two reference strains for food (ATCC 19111, WDCM 1/2a) and one strain isolated from the product. The working culture strains were prepared from reference stock following the procedures of EN ISO:11133:2014. Individual cheese samples in replicates were inoculated to achieve an initial concentration level of 4.04 logCFU/g. The aerobically packed, inoculated samples were stored at temperatures of 5°C and 15°C for 10 days. The sample's physicochemical properties were measured (pH, aw, lactic acid) on the first and last day. Enumeration was performed for L. monocytogenes and LAB on each sampling day (days 0, 2, 4, 7, 8, 10) by plating out the appropriate dilution on OCLA and MRS media (Oxoid, Basingstoke Hampshire, UK) respectively (Figure 1).
Figure 1.
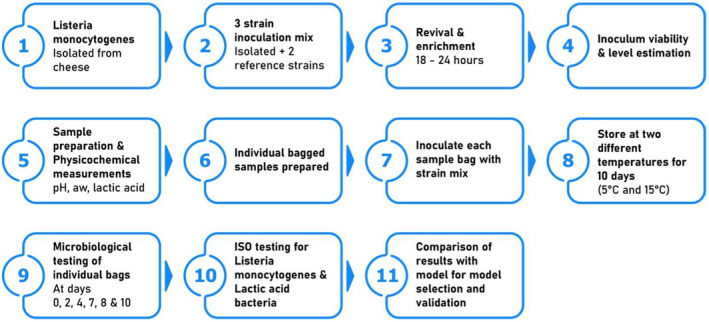
Inoculation study plan of Listeria monocytogenes on ser podpuszczkowy
Enumeration results were graphically compared for model selection, taking into account plate count uncertainty of 0.3–0.6 logCFU (Jarvis et al., 2007). The Food Spoilage and Safety Predictor (FSSP), developed by DTU, was selected as it included LAB and lactic acid concentration as an input in its L. monocytogenes cottage cheese model (‘Growth of Listeria monocytogenes in cottage cheese in combination with LAB’) (Østergaard et al., 2014) (Figure 2).
Figure 2.
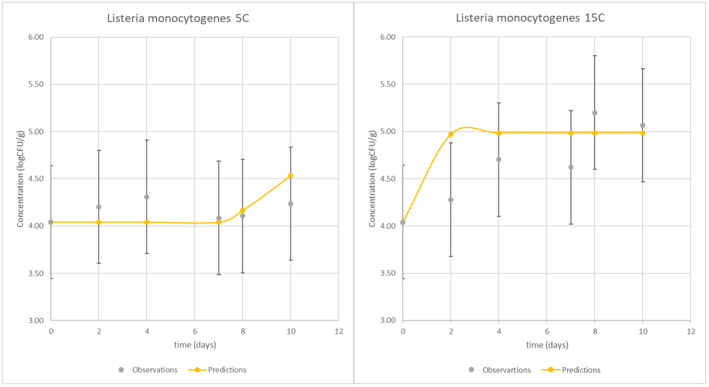
Graphical comparison of L. monocytogenes FSSP cottage cheese model prediction (line) and average observed counts (points) from the inoculation study at 5°C and 15°C
The relative error % (RE) for each set of predictions‐observations was calculated according to equation (1). At 5°C, all predictions were within the 10% RE zone while at 15°C 50% of the predictions were within the 10% RE zone, with one outside of the 20% RE zone.
| (1) |
2.2.6. QMRA in R
R is an open‐source programming language used for statistical analysis and graphics. The language has become a popular choice for risk assessors due to its flexibility and transparency, an important aspect of risk assessment. During the fellowship, the fellow learned to code in R and developed a custom R script for use in stochastic quantitative risk assessment of cheeses.
Probability distribution fitting scenarios of the collected data were statistically tested for goodness of fit. The distribution selection was made based on the chi‐square test, the Kolmogorov–Smirnov test, the Cramer–Von Mises test, the Anderson–Darling Test, the Akaike information criterion and the Bayesian Information Criterion. Based on the summary of these statistics, appropriate distributions were selected to describe the model inputs where possible. In cases where the data was insufficient or fitting based on different goodness of fit tests was not conclusive, variables were assigned distributions according to scientific literature. In the case of L. monocytogenes, initial concentration N0 was assumed to follow a beta‐general distribution as described in the EFSA opinion on L. monocytogenes contamination of RTE foods (Ricci et al., 2018). The variability in aw and pH values was described by applying the beta‐pert distribution based on the minimum, maximum and most likely values. The cumulative distribution function was used for the time of consumption and serving size to describe consumer behaviour. The mean lactic acid concentration was used to calculate the undissociated lactic acid (uLA) in mM, based on the product pH value according to equation (2).
| (2) |
For the prevalence of L. monocytogenes in the tested products a beta distribution was chosen to incorporate the uncertainty resulting from the limited sample size (Figure 3).
Figure 3.
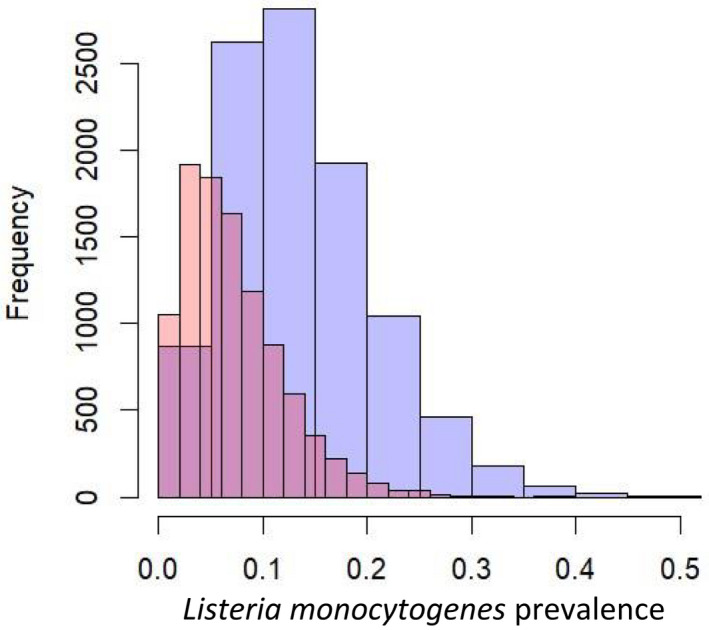
Estimated prevalence distribution of Listeria monocytogenes in raw‐milk twarog (pink) and ser podpuszczkowy (purple)
To predict the fate of Listeria in the contaminated products, the growth model of Østergaard et al from FSSP was coded into the R script (Østergaard et al., 2014). The FSSP model follows the Jameson effect approach, illustrating the inhibiting influence of mesophilic LAB on the growth of L. monocytogenes. This model also includes the term ξ for quantifying the effects of interactions between temperature, pH and organic acids on the growth/no growth limit (le Marc et al., n.d.). The model estimated the LAG time, maximum growth rate μmax, the growth rate of Listeria in the presence of LAB μlm and the final concentration N for each simulated scenario. A total of 10,000 simulations were run for the complete model, sampling from the inputted distributions for the Monte Carlo analysis, calculating the final concentration of L. monocytogenes at the time of consumption. These results were multiplied with the distribution of the single serving size resulting from the cumulative distribution, to estimate the dose ingested by consumers (lamda) (Figure 4, Table 1).
Figure 4.
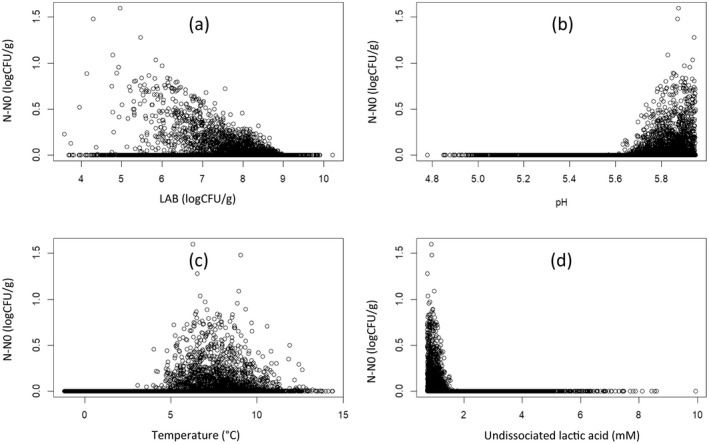
Ser podpuszczkowy L. monocytogenes growth in relation to LAB concentration (a), pH (b), Temperature (c) and undissociated lactic acid (d) for each of the 10,000 Monte Carlo simulations
Table 1.
Overview of the R stochastic model parameters for both products
| Model parameters | Description | Probability Distribution/Value | Units | Source | ||
|---|---|---|---|---|---|---|
| Twarog | Ser podpuszczkowy | |||||
| N0 | Initial concentration of Listeria monocytogenes | rbetagen(iterations, shape1 = 0.194, shape2 = 3.177, min = −1.69, max = 7) | logCFU/g |
EFSA Journal 2018;16(1):5134, 173 pp. Gombas et al. (2003) |
||
| LAB | Initial concentration of lactic acid bacteria | rweibull(iterations, shape = 7.659300, scale = 7.859085) | rweibull(iterations, shape = 10.066707, scale = 7.959734) | logCFU/g | Microbiological analysis | |
| pH | Product pH | rpert(iterations, min =3.98, max = 4.66, mode = 4.32) | rpert(iterations, min = 4.67, max = 5.95, mode = 5.84) | – | Physicochemical testing | |
| aw | Product water activity | rpert(iterations, min = 0.9550, max = 0.9985, mode = 0.9980) | rpert(iterations, min = 0.9696, max = 0.9951, mode = 0.9832) | – | Physicochemical testing | |
| LAC | Lactic acid concentration | Mean = 10,340 | Mean = 8,395 | ppm | Physicochemical testing | |
| Temp | Domestic refrigerator Temperature | Truncated normal distribution(TBP) | °C | Temperature survey | ||
| times | Time of consumption during product's shelf life | recdf(tofcon (a) , iterations) | Days | Consumption questionnaire | ||
| SSS | Single Serving Size | recdf(SS (b) , iterations) | g | Consumption questionnaire | ||
| lamda | Dose received | =10N × SSS | CFU | FAO/WHO (2004) | ||
| iterations | Number of iterations for the Monte Carlo analysis | 10,000 | – | – | ||
| P_Contam | Prevalence of Listeria monocytogenes in tested products | rbeta(iterations, s + 1 = 2, n‐s + 1 = 26) | rbeta(iterations, s + 1 = 3, n‐s + 1 = 19) | – | Microbiological testing | |
| P_ill | Probability of illness | = 1 – e(–r(c)×lamda) | – | FAO/WHO (2004) | ||
| Risk | Listeriosis risk for consumers of Polish raw‐milk cheeses produced in short supply chains |
|
– | – | ||
TBP: to be published.
tofcon:Time of consumption Data from Consumption questionnaire.
SS: Single Serving Data from Consumption questionnaire.
r =
The exposure assessment results were then inserted into the exponential dose response model of FAO/WHO (2004) for the hazard characterisation. Two scenarios were examined for listeriosis infection resulting from the consumption of fresh raw‐milk cheeses, one for the healthy general population and one for susceptible consumers with the r parameter values being and , respectively. The resulting probability of illness was a probability distribution which was used in the risk characterisation. The final risk to consumers of these products was calculated by multiplying the probability of illness by the prevalence of Listeria in each product.
The Monte Carlo analysis allowed for estimating the risk of foodborne illness per serving with the simulation model, drawing the values of input variables such as prevalence, initial L. monocytogenes concentration, initial LAB concentration, storage temperature, pH, aw, time of consumption and individual serving size from the described probability distributions (Figure 5).
Figure 5.
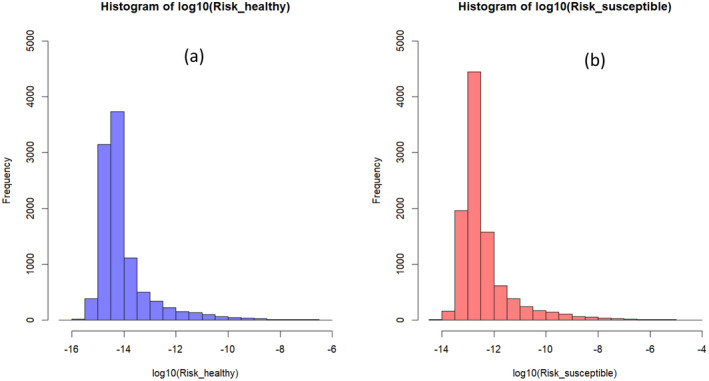
Probability distribution of Log10(Risk) of listeriosis associated with the consumption of one serving of raw‐milk ser podpuszczkowy cheese by (a) typical healthy consumers and (b) susceptible high‐risk consumers
3. Conclusions
The main aim of the project was the development and application of a stochastic quantitative microbial risk assessment model for estimating the health risk for listeriosis arising from the consumption of RTE animal origin products produced in small supply chains in Poland. The working programme enabled the fellow to gain expertise in the many components of a QMRA by applying the methodology on the specific food‐hazard combination of raw‐milk cheese and L. monocytogenes. In the course of the fellowship, the fellow became familiarised with appropriate data selection from scientific literature, collection of data to fill in identified case‐specific data gaps, designing a risk assessment pathway, mathematical modelling of bacterial growth and developing a complete QMRA model for risk estimation, taking into account input and output variability and uncertainty. A particularly valuable facet of this process has been the introduction to R language programming, which has allowed for the model to be conceived and designed probabilistically with the use of Monte Carlo analysis.
Based on 10,000 simulations of the developed model, Listeria did now grow in twarog but displayed limited growth in 1.17% of the ser podpuszczkowy simulations. The mean log10(Risk) per serving for healthy consumers of local raw‐milk twarog and ser podpuszczkowy was estimated −14.40 (min = −16.80, median = −14.66, max = −6.73, sd = 1.05) and − 14.05 (min = −16.08, median = −14.35, max‐6.11, sd = 1.02), respectively. In the case of susceptible consumers, the mean log10(Risk) was increased, at −12.75 (min = −15.84, median = −13.01, max = −4.77, sd = 1.04) for twarog and − 12.40 (min = −14.49, median = −12.70, max = −4.22, sd = 1.03) for ser podpuszczkowy. These estimates are due to the short shelf life, low pH values and high lactic acid concentration due to LAB the products. Thus, the risk of Listeriosis arising from the consumption of these products can be considered as very low, although translating the risk into qualitative terms should be done with caution when communicating with risk managers as there is no consensus on translating probabilities (Scientific Opinion on Risk Assessment Terminology, 2012).
The fellowship programme has been an invaluable opportunity for the fellow to obtain new scientific knowledge and skills, and join the thriving and growing network of the EU‐FORA fellows and alumni, promoting the dissemination of risk assessment expertise in Europe.
4. Additional activities during the EU‐FORA fellowship
The hosting site arranged a visit to the on‐farm facilities of the producer in the Silesian voivodeship participating in the project. During the visit, the facilities were inspected and samples from the production environment were taken and analysed for pathogen and spoilage microorganisms. Throughout the year the fellow was also given the opportunity to attend in person and virtually various scientific conferences and webinars to expand his knowledge in the fields of food safety and risk analysis.
Attended virtually the Recent Advances in Food Analysis (RAFA) 2021 conference.
Presented in person at the IAFP 2022 scientific conference in Munich on the topic of quantitative risk assessment of food‐borne viruses (QVRA).
Attended virtually the 4th BVL/MRI Course on Food Safety, Food Authenticity and Risk Management
Followed a presentation by researchers from the Institute Of Technology Of Agricultural Products ITAP of Greece on photometric methods for microbial spoilage assessment.
Attended virtually the EFSA ONE HEALTH conference 2022.
Took part in a 10‐h intensive QMRA in R workshop, PredMicro2022.
Participated in the setting up of a QDA sensory profiling of Polish cheeses.
Took Polish language classes at a Polish Language School for Foreign students.
Abbreviations
- ALOP
Appropriate Level of Protection
- CFU
colony forming units
- DTU
Danmarks Tekniske Universitet
- FAO
Food and Agriculture Organization
- FSSP
Food Safety and Spoilage Predictor
- HPLC
high‐performance liquid chromatography
- LAB
lactic acid bacteria
- MRS
de Man Rogosa and Sharpe agar
- OCLA
Oxoid Chromogenic Listeria Agar
- QMRA
quantitative microbiological risk assessment
- RA
risk assessment
- RASFF
Rapid Alert System for Food and Feed
- RTE
ready to eat
- uLA
undissociated lactic acid
- WHO
World Health Organization
Appendix A – Consumption Questionnaire


Suggested citation: Sefanou C‐R, Bartodziejska B and Szosland‐Fałtyn A, 2022. Quantitative microbiological risk assessment of traditional food of animal origin produced in short supply chains in Poland. EFSA Journal 2022;20(S2):e200921, 15 pp. 10.2903/j.efsa.2022.e200921
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements This report is funded by EFSA as part of the EU‐FORA programme.
Approved: 31 August 2022
References
- de Niederhäusern S, Camellini S, Sabia C, Iseppi R, Bondi M and Messi P, 2020. Antilisterial activity of bacteriocins produced by lactic bacteria isolated from dairy products. Foods, 9. 10.3390/foods9121757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombas DE, Chen Y, Clavero RS and Scott VN, 2003. Survey of Listeria monocytogenes in ready‐to‐eat foods. Journal of food protection, 66(4), 559–569. 10.4315/0362-028x-66.4.559 [DOI] [PubMed] [Google Scholar]
- Główny Inspektorat Sanitarny . https://www.gov.pl/web/gis/glowny‐inspektorat‐sanitarny (accessed 8 December 2021).
- Jarvis B, Hedges AJ and Corry JEL, 2007. Assessment of measurement uncertainty for quantitative methods of analysis: comparative assessment of the precision (uncertainty) of bacterial colony counts. International Journal of Food Microbiology, 116, 44–51. 10.1016/j.ijfoodmicro.2006.12.037 [DOI] [PubMed] [Google Scholar]
- le Marc Y, Huchet V, Bourgeois CM, Guyonnet JP, Mafart P Thuault D n.d. Modelling the growth kinetics of Listeria as a function of temperature, pH and organic acid concentration. Available online: www.elsevier.com/locate/ijfoodmicro [DOI] [PubMed]
- Lucey, J.A. (2011). Cheese ¦ Acid‐ and Acid/Heat Coagulated Cheese. Encyclopedia of dairy sciences, Vol. 1, Academic Press, London, pp. 350‐356. 10.1016/B978-0-12-374407-4.00083-2 [DOI] [Google Scholar]
- Mellefont LA, McMeekin TA and Ross T, 2008. Effect of relative inoculum concentration on Listeria monocytogenes growth in co‐culture. International Journal of Food Microbiology, 121, 157–168. 10.1016/j.ijfoodmicro.2007.10.010 [DOI] [PubMed] [Google Scholar]
- Østergaard NB, Eklöw A and Dalgaard P, 2014. Modelling the effect of lactic acid bacteria from starter‐ and aroma culture on growth of Listeria monocytogenes in cottage cheese. International Journal of Food Microbiology, 188, 15–25. 10.1016/j.ijfoodmicro.2014.07.012 [DOI] [PubMed] [Google Scholar]
- Pyz‐łukasik R, Gondek M, Winiarczyk D, Michalak K, Paszkiewicz W, Piróg‐Komorowska A, Policht A and Ziomek M, 2021. Occurrence of listeria monocytogenes in artisanal cheeses from poland and its identification by maldi‐tof ms. Pathogens, 10. 10.3390/pathogens10060632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernández Escámez PS, Girones R, Herman L, Koutsoumanis K, Nørrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, ter Kuile B, Threlfall J and Lindqvist R, 2018. Listeria monocytogenes contamination of ready‐to‐eat foods and the risk for human health in the EU. EFSA Journal, 16. 10.2903/j.efsa.2018.5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scientific Opinion on Risk Assessment Terminology , 2012. EFSA Journal, 10(5). 10.2903/j.efsa.2012.2664 [DOI] [Google Scholar]
- Sip A, Wieckowicz M, Olejnik‐Schmidt A and Grajek W, 2012. Anti‐Listeria activity of lactic acid bacteria isolated from golka, a regional cheese produced in Poland. Food Control, 26, 117–124. 10.1016/j.foodcont.2012.01.014 [DOI] [Google Scholar]
- Soroka A and Wojciechowska‐Solis J, 2019. Consumer awareness of the regional food market: the case of eastern European border regions. Foods, 8. 10.3390/foods8100467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoukis PS, Giannakourou MC, Koutsoumanis K Bakalis S n.d. Modelling the Effect of House Hold Chilled Storage Conditions on the Risk Distribution of Meat Products . Available online: http://smas.chemeng.ntua.gr
- Tasara T Stephan R 2006. Cold Stress Tolerance of Listeria monocytogenes: a review of molecular adaptive mechanisms and food safety implications. In Journal of Food Protection 69. Available online: http://meridian.allenpress.com/jfp/article-pdf/69/6/1473/1678286/0362-028x-69_6_1473.pdf [DOI] [PubMed] [Google Scholar]
- The European Union One Health 2019 Zoonoses Report , 2021. EFSA Journal, 19(2). 10.2903/j.efsa.2021.6406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The European Union One Health 2020 Zoonoses Report , 2021. EFSA Journal, 19(12). 10.2903/j.efsa.2021.6971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielicka A and Goryńska‐Goldmann E, 2005. World and Poland Per Capita Cheese Consumption. Journal of Agribusiness and Rural Development, 4(359), 157–166. Retrieved from. https://www1.up.poznan.pl/jard/index.php/jard/article/view/1010 [Google Scholar]
- WORLD AND POLAND PER CAPITA CHEESE CONSUMPTION . n.d. Available online: www.fil-idf.org
- World Health Organization & Food and Agriculture Organization of the United Nations . (2004). Risk assessment of Listeria monocytogenes in ready‐to‐eat foods: interpretative summary. FAO. https://apps.who.int/iris/handle/10665/42874


